[page I]
AN
ELEMENTARY INTRODUCTION
TO
MINERALOGY:
COMPRISING
A NOTICE OF THE CHARACTERS AND MINERALS;
WITH ACCOUNTS OF THE PLACES AND CIRCUMSTANCES IN WHICH THEY ARE FOUND.
BY WILLIAM PHILLIPS,
F.L.S. M.G.S.L. & C.
HON. MEMBER OF THE CAMBRIDGE AND YORKSHIRE PHILOSOPHICAL SOCIETIES.
FOURTH EDITION, CONSIDERABLY AUGMENTED,
BY ROBERT ALLAN,
F.R.S.E. M.G.S.L.&c.
LONDON:
LONGMAN, REES, ORME, BROWN, GREEN, & LONGMAN;
J. G. & F. RIVINGTON, WHITTAKER, & CO.; TEGG & SONS;
SIMPKIN, MARSHALL, & CO.; AND C. TILT.
M.DCCC.XXXVII.
[page II]
HAPVARD UNIVERSITY LIBRARY FEB 19 1968
EDINBURGH:
Printed by THOMAS ALLAN & Co.
265 High Street.
[page III]
TO
JOHN GEORGE CHILDREN, Esq.
ONE OF THE LIBRARIANS TO THE BRITISH MUSEUM,
SECRETARY TO THE ROYAL SOCIETY OF LONDON,
&C. &C. &C.
TO WHOSE SCIENTIFIC LABOURS THE LATE WILLIAM PHILLIPS
ACKNOWLEDGED HIMSELF INDEBTED FOR MANY
IMPORTANT OBSERVATIONS,
THIS FOURTH EDITION
OF THAT AUTHOR'S WORK
IS INSCRIBED
BY
HIS OBLIGED AND FAITHFUL FRIEND,
THE EDITOR.
[page IV]
[page V]
ADVERTISEMENT BY THE EDITOR.
IT is with extreme diffidence that I have undertaken a new edition of the Elementary Treatise on Mineralogy by the late WILLIAM PHILLIPS, being well aware of the difficulty of following up the ideas of any individual upon a subject of this nature, and at the same time conscious of my incapacity to do his work that justice which it merits. I have, however, endeavoured as far as possible to place myself in the position which the author might be expected to have assumed, had he been in existence to re-edit his valuable treatise at the present period. The nature of the subject does not admit of additions in the form of notes, and recourse has consequently been had to what may be considered unjustifiable freedoms with the late author's text. During the fourteen years which have intervened since Mr PHILLIPS'S last edition, the science of Mineralogy has undergone very great improvements; and although no treatise which has yet appeared on the subject has afforded more satisfaction, particularly in this country, than that author's, there have been several hundred new species described, and so many additional properties discovered in the old ones, as to render another edition of the work essentially necessary. These new
[page] VI
species I have endeavoured to bring into their proper places, and, by curtailing the descriptions of mere varieties, have succeeded, although with the addition of one hundred and fifty minerals and about sixty figures, in not greatly increasing the size of the volume. With the introductory portion of the book the same liberties have not been taken as with the descriptive, excepting only the chemical part; for the improved appearance of which, I am indebted to the able pen of a recently lamented and highly talented friend, whose name must long stand preeminent among British chemists. In the descriptive part of the treatise considerable extracts have been made from my own Manual of Mineralogy (Edinburgh, 1833), which I trust will not be unacceptable; and for the most recent discoveries in the science, I have drawn from the works of NECKER, BEUDANT, and ROSE, as well as from the various scientific journals.
In conclusion, I have only to express a wish that circumstances had of late afforded me better opportunities of doing greater justice to the work of Mr PHILLIPS. It is sufficiently difficult to explain one's own ideas on such a subject with perspicuity; but it must be allowed to be no easy task to engraft them on the work of another. If, however, I have succeeded in accommodating this original British Treatise to the rapidly advancing state of mineralogical science, and retaining the name of "PHILLIPS" in its former prominent position, I shall be fully repaid for the difficulties of my labour as an Editor.
ROBERT ALLAN.
Edinburgh, March 1837.
[page VII]
PREFACE TO THE THIRD EDITION.
THE present differs from the preceding edition in some respects which appear to require notice in this place.
The most important additions and improvements that have been made, consist, first, in the introduction of notices or descriptions of about eighty minerals, of which the greater part have been discovered since the publication of the preceding edition; secondly, in the insertion of the results obtained by a careful examination of most crystalline minerals, as regards their structure and cleavage; thirdly, in the addition of a figure to the verbal description of most substances found in a crystallized state, representing the primary form, and another the secondary planes in connection with those of the primary crystal, together with such measurements of the planes as I have been able to obtain, chiefly by means of the reflective goniometer of Dr Wollaston; in the fourth place, advantage has been taken of a translation of Berzelius's excellent work on "The Use of the Blowpipe in Chemical Analysis and the Examination of Minerals, by J. G. Children, F.R.S.L. & E. &c." in so far as relates to the more simple experiments with that useful assistant to the student in recognizing minerals; and, fifthly, the meanings of the names by which minerals are commonly known in this country are mostly given at the foot of the page containing the description, except where, being chemical, they manifestly have been derived from the composition of the substance.
In regard to arrangement, no alteration has been made in this edition, except where new and more satisfactory analyses demanded a change: on the subject of the arrangement, therefore, it seems requisite only to add, that, having in the first instance
[page] VIII
adopted it as being in my own estimation the most advantageous to the student that I could devise, the experience of its utility now induces me to recommend it to him as an instructive method of placing the minerals in his cabinet.
In pursuing the at once pleasant and laborious investigations connected with the important characters of cleavage, crystalline form, and measurement, and which were undertaken with the view of rendering the present edition more instructive to the student, it will be imagined that I have myself derived much information; and, although some new facts relative to these points have resulted, it must be acknowledged that much yet remains for future investigation.
If the more accomplished mineralogist should condescend to consult this little work, he will perceive that the measurements of the crystalline forms, and especially of the secondary planes, are not precisely exact, do not on all occasions relatively agree; for in no instance has it been attempted to correct the geometry of nature by a resort to the more rigid laws of calculation. It has been ascertained, by a comparison of the measurements taken from similar and brilliant planes of different crystals, that, owing to some natural inequality of surface, the same precise angle is rarely obtained, and hence those given in the succeeding pages cannot be expected to be absolutely exact. Experience, however, leads to the conclusion that the limit of error is considerably within one degree,—that it rarely exceeds 40 minutes, and that it is frequently confined to a minute or two. The measurements annexed to the figures will therefore be considered only as near approximations to the true value; but where those of the primary form have been obtained from planes produced by cleavage, which is generally noted, when that is the case, in the description of the mineral, they may be considered as approximating the truth much more nearly than when taken by means of the natural planes. A considerable proportion of the whole will perhaps be found sufficiently precise to form a basis for the calculations of the mathematician, and, together with the accompanying figures, to induce the student to examine the forms of crystals, and to delineate and measure the angles formed by the meeting of the planes by which the crystals in his own cabinet are bounded. If errors should be found in the following pages, greater than those above alluded to, they are to be attributed to my own want of exactness in noting the measurements obtained; for, although much care has on all occasions been taken to select the smallest and most brilliant crystals, and to note the results faithfully, it is scarcely to be hoped that errors of this nature have altogether been avoided.
It may perhaps be concluded, that by adopting at once the
[page] IX
figures and measurements given by Haüy, and other mineralogists, much chance of inaccuracy might have been prevented. But it must be observed, that where the primary form is not a regular geometrical solid, such as are the cube, regular octahedron, and six-sided prism, the means resorted to for determining the true measurements—namely, that of subjecting the planes obtained by cleavage to the reflective goniometer—is a more certain method than that adopted by Haüy; and it will be perceived that a very large proportion of all the primary forms are not regular geometrical solids, such, for instance, as the oblique and doubly oblique prisms, and the very numerous class of rhombic prisms. Where the cube, regular octahedron, six-sided prism, and other regular solids, are the primary forms, I have adopted the measurements given by Haüy, acknowledging them in all cases by annexing the letter H, or by some other mode; first, however, verifying them in most cases by the reflective goniometer. In a very few instances, the authority of the Comte de Bournon has been resorted to, but not without acknowledgment. It will of course be understood that where no authority is mentioned the measurements have been obtained by the reflective goniometer, and, from what is said above, that they must be considered only as approximations.*
In regard to the figures to which the measurements are annexed, it may be observed, that these are not in all cases the representatives of single crystals, for in some of them are associated the planes observed on two or three; thus occasionally rendering the form more complicated than any single crystal I have seen, but not more so than may probably be found hereafter. This mode has been adopted, as offering to the student the greatest assistance that I could devise, since it combines at one view all the observed planes, without increasing enormously the bulk and consequent expense of the work, as must have been the case if all the varieties of form had been given separately. As to the drawing of the figures, it remains to be added, that they are not given as the result of a laborious execution by the assistance of the rules of geometrical projection; but, in the general, only as diagrams, wanting the precision which in that case might have been claimed for them, and drawn without any other rule than such as the hand and eye could furnish.
The letters on each plane of the larger figures have been so
* In some, though comparatively few instances, the crystals of a substance have not been found sufficiently bright for the use of the reflective goniometer; the common goniometer has then been resorted to, and the measurements taken by it are always distinguished by having the letters c. g. annexed to them.
[page] X
placed, according to the system of notation adopted in the "Familiar Introduction to Crystallography, &c. by H. J. Brooke, F. R. S. &c." a work which may, without hesitation, be recommended strongly to the student, as being calculated to teach the interesting science on which it treats in its most pleasing form, and of which the first part is so simple that, without any reference to trigonometry, geometry, or algebraical calculation, it shows, by means of the figures of crystals and attendant explanations, the position on the primary forms of every secondary or modifying plane to which those forms are liable, and thereby the transitions of one form into-another; and here, if he be so inclined, the student may stop, after having gained all that a purely mechanical view of the subject will afford him, or he may proceed to the second part, in which crystallography is treated more scientifically; and it may be added, that the pupil may, in either case, attain such a knowledge of it, as will not fail to open to him new sources of delight in this interesting department of Mineralogy.
To the author of the fore-mentioned work I am under much obligation, for assistance on various points connected with the improvements which it is confidently hoped will be manifest in the present Edition. Often as his name occurs in its pages, I have been yet more often indebted to him, not only for the loan of specimens, amongst which were several that I could not otherwise have obtained, but for assistance in the clearing up of many difficulties which, without his help, would have been left in doubt, or would have terminated in error.
My acknowledgments and thanks are also thus publicly due to several others of my friends. To Thomas Allan, F.R.S.L. & E. for many useful criticisms, of which I have not failed to avail myself, as well as for the liberal transmission from Edinburgh of some rare and valuable minerals. To Ashhurst Majendie, M.G.S. for the loan of well-defined crystals of several scarce substances. To Samuel Luck Kent, M. G. S. for a free access to his cabinet upon all occasions, and for his cheerful permission to avail myself of the advantage in any manner that might tend to benefit the work, and even for the presentation to me of some rare substances. To Henry Heuland, For. Sec. G. S. for some valuable minerals presented to me in a manner consistent with his well-known liberality; a liberality which also I have experienced in numerous instances from G. B. Sowerby, F. L. S. to whom likewise I am greatly indebted for many valuable hints, and for the readiness with which he has upon all occasions endeavoured to promote my views.
In conclusion, if the utility of a nice investigation of the structure of crystallized minerals, and the measurement of their angles, should become an inquiry, it may be replied that they
[page XI]
often determine the differences existing between minerals which greatly resemble each other. This, as is observed more at large in the following 'Introduction,' is fully exemplified in the differences discovered by means of the reflective goniometer, between the measurements of the primary rhomboids of carbonate of lime, carbonate of lime and magnesia, and carbonate of iron; minerals which often so greatly resemble each other, that the difference between them can only be ascertained by a resort to chemistry, or the reflective goniometer. The utility of a close attention to this instrument has been further manifested since the foregoing was written, and in a very remarkable manner:—A mineral which has always been considered as bitterspar from the Tyrol, and of which the primary crystal is a rhomboid not distinguishable by the unassisted eye from that of either of the foregoing, was found by the reflective goniometer to afford measurements differing from them all; the cause of this became manifest by a resort to analysis, which proved it to be a new compound, namely, a carbonate of magnesia and iron. The reflective goniometer is moreover of great use to the geologist, who finds those rocks which are termed primitive, and many of those which are called transition, or the oldest secondary, to consist, not of one homogeneous mass, as is often the case with those of a newer origin, but of two or more minerals, so intermixed and associated that a reference to the chemist is of little avail to him: by such means he may indeed become informed whether a particular earth or alkali is to be found in the mass, but the various substances of which it is compounded are often too minute, and therefore too intimately associated with the others, to allow of a determination as to which of the component substances may contain the earth or the alkali so discovered. Hence structure, if it exist, becomes a character of essential importance; for it will be found that fragments far too minute for analysis will often afford brilliant planes, well adapted to the use of the reflective goniometer. A knowledge of Structure, therefore, and of the measurements of the primary forms of minerals, is very important to the geologist; but where structure does not exist, the examination of the various external characters of the minute portions forming the aggregate of the rock are often of singular advantage; and hence the geologist should become intimately acquainted with the external characters of at least all such substances as are found entering into the composition of rocks. Mineralogy, therefore, is in reality essential to the geologist; it is the very alphabet to the older rocks, and it is probably to be attributed in great measure to the want of due preparation for the study of these rocks, by an intimate acquaintance with minerals in the simple state, that the primary
[page] XII
and transition tracts of England and Wales have been investigated in a far less degree than those of a newer origin.
"It has been said of crystals," says the Abbé Haüy, "that they are the flowers of minerals; an observation concealing a very just idea beneath the air of a comparison which appears to be only ingenious." The importance of "form will become more evident," he further observes, "if, in pursuing our inquiries into the niceties of the mechanism of structure, we conceive all these crystals as the assemblages of integrant molecules perfectly resembling each other, and subject to the laws of regular arrangement. Thus, although by a superficial notice of crystals we might adjudge them to be only the sports of nature, a more intimate acquaintance with them leads to this conclusion,—that the Deity, whose power and wisdom prescribed the unerring laws of the planetary motions, has also established those which are obeyed with the same fidelity, by the molecules composing the various substances concealed in the recesses of the Earth."
May 10, 1823.
W. P.
[page XIII]
INTRODUCTION.
THE investigation of the structure of the earth belongs to the science of Geology. It may however be interesting to take a rapid survey of the present state of our knowledge respecting it, were it only for the sake of showing its intimate connection with mineralogical pursuits.
In speaking of the earth and of our knowledge of its nature, it is essential that the limited extent of that knowledge should always be had in remembrance. We are acquainted with it only to a very inconsiderable depth; and when it is recollected that, in proportion to the bulk of the earth, its highest mountains are to be considered merely as unimportant inequalities of its surface, and that our acquaintance does not extend in depth more than one fourth of the elevation of these mountains above its general level, we shall surely estimate our knowledge of the earth to be extremely superficial; that it extends merely to its crust.
The term 'Crust of the Earth,' therefore, relates only to the comparative extent of our knowledge beneath its surface. It is not used with the intention of conveying an opinion that the earth consists only of a crust, or that its centre is hollow; for of this we know nothing. The term may not be philosophical, but it is convenient.
The nature of the crust of the earth is most readily studied in mountains, because their masses are obvious; and also because, as they are the chief depositories of metalliferous ores, the operations of the miner tend greatly to facilitate their study. Mountains are composed of masses which have no particular or discernible shape; or, as is more commonly the case, of strata or beds, either horizontal or oblique, sometimes nearly vertical.
In these masses and beds different structures have been observed. Some of them are crystalline; that is to say, are composed
[page] XIV
of crystals deposited in a confused manner, as in granite; or of crystals imbedded in some other substance, as in porphyry. These crystalline rocks contain no organic remains; and, as they are always found beneath, never above, those which do contain them, they are considered to have been of earlier formation, and therefore have been termed Primitive rocks.
Other mountain rocks have no appearance of crystallization; but, on the contrary, seem rather to have been formed by the mere falling down, or settlement, of the substances of which they are composed, from the solution which contained them. These are always found above, never beneath, the crystalline rocks; and often include abundance of organic remains, both animal and vegetable. The more ancient of these, or such as contain the remains of animals of which the genera and species are extinct, are called Transition rocks; the more recent, or such as contain the remains of animals most nearly resembling those now inhabiting our oceans, are called Flœtz or Flat rocks, because their position is considerably or perfectly horizontal; the former have received the name of transition, as connecting the primitive with the flœtz rocks. By most geologists the transition and the flœtz are classed together under the name of Secondary rocks.
Primitive and secondary rocks have suffered considerable change and ruin from causes which it is not our present object to notice; and their disintegrated portions, having been formed anew, now constitute that peculiar description of deposit which is termed alluvial, or diluvial, and which therefore consists of the debris of rocks. Such are some clays, gravel, sand, &c. and these often contain the remains of land and amphibious animals, and of fish; they are found above the preceding, or sometimes resting immediately upon primitive rocks.
But there is still another and a very different kind of rock, abundantly found in certain countries, which may in great measure be considered, like the preceding, as resulting from the ruin of rocks, but from an opposite cause, or by an agent directly the reverse, viz. by fire; constituting those known by the name of Volcanic rocks. Many of these strongly bear the marks of heat, and even of fusion; some, on the contrary, offer no evidence of their having been subjected to heat.
Lofty mountains composed of primitive rocks usually present rugged and uneven summits, and steep acclivities on the sides, as though they had suffered by convulsion. Such as are wholly or externally composed of secondary beds or strata are less rugged, their summits are flattish or somewhat rounded, and their sides present acclivities more easily accessible.
Both primitive and secondary mountains, more particularly the former, are traversed in various directions by fissures of
[page] XV
different dimensions. These fissures are not often empty, but are partially, and sometimes, though but rarely, filled with stony or metalliferous substances. They are termed Mineral Veins; and from them a large proportion of the specimens composing the cabinet of the mineralogist are obtained; indeed almost all such as, from their rarity, brilliancy, or peculiarity of form and combination, possess the greatest attraction for the mere collector.
Mineralogy is a science of such interest, that it would be much to be regretted if its real objects and tendency were misunderstood, or suffered to degenerate into an avidity merely for the collecting of what is brilliant or rare. To the attainment of the science of geology, which is intimately connected with agriculture and the arts of life, that of mineralogy is essentially requisite. The study of mineralogy therefore does not include only a knowledge of the more rare and curious minerals: there is nothing in the mineral kingdom too elevated or too low for the attention of the mineralogist, from the substances composing the summits of the loftiest mountains, to the sand or gravel on which he treads. It is true that the aggregated masses of compound rocks are not arranged in a mineralogical collection; but it must be remembered that each of the substances of which such aggregated masses are constituted, are comprehended in a mineralogical arrangement, and therefore find their places in the cabinet. Granite, indeed, is not to be found there; but its components, quartz, felspar, and mica, are met with in every one.
Thus, then, by the study of what, in opposition to the terra aggregated rocks, may be termed simple minerals, the mineralogist becomes enabled to detect the substance with which he holds acquaintance by itself, when aggregated with others in a mass; and thus he becomes qualified for the more difficult and more important study of the science of geology, which embraces a knowledge of the nature and respective positions of the masses and beds composing mountains, and indeed of country of every description, whether mountainous or otherwise.
It is not, therefore, or at least it ought not to be, the sole object of the mineralogist to be able to distinguish the several genera and species of mineral substances; nor should his attention be confined to the mere task of recognising a mineral at first sight, or of being capable of at once assigning it a proper place in his cabinet. He should hold a more enlarged acquaintance with minerals, and with the circumstances attending them, in what may be termed their native places; he should know something of the positions they respectively bear towards each other in those places; he should become acquainted with their relative ages, deduced from the nature of the rocks in which they are found; their comparative scarcity or abundance; their com-
[page] XVI
binations; the countries in which they occur; and their characters, both internal and external.
This knowledge, it may be repeated, is the first and requisite step in the science of geology; not that it is essential to this science that every mineral should be accurately known: some are of comparatively little importance in a geological point of view, from their extreme scarcity; but it is essential to become acquainted with simple minerals in the general, because of some of them, many of the vast masses of the earth are composed.
Minerals which are found only in primitive rocks, are said to belong to primitive countries; by which name are designated such tracts as are chiefly composed of primitive rocks. The substance in or on which a mineral is found, is called its gangue or matrix; when in its natural place or position, a mineral is said to be in situ; when this place and position are known, we are acquainted with its habitat.
OF THE CHARACTERS OF MINERALS.
§ 1. It is one of the first, if not the first inquiry of those who are uninstructed in mineralogy, if a specimen, of quartz for instance, be shown them, how they may recognise it. The reply necessarily is, that it is essential to observe it closely, to study it, to mark with precision its characters;—that as minerals are not organized bodies, their characters are less defined, and therefore not so readily intelligible, as those of such bodies as possess regular organization;—that, in fact, there is no treatise, by a reference to which, the beginner is enabled, if he take up a mineral, to arrive at once at a knowledge of its nature;*— that therefore at present practical observation is the only mean of attaining this knowledge. It will be of advantage, then, that these characters, and the mode of observing them, should be pointed out.
§ 2. Although long experience and attention give a facility in recognising minerals by mere inspection, this facility can only be acquired by such means. There are certain minerals which may at once be detected by some simple experiment; that is to say, there exist a few possessing some one character which decides with precision what the mineral must necessarily be, because that character belongs to no other. For instance, there are three substances which often so nearly resemble each other, that simple inspection indicates no difference, even when re-
* The method proposed by Professor Mohs is perhaps the most systematic, and approaches nearer to this desirable point than any other,—more especially as regards crystalline minerals. E.
[page] XVII
duced by cleavage to the primary form. All may be cleaved into obtuse rhomboids, differing From each other in measurement. If the planes of one of them meet at the angles of 105° 5′ and 74° 55′, it is carbonate of lime; if the second measures 106° 15′ and 73° 45′, it is bitterspar; the third, measuring 107° and 73°, is carbonate of iron. But comparatively few substances can be known by so simple a process; some cannot be cleaved with regularity; we must then resort to other characters; and it is frequently only by a comparison of several of these that the desired object is attained. It is therefore essential that the characters belonging to each should be faithfully detailed in describing it, since there is no book to which the beginner can resort, that will enable him to distinguish the generality of minerals with facility.
§ 3. The characters belonging to most simple minerals may be said to be numerous. If its parts cohere, it possesses some degree of hardness; by trying its hardness, we may discover the ease with which it breaks, or its frangibility; and we may or may not perceive that it possesses a regular structure; if the structure be regular, we discover the forms into which it may be divided, and amongst, them, that from which all the rest are derived, or, its primary crystal. These regular forms may be termed the geometrical characters of the substance; although, along with numerous others, they are commonly included under the term of physical or external characters.
§ 4. These characters are extremely important; but with whatever precision they are given, we should still be far from a competent knowledge of the real nature of the substance, without the aid of the chemist. Hence the characters of minerals may be said to be of two kinds; Physical or external, and Chemical.
1. OF THE PHYSICAL CHARACTERS.
§ 5. These characters are numerous, and require to be well defined, in order that the same language may always convey the same definite idea: there exist, however, and often in the same substance, such very nice shades of difference in certain of them, that much at last is necessarily left to experience. The learner will find that, after a laborious endeavour to discover by written description what a mineral is, it will be much more easy to discover what it is not; and at all times he will reap an infinitely greater and more speedy advantage from personal instruction than from books. Such, however, as can resort only to the latter, will find that an attentive observation of the physical characters, and a comparison of them in different minerals, will forward the acquisition of knowledge.
[page] XVIII
These characters are comprehended in the following list.
| External form. | Double refraction. |
| Structure. | Touch, Taste, and Odour. |
| Fracture. | Streak. |
| Frangibility. | Powder. |
| Hardness. | Adhesion to the tongue. |
| Transparency. | Magnetism. |
| Lustre. | Electricity. |
| Colour. | Phosphorescence. |
| Flexibility. | Specific gravity. |
External Form.
§ 6. Only a small proportion of the specimens admitted into our collections can be said to possess precise external forms, since they mostly exhibit on one side or the other, and are sometimes entirely bounded by, surfaces produced by fracture; there are comparatively few minerals which are found in masses absolutely isolated.
§ 7. Nevertheless there are many minerals to which particular external forms belong; some few are found in single or separate crystals, and the surfaces of others are coated by them.
A crystal may be defined as a more or less symmetrical, geometrical solid, commonly bounded by plane surfaces, which in mineralogical language are termed planes or faces.
An edge is formed by the meeting of two planes.
A solid angle is a point formed by the meeting of three or more planes.
A prism is rarely found having only three sides, very commonly four, six, eight, or more sides; the sides, or lateral planes, surround its axis, which is an imaginary line passing down the middle of the prism, from the centre of the upper terminal plane to the centre of the lower; the terminal planes are also called the bases. But prisms are found both very long and very short; when long, and the crystals slender and curved, they are termed capillary; when straight, acicular; when the prism is short, the crystal is said to be tabular.
A pyramid is formed by the meeting of three or more planes at a point, which is termed the apex, each plane being bounded by edges; considered separately, a pyramid is supposed to have a base, which is the case in regard to the tetrahedron; but in respect of most other forms, it is only imaginary, as in the instance of the octahedron, which often is termed a double four-sided pyramid; and also the dodecahedron with triangular faces, which is frequently denominated a double six-sided pyramid.
[page] XIX
The prism and the pyramid are however often combined in the same crystal; which in that case is generally described as a prism of four, six, or eight sides, terminated by a pyramid of four or more sides.
§ 8. We have spoken of the edges and solid angles of crystals; these however are sometimes wanting; for instead of the edge or the solid angle, we find a plane; the edge or solid angle is then said to be replaced or truncated. These are, however, merely terms of convenience; neither the edges nor angles ever were on the crystal, and therefore could not have been replaced, truncated, or taken off. But when it is said that the lateral or terminal edges are replaced, or when a solid angle or an apex is described as being truncated, it is meant only by a single plane, unless expressed to the contrary.*
When the edges of a crystal are replaced by two planes, separated only by an edge, they are said to be bevelled.
§ 9. These truncations and bevelments are sometimes so slight as not to alter the general form of the crystal; but are often sufficiently deep to give it a perfectly different figure. Thus the octahedron passes into the cube, the cube into the octahedron, and the latter into the rhombic dodecahedron, as will presently be shown.
§ 10. These passages of one form into another,—and in many more respects than are above recited,—are constantly found to occur in certain mineral substances. This is not ideal. For not only may a series of crystals be observed which exhibit these transitions, but they may also be proved by evidence of the most convincing kind, arising out of an examination of these crystals by a method more decisive than that which depends on the accuracy of the eye. The fact here alluded to is, that the crystals of many substances may, by the application of force, be mechanically divided or cleaved, in the direction of the laminœ (along their natural joints), so as to reduce the one form into the other; but the consideration of this fact belongs properly to that division of the subject which may be denominated structure.
* We must however make certain exceptions to some of the preceding remarks in regard to prismatic crystals. Every crystal which has lateral and terminal planes is not considered as being prismatic; as, for instance, the cube, which is a solid of perfect proportion, all its sides being equal; so with the rhombic dodecahedron, which also appears in some points of view as having lateral faces; but as these solids are perfectly symmetrical, their apparently lateral and terminal faces are never so distinguished, and, when their edges are replaced, the fact is merely stated without distinction; indeed it commonly happens in these perfectly geometrical solids, and in others, as the regular octahedron and the tetrahedron, that when one edge is replaced, the others are so also.
[page] XX
Structure.
§ 11. Structure, when it exists in a mineral substance, arises from the particular arrangement of the minute portions or molecules of which it is composed.
§ 12. In some minerals this arrangement exists both in the regular crystals in which these occur, and in those masses which have no particular external form.
§ 13. Of the forms of those minute and imperceptible molecules which are aggregated by the law of attraction into masses, nothing is known with certainty. Conjecture has in some instances been allowed to supply the deficiency. The consequences of their arrangement, however, are very perceptible, and may be satisfactorily proved in some instances, by the rudest attempt; a slight blow, or letting fall a specimen of certain minerals on the floor or the pavement, will suffice to produce instantaneous conviction that this arrangement does exist; for by such means fragments of perfectly regular form may be obtained, and from the faces of these fragments may again be procured thin slices, of which the larger planes are perfectly parallel, and these slices may again be subdivided into regular forms, until the fragments are no longer perceptible without the aid of the microscope.
§ 14. Structure, then, it may be repeated, arises from the particular arrangement of the minute portions or molecules of which the mineral is composed.
If a mineral can be mechanically divided or cleaved in directions which produce only one particular form, that form is denominated its primary or primitive crystal. For instance, calcareous spar can only be cleaved into the form of an obtuse rhomboid of particular measurements, which therefore is termed its primary crystal; a rhomboid has six planes, which are parallel two and two. Calcareous spar therefore has three cleavages; it possesses natural joints in three directions: so has common salt, of which the primary form is the cube.
But some minerals are not so circumstanced. Fluor spar, which may be cited as yielding with ease to mechanical division, is an instance. It cleaves in four directions, and affords three different forms, a regular octahedron, a regular tetrahedron, and an acute rhomboid; of these, the first has arbitrarily been selected as the primary crystal, and convenience may be assigned as the reason for the preference.
Other substances are cleavable in a still greater number of directions; for instance, blende, from which may be extracted a rhombic dodecahedron, and from this an obtuse rhomboid, an octahedron, an acute rhomboid, and an irregular tetrahedron;
[page] XXI
in this mineral also, the choice of a primary crystal has been arbitrary, the rhombic dodecahedron having been selected.
Further instances might be cited, but these will suffice; they are particularly quoted because of the remarkable ease with which the learner may satisfy himself of the facts.
§ 15. Many minerals yield to cleavage with ease only in one direction, of which topaz is an instance. The structure of such is described as being perfectly crystalline or lamellar in one direction. Sapphire yields to cleavage in one direction with much ease; in the others with extreme difficulty.
§ 16. The arbitrary selections just noticed will suffice to induce the suspicion, that in this department Mineralogy has not yet attained perfection; and also to lead the pupil to investigate, as he advances in the science, rather than take for granted what is asserted without proving the facts.
§ 17. Other circumstances also exist sufficient to make us extremely cautious on this point.
Some minerals, to which primary forms have been assigned, do not yield, or have not yet been found to yield, to regular cleavage in more than one direction, or even not in any direction. ID these determinations one of two modes has been resorted to. In the first, thin fragments of the substance have been held up between the eye and the light; and by this means the Abbé Haüy was enabled in several instances to deduce the probable form of the primary, from the directions of the crevices, or appearances of natural joints, which are observable in the fragment; and, in many, these have afterwards proved to be correct. By the other mode, the primary form is determined by analogy, that is, by a comparison of the forms of the crystals of a mineral with those of other known substances; but this may in some cases prove a source of error.
§ 18. Cleavage can be accomplished in various ways, dependent on the nature of the substance. In some, as in blende, it is best effected by a sharp knife, when the mineral is held between the fingers, because of its numerous natural joints, which a blow might disturb in the wrong direction. In sulphate of strontian, it is done by the same means for another reason; namely, because it is easily cracked in directions contrary to the natural joints, even by a slight blow. Fluor is best cleaved by putting it on a table and placing the edge of a knife along the natural joints; a slight blow then separates them. The oxide of tin yields only to the pressure of the cutting pincers, when held in proper directions.*
* For farther practical hints on this subject, vide Mr Phillips' Communication to the Geological Society On the Primitive Crystals of certain Substances, and on the Modes of cleaving them, inserted in vol. iv. of its Transactions. E.
[page] XXII
§ 19. By one or other of the preceding methods, however, most minerals have had assigned to them some one solid, as the primary form of the several varieties of crystals in which they are found.
§ 20. The whole number of primary forms are comprehended in the regular tetrahedron, the cube, the rhombic dodecahedron, the octahedron, the six-sided prism, and the parallelopiped.
The first four may be termed regular geometrical solids, all the planes of each being equal and similar. To those octahedrons in which the two pyramids composing them are higher or lower than those of the regular octahedron, the term four-sided prism is applied; in these, the sides of the planes are not equal and similar. Six-sided prisms, as primary crystals, are of various lengths. The term parallelopiped includes all those solids whose bounding planes are parallel two and two; as, for instance, all the varieties of the rhomboid, both acute and obtuse; and all the prisms, both right and oblique,* of which the terminal planes are rhombic; and all the square and rectangular† prisms which do not possess the precise proportions of the cube.
§ 21. Whoever undertakes, for the first time, the examination of the crystalline forms of a mineral, will find, if they be numerous, that many of them seem to possess no mutual relation.
§ 22. He will, however, eventually discover that a substance, from whatever country it may be brought, always assumes crystals, which, if they yield readily to mechanical division, will always afford by it the same nucleus or primary form.
§ 23. Hence we have a right to conclude that the form of the molecules constituting these crystals must invariably resemble each other in the same substance, and that their arrangement must be invariable in regard to each other.
§ 24. How comes it, then, will be the inquiry, that so great a diversity of external forms should be produced by an invariable
* Being aware that the most accurate verbal description of crystalline forms conveys to the mind in most instances only a very imperfect idea of them, it would have seemed requisite here to give those which are above mentioned as the primitive or primary forms, if the figures annexed to the following descriptions of minerals were not calculated to speak intelligibly to the eye. Amongst them, however, there is one which demands an observation. The term oblique prism is usually and correctly employed to designate one in which, supposing the lateral planes to be held perpendicularly, the terminal planes are not at right angles to them, but are placed obliquely—at a greater or less angle than 90 degrees.
† A square prism is necessarily rectangular; but there are prisms of which the planes are at right angles one with another that are not square, a term which implies that all the planes are of equal width; when not of equal width, these prisms are simply termed rectangular.
[page] XXIII
internal arrangement? A satisfactory answer to this question cannot perhaps be given. We only know the fact; and are compelled in general terms to suppose it to be the consequence of affinity, or attraction, or polarity; of laws to which matter is subject. We must not, however, fail to notice the curious and important fact, that the crystals of a mineral, from what part of the world soever it may be brought, and however unlike each other at first sight in external form, are always found to possess such a mutual relation as will enable the observer to trace them to the same primary form.
§ 25. A few of the many minerals which may be cleaved with regularity have already been noticed (§ 14), and we have pointed out the manner in which the crystals of certain substances may be reduced to their primary forms (§ 18). Let us now attend to the manner in which the primary forms of certain minerals may be supposed to have increased, so as to assume external forms which appear to have little or no affinity with the primary.
§ 26. In examining a cubical crystal of fluor, we find that all its solid angles may readily be taken off by means of a knife; and that by thus displacing each angle, we produce eight triangular planes, which are smooth and brilliant; we moreover find that it cannot be cleaved, so as to produce a brilliant plane in any other direction.
Fig. 1.
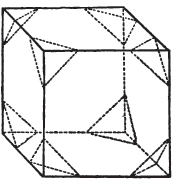
Fig. 2.
§ 27. Then let the lines of fig. 1 represent a cube, and the dotted lines, the triangular planes produced by cleavage.
§ 28. The cube having its solid angles displaced, is also represented in fig. 2. If however we pursue the cleavage by which we produced the triangular planes, that is, if layers or laminæ parallel with those planes are removed, we reduce the volume of the crystal by degrees, and finally change its form; for we ultimately find that the eight triangular planes of the cube become the eight triangular planes of the octahedron within it. Let us not fail to observe that the termination of each solid angle of the octahedron forms, as it were, a point in the centre of each plane of the cube.
[page] XXIV
§ 29. If we still go on, and remove from each plane of the octahedron other laminæ, we thereby reduce its size, but do not alter its form; hence the octahedron is considered to be the primary form of fluor.
§ 30. Every one of the laminæ taken off in this process may be again subdivided; it may be broken into octahedrons, tetrahedrons, and acute rhomboids (§ 14).
§ 31. Assuming, then, the octahedron to be the primary form of fluor (§ 14), and knowing that all its laminæ may be divided into regular forms, is it not reasonable to conclude that the whole cube upon which we first began to operate, is composed of minute solids of a definitive form, whatever that form may be; and since the cleavage is attainable only in the directions specified (§ 26), is there not reason for concluding that they must be arranged with perfect regularity?
§ 32. Hence the cube (which is therefore one of the secondary forms of fluor) appears to be the consequence of a regular arrangement, on the planes of the primary octahedron, of extremely minute solids, resembling each other in respect of form. We may assume this without pretending to decide the precise form of those molecules or integrant particles.
§ 33. When, therefore, we describe a crystal of fluor, as being a cube of which the solid angles are (naturally) replaced by triangular planes, we do not describe it either truly or philosophically; but we thus describe it from motives of convenience; we might more aptly term it a cubo-octahedron, because the triangular planes belong manifestly, from what has preceded, to the octahedron, and the larger planes to the cube (§ 28). But there are few crystals to which terms so convenient could be applied.
§ 34. Let us take one more example, in which the regular octahedron is the primary; this will also apply to fluor.
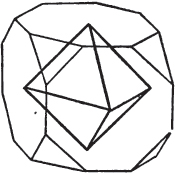
Fig. 1.
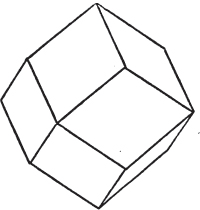
Fig. 2.
§ 35. Let fig. 1 represent a crystal of fluor in the form of the rhombic dodecahedron.
The same form is visible in fig. 2, and within it an octahedron; the lines of the latter being somewhat the darkest.
[page] XXV
§ 36. Now, by the latter figure, we perceive that the rhombic dodecahedron is the consequence of an accession of crystalline laminæ composed of molecules placed in regular succession on every plane of the primary; the laminæ regularly diminishing in size until they arrive at a point, and producing on every plane of the octahedron a low three-sided pyramid.
§ 37. On one plane of the octahedron in fig. 2, the laminæ, progressively diminishing and terminating in a point, are shown by lines, and these lines or striæ are often visible in the rhombic dodecahedron, when the primary is an octahedron. Whenever striæ are seen on the planes of a crystal, they generally denote that it may be cleaved along them. These may be observed in dodecahedrons of fluor and red oxide of copper, of which the primary is the regular octahedron; and if the substance does not yield to cleavage, they sometimes serve as a clue to the determination of the primary form.
But it may be asked, how does it happen, that if these laminæ progressively diminish, forming, as represented in fig. 2, a sort of step from one to the next, that the planes have sometimes a perfectly brilliant polish, without any of the roughness which in such a case might be expected. The answer is simple. The molecules composing the crystal may be termed almost infinitely small, since no limit has been found to mechanical division.
§ 38. Hitherto the octahedron has been assumed as the primary; let us now take the cube, and suppose the octahedron and rhombic dodecahedron to be its secondary crystals, as they are in several minerals. Afterwards the pentagonal dodecahedron will be considered as arising from the same primary form.
Fig. 1.
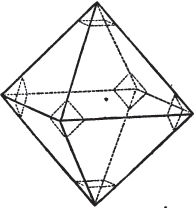
Fig. 2.
§ 39. Let the lines of fig. 1 be a regular octahedron, and let the square formed by dotted lines represent the planes which would be produced by replacing the solid angles.
[page] XXVI
§ 40. In fig. 2, the octahedron is represented as having its solid angles replaced, and a cube within it. On considering the relations of these two figures, it is manifest, that by pursuing the cleavage parallel with all the planes produced by displacing the solid angles of the octahedron, we ultimately convert that form into the cube. This might be performed in the instance of common salt, but octahedral crystals of salt are rare. If however we apply the knife, or the hammer, to each of the solid angles of an octahedron of galena, we find that they may readily be taken off, so as to obtain a brilliant cube.
§ 41. By pursuing the division still further, that is, by taking off laminæ in the same directions, we only reduce the volume of the cube, not alter its form.
§ 42. If then the cube, which in this case is the primary crystal, can only be cleaved into cubes (as is the case with common salt and galena), we conclude that the octahedron, which is only a secondary form, has arisen from an accession, on every plane of the primary cube, of crystalline laminæ composed of minute cubes; the points of the solid angles of the cube being, in the preceding figure, precisely in the centre of the planes of the octahedron.
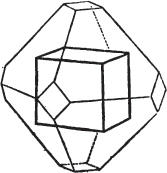
§ 43. Let us now consider the rhombic dodecahedron as arising from the cube.
This figure represents the rhombic dodecahedron, having within it a cube. On considering the relation of these two solids, we conclude that the rhombic dodecahedron, which is the secondary crystal, arises from the primary cube by an accession of crystalline laminæ on each plane of the cube, so as to form thereon a low quadrangular pyramid; and progressively diminishing in size, so as to terminate in a point. This pyramid, if the primary can only be cleaved into cubes, is assumed to be composed of cubic molecules, regularly arranged.
§ 44. These laminæ progressively diminishing, are represented on one plane of the primary nucleus, and the same observations
[page] XXVII
apply to the crystals thus formed as were made upon the rhombic dodecahedron arising out of the octahedron (§ 34). The striæ, it has been observed, sometimes denote the primary. In this case it will be seen that their direction is parallel with the lesser diagonals of the rhombic planes; and the existence of these striæ in the aplome, usually ranked as a variety of garnet, induced the Abbé Haüy to suspect its primary to be a cube.
§ 45. We now proceed to consider the trapezoidal dodecahedron as a secondary crystal of the cube, in other words, as arising from a regular deposition of crystalline laminæ on the planes of that solid.
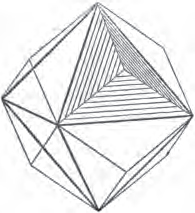
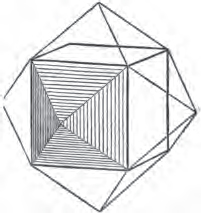
Fig. 1.
Fig. 2.
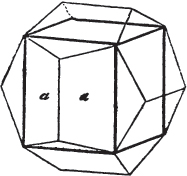
Fig. 1 represents a trapezoidal dodecahedron; a solid bounded by twelve equal and similar trapeziums; it is sometimes termed the pentagonal dodecahedron, all its planes being five-sided.
The same dodecahedron is also seen in fig. 2, having within it a nucleus in the form of a cube.
§ 46. We here observe, that on each plane of the cube there is an equal and similar pyramid; and that each pyramid is not, as in the instance of the rhombic dodecahedron just described, composed of equal and similar planes. But, in this instance, the planes of each pyramid are equal and similar two and two. a and a resemble each other; and the two small triangular planes, the one above, the other below the planes a, a, also resemble each other.
§ 47. Here, therefore, there must necessarily be an arrangement of the little cubic molecules of which the crystal is assumed to be composed, very different to that which, in the instance of the rhombic dodecahedron, produced a precise uniformity. Here, between a a we have a line, but the four planes on each surface of the cube terminated, in the rhombic dodecahedron, in a point —in a single cube.
§ 48. Let us observe whence this difference of form arises, on the assumption that the crystal is composed of cubic molecules.
[page] XXVIII
Let a b c d e f be the cubic nucleus, or primary crystal, composed of minute cubic molecules.
Then a f e h n will be one of the six pyramids on the planes of the cube.
§ 49. Now there is a remarkable difference in the arrangement of the cubic molecules on the two sides of this pyramid, which are obvious to us; the same difference will consequently exist between the other two. On the side a f h n, which resembles the steps of a stair-case, we observe that these steps are two ranges of molecules in breadth, and only one in height. But the very reverse of this is the case of the side f e h; for in this, the molecules are two ranges in height and only one in breadth.
§ 50. The consequence of this difference in the arrangement of the molecules is, that the quadrangular sides of the pyramid incline much more upon the upper plane of the cube than those which are triangular. Not so in the instance of the pyramids on the planes of the cube forming the rhombic dodecahedron (p. xxvi.); for in them, as in all the preceding figures, the superposition of molecules on the primary nucleus is on every side equal and similar, producing equal and similar planes, and precisely equal measurements in every direction. The structure in those crystals may therefore be termed simple: as the planes decrease equally to a point, they are said to arise from a simple decrement.
But the structure of the pentagonal dodecahedron may be termed compound, because its planes do not decrease equally on all sides; the decrement is compound. Of this species of structure there are several varieties.
§ 51. But it may be objected, that, since the molecules of which crystals are constituted, are too minute to be detected by the help of the most powerful glass, every thing which can be
[page] XXIX
said in regard to the form of these molecules must necessarily be theoretical.
§ 52. This of course will be granted. We are not specially contending for any peculiar form in the integrant particles of matter; but only for this,—that since the crystals of a substance yield to mechanical division in particular directions, and cannot be made to yield to it with regularity in other directions, these particles, whatsoever may be their form, must necessarily resemble each other, and be arranged with the utmost regularity; and also that this perfection of internal structure is the cause of regular external form.
§ 53. The planes of the rhombic dodecahedron (p. xxvi.) meet each other under an angle of 120°, and those of the pentagonal dodecahedron (p. xxvii.), under different angles.
In the determination of the value of these angles, calculation has been resorted to, for the purpose of confirming the measurements obtained by the goniometer: and thus it has been decided that the pyramid formed on each plane of the cube, in the instance of the rhombic dodecahedron (§ 43) (being composed of planes which are equal and similar, and the measurement of any one upon the next being uniformly the same), that those pyramids must be composed of laminæ superimposed in regular order on every side; namely, of one molecule in height, and one in breadth. But as the planes of the pyramid superimposed on each face of the cube are dissimilar and unequal (or similar and equal only two and two) in the pentagonal dodecahedron (§ 45), so they afford different results under the goniometer, which have been confirmed by calculation; for by calculation it has been determined that the angles under which these planes meet, could only be the consequence of a superposition* of laminæ on
* It is a conclusion necessarily arising from the structure of crystals, that those which are secondary result from a superposition on the primary nucleus, of laminæ, which are composed of regularly arranged molecules.
But the usual mode of describing the manner in which the secondary forms arise out of the primary, supposes the contrary to be the fact. The secondary crystal is described as arising out of the replacement of the edges or angles, or both, of the primary crystal.
Thus, in the instance of Red Copper, p. 317, it is said that the primary is an octahedron; that fig. 6 arises from the replacement of its edges: but fig. 6 is in reality the consequence of a very opposite cause—of an increase of laminæ-on the planes of the octahedron, the laminæ diminishing progressively in width. Fig. 7 is described as the consequence of a deeper, fig. 8 of a complete, replacement of its edges, by which the octahedron is converted into the rhombic dodecahedron—when, in fact, these crystals arise from an increase of laminæ on the planes, progressively diminishing to a point.
It may be inquired, why, in these descriptions, a mode is adopted which is diametrically opposed to fact. The reply is, that it is convenient. If the fact were adhered to, the descriptions would he long, and scarcely intelligible; and, in effect, it matters not which method is adopted, for the same consequence is arrived at in either case.
The beginner may convince himself of this assertion, by moulding or cutting a piece of wax or of soap into the form of the octahedron; then let each edge be equally cut away (replaced) by a knife, and the ultimate consequence will be the rhombic dodecahedron. Let then those solid angles of the dodecahedron which are formed by the meeting of three planes, be in like manner replaced by a knife, and the consequence will be, that in lieu of each there will be a triangular plane: if these triangular planes (eight in number) be increased by deeper replacements parallel with each, the rhombic dodecahedron will ultimately be converted into the octahedron. Thus the assertion is proved, that whether we describe this secondary crystal as arising from the replacement of the edges of the primary, or from increase on its planes, the effect is the same.
This practice is recommended to the beginner, not simply as regards the above fact, but also as a pleasing method of convincing himself of the transitions of crystalline forms.
[page] XXX
each plane of the cube, of two molecules in height and one in breadth on the one side, and of one in height and two in breadth on the other (§ 49). And whether we assume these molecules to be cubes, or any other form, we must assume them to be equal to each other; and if so, whatever may be their form, the same structure would ensue.
§ 54. By means of calculation the Abbé Haüy determined the angles under which the secondary planes meet, which result from an increase of laminæ on the cube and octahedron, and on other geometrical solids, considered as primary crystals: and thus, if we procure a portion of a crystal presenting only two planes of one of the varieties of those solids, we may decide which they are,—the use of the common goniometer will approximate the truth sufficiently to enable us to decide by a reference to his measurements; which may doubtless be relied on in every instance wherein the primary crystal is a perfectly geometrical solid, as the cube, the regular octahedron, the tetrahedron, the rhombic dodecahedron, and the six-sided prism: for, the angles formed by the meeting of any two planes of these solids being accurately known, it follows that the angles of the secondary planes may be accurately calculated.
§ 55. There are other primary forms which are not regular geometrical solids; for instance, all those varieties of the octahedron of which the sides of the planes are not equal and similar; the primary octahedron of the oxide of tin is flatter than the regular octahedron, and that of sulphur is more acute. The varieties of the parallelopiped also are not regular geometrical solids, amongst which are the several acute and obtuse rhomboids, and all those solids whose bounding planes are equal and similar two and two, as the several varieties of prisms.
[page] XXXI
The Goniometer.

The Common Goniometer, or that invented by Carangeau, consists of a brass or silver semicircle a b c, graduated into 180 degrees (in the above figure the graduation is only partial), each degree being marked on the instrument by a short line extending from the outer rim, to the circle which is about one-twentieth of an inch within it; d e and f g are two steel arms, the horizontal one being fixed, the vertical moveable; beneath the arm d e, there is a plate of steel or of brass, which is attached to the semicircle near c, and extends somewhat more than halfway towards a, its termination being connected with the semicircle by the bar h for the sake of firmness: i is the head of a pin in the centre of motion, which is precisely midway between the two extremities of the semicircle a e, and at the same distance from b as from a or c. The pin passes through both arms and the brass plate; and on this pin the arm f g is at pleasure moved by the finger. The upper part of the arm f g cuts the semicircle, in the above figure, precisely at 90 degrees, expressed by 90°; if then two faces of a cube were presented to the lower portions of the two arms g l and l e, it would be found to fit them accurately, since the planes of a cube always meet each other at the angle of 90°. But if the solid be less or more than that angle, the instrument may be accommodated to the angle at which the two planes meet, by altering with the finger the moveable arm f g, if applied near its termination f; and the value of the angle will be indicated by the edge of the moveable arm.
As this goniometer is here figured, it is adapted to the planes of a crystal free from it gangue; but if the crystal be small and
[page] XXXII
surrounded by obstruction, the two arms may be drawn by the ends d and f (the cavities in the arms permitting them to slide), so that the points g and e will be much nearer the pin which is the centre of motion. Sometimes however this goniometer is made in two parts; the semicircle being one of them, and the two arms, connected by the pin, the other. In that case, the arms are in some instances more conveniently applied to the planes of a crystal; which being accurately done, the pin is dropt into a small hole, made to receive it, and the arm f g indicates the angle on the semicircle; care being taken, that the relative position of the arms be not disturbed, after they have been adjusted to the planes of a crystal.
It must be obvious that the use of this instrument depends on its precise adjustment to the planes of the crystal to be measured; for unless the light be excluded from between the instrument and the crystal, the adaptation will not be complete. If this cannot be accomplished, it may be concluded that the crystal, how perfect soever its planes appear, is not sufficiently regular to be relied on, if perfect accuracy be required.
The Reflecting Goniometer, as invented by Wollaston, and improved by Sang, is a very superior instrument.
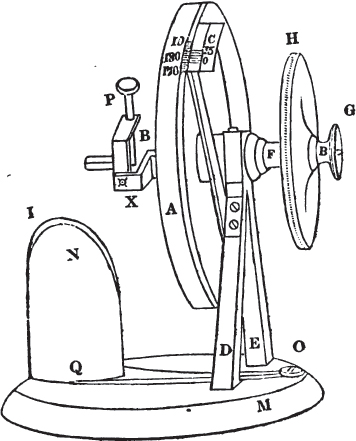
[page] XXXIII
A is a moveable circle graduated on one edge to half degrees, and divided for convenience into two parts of 180 degrees each (it is graduated only in part in the above sketch).
C is an immoveable brass plate screwed upon and supported by the pillar D, and graduated as a vernier.
F is the axis of the circle A, and passes through the upper part of two brass pillars D E, the lower ends of which are insert ed into a wooden base M.
B is an axis enclosed within F, and turned by means of the smallest circle G, which communicates a motion to all the apparatus to the left of A, without however moving that circle.
H is a circle to which is attached the axis of the principal circle A. If, therefore, we would move the latter, it is done by turning H; and as the axis of the principal circle includes that of the apparatus on the left of it, the whole instrument is necessarily put in motion, by moving the circle H.
L is a curved brass plate connected with the concealed axis B, and to which a motion is given by turning the small circle G; to L another curved plate is attached, but so as to admit of movement; and through the upper extremity of this last, passes the pin P, which is so adjusted as to allow of being moved either up or down or circularly.
By means of the several motions thus obtained, a crystal attached to the lower extremity of the pin P may be brought as nearly as possible on a line with the axis of the instrument.
I is a small mirror made of some substance which does not give a very bright reflection; black glass or obsidian for example. This is placed obliquely on a support N, at an angle towards the object of about 45°, immediately under the crystal (which in the present instance we shall assume to be a rhomboid of calcareous spar). The support is fixed to the wooden base M by a pin in the centre, which admits of its being turned for adjustment, and by a clamping screw O at the extremity of the arm Q O, which secures it in its proper position.
The use of this instrument depends on the reflecting power of the polish on the natural planes or fractured surfaces of minerals, which, in some cases, is very powerful. In adjusting it, the image of any object seen by reflection from the face of the crystal, is made to agree with the image of the same object seen by the help of the mirror I. In this way any object may be selected which has a well-defined outline; while the goniometer, like the common sextant, may be held in the hand.
If a distant object, say the moon, be used, the coincidence of its two images will indicate that the face of the crystal is parallel to the mirror I, so that, if the two faces be brought successively into that position, the angular motion of the divided circle must measure the inclination of these faces. In this, it is es-
B 2
[page] XXXIV
sential that the plane of the mirror I be parallel to the axis of motion. Its adjustment is thus effected. Select a thin plate of any mineral, say calcareous spar, of which the opposite faces are parallel, and cement it to the lower extremity of the pin P; then bring the image of any distant object seen in one of its faces, to agree with that seen in I; turn the instrument half round, so as to bring up the opposite face. If the images agree now, the mirror is correctly placed; if not, one half of the apparent error must be corrected by releasing the clamp nut O, and turning the mirror on the pin at Q, the other half by the motions of P; a second, perhaps a third trial must be made, to ascertain the adjustment completely; but, when this is once done, it need only be examined at long intervals, or after any accident.
The mirror having been thus adjusted, and the reflexions in both the surfaces whose inclinations are to be measured having been brought to agree with it, we have now to observe that the line at 180° or 0 forms a line with that at 0 on the vernier, at the same time that the double reflection of the distant object in the mirror, and in one of the faces of the crystal, appear exactly to agree. One movement more, and the measurement is completed. Turn the circle H, until the reflection of the same object, as seen on the adjoining plane of the crystal, appears exactly to cover the image in the mirror, and it is done.
We now observe what line of the principal circle touches that at 0 in the vernier, Suppose that 105° on the former be now on a line with 0 on the vernier;—it is the value of the angle. But suppose it to be a little more than 105°, and less than 105½°, it must then be observed which line of the vernier touches, or forms but one line with, another line on the principal circle; suppose it to be 5 on the vernier; the angle is then 105° 5′, which is the true value of the obtuse angle of a rhomboid of calcareous spar.
Mr Sang's* important improvement on Wollaston's goniometer consists principally in the apparently simple addition of the mirror I, which, however, renders it a vastly superior instrument, not only as regards precision and rapidity of measurement, but from its being readily used as a sextant, or rather as a repeating reflector, and thus enabling the expert mineralogist both to determine the angles of minute crystals, and to delineate the geographical features of the district which he may be exploring.
Of the comparative Value of the Common and Reflecting Goniometers.—The use of the common goniometer depends on two
* For Mr Sang's polite attention in explaining this valuable addition to Wollaston's goniometer, the editor feels himself deeply indebted.
[page] XXXV
circumstances; one, the perfection of the crystalline planes; the other, the steadiness and accuracy of the hand and eye.
We are but little acquainted with the works of nature in her more hidden processes, amongst which may be reckoned crystallization; but it can be demonstrated beyond dispute, that the surfaces of large crystals are not so uniformly even, however brilliant they may appear, as the surfaces of small ones. Now the larger ones are best adapted for the use of the common goniometer; hence, if the crystal to be measured be not selected with the utmost care, and if the hand and eye be not steady and accurate, we cannot hope for precision in the use of it; we cannot expect that precision which ought to exist, since this mechanical operation is to form the foundation for calculation.
That the planes of small crystals are more perfect than those of large ones, is proved by the use of the reflecting goniometer, which depends on the perfection of these planes, and on their brilliancy. Even minute crystals, which generally are the most perfect, rarely agree in the angles they afford; but this disagreement is commonly too small to be detected by the common goniometer; a fact which clearly proves that its use cannot be relied on as a foundation for calculation. When, therefore, we would arrive at the greatest precision, we shall prefer the reflecting goniometer, and the reflections from the planes of minute crystals, in preference to those of large ones, but, above all, from planes produced by cleavage, whenever they can be obtained.
Now the surfaces produced by cleavage are sometimes very small, and therefore are not adapted to the common goniometer; while for the reflecting goniometer, it matters not if the surface be small, provided it be perfect and brilliant; a surface of the 100th part of an inch in length and breadth will suffice.
§56. Hitherto we have been treating chiefly of that structure which may be termed perfectly crystalline; this exists in such minerals as admit, in various directions, of regular cleavage. There are, however, other kinds of structure observable. In some minerals the natural joints are scarcely attainable, or, when attained, are only perceptible by the assistance of a microscope; in these the structure is said to be imperfectly lamellar, and this effect may be supposed to arise either from the brittleness of the substance, or from the strong cohesion existing between the laminæ. Such minerals may be said to be imperfectly crystalline, as may those also of which the planes obtained by cleavage are curved or undulating. It has already been observed, that some minerals are perfectly lamellar in one direction only,—the topaz, for instance; others cleave readily in one direction, with difficulty in another, as the sapphire.
[page] XXXVI
§ 57. The fibrous structure which some minerals assume may in most cases be considered only as resulting from the close longitudinal adherence of small, or of extremely fine acicular crystals; for the terminations of the crystals are often observable on the exterior of the mass.
§ 58. Perhaps also under the head of Structure may be classed the variety of appearances assumed by the aggregation of small crystals. When merely collected, as it were, into a bundle, they are said to be fasciculated; when they are fasciculated, and diverge from a common centre, they are said to be scopiform; but when the divergence surrounds the centre, they are said to be radiated, or stellated.
§ 59. The term slaty, as it regards structure, is rarely applied to those minerals of which we have been treating, even when they are separable only in one, or at most two, directions. This term is more commonly applied to such substances as consist of parallel layers which are thick and coarse.
§ 60. The granular structure arises from an aggregation of small particles, frequently of laminæ which separately are lamellar, intercepting each other in every direction. And in proportion to the fineness of these particles, a mineral is termed coarse-grained or fine-grained. If the particles are only perceptible by the microscope, the mineral is said to be fine-grained; but if the parts of which a mineral is constituted be not thus apparent, it is termed compact.
Fracture.
An important part of this subject has already been considered under the head of structure; namely, that which treats of the geometrical forms into which some minerals may be cleaved; and the means (p. xx.) of attaining this have been adverted to.
But when such minerals as may be mechanically divided along their natural joints are broken in directions contrary to those joints, the surfaces so produced are not plane; they are said to be conchoidal when the surface more or less resembles the appearance of a shell; thus, we have the perfect, imperfect, large, small, and flat conchoidal. These varieties of fracture also exist in minerals which appear not to possess any regular internal structure. There are also other kinds of fracture; as the even, when the surface is nearly flat; the uneven, when it is not flat; the splintery, &c.
When a mineral breaks with a peculiarly uneven surface, somewhat similar to pure copper, for instance, the fracture is said to be hackly.
[page] XXXVII
Frangibility.
The frangibility of some minerals may in a measure be said to depend upon their structure; in all, it is probably dependent on some peculiarity in the arrangement of the molecules or particles of which a mass or crystal is composed. From whatever cause it proceeds, this quality varies greatly in different substances, ranging through all the intermediate degrees, from very brittle to very tough.
Some few minerals, as sulphur, are so brittle, that a fragment is easily detached by the pressure of the nail on the edge of a broken surface; but as this may be produced in any direction, it cannot be said to depend on the structure of the substance.
The laminæ of selenite are readily separable in one direction; and, if very thin, are brittle in another direction, while in the same, if the specimen be a line or more in thickness, it is tough; heavy spar is easily frangible in every direction; so also are calcareous spar and fluor. But frangibility, strictly speaking, ought not to be considered as connected with the ease or difficulty with which minerals yield in directions parallel to their natural joints: it seems rather applicable to their property of yielding to mechanical force in other directions. If this quality depended on regular cleavage, we should say that corundum is very brittle, because it yields along its natural joints with ease; and we should characterize the diamond as moderately brittle, because it can be cleaved with but little force: but in contrary directions these substances are far removed from either brittleness or toughness.
Sulphur, and the sulphate of lead, are very brittle; carbonate of lead, red silver, grey copper, and others, are moderately brittle, and easily frangible in every direction. From these, fragments are readily detached by the pressure of the knife; other minerals yield only to a blow with the hammer. Others again are said to be tough, because, instead of breaking, their particles only yield to the force, and by sliding, as it were, over one another, suffer depression without producing fragments. Granular selenite is considerably tough; massive hornblende is very tough. In using the hammer, it will be found that a smart blow from a small one will produce more effect, and better surfaces, than a heavy blow with a large one.
It may be observed that most of the porous minerals, and perhaps there are few which are not so, are much more frangible when first taken from their native bed than after exposure. Of this, common flint, in which no regular structure has been observed, is a remarkable instance. This circumstance is doubtless owing to the water which fills its pores when in its native place, but which evaporates on exposure.
[page] XXXVIII
Hardness.
Hardness is a very useful property in determining minerals; and we are indebted to Professor Mohs for a scale easily formed, and at the same time distinct and accurate. The means of applying it also are within the reach of every mineralogist. It consists of
1. Talc, of a white or greenish colour.
2. Rock salt, a pure cleavable variety; or gypsum uncrystallized, and only semi-translucent.
3. Calcareous spar, any cleavable variety.
4. Fluor spar, presenting good cleavage.
5. Apatite, the asparagus stone from Saltzburg.
6. Adularia, any perfectly cleavable variety.
7. Rock crystal, limpid and transparent.
8. Topaz, any simple variety.
9. Corundum stone from Bengal, which affords smooth surfaces when fractured.
10. The diamond.
In employing this scale, we endeavour to find the degree of hardness of a given mineral by trying which number of the series is scratched by it; or, still better, by passing with the least possible force the specimens under comparison over a very fine file. Every person will observe a marked difference on comparatively trying in this way any two consecutive numbers of the above scale, and by a little experience he will soon acquire the manual skill necessary for nice discrimination.
From the resistance these bodies afford to the file, from the noise occasioned by their passing over it, and from the quantity of powder left on its surface, their mutual relations in respect to hardness are deducible with great correctness. When, after repeated trials, we are satisfied which member of the series our mineral is most closely allied to, we say its hardness (suppose it to be calc-spar) is equal to 3, and write after it H. = 3·0. If the mineral do not exactly correspond with any member of the series, but is found to be between two of them, we say H. = 3·5, or 3·75 if it approximate to the higher number. Care, however, must be taken to employ specimens of each which nearly agree in form and size, and correspond as much as possible in the shape of their angles. They must likewise possess perfect purity, as the degrees of hardness can no more be correctly ascertained than the specific gravity, if impure substances are made use of. The file required for this purpose should be cut fine, and, if possible, of the hardest steel. The latter property, however, is of less moment, as it is not the hardness of the tool with which we are to compare that of the mineral, but the relative degrees of hardness of minerals, which are to be ascertained through the medium of the file (Introduction to Allan's Manual.)
[page] XXXIX
Transparency.
This is not an essential physical character, inasmuch as the degree in which light is transmitted through a mineral often varies greatly in the same substance, and even in the same specimen. In description, however, a mineral is said to be transparent when objects can be distinctly and clearly perceived through it, semi-transparent when they are imperfectly seen, translucent when they are scarcely or not at all visible; but when, from various causes, a mineral appears not to suffer the transmission of light, we may perceive, on holding it between the eye and the light, that it is translucent on the edges; when this does not exist, the substance is termed opake.
Lustre.
Lustre is a character of considerable importance. It is of several kinds; and the same lustre which a mineral presents internally is usually exhibited throughout the species, although in crystallized substances it often differs very much externally, even on the same specimen.
That which is peculiar to the metals in their pure state is termed the metallic lustre; this belongs chiefly to opake minerals, such as plumbago, among amorphous substances; and specular iron and grey copper among those which are crystallized: but this kind of lustre is not equally intense in all those minerals which possess it, inasmuch as it varies from shining to dull. In some minerals, however, there is a species of metallic lustre which is perceptible only when the substance is held towards the light in some particular direction, as in bronzite; it is then termed pseudo-metallic.
Adamantine lustre will be better understood by a reference to those substances to which it belongs, than by any description. It exists in the diamond, some varieties of corundum, in sulphate of lead, &c. It belongs only to such species as possess a greater or less degree of translucency; and being dependent on their capabilities of reflecting and of refracting light, it is supposed in some degree to depend on their structure.
Pearly lustre, more or less distinct, is peculiar to several species, though sometimes only in a particular direction; it rarely exists but in lamellar minerals.
The silky lustre is particularly observable in satin spar, malachite, and in other species of which the structure is fibrous; and the changeable play of light sometimes visible on an alteration of position in the mineral, induces the conclusion that the fibres of which such substances are composed are in reality regular crystals, from the surfaces of which a reflection may be supposed to arise. The chatoyement of the cat's eye is believed
[page] XL
to arise from fine fibres of asbestus or amianthus included in it. But no adequate cause has been assigned for that changeable play of light so beautifully displayed in moonstone and chrysoberyl; or the still more beautiful colours of noble opal and labradorite.
The resinous lustre in minerals resembles that which is observable on the fractured surfaces of resins; the vitreous exhibits that of broken glass: these belong chiefly to the surfaces produced by fracture in directions contrary to those of the laminæ, if the mineral possess regular structure; some varieties of pitchstone are instances of resinous, quartz of vitreous lustre. Waxy lustre is observable in leelite, the newly broken surfaces of which possess that lustre which belongs to bees' wax: it is rarely, if ever, observable where regular structure exists.
When no particular lustre is observable, except such as arises from the mere polish of the natural surfaces, or of those produced by fracture, a mineral is described according to the intensity, as being splendent, shining, glistening, or glimmering; but a glistening or glimmering lustre only, often arises from the fractured surfaces of some minerals, merely because those surfaces are uneven, and consist of minute irregularly-disposed planes, from which the light is unequally reflected.
In the absence of lustre, a mineral is described as being dull.
Colour.
It seems requisite to notice colour, though in reality it cannot be considered of importance among the characters of minerals, since there are few earthy substances, except the emerald, in which it is characteristic, and even that substance exhibits several shades of the same colour. But in fluor, which is found of almost every hue, it would be absurd to quote its colours among its characters. In some instances we have varieties of a mineral under names very different from that of the mineral itself, merely from the colour, as in prase as a variety of quartz, chrysoprase of calcedony. The colour of the former is by some supposed to arise from an intimate mixture of another substance in the mass; that of the latter is derived from a metallic oxide; and these oxides are the principal colouring matter of earthy minerals, the earths being all white and colourless when chemically produced in a pure state.
When a crystallized mineral includes a metallic oxide, or any other substance which produces no alteration in the crystalline forms assumed by the mineral in its pure state, such an ingredient, whether it be the colouring matter or not, is considered to be only accidental.
In some of the metalliferous ores, however, where it depends on the nature of the mineral, and is therefore nearly uniform, olour constitutes a more important characteristic.
[page] XLI
Flexibility and Elasticity.
Flexibility serves as one among the distinctive characters of the few minerals which possess it. That substance is said to be flexible which, being bent, does not of itself resume its former shape, but continues in the form forcibly given to it. Talc is flexible; as is the Cornish phosphate of iron, while that of New Jersey is brittle. Those minerals, on the other hand, are termed elastic which, after being bent, spring back to their former position. Mica is very elastic, and may by this character alone be distinguished from talc, which is only flexible.
Double Refraction.
Recent discoveries have so widely extended our information on this subject, that any adequate explanation of its details would be quite unsuitable in this place. The reader, therefore, who desires to make himself acquainted with this most interesting and curious department of mineralogical science, is referred to the able papers of Sir David Brewster, in the Transactions of the Royal Societies of London and Edinburgh; to the two numbers of the Library of Useful Knowledge on Double Refraction and Polarization; and, for a brief outline of the subject, to the article on the Structure of Minerals as exhibited in their Double Refraction, which forms a prominent part of the Introduction to Allan's Manual of Mineralogy.
Touch.—Taste.—Odour.
The touch, or feel, is very characteristic in a few minerals. Soapstone is unctuous to the touch. Chalk is said to be meagre, being dry and without absolute harshness. It is principally in these two respects that this character is used in description.
Taste is employed as a discriminating property in most saline minerals, of which water is a solvent; in this case the palate may be resorted to as a test of their nature.
The odour of a mineral is a character of very restricted use. When swinestone is struck forcibly, or rubbed against another and a harder substance, it emits a peculiarly fœtid odour; and some argillaceous minerals give out a smell of clay when breathed upon.
Streak.
This is a very important character. The colour of a mineral and that of its powder are frequently different; and as the particular hue of the latter is most easily obtained by rubbing or streaking the specimen under examination on a slab of porcelain biscuit, the colour of the powder of a mineral is thence de-
[page] XLII
nominated its streak. The streak of white minerals is usually white, that of coloured ones paler than the mass; but when it corresponds with the colour of the mineral, it is said to be unchanged. This is a more constant property than colour; and among the metals (the magnetic and specular iron ores for instance) is perfectly characteristic.
Adhesion to the Tongue.
This character depends on the disposition of a mineral to imbibe moisture. Lithomarge adheres strongly to the tongue; as do some substances which are supposed to be in a state of decomposition, as several varieties of calcedony and opal.
Magnetism.
This character is confined, with little exception, to some of the ores of iron, amongst which there are very perceptible degrees of difference in their power of attracting the magnet; dependent on their several states of oxidation, or upon their being constituted of iron differently oxidized. Oxydulated iron is strongly magnetic, and possesses polarity; the specular is magnetic in a less degree; and red hæmatite is sometimes feebly so. Carbonate of iron is considerably magnetic. Iron, cobalt, and nickel, are the only metals which possess magnetism; and whenever other substances present this property, it arises from the presence of iron.
A common magnet has two poles, a north and a south. If the north poles of two magnets be brought in contact, they repel one another, and the same effect ensues if the south poles are presented together. But the north pole attracts the south pole, and the south the north; and hence, when a mineral is presented to the magnet, which attracts the one and repels the other, it is said to possess polarity.
But in order to determine this point, it is advantageous to employ a needle of feeble power; for if the magnetic power of the needle be greatly superior to that of the mineral, the latter will attract both poles of the magnet: which has been explained in this manner: it is said that the superior power of the needle produces in the mineral a polarity contrary to its own.
Electricity.
It will be recollected, that there are two kinds of electricity, which are called positive and negative, or vitreous and resinous, according as they are produced by exciting smooth glass, or any resinous substance. It will also be recollected, that when two
[page] XLIII
bodies possess the same kind of electricity, whether positive or negative, they repel each other; but if one possess positive electricity and the other negative, they attract each other.
A considerable number of minerals may be rendered electric by friction with the hand or woollen cloth; and when thus excited, they are capable of attracting light bodies, or of moving a delicate electrometer.
Among the minerals which are capable of exhibiting electric properties, there are a few which acquire electricity by being heated, either by simple exposure to a fire, or by immersion in hot water. But those substances which are excited by heat, acquire at the same time both positive and negative electricity; but so separated, that, on whatever part of the mineral the positive may appear, the negative will be found on the part diametrically opposite. Thus, if positive electricity appear on one side, or at one extremity of a crystal, negative electricity will exist on the opposite side, or at the other extremity. And it is very remarkable, that, in crystallized minerals, excitable by heat, the opposite parts of the crystal on which the two electricities appear, are almost always different from each other in their configuration, or number of sides, although similarly situated in reference to the crystal itself. Thus, if it be a prismatic crystal of tourmaline, and if the two electricities appear at the two extremities or summits of the prism, these two summits will differ from each other in the number or situation of their planes.* Most frequently that part of the crystal which possesses positive electricity presents the greater number of faces; and, on the contrary, when a crystal does not become electric by heat, the opposite parts are usually similar. Sometimes certain angles or faces possess positive electricity, while the opposite angles or faces exhibit negative.
It may be stated as a general fact, with very few exceptions, that earthy minerals and salts, possessing a considerable degree of purity, and having their surfaces polished, acquire positive electricity; but if their surfaces are not smooth and polished, they acquire negative electricity, as is the case with rough glass.
Combustibles, the diamond excepted, become negatively electric by friction. The diamond, whether polished or unpolished, always becomes positive.
Ores are usually conductors of electricity, with the exception of some metallic salts, which become positive by friction.
* The different configuration of the opposite parts of a crystal, exhibiting the two kinds of electricity, has been supposed to be a uniform fact. But more extensive observations seem to show that it is not always the case. Some tourmalines from Pegu and Ceylon, which possessed both electricities, appear to have both summits perfectly regular and similar Another exception appears in the dodecahedral crystals of boracite.—(Bournon.)
[page] XLIV
For observing the electricity of minerals, the electrometer is the most convenient instrument
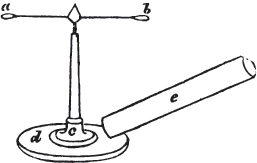
In this figure a b is a needle of copper, terminated at each extremity by a small ball, and moving very easily on a pivot at the centre. At c the instrument has a metallic base. If a mineral, which has been excited, either by friction or heat, be presented near to one of the balls, the needle turns, whether the electricity be positive or negative; and the force of the electricity may be estimated by the distance at which the needle begins to move.
To determine which kind of electricity a mineral possesses, the needle must previously be electrified, either positively or negatively; which may be done in the following manner. Let the instrument be insulated by placing it on d, a plate of glass or resin. Having excited a tube of glass, or a stick of sealing wax, place one finger on the metallic base c of the electrometer, and then bring the excited glass or sealing-wax e within a small distance of one of the balls of the needle. When the needle is sufficiently electrified, first withdraw the finger, and then remove the glass or sealing-wax. If now an excited mineral be presented to the needle, they will repel or attract each other, according as they possess the same or opposite kinds of electricity. But as the electricity of the needle is known, that of the mineral may be determined.
To ascertain the electric poles, or those parts of a crystal which possess contrary electricities, let a thread of silk about one fourth of an inch in length be connected to one extremity of a rod of sealing-wax, which must then be excited. To this thread of silk, which of course is negative, let the sides, angles, or summits of the mineral under examination be successively presented; and the attraction or repulsion observed will indicate those parts of the crystal where the two electricities reside.
Sealing-wax, when rubbed by most minerals, becomes negative. There are, however, a few minerals, of which sulphuret of molybdena is one, which, being rubbed on sealing-wax, communicate to it positive electricity. In these experiments both the wax and mineral should possess smooth surfaces of considerable extent. (Introduction to Cleaveland's Mineralogy.)
[page] XLV
Phosphorescence.
This is a curious property rather than a useful character in the few minerals which possess it. A mineral which emits a light, either by heat or friction, is said to be phosphorescent. Fluor, and particularly that variety of it termed chlorophane, is an instance of the first, and a variety of blende of the last; if two pieces of quartz, or of the calcareous spar from Huel Goet in Brittany, be rubbed together, they emit sparks of light.
This character is of the less importance, because it does not seem to be essential to all such minerals as possess it even in the greatest degree; for, according to Bournon, some varieties of fluor are not phosphorescent.
The light emitted by phosphorescent substances is extremely variable in respect of colour. The best mode of exhibiting it in those which become so by heat, is by first pounding them, and then ejecting the powder on a shovel not quite red hot, in a dark room. Whatever colour a phosphorescent mineral may possess, it is generally lost by repeatedly subjecting it to heat; and the property of phosphorescing is also gradually diminished, and ultimately destroyed.
This property, however, does not appear to be dependent on colour, or even connected with it, since the most perfectly colourless and transparent fluor, when powdered and thrown on live coal, emits a brilliant blue light.
Specific Gravity.
Two masses exactly corresponding in size, but consisting of different substances, are found in most cases to disagree in weight. If the weight of one of these be considered as unity, the proportional weight of the other is termed its specific gravity. For example, suppose a cube of water to weigh exactly a pound, a similar sized cube of calc-spar will weigh two pounds and nearly seven tenths of a pound, a sum which is represented in decimals as equal to 2·7.
This is one of those physical properties which are extremely useful in acquiring a knowledge of the inorganic productions of nature, as it can not only be easily ascertained to a considerable degree of accuracy, but is constant, or at least ranges within very narrow limits, in minerals of the same species.
The instruments used for ascertaining the specific gravity of solid bodies, are the hydrostatic balance and the areometer. The former allows of very high degrees of accuracy, and is most conveniently used in the following manner:—One of the scales of a very fine balance being elevated considerably above the
[page] XLVI
other, a small hook is attached to its lower surface, from which a watch-glass is suspended by means of a hair or a fine fibre of silk. The mineral is then placed in the watch-glass, and along with it immersed in water. Thus the difference indicated in the weight of the mass, before and after its immersion in the fluid, amounts to the weight of the quantity of water displaced by the bulk. When an experiment is to be performed, this glass is immersed in water, and the weight of the specimen placed in the elevated scale first ascertained. For instance, a piece of metal is found to weigh in the elevated scale 2·645 grains; but when put into the watch-glass and immersed in water, its weight amounts only to 0·295 grains. Then, as 0·295 is to 2·645, so is unity to the specific gravity of the metal, which in this case will be found to amount to 8·966.
The delicacy of the hydrostatic balance occasions it to be affected by the slightest current of air, and it therefore requires, when used, to be enclosed in a glass-case. For this reason, in performing common experiments, and in most cases for obtaining the required results with quite sufficient accuracy, the areometer will be found preferable. This instrument has also the advantage of cheapness and portability. Its form resembles the accompanying figure. The body consists of a hollow cylinder or tube, the lower portion of which terminates in a point. A certain quantity of lead is melted into the bottom of this at D, so as to give it, when placed in water, an upright position, and admit at same time of a portion of the cylinder rising above the surface, as may be supposed to the line F. Weights are then placed in the cup A, until the whole instrument is depressed in the water to the point B, marked upon the wire-index which supports the cup. This gives the normal or standard weight. The mineral under examination is then placed in the cup A, and so much of the weight at the same time removed from it, as to raise the whole again to the index-point B marked on the wire. By this means the actual weight of the specimen is obtained; but when it is removed from the cup A, and placed on the top of the cylinder, which is a little hollowed at C, the instrument will be found to rise considerably in the water, and
[page] XLVII
a certain weight will then be requisite to depress it to the above-mentioned point B. This last weight then is required to counterbalance the loss sustained by the mineral in water, and is equivalent to the weight of the volume of water displaced by the mineral. The second weight subtracted from the first or normal weight, leaves the absolute weight of the mineral; the second weight deducted from the third, leaves the weight of an equal volume of water; and from these results, as on the former occasion, the specific gravity of the mineral may be reckoned. The normal weight, for instance, is twenty grains, that is, it requires 20·000 to depress the instrument to the point B. Suppose the same piece of metal as in the former experiment to be used: it is placed in the cup A along with 17·355 grains, in order to bring the instrument to the same depth. When removed from the cup A to that at C, 17,650 grains are found requisite to produce the same effect. And now, to find the specific gravity, divide 20·000—17·355, or 2·645, by 17·650—17·355, or 0·295, from which, as in the foregoing case, the specific gravity of the mineral will be found to amount to 8·966.
A considerable degree of attention is necessary both in selecting the specimens and in performing the operation of weighing. The minerals intended for examination require to be perfectly pure; and, previous to commencing, the greatest care must be taken to remove whatever foreign matter may adhere to the specimen. All the vacuities or empty spaces are to be carefully opened, or the mineral broken down, not into powder, but into fragments; and distilled water must be used, of a temperature not differing much from 60° Fahr. When the mineral, during the process, is supposed to have absorbed water, the weight of what is imbibed may be ascertained by again weighing the mineral in air, and adding the amount to the first term of the proportion, or, what will generally suffice, it may be varnished before being subjected to such examination. (Introduction to Allan's Manual.)
2. CHEMICAL CHARACTERS.
Although the chemical characters of a mineral are most fully and completely understood by its analysis, there are other means of arriving at some knowledge of its component principles; and, therefore, although the methods about to be described do not make us well acquainted with all that is to be known, they serve now and then to detect an important ingredient, and therefore add, by very simple processes, to the distinctive characters: the means are chemical, inasmuch as they produce a change, or partial decomposition.
[page] XLVIII
Action of the Blowpipe.
The use of this instrument is somewhat difficult of attainment; supposing it to be applied to the mouth (which is always understood unless the contrary be expressed), its effect depends on the power of producing a constant and pretty uniform stream of air. This current is not supplied at once from the lungs. The mouth being filled, the communication between it and the lungs is to be closed by a peculiar action of the tongue, which is to be drawn back against the orifice, while the lungs are replenished through the nose; as the mouth becomes empty, it is again to be filled from the lungs, and the communication closed as before, while the lungs are filling through the nostrils. The best mode of attaining the use of the blowpipe is, perhaps, to sit down to it with no other object at first than that of producing from the flame of a common candle a steady stream of flame; taking care that the wick be of a moderate length, and the top of it bent in the direction of the blast. The best blowpipes are formed of silver: those made of brass are apt to get out of order, and glass does not stand the necessary heat.
The use of this instrument is highly interesting. If fusion be not produced, we have at least the advantage of seeing the impression made by very powerful heat; of noting the appearances and consequences which gradually take place, and which often are very characteristic.
It will be observed that there are two cones of flame projected from the pipe; the outer yellow, the inner blue—more properly designated as the oxidating and reducing flames of the blowpipe. The heat of the outer cone is less than that of the inner, and the most intense heat of the blue flame is near its point.
The substance to be acted on ought not to exceed the size of a grain of pepper; for if too large, a part of it will be without the focus of the heat, to which every part ought to be subjected alike. In most cases it will be advantageous to expose the mineral at first to the heat only of the outer flame.
Various methods, depending on the nature of the mineral, must be employed for supporting the fragment before the flame. Very small forceps will be sufficient, when the mineral has but little fusibility. For substances easily fusible, a small platina or silver spoon may be employed. It is important that these metallic supports should be very small, that they may not absorb too much heat. When metallic oxides are to be reduced, a piece of compact charcoal forms the best support. A small cavity is made in the charcoal, in which even minerals in a state of powder may be conveniently examined, especially if the cavity be partly covered by another piece of charcoal. Minerals, while exposed to the action of the blowpipe, exhibit
[page] XLIX
very different appearances, which, being directly before the eye, are easily observed, and should be minutely described. Sometimes their colour is changed, or entirely disappears. Some minerals decrepitate, others divide or exfoliate, when exposed to the flame. Some indurate and contract their bulk; others effervesce, or, rising in little blisters, melt with intumescence.
On some minerals the blowpipe produces no effect whatever; others are partially fused; and others again melt with facility. The results of fusion may depend in some degree on the intensity or continuance of heat, as well as on the nature of the substance. Some minerals by the action of the blowpipe are merely softened, and alter their shape; or, if in loose grains, they become agglutinated. Others are converted into a kind of porcelain, in which only a few points are vitrified. Some melt into a slag, which is a compact substance, containing metallic matter; others yield a tumefied mass, or are reduced into a scoria, which is light and porous; and others give an enamel, which has a vitreous aspect, but is not transparent; sometimes the enamel is only superficial.
Many minerals, when melted, yield a globule of perfect glass, which, in different substances, has various colours, and possesses different degrees of transparency. Both enamels and glasses are sometimes porous or vesicular.
These and all similar changes must be carefully noted, even the vapour or odour evolved during the experiment, the colour which some minerals communicate to the flame, their phosphorescence, and every other phenomenon which may lead to the detection of elements whose presence was not anticipated, or perhaps even suspected.
After having observed the alteration which the substance undergoes by the mere action of heat, it will be necessary to examine what farther change takes place when it is melted with various fluxes, and how far it is capable of reduction to the metallic state. Of these fluxes, or re-agents as they are termed, the most invaluable for their respective purposes are the three proposed (we may truly say) in the infancy of the science by Cronstedt. These are,
1. The carbonate of soda, which is used for ascertaining by its means whether bodies be fusible or not, and for assisting the reduction of metallic oxides.
2. Borax, which is employed in effecting the fusion of a great number of substances.
3. Salt of phosphorus, or microcosmic salt, a compound of phosphoric acid, soda, and ammonia, which, as it exhibits the action of acids on the assays, is particularly applicable to the examination of metallic oxides.
There are of course a variety of other uses to which each of
C
[page] L
these may be rendered subservient, and of which the skilful operator with the blowpipe will soon learn to avail himself; but as in some cases effects are required to be produced, which do not come within the reach of any of them, the test-box should also contain compartments for the following substances:
4. Oxide of copper, to detect the presence of muriatic acid.
5. Iron, in the state of very thin wire, for ascertaining the presence of phosphorus.
6. Tin, in the form of foil, for promoting reduction in the fused vitreous compounds.
7. and 8. Gypsum and fluor spar, which, when well dried, are used mutually to detect each other.
9. Bisulphate of potash and fluor, mixed in the proportion of four and a half of the former to one of the latter, for ascertaining the presence of boracic acid.
10. Another very useful re-agent is a solution of the nitrate of cobalt in water, which, when concentrated, is employed to ascertain the presence of alumina and magnesia, affording with the former a fine blue, and with the latter a pale rose colour.
It is unnecessary, in a treatise like the present, to enter more at length on the subject of the blowpipe. Suffice it to say, that the above re-agents are of the highest importance in the examination of metallic minerals. The ores of the difficultly reducible metals, such as manganese, cobalt, chrome, and titanium, are characterized by the colours which their oxides give to glass. In all these cases, therefore, glassy fluxes must be largely employed, both to dissolve the earthy matter with which the oxides are generally combined, and to furnish a body with little or no colour of its own, which may receive and sufficiently dilute the inherent colour of the oxide. When the colour thus produced is so intense as to appear opake, it is requisite to flatten the glass, before it becomes solid, between a pair of forceps, or to draw it out into a thread at the instant it begins to cool.
A description of the comportment of each species, when exposed to the action of the blowpipe, is shortly mentioned in the body of the work, under their respective heads; and for further instruction, both as to the mode of performing experiments, the phenomena presented, and the results afforded by them, the reader is referred to the excellent work of Berzelius on the use of the blowpipe, as translated by Mr Children.
Action of Acids.
Although complete analysis be not the object in subjecting minerals to the action of acids, yet we may thereby obtain characteristic information in regard to many species, especially the acidiferous, and some of the alkalino-earthy minerals.
[page] LI
In this process it will often suffice that a small fragment of the mineral, or a portion of it reduced to powder, should be placed in a concave receiver, a watch-glass for instance, and that it be covered with diluted acid; for this purpose the muriatic is commonly used, but the nitric or sulphuric is sometimes employed. When effervescence ensues, it is important to notice the rapidity and degree of effervescence; in some minerals it is great and rapid, in others slow, and not very apparent: sometimes the solution is complete; sometimes a residue is left, and occasionally, as in some of the alkalino-earthy substances, the mineral becomes gelatinous. In most cases, the process is carried on at the common temperature of the air; in others, by the application of a gentle heat.
Hence it will be concluded, that, in more than a few instances, the consequences of the action of acids form an important feature among the characters of minerals.
Analysis.
It forms no part of our present object to describe the manner in which the chemist pursues his researches. We look only to the results;—to the information which is to be derived from the labours of the chemist in regard to the number and nature of all the chemical elements of minerals which have hitherto been analysed.
Minerals have been considered as either simple or compound. Strictly speaking, very few minerals are found in a simple form; for if, by that expression, be understood the ultimate elements into which a body has been resolved, only a small number of the native metals will fall within that definition. The true chemical elements which constitute, by their various proportions and modes of combination, the immense variety of mineral bodies which nature presents to our observation, do not exceed fifty two in number. They are the following:—
NON-METALLIC.
| Oxygen. | Nitrogen. | Silicon. |
| Chlorine. | Hydrogen. | Sulphur. |
| Iodine. | Boron. | Selenium. |
| Bromine. | Carbon. | Phosphorus. |
| Fluorine. |
METALLIC.
ORDER 1.—Bases of the Alkalies and Earths.
| Potassium. | Strontium. | Glucinium. |
| Sodium. | Calcium. | Yttrium. |
| Lithium. | Magnesium. | Thorium. |
| Barium. | Aluminum. | Zirconium. |
[page] LII
ORDER 2.—Metals which decompose Water, and retain Oxygen, at a red heat.
| Manganese. | Tin. | Cobalt. |
| Zinc. | Cadmium. | Nickel. |
| Iron. |
ORDER 3.—Metals which do not decompose Water at any temperature, and whose Oxides are not reducible by heat.
| Arsenic. | Columbium. | Titanium. |
| Chromium. | Antimony. | Tellurium. |
| Vanadium. | Uranium. | Copper. |
| Molybdenum. | Cerium. | Lead. |
| Tungsten. | Bismuth. |
ORDER 4.—Metals the Oxides of which are reducible by a red heat.
| Mercury. | Platinum. | Osmium. |
| Silver. | Palladium. | Iridium. |
| Gold. | Rhodium. |
To describe at length the properties and combinations of these fifty-two simple bodies, would be foreign to the purpose of this work, and is indeed within the province of treatises on Chemistry. A concise enumeration of their leading characters, especially as respects their relation to the mineral kingdom, is all that is suitable to this place.
I.—NON-METALLIC BODIES.
OXYGEN has never yet been obtained in a state of complete insulation. Its most simple form is that of an elastic fluid or gas, resembling, in mechanical properties, the air of our atmosphere, and not condensible into a liquid by any known degree of cold. Oxygen gas was first obtained by Priestley in 1774, and may be separated by heat from black oxide of manganese, chlorate of potassa, &c. Its specific gravity, that of air being 1 at mean temperature and pressure, is 1·1026; hence 100 cubic inches weigh 34·1935 grains. Oxygen unites with some bodies slowly and imperceptibly; with others rapidly, and with the extrication of heat and light; and the resulting compounds are sometimes gaseous, sometimes fluid, at others solid. In the compounds thus generated new properties are apparent; some being acid, others alkaline, others, which are neither acid nor alkaline, being called oxides. Among the non-metallic bodies, hydrogen, carbon, and silicon, are those, in union with which oxygen is most abundantly diffused through the mineral kingdom, under the forms of water, carbonic acid, and silica. Of its combinations with metals, the most universal are those with calcium,
[page] LIII
aluminum, and iron. Oxygen gas constitutes about four fifths in volume of the air of our atmosphere, to which it imparts the properties of being respirable, and supporting combustion. Its combining proportion or equivalent number, that of hydrogen being taken as unity, is 8; its symbol O.
CHLORINE, discovered by Scheele in 1774, is a gas of a yellowish-green colour, not capable of being permanent over water, which absorbs twice its volume. Its specific gravity is 2·47, which gives 75·67 as the weight of 100 cubic inches. It is condensible, by a pressure of four atmospheres, into a yellowish liquid; is not respirable, and, if breathed unmixed, produces suffocation. It unites with an equal volume of hydrogen gas, giving two volumes of hydro-chloric or muriatic acid, which acid is absorbed to a great extent by water. Chlorine combines also with most of the metals, with some exhibiting the appearance of a brilliant inflammation. In nature it exists most abundantly in common salt (chloride of sodium). Its equivalent number is 35·42; its symbol CI.
IODINE was discovered in 1812, by M. Courtois of Paris. It may be ranked among mineral products, inasmuch as it exists in sea-water, and in the water of several natural springs. The process for obtaining it is too complicated to be described here; and it may be purchased ready prepared. It occurs in shining scales, having the lustre and colour of steel, or rather of micaceous iron ore, the specific gravity of which is 4·948. It is crystallizable; and the crystals have, for their primitive form, a rhombic octahedron. At 225° Fahrenheit it fuses, and at 347° forms a rich violet-coloured vapour, of specific gravity 8·7012; hence 100 cubic inches must weigh 269·84 grains. Iodine possesses an extensive range of combination, and forms acids both with oxygen, hydrogen, and chlorine; but, compared with any of these elements, it is a very rare production of nature.
BROMINE, discovered by M. Balard of Montpellier in 1826, exists in sea-water in the state of bromide of sodium or bromide of magnesium, but in very minute quantity; and sparingly also in several mineral springs. At common temperatures it is liquid; dark red by reflected, hyacinth-red by transmitted light; its odour is strong and unpleasant; its taste acrid. At 116° Fahrenheit it boils; between 0 Fahrenheit and — 4° it congeals. The density of its gas is 5·54; its equivalent 126·3; its symbol Br. It acts powerfully on animal substances, and is extremely poisonous. It unites with all the simple bodies that have been enumerated, and with the metals, forming, with the latter, a class of compounds called bromides.
[page] LIV
FLUORINE has never yet been obtained insulated. From analogy it is believed to constitute, with hydrogen, hydro fluoric acid, which was first described by Gay-Lussac and Thenard in 1810. The estimated proportion is 1 by weight of hydrogen to 18·68 fluorine, which number therefore expresses its equivalent. The great repository of this element in nature is the mineral called fluor spar, from which hydro-fluoric acid is obtained by distillation with sulphuric acid in a leaden vessel. The acid is gaseous over mercury, but act powerfully in glass vessels, which, to contain it, must be coated internally with bees' wax. The gas is copiously absorbed by water; and the liquid may be kept in leaden vessels well stopped, at temperatures under 60° Fahrenheit. A strong solution corrodes and destroys animal substances; and, when applied to the human skin, produces deep ulcerations. The gas unites with silica, and forms fluo-silic acid. The symbol of fluorine is F.
NITROGEN is not known to us separately in a solid or liquid form. The great repository of it is the atmosphere, of the whole volume of which it forms about four-fifths. It was discovered by Professor Rutherford of Edinburgh in 1772, and may be obtained by several processes, the object of most of which is to take away the oxygen gas from atmospheric air. It is a colourless gas, incapable of supporting respiration or combustion; tasteless, and free from smell; its specific gravity 0·976; sparingly absorbable by water; and in its gaseous state not disposed to enter readily into combinations. It is the base of nitric acid, an acid which enters into the composition of nitrate of potassa and nitrate of soda, both found in the mineral kingdom. The combining number of nitrogen is considered by some chemists to be 14·15, by others half of that number, according to their respective theoretical views. Its symbol is N.
HYDROGEN, in its simplest form, is a gas. It is obtained by the action of iron or zinc on dilute sulphuric acid. It is permanent over water; destitute of colour, and, when pure, of smell; combustible; and the lightest of all known bodies, its specific gravity being to air as 0·0687 to 1. With half its volume of oxygen gas it combines and forms water; with an equal volume of chlorine gas it forms hydrochloric acid; and it composes analogous acids with iodine, bromine, and fluorine. Its presence in the mineral kingdom is therefore very extensive, perhaps more so than that of any other element except oxygen. Its symbol is H.
BORON is an artificial product, obtained by the action of potassium on boracic acid, which acid is found in nature both se-
[page] LV
parate, and in union with soda. Boron is a dark olive-coloured solid, possessing neither taste nor odour; about twice the weight of water; not fusible when intensely heated in a close vessel, but, when exposed at the temperature of 600° to the atmosphere, taking fire, burning, and being converted into boracic acid. Its equivalent is 10·9, and that of boracic acid 34·9. The symbol of boron is B.
CARBON, or pure charcoal. In its ordinary form, this substance is best represented by the charcoal prepared by exposing wood to a red heat in close vessels. The diamond is a much purer variety. Charcoal is highly combustible, and has extensive powers of combination. When burned in oxygen gas, it does not, under circumstances favourable to such a result, alter the volume of the gas, but gives precisely an equal bulk of carbonic acid gas. This acid gas, if pure, has the specific gravity 1·5277; hence 100 cubic inches weigh 47·262 grains, a specific weight which, in some subterraneous places, occasions it to occupy a situation nearest the ground. It is non-respirable, and is incapable of supporting combustion. Carbon is most extensively diffused through the mineral kingdom, especially, as will afterwards appear, in the compounds of carbonic acid with various bodies, and in the several varieties of coal. Its equivalent is 6·12; that of carbonic acid 14·12.
SILICON is also an artificial product, obtainable from the earth called silex or silica, which enters into a very great number of mineral bodies, and in large proportions. Silicon was discovered by Berzelius in 1824. At first it was considered to be a metal, but it has since been thought to bear a more striking analogy to boron, carbon, &c. It is of a dark nut-brown colour, without any metallic lustre; incombustible in air or in oxygen gas; but oxidizable by circuitous methods, which convert it into silica, now more properly called silicic acid. Its equivalent is 7·5, and its symbol Si.; that of silicic acid is 15·5, and it is denoted by Si.
SULPHUR is an abundant product of the mineral kingdom, not only in a nearly pure state, but also in combination. As met with in commerce, it is chiefly the product of volcanoes; and it is also obtained from pyrites, a compound of iron or copper, and sulphur. Its colour is yellow; its specific gravity 1·99; it begins to fuse at 216° F., and becomes more and more fluid up to 280°. At 550° or 600° F., it is volatilized, and gives a vapour, the specific gravity of which is between 6·5 and 6·6. This vapour, when condensed, forms flowers of sulphur; and the flowers, when melted and cooled, become roll or stick sulphurs. By slow cooling, sulphur takes a regular crystalline form. When heated to 300°,
[page] LVI
or a little more, in the open air, it takes fire and burns, with a blue flame of suffocating smell.
Sulphur unites with oxygen in various proportions; but its most important compound with that basis is sulphuric acid. With hydrogen it forms sulphuretted hydrogen gas, a natural product; and with the metals it constitutes the important class of mineral compounds called sulphurets. Its equivalent number is 16·1; its symbol S.: the equivalent of sulphuric acid is 40·1, and its symbol .
SELENIUM, first made known by Berzelius in 1818, is rather a rare substance. It was first obtained from some varieties of Swedish iron-pyrites, and has since been found combined with lead, cobalt, silver, mercury, and copper. Its chemical habitudes approach most nearly to those of sulphur, from which, however, it is readily distinguishable. It has, when in mass, a metallic lustre and the aspect of lead; and, when pulverized, exhibits a deep-red colour; its specific gravity is 4·3; at 212° F. it softens, and may be drawn into fine threads, which are red by transmitted light. It becomes fluid a little above 212°, and at 650° is converted into a deep-yellow vapour. It unites with oxygen in various proportions, forming acids; and with hydrogen constitutes a gas which powerfully affects the nose and eyes, and has an acid re-agency. Its equivalent is 39·6, and its symbol Se.
PHOSPHORUS is an artificial solid, obtained most abundantly, by a circuitous process, from bones, but also derivable from the mineral kingdom. It is fusible at 108° F.; volatile at 550°. It is highly combustible, both in air, in oxygen gas, and in chlorine. According to the proportion of oxygen, it forms different acids; and one of these, the phosphoric acid, in union with lime, exists in the mineral kingdom. It unites also with hydrogen. Its equivalent is 15·7; its symbol P.
II—METALS.
ORDER I.
POTASSIUM was discovered by Sir H. Davy in 1807. It does not exist in nature as a metal, but is obtained by artificial methods, all of which have in view the decomposition of potash or potassa. It is soft and solid at common temperatures, and yields like wax to pressure; it begins to melt at 70°, and is quite fluid at 150° F. It rises into vapour when heated in a vessel from which atmospheric air is excluded. In colour and lustre it resembles quicksilver; is quite opake; conducts heat and electricity, and
[page] LVII
has the specific gravity 0·865, or it is considerably lighter than water. It is highly oxidable, and even takes oxygen from water, on the surface of which it burns with a bright flame by a succession of explosions. In the proportion of 39·15 to 8 parts of oxygen, it constitutes potassa. The former number therefore represents its equivalent, and 47·15 that of potassa. Its symbol is K, the first letter of Kalium, by which name it has been distinguished by foreign chemists.
SODIUM, in its external properties, resembles potassium; but it has greater specific gravity, viz. 0·972. It fuses at 200° F., and is not volatilized by a heat under redness. When united with oxygen in the proportion of 23·3 by weight to 8 oxygen, it constitutes soda, an important ingredient of several mineral substances, such as the chloride and the carbonate of that earth.
LITHIUM. The oxide of this metal, lithia, was discovered by M. Arfwedson in 1818, in the mineral called petalite; and it has since been extracted from spodumene, lepidolite, and some kinds of mica, and also from the waters of Carlsbad, by Berzelius. From lithia, the metal is obtained by de-oxidizing processes; but if placed in contact with air, it returns to the state of lithia too rapidly to admit its accurate examination. The precise proportion in which lithium unites with oxygen is not known. Indirectly its equivalent has been estimated at 10·1, and that of lithia at 18·1. In its obvious properties, lithia approaches to potassa and soda, but has a greater neutralizing power.
BARIUM. Baryta, the source of barium, a metal which can only be got by chemical operations, was discovered by Scheele in 1774. Barium has a dark-grey colour, and a lustre resembling that of cast iron. It is much denser than water, and even than sulphuric acid. It attracts oxygen with avidity, and is re-converted into baryta, of which 76·7 parts contain 68·7 barium and 8 oxygen, numbers indicating the equivalents of these bodies.
Baryta is pretty extensively diffused through the mineral kingdom, chiefly in combination with the carbonic and sulphuric acids.
STRONTIUM. The source of this metal, which does not exist as such in nature, is strontia. The carbonate of that earth was first accurately examined, and the peculiarities of its earthy base established, by Professor Hope of Edinburgh in 1792. Little is known of the properties of strontium in its metallic form; and the composition of the earth which it forms with oxygen has been deduced chiefly from the indirect experiments of Stromeyer. These render it probable that 43·8 parts of strontium unite with 8 parts of oxygen, and give 51·8 of strontia. The symbol of stron-
C 2
[page] LVIII
tium is Sr., of strontia Ṡr. In nature, the carbonate and the sulphate of strontia are not very uncommon productions.
CALCIUM, the metallic base of the well-known earth lime, is of a whiter colour than barium or strontium, and is rapidly converted back again into lime, of which 20·5 parts contain 8 oxygen and 20·5 calcium. Lime, or protoxide of calcium, forms a very large proportion of the crust of our globe, chiefly in the shape of carbonate of lime, which constitutes whole mountains and extensive strata; and also of sulphate of lime. Lime, it is well known, heats violently on the addition of water; is sparingly soluble in that fluid; has an alkaline re-agency, and enters into energetic combination with a great variety of bodies. The symbol of calcium is Ca.
MAGNESIUM, or rather its oxide, magnesia, is pretty extensively diffused as a constituent of mineral bodies. The equivalent of the metal, approximated indirectly, is 12·7; and this, with one proportion of oxygen, forms 20·7 of magnesia. The chief natural compounds of magnesia are with sulphuric, muriatic, silicic, and carbonic acids; and with alumina and other earths.
ALUMINUM or ALUMINIUM is the metallic base of alumine, or pure argillaceous earth or clay, than which, few substances are more extensively diffused throughout the mineral kingdom. Aluminum, artificially obtained, presents the appearance of a grey powder, very similar to that of platinum. It requires an intense heat for its fusion, and shows a feeble affinity for oxygen, so far as is indicated by direct combination. Alumina is viewed by Berzelius and others as a sesquin-acid, consisting of two equivalents of aluminum (27·4), and three equivalents of oxygen, (24), making up the number 51·4 for the equivalent of that earth.
GLUCINIUM. Its source, glucina, was identified as a distinct earth by Vauquelin in the year 1798. At first it was found only in euclase, beryl, and emerald, and may still be considered a very rare product of nature. Its name was derived from a Greek word signifying sweet, a property observed in all its salts. Glucina itself is white, insipid, and insoluble in water. Its equivalent number is not accurately ascertained.
YTTRIUM is the base of the earth yttria, which was discovered by Professor Gadolin in 1794, in a mineral found at Ytterby in Sweden, since called gadolinite. Yttria bears a considerable resemblance in its properties to glucina. The equivalent of yttrium deduced by Berzelius is 32·2, and that of yttria 40·2.
[page] LIX
THORIUM. The earth thoria has only been obtained by Berzelius from a rare Swedish mineral, now called thorite. The high specific gravity of this earth, 9·402, is its most remarkable characteristic. Its equivalent is not known, but is supposed to be about 67·6.
ZIRCONIUM is the base of the earth zirconia, which was discovered in 1789 by Klaproth, in the jargon or zircon of Ceylon, and since in the hyacinth of France. The nearest approximation to its equivalent is probably, according to Berzelius, between 30 and 33.
ORDER II.
MANGANESE was first obtained in a metallic form by Gahn, from the black oxide of manganese, a substance first investigated by Scheele. As a metal, it possesses so powerful an affinity for oxygen, that it never occurs native. It is of a grey colour; has a granular texture; a specific gravity of about 8; is hard and brittle; and is very difficult of fusion. Its oxides are, 1st, the protoxide, which is the base of all the salts of manganese. This is of a light-green colour, is composed of 27·7 parts by weight of metal, and 8 of oxygen; and has so strong an attraction for a further proportion of that basis, as to take fire and burn when heated to about 600° F. in the open air. 2d, The sesqui-oxide, which may be obtained artificially, is also found in nature, combined only with water, and constituting prismatic crystals. It is the sesqui-oxide which remains after heating the next oxide to redness. It consists of two equivalents of manganese and three of oxygen. 3d, The per-oxide, the well-known black ore used in preparing chlorine, consists of 27·7 manganese and 16 oxygen, or of one equivalent of metal and two equivalents of oxygen. Besides these well-characterized oxides, there are two others which occur native, but each of which may perhaps be properly regarded as compounded of two of those already described. There are also two other oxides, the red oxide, and the mineral called varvicite. Besides these, the sulphuret is the only natural compound of manganese.
IRON is a metal too well known to need description. Of all the metals, it is the one which is most abundant in the mineral kingdom. It is malleable and ductile; its specific gravity, which varies according to the processes it has undergone, is about 7·7; it powerfully attracts oxygen, and in oxygen gas even burns with brilliant corruscations. There are two distinctly characterized oxides of iron; the one, which is black, but affords green salts with acids, is constituted of twenty-eight parts
[page] LX
of iron and eight of oxygen, and is called the protoxide; the other, named peroxide, may be viewed as composed of two equivalents (28 × 2) = 56 of iron, and three equivalents of oxygen. The colour of the peroxide is red, and it imparts that colour to its saline combinations. Besides these two oxides, there is also a native black compound of iron and oxygen, which is probably composed of the protoxide and peroxide in atomic proportions. It is found in regular octahedral crystals, which not only affect the magnet, but are sometimes magnetic. Iron also enters very extensively into combinations with other simple and compound bodies. Its compounds with sulphur are, next to its oxides, the most important to the mineralogist. The proto-sulphuret, both artificially prepared and occurring native, consists of 28 iron and 16 sulphur; the sesqui-sulphuret is an artificial product, of 56 iron and 48 sulphur; and the bi-sulphuret (native iron pyrites) consists of 28 iron and 32 sulphur. Besides these, there are other sulphurets formed by the union of the preceding ones in different proportions. With carbon, iron forms that useful mineral product plumbago, or graphite. The symbol of iron is Fe.
CADMIUM was discovered by Stromeyer in 1817, in an oxide of zinc. It may be obtained from the sublimate which rises from calamine. Cadmium is both ductile and malleable; resembles tin in appearance and fusibility; is nearly as volatile as mercury; and its vapour, which is free from odour, condenses into shining drops. Its specific gravity is about 8·6. When heated in the open air it is readily oxidized. Its only oxide is of an orange yellow, and consists of 55·8 cadmium and 8 oxygen. Its symbol is Cd.
TIN, in the form of pure grain tin, is of a white colour, resembling silver, and has a similar lustre. It is malleable and ductile, the latter in a less degree than some other metals. Its specific gravity is about 7·9; it fuses at 442° F.; and, when more strongly heated, takes fire and burns into protoxide, which consists of 57·9 tin and 8 oxygen. This oxide is combustible, and, when touched by a red-hot body, burns in the air with peroxide, consisting of the same weight of metal and 16 oxygen. Besides these, an artificial compound may be formed of two equivalents of tin and 3 of oxygen, which has been termed sesqui-oxide. Analogous to these are the three sulphurets, the proto-sulphuret, bi-sulphuret, and sesqui-sulphuret. The symbol of tin is Sn, from its Latin name Stannum.
COBALT is principally obtained from an ore of arsenic, and is found in small proportion in meteoric iron. It is a brittle metal, of a reddish-grey colour, and feeble lustre; its specific gravity
[page] LXI
about 7·8; fusible at a heat rather below that at which iron melts; attractable by the magnet; and readily oxidable. It constitutes, with oxygen, two distinct oxides, one consisting of 29·5 cobalt and 8 oxygen, which is the protoxide; the other, of 59 metal and 24 oxygen, is the peroxide. There is also an intermediate compound between these two. The distinguishing character of this metal is, that in solution it forms the basis of the best sympathetic inks. Sulphur also unites with cobalt in three proportions. Its symbol is Co.
NICKEL is obtained from the ore of cobalt. It has the specific gravity of about 8·5 to 9; is ductile and malleable, and is not only attracted by the magnet, but capable of being itself rendered magnetic. It is very infusible; is not altered by the air at common temperatures, but absorbs oxygen at a red heat. The protoxide consists of 29·5 metal and 8 oxygen, the peroxide of 59 metal and 24 oxygen. The solutions of this metal in acids have, for the most part, a beautiful green colour.
ORDER III.
ARSENIC, in its metallic form, has a strong metallic lustre; is brittle, and reducible to powder; is volatilized, without fusing, at 356° F.; and, in close vessels, condenses into a brilliant solid. The specific gravity of metallic arsenic is 5·88. Its vapour is characterized by a strong odour, resembling that of garlic. Arsenic readily combines with oxygen, and forms two compounds. The first, known as common arsenic, or white oxide of arsenic, but now more properly termed arsenious acid, is of a white colour, sparingly soluble in water, and intensely poisonous. It consists of 75·4 arsenic and 24 oxygen. The second, arsenic acid, is the result of chemical operations, but is also found native in combination. It consists of 75·4 metal and 40 oxygen. Of the sulphurets there are three, constituted of one equivalent of arsenic, with one, three, and five equivalents of sulphur respectively.
CHROMIUM was discovered by Vauquelin in 1797, in a beautiful red mineral, then called chromate, more properly dichromate, of lead. Chromium is a brittle infusible metal, of specific gravity about 5; capable of uniting with oxygen, and of forming two distinct compounds. The green sesqui-oxide consists of 56·2 chromium (two equivalents) and 24 oxygen (three equivalents). The chromic acid of 28·1 (one equivalent) of metal, and 24 (three equivalents) of oxygen. All the compounds of chromium are distinguished by their brilliant colours, from whence is derived the name of this metal.
[page] LXII
VANADIUM was discovered in 1830, by Sefström, and has since been found by Professor Johnston in vanadiate of lead. It is extracted by complicated processes; and is but indistinctly characterized as a metal. In colour it resembles silver, or rather molybdenum; it is extremely brittle, and is not readily oxidized. When, however, this has been effected by circuitous processes, it forms two distinct compounds, the protoxide, composed of 68·5 vanadium and 8 oxygen; the peroxide, of 68·5 metal and 16 oxygen.
MOLYBDENUM is a white, brittle, and very infusible metal; its specific gravity about 8·6; it is easily oxidizable, and has three degrees of oxidation, one of which is acid. In the protoxide, peroxide, and molybdic acid, 47·7 molybdenum are respectively united with 8, 16, and 24 oxygen. It combines also in three proportions with sulphur.
TUNGSTEN is a metal, not found pure in nature, but obtainable by chemical operations; it is of a greyish-white colour; has considerable lustre; is brittle, and infusible except at an intense heat. With oxygen it forms two compounds, the dark brown oxide, consisting of 99·7 tungsten and 16 oxygen, and the yellow acid, constituted of the same proportion of metal and 24 oxygen. There are also corresponding sulphurets.
COLUMBIUM, or TANTALUM, discovered by Mr Hatchett in 1801, is a very rare metal, existing chiefly in tantalite and yttro-tantalite. When extracted by chemical processes, it has the form of a grey powder, which, by pressure, acquires a metallic lustre, and then exhibits an iron-grey colour. This metal takes fire when heated in contact with air, and burns into columbic acid, which is constituted of 185 metal and 24 oxygen. There is also an oxide, composed of the same proportion of columbium united with 16 oxygen.
ANTIMONY has been known ever since the fifteenth century, when it was discovered by Basil Valentine. It is principally obtained from the native sulphuret called crude antimony. It is brittle, of a white colour, with a shade of bluish grey. Its specific gravity is 6·7: it is fusible at 810° F., and on cooling sometimes forms crystals. If heated out of contact with atmospheric air, it is not volatile; but when air is present, it inflames at a white heat, and forms an oxide, which condenses in white needles, formerly called flowers of antimony, but now considered and named as a sesqui-oxide. In this, 125·2 parts by weight of antimony (two equivalents) are united with 24 (three equivalents) of oxygen. In antimonious acid, the same proportion of
[page] LXIII
metal is combined with 32 oxygen, and in antimonic acid with 40 of oxygen. There are three sulphurets, the proportions of which correspond with those of the oxides.
URANIUM. Klaproth first pointed out this metal in a mineral found in Saxony, called pitchblende, from which it may be extracted by chemical processes. Its metallic properties are faintly marked, but it has some lustre. It is not changed by air at common temperatures; but, when heated in an open vessel, it absorbs oxygen, and is converted into protoxide. The equivalent number of the metal, arrived at by indirect processes, is 217.
CERIUM was discovered in 1803, by Hisinger and Berzelius, in a rare mineral called cerite, and subsequently by Dr Thomson, in a mineral called allanite, in honour of the late Mr Allan, who first showed it to be a distinct species. In a metallic state, the properties of cerium are very imperfectly known. Its equivalent number has been stated at 46; and it is believed to form two oxides, the protoxide, of a white colour, consisting of one equivalent with one of oxygen; the peroxide, of a fawn red, composed of the same proportion of cerium with three equivalents of oxygen.
BISMUTH is a well-characterized metal, and can be obtained in considerable quantity. It is brittle when cold. Its colour is reddish white, and it has considerable lustre; its density about 10. It fuses at 476° F., and, when slowly cooled, crystallizes in octahedrons. In close vessels it sublimes, but not under a red heat. In open vessels it burns at that temperature into a white volatile oxide, in which 71 of metal are united with 8 of oxygen. By circuitous processes, 2 equivalents of bismuth (= 142) may be brought to unite with 3 equivalents of oxygen. The sulphuret is a compound of one equivalent of each of its elements.
TITANIUM was first found by Mr Gregor of Cornwall, in a mineral called menaccanite, and has since been detected in several other minerals. In 1822 Dr Wollaston remarked it forming very small but perfect cubes in an iron slag, and it appears not to be uncommon in the refuse of iron furnaces. These small cubes have a specific gravity of 5·3; they are exceedingly hard and infusible. They resist the action of solvents applied in the usual way, but, by particular management, the metal may be oxidized. The equivalent of the metal is probably near 24·3. It appears to be susceptible of uniting with one equivalent of oxygen, and also with two; the latter constituting titanic acid.
TELLURIUM was discovered by Klaproth, about the year 1798,
[page] LXIV
in an ore of gold. When extracted by artificial methods, and metallized, it is of a tin-white colour, verging to lead grey; has considerable lustre, and a foliated or scaly fracture. It is very brittle; fusible below ignition; and, excepting osmium and mercury, is the most volatile of all metals. Its specific gravity does not exceed 6·185. It is susceptible of two degrees of oxidation, both of which exhibit acid properties. The one, consisting of 64·2 metal and 16 oxygen, formerly called oxide of tellurium, has a feeble acid re-agency, and is now termed tellurous acid; the other,telluric acid, is constituted of the same proportion of metal and 24 oxygen. The bi-sulphuret and ter-sulphuret of tellurium are analogous compounds; the former of two equivalents, the latter of three of sulphur to one of tellurium.
COPPER has been known from the most ancient times, and, next to iron, constitutes one of the most valuable and abundant mineral treasures of this island. In its metallic state it is of a fine red colour; it is capable of considerable lustre; is both malleable and ductile; and has a specific gravity, varying with its purity and the processes it has undergone, from 8·434 to 9·0. At 27° of Wedgewood's pyrometer it melts, and emits fumes. It has a great affinity for oxygen, and may be converted into black oxide by long exposure to a sufficient heat with contact of air. This compound, consisting of 64 parts of copper and 16 oxygen, was considered by some chemists as the peroxide, and as constituted of one equivalent of metal and two equivalents of oxygen, which would make the number for copper 64; but it is now most commonly regarded as the true binary oxide, constituted of an atom of each of its elements, which fixes the equivalent of copper at 32. There is also an orange-red oxide of copper found native in octahedrons, and obtainable by chemical processes, which consists of 64 metal and 8 oxygen. This may be regarded as constituted of two atoms of metal (32 × 2 = 64), and one atom of oxygen, i.e. as a suboxide or dioxide. This suboxide is permanent at ordinary temperatures, but at a red heat is converted into the black oxide or peroxide. There are also chlorides and sulphurets of copper, analogous in atomic proportions to the oxides. Copper glance (the equivalent of copper being taken at 32) is a disulphuret, consisting of two atoms of base and one atom of sulphur. The true sulphuret, consisting of one equivalent of each element, is one of the ingredients of copper pyrites, in which it exists along with protosulphuret of iron.
LEAD. This well-known metal is of a bluish-grey colour, of specific gravity 11·381, very malleable, and ductile in a small degree. It melts at 612° F., and, when slowly cooled, shoots
[page] LXV
into octahedral crystals. It is readily oxidized when exposed at high temperatures to the air. Its protoxide, which is yellow, and known under the name of massicot, is constituted of 104 lead and 8 oxygen. The peroxide is of a puce colour, and is constituted of the same proportion of metal with 16 oxygen. The beautiful red compound, called minium, or red lead, is not a true atomic compound; but is variable as to the proportions of protoxide and peroxide which constitute it. The sulphuret, which is the most abundant source of the lead of commerce, consists of one equivalent of lead, and one equivalent of sulphur.
ORDER IV.
MERCURY or QUICKSILVER, as is well known, is fluid at common temperatures. Its specific gravity at 47° F. is 13·545. At 39° or 40° below 0 F. it becomes a solid, which may be flattened by the hammer or cut with a knife, and has the specific gravity 15·612. At a temperature variously stated between 656° and 680° F. it boils, and is convertible into a vapour, the specific gravity of which exceeds, by very nearly seven times, that of atmospheric air. Mercury has two distinct oxides; the protoxide, of a black colour, consists of 202 (1 equivalent) of mercury and 8 oxygen; the second, or peroxide, of a fine red colour, is obtained by long-continued calcination, and consists of 202 mercury and 16 oxygen. Mercury forms also, with sulphur, two definite compounds; the first, of a black colour, consists of an equivalent of each element; the second, cinnabar, which is of a beautiful red colour when powdered, and is then well known as vermilion, occurs native, and is also an artificial product. The latter consists of 202 mercury and 32 (two equivalents) of sulphur.
SILVER has a beautifully white colour, and is inferior in lustre only to polished steel. Its specific gravity, after being hammered, is 10·51. In malleability and ductility it is superior to all the metals except gold. At 22° of Wedgewood's pyrometer it fuses, and by slow cooling forms crystals. It does not, like most other metals, enter in several proportions into union with oxygen, chlorine, or sulphur, but forms only one compound with each of those elements. In the proportion of 108 by weight to 8 oxygen, it constitutes the oxide of silver; with 35·42 chlorine, the chloride; and with 16 sulphur, the sulphuret. When alloyed with copper, in the proportion of about one-twelfth of the weight of the latter metal, it constitutes the standard silver of this country, which, though differing little in colour from pure silver, is much harder, and less liable to wear.
[page] LXVI
GOLD is the only metal which has a yellow colour. Its specific gravity varies with the processes which it has undergone; but may be stated, on an average, at 19·3. It surpasses all metals in malleability and ductility. At a heat of about 32° Wedgewood it fuses, and, on cooling slowly, shoots into quadrilateral pyramids. It is not volatile at any known temperature. One of its most valuable properties is, that it may be exposed to the air for ages without change. It may, however, be oxidized by chemical processes, and unites in the proportion of 199·2 gold to 8 oxygen, forming protoxide; and of the same proportion of gold to 24 oxygen, constituting the peroxide of gold. The intermediate compound, or deutoxide, is supposed to be the purple substance which is formed when gold is burnt by intense heat or galvanic electricity. Only one sulphuret is known, constituted of 199·2 gold and 48·3 (3 equivalents) of sulphur.
PLATINUM. This metal, which, if it were more plentiful and cheap, would be applicable, on account of its infusibility and property of resisting most chemical agents, to a variety of valuable purposes, is inferior in beauty and lustre to silver, but exceeds that metal and all others in specific gravity, which is between 21 and 22. Among the metals it is one of the slowest conductors of heat, and is less expansible than most of them by that agent. It is both highly malleable and ductile. It is not oxidizable, even by the long-continued action of heat and air, but may be brought to combine with oxygen by circuitous processes, which afford two well-characterized oxides. The protoxide consists of 98·8 (1 equivalent) of platinum and 8 oxygen; the peroxide, of the same weight of metal and 16 oxygen; and there appears also to be an intermediate oxide, consisting of 2 equivalents (197·6) of platinum and 3 equivalents of oxygen. There are also chlorides and sulphurets of platinum corresponding with the above as to equivalent proportions of their elements.
PALLADIUM was discovered by Dr Wollaston in 1803, forming distinct small fragments in the native ore of platinum. He extracted it also by complex chemical processes, which he has described in the Phil. Trans. for 1804. In colour it resembles platinum, but is of a duller white. It is malleable and ductile; its specific gravity varies from 10·972 to 11·482. It is not fusible or oxidizable at a degree of heat sufficient to melt gold, but at a stronger heat melts, and, on cooling, affords a mass, of specific gravity 11·871. By indirect methods it combines with oxygen. Its protoxide is black, and consists of 53·3 (1 equivalent) of palladium and 8 (1 equivalent) of oxygen; its peroxide, also black, is constituted of 1 equivalent of metal and 2 equivalents of oxygen.
[page] LXVII
RHODIUM is also a discovery of Dr Wollaston, made at the same time, and from the same source, as palladium. It has a white colour, a metallic lustre, is brittle, and extremely hard, and has a specific gravity of about 11. It attracts oxygen from the air when heated to redness; but resists, unless when alloyed, the action of acids. Two oxides are known, the protoxide, which is black, and enters into the salts of rhodium, is constituted of 52·2 (1 equivalent) of metal, and 8 (1 equivalent) of oxygen; the peroxide, of 2 equivalents of metal, and 3 equivalents of oxygen.
OSMIUM is another ingredient of the ore of platinum, in which it was discovered in 1803 by Mr Smithson Tennant. It can only be extracted by complicated methods, which present it in the form of a black powder, susceptible of metallic lustre by friction; of the specific gravity of about 7. It takes fire in the open air, and its oxide, which is volatile, has an acrid and suffocating odour. From the experiments of Berzelius, it seems to be capable of entering into combination with several proportions of oxygen. Its equivalent number is probably about 99·7.
IRIDIUM is another elementary metal (the fifth) which enters into the crude ore of platinum. It is evolved in the process by which osmium is separated from the same ore. Its distinguishing property is the variety of colours which it exhibits (from iris, the rainbow). It is a very brittle metal, susceptible, when carefully burnished, of considerable polish. It is very difficult of fusion; but when fused in Mr Children's experiments, with the aid of a powerful galvanic battery, it had the specific gravity of 18·68. It is oxidized by a red heat, but only when finely divided; and it is not easily acted upon by acids. From the researches of Berzelius, who estimates the equivalent of iridium at 98·8, it appears to have three degrees of oxidation; and it is the rapid transition of these oxides into each other, that occasions the variable tints of iridium.
Such are the comparatively few elements constituting the immense variety of bodies that are objects of classification and description to the mineralogist. That these elements are combined together in definite proportions (at least in all well-characterized, and especially in crystallized minerals), does not admit of the smallest doubt. In a great number of instances, the results of analysis fully bear out this general proposition. But minerals that are less distinctly characterized (all those, for example, that are called amorphous), are also less uniform in the
[page] LXVIII
proportions of their constituents, probably from the admixture of foreign matters. We may, however, reasonably entertain sanguine hopes of great advantages from the alliance of chemical with mineralogical science; and may confidently expect that the former will in time furnish data for a truly philosophical arrangement of mineral bodies.
[page LXIX]
EXPLANATION OF TERMS
Used in Mineralogical Descriptions.
Acicular. Long, slender, and straight prisms, or crystals, are termed acicular, from the Latin, acicula, a little needle.
Aggregated. A mineral or rock is said to be aggregated when the several component parts only adhere together, and may be separated by mechanical means: the felspar, quartz, and mica, constituting granite, may be separated mechanically. Granite is an aggregated rock.
Alliaceous. The odour given out by arsenical minerals, when exposed to the blowpipe or struck by the hammer, resembles that of garlic, in Latin, allium; whence alliaceous.
Alloy. A natural combination of two or more metals in the metallic state.
Amalgam. A natural combination of two metals, of which mercury is one.
Amorphous. Without form; of undefinable shape; from the Greek, αμοζϕοε having that signification. Amorphous minerals are sometimes described as being of indeterminate or indefinite forms.
Anhydrous, from the Greek ανύδζο, signifying without water.
Arborescent. From the Latin arboresco, to grow like a tree. See Dendritic.
Arseniate. A term applied to a mineral consisting of arsenic acid united with a base.
Base. A term denoting the substance to which an acid is united; in the arseniate of copper, the copper is the base.
Borate. A mineral in which boracic acid is combined with a base.
Botryoidal. From the Greek βοτζυδηο, signifying hung with clusters of grapes or berries. So a mineral presenting an aggregation of large sections of numerous small globes is termed botryoidal; but when the globes are larger, and the portions are less and separate, the appearance is expressed
[page] LXX
by the term mammillated. These forms may be observed in certain ores of cobalt, copper, and manganese, and often in calcedony.
Brittle. This character of mineral bodies does not depend upon their hardness; those of which the particles cohere in the highest degree, and are immoveable one among another, are the most brittle. The diamond, quartz, sulphate of barytes, and sulphur, vary greatly as to hardness; they are all brittle, the first only in particular directions.
Capillary, derived from the Latin capillus, a hair, is chiefly used to express the long, tortuous, hair-like appearances observable in native gold, silver, and some other minerals. Crystals are sometimes termed capillary when long and slender; but when also straight, they are more properly designated acicular.
Carbonate. A mineral in which carbonic acid is combined with a base.
Cellular. This term was used by Werner in the description of such minerals as exhibit cells formed by the crossing and intersecting of the lamellæ of which they are constituted; commonly, any mineral presenting numerous small cells or cavities is termed cellular. See vesicular.
Chatoyant has been adopted from the French, who use it to express the changeable light resembling that observable in the eye of a cat, exhibited by certain minerals.
Chromate, a mineral in which chromic acid is united with a base.
Cleavage. This term is most commonly used in relation to the fracture of those minerals which, having natural joints, possess a regular structure, and may be cleaved into more or less geometrical fragments; as, into varieties of the parallelopiped, the rhomboid, &c.
Coherent. In minerals that are brittle, the particles are strongly coherent; in such as are friable they are slightly coherent.
Columnar distinct concretions; a term used to express the great and small columns in which certain iron ores and other minerals are found.
Compact. A mineral is compact when no particular or distinct parts are discernible; a compact mineral cannot be cleaved or divided into regular or parallel portions. It is too often confounded with the term massive.
Concentric lamellar. This relates to structure, and is used in the description of such minerals as, being of a spherical form, have received successive coatings or depositions. An onion cut in two exhibits the concentric-lamellar appearance in perfection.
Conchoidal relates only to fracture, and is derived from the
[page] LXXI
Latin conchoides, signifying like a shell. Many of the brittle minerals exhibit this appearance, and occasionally in great perfection, as quartz, sulphur, &c.
Concretion generally signifies a small and distinct mass.
Coralloidal, resembling branches of coral.
Cuneiform, wedge-shaped; cuneus, in Latin, signifies a wedge.
Decomposed. This term, when used strictly in a mineralogical sense, imports the consequence of the chemical action which takes place naturally in some minerals. Certain ores of iron, &c. in which sulphur predominates in an unusual degree, decompose by exposure to air.
Decrement. This term relates to structure. See p. xxviii.
Decrepitate. A mineral is said to decrepitate on exposure to heat when it flies with a crackling noise similar to that made by salt when thrown into the fire.
Dendritic, derived from the Greek δενδιτις, signifying, like the growth of a tree. The terms arborescent and dendritic are used synonymously: they are alike applied to the tree-like appearance in which native silver and native copper are sometimes found; to the delineations seen on the surfaces of certain minerals; to the appearance in the mocha-stone, &c.
Dentiform, in the shape of teeth; dens being the Latin for a tooth.
Disseminated. When a mineral, whether crystallized or otherwise, is found here and there imbedded in a mass of another substance, it is said to be disseminated in the mass.
Disintegrated is generally used to express the falling to pieces of any mineral, without any perceptible chemical action.
Diverging, or Divergent. When the structure is fibrous, and the fibres are not parallel, they usually diverge in part, but not wholly, around a common centre; as in certain zeolites, and hæmatitic irons. The crystals of some substances assume a diverging position.
Drusy has been adopted from the German term drusen, for which we have no English word. The surface of a mineral is said to be drusy when composed of small prominent crystals, nearly equal in size; it is often seen in iron-pyrites.
Efflorescence is the consequence of chemical action; it is applied to such minerals as are found in extremely minute fibres on old walls, &c.
Elastic. A mineral which, after being bent, springs back to its original form, is elastic. Mica is elastic; talc, which greatly resembles mica, is only flexible.
Earthy. This term relates to fracture and to texture. Chalk,
[page] LXXII
and certain of the ores of iron and lead, are notable instances of the earthy fracture or texture.
Fasciculated. When a number of minute fibres or acicular crystals occur in small aggregations or bundles, they are said to be fasciculated; from the Latin fasciculus, a little bundle.
Fibrous. This term relates both to form and structure. Certain minerals, as amianthus, gypsum, &c. occur in distinct fibres. Asbestus, red hæmatite, &c. are found massive, and present a parallel fibrous structure; others are of a radiating fibrous structure, when the fibres diverge from a common centre.
Filament. A mineral is said to occur in filaments when it is found in slender thread-like or hair-like portions. It is therefore nearly synonymous with the term capillary.
Filiform is used in the same sense as the preceding, but Werner employed it to express the appearance of certain metals which occur in the form of wire, as native silver and native copper. Filum, in Latin, signifies thread; filum metalli, wire.
Flexible. Talc is flexible; it readily bends, but does not return of itself to its original form. Mica is both flexible and elastic.
Fluate. This term designates a mineral in which fluoric acid is combined with a base.
Foliated. This term, from the Latin foliatus, having or consisting of leaves, is used by Werner to express the structure of all minerals that may be divided or cleaved regularly, and are therefore by him said to consist of folia or leaves. The structure of such minerals is more commonly and better expressed by the term lamellar; and they are said to consist of laminæ.
Fracture is a term chiefly employed in designating the appearance of minerals which have no regular structure when they are broken; such minerals present an earthy, even, uneven, or conchoidal fracture, &c.
Frangible. This term relates to the susceptibility of minerals to separate into fragments by force: it is a quality not dependent on hardness; the structure of some, and the brittleness of other minerals, render them easily frangible; while many, from their softness, and the ease with which their particles or molecules yield or slide over one another, are much more difficultly frangible; these possess the character of toughness. Quartz is easily broken, asbestus is tough.
Friable. A mineral whose portions or particles slightly cohere, and which is therefore easily crumbled or broken down, is said to be friable.
[page] LXXIII
Fungiform. Certain substances, as, for instance, calcareous stalactites, are occasionally met with, having terminations similar to the head of a fungus; whence the term.
Gangue, from the German. The gangue or matrix is the substance in or upon which a mineral is found.
Geode. This also is derived from the German; a geode is a hollow ball, generally lined with crystals.
Glance is a German word, meaning shining; thus, glance-coal, copper-glance, &c.
Globular distinct concretion is used to designate the form of any mineral which occurs in small round or roundish masses: pea-stone and roe-stone are examples of it.
Granular. The structure of a mineral is said to be granular when it appears to consist of small grains or concretions, which sometimes are, sometimes are not, discernible; we have therefore the fine granular and the coarse granular structure.
Greasy is used in relation to lustre.
Hackly. This term relates to a fracture which is peculiar to the malleable metals; which, when broken, present sharp protruding points.
Hæmatite is derived from the Greek άιματιτις, signifying blood-red. It was first applied to the variety of iron ore which is called Red Haematite; but has since been extended to other iron ores of the same structure, but differing in colour.
Hepatic, from the Latin hepar, the liver: it is applied either to colour or form.
Hydrate is derived from the Greek υδωζ, water; and is applied to those minerals of which water forms an ingredient in large proportion.
Imbedded. A mineral found in a mass of another substance is said to be imbedded in it.
Incrusted. Any substance covered by a mineral is said to be incrusted by it.
Interlacing. When fibres or crystals of a mineral are found intermingling with each other in various directions, they are said to be interlacing.
Investing. A mineral coating or covering another is sometimes described as investing it.
Iridescent. This term relates only to the colour with which the surfaces of some metallic species are naturally tarnished.
Irisated. A mineral is described as irisated which exhibits the prismatic colours either externally or internally: the latter is generally the consequence of some injury sustained by the mineral.
[page] LXXIV
Lamella, Lamellar, relate to structure. When a mineral can be fractured or cleaved into regular and parallel plates, its structure is said to be lamellar; and the portions thus obtained are termed laminæ or lamellæ. These terms have been adopted from the Latin, in which they were almost synonymously used to express thin plates of any substance.
Lamellar distinct concretions. This is used to denote the form of certain minerals consisting of separate tabular crystals.
Lamelliform. A mineral consisting of lamellæ is said to be lamelliform.
Lenticular is employed to express the forms of certain crystals which are nearly flat, and convex above and beneath; and which consequently resemble a common lens.
Malleability. Some of the metals suffer extension when beaten with a hammer, and are therefore termed malleable metals. Native gold and native silver are very malleable metals.
Mammillated. See Botryoidal.
Massive. This term is sometimes used in describing a substance of indeterminate form, whatever may be its internal structure; but is more commonly applied to those minerals which possess regular internal structure, without any particular external form.
Matrix. See Gangue.
Meagre. This term relates to the touch or feel of a mineral. It belongs chiefly to some of those species which possess an earthy texture. Chalk is remarkably meagre to the touch.
Natural joints. Such minerals as can be broken into regular forms, as the cube, rhomboid, &c. are cleavable into those forms only in the direction of or along their natural joints. In some species these natural joints are perceptible by the assistance of a strong light.
Nacreous relates to lustre, and is employed to express the lustre of some minerals which greatly resembles that of pearl.
Nodular. A mineral which presents irregularly globular elevations is termed nodular.
Opake. Those minerals are opake which do not transmit a perceptible ray of light even through the thinnest and smallest pieces.
Pass into. One mineral is said to pass into another, when both are found so blended in the same specimen that it is impossible to decide where the one terminates and the other begins. Flint is found passing into calcedony.
Porous. A mineral is said to be porous when it is traversed in different directions with communicating holes which pass through the substance.
[page] LXXV
Pseudomorphous. Minerals exhibiting impressions of the forms peculiar to the crystals of other substances are said to be pseudomorphous. ΨενΔοζ, in Greek, signifies false; μοζΦη, form or figure.
Pulverulent. When the particles of a mineral are minute, and cohere very slightly, or not at all, it is said to be pulverulent, or in the pulverulent state.
Radiated. Radiatus, in Latin, signifies beset with rays. When the crystals of a mineral are so disposed as to diverge from a centre, they are said to be radiated.
Ramose. Ramus, in Latin, signifies the branch of a tree. A mineral having that appearance is described as being ramose.
Refractoriness. The term is used both chemically and mechanically in relation to minerals. It is sometimes applied to those which strongly resist the application of heat, and occasionally to some whose toughness enables them to resist repeated blows.
Reniform, kidney-shaped. Ren, in Latin, signifies kidney.
Reticulated. Minerals occurring in parallel fibres, crossed at right angles by other fibres which also are parallel, exhibit squares like the meshes of a net. Retis, in Latin, signifies a net.
Schistose or slaty structure. Minerals which split only in one direction, and present fragments which are parallel, but of unequal thickness, which also are not smooth and even, and are without lustre, are said to possess a schistose structure.
Scopiform. If a number of minute crystals or fibres be closely aggregated into a little bundle, with the appearance of diverging slightly from a common centre, they are said to be scopiform. Scopa, in Latin, signifies a broom or besom.
Sectile. The term sectile is derived from the Latin seco, to cut. Those minerals are termed sectile which are midway between the brittle and the malleable. A slice or portion cut from a sectile mineral is fragile, and the new surface on the mass is smooth and shining.
Semi-transparent. A mineral is said to be semi-transparent when an object is not distinctly seen through it.
Specular Minerals are those which present a smooth and brilliant surface which reflects light. Speculum, in Latin, signifies a looking-glass.
Splintery fracture belongs to imperfectly crystalline minerals.
Stalactitiform. ΣταλáΓμα signifies a drop, an icicle, which stalactitiform minerals greatly resemble in shape.
Stalagmite. A stalagmite is the deposition afforded by the water dropping from a stalactite, as on the floor of a cavern.
[page] LXXVI
Stellated. When the crystals or fibres of a mineral diverge all round a common centre, it is said to be stellated. Stella, in Latin, signifies a star.
Striæ, Striated. The slight channels occasionally observable on the planes of crystallized minerals are termed striæ, and the crystals which exhibit them are said to be striated. The striæ are commonly parallel, and generally indicate the direction in which crystals may be cleaved. Stria, in Latin, signifies a groove or channel.
Sulphuret. A metallic mineral in which the metal is combined with sulphur. In these minerals the metal is not in the state of an oxide, but in the metallic state.
Supernatant. Such minerals as are lighter than water, and consequently swim upon it, are said to be supernatant; from the Latin.
Tabular. When this term is used in relation to structure, it is nearly allied to the schistose or slaty. It is used more generally to express the external form of such crystals as are nearly flat; these are termed tabular crystals; from the Latin, tabula, a table.
Toughness relates to internal texture. Those minerals which are bruised, or suffer depression, by repeated blows in the attempt to fracture them, are esteemed to be tough.
Translucent. A mineral through which an object cannot be seen, but which transmits some light, is termed translucent. Many minerals are translucent only on the edges.
Transparent. Those minerals are transparent through which an object may be clearly seen.
Tubercular. A mineral whose unevenness of surface arises from small and somewhat round elevations is said to be tubercular.
Vesicular. A mineral is said to be vesicular when it has small and somewhat round cavities, both internally and externally. Lava, pumice, limestone, &c. are sometimes vesicular. From the Latin, vesicula, a little bladder.
Vitreous. Minerals having the lustre of glass are said to possess the vitreous lustre.
Unctuous. This term relates to the touch. Plumbago and soap-stone are very unctuous.
[page] LXXVIII
OF THE ARRANGEMENT
IN WHICH MINERALS ARE DESCRIBED IN THE FOLLOWING PAGES.
IN the absence of that organization which so admirably serves as a guide to the generic differences in animals and plants, we must seek for some other basis on which to found an arrangement of minerals. No one has yet been, nor does it seem possible that one should be, constructed, that is altogether satisfactory;—one in which there is not much that is arbitrary.
The characters of minerals are of two kinds, Physical and Chemical: every system must be founded on one or other of these, or upon their combination.
Of the Physical characters, the most valuable, because the most certain, when it exists, is Structure; and since by it alone we may often recognise a mineral, it is highly deserving of the earliest attention of the student. There are, however, many minerals in which no regular structure is visible. If, therefore, we would depend on the physical characters, we must look for some other amongst them; but there is none so invariable as structure. Therefore any arrangement that is made to depend on the physical characters can only be founded on a comparison of a number of them; but many, if not most, of these characters, are subject to some, and often to a considerable degree of variation, even in the same substance.
The physical characters, therefore, are not of that precise, invariable, and universal application which alone would justify their adoption as the basis of an arrangement.
One of the chief difficulties attendant on the plan of arranging minerals according to their composition is, the uncertainty which exists in particular substances, as to what their absolutely essential constituents are. It may, however, be understood, that whatsoever enters into the composition of a mineral, that does not alter the external form and internal structure of that substance in its purer state, is not an essential element, but an accidental ingredient. Thus, among earthy minerals, the various colouring
[page] LXXVIII
matters of quartz, and in the rhombic calcareous spar of Fontainbleau, the sand it encloses, which is said to amount to one-fifth of the whole weight of the mineral, are only accidental ingredients. Among metalliferous minerals, grey copper or fahlerz may be cited as an instance of remarkable diversity of composition, without any alteration of external form; for, besides copper, iron, and sulphur, it sometimes includes a proportion of arsenic, lead, silver, or antimony. Many other instances might be cited.
Another difficulty arises from the still progressive state of chemistry. Hence new analyses, whenever they offer new results, tend to a perplexity of choice, which can only be terminated by selecting that analysis which has resulted from the labours of the most eminent analyst.
But although these difficulties are attendant upon a reliance on the chemical characters as the basis of an arrangement, such an arrangement appears to be equally certain, more instructive, of more universal application, and therefore far more intelligible to the beginner, than one founded upon physical characters.
Assuming, then, a chemical basis for the arrangement, another point is still open for determination, namely, where to begin. Hence it becomes requisite to seek a sufficient reason for establishing (instead of beginning arbitrarily without any apparent motive) some precise order of description, founded upon an intelligible principle, and such an one as should begin with the most simple, and terminate with the most compound substances. And viewing the intimate connection existing between mineralogy and geology, it seemed that a sufficient motive might be found in this connection to determine a preference.
The localities of minerals tend to show that there does exist a more or less certain criterion for determining the relative ages of the earths and the metals.
Some of the earths chiefly constitute those rocks which are esteemed to be of the oldest formation, while others do not enter into the composition of rocks; being found only in the veins which traverse them; these therefore may be estimated as of later origin than the former.
Of the alkalies and acids as mineral constituents, either combined with the earths or with each other, the former claim the precedence, as entering into the composition of the oldest rocks.
Two or three of the metals occur in small quantity in the masses of some of the earlier rocks; but in general the metals are found in veins; some in veins traversing the older rocks, and rarely or never in those of a more recent description; others most abundantly or only in those of newer formation.
As rocks are constituted chiefly of earths, and metals are principally found in veins, earthy minerals may be assumed to be of
[page] LXXIX
earlier origin than the metalliferous; hence minerals appear to possess a claim to a somewhat natural order of succession in our cabinets.
Thus siliceous minerals are first described, because it is estimated that silica forms the largest proportion of the oldest and most abundant primitive rocks, and all earthy minerals of which silica is the largest ingredient are arranged under that head; beginning, chemically, with silica in its purest form, and proceeding to such as consist of that and another earth, as silica and alumina, then to those consisting of silica and lime, &c. and afterwards to such minerals as are chiefly constituted of three or more earths, terminating with the most compound; and regarding the iron, manganese, &c. involved in many of them, only as accidental ingredients. The other earthy minerals are proceeded with in like manner; arbitrarily selecting such as contain the rare earth glucina, and placing them under that head, except that the gadolinite, which also contains the still more rare earth yttria, is placed under the latter.
Next after those minerals which consist only of one or more of the earths, succeed those in which one or other of the alkalies is found; to these, such of the acids as occur in the concrete state; then those minerals which are primarily constituted of one or more earths and an acid; and after these those consisting of an alkali and an acid; and, finally, the very few in which an earth, an alkali, and an acid, are combined together.
The native metals and metalliferous minerals succeed, arranged according to the order of age and formation, subordinately beginning with the metal in its native state, when it so occurs; then its combination with other metals, when in the state of a natural alloy; then combined with sulphur, with oxygen, and finally, as an oxide combined with an acid.
The combustibles follow, beginning with sulphur, to which succeeds carbon in its purest form, and afterwards its several combinations with other bodies, as the base of the greater part of all the substances belonging to this class.
The order of arrangement is therefore as follows:
Earthy Minerals.
Alkalino-earthy Minerals.
Acids.
Acidiferous Earthy Minerals.
Acidiferous Alkaline Minerals.
Acidiferous Alkalino-earthy Minerals.
Native Metals and Metalliferous Minerals.
Combustibles.
[page LXXX]
THE object of the following Tables is principally that of exhibiting the Mineralogical Arrangement adopted in this work, by at once presenting the distinguishing constituents of the various species. Perfect accuracy in respect of the relative quantity of these constituents is out of the question, but the most approved analyses have been invariably followed; and for more ample details the student is referred to the body of the work.
N. B.—The proportions are indicated by figures, where ascertained; when doubtful, are marked thus —.
EARTHY MINERALS.
| Silica. | Alumina | Lime. | Water. | Iron. | ||
| Silica. | . | . | . | . | . | . |
| Quartz, | 100 | . | . | . | . | |
| Opal, | — | . | . | . | . | |
| Flint, | 98 | 2 | . | . | . | |
| Calcedony, | 84 | 16 | . | . | . | |
| Jasper, | — | — | . | . | — | |
| Hornstone, | 74 | 16 | . | . | 10 | |
| angan | ||||||
| Leelite, | 75 | 22 | . | . | . | 3 |
| Karpholite, | 37 | 29 | . | 11 | 3 | 20 |
| Alumo-calcite, | 86 | 3 | 7 | 4 | . | |
| Garnet, | 43 | 16 | 20 | . | 21 | |
| Cinnamon stone, | 40 | 23 | 32 | . | 5 | |
| Idocrase, | 40 | 33 | 22 | . | 5 | |
| Gehlenite, | 29 | 24 | 35 | 5 | 7 | |
| Prehnite, | 44 | 28 | 20 | 5 | 3 | |
| Stilbite, | 58 | 17 | 9 | 16 | . | |
| Heulandite, | 59 | 15 | 12 | 14 | . | |
| Dipyre, | 62 | 25 | 11 | 2 | . | |
| Davyne, | 45 | 34 | 13 | 8 | . | |
| Laumonite, | 50 | 22 | 12 | 16 | . | |
| Zoisite, | 43 | 31 | 22 | . | 4 | |
| Epidote, | 40 | 28 | 15 | . | 17 | |
| Axinite, | 50 | 16 | 17 | . | 9 | 8 |
| Isopyre, | 48 | 14 | 16 | . | 22 | |
| Indianite, | 43 | 38 | 15 | . | 4 | |
| Xanthite, | 35 | 14 | 38 | . | 13 | |
| . | . | . | . | . | . | Magnesia |
| Anthophyllite, | 63 | 14 | 4 | 2 | 13 | 4 |
| Amphodelite, | 46 | 36 | 10 | 2 | 1 | 5 |
| Smaragdite, | 50 | 21 | 13 | . | 13 | 3 |
| Anorthite, | 45 | 34 | 15 | . | 1 | 5 |
| Clays, | 75 | 10 | 5 | . | 3 | 2 |
| Kerolite, | 39 | 13 | . | 31 | . | 17 |
[page] LXXXI
EARTHY MINERALS.—(Continued.)
| Silica. | Alumina | Lime. | Water. | Iron. | Magnesis. | |
| Silica. | ||||||
| Pyrophyllite, | 60 | 30 | . | 5 | 1 | 4 |
| Fahlunite, | 48 | 29 | . | 13 | 5 | 5 |
| Chiastolite, | 67 | 30 | . | . | . | 3 |
| Iolite, | 50 | 34 | . | . | 5 | 11 |
| Sordawalite, | 50 | 15 | . | 5 | 19 | 11 |
| Bar. and Strontia. | . | . | ||||
| Harmotome, | 47 | 18 | 20 | 15 | . | |
| Brewsterite, | 54 | 17 | 15 | 13 | 1 | |
| . | . | Lithia. | . | . | ||
| Petalite, | 78 | 17 | 5 | . | . | |
| Spodumene, | 66 | 25 | 8 | . | 1 | |
| . | . | Lime. | . | . | Mangan. | |
| Jeffersonite, | 58 | 2 | 16 | . | 10 | 14 |
| Tabular spar, | 52 | . | 46 | . | 2 | |
| Okenite, | 57 | . | 27 | 16 | . | |
| . | . | . | Titan. | . | Magnesia. | |
| Mellilite, | 40 | 3 | 20 | 4 | 14 | 19 |
| Gismondine, | 43 | 3 | 48 | . | 4 | 2 |
| Augite, | 53 | . | 22 | . | 17 | 8 |
| Diopside, | 58 | . | 17 | . | 6 | 19 |
| Babingtonite, | . | . | . | . | . | |
| Bucklandite, | . | . | . | . | . | |
| Hornblende, | 59 | . | 14 | . | 7 | 20 |
| Arfwedsonite, | . | . | . | . | . | |
| Hypersthene, | 56 | 3 | 1 | . | 26 | 14 |
| . | . | . | Water. | . | ||
| Schiller spar, | 43 | . | 1 | 13 | 14 | 29 |
| Bronzite, | 60 | . | . | . | 8 | 32 |
| Thulite, | 47 | 28 | 23 | . | . | 2 |
| Alumina. | Alumin. | Water. | Silica | Iron. | . | |
| Corundum, | 98 | . | . | 2 | . | |
| Diaspose, | 85 | 14 | . | 1 | . | |
| Gibbsite, | 65 | 35 | . | . | . | |
| Calaite, | 74 | 19 | . | 7 | . | |
| Hydrate of alumina, | 45 | 40 | 15 | . | . | |
| Allophane, | 34 | 42 | 24 | . | . | |
| Scarbroite, | 43 | 48 | 8 | 1 | . | |
| Halloysite, | 34 | 26 | 40 | . | . | |
| Worthite, | 54 | 5 | 41 | . | . | |
| Fibrolite, | 58 | . | 38 | 4 | . | |
| Sillimanite, | 55 | . | 43 | 2 | . | |
| Kyanite, | 64 | . | 34 | 2 | . |
D 2
[page] LXXXII
EARTHY MINERALS.—(Continued.)
| Alumin. | Magnesia. | Silica. | Iron. | Fluor. A. | ||
| Alumina. | ||||||
| Staurolite, | 52 | . | 30 | 18 | . | |
| Ox of zinc. | ||||||
| Automalite, | 60 | 3 | 4 | 9 | . | 24 |
| Fluellite, | — | . | . | . | — | . |
| Topaz, | 58 | . | 35 | . | 7 | . |
| Chrysoberyl, | 81 | . | 19 | . | . | . |
| Spinel, | 74 | 8 | 15 | 3 | . | |
| Sapphirine, | 64 | 17 | 15 | 4 | . | |
| Pleonaste, | 67 | 14 | 3 | 16 | . | |
| Lime. | ||||||
| Turnerite, | — | — | . | — | . | — |
| Magnesia, | Magnes. | Water. | Silica. | Iron. | Alumin. | |
| Hydrate of magnesia, | 70 | 30 | . | . | . | |
| Chrysolite, | 43 | . | 38 | 19 | . | |
| Olivine, | 38 | . | 50 | 12 | . | |
| Ligurite, | ||||||
| Forsterite, | ||||||
| Condrodite, | 56 | . | 38 | 6 | . | |
| Humite, | ||||||
| Tautolite, | — | . | — | — | . | |
| Serpentine, | 40 | 15 | 42 | 3 | . | |
| Soapstone, | 25 | 19 | 46 | 1 | 9 | |
| Steatite, | 32 | 7 | 59 | 2 | . | |
| Potstone, | 30 | 3 | 49 | 12 | 6 | |
| Nephrite, | 31 | 3 | 50 | 6 | 10 | |
| Nemalite, | 52 | 29 | 13 | 6 | . | |
| Marrnolite, | 42 | 15 | 42 | 1 | . | |
| Picrolite, | 38 | 12 | 41 | 9 | . | |
| Picrosmine, | 35 | 8 | 55 | 2 | . | |
| Zirconia. | Zirconia. | Water. | Silica. | Iron. | . | |
| Zircon, | 69 | . | 29 | 2 | . | |
| Ostranite, | . | . | . | . | . | |
| Glucina. | Glucina. | Water. | Silica. | Iron. | Alumin. | |
| Euclase, | 22 | . | 44 | 3 | 31 | |
| Emerald, | 15 | . | 68 | 1 | 16 | |
| Yttria. | Yttria. | Glucina. | Silica. | Iron. | Cerium. | |
| Gadolinite, | 38 | 5 | 25 | 16 | 16 | |
| Tkorina. | Thorina. | Lime. | Silica. | Iron, &c. | Water. | |
| Thorite, | 58 | 3 | 20 | 9 | 10 |
[page] LXXXIII
ALKALINO-EARTHY MINERALS.
| Potash. | Silica. | Alumina. | Water. | Magnes. | Iron. | ||
| Potash. | |||||||
| Mica, | 10 | 46 | 14 | . | 10 | 20 | |
| 8 | 48 | 25 | 4 | . | 15 | ||
| Lime. | |||||||
| Rubellane, | 10 | 45 | 10 | 5 | 10 | 20 | |
| Margarite, | 2 | 40 | 42 | 1 | 10 | 5 | |
| Leucite, | 21 | 56 | 23 | . | . | ||
| Herschellite, | . | . | . | . | . | ||
| Andalusite, | 4 | 36 | 55 | . | . | 5 | |
| Bucholzite, | 2 | 46 | 50 | . | . | 2 | |
| Phillipsite, | 7 | 48 | 23 | 16 | 6 | ||
| Apophyllite, | 5 | 52 | . | 18 | 25 | ||
| Dysclasite, | 2 | 58 | . | 14 | 26 | ||
| . | . | . | . | Magnes. | |||
| Nacrite, | 18 | 50 | 26 | . | 1 | 5 | |
| . | . | . | Sulp. A. | . | |||
| Hauyne, | 16 | 38 | 19 | 13 | 12 | 2 | |
| . | . | . | Water. | . | |||
| Weissite, | 6 | 55 | 23 | 4 | 9 | 3 | |
| Pearlstone, | 4 | 76 | 12 | . | 5 | 3 | |
| Giésèckite, | 7 | 48 | 36 | 5 | . | 4 | |
| Pinite, | 9 | 56 | 25 | . | . | 10 | |
| Pyrargyllite, | 3 | 44 | 29 | 16 | 3 | 5 | |
| Felspar, | 14 | 67 | 19 | . | . | ||
| Lime. | |||||||
| Latrobite, | 7 | 45 | 37 | 2 | 9 | ||
| Agalmatolite, | 7 | 56 | 29 | 5 | 2 | 1 | |
| Magnes. | |||||||
| Chlorite, | 7 | 52 | 10 | 6 | 12 | 13 | |
| Killinite, | 6 | 56 | 27 | 8 | . | 3 | |
| Lime. | |||||||
| Couzeranite, | 10 | 53 | 24 | . | 13 | ||
| Glaucolite, | 5 | 52 | 29 | . | 14 | ||
| & Flu.A. | Lithia. | ||||||
| Lepidolite, | 9 | 50 | 29 | 5 | 5 | 2 | |
| . | . | . | . | . | |||
| Soda. | Soda. | Silica. | Alumina. | Water. | Lime. | ||
| Mesotype, | 16 | 48 | 27 | 9 | . | ||
| Thomsonite, | 5 | 38 | 30 | 13 | 14 | ||
| Mesole, | 8 | 42 | 28 | 11 | 11 | ||
| Needlestone, | 6 | 47 | 26 | 12 | 9 | ||
| Brevicite, | 10 | 44 | 29 | 10 | 7 | ||
| Gmelinite, | 5 | 50 | 20 | 21 | 4 | ||
| Comptonite, | . | . | . | . | . | ||
| Magnesia. | Phos. A. | ||||||
| Ledererite, | 4 | 50 | 22 | 9 | 12 | 3 | |
| Alumina. | |||||||
| Hypostilbite, | 2 | 53 | 18 | 19 | 8 |
[page] LXXXIV
ALKALINO-EARTHY MINERALS.—(Continuéd.)
| Soda. | Silica. | Alumina. | Magnes. | Lime. | Iron. | |
| Soda. | ||||||
| Epistilbite, | 2 | 59 | 17 | 14 | 8 | |
| Spherostilbite, | 1 | 56 | 17 | 17 | 9 | |
| &Potash | Magnes. | |||||
| Erlauiite, | 3 | 54 | 15 | 5 | 15 | 8 |
| Humboldtilite, | 5 | 44 | 11 | 6 | 31 | 3 |
| Lapis-lazuli, | 9 | 51 | 12 | 6 | 17 | 5 |
| Nepheline, | 20 | 44 | 34 | . | 2 | |
| Water. | ||||||
| Ittnerite, | 14 | 34 | 30 | 12 | 10 | |
| Elaolite, | 21 | 46 | 32 | . | . | 1 |
| Nuttalite, | 8 | 38 | 26 | 8 | 19 | 1 |
| Soda. | Mur. A. | |||||
| Sodalite, | 26 | 36 | 32 | 6 | . | |
| Cancrinite, | 26 | 41 | 33 | . | . | |
| Sul. A. | Iron, &c. | |||||
| Spinellane, | 18 | 41 | 29 | 5 | 2 | 5 |
| Pericline, | 10 | 70 | 20 | . | . | |
| Labradorite, | 4 | 56 | 27 | . | 11 | 2 |
| Albite, | 11 | 70 | 19 | . | . | |
| Water. | ||||||
| Analcime, | 14 | 55 | 23 | 8 | . | |
| Sarcolite, | . | . | . | . | . | |
| Pitchstone, | 3 | 73 | 12 | 8 | 1 | 3 |
| Pumice, | 3 | 77 | 18 | . | . | 2 |
| Obsidian, | 10 | 75 | 12 | . | . | 3 |
| Spherulite, | 4 | 79 | 12 | 2 | . | 3 |
| Magnes. | ||||||
| Saussurite, | 6 | 49 | 24 | 3 | 10 | 8 |
| Water. | ||||||
| Scapolite, | 1 | 45 | 35 | 2 | 17 | |
| Ekebergite, | 5 | 47 | 29 | 3 | 14 | 2 |
| Pectolite, | 10 | 51 | 1 | 4 | 34 | |
| Chabasie, | 2 | 51 | 18 | 19 | 10 | |
| Levyne, | . | . | . | . | . | |
| Bor. A. | Magnes. | |||||
| Tourmaline, | 3 | 36 | 35 | 4 | 6 | 16 |
| Lime. | ||||||
| Meionite, | 2 | 40 | 32 | . | 24 | 2 |
| Edingtonite, | 3 | 54 | 14 | . | 21 | 8 |
| Water. | Magnes. | |||||
| Krokydolite, | 8 | 51 | . | 4 | 3 | 34 |
| Achmite, | 11 | 56 | . | . | . | 33 |
| Cummingtonite, | 9 | 59 | . | 2 | . | 30 |
| Zirconia. | Lime. | |||||
| Eudyalite, | 14 | 54 | 11 | 3 | 10 | 8 |
[page] LXXXV
ACIDS.
| Sulphur. | Oxygen. | ||
| Sulphuric acid, | 40 | 60 | |
| Borax. | Oxygen. | ||
| Boracic acid, | 26 | 74 |
ACIDIFEROUS EARTHY MINERALS.
| Sul. A. | Alumina. | Water. | Silica. | Iron. | ||
| Alumina. | ||||||
| Subsulphate of alum., | 24 | 30 | 46 | . | . | |
| Sulphate of alumina, | 36 | 16 | 48 | . | . | |
| Phos. A. | ||||||
| Wavellite, | 35 | 37 | 28 | . | . | |
| Kakoxene, | 18 | 10 | 26 | 10 | 36 | |
| Lithia. | ||||||
| Amblygonite, | 54 | 39 | . | . | . | 7 |
| Childrenite, | — | — | . | . | — | |
| Magnesia. | ||||||
| Azurite, | 43 | 35 | 6 | 3 | 3 | 10 |
| Lime. | Carb. A. | Lime. | Water. | Silica. | Iron. | |
| Carbonate of lime, | 44 | 56 | . | . | . | |
| Strontia. | ||||||
| Arragonite, | 44 | 54 | 1 | . | . | 1 |
| Magnesia. | ||||||
| Bitter spar, | 50 | 34 | 2 | . | . | 14 |
| Ankerite, | 35 | 50 | . | . | 3 | 12 |
| Lead. | ||||||
| Plumbo-calcite, | 43 | 54 | . | . | . | 3 |
| Phos. A. | ||||||
| Apatite, | 44 | 56 | . | . | . | |
| Herderite, | . | . | . | . | . | |
| Fluor.A. | ||||||
| Fluor spar, | 28 | 72 | . | . | . | |
| Sul. A. | ||||||
| Anhydrite, | 58 | 42 | . | . | . | |
| Gypsum, | 46 | 33 | 21 | . | . | |
| Nit. A. | ||||||
| Nitrate of lime, | 66 | 34 | . | . | . | |
| Bor. A. | ||||||
| Datholite, | 22 | 36 | 5 | 37 | . | |
| Arsen. A. | ||||||
| Pharmacolite, | 50 | 26 | 24 | . | . | |
| Haidingerite, | 57 | 29 | 14 | . | . | |
| Tung. A. | ||||||
| Tungstate of lime, | 80 | 20 | . | . | . |
[page] LXXXVI
ACIDIFEROUS EARTHY MINERALS.—(Continued.)
| Carb. A. | Magnesia. | Water. | Iron. | ||
| Magnesia. | |||||
| Carbonate of magnesia, | 50 | 48 | 2 | . | |
| Breunnerite, | 49 | 42 | . | 9 | |
| Lime. | |||||
| Conite, | 49 | 33 | . | 3 | 15 |
| Sul. A. | |||||
| Sulphate of magnesia, | 33 | 16 | 51 | . | |
| Nit. A. | |||||
| Nitrate of magnesia, | 72 | 28 | . | . | |
| Phos. A. | Flour. A. | ||||
| Wagnerite, | 42 | 47 | . | 4 | 7 |
| Borac. A. | |||||
| Boracite, | 69 | 31 | . | . | |
| Lime. | |||||
| Hydro-boracite, | 50 | 11 | 26 | . | 13 |
| Baryta. | Carb. A. 22 | Baryta. | |||
| Witherite, | 22 | 78 | . | . | |
| Lime. | |||||
| Baryto-calcite, | 31 | 51 | . | . | 18 |
| Sul. A. | |||||
| Barytes, | 34 | 66 | . | . | |
| Strontia. | Carb. A. | Strontia. | Sul. A. | Baryta. | |
| Strontites, | 30 | 70 | . | . | |
| Lime, &c. | |||||
| Barystrontianite, | 22 | 48 | 9 | 18 | 3 |
| Sul. A. | |||||
| Celestine, | 44 | 56 | . | . | |
| Yttria. | Phos. A. | Yttiia. | Iron. | ||
| Phosphate of yttria, | 35 | 63 | . | . | 2 |
ACIDIFEROUS ALKALINE MINERALS.
| Nitric Acid. | Potash. | Water. | |
| Potash. | |||
| Nitrate of potash, | 54 | 46 | . |
| Sulp, Acid. | |||
| Sulphate of potash, | 46 | 54 | . |
| Soda. | Carbon. Acid. | Soda. | |
| Carbonate of soda, | 35 | 50 | 15 |
| Trona, | 39 | 38 | 23 |
[page] LXXXVII
| Sulph. A. | |||
| Sulphate of soda, | 45 | 35 | 20 |
| Nitric A. | |||
| Nitrate of soda, | 63 | 37 | |
| Boracic A. | |||
| Borate of soda, | 37 | 15 | 48 |
| Muriatic A. | |||
| Muriate of soda, | 47 | 53 | |
| Ammonia. | Sulph. A. | Ammonia. | |
| Sulphate of ammonia, | 53 | 23 | 24 |
| Muriatic A. | |||
| Muriate of ammonia, | 51 | 32 | 17 |
ACIDIFEROUS ALKALINO-EARTHY MINERALS.
| Sul. A. | Potash. | Alumina. | Lime. | Water. | ||
| Potash. | ||||||
| Alum, | 34 | 10 | 11 | . | 45 | |
| Alum-stone, | 36 | 10 | 40 | . | 14 | |
| Magnesia | ||||||
| Polyhallite, | 53 | 15 | 19 | 6 | 7 | |
| Soda. | Fluor. A. | Soda. | ||||
| Cryolite, | 44 | 32 | 24 | . | . | |
| Sul. A. | ||||||
| Glauberite, | 57 | 22 | 21 | . | ||
| Mur. A. | Magnesia. | |||||
| Reussite, | 57 | 29 | . | 2 | 12 | |
| Water. | ||||||
| Soda-alum, | 38 | 8 | 12 | . | 42 | |
| Carb. A. | ||||||
| Gaylussite, | 29 | 20 | 1 | 18 | 32 | |
| Iron, &c. | ||||||
| Native carbon. of lime and soda, | 37 | 9 | 39 | 10 | 5 | |
| Ammonia. | Sul. A. | Ammo. | Magnesia. | |||
| Sulp. of alumina and ammonia, J | 37 | 5 | 12 | . | 45 | 1 |
[page] LXXXVIII
METALLIFEROUS MINERALS.
| Iron. | Sulphur | Water. | Silica. | Lime. | ||
| Iron. | Nickel. | |||||
| Native iron, | 97 | . | . | . | . | 3 |
| Iron pyrites, | 47 | 53 | . | . | . | |
| White iron pyrites, | 46 | 54 | . | . | . | |
| Magnetic iron pyrites | 61 | 39 | . | . | . | |
| Arsenic. | ||||||
| Arsenical iron, | 36 | 21 | . | . | . | 43 |
| Oxygen. | ||||||
| Oxydulated iron, | 72 | 28 | . | . | . | |
| Specular iron, | 69 | 31 | . | . | . | |
| Red hæmatite, | 65 | 29 | 3 | 2 | 1 | |
| Mangan. | Zinc. | |||||
| Franklinite, | 46 | 30 | . | . | 10 | 14 |
| Hydrous ox. of iron, | 57 | 26 | 14 | 2 | 1 | |
| Goethite, | 61 | 28 | 11 | . | . | |
| Brown hæmatite, | — | — | — | . | . | |
| Stilpnosiderite, | 56 | 25 | 16 | 3 | . | |
| Magnes. | ||||||
| Cronstedtite, | 42 | 18 | 11 | 22 | 2 | 5 |
| Alumina. | ||||||
| Pinguite, | 25 | 11 | 26 | 37 | 1 | |
| Anhydrous silicate of iron, | 51 | 19 | . | 29 | 1 | |
| Alumina. | ||||||
| Chloropal, | 24 | 11 | 18 | 44 | 2 | 1 |
| Chamoisite, | 42 | 19 | 17 | 14 | . | 8 |
| Siderochisolite, | 53 | 20 | 7 | 16 | . | 4 |
| Hisingerite, | 37 | 15 | 12 | 29 | 1 | 6 |
| Lime. | ||||||
| Yenite, | 39 | 16 | 1 | 30 | 2 | 12 |
| Sulp. A. | Arsen. A. | |||||
| Pitchy iron ore, | 24 | 11 | 29 | . | 10 | 26 |
| Mur. A. | Mangan. | |||||
| Pyrosmalite, | 24 | 10 | 7 | 36 | 7 | 16 |
| Carb. A. | ||||||
| Spathose iron, | 46 | 14 | . | . | 40 | |
| Phos. A. | ||||||
| Phosphate of iron, | 32 | 10 | 27 | . | 31 | |
| Mangan. | ||||||
| Hétéposite, | 32 | 11 | — | . | 48 | 9 |
| Karphosiderite, | — | — | — | . | — | |
| Sulp. A. | ||||||
| Sulphate of iron, | 19 | 7 | 45 | . | 29 | |
| Botryogene | 25 | 10 | 33 | . | 32 | |
| Misy, | — | — | — | . | — |
[page] LXXXIX
| Iron. | |||||||
| Arseniate of iron, | 29 | 13 | 20 | . | 38 | ||
| Oxal. A. | |||||||
| Oxalate of iron, | 41 | 13 | . | . | 46 | ||
| Tung.A. | Mangan. | ||||||
| Tungstate of iron, | 14 | 9 | . | 2 | 71 | 4 | |
| Manganese. | Mangan. | Oxygen. | Water. | Silica. | Iron. | Baryta. | |
| Hausmannite, | 78 | 22 | . | . | . | ||
| Braunite, | 68 | 29 | 1 | . | . | 2 | |
| Pyrolusite, | 66 | 31 | 2 | 1 | . | . | |
| Grey oxide of manganese, | 68 | 22 | 10 | . | . | . | |
| Psilomelane, | 55 | 23 | 6 | . | . | 16 | |
| Silica, &c. | |||||||
| Wad, | 48 | 21 | 17 | 10 | 4 | ||
| Copper. | |||||||
| Cupreous mangan. | 53 | 23 | 20 | 1 | 3 | ||
| Sulph. A. | Iron. | Glucina. | |||||
| Helvine, | 34 | 8 | 5 | 38 | 6 | 9 | |
| Siliciferous oxide of manganese, | 36 | 17 | Water. 3 | 40 | 4 | ||
| Hydrosilicate of manganese, | — | — | — | — | . | ||
| Knebelite, | 27 | 15 | . | 33 | 25 | ||
| Lime. | |||||||
| Bustamite, | 28 | 8 | . | 49 | . | 15 | |
| Sulphuret of manganese, | 66 | 18 | . | Sulphur. 5 | . | Carb. A. 11 | |
| Lime. | |||||||
| Carb. of mangan. | 39 | 17 | . | 1 | 38 | 5 | |
| Pelokonite, | — | — | — | . | Iron. | Phos. A. | |
| Huraulite, | 24 | 14 | 16 | . | 8 | 38 | |
| Lime. | |||||||
| Phos. of mangan. | 25 | . | 2 | 25 | 34 | ||
| Molybdena. | Molybd. | Sulphur. | |||||
| Sulphuret of molybdena, | 60 | 40 | . | . | |||
| Oxygen. | |||||||
| Oxide of molybdena, | 85 | 15 | . | . | |||
| Tin. | Tin. | Oxygen. | Copper. | Iron. | |||
| Oxide of tin, | 79 | 21 | . | . | |||
| Sulph. | |||||||
| Sulphuret of tin, | 36 | 26 | 36 | 2 |
[page] XC
| Tungst. | Oxygen. | |||||
| Tungsten. | ||||||
| Oxide of tungsten, | 86 | 14 | . | . | . | |
| Titanium. | Titan. | Oxygen. | Iron. | Silica. | Lime. | |
| Anatase, | — | — | . | . | . | |
| Rutile, | — | — | . | . | . | |
| Iserine, | 45 | 16 | 36 | 3 | . | |
| Brookite, | — | — | — | . | . | |
| Crichtonite, | — | — | — | . | . | Mangan. |
| Ilmenite, | 59 | 10 | 30 | . | . | 1 |
| Mohsite, | — | — | — | . | . | |
| Sphene, | 33 | . | . | 34 | 33 | |
| Tit. A. | Water. | Cerium. | Uran. | |||
| Pyrochlore, | 63 | 10 | 4 | 5 | 13 | 5 |
| Iron, &c. | Zircon. | |||||
| Æschynite, | 56 | 4 | 4 | 12 & Yttria. | 4 | 20 |
| Polymignite, | 53 | . | 12 | 16 | 4 | 15 |
| Cerium. | Cerium. | Oxygen. | Silica. | Water. | Lime. | Iron. |
| Cerite, | 54 | 15 | 18 | 10 | 2 | 1 |
| Silicate of cerium, | — | — | — | . | . | |
| & Alum. | ||||||
| Allanite, | 19 | 15 | 33 | 3 | 26 | 4 |
| Torrelite, | 11 | 8 | 33 | 4 | 28 | 16 |
| &Yttria. | ||||||
| Orthite, | 16 | 6 | 36 | 8 | 24 | 10 |
| & Carbon.] | ||||||
| Pyrorthite, | 12 | 4 | 11 | 27 | 5 | 41 |
| Carb. A. | ||||||
| Carbon. of cerium, | 60 | 16 | . | 13 | 11 | |
| Lime. | Fluor. A. | Yttria. | ||||
| Yttro-cerite, | 14 | 4 | 47 | . | 25 | 10 |
| Fluate of cerium, | 66 | 17 | . | . | 16 | 1 |
| Uranium. | Uran. | Oxygen. | Silica. | Iron. | Water. | Sulp. Lead |
| Pitchblende, | 82 | 5 | 5 | 3 | . | 5 |
| Lime, &c. | Phos. A. | |||||
| Uranite, | 55 | 7 | 7 | 1 | 15 | 15 |
| Copper. | ||||||
| Chalkolite, | 55 | 8 | . | 6 | 15 | 16 |
| Carb. A. | ||||||
| Carbon. of uranium, | — | — | . | . | . | |
| Sulp. A. | ||||||
| Johannite, | — | — | . | . | — | |
| Tantalum. | Tantal. | Oxygen. | Iron. | Mangan. | Yttria. | |
| Tantalite, | 81 | 10 | 4 | 5 | . | |
| Lime. | ||||||
| Yttrotantalite, | 51 | 4 | 4 | . | 37 | 4 |
| Cerium. | Tin, &c. | Zirconia | ||||
| Fergusonite, | 44 | 5 | 3 | 3 | 42 | 3 |
[page] XCI
METALLIFEROUS MINERALS.—(Continued.)
| Chrome. | Oxygen. | Iron. | |||||
| Chrome. | |||||||
| Oxide of chrome, | 70 | 30 | . | . | . | Alumina. | |
| Chromate of iron, | 39 | 28 | 26 | . | . | 7 | |
| Bismuth. | Bismuth. | Sulphur. | Copper. | Lead. | |||
| Native bismuth, | 100 | . | . | . | . | ||
| Sulphuret of bismuth, | 81 | 19 | . | . | . | ||
| Cupreous bismuth, | 49 | 13 | 38 | . | . | ||
| Needle ore, | 43 | 15 | 12 | 30 | . | ||
| Oxygen. | |||||||
| Oxide of bismuth, | 90 | 10 | . | . | . | ||
| Silica. | Iron. | Phos. A. | |||||
| Bismuth-blende, | 62 | 9 | 23 | 2 | . | 4 | |
| Tellur. | Sulphur & Selen. | Silver. | |||||
| Telluric-bismuth, | 64 | . | . | 31 | 3 | 2 | |
| Arsenic. | Arsenic. | Oxygen. | Iron. | Antimony | |||
| Native arsenic, | 96 | . | 1 | . | . | 3 | |
| Oxide of arsenic, | 76 | 24 | 30 | . | . | . | |
| Sulphur. | |||||||
| Sulphuret of arsenic, | 70 | 30 | . | . | . | ||
| Nickel. | |||||||
| Arsenical pyrites, | 65 | 5 | 28 | . | . | 2 | |
| Cobalt. | Cobalt. | Arsenic. | Sulphur. | Water. | Iron. | ||
| Bright white cobalt, | 34 | 44 | 20 | . | 2 | ||
| Tin-white cobalt, | 22 | 75 | . | . | 3 | ||
| Bismuth. | |||||||
| Bismuth cobalt ore, | 10 | 78 | 3 | . | 5 | 4 | |
| Copper. | |||||||
| Sulphuret of cobalt, | 44 | . | 39 | . | 4 | 13 | |
| Oxygen. | |||||||
| Earthy cobalt, | 61 | 16 | . | 23 | . | ||
| Arsen.A. | |||||||
| Cobalt bloom, | 31 | 9 | 38 | 22 | . | ||
| Lime. | Magnesia. | ||||||
| Roselite, | — | — | — | — | — | — | |
| Sulph. A. | |||||||
| Sulphate of cobalt, | 23 | 6 | 30 | 41 | . | ||
| Nickel. | Nickel | Sulphur. | Antim. | Water. | Alumin. | ||
| Sulphuret of nickel, | 65 | 35 | . | . | . | ||
| Antimonial nickel, | 28 | 16 | 56 | . | . | ||
| Arsenic. | |||||||
| Arsenical nickel, | 44 | 1 | 55 | . | . | ||
| Oxygen. | Arsen.A | ||||||
| Nickel ochre, | 30 | 8 | 37 | 25 | . | . | |
| Silica. | Lime, &c | ||||||
| Pimelite, | 13 | 3 | 35 | 38 | 6 | 5 |
[page] XCII
| Silver. | Sulphur. | Antim. | Iron. | Copper. | ||
| Silver. | ||||||
| Native silver, | 100 | . | . | . | . | |
| Antimonial silver, | 84 | . | 16 | . | . | |
| Tellur. | ||||||
| Telluric silver, | 63 | . | 37 | . | . | |
| Sulphuret of silver, | 87 | 13 | . | . | . | |
| Flexible sul. of silver, | . | . | . | . | . | |
| Sternbergite, | 33 | 33 | . | 34 | . | |
| Antim. | ||||||
| Brittle sul. of silver, | 68 | 16 | 14 | 2 | . | |
| Sul. of silver and antimony, | ||||||
| Polybasite, | 65 | 17 | 5 | . | 9 | 4 |
| Red silver, | 60 65 | 23 20 | 17 | . | . | 15 |
| Miargyrite, | 37 | 22 | 40 | . | 1 | |
| Sul. of silver and copper, | 53 | 16 | . | 31 | ||
| Bismuth | Lead. | |||||
| Bismuthic silver, | 15 | 17 | 28 | 6 | . | 34 |
| Selenium. | ||||||
| Seleniuret of silver, | 69 | 26 | . | . | . | 5 |
| Seleniuret of silver and copper, | 39 | 28 | . | . | 24 | Carb.A.&c 9 |
| Iodine. | ||||||
| Iodic silver, | . | . | . | . | ||
| Antim. | Carb. A. | |||||
| Carbonate of silver, | 73 | . | 15 | . | . | 12 |
| Mur. A. | ||||||
| Muriate of silver, | 72 | . | . | 6 | . | 22 |
| Arsen. A. | ||||||
| Gänseköthig-erz, | . | . | . | . | . | . |
| Copper. | Copper. | Sulphur. | Iron. | Arsenic. | ||
| Native copper, | 100 | . | . | . | ||
| Sulphuret of copper, | 78 | 19 | 3 | . | ||
| Kupferindig, | 65 | 33 | 2 | . | ||
| Bi-sulph. of copper, | 67 | 33 | . | . | ||
| Purple copper, | 62 | 23 | 15 | . | ||
| Grey copper, | 48 | 13 | 25 | 14 | ||
| Copper pyrites, | 33 | 36 | 31 | . | ||
| Selen. | ||||||
| Seleniuret of copper J | 60 | . | . | 40 |
[page] XCIII
| Copper. | Oxygen. | Iron. | Carb. A. | Water. | |||
| Copper. | |||||||
| Red oxide of copper, | 89 | 11 | . | . | . | ||
| Black copper, | 80 | 20 | . | . | . | ||
| Blue carb. of copper, | 55 | 14 | . | 26 | 5 | ||
| Green carb. of copper, | 57 | 15 | . | 19 | 9 | ||
| Silica. | |||||||
| Chrysocolla, | 35 | 9 | . | . | 20 | 36 | |
| Dioptase, | 38 | 11 | . | . | 14 | 37 | |
| Sulph.A. | |||||||
| Sulphate of copper, | 25 | 7 | . | 32 | 36 | ||
| Tin, &c | |||||||
| Brochantite, | 53 | 15 | . | 17 | 12 | 3 | |
| Zinc. | |||||||
| Kupfersammterz, | — | — | — | — | . | ||
| Mur A | |||||||
| Muriate of copper, | 57 | 15 | . | 11 | 17 | ||
| Phosphate of copper, | 51 | 14 | . | 28 | 7 | ||
| Hydrous phosph. of copper, | 50 | 13 | . | 22 | 15 | ||
| Arsen.A. | |||||||
| Arseniate of copper, | 29 | 8 | . | 28 | 35 | ||
| Euchroite, | 38 | 10 | . | 33 | 19 | ||
| Carb. of Lime. | |||||||
| Kupferschaum, | 37 | 9 | . | 26 | 18 | 10 | |
| Alumina. | |||||||
| Erinite, | 47 | 12 | . | 34 | 5 | 2 | |
| Iron. | |||||||
| Skorodite, | 18 | 10 | 21 | 32 | 19 | ||
| Gold. | Gold. | ||||||
| Native gold, | 100 | ||||||
| Platina. | Platina. | ||||||
| Native platina, | 100 | ||||||
| Palladium. | Pallad. | ||||||
| Native palladium, | 100 | ||||||
| Iridium. | Iridium. | ||||||
| Native iridium, | 100 | . | . | . | |||
| Iridium. | Osmium. | Rhod. | Iron. | ||||
| Iridium and osmium, | 47 | 49 | 3 | 1 | |||
| Tellurium. | |||||||
| Tellur. | Gold. | Silver. | Iron. | Sulphur. | |||
| Native tellurium, | 92 | 1 | . | 7 | |||
| Lead. | |||||||
| Graphic tellurium, | 58 | 28 | 12 | 2 | . | ||
| Yellow tellurium, | 45 | 27 | 8 | 20 | . | ||
| Antim. | |||||||
| Black tellurium, | 16 | 6 | . | 63 | 11 | 4 |
[page] XCIV
| Antim | Sulphur | Iron. | Lead. | ||||
| Antimony. | |||||||
| Native antimony, | 100 | . | . | . | . | ||
| Berthierite, | 53 | 31 | 16 | . | . | ||
| Sulphuret of antimony, | 74 | 26 | . | . | . | ||
| Jamesonite, | 35 | 23 | 2 | 40 | . | ||
| Plagionite, | 38 | 22 | . | 40 | . | ||
| Zinkenite, | 45 | 23 | . | 32 | . | ||
| Oxygen | |||||||
| Red antimony, | 75 | 20 | . | . | 5 | ||
| Oxide of antimony, | 84 | . | . | . | 16 | ||
| Water. | |||||||
| Antimonial ochre, | — | . | . | . | . | ||
| Antimonphyllite, | — | . | . | . | . | ||
| Lead. | Lead. | Sulphur. | Water | Antim. | |||
| Native lead, | |||||||
| Sulphuret of lead, | 84 | 16 | . | . | . | ||
| Copper. | |||||||
| Bournonite, | 41 | 20 | . | . | 26 | 13 | |
| Arsen&c | |||||||
| Prism. copper glance, | 33 | 10 | . | 18 | 19 | 20 | |
| Native minium, | . | . | . | . | |||
| Selen. | Cobalt. | ||||||
| Seleniuret of lead, | 70 | 28 | . | 2 | . | ||
| Oxygen. | Alumin. | ||||||
| Plombgomme, | 40 | 3 | 19 | 38 | . | ||
| Carb. A. | |||||||
| Carbonate of lead, | 74 | 10 | . | . | 16 | ||
| Sulphato-carbonate of lead, | 82 | . | . | . | 4 | Sulp. A. 14 | |
| Sulphato-tri-carb. of lead, | 88 | . | . | . | 5 | 7 | |
| Cup. sul. carbonate of lead, | 72 | . | . | Copper. 7 | 6 | 15 | |
| Mur.A. | |||||||
| Muriate of lead, | — | . | . | . | — | — | |
| Cotunnite, | 75 | . | . | . | . | 25 | |
| Murio-carb. of lead, | 80 | 6 | . | . | 6 | 8 | |
| Phosphate of lead, | 76 | 6 | . | . | 16 | 2 | |
| Magnesia. | |||||||
| Polysphärite, | — | — | — | — | — | — | |
| Arsen.A. | Mur. A. | ||||||
| Arseniate of lead, | 72 | 6 | . | 13 | 7 | 2 | |
| Sulp. A. | |||||||
| Sulphate of lead, | 65 | 7 | 2 | . | 26 | . |
[page] XCV
| Lead. | Oxygen. | Water | Copper. | Sulp. A. | ||
| Lead. | ||||||
| Cupreous sul. of lead, | 56 | 4 | 5 | 15 | 20 | |
| Molyb. A. | ||||||
| Molybdate of lead, | 57 | 4 | . | . | 39 | |
| Chrom. | ||||||
| Chromate of lead, | 63 | 5 | . | . | 32 | |
| Melanochroite, | 71 | 6 | . | . | 23 | |
| Vauquelinite, | 57 | 6 | . | 9 | 28 | |
| Tung. A. | ||||||
| Tungstate of lead, | 44 | 4 | . | . | 52 | |
| Vanad.A | Mur. A. I | |||||
| Vanadiate of lead, | — | . | . | . | — | — |
| Zinc. | Zinc. | Sulphur. | Iron. | Silica. | Water. | |
| Sulphuret of zinc, | 63 | 33 | 4 | . | . | |
| Oxygen. | ||||||
| Red oxide of zinc, | 74 | 18 | 8 | . | . | |
| Siliceous ox. of zinc, | 54 | 13 | . | 25 | 8 | |
| Carbonate of zinc, | — | — | — | . | . | |
| Carb. A. | ||||||
| Willelmine, | 52 | 13 | . | 35 | . | |
| Sulp. A. | ||||||
| Sulphate of zinc, * | 22 | 6 | . | 30 | 42 | |
| Hopeite, | — | . | . | . | — | |
| Mercury. | Mercury | Silver. | ||||
| Native quicksilver, | 100 | . | . | . | . | |
| Native amalgam, | 85 | 15 | . | . | . | |
| Chlorine. | ||||||
| Muriate of Mercury, | 85 | 15 | . | . | . | |
| Iodine. | ||||||
| Iodic mercury, | — | — | . | . | . |
[page] XCVI
COMBUSTIBLE MINERALS.
| Sulphur. | Iron. | Silica. | ||
| Sulphur, | 100 | . | . | |
| Carbon. | ||||
| Diamond, | 100 | . | . | |
| Plumbago, | 92 | 8 | ||
| Anthracite, | 72 | 4 | 24 | |
| Hydrogen. | Oxygen. | Azote. | ||
| Naphtha, | 88 | 12 | . | . |
| Bitumen, | 53 | 7 | 40 | . |
| Coal, | 75 | 6 | 5 | 14 |
| Dysodile, | — | — | ||
| Amber, | 81 | 12 | 7 | |
| Hatchetine, | — | — | ||
| Schererite, | 76 | 24 | ||
| Ozokerite, | — | — | ||
| Mel. A. | Alumin. | Water. | ||
| Mellite, | 41 | 15 | 44 | |
| Retinasphalt, | ||||
| Fossil copal, |
APPENDIX.
| Arsenical antimony | Monticellite |
| Arseniuret of manganese | Mysorine |
| Atelestite | Neoctese |
| Batrachite | Nontronite |
| Berzeline | Osmelite |
| Beudantite | Phenakite |
| Biotine | Poonahlite |
| Breislakite | Protheite |
| Chelmsfordite | Pyrosklerite |
| Chlorophæite | Somervillite |
| Chonikrite | Steinmannite |
| Cobaltic galena | Stilpnomelan |
| Dermatine | Sylvyne |
| Green iron earth | Tephroite |
| Herrerite | Thenardite |
| Marceline | Uwarowite |
| Microlite | Zurlite |
| Monazite |
[page 1]
EARTHY MINERALS.
UNDER this head are included such minerals as consist of one earth or more, together with such occasional and variable proportions of iron, manganese, water, and even of acids, as induce the conclusion that they are only accessory constituents. We shall begin with silica in its purest form, as being the oldest and most abundant mineral, and as affording the most simple arrangement; and then proceed to such as, by the most authentic analyses, appear to consist chiefly of silica. Those of which alumina forms the greatest proportion succeed, as being the earth next in age and abundance. Magnesia follows; then such minerals as consist primarily or in part of zirconia, or glucina, or lastly of yttria.
QUARTZ.
Quarz, W. and H. Rhombohedral Quartz, M.
Pure silica accidentally mixed with minute proportions of metallic oxides,—from whence the fine colours of this species are derived.
| Rock Crystal. | Amethyst. | |
| Silica 96·375 | 97·50 | |
| with a trace of ferriferous alumina | Oxide of iron 0·75 | |
| and manganese. Bucholz. | Alumina 0·25 Rose. | |
Sp. Gr. 2·6. H. = 7·0.
Of quartz there are many varieties, most of which the older mineralogists described as distinct species. Some of these differ considerably in their external characters; others nearly agree: they are all sufficiently hard to scratch glass, and, when compact enough, they give fire with steel; they do not yield to the knife; and alone are infusible before the blowpipe, by exposure to which the coloured varieties generally lose their colour.
Quartz occurs massive and crystallized; also stalactitic, pseudomorphous, spongiform, granular, compact, &c.
A
[page] 2
CRYSTALLIZED QUARTZ. Berg-crystal, Gemeiner Quarz, W. Quarz Hyalin,* H. There is no specific difference between common quartz and rock crystal. The latter term was formerly confined to the large transparent crystals of Madagascar, the Alps, &c.; but the small and even minute transparent crystals occurring in almost all metalliferous veins do not differ from rock crystal, either in chemical or external characters.
The common form of crystallized quartz is a six-sided prism terminated by six-sided pyramids (fig. 6); the two pyramids joined base to base (fig. 3) without an intervening prism are less frequent. The crystals may be cleaved, presenting brilliant surfaces, parallel to all the planes of the six-sided pyramid (fig. 3), which might therefore be considered as the primary form of quartz, but the obtuse rhomboid has been adopted as more simple: the connection between this rhomboid and the six-sided pyramid will be perceived by consulting figs. 1, 2, and 3. The angles of the primary rhomboid are 94° 15′ and 85° 45′, according to coinciding measurements by the reflecting goniometer, taken both on cleavages and natural planes. The cross fracture is often perfectly conchoidal. From two pieces rubbed together in the dark, a phosphorescent light is produced, and a faint empyreumatic odour is at the same time emitted.

Fig. 1, the primary rhomboid. Fig. 2, the lateral angles replaced by triangular planes; which are complete in fig. 3, having converted the rhomboid into a dodecahedron with triangular planes. Fig. 4 shows the small triangular planes connected with four-sided planes, which are parallel with the axis of the crystal. Fig. 5, the four-sided plane complete. Fig. 6, the four-sided plane elongated, being the common crystal of quartz. Fig. 7 shows a rhombic plane on each of the lateral angles of the dodecahedron; this very rare form has been observed among the
* The meaning of the term Quartz is not known; Hyalin, from its vitreous aspect.
[page] 3
Bornholm diamonds. Fig. 8 exhibits an irregular four-sided plane on the upper left corner of each plane of the prism of a common crystal; if the crystal were drawn complete, the same plane would be shown on the lower right corner. Fig. 9 presents the same plane on the upper right corner, which frequently occurs: these planes are mostly seen in combination with the rhombic planes of fig. 7. Fig. 10 shows the replacement of the edges between the lateral and terminal planes, by four planes: these mostly occur in combination with the four-sided planes of figs. 8 and 9. Fig. 11 presents one of the four planes increased; tending to a very acute six-sided pyramid, capped by the common pyramid. Fig. 12 shows the replacement of the apex of the common pyramid by six planes, forming a very obtuse pyramid.

The planes b b tend to an obtuse rhomboid, or, in connexion with c, to an obtuse six-sided prism.
The planes k 1 to 4 to acute rhomboids, or, in connexion with g 2 to 5, to six-sided pyramids more acute than the common one.
The planes h 1 to 5 and i 1 to 6 occasionally, though rarely, occur on the same crystal.
The planes n and o are always convex: d d are always minute and rough.
The planes o o sometimes replace only the alternate edges of the prism.
The finest specimens of crystallized quartz, or rock crystal, occur in Savoy, Dauphiné, and elsewhere among the Alps, forming drusy cavities in mica slate; gigantic crystals have been brought from Madagascar and the Brazils; while the smaller, but often most transparent and perfect, are of frequent occurrence in metalliferous veins. Beautifully limpid crystals occur imbedded in primitive marble at Carrara, and very brilliant specimens at Cape Diamond, near Quebec. The species quartz does not by any means abound in varieties of crystalline form, and yet, owing to the disproportionate size of the faces, its crystals offer considerable diversity of appearance. Rock crystals sometimes present beautiful iridescences, both superficially and internally; when
[page] 4
external, it is supposed to arise from the deposit of some metallic oxide, probably of iron; when internal, it is the refraction of light in consequence of fissures in the crystal; and it will often be found that these fissures are straight, and parallel with one or other of the planes of the pyramid. This appearance may be produced by plunging a heated crystal into cold water; the laminæ then partially separate parallel to the natural joints. Another interesting peculiarity of quartz is its frequent occurrence in pseudomorphous crystals; at Schneeberg in Saxony, it assumes the forms of various calc-spars; at Beeralstone in Devonshire, those of certain fluors; at Montmartre, near Paris, that of lenticular gypsum, &c.
In the massive state, quartz is a most abundant mineral, forming extensive veins in primitive and transition rocks, and being consequently diffused over almost every quarter of the globe. Sandstone, or granular quartz, is almost pure silica, any minute portions of foreign matter united with it being merely a mechanical admixture. Near Villa Ricca in Brazil, a variety of sandstone occurs in thin strata, which are remarkable for their flexibility, a property apparently arising from small scales of mica disposed throughout its mass. The minute particles of pure white sand are likewise quartz in a state of disintegration, resulting from the destruction of this species. Such are the sands of the coast of Norfolk, and of Alum Bay in the Isle of Wight, so largely used in the making of glass: a somewhat similar sand is found in the caverns of Reigate in Surrey.
Crystals of quartz are occasionally found enclosing foreign substances—as water, air, bitumen, schorl, asbestus, actynolite, crystallized titanite, and oxide of iron.
The following sub-species have been distinguished.
Avanturine. Quarz hyalin avanturine, H. A variety of quartz rock, including small laminæ of mica, which, when polished, presents a shining spangle-like appearance. The most common colour of the base is brown, or reddish brown, enclosing spangles of a gold colour, as in the variety from Cape de Gatte in Spain; occasionally it exhibits a fine green tint.
Prase. Quarz hyalin vert-obscur, H. This variety derives its dark leek-green colour (whence its name from the Greek) from an admixture of amphibole. It only occurs massive, and that principally in the iron mines of Breitenbrunn, near Schwartzenberg, in Saxony.
Milk Quartz. Quarz hyalin laiteux, H., and Rose Quartz. Quarz hyalin rose, H. Occur massive, and are only distinguished by their colour; the former, as its name denotes, presents a milky aspect, while the latter has frequently a fine rose-red tint, which is supposed to be derived from a minute admixture of
[page] 5
manganese. It occurs in granite at Rabenstein in Bavaria, at Abo in Finland, and near Connecticut in America; when cut and polished, it forms a handsome ornamental stone.
Violet Quartz. Amethyst. Quarz hyalin violet, H. Amethyst chiefly differs from common quartz in its colour, which is purplish violet, supposed to be derived from a minute proportion of iron and manganese, which it contains; it becomes white and opalescent by long exposure to heat. The best amethysts are brought from Cambay in India, from Siberia, Ceylon, and Persia, where they are found both lining the cavities of geodes, and in rolled masses. Of inferior transparency and hue, they occur in Sweden, the Hartz, Bohemia, Transylvania, in agate balls at Oberstein in Germany, in large crystalline groups near Cork, and in several parts of the United States.
Yellow Quartz. Quarz hyalin jaune, H. Is of various shades of yellow, and nearly transparent. Splendid specimens have been found at Cairngorum in Inverness-shire, whence the trivial name frequently applied by lapidaries to this variety.
Brown Quartz. Quarz hyalin enfumé, H. Closely allied to the last, but less transparent, and more common.
Ferruginous Quartz. Eisenkiesel, W. Quarz hyalin rubi-gineux, H. Iron flint, J. This variety presents several shades of yellow and red, and occurs both massive and crystallized. It is opake; gives sparks with steel; and consists chiefly of silica, with about 5 per cent. of iron. Eisenkiesel is found in Bohemia, in iron-stone veins in the Hartz, and at Altenberg in Upper Saxony.
Radiated Quartz occurs in crystals which are closely aggregated, and which radiate from a point.
Fibrous Quartz is produced when the composition presents thin columnar particles. The Cat's Eye is a variety of this, interspersed with thin filaments of asbestus, which, when the stone is cut en cabochon, presents a peculiar opalescent, or (as the French term it) chatoyant streak of light. It is usually of a greyish or greenish colour, but often brown and red; it contains small portions of alumina and lime, derived from the asbestus which traverses its mass; is generally translucent, and is frequently employed as an ornamental stone. Its principal localities are Ceylon and the Malabar coast.
Spongiform Quartz. Schwimmstein, W. Float-stone, J. The quarz nectique of Haüy is described as occurring in beds of flint in chalk, at St Ouen near Paris. It presents a spongy or porous appearance; consists of numerous minute white or greyish-white crystals; scratches glass; and, as its name indicates, possesses the property of swimming on water, at least until the air contained in its numerous cavities is displaced. It has also been observed under different forms in some of the Cornish mines. It
[page] 6
consists, according to Vauquelin, of 98 silica, and 2 carbonate of lime.
Hyalite.* Muller's Glass. Quarz hyalin concretionné, H. It occurs in white and transparent botryoidal masses, or in stalactites; has a vitreous lustre, is brittle, but is as hard as quartz. Specific gravity about 2·4. It is infusible by itself before the blowpipe; and consists, according to Bucholz, of silica 92, water 6·3, with a trace of alumina. This singular mineral is chiefly found investing or lining the cavities of trap or basaltic rocks. It occurs in amygdaloid near Frankfort-on-the-Maine, at Schemnitz in Hungary, and imbedded in clinkstone at Waltsch and other places in Bohemia.
Siliceous Sinter. Kieselsinter, W. Quarz agathe concretionnée thermogéne, H. Consists of silica 98·0, alumina 1·5, iron 0·5—Klaproth. Sp. Gr. about 1·8. The common colours of this mineral are white, greyish-white, and yellow. It is light, brittle, dull, commonly porous, with a fibrous texture, although sometimes sufficiently compact to admit of a conchoidal fracture; lustre pearly. Per se infusible before the blowpipe. It occurs abundantly around, and is deposited by, the hot springs of Iceland.
A variety of this is the Pearl sinter or Fiorite, which occurs in stalactitical, cylindrical, botryoidal, and globular masses, of a white, yellowish-white, or greyish colour; externally it is smooth and shining, internally glistening with a pearly lustre; fracture flat conchoidal; translucent on the edges; not so hard as quartz; and infusible before the blowpipe without addition. It consists of 96 silica, 2 alumina, and 2 lime. It occurs in volcanic tufa and pumice, in the Vicentine; the Florentine dominions; and in other volcanic districts of Italy.
OPAL.
Opal, W. Quarz Resinite, H. Silex Opale, Bt. Uncleavable Quartz, M. Sp. Gr. 2·09.
Opal, like quartz, consists chiefly of silica and water; but analysis generally indicates a greater quantity of the latter than in quartz. Its occasional resinous lustre is probably the origin of Haüy's appellation. None of its varieties are sufficiently hard to give fire with steel.
1. PRECIOUS OPAL. NOBLE OPAL. Edler opal, W. Quarz resinite opalin, H. This beautiful mineral is of a white, bluish, or yellowish-white colour, and when viewed by transmitted light
* From the Greek, in allusion to its glassy appearance.
[page] 7
is yellow. It exhibits brilliant and changeable reflections of green, blue, yellow, and red. This play of colours has not been satisfactorily accounted for; Sir D. Brewster supposes it to be owing to the refraction and reflection of light in certain openings in the interior of the mass, which are not fissures, but possess an uniform shape. It is translucent; fracture conchoidal, with a vitreous or resinous lustre; easily broken, but scratches glass. Before the blowpipe it decrepitates, and loses its colours. The Hungarian consists, according to Klaproth, of 90 silica, and 10 water: this water, however, is believed (in common with the water found in other siliceous minerals) to be purely hygrometric, and therefore to vary with the state of the atmosphere. It occurs, accompanied by common opal, imbedded in porphyry, at Czervenitza in Hungary; in trap-rocks in the Faroe Islands; at Freyberg in Saxony; and at Gracias a Dios, in the province of Honduras, in America, whence it has been brought in specimens of considerable size, and of great splendour.
2. FIRE OPAL. Quarz resinite girasol, H. Silex girasol, Bt. Is found with the noble opal in Hungary, but is much scarcer. It differs from the precious in possessing only bright hyacinth-red and yellow tints when turned towards the light. It occurs principally at Zimapan in Mexico, and in the Faroe Islands.
3. COMMON OPAL. Gemeiner opal,W. Quarz resinite commun, H. Silex resinite, Bt. Presents various shades of white, green, yellow, and red, but is entirely devoid of the play of colours peculiar to noble opal. In hardness, translucency, fracture, and specific gravity, it corresponds; and its constituents are, 92·0 silica, 7·75 water, and 0·25 oxide of iron. It occurs abundantly at Telkobanya and elsewhere in Hungary, forming short irregular beds which traverse porphyry; in Faroe occupying the cavities of amygdaloidal rocks; in Ireland, at the Giant's Causeway; and in many parts of the Hebrides.
4. SEMI-OPAL. Halb-opal, W. Quarz resinite hydrophane, H.? Differs from the last in being more opake; the translucency, however, of some varieties is increased by immersion in water, particularly when in thin fragments. It occurs of various shades of white, grey, yellow, brown, and green. When compact, the fracture is flat conchoidal. According to Klaproth, it consists of 85.0 silica, 1.0 carbon, 1.75 oxide of iron, 8 ammoniacal water, and a small portion of bitumen. It is found in amygdaloid in the Faroe Isles, Iceland, &c. associated with common opal; also near Frankfort; and in some of the metalliferous veins of Cornwall.
5. WOOD-OPAL. Holz-opal, W. Quarz resinite xyloide, H. This variety is remarkable for its ligneous appearance. It presents several tints of white, grey, brown, and black; and in fracture, translucency, and lustre, does not materially differ from semi-opal, although somewhat harder. It occurs occasionally
[page] 8
forming large trees, in the pumice conglomerates of Neusohl and Kremnitz in Hungary; in trap-rocks in Transylvania and the Faroe Islands; and in Van Diemen's Land.
6. FERRUGINOUS OPAL. Opal jasper, W. Jaspe-opal, Br. Jasper-opal, J. The ferruginous is distinguished from common opal by its colours, which are deep shades of red, yellow, and grey and by being opake, or only feebly translucent on the edges. Its fracture is flat conchoidal. It does not become white before the blowpipe. A variety from Telkobanya yielded to Klaproth silica 43·5, oxide of iron 47.0, water 7.5. It occurs in porphyry near Telkobanya and Tokay in Hungary; in the Saxon Erzgebirge; at Dominica; and in St Helena.
7. HYDROPHANE. Quarz resinite hydrophane, H. A variety of opal devoid of transparency, but assuming it when immersed in water or any other transparent fluid (whence its name, from the Greek); emitting at same time numerous globules of air, and becoming considerably heavier. It adheres to the tongue; and consists, according to Klaproth, of 93·1 silica, 1·6 alumina, 5·0 water and inflammable matter. It occurs in Hungary, in Bucharia, and at the Giant's Causeway, in small masses resembling mountain-cork, which are quite opake until immersed in water, when they dilate and become translucent.
8. MENILITE. Quarz resinite subluisant, brunatre, H. Menilite is a variety of semi-opal, occurring in compact reniform masses of a brown colour; structure slaty; somewhat translucent; and found in beds of adhesive slate at Menil-montant, near Paris. From the resemblance of its darker varieties to pitch, it is sometimes called the Pitchstone of Menil-montant. It consists, according to Klaproth, of 85·5 silica, 1 alumina, 11 water and inflammable matter, with small portions of lime and oxide of iron.
FLINT.
Feuerstein, W. Quarz Agathe Pyromaque, H. La Pierre à Feu, Br.
Consists of about ninety-eight per cent. of silica, with minute proportions of oxide of iron, lime, alumina, and water.—Klaproth.
Sp. Gr. about 2.59.
Flint is of various shades of grey, yellow, and black. It is somewhat harder than common quartz, which it scratches; is rarely laminated, and therefore may be broken with nearly equal ease in every direction: is brittle when first taken from the native bed, but becomes harder by exposure; has a flat conchoidal fracture, and feeble lustre: thin fragments of the black varieties are very translucent, and sharp. It is infusible, but whitens and becomes opake when exposed to heat.
[page] 9
In the chalk formation it occurs in regular beds, and consists either of nodules or flat tabular masses; the cliffs near Dover present fine examples of this. Flint is also abundant in alluvial deposits in the neighbourhood of chalk, as in France and the north of Ireland. It is often found enclosing sponges, alcyonia, echinites, and other fossil remains. By exposure to the action of air and water it becomes yellow, and is then termed ferruginous flint; such for the most part are the flints of our gravel beds, which have been rounded by attrition.
CALCEDONY.*
Kalzedon, W. Quarz Agathe Calcedoine, H. Calcedoine, Br.
Consists of silica and alumina.
| Faroe. | |
| Silica | 84·0 |
| Alumina | 16·0—Bergman. |
| Sp. Gr. about 2·6. | |
Calcedony presents various shades of white, grey, yellow, brown, green, and blue,—the colour for the most part being uniform. It occurs massive; forming veins; in nodules; and also botryoidal and stalactitic; but never crystallized. It is commonly semi-transparent; has an even or very flat conchoidal fracture; is harder than flint; and is infusible.
Splendid stalactitic specimens of this mineral were at one time found in Trevascus mine in Cornwall; Iceland and the Faroe Islands are now, however, its best known localities. It also occurs in Ceylon, in several parts of India, in Siberia, in Carinthia, Hungary, and many of the Hebrides. Pednandræ mine in Cornwall has yielded it of a smalt-blue colour; and a variety presenting the form of hexahedral crystals, which, however, are certainly pseudomorphous, has been brought from Tresztyan in Transylvania.
The following are considered varieties of calcedony.
Onyx.† Consists of alternate layers of brown and opake white calcedony, and is that variety specially used in the formation of cameos.
Plasma‡ is grass-green and semi-transparent, with a glistening
* So named from the town of Calcedon in Upper Asia, where it was collected by the ancients.
† According to Brongniart, "onyx veut-dire ongle" (the nail); inferring that this stone received its name from the two colours sometimes observable on the human nail.
‡ Plasma, Greek, engraving; the stone having been used by the ancients for that purpose.
[page] 10
lustre. It occurs principally in India and China, and is brought to this country in the shape of beads and other ornaments; occasional specimens are found among the ruins of Rome.
Heliotrope or Bloodstone is usually deep green, and translucent; with yellow or blood-red spots interspersed through the mass. Its colour is supposed to be derived from green earth. It is found in Siberia, Iceland, in the Isle of Rum, and elsewhere. Among lapidaries it is in considerable request.
Chrysoprase is of an apple-green colour; translucent; massive; internally somewhat glimmering, or not quite dull; and its fracture is even, rarely approaching the conchoidal. Before the blowpipe it loses its colour, becomes opake, but is infusible without addition. According to Klaproth, it consists of silica 96·16, oxide of nickel 1·0, and minute portions of lime, magnesia, alumina, and oxide of iron,—its colour being attributed to the nickel. It has been found extensively at Kosemutz in Silesia, in veins traversing serpentine, and accompanied by calcedony, opal, quartz, and pimelite; also at New Fane, in Vermont, North America. It is much prized by jewellers, and is usually cut into a convex form.
Cacholong is opake, of a milk or yellowish-white colour; externally dull, but internally of a somewhat pearly lustre; it sometimes disintegrates by exposure, when it adheres to the tongue; and before the blowpipe is infusible. Its specific gravity is about 2·2; and it appears to be closely allied to hydrophane.
It occurs in loose masses on the borders of the river Cach,* in Bucharia; also with calcedony in the trap-rocks of Iceland; in the Faroe Islands; and in Greenland.
Carnelian.† Karneol, W. Quartz agathe cornaline, H. Carnelian and agate do not materially differ. The former is principally from Arabia, India, and Surinam, where it is found in nodules of a dark-grey colour. These are first exposed to the sun for some weeks, and then placed in earthen pots and subjected to heat, which gives them those hues which constitute their value in jewellery.
Agate is an impure variety of calcedony, of frequent occurrence in the vesicular cavities of amygdaloidal rocks. It presents the most brilliant and the most varied colours, and, from its hardness and compact structure, being capable of receiving a high polish, occupies a distinguished position in most collections. The parallel lines which some agates exhibit, when cut and polished, much resemble those of a fortification. In others the colours alternate, as in certain ribbons. A variety from Saxony presents
* Hence its name of Cacholong.
† From the resemblance of its colour to that of flesh.
[page] 11
a brecciated structure; and so forth. When translucent, and containing appearances of aborization,or vegetable filaments, but which are ascribed to the infiltration of iron or manganese, it is termed Mocha-stone; this is principally brought from Arabia. Moss agate is calcedony containing delicate vegetable ramifications of different shades, and occasionally traversed by irregular veins of red jasper.
The most beautiful agates are found in the trap rocks of Oberstein; some are hollow, and are lined with crystals of amethyst; others also include harmotome. Agate is also found in Saxony, Bohemia, Silesia, Italy, Siberia, and India. The Hill of Kinnoull and the Isle of Skye are well-known Scotch localities. Agate is used both as an ornamental stone, and for cups and mortars by the chemist. The facility with which certain of its lines imbibe moisture enables the lapidary, by boiling it first in oil and then in sulphuric acid, to give it much the resemblance of the onyx.
JASPER.
Jaspis, W. Jaspe, H.
Jasper, in a chemical point of view, only differs from agate in containing a larger portion of iron; mineralogically, it is for the most part readily distinguished by its opacity. Its prevalent colours are yellow, brown, and red of various shades, and sometimes green; occasionally intermixed, or in spots and irregular veins. It has often a resinous lustre, but is sometimes dull; its fragments are rarely translucent on the edges; is hard, and frequently brittle; and does not fuse before the blowpipe. It occurs in many parts of the Continent, in veins and beds both in primitive and secondary mountains; also in Cornwall; in the Pentland and Moorfoot Hills, and in other parts of Scotland.
Striped Jasper, or Ribbon Jasper, presents green, yellow, and red colours, of various shades, the most beautiful variety being composed of equal and parallel layers of these colours. It occurs principally in the Ural Mountains of Siberia, in Saxony, and in Devonshire.
Egyptian Jasper, or Egyptian Pebble, occurs in roundish masses which are externally rough, and generally of a brown colour. Internally it exhibits a lighter hue, which sometimes approaches to that of cream, around which are disposed irregular zones or bands of brown, sometimes intermixed with nearly black spots, and also with dentritic appearances. It is found, according to Dr Clarke, in abundance, together with masses and detached fragments of petrified wood, scattered over the surface of the sandy desert eastward of Grand Cairo, even to the borders of the Red Sea.
[page] 12
Porcelain Jasper, or Porcellanite, is also by some mineralogists considered a variety of this species; but it is merely clay indurated by heat. It is compact, massive, and opake; presents sometimes a slaty structure, with a vitreous, uneven, conchoidal fracture, and has either a bluish-grey or a light-fawn colour. It melts before the blowpipe into a semi-transparent enamel, and is therefore quite distinct from any of the jaspers. It occurs principally in the neighbourhood of coal seams which have been in a state of combustion, and is abundant around Carlsbad in Bohemia. (Manual.)
HORNSTONE.
Hornstein, W. Quarz Agathe Grossier, H.
A green-coloured soft hornstone, used for the setting of lancets, yielded to Faraday, silica 71·3, alumina 15·3, protoxide of iron 9·3, and a trace of lime.
This substance occurs massive, in nodules, and pseudomorphous. The massive has a splintery or somewhat conchoidal fracture, and is translucent or opake; is dull, or has a glimmering lustre; is scarcely so hard as quartz; and is infusible. Its general colour is grey, which is tinged blue, green, brown, red, or yellow. In appearance it closely resembles compact felspar: hornstone, however, is infusible; felspar is fusible. Hornstone is found in round masses in limestone in the Tyrol, forming veins in Hungary and Sweden, and presenting remarkable pseudomorphous crystallizations in Saxony and Bohemia. It is by most mineralogists classed with flint.
LEELITE.*
Leelite—the helleflinta of the Swedes—is found compact and massive, of a deep flesh-red colour, at Gryphytta in Westmania, in Sweden. It possesses a peculiarly wax-like texture, and about the same lustre and translucency as horn. The fracture resembles that of flint. Sp. Gr. 2·71. This mineral was first noticed by Dr Clarke of Cambridge, to whom it yielded silica 75, alumina 22, manganese 2·50, water 0·50.
* In honour of J. F. Lee, LL.D. of St John's College, Cambridge.
[page] 13
KARPHOLITE.
Strohstein, W. Carpholite, N.
Silica 36·15, alumina 28·67, protoxide of manganese 19·16, protoxide of iron 2·29, lime 0·27, fluoric acid 0·47, water 10·78 —Stromeyer.
Sp. Gr. 2·935.
The colour of this mineral is generally wax or straw yellow (hence its name). It occurs in tufts of minute, fibrous, imperfectly-formed crystals; also massive, with a fibrous and frequently radiated structure, which is rather incoherent; and in an earthy state, probably from disintegration. Opake; with a silky lustre; and very brittle. Under the blowpipe on charcoal it intumesces, whitens, and fuses slowly into a brown opake glass; with borax it forms a transparent glass, which in the outer flame presents the amethystine tinge of manganese, and in the reducing flame becomes green.
It is found disposed on granite, with fluor and quartz, in the tin mines of Schlackenwald, in Bohemia.
ALUMOCALCITE.
Silica 86·60, alumina 2·25, lime 6·25, water 4·0—Kersten.
Sp. Gr. 2·174. May be crushed between the fingers.
Colour milk-white, inclining to blue. Streak the same. Fracture conchoidal. Adheres strongly to the moistened lip. Yields water in the glass tube. Becomes opake and grey coloured when exposed to heat in the platina forceps. With borax forms a colourless glass. In salt of phosphorus is soluble, with the exception of a silica skeleton. In concentrated muriatic acid it forms a transparent jelly.
This substance occurs in the clefts of ironstone veins at Eyben-stock in the Erzgebirge. Breithaupt separated it from opal, with which it had previously been confounded; and to him we are indebted for the above description.
GARNET.*
Dodecahedral Garnet, M.
Under this term are included several substances, consisting
* Grenat, Fr.; of the colour of pomegranate seeds.
[page] 14
principally of the same elements in variable proportions, but several include other constituents; they agree so far in external character, that all such as are found regularly crystallized occur in the rhombic dodecahedron and its varieties.
1. ALMANDINE. PRECIOUS GARNET. Edler granat, W. Grenat, H. Grenat noble, Br. Bt. Combination of a silicate of the protoxide of iron with silicate of alumina.
| Bohemia. | New York. | |
| Silica | 33·75 | 42·51 |
| Alumina | 27·25 | 19·15 |
| Oxide of iron | 36·00 | 33·57 |
| Oxide of manganese | 0·25 | 5·49 |
| Lime | 0·00 Klaproth. | 1·07 Wachtmeister. |
Sp. Gr. 4·2. H. between 6·5 and 7·5.
The principal colour of this beautiful mineral is red of various shades, having sometimes a tinge of yellow or blue, or a smoky aspect: it is commonly translucent, often transparent. It occurs crystallized in the rhombic dodecahedron, and may sometimes be cleaved, though not without difficulty, parallel to the planes of that solid,—this is therefore considered its primary form. Fracture conchoidal, with a shining, vitreous lustre. Before the blowpipe per se it fuses into a black globule, which acts upon the magnet, and with borax melts slowly into a dark glass tinged by iron. Insoluble in acid.
The almandine is much esteemed as a precious stone. Its principal localities are Ceylon and Pegu, where it occurs in alluvial deposits; and Greenland, whence many fine stones have been obtained for the purposes of the lapidary. In smaller but most beautiful crystals, it is found accompanying diopside and talc, at Ala in Piedmont; sometimes near Ely in Fifeshire; and in several parts of Bohemia. It is believed to be the Carbuncle of the ancients.
2. COMMON GARNET. Gemeiner granat, W. Grenat brun, &c. H. Colour reddish-, yellowish-, greenish-, or blackish-brown, and it differs from precious garnet in being commonly opake, or only translucent. It is found in granular masses, and crystallized in dodecahedrons, which are often considerably modified. Not quite so hard as almandine; fracture sometimes uneven, sometimes lamellar.
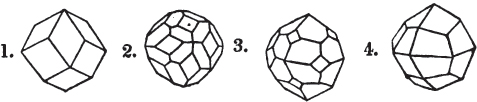
Fig. 1, Primary rhombic dodecahedron. Fig. 2 the same, all the edges being replaced by 6-sided planes, which, being further advanced
[page] 15
in fig. 3, reduce those of the primary crystal to small rhombs; and being complete in fig 4, assume the form of trapeziums, forming the trapezoidal garnet, in which no portion of the primitive planes is visible.
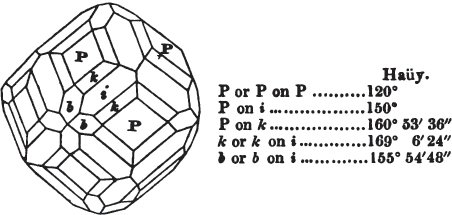
Common garnet is abundantly disseminated in some of the older rocks, as mica-slate, serpentine, and gneiss; and sometimes in granite. It is met with in most countries in which those rocks occur, and is often so abundant as to form an important ore to the iron smelter. Regular dodecahedrons of several pounds weight have been found at Fahlun in Sweden; also at Arendal and Kongsberg in Norway; and in the Zillerthal, Tyrol. In the mica-slates of Perthshire, Inverness-shire, Shetland, and the Isle of Mull, it is of frequent occurrence. At Schwartzenberg in Saxony, and in the Bannat, it presents peculiarly green-coloured crystals; and at Vesuvius occupies the cavities of ejected debris.
3. PYRENEITE. Pyrenit, W. Pyreneite, J. This variety of garnet is black, and occurs in minute but very symmetrical rhombic dodecahedrons, which glisten externally. The fracture is uneven. It is opake; hard; and before the blowpipe loses its colour, and melts easily into a porous black slag. It occurs imbedded in primitive limestone, in the Pic Eres-Lids near Bareges in France.
4. GROSSULAR. Combination of silicate of lime and silicate of alumina.
| Siberia. | |||
| Silica | 44·0 | 40·55 | |
| Alumina | 8·5 | 20·10 | |
| Lime | 33·5 | 34·86 | |
| Oxide of iron | 12·0 | 5·00 | |
| Oxide of manganese | 2·0 Klaproth. | 0·48 Wachtmeister. | |
| Sp. Gr. 3·372. | |||
This rare mineral generally assumes the trapezoidal form of crystallization. It is light olive-green;* translucent or semi-
* Grossulaire, Fr. gooseberry, from its green colour.
[page] 16
transparent; hard; and brilliant externally. Its fracture is conchoidal, and its fragments possess a vitreous lustre. Its comportment before the blowpipe is similar to that of almandine, only that the glass produced is of a brownish colour. It occurs, with idocrase, in a greenish-grey argillaceous rock, near the river Wiloui in Siberia.
5. APLOME. Aplome, H. Combination of silicate of lime and silicate of the peroxide of iron.
| Altenau. | |
| Silica | 35·64 |
| Lime | 29·22 |
| Oxide of iron | 30·00 |
| Oxide of manganese | 3·01 |
| Potash | 2·35—Wachtmeister. * |
Sp. Gr. 3·44.
It is usually considered a variety of garnet, with which it agrees in external form as well as in general aspect; but differs in this respect, that although it commonly occurs in rhombic dodecahedrons, its planes are striated parallel with their lesser diagonal, which, in Haüy's opinion, indicates its primary not to be a rhombic dodecahedron, but a cube.* It is usually of a deep-brown or orange-brown colour; is opake, and somewhat harder than quartz. It is fusible into a black glass.
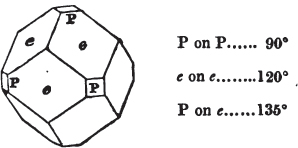
Aplome is found on the banks of the river Lena in Siberia; in the Bannat; and at Schwartzenberg in Saxony.
6. MANGANESIAN GARNET. Grenat manganesie, Bt. Spessartine, Beudant. Of which the protoxide of manganese forms from twenty to thirty-five per cent.
| Spessart. | Brodbo. | ||
| Silica | 35·00 | 39·00 | 35·83 |
| Alumina | 14·25 | 14·30 | 18·06 |
| Peroxide of iron | 14·00 | 0·00 | 0·00 |
| Protoxide of iron | 00·00 | 15·44 | 14·93 |
| Protox. of mangan. | 35·00 Klap. | 27·90 D'Ohson. | 30·96 Seybert. |
This mineral occurs massive, and in dodecahedral crystals va-
* Whence Aplome, in allusion to the ready transition of the cube into the rhombic dodecahedron.
[page] 17
riously modified; it is slightly translucent on the edges. It does not appear to possess any regular structure; fracture commonly vitreous and imperfectly conchoidal; colour deep hyacinth or brownish red. It is fusible before the blowpipe; and, by the decided green colour it exhibits with soda on platina foil, marks distinctly the presence of manganese.
It occurs in granite near Aschaffenberg, in Franconia; massive in the United States, at Jones's Eddy near Bath; at Finbo and Brodbo near Fahlun in Sweden; and elsewhere.
7. MELANITE. Grenat noir, H. Grenat melanite, Br. Bt.
| Frescati. | |
| Silica | 35·50 |
| Alumina | 6·00 |
| Lime | 32·50 |
| Oxide of iron | 25·25 |
| Ox. of manganese | 0·40—Vauquelin. |
Sp. Gr. 3·7.
Melanite is usually quite black,* and completely opake. It occurs in rhombic dodecahedrons, whose edges are replaced. Before the blowpipe it fuses alone into a brilliant black globule; with borax difficultly into an impure green glass coloured by iron.
It has been found in the neighbourhood both of Naples and Rome, imbedded in ancient lava.
8. COLOPHONITE. Grenat resinite, H. This mineral, composed of round particles, which may be separated with facility, is of a greenish, yellowish-brown,† or orange-red colour; and presents, both superficially and when fractured, a shining vitreous lustre. Its specific gravity is 3·5. It consists, according to Simon, of 37 silica, 13·5 alumina, 29·0 lime, 6·5 magnesia, 7·5 oxide of iron, 4·75 oxide of manganese, 0·5 oxide of titanium, and 1·0 water.
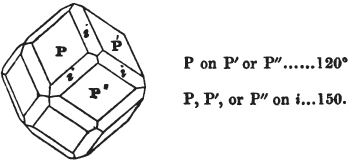
It is found at Arendal in Norway.
9. ALLOCHROITE is of a greyish, dingy yellow, or reddish co-
* Whence Melanite, from the Greek.
† Colophonite, from the Greek, in allusion to its resin colour.
[page] 18
lour,* and opake; its composition is impalpable, or the particles so intimately connected that they cannot be distinguished; not so hard as quartz, but gives fire with steel; fracture uneven. Before the blowpipe comports itself like melanite. Contains silica 35·0, alumina 8·0, lime 30·0, oxide of iron 17·0, oxide of manganese 3·5—Vauquelin. The allochroite is principally found in an iron mine near Drammen in Norway.
10. PYROPE.† Pyrop, W. Grenat, rouge de feu, granuliforme, H. Is nearly allied to almandine, with the exception that part of the protoxide of iron is in pyrope replaced by magnesia.
| Silica | 40·00 |
| Alumina | 28·50 |
| Lime | 3·50 |
| Magnesia | 10·00 |
| Oxide of iron | 16·50 |
| Oxide of manganese | 0·25 Klaproth. |
Sp. Gr. 3·8.
It is found only in rounded or angular grains, of a red colour, which is sometimes clouded with yellow: never crystallized. It is transparent; has a conchoidal fracture; and vitreous lustre; scratches glass. That of Ceylon before the blowpipe fuses into a brilliant black globule, with borax into a chrome-green glass.
Pyrope is found imbedded in serpentine at Zoeblitz in Saxony, and in wacke in Bohemia, but is more common in the latter country; and in Ceylon, in alluvial deposits, accompanied by hyacinths and sapphires.
11. TOPAZOLITE.‡ Topazolite, Bonvoisin. This variety of garnet occurs in remarkably well-defined dodecahedrons, of a topaz-yellow colour, either perfect, or more commonly exhibiting a very low four-sided pyramid on each plane; occasionally also of an olive green; translucent. It consists of silica 37, alumina 2, lime 29, glucina 4, iron 25, manganese 2—Bonvoisin.
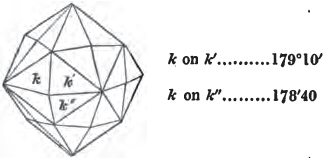
It is found at Mussa in Piedmont, sometimes upon mussite.
* Whence probably its name, from two Greek words signifying of various colours.
† From the Greek, signifying a fire-red colour.
‡ So named from its colour being of a nearly topaz yellow.
[page] 19
The Succinite of Bonvoisin is considered to be an amorphous variety of topazolite. It is amber yellow,* and translucent; and occurs in small rounded masses: it is not hard enough to scratch glass, but easily pulverizes, and melts into a blackish globule under the blowpipe.
Its locality is a serpentine rock in the Vin valley, forming part of the great valley of Lans in Piedmont
CINNAMON STONE. †
Kaneelstein, W. Essonite, H.
Combination of silicate of alumina, silicate of lime, and silicate of the peroxide of iron.
| Ceylon. | Ceylon. | Malsjo. | |
| Silica | 38·80 | 40·00 | 41·87 |
| Alumina | 21·20 | 22·99 | 20·57 |
| Lime | 31·25 | 30·57 | 33·94 |
| Oxide of iron | 6·50 Klaproth. | 3·66 Gmelin | 3·93 Arfwedson. |
with minute proportions of potash and magnesia.
Sp. Gr. 3·5—3·6.
This mineral commonly occurs in masses which are full of fissures. Its general colour is red, with occasionally a brown or orange-yellow tinge; translucent, rarely transparent; fracture flat conchoidal; lustre vitreo-resinous; scratches quartz with difficulty. Before the blowpipe it is fusible with ebullition into a darkish-green glass; and with borax melts very readily into a transparent glass, more or less feebly tinged by iron.
It has been found in considerable masses in some of the primitive rocks of Ceylon, and imbedded in limestone at Malsjo in Sweden; but is most commonly met with in grains among the sand of certain rivers, both in Ceylon and in Brazil.
The Romanzovite of Nordenskiold is considered a variety of cinnamon stone. Its colour is brown or brownish-black; and it is described as occurring either compact or in crystalline plates which indicate the rhombic dodecahedron; fracture resinous, with a greasy lustre; brittle, but hard enough to scratch felspar; streak light yellow. Before the blowpipe it melts without ebullition. Contains silica 41·24, alumina 24·08, lime 24·76, oxide of iron 7·02, magnesia and oxide of manganese 0·92, volatile parts and loss 1·98—Nordenskiold. It occurs at Kimito in Finland.
* Succin, Fr. Amber; whence Succinite.
† Its name is probably derived from the resemblance of its colour to that of cinnamon.
[page] 20
IDOCRASE.
Vesuvian, W. Idocrase, H. Pyramidal Garnet, M.
Combination of silica, alumina, and lime.
| Vesuvius. | Siberia. | Egeran, Bohemia. | |
| Silica | 35·50 | 42·00 | 41·0, |
| Alumina | 33·00 | 16·25 | 22·0 |
| Lime | 22·25 | 34·00 | 22·0 |
| Oxide of iron | 7·50 Klap. | 5·50 Klap. | 6·0 Borkowsky. |
with traces of oxide of manganese.
Sp. Ur. 3·08—3·4. H. 6·5.
Idocrase occurs crystallized, either solitary or in groups; and massive. The general form* of the crystals is a quadrangular prism, which sometimes is terminated by planes, and the edges of the prism are often replaced. Primary form a right prism with square bases; yielding to cleavage readily parallel to all its planes, with sufficient brilliancy to obtain incidences of 90° by the reflective goniometer in every direction; the prism is also divisible parallel to both its diagonals, though not easily: fracture small conchoidal, and shining. Idocrase is mostly brownish- or yellowish-green, sometimes orange, rarely black; generally translucent, sometimes nearly transparent; possesses double refraction; is fusible with ebullition into a yellowish translucent globule, and forms with borax a diaphanous glass tinged green by iron.
Fig. 1, the primary; a right prism with square bases. In fig. 2 the lateral edges of the prism are replaced by quadrangular planes, and a portion of the summit is replaced by four six-sided planes. In fig. 3 the quadrangular planes replacing the edges of the prism are very much increased, so as greatly to reduce the lateral primary planes.
* Idocrase, in allusion to its form; a mixed figure. Vesuvian, from its having been first discovered on Vesuvius.
[page] 21
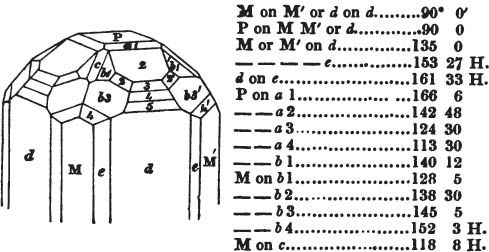
Idocrase is met with both in volcanic and primitive countries. It occurs in the ejected masses of Vesuvius, where its crystals line the cavities of volcanic rocks, accompanied by garnet, hornblende, melanite, mica, and icespar. Crystals, occasionally of large dimensions, are found at this locality; but their forms are complicated. The above fig. 3 is the usual crystallization of the Siberian idocrase, which is met with in a greenish-white serpentine, near the Lake Baikal, and on the banks of the Wiloui; but by far the finest specimens come from Ala in the Val di Brozzo, in Piedmont: these are in general semi-transparent, present fine olive-green, hair-brown, and, though rarely, perfect black colours; are deeply streaked longitudinally, and are as remarkable for lustre and brilliancy as for the symmetry and perfection of their crystalline forms. Large, well-defined, opake crystals, often exceeding four or five inches in diameter, occur at Egge near Christiansand in Norway.
1. CYPRINE, or cupreous idocrase, from the vicinity of Telle-marken in Norway, exhibits occasionally crystalline faces, and has a fine smalt-blue tinge, arising, it is supposed, from a minute portion of copper. It fuses readily, with effervescence, into a globule, which becomes black in the oxydizing flame and red in the reducing one.
2. EGERAN. It occurs in diverging groups of deeply streaked translucent crystals, of a liver-brown colour, whose form is that of a right rectangular prism, having its lateral edges replaced. Before the blowpipe it melts with intumescence into a greenish blebby glass. It occurs at Haslau, near Eger* in Bohemia, and is sometimes accompanied by quartz and tremolite.
* Whence Egeren.
[page] 22
GEHLENITE.*
Combination of silica, alumina, lime, and oxide of iron.
| Tyrol. | Tyrol. | |
| Silica | 29·64 | 29·50 |
| Alumina | 24·80 | 14·50 |
| Lime | 35·30 | 27·55 |
| Oxide of iron | 6·56 | 12·20 |
| Water | 3·30 | 6·00 |
| Soda and loss | 0·00 Fuchs. | 10·00 Clarke. |
Sp. Gr. 2·98 to 3·02. H. 5·5 to 6·0.
Gehlenite occurs in rectangular four-sided prisms, nearly approaching in their dimensions the form of the cube; sometimes isolated, generally invested by calcareous spar, aggregated irregularly in groups; or massive, including pleonaste. Usual colour grey, but frequently having a greenish or yellowish tinge. Surface commonly rough and dull; when sufficiently brilliant for the use of the reflective goniometer, the crystals afford angles of 90° in every direction. Fracture uneven, passing into splintery; opake, the fragments feebly translucent on the edges. Before the blowpipe Gehlenite suffers little change when alone; with borax it melts with difficulty into a glass coloured by iron. It gelatinizes in heated muriatic acid.
Mount Monzoni, in the valley of Fassa, is its only well-authenticated locality, although Monticelli mentions it as occurring indistinctly crystallized, among the productions of Vesuvius.
PREHNITE.†
Prehnite, W. H. Axotomous Triphane Spar, M.
Combination of silica, alumina, lime, and water.
| Cape of Good Hope. | Dumbarton. | Reichenbach. | |
| Silica | 43·80 | 43·60 | 42·50 |
| Alumina | 30·88 | 23·00 | 28·50 |
| Lime | 18·33 | 22·33 | 20·40 |
| Oxide of iron | 5·66 | 2·00 | 3·00 |
| Water | 1·83 | 6·40 | 2·00 |
| Klaproth. | Thomson. | Laugier. |
Sp. Gr. 2·926. H. 6·0 to 7·0.
Generally of a pale greenish or yellowish colour; with a vi-
* Gehlenite, in honour of the chemist Gehlen.
† In honour of Colonel Prehn, its discoverer.
[page] 23
treous or pearly lustre; and somewhat translucent; becomes electric by heat, and is slowly soluble in dilute muriatic acid. It occurs fibrous, massive, and in crystals, which are for the most part closely aggregated; their primary form is a right rhombic prism of about 100° and 80°, but the crystals are subject to modification by two planes on the summit, a1 a1', of the following figure. It presents several varieties of form. Cleavage distinct parallel to P, less so parallel to M. Per se it fuses with intumescence into a white or pale-yellowish frothy glass; and with borax forms a transparent bead.
Koupholite,* a variety in small transparent rhombic tables (fig. 1) from Barèges in the Pyrenees; has a white or yellowish-white colour, and a glistening pearly lustre. It consists, according to Vauquelin, of 48 silica, 24 alumina, 23 lime, and 4 oxide of iron; and it is fusible into a white frothy glass.
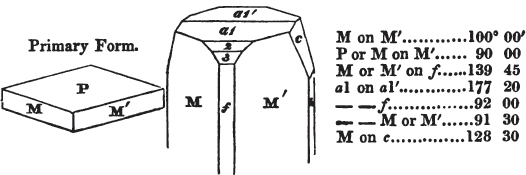
Crystallized prehnite in considerable quantity, and of a purer green than that of Europe, is found at the Cape of Good Hope; it occurs in the Fassa valley, Tyrol, of a peculiar bluish-green hue; with axinite and epidote, at St Christophe in Dauphiné; in large, well-formed, pale green coloured crystals in Massachusetts; in Carinthia, Sweden, and elsewhere. In England it has been noticed in Staffordshire, at Woodford in Gloucestershire, and at Botallack near the Land's End, Cornwall. In Scotland it is abundant in veins traversing trap near Dumbarton; at Hartfield Moss, near Paisley; at Frisky Hall, near Glasgow; at the Castle Rock and Salisbury Crag, near Edinburgh; in the Isles of Mull and Skye; &c. The Scotch varieties in general exhibit radiated, botryoidal, orbicular, or mammillated masses of various colours, from apple-green to straw-yellow, the latter particularly at Salisbury Crag; sometimes translucent and colourless, as at the Castle Rock; and very often white and opake, as in Dumbartonshire.
* From two Greek words, signifying a light stone.
[page] 24
STILBITE.
Strahl-Zeolith, W. Stilbite, H. Bt. Radiated Zeolite, J. Prismatoidal Kouphone Spar, M.
| Iceland. | Faroe. | Red from Dumbarton. | ||
| Silica | 58·0 | 59·25 | 58·3 | 52·50 |
| Alumina | 16·1 | 15·00 | 17·5 | 17·37 |
| Lime | 9·2 | 5·35 | 6·6 | 11·52 |
| Water | 16·4 | 16·00 | 17·5 | 18·45 |
| Potash | 0·0 Hisin. | 4·75 Duménil. | 0·0 Meyer. | 0·00 Thom. |
Sp. Gr. 2·0—2·2. H. 3·5 to 4·0.
Its colours are white, grey, red, and brown; translucent; lustre vitreous, except on the faces T, which exhibit a peculiar glistening or shining pearly appearance.* Primary form a right prism with rectangular bases, in which it sometimes occurs; but it is more frequently found in prisms of which the edges are replaced, and which are terminated by tetrahedral summits; the planes forming the pyramids being placed on the angles of the prism. Cleavage parallel to the planes T and M, the latter only being perfect. It intumesces before the blowpipe, and runs into a blebby colourless glass.
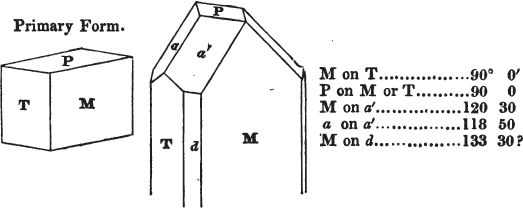
Stilbite is met with occasionally in the fissures of primitive rocks, and in mineral veins; but its principal repositories are the cavities of trap. The Faroe Islands and Iceland are the great localities of this beautiful mineral. Splendid specimens have also been brought from Indore in the Vendayah Mountains of Hindustan. And very beautiful crystals, of a brick-red colour, occur in porphyritic amygdaloid, near Kilpatrick in Dumbartonshire; and in the Fassa valley, Tyrol. Dauphiné, Andreasberg in the Hartz, Arendal in Norway, Gustafsberg near Fah-
* Whence Stilbite, signifying a particular lustre.
[page] 25
lun in Sweden, and the island of Arran, are also well-known localities of stilbite; at most of the latter being found in granite and primitive rocks.
HEULANDITE.*
Blätter Zeolith, W. Var. de Stilbite, H. Foliated Zeolite, J. Heulandite, Brooke. Hemi-prismatic Kouphone Spar, M.
Combination of silica, alumina, lime, and water, with occasionally a little oxide of iron.
| Red, Campsie. | Red, Edelfors. | ||
| Silica | 59·90 | 60·28 | 52·6 |
| Alumina | 16·83 | 15·41 | 17·5 |
| Lime | 17·19 | 8·18 | 9·0 |
| Water | 13·43 | 11·07 | 18·5 |
| Oxide of iron | 0·00 Walmstedt. | 4·16 Retzius. | 0·0 Vauq. |
Sp. Gr. 2·20. H. 3·5—4·0.
This mineral commonly occurs crystallized in right oblique-angled prisms (two of its opposed lateral planes being longer than the other two), generally modified on the angles and one lateral edge. Cleavage parallel only to the terminal plane P of the following figures, highly perfect. Lustre vitreous, except P, which possesses high degrees of pearly lustre, both as faces of cleavage and of crystallization. Generally translucent, but the crystals are often nearly transparent when colourless; from which it varies to white, yellow, brownish, and deep red. It also occurs massive; frequently in a globular form; and is easily frangible. Before the blowpipe it melts with intumescence, emitting at the same time a phosphorescent light. With acids it does not gelatinize.
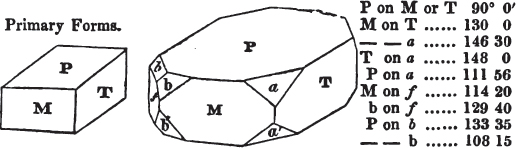
The varieties of heulandite are usually found accompanied by stilbite in the vesicular cavities of amygdaloidal rocks, and in certain metalliferous veins. The Faroe Isles and Iceland afford the finest crystals; but splendid specimens have also been brought from the Vendayah Mountains in Hindustan, and Cape
[page] 26
* Heulandite, as a testimony to the liberality and zeal of M. Heuland.
Blomidon in Nova Scotia. Campsie, near Dumbarton, and the Fassa Valley, Tyrol, are the localities of the red variety.
DIPYRE.
Schmelztein, W. Dipyre, H.
Consists of 60 silica, 24 alumina, 10 lime, and 2 water, loss 4. Its specific gravity is about 2·7. H. = 6·0.
This rare substance occurs in slender indistinctly formed prisms, of a greyish, or reddish-white colour, fasciculated into masses. It has been observed in the form of a hexahedral prism terminated by a low pyramid. Lustre vitreous; translucent; hard enough to scratch glass; and becomes slightly phosphorescent by the application of heat. Before the blowpipe it fuses with effervescence into a blebby colourless glass.* It is found in the torrent of Mauléon, in the Western Pyrenees, imbedded in a kind of soft slate. It is generally classed with scapolite.
DAVYNE.
Davina, Monticelli. Davytic Kouphone Spar, Haidinger.
Contains silica 42·91, alumina 33·28, lime 12·02, iron 1·25, water 7·43, loss 3·11—Covelli.
Sp. Gr.2·4. H. = 5·0—5·5.
Colour white or yellowish; transparent, translucent, or opake,— the lustre inclining to opalescent in the first case, to pearly when opake; and the colour to grey when transparent,—to whitish when opake. Cleavage highly perfect parallel to M; fracture conchoidal.
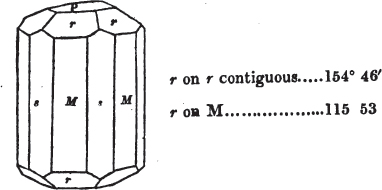
With nitric acid it effervesces and gelatinizes; and alone before the blowpipe fuses with effervescence into a white opake
* Dipyre, from the Greek, signifying the double effects of fire, in allusion to its phosphorescence and fusibility by heat.
[page] 27
somewhat porous globule. Laminæ exposed to the flame of a candle do not lose their transparency. With boracic acid on platina wire, it affords a limpid colourless globule; and with salt of phosphorus in proper proportions, yields a pearly bead, which appears milky and opake when hot, but becomes translucent on cooling.
This mineral was described by Monticelli and Covelli, in their Prodromo della Mineralogia Vesuviana, and named by these mineralogists in honour of our illustrious countryman Sir Humphry Davy. It occurs in the more ancient rocks of Vesuvius, accompanying garnet, mica, Wollastonite, &c. It may be distinguished from nepheline by the length of its crystals invariably exceeding their breadth, the reverse of which is the case in that mineral; its specific gravity is also much lower; and nepheline is not acted upon by acid as this is. (Manual.)
LAUMONITE.*
Lomonit, W. Diatomous Kouphone Spar, M.
Combination of silica, alumina, lime, and water.
| Huelgoet. | Skye. | ||
| Silica | 48·3 | 49·0 | 52·04 |
| Alumina | 22·7 | 22·0 | 21·14 |
| Lime | 12·1 | 9·0 | 10·62 |
| Water | 16·0 | 17·5 | 14·92 |
| Carbonic acid | 0·0 Gmelin. | 2·5 Vogel. | 0·00 Connell. |
Sp. Gr. 2·3. H. above 4·0 when fresh.
This mineral occurs in aggregated crystalline masses, deeply striated; or in separate crystals of several varieties of form, and sometimes in that of its primary crystal,—an oblique rhombic prism, of which the inclination of the terminal plane is from one acute angle to the other: this prism yields to cleavage parallel to its lateral planes and both diagonals. It is white, or yellowish white, sometimes with a tinge of red; and is transparent or translucent. Before the blowpipe with borax it intumesces, and fuses into a colourless glass: per se, it first forms a white spumous enamel, and on continuing the heat, becomes translucent. It gelatinizes with nitric or muriatic acid.
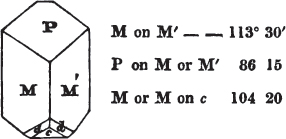
* In honour of Gillet de Laumont, its discoverer.
[page] 28
Laumonite was formerly termed the efflorescent zeolite, on account of its losing its water on exposure to the air; in consequence of which it becomes opake, of a shining white colour, and pearly lustre; and eventually falls into a white powder similar to that resulting from the decomposition of Glauber's salt.*
This mineral was first discovered in the lead mine of Huelgoet in Brittany, lining the cavities of the veins. It has since been found in trap in Iceland and Faroe; at St Gothard; forming large masses, which exhibit a radiating and divergent structure, in the Fassa-thal, Tyrol; near Paisley in Renfrewshire; at the Kilpatrick Hills, Dumbartonshire; near Loch Enort in the Isle of Skye, accompanying stilbite; and at Port Rush in Ireland with analcime and stilbite in trap rocks. More recently it has been discovered in the amygdaloid of Connecticut, and in Nova Scotia.
ZOISITE.†
Zoisit, W. Var. d'Epidote, H.
Combination of silica, alumina, and lime, with a little protoxide of iron.
| Styria. | Bayreuth. | Valais. | |
| Silica | 43·0 | 40·25 | 37·0 |
| Alumina | 29·0 | 30·25 | 26·6 |
| Lime | 21·0 | 22·50 | 20·0 |
| Protox. of iron | 3·0 Klaproth. | 4·50 Bucholz. | 13·0 Laugier. |
Sp. Gr. 3·2 to 3·3. H. = 6·0—7·0.
It occurs in rhombic prisms, of a grey, greyish-yellow, or brown colour, but which are rarely perfect, owing to deep longitudinal striæ. The obtuse lateral edges of the prism are often rounded, and the terminations incomplete. It also occurs massive, and cleaves parallel to the sides and both diagonals of a right rhombic prism of about 60° and 120°, but not with brilliant surfaces. It has a pearly lustre; and is translucent. Alone, before the blowpipe, it fuses on the outer edges into a yellowish transparent glass, but finally into a vitreous scoria; with borax intumesces, and forms a diaphanous glass.
It is met with in the Bacher Mountain and the Sau Alp in Styria, in a rock composed of kyanite, garnet, and augite; in granite in Bayreuth; also in Bavaria, Salzburg, the Tyrol, and the Vallais. It is mentioned by Jameson as occurring at Glenelg in Inverness-shire, and in Shetland.
This species is by Mohs united with epidote, from which it principally differs in colour.
* This may to a considerable extent be counteracted, by dipping the specimens, when fresh, into a thin solution of gum arabic.
† Zoisite, after the Baron de Zois.
[page] 29
EPIDOTE.
Prismatoidal Augite Spar, M. Pistacit, W. Epidote, H. Thallite, Karsten.
Combination of silica, alumina, protoxide of iron, and lime.
| Pistaxite, Isère. | St Marcel. | Crystallized Epidote. | Isle St John. | |
| Silica | 37·0 | 33·5 | 37·0 | 40·9 |
| Alumina | 27·0 | 15·0 | 21·0 | 28·9 |
| Lime | 14·0 | 14·5 | 15·0 | 16·2 |
| Oxide of iron | 17·0 | 19·5 | 24·0 | 14·0 |
| Ox. of mangan. | 1·5 | 12·0 | 1·5 | 0·0 |
| Descortes. | Cordier. | Vauquelin. | Beudant. |
Sp. Gr. 3·42. H. = 6·0—7·0.
This mineral is found granular, massive, and in prismatic crystals, variously terminated and longitudinally striated. Colour green of different shades, occasionally almost black, rarely brown or reddish. It has a shining lustre; and is somewhat transparent. The primary crystal is a right oblique angled prism, of about 115° 30′ and 64° 30′; and it cleaves with brilliant surfaces, parallel to the sides and lesser diagonal of the prism. Before the blowpipe it intumesces, but does not, even by a strong heat, completely melt; with borax it intumesces, and then fuses into a glass coloured by iron—unless manganese predominate, in which case it assumes in the oxidating flame an amethystine tinge.
Fig. 1. The primary; a right prism of which the bases are oblique-angled parallelograms. Of this there are several modifications, which commonly do not appear to have much direct affinity with the primitive: Fig. 2 exhibits one of the most simple.
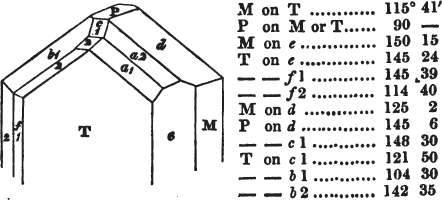
[page] 30
Epidote is not often found massive, but chiefly in crystals, varying in size from the acicular to near an inch in diameter, and several inches in length; the acicular are met with in the department of Isère in France, at Bourg d'Oisans in Dauphiné, in the Alps, &c. (Thallite of Karsten). The larger occur at Arendal in Norway, and Normark in Sweden (Acanticonite of Dandrada, or Arendalite): the magnificent crystals from these localities consist of concentric coats, the exterior of which admit of removal, and thus out of a large imperfect crystal, one of smaller size, but more completely formed, may be produced with facility. It belongs chiefly to primitive rocks, but is only found in veins and fissures, among which, in small quantities, it occurs in many countries; magnetic iron, garnet, felspar, adularia, axinite, and asbestus, are the minerals which chiefly accompany it.
Granular epidote. Scorza, Br. Appears from its analysis to be epidote reduced to small grains by attrition. It occurs on the banks of the river Arangos, near Muska in Transylvania: and is called Scorza by the inhabitants of the country.
Manganesian epidote. Manganèse oxydé silicifère, H. Epidote violet, Bt. Occurs in small prismatic crystals of a violet or reddish-brown colour, which are generally associated in groups, sometimes imbedded in asbestus. It is opake, and yields to the knife; contains about 12 per cent. of the oxide of manganese, and before the blowpipe fuses easily into a black glass,—with borax into a transparent one, exhibiting in the oxidizing flame the amethystine tinge of manganese.
It occurs at St Marcel, in the valley of Aosta, in Piedmont, in gneiss accompanied by oxide of manganese, quartz, asbestus, &c.
AXINITE.*
Prismatic Axinite, M. Axinit, W. Axinite, H. Thumerstone, J.
Combination of silica, alumina, lime, oxide of iron, and manganese.
Dauphiné.
| Silica | 50·50 | 44·0 | 45·00 |
| Alumina | 16·00 | 18·0 | 19·00 |
| Lime | 17·00 | 19·0 | 12·50 |
| Oxide of iron | 9·50 | 14·0 | 12·25 |
| Oxide of manganese | 5·25 | 4·0 | 9·00 |
| Magnesia | 0·00 | 0·0 | 2·25 |
| Boracic acid | 0·00 | 0·0 | 2·00 |
| Klaproth. | Vauquelin. | Wiegmann. |
Sp. Gr. 3·27. H. = 6·5—7·0.
* In sharpness like the edge of a hatchet, whence Axinite.
[page] 31
This mineral rarely occurs massive, more frequently in flat oblique rhomboidal prisms, whose edges are remarkably sharp. Common colour violet or clove-brown, inclining to plumb-blue and pearl-grey; also yellow and green, from an admixture of chlorite; occasionally nearly colourless, and transparent. The crystals do not appear to possess regular cleavages, their primary therefore has not been determined; their general form is that of a doubly oblique prism, which is assumed as the primary in the following figure. Externally the crystals are very brilliant; fracture small and conchoidal; becomes electric by exposure to heat; before the blowpipe intumesces, and fuses readily into a dark green glass, which changes to black in the oxidating flame; with borax into a glass, also coloured by iron; is not acted upon by acid.
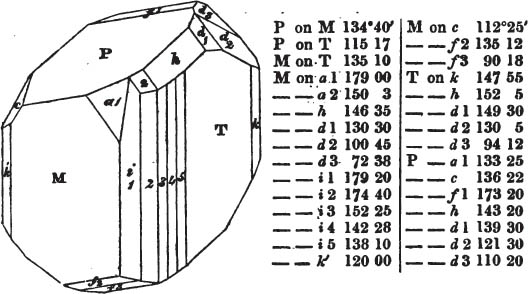
It occurs in beds at Thum* in Saxony; at Barèges in the Pyrenees, upon a gangue of quartz; in splendid crystals, as remarkable for the brilliancy of their lustre as for their size and symmetry of form, at St Christophe, near D'Oisans in Dauphiné; near Kongsberg in Norway, in a white laminated calcareous rock, accompanied by black mica, quartz, and native silver; at Arendal, with felspar, epidote, &c.; on mica slate in Savoy; in several places in the Hartz; and in killas at Botallack near the Land's End, Cornwall, where it occurs both in well defined though rather complex crystals, and massive and compact entering into the composition of a rock with tourmaline and garnet.
* Whence its designation of Thumite or Thumerstone.
[page] 32
ISOPYRE.
Isopyric Quartz, Haidinger.
Contains silica 47·09, alumina 13·91, peroxide of iron 20·07, lime 15·43, peroxide of copper 1·94—Turner.
Sp. Gr. 2·9—3·0. H. = 6·0—6·5.
Occurs in compact masses of a velvet-black colour, occasionally dotted with red, as in heliotrope. Opake, or faintly translucent on its thinnest edges, with a dark liver-brown tint. Brittle. Lustre vitreous. Cleavage not perceptible. Fracture flat con-choidal, highly perfect when the mineral is pure. Acts slightly on the magnetic needle. It fuses before the blowpipe without emitting any gaseous matter. Acids act upon it with difficulty, but it is easily and completely decomposed by alkaline carbonates.
This mineral much resembles obsidian, but was distinguished by Haidinger in consequence of its fainter and less vitreous lustre. It is perfectly black, and forms compact masses occasionally two inches in diameter, in the granite of St Just near Penzance, where it occurs associated with tin and tourmaline. Breithaupt's Tachylite appears to be the same mineral; its specific gravity is stated somewhat lower, but in other repects it is identical; it forms small masses in basalt and wacke, at Säsebühl near Göttingen.
INDIANITE.
Bournon.
| Red. | White. | ||
| Silica | 42·5 | 42·0 | 43·0 |
| Alumina | 37·5 | 34·0 | 34·5 |
| Lime | 15·0 | 15·0 | 15·6 |
| Oxide of iron | 3·0 | 3·2 | 1·0 |
| Soda | 0·0 Chenevix. | 3·3 Laugier. | 2·6 Laugier. |
with a trace of manganese.
Sp. Gr. 2·74. Scratches glass.
In granular masses, of a white or greyish colour, with a shining lustre, sometimes tinged brown by a mixture of garnet. It is translucent. It cleaves, according to Brooke, into rhombic prisms of 95° 15′ and 84° 45′; is infusible before the blowpipe; and when digested in acids, becomes gelatinous. This substance was described by Bournon. It forms the gangue of corundum from the Carnatic;* and occurs associated with garnet, felspar, fibrolite, and hornblende.
* Whence Indianite.
[page] 33
XANTHITE.
Mather and Thornton.
| Contains Silica | 32·71 |
| Alumina | 12·28 |
| Lime | 36·31 |
| Peroxide of iron | 12·00 |
| Protoxide of manganese | 3·68 |
| Water | 0·60—Thomson. |
Sp. Gr. 3·20. Easily crushed by the nail.
Consists of a congeries of small rounded grains easily separable fromeach other. Colour light greyish or yellow. Translucent or transparent. Lustre splendent or shining, inclining to resinous. Cleavage parallel to the sides of a doubly oblique prism, and forming angles of 97° 30', 94°, and 107° 30'. It is fusible in small particles on a fine slip of platinum foil; when in fusion it intumesces, and forms a greenish translucent bead, which is slightly attractable by the magnet. With borax it produces a glass which is yellow when hot, but becomes colourless on cooling.
It occurs imbedded in calcareous spar at Amity, in Orange County, U. S. The rounded grains of which it is composed, seem when examined by the microscope to consist of imperfect crystals having a foliated texture.
ANTHOPHYLLITE.*
Strahliger-Anthophyllite, H. J. Prismatic Schiller Spar, M.
Combination of silica, alumina, magnesia, and protoxide of iron.
| Silica | 62·66 |
| Alumina | 13·33 |
| Magnesia | 4·00 |
| Lime | 3·33 |
| Oxide of iron | 12·00 |
| Oxide of manganese | 3·25 |
| Water | 1·43—John. |
Sp. Gr. 3·0 to 3·3. H. = 5·0 to 5·5.
Anthophyllite has a grey or clove-brown colour; with an occasional blue tinge; and a glistening, pearly, pseudo-metallic lustre. It occurs massive, the mass consisting of crystals or crystalline fibres, often disposed in a radiating form: these may be cleaved parallel to the lateral planes of a rhombic prism of about 125° and 55° (73° 44′ and 106° 16′ according to Necker), and both its
* From its resemblance in colour to the flower Anthophyllum.
B2
[page] 34
diagonals; the latter are not brilliant. The prism is generally traversed by natural crevices nearly at right angles to its axis; translucent on the edges. It is infusible before the blowpipe per se; with borax it melts with difficulty into a glass coloured by iron; and with salt of phosphorus decomposes slowly, and yields a skeleton of silica.
It occurs at Kongsberg in Norway, with hornblende; translucent, and of a rich clove-brown colour, at Ujordlersoak in Greenland; in foliated masses with mica, at Snarum, near Modum in Norway; and associated with tourmaline and iolite at Haddam in Connecticut.
AMPHODELITE.
Nordenskiold.
Contains silica 45·80, alumina 35·45, lime 10·15, magnesia 5·05, oxide of iron 1·70, water 1·85.
Sp. Gr. 2·76. H. = 4·5.
Crystalline form, resembling that of felspar. Colour light red; similar to scapolite in its fracture, and possessing two cleavages which meet at an angle of 94° 19′.
Occurs in the limestone quarry of Lojo in Finland.
SMARAGDITE.*
Smaragdit, Saussure. Diallage verte, H. Diallage, J.
It contains silica 50, alumina 21, lime 13, magnesia 3, oxide of chrome and oxide of iron 13—Vauquelin. Sp. Gr. 3·0.
Smaragdite has a brilliant or emerald-green colour, and a silky or pearly lustre; is transparent on the edges, or opake; is scarcely so hard as glass, and yields to the knife; has a laminated structure, with cleavage parallel to the sides and diagonals of a slightly rhombic prism. It fuses into a grey or greenish enamel.
It is found massive, or disseminated in Saussurite, near Geneva, and on Monte Rosa in Switzerland; also in Corsica imbedded in felspar. Haidinger considers it to be a compound of laminæ of hornblende, alternating with laminæ of augite, both frequently of bright-green colours. Vol. x. of Edinburgh Royal Society Transactions.
* From the Greek, signifying a green stone—an emerald.
[page] 35
ANORTHITE.
Anorthotomous Feldspar, M. Christianite, Monticelli. Anorthite, Rose.
Combination of silica, alumina, lime, magnesia, and oxide of iron. Silica 44·49, alumina 34·46, lime 15·68, magnesia 5·26, oxide of iron 0·74—Rose.
Sp. Gr. 2·65. H. = 6·0.
Primary form an oblique rhombic prism. Cleavage perfect parallel to P and M. Occurs in white, translucent or transparent crystals, which present a vitreous lustre, inclining to pearly on the planes of cleavage; fracture conchoidal; streak white. Is entirely decomposed in concentrated muriatic acid. Before the blowpipe on charcoal, it assumes first a vitreous and translucent aspect, and then fuses with difficulty on the edges into a blebby and semi-transparent glass; with salt of phosphorus is decomposed, with the exception of a silica skeleton, and yields a glass which becomes opaline on cooling.
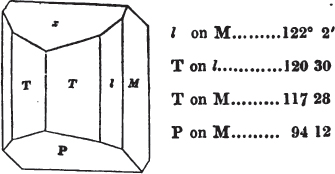
This species was simultaneously described by Rose under the title of anorthite, and by Monticelli under the denomination of Christianite.* Its principal locality is Vesuvius, or rather Monte Somma, the ancient crater of that volcano; it generally occupies the cavities of chloritic masses, and is associated with ice-spar, augite, mica, and idocrase. Its infusibility before the blowpipe serves to distinguish this mineral from any of the zeolites, as well as from nepheline and leucite.
CLAYS.
Thon, W. Argile, H.
The substances comprehended in the term Clay admit of no general description, but most of them agree in possessing an earthy texture, and emit an argillaceous odour when breathed on. They consist chiefly of silica, with a variable proportion of alumina, and a small quantity of lime or magnesia, occasionally of
* In compliment to Prince Christian of Denmark.
[page] 36
alkali. They all appear to be mechanical deposits, not one of them occurring crystallized, or with a crystalline structure; some are slaty.
1. SLATE-CLAY. SHALE. Schieferthon, W. Argile schisteuse, Br. Shale occurs only massive; its general colour is grey, which sometimes is bluish, yellowish, or blackish; in one direction its structure is slaty, in the other earthy; it is easily broken; it usually adheres to the tongue, and yields to the nail; is opake, meagre to the touch, and dull, except from casually imbedded mica, which sometimes imparts a glimmering lustre: its specific gravity is about 2·6. It is found resting upon, as well as interposed between, beds of coal, which it invariably accompanies. It often contains impressions of reeds and of ferns.
Black Bituminous Shale has a slaty structure; when put into the fire, it blazes, crackles, and emits a black smoke and bituminous odour, loses a considerable portion of its weight, and is converted into a whitish or reddish flaky ash. It is found in common coal, being generally more or less mixed with it.
Brown Bituminous Shale is met with at Kimmeridge in Hampshire; and from its giving out a bituminous odour when placed in the flame of a candle, or in the fire, is termed Kimmeridgecoal. Its colour is greyish brown; it has a somewhat slaty texture, and occasionally a large flat conchoidal fracture: it yields easily to the knife, and acquires lustre by the pressure of the nail. On exposure to a considerable heat, the bituminous part is consumed, and it is reduced to a grey earthy ash.
Rottenstone. Its colour is dirty grey, or reddish brown, passing into black: it is dull, earthy, soft, meagre to the touch, and emits an unpleasant odour when rubbed. According to the analysis of R. Phillips, it consists of 86 alumina, 4 silica, and 10 carbon. Occurs at Bakewell in Derbyshire, and is believed to arise from the decomposition of the shale of that country; also at Albany near New York.
2. ADHESIVE SLATE. Schiste à polir, Br. Adhesive slate, J. Contains silica 86·50, alumina 7·00, magnesia 1·50, lime 1·25, oxide of iron 2·50—Klaproth. Is found massive, and possesses a slaty texture, which becomes visible by exposure; but if the mass be immersed in water, it resumes its former appearance. Has a yellowish or smoke-grey colour; is very soft, splits easily, adheres strongly to the tongue (whence Adhesive slate), and is opake. Its specific gravity is 2·08; and it is infusible before the blowpipe. On exposure to a red heat, it becomes brownish, and loses weight. It absorbs water with avidity, but does not fall to pieces. It has hitherto been found only in the gypsum formation around Paris, and is the imbedding substance of the Menilite.
3. POLISHING SLATE. Polier schiefer, W. Sp. Gr. 0·59—0·60. Is of a white, yellowish-white, or yellow colour; massive, with
[page] 37
a slaty texture; is opake,brittle, and so light as to swim on water. One variety yielded to Bucholz, silica 83·50, alumina 4·0, lime 8·50, oxide of iron 1·60, water 9·0. It imbibes water; and when burnt becomes red, but does not fuse. It is found near Bilin in Bohemia, in a bed resting on marl; also at Zwickau in Saxony, and in Auvergne, and is supposed to be a volcanic production. It is used for polishing glass, marble, and metals.
4. LITHOMARGE. Steinmark, W. Argile lithomarge, H. Sp. Gr. 2·43. Is yellowish or reddish-white; also grey or bluish, and is frequently spotted internally. It is massive; soft; adheres strongly to the tongue; has a greasy feel; gives a shining streak; and is commonly opake, occasionally translucent; texture earthy, but has a large conchoidal fracture. It is infusible before the blowpipe; sometimes phosphoresces when heated; and hardens if exposed to a high temperature. It occurs at Ehrenfriedersdorf and Altenberg in Saxony; at Planitz, near Zwickau in Bohemia; and has been noticed in small quantities in the tin and copper veins of Tin Croft and Cook's Kitchen mines near Redruth, which traverse both granite and schiste.
Friable Lithomarge, in scaly, glimmering particles, which are phosphorescent in the dark, occurs in the tin veins of Ehrenfriedersdorf in Saxony, and some other places. Klaproth found it to consist of silica 32, alumina 26·50, iron 21, water 17, and muriate of soda 1·50.
5. FULLER'S EARTH. Walkerde, W. Argile smectique, H. Sp. Gr. 1·82 to 2·19. Occurs massive, and is usually of a greenish-brown colour, sometimes nearly that of slate; it is opake, soft, dull, possesses an earthy fracture and a greasy feel, and yields to and receives a polish from the nail, but scarcely adheres to the tongue; in water it becomes translucent, and falls into a pulpy impalpable powder. A variety from England yielded 53 silica, 10 alumina, 0·5 lime, 1·25 magnesia, 9·5 oxide of iron, 1 muriate of soda, and 24 water. It is fusible into a porous slag, and ultimately forms a white blebby glass.
At Nutfield near Riegate, in Surrey, it occurs in regular beds near the summit of a hill of considerable elevation, between beds of sand or sandstone containing fossil wood and impressions of the nautilus and other sea-shells. There are two distinct beds of fuller's earth; the upper, of a greenish clay colour and five feet in thickness, rests upon the other, which is of a light slate blue, and eleven feet thick; in these beds, but principally in the latter, are found considerable masses of sulphate of barytes, sometimes exhibiting regular crystallizations. Fuller's earth is also found at Deptling, near Maidstone in Kent; and at Aspley, near Woburn in Bedfordshire, under nearly the same circumstances as at Nutfield. Also at Old Down near Bath; near Nottingham; in Sussex; and at Rosswein in Saxony. It
[page] 38
occurs among primitive rocks, and is supposed to originate from their decomposition. From its property of absorbing oil and greasy matter, this substance was formerly much used in the fulling of cloth (whence its name), and was forbidden to be exported under severe penalties: soap is now generally substituted.
6. TRIPOLI. Tripel, W. Quarz aluminifère Tripoléen, H. Sp. Gr. 1·86—2·2. This mineral has generally an argillaceous aspect. It occurs massive, with a coarse, dull, earthy fracture; it is meagre and rough to the touch, does not adhere to the tongue, and yields easily to the nail. Presents various shades of grey, yellow, and red; and yielded to Haase 90 silica, 7·0 alumina, and 3·0 iron. It imbibes water, which softens it; when burnt, becomes white and is hardened; but is very difficultly fusible. It was first brought from Tripoli in Africa, but has since been noticed in the Puy de Dome, in Tuscany, near Prague, at Arnberg in Bohemia, and many other places; and appears to be merely a fine arenaceous variety of quartz, accidentally mixed with clay. It is used in polishing metals, marble, glass, and other hard bodies.
7. BOLE. Bol, W. Bole, J. Sp. Gr. 1·60 to 1·97. Bole occurs in solid amorphous masses of a yellow, red, or brownish-black colour. The yellow is translucent on the edges, the red is nearly translucent, and the brownish-black opake. It yields to the nail, exhibits a conchoidal fracture; gives a shining streak; adheres to the tongue; has a greasy feel; and fuses into a slag. Immersed in water, it emits a crackling noise, and breaks in pieces.
This substance is found in irregular beds or disseminated masses in wacke and basalt, from the decomposition of which it is supposed to arise. It occurs at Striegau in Silesia, at the Habichtswald in Hessia, and near Sienna in Italy.
8. LEMNIAN EARTH is yellowish grey, or white, frequently with ochreous spots on the surface. The fracture is earthy; it is dull; has a meagre feel; adheres slightly to the tongue; and, when immersed in water, falls to pieces, evolving numerous air-bubbles. Klaproth found it to consist of silica 66, alumina 14·50, oxide of iron 6, water 8·50, together with very minute portions of lime and magnesia, and 3·50 of soda.
It is dug once a year with much ceremony in the isle of Lemnos,* in the Mediterranean, where only it is found. It was formerly used in medicine.
9. CIMOLITE is of a light greyish-white, inclining to pearl-grey, but by exposure it acquires a reddish tint; it occurs
* Whence Lemnian earth.
[page] 39
massive, and exhibits a somewhat slaty texture; is opake, dull, and has an earthy fracture; yields to the nail, and adheres to the tongue. It often encloses small grains of quartz. It consists of 63 silica, 23 alumina, 1·25 oxide of iron, and 12 water. Sp. Gr. 2. It is infusible.
It abounds in the island of Cimola,* now called Argenteria, situated near that of Milo. It was employed by the ancients, and still is by the inhabitants of the island, for some of the purposes to which fuller's earth is applied.
10. MOUNTAIN-MEAL. Bergmehl, Fabbroni. This singular mineral was found in the form of a bed by Fabbroni, at Santa Fiora, between Tuscany and the Papal dominions; it is manufactured into bricks so light as to swim on water. It consists of silica 79, alumina 5, oxide of iron 3, water 12—Klaproth.
11. BLACK CHALK. Zeichenschiefer, W. Argile achisteuse graphique, H. Schiste à dessiner, Br. Ampelite graphique, Bt. This mineral is greyish or bluish black; has a slaty texture; is meagre to the touch; and soils the fingers. Exposed to heat it becomes red. It is found in primitive mountains, accompanying argillaceous schiste, particularly the aluminous, to which it is nearly allied. It is met with in France, Spain, Italy, and in Bayreuth. It is used both in drawing and painting; its streak on paper is quite black. The variety from Bayreuth contains silica 64·50, alumina 11·25, oxide of iron 2·75, carbon 11·00, water 7·50—Wiegleb. Sp. Gr. 2·11—2· 18.
12. PIPE-CLAY. Has a greyish or yellowish white colour; an earthy fracture; and smooth greasy feel; it adheres pretty strongly to the tongue, is very plastic and tenacious, and is infusible. It is manufactured into tobacco-pipes, and is the basis of the queen's-ware pottery. An extensive stratum of pipe-clay lies in a horizontal position above the chalk extending from Hand-fast Point to beyond Corfe Castle in Dorsetshire. It may be traced in the hills near Poole, and is found in many parts of that extensive tract called the Trough of Poole.
13. POTTER'S CLAY is plastic, and disintegrates by exposure; is generally of a reddish, bluish, or greenish colour, and has a soft and often greasy feel. When mixed with sand, it is made into bricks and tiles. A variety found in the forest of Dreux in France (employed, on account of its infusibility, in the making of tiles for the porcelain furnaces), consists of 43 silica, 33 alumina, 3 lime, 1 iron, and 18 water. Most of the clay used in the Staffordshire potteries is brought from Devonshire.
* Whence Cimolite.
[page] 40
KEROLITE.
Cerolite, Kerolite, L. Breithaupt.
Contains silica 37·95, alumina 12·18, magnesia 16·02, water 31·00—Pfaff.
Sp. Gr. 2·0—2·2. H. about 2·0.
Is found in kidney-shaped masses, which have a lamellar or compact structure, and a white, yellow, or green colour. Lustre vitreous or resinous; transparent or translucent; fracture con-choidal; feels greasy, but does not adhere to the tongue.
It occurs at Frankenstein in Silesia, and at Zöblitz in Saxony, in both localities associated with serpentine.
PYROPHYLLITE.
Hermann of Moscow.
Silica 59·79, alumina 29·46, magnesia 4·0, oxide of iron 1·8, water 5·62—Hermann.
Sp. Gr. 2·8. H. = 1·5.
Occurs in fibrous radiating masses, and small elongated prisms, sometimes with terminations, but whose form is nevertheless not ascertained. Of a light green colour; lustre pearly; in thin laminæ, transparent. This mineral used to be considered a radiated variety of talc, but its comportment before the blowpipe is peculiar. Heated per se, it exfoliates into white leaves, and increases to about twenty times its original size; but does not fuse. With borax it forms a green transparent glass, which on cooling loses its colour; with salt of phosphorus is decomposed into a colourless glass and a skeleton of silica; with soda fuses with effervescence into a transparent yellow glass; and heated with a solution of cobalt, it assumes a blue tinge.
It occurs near Beresof, in the Ural Mountains of Siberia.
FAHLUNITE.
Tricklasite, Leonhard.
Combination of silica, alumina, and water, mixed with magnesia, oxide of iron, and manganese.
| Green. | Black. | ||
| Silica | 46·79 | 44·95 | 44·60 |
| Alumina | 26·73 | 30·70 | 30·10 |
| Magnesia | 2·97 | 6·04 | 6·75 |
| Protoxide of iron | 5·01 | 7·22 | 3·86 |
| Ox. of manganese | 0·43 | 1·90 | 2·24 |
| Water | 13·50 | 8·65 | 9·35 |
| Potash | 0·00 Hising. | 1·38 Wacht. | 1·98 Wacht. |
Sp. Gr. 2·6—2·7. H. = 3·0.
[page] 41
Primary form an oblique rhombic prism of 109° 28′ and 70° 32′. Occurs massive, and in six-sided prisms; the crystals, however, from their highly perfect cleavage, almost invariably fracture in parallel position with the slate in which they occur, and thus present only sections of their form. Cleavage perpendicular to the axis of the prism; lustre resinous. Before the blowpipe it becomes grey, and fuses on its thinnest edges; but with borax melts slowly into a glass slightly coloured by iron. Colour dark-reddish brown; occasionally green or black, and opake; but when reduced to small fragments, translucent, and yellowish brown by transmitted light.
It occurs in the copper mine of Eric Matts at Fahlun in Sweden, imbedded in chlorite slate.
CHIASTOLITE.*
Hohlspath, W. Macle, H.
Sp.Gr. 2·9—3·0. H. = 5·0—5·5.
Occurs crystallized in rectangular prisms, which present a black cross in their transverse section. Colour white or grey, the dark portion black or bluish-black. Translucent. Lustre vitreous, though indistinct. Streak white. Cleavage imperfect. Fracture splintery. Contains silica 68·49, alumina 30·17, magnesia 4·12, oxide of iron 2·7, water 0·27—Landgrabe; which corresponds with the researches of Berzelius, who ascertained it to be a compound of silica and alumina. Before the blowpipe the white portion becomes still whiter, but does not fuse; while the black melts into a dark-coloured glass. With either borax or salt of phosphorus it is difficultly fusible, forming a transparent glass; but it effervesces, and is entirely soluble, in nitric acid.
Chiastolite occurs imbedded in clay-slate or schiste in many places, particularly near Barèges in the Pyrenees; at St Jago di Compostella in Spain; in clay-slate near Santa Elena in the Sierra Morena, as observed by Dr Traill; at Bretagne in Normandy; on Skiddaw in Cumberland; and at Agnavanagh in Wicklow. Haüy supposes the crystals to be produced by the union of two individuals similarly crystallized, the one in a state of purity, the other a mixture.
* Chiastolite, from the Greek, in allusion to its being marked with the form of an X, in dark lines, visible on the summits of the crystals.
[page] 42
IOLITE.*
Dichroite, Cordier. Cordierite, Leonhard. Prismatic Quartz, M. Iolite, H.
Combination of silica, alumina, magnesia, oxide of iron, and manganese.
| Simitok. | Bodenmais. | Orijerfwi. | Fahlun. | |
| Silica | 49·17 | 48·35 | 49·95 | 50·24 |
| Alumina | 33·10 | 31·70 | 32·88 | 33·42 |
| Magnesia | 11·48 | 10·15 | 10·45 | 10·84 |
| Oxide of iron | 4·33 | 8·31 | 5·00 | 4·00 |
| Manganese | 0·00 | 0·33 | 0·03 | 0·68 |
| Water | 1·20 Strom. | 0·59 Strom. | l·75 Bonsd. | 1·66 Strom. |
Sp. Gr. 2·56—2·6. H. = 7·0—7·5.
This mineral has a dark-blue colour, sometimes with a tinge of black; but when viewed by transmitted light at right angles to the axis of the prism, it appears brownish-yellow. It occurs massive, and crystallized in six or twelve-sided prisms; its primary being the six-sided prism. Transparent or translucent; with a shining vitreous lustre; and an uneven or somewhat conchoidal fracture. Alone, before the blowpipe, in a strong heat, the edges fuse into a blue glass; with borax it melts slowly into a diaphanous glass. Not affected by acid.
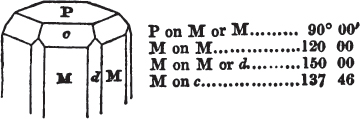
It is found at Cape de Gatte in Spain, imbedded in granite; in very large individuals engaged in quartz at Ujordlersoak and Simitok, in Greenland; and in distinct crystals, with magnetic pyrites, at Bodenmais, in Bavaria. It is more common massive, being found in that state among the primitive rocks of Arendal in Norway; Orijerfwi in Finland, &c.
1. PELIOM† is a name occasionally given to the Bodenmais variety, which, from its containing a larger proportion of iron, is somewhat heavier.
2. STEINHEILITE,‡ again, refers to that from Finland. Both of these, however, are perfectly identical with Iolite.
* Iolite, from its bluish violet colour in one direction; Dichroite, from two Greek words, signifying of two colours.
† From the Greek, signifying bluish colour or blackish.
‡ So named after Count Steinheil.
[page] 43
3. THE HARD FAHLUNITE of Berzelius, from Fahlun in Sweden, is merely a brownish-yellow variety of this species, which derives its peculiar colour and opacity from accidental admixture. In other respects it is similar. The Sapphire d'eau of jewellers, is a transparent Iolite from Ceylon.
SORDAWALITE.
Contains silica 49·40, alumina 13·80, peroxide of iron 18·17, magnesia 10·67, phosphoric acid 2·68, water 4·38—Nordenskiold.
Sp. Gr. 2·53—2·58. H. = 2·5—3·0.
Occurs in opake, greyish or bluish-black coloured masses, which do not exhibit traces of cleavage; lustre vitreous; fracture conchoidal; streak liver-brown; brittle. Before the blowpipe per se it fuses with difficulty into a dark-coloured globule, and with borax forms a green glass; with a small quantity of soda it yields a blackish-green globule, and with a larger quantity a rough slaggy mass. It is partly soluble in muriatic acid; and becomes red on exposure to the atmosphere.
This mineral was discovered and analysed by Nordenskiold, who found it near the town of Sordawala in Finland, forming thin layers in a primitive rock. It occurs also with magnetic pyrites at Bodenmais in Bavaria.
HARMOTOME.
Kreutzstein, W. Harmotome, H. Pierre Cruciforme, Br. Cross-stone, J. Paratomous Kouphone Spar, M.
Combination of silica, alumina, barytes, and water, with occasional small proportions of lime, soda, and potash.
| Schiffenburg. | Andreasberg. | Oberstein. | Strontian. | ||
| Silica | 44·79 | 49·0 | 56·30 | 47·5 | 47·04 |
| Alumina | 19·28 | 16·0 | 14·50 | 19·5 | 15·24 |
| Barytes | 17·59 | 18·0 | 17·52 | 16·0 | 20·85 |
| Lime | 1·08 | 0·0 | 1·00 | 0·0 | 0·10 |
| Soda or potash | 0·00 | 0·0 | 1·25 | 0·0 | 0·88 |
| Water | 15·32 | 15·0 | 11·69 | 13·5 | 14·92 |
| Wernekinck. | Klapr. | Gmelin. | Tassaert. | Connell. | |
Sp. Gr. 2·35—2·4. H. = 4·5.
Harmotome sometimes occurs in flattish quadrangular prisms, terminated by rhombic planes, replacing the solid angles of the
[page] 44
prism; these crystals often cross* each other length wise and at right angles, so that their axes coincide. The crystals yield to cleavage parallel to the planes and both diagonals of a right rectangular prism, which is the primary form. The usual colour of this mineral is greyish-white; it is translucent, and has a somewhat pearly lustre. Before the blowpipe it fuses easily, without intumescence, into a diaphanous glass; and is scarcely affected by acids, unless they are heated.
Fig. 1. The primary form, a right rectangular prism, of which certain of the angles are replaced in fig. 2, reducing it, when placed in another direction, to a six-sided prism, of which the edges are modified in fig. 3 by narrow planes, which are increased in fig. 4, and complete in fig. 6, and so increased in fig. 7 as greatly to reduce the primary planes, and to give a nearly octahedral form to the crystal. Fig. 5 represents two crystals of the same form as fig. 6, but flatter, crossing each other.
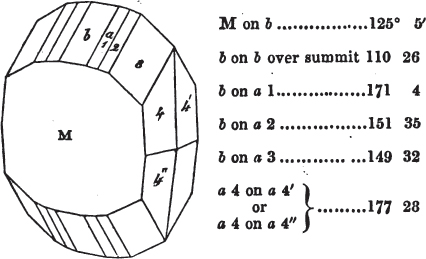
Strontian, in Argyleshire, produces the finest specimens of the simple crystal; while the cruciform varieties are best known in metalliferous veins, traversing grawacke, at Andreasberg in the Hartz. It is also met with in the Kilpatrick Hills, Dumbartonshire, accompanying analcime; on gneiss at Kongsberg in Norway; and in the cavities of siliceous geodes at Oberstein in Deuxponts.
* Harmotome signifies that which divides along the joints; alluding probably to the easy separation of the cross crystals from each other, where they join.
[page] 45
BREWSTERITE.*
Brewsterite, Brooke. Brewsteritic Kouphone Spar, Haid.
Combination of silica, alumina, strontia, baryta, lime, and water, with a little oxide of iron. Silica 53·66, alumina 17·49, strontia 8·32, baryta 6·75, lime 1·34, water 12·58, oxide of iron 0·29— Connell. Sp. Gr. 2·1—2·4. H. = 5·0—5·5.
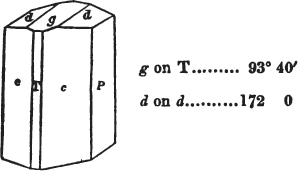
Primary form, an oblique rectangular prism. In small white or yellowish coloured crystals, whose cleavage is highly perfect parallel to P. Lustre vitreous, except on the faces of cleavage, which are pearly; transparent or translucent; fracture uneven. It gelatinizes with acids; and before the blowpipe loses its water, becomes opake, froths, and intumesces, but does not fuse; with salt of phosphorus it melts easily, leaving a skeleton of silica.
Brewsterite was first observed at Strontian in Argyleshire, where it generally occurs associated with calcareous spar; but has latterly also been met with coating the cavities of amygdaloidal rocks at the Giant's Causeway; in the lead mines of St Turpet, near Freiburg in the Brisgau; in the department of Isère in France; and in the Pyrenees. (Manual.)
PETALITE.†
Petalite, Br. Berzelite. Prismatic Petaline Spar, M.
Combination of silica, alumina, and lithia.
| Silica | 79·21 | 74·17 |
| Alumina | 17·22 | 17·41 |
| Lithia | 5·76 | 5·16 |
| Lime | 0·00 Arfwedson. | 0·32 Gmelin. |
Sp. Gr. 2·44. H. = 6·0.
Colour white, with frequently a reddish tinge; its structure is perfectly lamellar in one direction, and it admits of mechani-
* In honour of Sir David Brewster.
† Petalite, from the Greek, signifying of perfectly lamellar structure (in one direction).
[page] 46
cal division with some difficulty parallel to the sides and both diagonals of a rectangular but not square prism, apparently with oblique summits. It is translucent, and has a glistening lustre, approaching to pearly on the perfect faces of cleavage; but is not affected by acids. Alone on charcoal, before the blowpipe, it fuses on the edges with difficulty into a blebby semi-transparent glass; with borax into a diaphanous glass.
Petalite has hitherto been met with only at the iron mine of Uton, an island about thirty-five miles south-east of Stockholm, where it was first noticed by D'Andrada, accompanying lepidolite, tourmaline, spodumene, and quartz. The pink tinge which it occasionally presents, denotes a minute admixture of oxide of manganese.
SPODUMENE.
Prismatic Triphane Spar, M. Spodumene, D'Andrada. Triphane, H.
Combination of silica, alumina, and lithia.
| Uton. | Uton. | Killiney. | |
| Silica | 66·40 | 63·29 | 63·31 |
| Alumina | 35·30 | 28·78 | 28·51 |
| Lithia | 8·85 | 5·63 | 5·66 |
| Oxide of iron | 1·45 Arfwedson. | 0·79 Strom. | 0·83 Thorn. |
Sp. Gr. 3·0—3·2. H. = 6·5—7·0.
This mineral occurs massive; its structure is lamellar, with cleavage parallel to the sides and the shorter diagonal of a rhombic prism of about 100° and 80°; lustre shining and slightly pearly; cross fracture fine grained and uneven, with a glimmering lustre; colour greyish or light-green; it is translucent, scratches glass, and is brittle. It becomes colourless and opake when exposed to a red heat. Before the blowpipe, on charcoal, it intumesces, and fuses into an almost transparent glass.
It occurs in the iron mine of Uton, in Sweden, in a gangue of red felspar, quartz, and tourmaline; also in the Tyrol, near Sterzing; and, having a pale green or yellowish tinge, in granite, with killinite, at Killiney near Dublin.
JEFFERSONITE.*
Polystomous Augite Spar, Keating.
This mineral contains silica 56·0, lime 15·1, alumina 2·0, protoxide of manganese 13·5, peroxide of iron 10·0, oxide of zinc 1·0—Keating.
Sp. Gr. 3·51—3·55. H. about 4·5.
* Jeffersonite, in honour of Jefferson, President of the United States.
[page] 47
Occurs in lamellar or crystalline masses of a dark olive-green colour passing into brown; translucent on the edges; and yielding to mechanical division in three directions. On the planes of cleavage the lustre is semi-metallic; on the cross fracture resinous. It fuses readily before the blowpipe into a black globule, but does not act upon the magnet. In heated muriatic acid a portion is dissolved. It occurs with Franklinite and garnet, at the Franklin iron-works, near Sparta in Sussex county, New Jersey.
TABULAR SPAR.
Prismatic Augite Spar, M. Wollastonite, Necker. Schaalstein, W. Spath en Tables, H.
Combination of silica and lime.
| Cziklowa; | Pargas. | Perheniémi. | ||
| Silica | 51·44 | 53·1 | 52·58 | 51·60 |
| Lime | 47·41 | 45·1 | 44·45 | 46·41 |
| Magnesia | 0·00 | 1·8 | 0·68 | 0·00 |
| Ox. of iron | 0·40 Strom. | 0·0 Beud. | 1·13 Bonsd. | traces. Rose. |
Sp. Gr.2·86. H. = 4·5—5·0.
Tabular spar generally occurs in fibrous masses of a greyish-, yellowish-, greenish-, or reddish-white colour; with a shining and somewhat pearly lustre; translucent; often friable. Primary form a right or oblique rhombic prism. Principal cleavages are parallel to the planes of the primary. It is phosphorescent when scratched with a knife, as well as when heated. A fragment placed in nitric acid effervesces quickly at first, and then falls into powder. On charcoal it melts on the edge into a semi-transparent colourless glass, but requires a very strong heat for its perfect fusion; with borax it melts easily into a transparent glass.
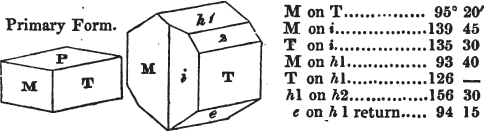
This mineral has been found in small, extremely fragile, tabular-shaped crystals, in the ejected stones of Vesuvius; in fibrous masses, with apophyllite, at Cziklowa and Dognatska in the Bannat of Temeswar; in cinnamon-stone from Ceylon; with co-lophonite in North America; and in fibrous radiated masses in basalt at the castle rock of Edinburgh. It frequently bears considerable resemblance to some varieties of tremolite. By fusing lime and silica in the required proportions, cleavable masses have been obtained.
[page] 48
OKENITE.
Kobell.
| Silica | 56·99 | 55·64 |
| Lime | 26·35 | 26·59 |
| Water | 16·65 | 17·00 |
| Alumina, oxide of iron, and traces of potash | 0·53—Kobell. |
Sp. Gr. 2·28. H. = 5·0—6·0.
Occurs in delicately fibrous and sometimes radiating masses, having a glimmering or pearly lustre. Colour white, with a shade of yellow or blue; translucent on the edges. Before the blowpipe in the matrass it affords much water slightly alkaline; alone on charcoal it fuses readily, intumesces, and forms a porcelain-like mass. With borax it melts into a limpid and colourless glass; but is difficultly and imperfectly soluble in salt of phosphorus.
It occurs at Disco Island and Tupaursak in North Greenland, in amygdaloid.
MELLILITE, H. Bt.*
Combination of silica, lime, magnesia, alumina, and the oxides of iron, manganese, and titanium.
Silica 38·0, lime 19·6, magnesia 19·4, alumina 2·9, oxide of iron 12·1, oxide of manganese 2·0, oxide of titanium 4·0—Carpi.
Sp. Gr. 3·24—3·28. Gives sparks with steel.
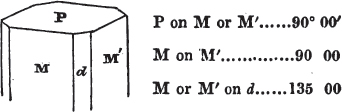
This mineral occurs in small square prisms, whose lateral edges are mostly replaced. Internally the crystals are of a honey-yellow or orange colour; externally they are usually coated by oxide of iron of a brown hue. Before the blowpipe it melts without effervescence into a greenish glass; and, when reduced to powder, gelatinizes with nitric acid.
It has only been found at Capo di Bove, near Rome, in the fissures of a compact black lava, with nepheline, pleonaste, and other volcanic minerals.
* Mellilite, from its being of a honey yellow.
[page] 49
GISMONDINE.
Abrazite, Brocchi. Zeagonite, Gismondi. Gismondine, L.
| Silica | 57·45 | 41·4 |
| Alumina | 7·36 | 2·5 |
| Lime | 25·30 | 48·6 |
| Magnesia | 2·56 | 1·5 |
| Oxide of iron | 3·00 | 2·5 |
| Oxide of mangan. | 0·50 Viviani. | 0·0 Carpi. |
Sp. Gr. 2·16—2·2. H. = 7·0—7·5.
Primary form, a right square prism; secondary, the same, surmounted by four-sided pyramids.
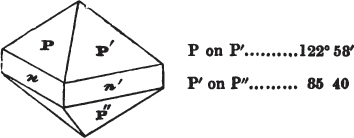
Occurs in white translucent crystals, having an adamantine lustre, and presenting an imperfect cleavage parallel to n. Fracture conchoidal. Before the blowpipe it phosphoresces, and becomes friable, but does not melt. It gelatinizes in acids without effervescence.
This species occurs at Capo di Bove near Rome, coating the cavities of ancient lava, along with other volcanic minerals.
AUGITE. PYROXENE.*
Augit, W. Pyroxène, H. Paratomous Augite Spar, M.
Combination of silica, lime, protoxide of iron, and sometimes alumina.
Analyses of black varieties.
| Taberg. | Frascati. | Etna. | |
| Silica | 53·36 | 48·00 | 52·00 |
| Lime | 22·19 | 24·00 | 13·20 |
| Protox. of iron | 17·38 | 12·00 | 14·66 |
| Magnesia | 4·99 | 8·75 | 10·00 |
| Alumina | 0·00 | 5·00 | 3·34 |
| Manganese | 0·00 Rose. | 1·00 Klaproth. | 2·00 Vauquelin. |
Sp. Gr. 3·10 to 3·15. H. = 5·0 to 6·0.
* Augite, from the Greek, splendour, in allusion to the brilliancy of its crystals; Pyroxène, signifying a guest in the domain of fire,—unaltered by heat.
C
[page] 50
Augite occurs crystallized, also in grains, and amorphous; the crystals generally small, and often hemitrope or macled. Its colour is green, brown, or brownish-black, sometimes black; with vitreous or resinous lustre; and opake. It cleaves parallel to the sides of an oblique rhombic prism of 87° 5′ and 92° 55′ by the reflective goniometer, which therefore is its primary form. Before the blowpipe it fuses, emits a few bubbles, and finally yields a glassy globule, more or less tinged by iron; it is readily soluble with borax. Several varieties of augite have been obtained artificially by means of fusion.

Augite is a common volcanic production; but that it existed prior to its matrix being subjected to volcanic action there is no doubt. The debris of the Monti Rossi on Etna is full of detached crystals of black augite; and in the volcanic regions of Vesuvius, Stromboli, Auvergne, Teneriffe, and Bourbon, they are also of frequent occurrence. Augite is likewise met with, imbedded in basalt, at Aussig and Toplitz in Bohemia; in Hungary, Transylvania, Hessia, and elsewhere on the continent; occasionally also in primitive rocks, as in Greenland, and in the iron mines of Arendal in Norway. The crystals met with in basalt are generally larger than those found in lava.
[page] 51
The five following substances are varieties of augite.
1. DIOPSIDE.* Diopsid, W. Var. de Pyroxène, H. Mussite, Alalite.
Combination of silica, lime, magnesia, and a little protoxide of iron.
The Piedmontese variety contains silica 57·5, lime 16·5, magnesia 18·5, oxides of iron and manganese 6·0—Laugier.
Sp. Gr. 3·31. Scarcely scratches glass.
Diopside occurs in prismatic crystals, which are colourless, or green of various shades; and translucent or transparent. Their primary is an oblique rhombic prism, of the same form and measurement as that of augite. The crystals are generally striated longitudinally, have a shining lustre, and may be cleaved parallel with the planes of the primary prism. Before the blowpipe it fuses alone into a colourless semi-transparent mass; with borax into a diaphanous glass.

It was first discovered by Bonvoisin, in veins traversing serpentine, at Ala in Piedmont (hence Alalite), where it occurs in translucent crystals, accompanied by epidote, hyacinth, red garnet, and crystallized green talc; and latterly has been obtained in large individuals and crystalline masses, sometimes of a fine pistachio-green colour, at the Rothenkopf in the Zillerthal, Tyrol.
2. PYRGOM. Fassaite.† Is generally of a dingy-green colour; assumes nearly the same crystalline form, and readily
* From the Greek, signifying transparency, in allusion to the occasional transparency of its crystals.
† Fassaite, from its locality, the valley of Fassa.
[page] 52
yields to mechanical division parallel to the lateral planes of a prism of the same measurements as that of augite.
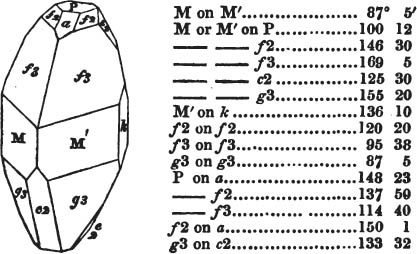
It is found in the valley of Fassa in the Tyrol.
3. SAHLITE. Sahlit, W. Pyroxene laminaire gris-verdatre, H. Malacolithe, Bt. Baikalite.
| Finland. | Siberia. | ||
| Silica | 50·0 | 54·83 | 44·0 |
| Lime | 20·0 | 24·76 | 20·0 |
| Magnesia | 19·0 | 18·55 | 30·0 |
| Oxide of iron and manganese | 4·0 | 0·99 | 6·0 |
| Alumina | 3·0 Vauquelin. | 0·28 Bonsdorff. | 0·0 Lowitz. |
Sp. Gr. 3·256.
Sahlite occurs in prismatic crystals of four or eight sides, and generally with inclined summits; it is greenish-grey, feebly translucent, and scarcely hard enough to scratch glass. It also occurs massive. The structure is lamellar, with joints parallel to the planes of an oblique rhombic prism, of the same measurements by the reflective goniometer as that of augite; the primary form of the two substances is therefore the same, but sahlite readily allows of mechanical division parallel to the oblique terminal planes of the prism, which augite rarely does. Before the blowpipe it melts per se, with slight effervescence, into a translucent glass; and is soluble in borax, salt of phosphorus, and soda, forming with them a clear glass.
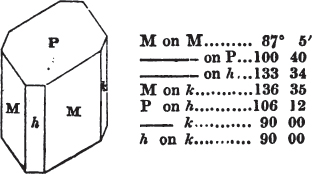
[page] 53
Sahlite occurs principally in the silver mines of Sahla* in Sweden, and at Arendal in Norway; the variety termed Baikalite is found in granite at the mouth of the Sljumanka river, which falls into Lake Baikal in Siberia.
4. COCCOLITE. Kokkolith, W. Pyroxène granuliforme, H. Consists of 50 silica, 1·5 alumina, 24 lime, 10 magnesia, 7 oxide of iron, and 3 oxide of manganese—Vauquelin. Specific gravity 3·3. It presents various shades of green and bluish green, and occurs in small translucent masses or grains† of irregular shapes, which are very slightly coherent; but sufficiently hard to scratch glass; structure lamellar, and lustre vitreous. It occurs principally in the iron mines of Arendal in Norway
BABINGTONITE.‡
Axotomous Augite Spar, M. Babingtonite, Levy.
Sp. Gr. 3·4—3·5. H. 5·5—6·0.
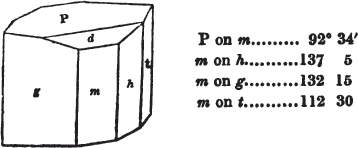
Sometimes the faces marked m are awanting. Colour dark-greenish black; the splinters faintly translucent, and appearing green perpendicular to P, brown parallel to it. Lustre vitreous. Surface brilliant. Cleavage perfect parallel to P, less so to t. Fracture imperfect conchoidal.
Before the blowpipe it fuses on the surface into a black enamel, and with borax gives a transparent amethystine coloured globule, which in the reducing flame becomes bluish-green.
Babingtonite resembles certain dark-coloured varieties of augite, from which it was first distinguished by Levy. According to Children, it is composed of silica, iron, manganese, and lime, with a trace of titanium. It occurs in very distinct crystals at Arendal in Norway, associated with epidote and massive garnet; and in the Shetland Isles imbedded in white quartz. (Manual.)
* Whence Sahlite.
† Whence Coccolite, from the Greek, signifying a granular stone.
‡ Named in honour of Dr Babington.
[page] 54
BUCKLANDITE.*
Dystomic Augite Spar, Haid. Bucklandite, Levy.
Sp. Gr. 3·94. Harder than augite.
Primary form an oblique rhombic prism of 109° 20′ and 70° 40′.
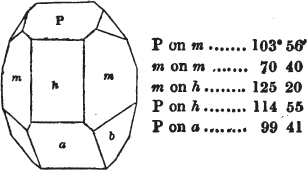
Colour dark brown, nearly black. Opake. Lustre vitreous. Cleavage not observable. Fracture uneven.
This very rare mineral bears much general resemblance to augite. It was distinguished and described by Levy, to whom we are indebted for the above measurements. It occurs at Aren-dal in Norway, with black hornblende, felspar, and apatite; and in minute brilliant crystals in lava, at the lake of Laach on the Rhine. (Manual.)
HORNBLENDE.
Hornblende, W. Var. de Amphibole, H. Hemi-prismatic Augite Spar, M.
Hornblende occurs crystallized, massive, and slaty. Combination of silica (partly replaced by alumina), protoxide of iron, magnesia, and lime.
| Black, Pargas. | Deep Green, Aker. | |
| Silica | 45·69 | 47·21 |
| Lime | 13·85 | 12·73 |
| Magnesia | 18·79 | 21·86 |
| Protox. of iron | 7·32 | 2·28 |
| Alumina | 12·18 | 13·94 |
| Fluoric acid | 1·50 Bonsdorff. | 0·90 Bonsdorff. |
Sp. Gr. 3·0 to 3·1. H. = 5·0—6·0.
Crystallized Hornblende is found in prismatic crystals, occasionally isolated, but more often confusedly aggregated, and frequently macled. The crystals cleave readily and with brilliant
* In compliment to Professor Buckland of Oxford.
[page] 55
surfaces parallel to the sides of a rhombic prism of 124° 30′ and 55° 30′ by the reflective goniometer, but not parallel to the terminal planes, Which are assumed to be oblique to the axis of the prism; and hence the primary crystal is an oblique rhombic prism, the declination of the terminal planes being from one obtuse angle of the prism to the other. Colour dark bottle-green or brownish-green, or brown approaching to black, but, when pulverized, of a greenish-grey: lustre vitreous; yields pretty easily to the knife; opake, or presenting on the thinnest edges a fine red colour by strong transmitted light; when massive, tough, and difficultly frangible. The black varieties invariably contain more iron than those of a lighter colour, as may be ascertained by bringing them in contact with the magnetic needle; some of them have been found to contain about nineteen per cent. Dark-green lamellar hornblende fuses alone before the blowpipe, with effervescence and intumescence, into a black brilliant glass. It affords with borax a transparent globule, and with salt of phosphorus a glass which becomes opaline on cooling.
The appellation of Basaltic has been given particularly to those cleavable and highly crystalline black hornblendes which occur in basaltic and amygdaloidal rocks. Hedenbergite is a variety from Tunaberg in Sweden, containing a large proportion of iron; and Carinthin applies to one of a green colour from the Sau Alpe in Carinthia.
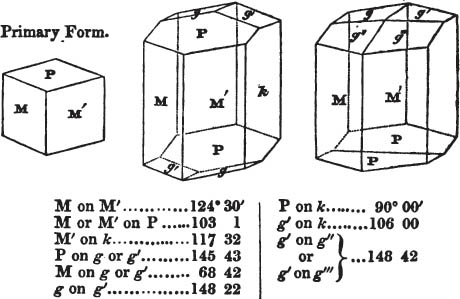
The 3d fig. represents a hemitrope, in which one half of the crystal is turned round, and is thus attached to the other half.
Massive Hornblende has a crystalline structure, consisting of minute and often of long crystals intersecting each other, sometimes confusedly radiating. Superficially it frequently assumes
[page] 56
a ferruginous brown from decomposition; is very tough, and difficult to break.
Hornblende-slate, Hornblende Schiefer, W. Is commonly of a greenish-black colour; and, except that it has a slaty or schistose structure, agrees in all its characters with the massive.
Hornblende is a very abundant mineral, being an essential ingredient of syenite and greenstone; and occurring frequently in granite, gneiss, basalt, and lava. It is therefore found in almost every country, but more particularly in the repositories of magnetic iron at Arendal, and other mining districts of Norway and Sweden; in large, frequently hemitrope crystals, in amygdaloid, near Teising and Toplitz, in Bohemia; in crystals occupying the drusy cavities of Vesuvian minerals; and in Greenland of a peculiar asparagus-green colour. Massive hornblende is met with on the Sau Alpe in Carinthia, and in several parts of Saxony; while hornblende-slate forms beds in gneiss, mica-schiste, and other primitive rocks.
The following are considered varieties of hornblende:
1. PARGASITE. Contains silica 46·26, magnesia 19·03, lime 13·96, alumina 3·43, fluoric acid 1·60. It occurs disseminated in somewhat round semi-crystalline masses, and in six-sided crystals with diedral summits. It yields to cleavage parallel to the lateral planes of a rhombic prism of the same measurements as the oblique rhombic prism of hornblende; but not parallel to the terminal planes. Colour, however, is the principal difference between hornblende and pargasite, the latter being somewhat translucent, and of a lighter green, or more generally of a bottle-green hue. It is harder than fluor, but is scratched by quartz. Specific gravity 3·11. Its comportment before the blowpipe is the same as black crystallized hornblende, except that the glass is less coloured.
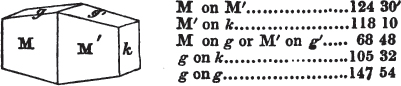
It is found at Pargas,* near Abo in Finland, in calcareous spar.
2. TREMOLITE.† Tremolith, W. Var. de Amphibole, H. Tremolite, Br. Grammatite, Bt. Common Tremolite, J.
Combination of silica, magnesia, and lime.
* Whence Pargasite.
† Tremolite, from the valley of Tremola, where it was first discovered.
[page] 57
| Gulsjo. | Fahlun. | Aker. | Cziklowa. | |
| Silica | 59·75 | 60·10 | 47·21 | 59·5 |
| Magnesia | 25·00 | 24·31 | 21·86 | 26·8 |
| Lime | 14·11 | 12·73 | 12·73 | 12·3 |
| Alumina | 0·50 | 00·42 | 13·94 | 1·4 |
| Protoxide of iron | 0·00 | 1·00 | 2·28 | 0·0 |
| Fluoric acid | 0·94 Bons. | 0·83 Bons. | 0·90 Bons. | 0·0 Beud. |
Sp. Gr. 2·9—3·1.
Its colour is white (Amphibole blanc, H.), occasionally grey with a greenish tinge. It occurs in masses composed of delicate crystalline fibres, which sometimes radiate; and in flat deeply striated four, six, or eight sided prisms, terminated, though rarely, by diedral summits. It cleaves with brilliant surfaces parallel to the sides of a rhombic prism of the same measurement as that of hornblende; its crystals often exhibit the appearance of fissures which are oblique to the axis of the prism. Semi-transparent or translucent, and hard enough to scratch glass, but very brittle; it becomes phosphorescent both by heat and friction. Before the blowpipe it fuses, in a very strong heat, into an almost opake greyish-white mass. With borax forms a transparent colourless globule.
Asbestiform Tremolite occurs in masses consisting of fasciculated groups of minute diverging, occasionally radiating fibres; its fracture exposes a delicately fibrous texture, with a glistening pearly or silky lustre. It becomes phosphorescent by friction, which is not the case with common asbestus, a mineral it otherwise much resembles. Before the blowpipe, that from Sheffield in Massachusetts bubbles and fuses with great difficulty into a vitreous mass.
Tremolite occurs in dolomite, at St Gothard in Switzerland; at Sebes in Transylvania; and in the United States; in Corsica in talc; near Nantes in granite abounding in felspar; the fibrous variety occurs snow-white and translucent in a bed of limestone at Gulsjo in Sweden; in Scotland, in primitive limestone in Aberdeenshire and Iona, and in the marble of Glen Tilt. Asbestiform tremolite forms masses of thin capillary crystals in Switzerland, the Tyrol, the Bannat, and other places; some beautiful specimens are met with at St Gothard in dark-green groups.
Catamite.* It occurs in rhombic prisms of a light asparagus-green colour, translucent, striated longitudinally, and yielding to mechanical division readily, parallel to the sides of a rhombic prism of the same measurements as that of hornblende. This mineral is soft; in form it resembles tremolite.
It occurs imbedded in serpentine with magnetic iron and calcareous spar, at Normark in Sweden.
* Calamite; catamus (Lat.), a reed; from the appearance of its crystal.
C 3
[page] 58
3. PYRALLOLITE.* Tri-silicate of magnesia, mixed with hydrate of magnesia. Contains silica 56·62, magnesia 23·38, alumina 3·38, lime 5·58, peroxide of iron 0·99, protoxide of manganese 0·99, water 3·58—Nordenskiold. Sp. Gr. 2·55—2·6. H. = 3·5. It occurs both massive, and crystallized in flat rhombic prisms greatly resembling those of tremolite, and is divisible parallel to the planes of a rhombic prism, but it cleaves more readily in one direction than the other; apparently also to its lesser diagonal, but not with brilliant surfaces; the prisms are generally above an inch in length, have occasionally a greenish tinge, but by exposure become pale yellow, and are then very soft and friable. Opake in the mass, but when reduced to thin laminæ parallel to the principal cleavage, translucent. If thrown in the state of powder upon a red-hot iron, it gives out a bright bluish phosphorescence. Before the blowpipe it first becomes blackish, afterwards white, and the edges are reduced to a white enamel; with borax it fuses with facility into a diaphanous glass.
It is found at Storgard in the parish of Pargas in Finland, in calcareous spar, with augite, felspar, and scapolite.
4. ACTYNOLITE. Strahlstein, W. Var. de Amphibole, H. This mineral is green of different hues, sometimes almost black, more or less translucent, and by reflected light yellowish or brownish. It may be divided into three varieties,—crystallized, asbestiform, and glassy.
Combination of silica, magnesia, protoxide of iron, and lime.
| Taberg. | Zillerthal. | Zillerthal. | |
| Silica | 59·75 | 53·1 | 53·1 |
| Lime | 14·25 | 11·4 | 10·6 |
| Protoxide of iron | 3·95 | 25·6 | 21·8 |
| Magnesia | 21·10 | 7·8 | 10·4 |
| Alumina | 0·00 Arfwed. | 1·7 Beudant. | 4·1 Beudant. |
with occasional traces of manganese, potash, and fluoric acid.
Sp. Gr. 3·0.
Crystallized Actynolite generally occurs in acicular hexahedral prisms, which are not regularly terminated, but which yield by mechanical division a prism of the same measurements as that of common hornblende. It has a shining lustre, and is translucent or transparent. Occasionally it appears in fine fibres having a silky lustre, and sometimes disposed in a radiating form.† It is hard enough to scratch glass. Per se it fuses, after becoming white, into an opake yellowish or brownish coloured glass.
Asbestiform Actynolite presents a green, greenish-grey, or brownish-green colour; and occurs both massive, and in capillary crystals
* From the Greek, πυζ, αλλος, λιθος, in allusion to the change of colour it presents when exposed to the action of fire.
† Whence Actynolite, from the Greek, in allusion to the sun's rays.
[page] 59
which are elastic. The crystals are sometimes disposed in wedge-shaped masses, or in radii promiscuously aggregated; they are opake or slightly translucent on the edges. It melts before the blowpipe into a yellowish-brown opake glass. The Byssolite of Saussure appears, from its analysis and principal characters, to be the same mineral.
Glassy Actynolite differs from the preceding in possessing an external lustre, which is vitreous inclining to pearly; and in being translucent and brittle.
Actynolite is chiefly found in primitive rocks; as gneiss, mica-slate, and limestone. It occurs in long six-sided prisms, imbedded in white talc, at Greiner in the Zillerthal, Tyrol; at St Gothard; near Salzburg; in Norway; and in Piedmont. In Britain, it has been noticed in the copper veins of the Maudlin mine, near Lost-withiel in Cornwall; in Glen Elg, Inverness-shire; in Skye, and elsewhere, in small quantities.
5. Of ASBESTUS there are several varieties, which generally present a fibrous texture, but vary in respect of flexibility. The fibres have not yet been seen in any very determinate form, but Haüy regarded some which fell under his observation as rhombic prisms. Asbestus* is extremely difficult of fusion in the mass.
According to Dr Thomson, its varieties contain the following proportions:
| White Amianthus, | Rockwood | Mountain Leather, | Mountain Cork, | |
| Sardinia. | Tyrol. | Strontian. | Piedmont. | |
| Silica | 55·91 | 54·92 | 57·65 | 57·75 |
| Magnesia | 27·07 | 26·08 | 2·06 | 10·85 |
| Lime | 14·63 | 0·00 | 10·00 | 14·05 |
| Alumina | 1·82 | 1·64 | 9·50 | 1·95 |
| Protoxide of iron | 6·52 | 12·60 | 5·80 | 18·90 |
| Water | 0·00 | 5·28 | 21·70 | 0·00 |
| Prot. of manganese | 0·00 | 0·00 | 0·00 | 1·85 |
Amianthus. Amianth, W. Asbeste flexible, H. Amianthus occurs in long and extremely slender fibres, longitudinally cohering with each other, and easily separated; these are more or less flexible and elastic, and of a whitish, greenish, or reddish colour. It is somewhat unctuous to the touch; has a shining or silky lustre; and is slightly translucent In mass it fuses though with difficulty into a white enamel; but when in single fibres it melts at the flame of a candle.
It usually occurs in serpentine; and is found in the Tarentaise in Savoy, in the longest and most beautiful fibres: that of Corsica is so abundant that Dolomieu used it for packing his mi-
* Its name is derived from a Greek word signifying imperishable, or, according to some, unstained, unsoiled.
[page] 60
nerals. It occurs in Dauphiné, and at St Gothard in veins in mica-slate; in serpentine in several of the United States; in Saltzburg; the Tyrol; St Keverne, in Cornwall; Portsoy, in Aberdeenshire; and in the Shetland Isles of Unst and Fetlar.
Amianthus was woven by the ancients into a kind of cloth, in which, being incombustible, they wrapped the bodies of their dead, before being placed on the funeral pile, that their ashes might be collected free from admixture.
Common Asbestus. Asbest dur, H. Common asbestus is much heavier than the preceding variety, its specific gravity being nearly 3·0. It occurs in masses consisting of fibres of a dull greenish colour, with occasionally a somewhat pearly lustre; and yields splintery fragments. It is scarcely or not at all flexible, and thereby is distinguishable from amianthus. It is somewhat unctuous to the touch; and is easily fusible before the blowpipe into a slightly greyish enamel. It is of more frequent occurrence than amianthus; is usually found in veins in serpentine; and is met with in Sweden, Hungary, Dauphiné, the Ural Mountains, &c.; also in serpentine at Portsoy, in Anglesey, and at the Lizard in Cornwall.
Mountain Leather. The principal difference between this and the foregoing variety appears to be the position of its fibres. In common asbestus, they are generally even and parallel: in mountain leather, they are interwoven or interlaced. It occurs in flexible flat pieces, having much the aspect of leather; but when very thin has been termed mountain paper. It is commonly of a whitish or yellowish-white colour, and is meagre to the touch. It occurs at Strontian in Argyleshire, and at the Lead Hills in Lanarkshire.
Mountain Cork. Berg Cork, W. Asbeste tressée, H. Rock Cork, J. Mountain cork has a fibrous texture, the fibres being interlaced so intimately as not to be recognisable, or capable of separation. It is opake, has a meagre feel, somewhat resembling that of common cork; about the same hardness; is sectile like that substance; rather elastic, and swims on water. It forms veins in serpentine, and is met with in Norway, Saxony, Spain, &c.; and at Portsoy and the Lead Hills in Scotland.
Mountain Wood. Berg-holz, W. Asbeste Ligniforme, H. Rock Wood, J. Is generally of a brownish colour and massive, and has somewhat the aspect of wood, being occasionally so hard and compact as to resemble petrified wood. It breaks into long masses in the direction of the fibres, which are sometimes curved, and separable with ease. It is opake; the fibres rarely elastic. It is fusible into a black slag; and is about twice the weight of water. It occurs at Schneeberg near Sterzing in the Tyrol, with asbestus and other minerals; in Dauphiné; Styria; in Maryland, North America; and at Portsoy in Scotland.
[page] 61
ARFWEDSONITE,*
Brooke.
Sp. Gr. 3·4—3·5. H. = 6·0.
This mineral has been separated from hornblende (of which it was commonly assumed to be a ferriferous variety), owing chiefly to the measurements which its cleavages afford; like that mineral, it yields to cleavage only parallel to the lateral planes of a rhombic prism, of which the measurements are 123° 55′, while those of hornblende are 124° 30′. Its colour is black without a shade of green; and opake. It has not been observed regularly crystallized; its cleavage planes are very brilliant,—much more so than those of hornblende.
According to Thomson, it contains upwards of 35 per cent. of iron; and by Children is described as fusing readily before the blowpipe into a black globule, yielding with borax a glass coloured by iron, and with salt of phosphorus the same, but paler, which becomes colourless on cooling, and leaves a silica skeleton undissolved.
It occurs with sodalite and eudialite, at Kangerdluarsuk in Greenland.
HYPERSTHENE.†
Labradorische Hornblende, W. Var. de Diallage Metalloide, H. Paulite. Hypersthène, Bt. Prismatoidal Schiller Spar, M.
Combination of silica, magnesia, and protoxide of iron.
| Silica | 54·25 |
| Magnesia | 14·00 |
| Oxide of iron | 24·50 |
| Lime | 1·50 |
| Alumina | 2·25 |
| Water | 1·00—Klaproth. |
Sp. Gr. 3·3 to 3·4.
Hypersthene is met with either massive, or imbedded in rocks. Its colour is dark brown, or greenish black; it has a lamellar structure parallel with the diagonals and sides of a rhombic prism of about 87° and 93°.‡ The cleavage of one side of the prism is more easily obtained than the other. When fractured, it exhibits reflections which are strongly metallic, and sometimes greenish,
* Named by Brooke, in honour of Professor Arfwedson.
† From the Greek, in allusion to its difficult frangibility.
‡ 98° 12′ and 81° 48′, according to Necker.
[page] 62
sometimes of a copper-red colour: this lustre is observable in one direction but not in the other; when reduced to very thin laminæ, it is translucent, with a slight tinge of green in one direction, but opake in the other; when pulverized it is dark grey. Before the blowpipe, on charcoal, it fuses easily into a greyish-green opake glass; with borax into a greenish glass.
It is found at the island of St Paul, on the coast of Labrador, chiefly in rolled masses, but also as a constituent of a syenitic or greenstone rock; likewise in Greenland.
SCHILLER SPAR.*
Schillerstein, W. Var. de Diallage Metalloide, H. Schillerspath, Br. Diallage Chatoyante, Bt. Diatomous Schiller Spar, M.
Combination of bisilicate of magnesia, protoxide of iron and lime, with hydrate of magnesia.
| Silica | 41·0 | 43·90 |
| Magnesia | 29·0 | 25·85 |
| Alumina | 3·0 | 0·00 |
| Lime | 1·0 | 2·64 |
| Oxide of iron | 14·0 | 13·21 |
| Water | 10·0 Drapier. | 14·43 Köhler. |
Sp. Gr. 2·6—2·8. H. = 3 ·5—4·0.
Cleavage in two directions, forming together an angle of about 135°; one of these cleavages highly perfect and easily obtained, the other appearing only in traces. Colour olive-green, occasionally pinchbeck-brown; with a shining metallic lustre on the faces of cleavage; opake; and yields to the knife. Streak greyish or yellowish-white. Becomes hard when exposed to heat; and before the blowpipe assumes a metallic aspect. Is with difficulty soluble in borax, exhibiting the re-action of iron; and with salt of phosphorus leaves a skeleton of silica. Reduced to powder, it is readily acted upon by sulphuric or muriatic acid.
It is found in serpentine and greenstone, at Baste in the Hartz.
BRONZITE.
Hemi-prismatic Schiller Spar, M. Bronzit, Karsten. Var. de Diallage Metalloïde, H.
Combination of silica, magnesia, lime, and the protoxides of iron and manganese.
* From the German, signifying Chatoyant Spar.
[page] 63
| Marburg. | Ulten-Thal. | |
| Silica | 57·19 | 56·81 |
| Magnesia | 32·67 | 29·67 |
| Lime | 1·29 | 2·19 |
| Protoxide of iron | 7·46 Kohler. | 8·46 Kohler. |
with small proportions of protoxide of manganese, alumina, and water.
Sp. Gr.3·3. H. between 4·0 and 5·0.
Its colour is brown, dark green, or ash-grey. It has a pseudo-metallic lustre, frequently approaching that of bronze; structure lamellar. Primary form an oblique four-sided prism. Cleavage very distinct, and readily obtained, parallel to the lateral planes of this prism. Frequently thin layers of calcareous spar appear between the laminæ; surface striated; opake when in mass; translucent if reduced to thin laminæ.
It is found in imbedded crystalline masses in serpentine, near Kraubat in Upper Stiria; very abundantly on the Monte Bracco, near Sestri, in Piedmont; imbedded in greenstone at the Baste, in the Hartz; near Hoff, in Bayreuth; at Stempel near Marburg; in the Ulten-Thal, Tyrol; in the Lizard district of Cornwall; and elsewhere.
WITHAMITE*
Sp. Gr. 3·1—3·3. H. = 6·0—6·5.
Occurs in minute, translucent, brilliant carmine-red crystals, which in form bear considerable resemblance to epidote. Lustre vitreous; streak white. Before the blowpipe intumesces, and fuses with difficulty into a dark greenish-grey scoria; with salt of phosphorus forms a globule, which contains a skeleton of silica, and becomes opake on cooling. Is not affected by acids; but silica, iron, and manganese, are unequivocally indicated among its constituents.
It occurs in Glenco in Argyleshire, both crystallized and massive, filling small cavities in a species of compact reddish trap.
THULITE.
Brooke.
H. = 6·0.
Contains silica 42·5, alumina 25·1, lime 19·4, magnesia 0·6—Beudant.
In crystalline masses of a rose-red colour; the form, when visible, resembling that of epidote. Translucent. Streak greyish-
* Named by Sir David Brewster, in honour of its discoverer, Henry Witham, Esq.
[page] 64
white. Cleavage in two directions parallel to the sides of a rhombic prism of 92° 30′ and 87° 30′; no distinct cleavage transverse to the axis of this prism.
Thulite occurs at Tellemarken in Norway, associated with quartz, fluor, and the variety of idocrase termed Cyprine. Like the preceding, it is generally considered an indistinct variety of epidote.
CORUNDUM.*
Rhombohedral Corundum, M.
This species includes sapphire, corundum-stone, and emery. It consists of pure alumina, coloured from admixture with oxide of iron.
| Blue Sapphire, | Red Sapphire. | Corundum, | Emery, | ||
| China. | Bengal. | Naxos. | |||
| Alumina | 98·5 | 84·0 | 90·0 | 89·50 | 86·0 |
| Lime | 0·5 | 0·0 | 0·0 | 0·00 | 3·0 |
| Silica | 0·0 | 6·5 | 7·0 | 5·50 | 3·0 |
| Oxide of iron | 1·0 | 7·5 | 1·2 | 1·25 | 4·0 |
| Klaproth. | Chenevix. | Klaproth. | Tennant. | ||
1. SAPPHIRE.† Saphir, W. Corindon hyalin, H. Perfect Corundum, Bournon. This consists of two varieties, the sapphire properly so called, and the oriental ruby, whose chief difference consists in their colour, although the specific gravity of the latter is also distinctly lower. They assume crystalline forms, which are derived from the same primary crystal, a slightly acute rhomboid, by the reflective goniometer of 86° 4′ and 93° 56′, in which measurements brilliant fragments of the sapphire and corundum-stone perfectly agree. It possesses double refraction. Alone before the blowpipe it suffers no change whether in fragments or powder; with borax fuses slowly, but perfectly, into a colourless glass. It is not acted upon by acids; but becomes electric when rubbed, a peculiarity which the transparent polished specimens preserve for a considerable time.
The sapphire is only inferior in hardness to the diamond; it occurs crystallized, in six-sided prisms variously terminated; and in rolled masses, which are colourless, or of a blue, yellow, or yellowish-green tinge, and transparent or translucent. The crystals yield to cleavage pretty readily in one direction, with a most brilliant surface; but they are extremely difficult to cleave pa-
* Corundum is the name given to common corundum by the inhabitants of India.
†Sappheiros, Greek, its ancient name.
[page] 65
rallel with the other planes of the primary rhomboid. The fracture is conchoidal.
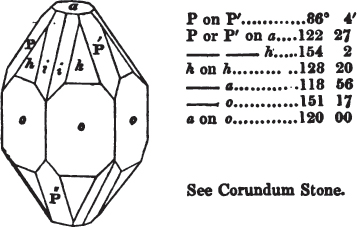
Sapphire has obtained several names dependent on its colour and lustre: the transparent or translucent, white sapphire; the blue, oriental sapphire; with pearly reflections, the chatoyant or opalescent sapphire; when transparent, and with a pale reddish or bluish reflection, girasol sapphire. Some, when cut en cabochon. present a silvery star of six rays, in a direction perpendicular to the axis; this variety is termed Asteria. The same crystal occasionally exhibits an union of two or three of these different colours.
The Oriental Ruby* (improperly so called) is of a blood red, or occasionally a rose-red colour; and chiefly occurs in the general form of six-sided prisms.
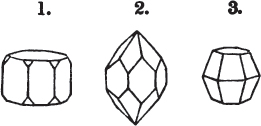
For an illustration of the passage of the primary rhomboid into a six-sided prism, see page 66. In fig. 1 and 2 the alternate triangular planes are the small remains of the primary rhomboid; the alternate planes of fig. 3 are also those of the rhomboid.
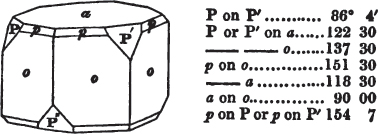
* Ruby, from the Latin ruber, red.
[page] 66
Blue sapphires are principally brought from Ceylon, either in six-sided prisms variously terminated, or in rolled masses from the beds of torrents; perfect specimens have been found upwards of three inches in diameter. The finest red sapphires are found in the Capelan Mountains, twelve days' journey from Sirian, a city of Pegu; it also occurs near Billin and Meronitz, in Bohemia; in the sand of the brook Expaillie, in France; at Brendola, in the Vicentine; on Mont St Gothard; and in Portugal. It is a valuable gem when obtained of some size.
2. CORUNDUM-STONE, or Common Corundum, has, probably from its texture, received the name of imperfect corundum, and from its hardness, or from its occasional peculiar lustre, that of Adamantine Spar. It is sometimes nearly colourless, and somewhat translucent; but more often has a greyish or greenish tint, occasionally reddish; also brown, with a metallic chatoyant lustre; more rarely blue, yellow and transparent, or black and opake. The common form of its crystal is the six-sided prism, which rarely exhibits a tendency to flat triedral terminations; it occurs also in obtuse and in acute hexahedral pyramids. Is likewise found granular or compact. The form of the primary rhomboid, which perfectly agrees with that of sapphire, is pretty easily obtained by cleavage, because some foreign substance is commonly interposed between the laminæ. Before the blowpipe this substance comports itself like the sapphire.
Granular corundum has the general appearance of a rough, purplish-coloured jasper; but it consists of grains, here and there of a rose-colour, closely associated with fibrolite; it is described by Bournon as compact corundum.
Fig. 1 is the primary rhomboid. Fig. 2 represents a rare variety, in which all the lateral edges of the primary rhomboid are deeply replaced by planes, tending to a six-sided prism, but terminated by portions of the planes of that rhomboid. Fig. 3 is a six-sided prism, arising from the complete replacement of the summits of fig. 2. Fig. 4, a very acute double six-sided pyramid; of these there are several varieties; as well as of obtuse six-sided pyramids, fig. 5. The two latter are rarely found presenting both pyramids.
[page] 67
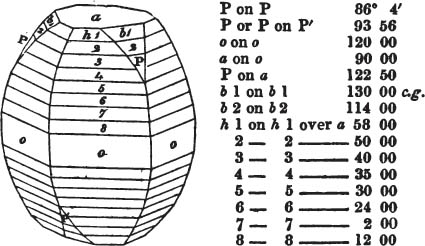
The planes o o o in conjunction with a tend to the production of a regular six-sided prism.
——— b 1 and b 2 to obtuse rhomboids, by planes situated on those of the primary rhomboid, and inclining on its summit.
——— h 1-8 to double six sided pyramids with triangular planes; they do not however occur as above represented on the same crystal.
Common corundum occurs in granitic rocks, accompanied by fibrolite, talc, garnet, zircon, and magnetic iron; in China; in the kingdom of Ava; on the coast of Malabar; and in the Carnatic. In smaller quantities also imbedded in magnetic iron at Gellivara in Sweden; near Mozzo in Piedmont in compact felspar; and at St Gothard of a red or blue tinge in dolomite.
In the East Indies it is used for polishing steel, and cutting gems; but the lapidaries of Europe prefer diamond-powder, on account of the greater rapidity with which it works.
3. EMERY. Schmiergel,W. Corindon granulaire, H. Emeril, Bt. Emery, though it bears little resemblance, is, from its hardness and analysis, considered to be a variety of the preceding. It usually occurs in masses of a blackish or bluish-grey colour, having the aspect rather of a fine-grained rock, than of a simple mineral. It occurs both massive and disseminated, with a somewhat glistening lustre; and is extremely tough and difficult to break. Its specific gravity is 3·66. In the Isle of Naxos, emery is found in rounded masses at the foot of primitive mountains. It occurs also near Smyrna; in Italy; and in Spain; but that of Ochsenkopf, near Schneeberg in Saxony, seems to be the only variety which has been seen in situ: it there occurs with talc-slate, is of a dark blue or black colour, and has much resemblance to fine-grained basalt.
[page] 68
DIASPORE.
Euklastic Disthene Spar, Haid. Diaspore, H. Bt.
Combination of alumina and water; often mixed with hydrate of iron.
| Ural. | |||
| Alumina | 85·14. | 80·0 | 76·06 |
| Water | 14·56 | 17·0 | 14·70 |
| Oxide of iron | 0·00 Hess. | 3·0 Vauq. | 7·78 Children. |
Sp. Gr. 3·43.
Diaspore is yet a scarce mineral. It occurs massive, in slightly curvilinear laminæ of a shining pearly lustre and greenish-grey colour, and which may be readily separated; also in cellular masses, constituted of slender crystals, which have a pearly lustre, and intercept each other in every direction, of a brown hue externally, but perfectly transparent and colourless when reduced to thin laminæ; rarely also in separate crystals, in the form of a doubly oblique prism.* It scratches glass. Exposed to heat in a matrass, it decrepitates violently, is dispersed (hence its name, from the Greek), and splits into small white brilliant scales, which, before the blowpipe with borax fuse readily into a colourless glass. When digested in muriatic acid, it becomes colourless, the oxide of iron being dissolved, but the mineral itself remaining unchanged.
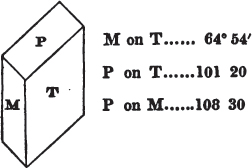
It is described as occurring only near the village of Kosoibrod in the Orenbourg government of Asiatic Russia, where it forms veins in primary limestone. Its superior lustre distinguishes it from the last species, some varieties of which it nearly resembles.
GIBBSITE.
Gibbsite, Cleaveland.
Combination of alumina and water. Analysis by Torrey: Alumina 64·8, water 34·7. Specific gravity 2·4.
* According to Mohs, it is a rhombic prism of about 130°.
[page] 69
It is described as commonly occurring in aggregations of irregular stalactites from one to three inches in length, and not less than an inch in diameter. Structure indistinctly fibrous, the fibres radiating from the centre; a little harder than calcareous spar, but easily reduced to powder; slightly translucent, and lustre faint; colour greenish- or greyish-white. Before the blowpipe it whitens but is infusible; yields in the matrass much water, but does not effervesce with acids.
It is found at Richmond in Massachusetts, North America, in a neglected mine of brown hæmatitic iron.
CALAITE.
Calaite, Fischer. Odontalite. Turquoise. Uncleavable Azure Spar, M.
Consists, according to Berzelius, of phosphate of alumina and phosphate of lime, silica, the oxides of iron and copper, and a little water; while John noticed only
| Alumina | 73·0 |
| Oxide of copper | 4·5 |
| Oxide of iron | 4·0 |
| Water | 18·0 |
Fischer considers it merely clay, coloured by oxide of copper.
Sp. Cr. 2·8—3·0. H. = 5·0—6·0.
It occurs in reniform masses, which are either botryoidal or mammillated; has a peculiar greenish-blue colour, but of various shades, passing on the one hand into sky-blue, and on the other into apple-green; and is dull internally; but occasionally the lustre is waxy, rarely splendent; fracture conchoidal; rough and uneven, frequently scaly. It is commonly opake; rarely translucent on the edges; streak white. The decomposed specimens resemble porcelain-clay. In the reducing flame of the blowpipe it becomes brown, and colours it green, but does not fuse; with borax it melts readily into a limpid glass.
The oriental calaite occurs in alluvial clay in the neighbourhood of Nishapuri and Firuzkuh in the Persian Korassan; and is found on sale in most of the cities of Persia.
Malachite, with which turquoise may sometimes be confounded, yields a green streak, while that of calaite is white.
The occidental turquoise found near the town of Simor, in Lower Languedoc, is merely tooth or bone, coloured with phosphate of iron. Analysis by La Grange: Phosphate of lime 80, carbonate of lime 8, phosphate of iron 2, phosphate of magnesia 2, alumina 1·5, water 1·6.
[page] 70
SILICIFEROUS HYDRATE OF ALUMINA.
Kollyrite, L. Alumine Hydraté Silicifère, Levy.
| Alumina | 45·0 | 44·5 |
| Silica | 14·0 | 15·0 |
| Water | 40·0 Klaproth. | 40·5 Berthier. |
Sp. Gr. 2·06 to 2·11.
This mineral occurs in white and nearly opake masses, which are perfectly sectile. When broken, it presents an earthy fracture, with a somewhat vitreous lustre.
It dissolves without effervescence in nitric acid; but is not affected by the blowpipe. When calcined, it gives off much water, separates into columnar masses like starch, and loses weight; adheres to the tongue; absorbs water with a slight noise, and becomes almost transparent.
It occurs at Schemnitz in Hungary, and in the gallery of a lead mine on the bank of the river Oo, in the Pyrenees.
The following are similar compounds, and most probably mere varieties of this species.
1. SEVERITE. Analysis by Pelletier: Alumina 22, silica 50, water 26, loss 2. It occurs in small masses of a white colour, without lustre, but possessing a slight degree of translucency; occasionally it is semi-transparent. It is a little harder than lithomarge, which it somewhat resembles. The surfaces produced by fracture are dull; it is extremely brittle, and yields easily to the knife; is soft, but receives a high polish by friction; adheres strongly to the tongue, and emits no argillaceous dour when breathed on. It does not effervesce with acids, nor form a paste with water. Its colour does not change by exposure to heat, and it is said to diffuse a smell like that of apples, when newly fractured.
It was found by M. Dufour in the neighbourhood of St Sever* in France, in a gravelly soil, in masses from two to four or five inches in diameter.
2. LENZINITE.† John. Has been divided into two varieties, the opaline and the argillaceous, which are described as follows:
Opaline. Consists of alumina 37·5, silica 37·5, water 25·0, and a trace of lime—John. Of a milk-white colour; to the touch smooth and slightly greasy; surface not shining; frac-
* Whence Severite.
† Named in honour of Lenz, a German mineralogist
[page] 71
ture large and flat conchoidal; translucent, or transparent on the edges; sectile; easily reduced to a white powder; adheres to the tongue. Specific gravity 2·1. In water it separates into numerous pieces, which are nearly transparent, and which, on the slightest touch, fall into small hard grains. Exposed to red heat, it loses 25 per cent. (water), and becomes hard enough to scratch glass.
Argillaceous, Consists of alumina 35·5, silica 39·0, water 25·0, lime a trace—John. Colour snow-white; occasionally tinged yellow by oxide of iron; dull, with an earthy fracture, and slight coherence. In minute pieces only it is slightly translucent; it becomes shining and unctuous by friction, and strongly adheres to the tongue. Its specific gravity is 1·80. Placed in water, it breaks down with much sediment, but less than that. of the opaline, without increasing its transparency. Exposed to a red heat, it becomes hard enough to scratch glass; but undergoes no other change. Both varieties occur at Kall in Eifeld.
4. ALLOPHANE. Stromeyer.
| Saalfeld. | Black Forest. | |
| Alumina | 32·20 | 38·76 |
| Silica | 21·92 | 24·11 |
| Water | 41·30 | 35·75 |
| Carbonate of copper | 3·06 | 0·00 |
| Oxide of copper | 0·00 | 2·33 |
| Hydrate of iron | 0·28 Stromeyer. | 0·00 Walchner. |
Sp. Gr. 1·8—1·9. H. = 3·0.
This mineral occurs in translucent masses, possessing a somewhat vitreous lustre, and a pale blue, green, or brown colour; it is extremely brittle, but may occasionally be cleaved into prisms which apparently are rectangular. Before the blowpipe it intumesces without fusing, and falls into powder, communicating to the flame a green tinge; with borax it melts into a colourless glass; and in acid it gelatinizes. It occurs at Saalfeld in Thu-ringia; at Gersbach in the Black Forest; in the Upper Palatinate; and at Schneeberg in Saxony.
5. SCARBROITE. Contains alumina 42·75, silica 7·90, water 48·55, peroxide of iron 0·80—Vernon. Sp. Gr. 1·48. Easily scratched by the knife. Massive. Colour pure white. Devoid of lustre. Fracture conchoidal. Highly adhesive to moist surfaces, and polished by the nail. Breathed upon, it emits a strong earthy smell; and when immersed in water neither becomes translucent nor falls to pieces, but gains considerably in weight. It occurs in a calcareous rock on the Yorkshire coast, near Scarborough, between septæ of oxide of iron.
[page] 72
6. HALLOYSITE. Consists of alumina 34·0, silica 39·5, water 26·5—Berthier. Sp. Gr. 1·8—2·1. In compact amorphous masses, having the aspect of steatite. Colour white, generally with a slightly bluish tint; translucent on the edges; fracture conchoidal, like that of wax; imbibes water, giving off numerous globules of air, and becoming more translucent. Adheres to the tongue; yields to the nail, and is polished by it. When exposed to a high temperature it loses in weight, but acquires much hardness, and its colour becomes milk-white. Sulphuric acid decomposes it readily, dissolving the alumina, and leaving the silica in a gelatinous state.
It occurs along with ores of zinc, iron, and lead, in the vicinity of Liege and Namur; and, according to Boussingault, also in the province of Bogota, in New Granada. It was described as a new species by Berthier, and named by him in honour of his uncle M. Omalius d'Halloy, who first noticed it. (Manual.)
7. WORTHITE. Alumina 54·45, silica 40·79, water 4·76— Hess. In foliated crystalline masses of a white colour; transparent; lustre vitreous; scratches quartz. Before the blowpipe in the matrass gives off water, and loses its transparency; alone upon charcoal it is infusible; with borax and salt of phosphorus undergoes no change; and with soda effervesces but does not melt. Occurs in bouldars in Sweden or Finland.
FIBROLITE.
Bournorn.
| Carnatic | China. | |
| Alumina | 58·25 | 46·0 |
| Silica | 38·00 | 33·0 |
| Iron | 0·75 Chenevix. | 13·0 Chenevix. |
Sp. Gr. 3·214.
Fibrolite is white, or of a greenish-grey colour; it is fibrous;* and rather harder than quartz, giving sparks with the steel. The fibres of which it is composed are rarely so large as to present any very determinate form, and are obliquely traversed by cracks; but Bournon describes some as right prisms with rhombic bases, of which the angles are 100° and 80°. It is infusible; acquires a sensibly resinous electricity by friction, and emits a reddish phosphoric light when two pieces are rubbed together.
It is found accompanying crystals of corundum in the Carnatic, and as a component part of the granite which is the matrix of that of China.
* Whence Fibrolite.
[page] 73
SILLIMANITE.
Bowen.
Alumina 54·11, silica 42·67, oxide of iron 2·00, water 0·51—Bowen.
Sp. Gr. 3·41. H. = 6·0—6·5.
Primary form an oblique rhombic prism of 106° 30′, the inclination of the base to the axis being 113°. Occurs imbedded in quartz in bent and twisted crystals, whose planes being dull and somewhat convex, seldom admit of accurate measurement. Colour dark grey, passing into clove brown; translucent on the edges. Lustre vitreous, considerable on the face of cleavage, which is parallel to the shortest diagonal of the prism. Fracture uneven, splintery. Brittle, and easily reduced to powder. It occurs in the county of Saybrook, Connecticut, and used to be considered a variety of anthophyllite, which in several respects it closely resembles, but from which it may easily be distinguished by its superior hardness.
KYANITE.
Sappare, Saussure. Kyanit, W. Disthène, H. Prismatic Disthene Spar, M. Rhætizite.
Combination of alumina and silica.
| St Gothard. | White, Zillerthal. | |||
| Alumina | 54·50 | 55·0 | 64·39 | 67·8 |
| Silica | 30·62 | 29·2 | 34·33 | 31·6 |
| Oxide of iron | 6·00 | 6·65 | 0·0 | 0·0 |
| Lime | 2·02 | 2·25 | 0·0 | 0·2 |
| Magnesia | 2·30 | 2·00 | 0·0 | 0·0 |
| Water | 4·56 | 4·09 | 0·0 | 0·0 |
| Saussure. | Arfwedson. | Beudant. |
Sp. Gr. 3·5—3·7. H. = 5·0—7·0.
Primary form a doubly oblique prism, of which the terminations are nearly rhombs; cleavage parallel to the planes of the prism, with difficulty parallel to those which may be considered as the terminal. The angles of the prism are 106° 15′ and 73° 45′; of the terminal plane on the prism, in one direction 100° 50′ and 79° 10′, and in the other 93° 15′ and 86° 45′. Generally occurs in irregularly terminated four-sided prisms. Its colours are white, grey, and blue:* it has sometimes a greenish
* Whence Kyanite, from the Greek, signifying blue.
D
[page] 74
tinge; the grey and blue are often intermixed in the same crystal; lustre pearly; the edges of the crystals scratch glass, but the broad surfaces yield to it. Some crystals by friction acquire negative electricity, others positive.* Before the blowpipe even its powder is infusible, and it remains unaltered in very high degrees of temperature; with borax it fuses slowly into a transparent colourless glass, and with salt of phosphorus forms a translucent silica skeleton, and a glass which does not become opaline on cooling. Is not acted upon by acids.
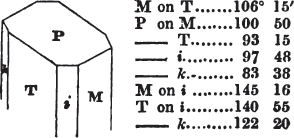
It occurs only in primitive rocks. In St Gothard in mica slate, associated with garnet, staurolite, and quartz; in the Sau-alp in Carinthia, with garnet, actynolite, &c.; in the Tyrol with quartz and hornblende; in very large crystals in Bohemia; at Villa Ricca in South America, and in Massachusetts. In Scotland, it occurs at Botrifny in Banffshire in gneiss; in primitive rocks near Banchory in Aberdeenshire; and in mica-slate in Mainland, Shetland.
Rhætizite is a nearly white or somewhat reddish variety, in aggregated masses, from the Pfitsch-thal in the Tyrol.
When in sufficiently large masses, of a fine blue colour and transparent, this species is cut and polished as an ornamental stone, bearing some resemblance to sapphire.
STAUROLITE.
Grenatit,† W. Staurotide, H. Prismatoidal Garnet, M.
Combination of alumina, silica, and oxide of iron.
| St Gothard. | |||
| Bretagne. | Reddish-brown. | Blackish-brown. | |
| Alumina | 44·0 | 52·25 | 41·00 |
| Silica | 33·0 | 27·00 | 37·50 |
| Oxide of iron | 13·0 | 18·50 | 18·25 |
| Oxide of manganese | 1·0 | 0·25 | 0·50 |
| Lime | 3·8 Vauq. | 0·00 Klap. | 0·00 Klap. |
Sp. Gr. 3·3 to 3·9. H. = 7·0 to 7·5.
* Hence the name Disthene was given by Haüy to this mineral, on account of its double electric powers.
† Staurolite, from the Greek, signifying a cross stone. Grenatite, in allusion to its (occasional) garnet colour.
[page] 75
Staurolite presents a reddish-brown colour, and occurs sometimes in rhombic prisms, of which the acute edges are frequently replaced, thus converting them into six-sided prisms. The crystals often intersect and cross each other at particular angles, and are then superficially of a dull brown; the primary crystal is a right rhombic prism of 129° 20′ and 50° 40′, by the reflective goniometer; it is divisible parallel to its sides and diagonals, the latter with the greatest brilliancy. Staurolite is opake or translucent; has a vitreous or resinous lustre; and in the apparently pure varieties, a conchoidal fracture. Before the blowpipe it assumes a darker hue, but per se does not fuse; with borax it melts slowly into a transparent deep-green coloured glass.
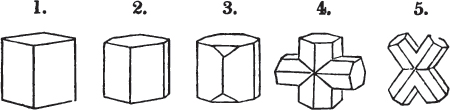
Fig. 1, the primary form; a right rhombic prism. Fig. 2, the same, of which the acute edges are replaced by planes, forming a six-sided crystal. Fig. 3 differs only from fig. 2 in having the obtuse solid angles replaced by triangular planes. Fig. 4, a macle, consisting of two crystals resembling fig. 2, crossing each other at right angles. Fig. 5, a macle, in which the crystals cross each other at a different angle.
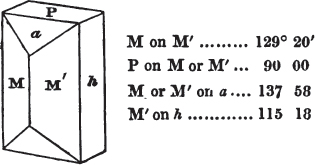
The staurolite belongs to primitive countries. It occurs in the form of fig. 2 at St Gothard in Switzerland, accompanying kyanite, and imbedded in talc slate; also in the Greiner Mountain, Tyrol: macles of considerable size, superficially of a dull brown colour, and opake, are met with in Bretagne in micaceous clay, considered to be the debris of a primitive rock. Several other varieties occur at Compostella in Spain, in some of the Hebrides, and in North America, generally dark coloured and opake.
[page] 76
AUTOMALITE.
Automalith, W. Spinelle Zincifère, H. Octahedral Corundum, M. ahnite.
It is an aluminate of zinc and of iron.
| Fahlun. | America. | ||
| Alumina | 60·00 | 55·14 | 57·09 |
| Silica | 4·76 | 3·84 | 1·22 |
| Oxide of zinc | 24·25 | 30·02 | 34·80 |
| Oxide of iron | 9·25 | 5·85 | 4·55 |
| Magnesia | 0·00 Eckeberg. | 5·25 Abich. | 2·22 Abich. |
Sp. Gr. 4·1 to 4·3. H. = 8·0.
Automalite by some mineralogists is considered a variety of spinel; and as it contains so large a proportion of the oxide of zinc, it has been designated Zinciferous Spinel; sometimes it is called Gahnite, in honour of Gahn, its discoverer. It occurs in regular octahedrons, which may be cleaved parallel with all its planes; it also occurs in tetrahedrons of which the angles are replaced; and in macles. It is much heavier than spinel, from which it also differs in being nearly opake, and of a dark bluish-green colour by transmitted light, as well as essentially in respect of composition. Before the blowpipe it is unalterable alone, and nearly so with salt of phosphorus or borax.
It occurs in a talcose rock at Fahlun in Sweden; in crystals of a large size at the Franklin iron works, Sussex county, New Jersey, accompanying quartz, felspar, and Jeffersonite; and in granite associated with chrysoberyl, garnet, and tantalite, at Haddam in Connecticut.
FLUELLITE.
Levy. Wollaston.
Combination of alumina and fluoric acid.
In small acute rhombic octahedrons, whose angles are 109° 82′, and 144°; the acute solid angles generally replaced. White and transparent; lustre vitreous. Occurs with wavellite and chalkolite on quartz at Stenna-gwyn in Cornwall, but is an extremely rare mineral.
It was discovered by Levy, but examined and named by Dr Wollaston.
[page] 77
TOPAZ*
Topaz, W. Silice Fluatée Alumineuse, H. Prismatic Topaz, M.
Combination of alumina, silica, and fluoric acid.
| Brazil. | Saxony. | |||
| Alumina | 47·5 | 58·38 | 59·0 | 57·45 |
| Silica | 44·5 | 34·01 | 35·0 | 34·24 |
| Fluoric acid | 7·0 | 7·79 | 5·0 | 7·75 |
| Klaproth. | Berzelius. | Klaproth. | Berzelius. | |
Sp. Gr. 3·49 to 3·56. H. = 80.
The topaz occurs massive, in rounded pieces, and crystallized.
General form prismatic, variously and dissimilarly terminated; the prism usually striated longitudinally and modified. Its structure is lamellar at right angles to the axis of the prism; it also cleaves, though with difficulty, parallel to the sides of a right rhombic prism of about 124° 22′ and 55° 38'; and it appears to yield to mechanical division on all the angles of the prism; cross fracture conchoidal, with a shining vitreous lustre. It is sometimes limpid and nearly transparent; or of various shades of yellow, green, blue, or red, and translucent. It becomes electric by heat, with polarity; and is easily excited by friction, the opposite terminations of the crystals presenting opposite kinds of electricity. Fragments exposed to heat burn with a blue, green, or yellowish phosphoric light. The pale-greenish and almost transparent topaz of Siberia becomes electric by heat, not by friction; the Saxon topazes, of a pale yellow colour, become electric by friction, not by heat, but lose their colour when exposed to fire; the deep-yellow topaz of Brazil becomes electric by heat, and red when placed in the fire. Before the blowpipe on charcoal it does not fuse; but the faces of crystallization appear covered with minute blisters which crack as soon as formed; with borax it melts slowly into a transparent glass.
Fig. 1, the primary; a right rhombic prism. Fig. 2, the same, terminated by four planes, of which two replace the acute solid angles, and the other two the obtuse solid angles. In fig. 3 each acute solid angle
* The name of the island whence this mineral was procured by the ancients.
[page] 78
is replaced by one plane, and each obtuse solid angle by two planes, leaving apparent a portion of the flat summit of the primary prism: there are several modifying planes on the prism itself. The crystals are rarely pyramidal at both ends; when they are so, the terminations are dissimilar.
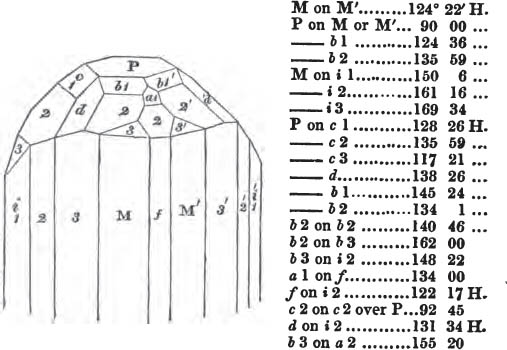
Topaz is almost peculiar to primitive countries. It is not, like quartz, a component part of any particular rock; but at Schneckenstein in Saxony, and in several parts of Cornwall, it occurs associated with tourmaline, quartz, and lithomarge, producing the mixture named by Werner, topaz-rock. Its usual matrix is granite, accompanying beryl, mica, tourmaline, fluor, apatite, and tin.
The district of Cairngorm in Aberdeenshire has produced the largest and most magnificent crystals of topaz. Jameson mentions one which weighed nineteen ounces. They are usually of a fine sky-blue colour, except on the edges of the prism, which appear pale brown. Topazes from this locality, however, are rare. In the Ural and Altai Mountains of Siberia they are more common; in Brazil they occur imbedded in an argillaceous earth, resulting, it is believed, from the decomposition of primitive rocks. In smaller crystals topaz is met with in the tin mines of Schlaggenwald and Zinnwald in Bohemia; at Schnecken-stein, Ehrenfriedersdorf, and Eibenstock, in Saxony; at St Michael's Mount in Cornwall, and in other places, with tin. In the Mourne Mountains of Ireland it is found in small but extremely perfect limpid crystals, associated with beryl, albite, and mica, in the drusy cavities of granite. The finest natural stones employed by the lapidary are those which, from their peculiar limpidity, are termed goutte d'eau, from Minas Novas in Brazil;
[page] 79
the larger portion of such as are used in jewellery being more or less altered in colour by exposure to heat.
1. PYROPHYSALITE. PHYSALITE. Pyrophysalith, Hisinger. Contains alumina 57·74, silica 34·36, fluoric acid 7·77, being the same ingredients as in topaz, and with very little variation of relative quantity, according to Berzelius. It is in fact a coarse opake variety of topaz found occasionally in yellowish-white crystals of considerable dimensions, and resembling that mineral in form. Its structure is lamellar in one direction, and splendent; the cross fracture glimmering and uneven. It is translucent on the edges; and not so hard as topaz. Specific gravity 3·41. It intumesces when heated,* and gives out a greenish phosphoric light.
It is found at Finbo, near Fahlun in Sweden, in a granite composed of white quartz, felspar, and silvery-white mica. A single well-pronounced crystal, weighing eighty pounds, is preserved in the college of mines at Stockholm.
2. PYCNITE. Schorlartiger beryl, W. Var. de silice fluatée alumineuse, H.
| Altenberg in Saxony. | ||
| Contains Alumina | 51·00 | 49·5 |
| Silica | 38·43 | 43·0 |
| Fluoric acid | 8·84 Berzelius. | 4·0 Klaproth. |
Sp. Gr. 3·51.
Pycnite is a variety of topaz which occurs in long six-sided prisms, deeply striated longitudinally, often closely aggregated† laterally, and exhibiting transverse rents, but without any apparent regular structure. It is usually of a dull yellowish or reddish-white colour, and translucent; is brittle, and may be readily broken across the prism; in other directions its fracture is imperfectly conchoidal, with a shining lustre; scratches quartz. Before the blowpipe on charcoal it does not fuse; with borax it melts slowly into a transparent glass. Becomes electric on exposure to heat.
It is found entering into the composition of a rock, chiefly consisting of quartz and mica, at Altenberg in Saxony; it is said also to occur in Bavaria, and other places; but Altenberg is its most noted locality.
* Whence Pyrophysalite, from the Greek, in allusion to the effect of heat on it.
† Whence Pycnite, from the Greek, signifying closely aggregated.
[page] 80
CHRYSOBERYL*
Krysoberyll, W. Cymophane, H. Bt. Prismatic Corundum, M. Chrysoberil, Br.
According to Berzelius, a combination of subsilicate of alumina with subsilicate of lime.
| Brazil. | Brazil. | |
| Alumina | 71·5 | 81·43 |
| Lime | 6·0 | 0·00 |
| Silica | 18·0 | 18·73 |
| Oxide of iron | 1·5 Klaproth. | 0·00 Arfwedson. |
(Seybert's and Thomson's analyses of chrysoberyl indicate from 15 to 18 per cent. of glucina, no lime, and an occasional quantity only of silica).
Sp. Gr. 3·65 to 3·8. H. = 8·5.
This substance occurs crystallized, and in rolled fragments in the alluvial deposits of rivers; its colour is green, sometimes with a yellow or brownish tinge, and occasionally presenting internally an opalescent bluish-white light. The primary form of its crystal is a right rectangular prism. The crystals yield to mechanical division readily and with brilliant surfaces parallel to the plane M of the following figures, and with difficulty also parallel to the plane T, and to the longer diagonal of the prism; the fracture is perfect conchoidal, with a splendent resino-vitreous lustre. It becomes electric by friction, but is not affected by heat. Before the blowpipe it suffers no change alone; but with borax fuses slowly into a transparent glass.
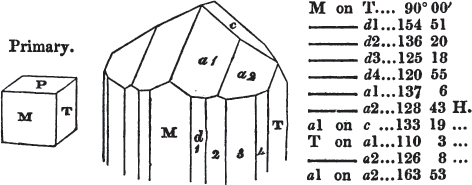
At Haddam in Connecticut it occurs in granite, in six-sided tables and prisms, with garnet, beryl, automalite, and tantalite. The finest specimens for lapidaries' purposes are, however, from
* Chrysoberyl, from the Greek, denoting a superior kind of beryl. Cymophane, from the same, signifying a floating light, in allusion to its opalescence.
[page] 81
Brazil, where, as in Ceylon, it occurs in the alluvial deposits of rivers, and consequently in rolled, and generally much rubbed, masses. When transparent and free from flaws, it forms a handsome gem.
SPINEL.
Spinell, W. Spinelle, H. Dodecahedral Corundum, M.
Combination of alumina and magnesia, coloured red by a minute portion of chromic acid, or blue by the protoxide of iron.
| Red. | Blue, Aker. | |
| Alumina | 74·50 | 72·25 |
| Magnesia | 8·25 | 14·63 |
| Silica | 15·50 | 5·45 |
| Lime | 0·75 | 0·00 |
| Protoxide of iron | 1·50 Klaproth. | 4·26 Berzelius. |
Sp. Gr; 3·5. H. = 8·0.
Spinel occurs crystallized either in regular octahedrons, occasionally having their edges replaced, or in macles presenting very different forms. It exhibits various shades of red, violet, or yellow; more rarely black. Its structure is lamellar, though not very distinctly so; but it yields to mechanical division parallel to the faces of the octahedron. Its fracture is commonly flat conchoidal, with a splendent vitreous lustre. It scratches quartz easily, but is not so hard as the oriental ruby, from which it is readily distinguished both by its colour and crystallization. It is infusible per se; the red varieties become brown and even black and opake as the temperature is increased, but on cooling they appear first green, then almost colourless, and at last resume their red hue. With borax they are difficultly fusible, but more easily so with salt of phosphorus.
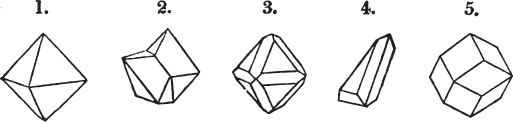
Fig. 1, the primary; the regular octahedron. Fig. 2, a macled crystal, in such a position as shows it to be composed of about equal parts of an octahedron (fig. 1), of which one half is half turned round. Fig. 3, the octahedron with its edges replaced. Fig. 4, a macle consisting of two equal and similar portions of a crystal resembling fig. 3, being sections, parallel with two opposite primary planes, placed base to base. Fig. 5, the rhombic dodecahedron, resulting from the replacement of all
D 2
[page] 82
the edges of the octahedron, by planes similar to those of fig. 3, but deeper.
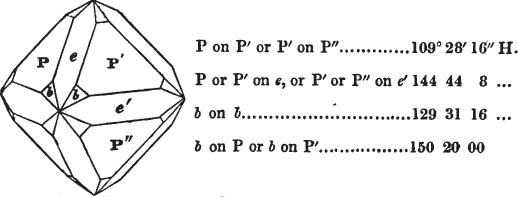
Spinel is principally found in Ceylon, Siam, and other eastern countries, where it occurs, like most other gems, in isolated and rolled crystals in the channels of rivers. The pale blue and pearl-grey varieties occur imbedded in calcareous spar at Aker in Sudermannland, Sweden.
By lapidaries the scarlet coloured is termed Spinel Ruby; the rose red, Balas Ruby; the yellow or orange red, the Rubicelle; and the violet coloured, Almandine Ruby.
SAPHIRINE.
Stromeyer. I.evy.
Sp. Gr. 3·42. H. = 7·0—8·0.
Alumina 63·11, silica 14·50, magnesia 16·85, lime 0·38, oxide of iron 3·92, oxide of manganese 0·53, water 0·49—Stromeyer.
Occurs disseminated in translucent grains of a pale-blue or green colour. Lustre vitreous. Streak white. Fracture imperfect conchoidal. Is not affected by the blowpipe, either alone or with borax.
This mineral was discovered by Giesècké, associated with mica and fibrous brown anthophyllite, at Akudlek in Greenland.
PLEONASTE.
Zeylanite, W. Pleonaste, H. Ceylanite, J. Candite, Bournon.
Aluminate of the protoxide of iron and magnesia.
| Alumina | 68·0 | 65·0 | 57·2 |
| Silica | 2·0 | 2·0 | 3·1 |
| Magnesia | 12·0 | 13·0 | 18·3 |
| Lime | 0·0 | 2·0 | 0·0 |
| Oxide of iron | 16·0 Descotils. | 16·5 Laugier. | 20·5 Gmelin. |
Sp. Gr. 3·64.
Pleonaste is frequently considered a variety of spinel; but its specific gravity is somewhat higher, and it differs both in colour
[page] 83
and composition. Pleonaste appears nearly black, and opake; but by transmitted light is feebly translucent on the thinnest edges, and has a green or blue tint. It occurs in crystals, whose primary is considered to be the regular octahedron. Fracture flat conchoidal; lustre splendent. Before the blowpipe, alone, it suffers no change, except that when strongly heated it becomes blue; with borax it fuses into a dark- green transparent glass.
Fig. 1, the primary octahedron. Fig. 2, the same, of which the edges are deeply replaced; the triangular planes being portions of the primary octahedron. This shows the passage of the octahedron into the rhombic dodecahedron, fig. 3.
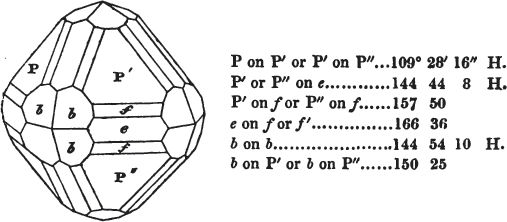
The geological situation of pleonaste differs materially from that of spinel. Pleonaste is found, accompanied by tourmaline, &c. in the rivers and alluvial country around Candy in Ceylon (hence Bournon's name of Candite for this variety). It also occurs in small but very perfect splendent crystals, coating, with mica and idocrase, the drusy cavities of ancient scoria at Monte Somma; imbedded in compact gehlenite at Monzoni in the Fassathal, Tyrol; in the volcanic rocks of Laach near Andernach on the Rhine; and in distinct octahedrons two or three inches in diameter, associated with chondrodite, at Amity in the Orange county, United States.
TURNERITE.
Turnerite, Levy.
H. above 4·0.
This rare mineral occurs in small crystals of a yellowish or brownish-yellow colour; brilliant externally; and translucent,
[page] 84
approaching to transparent. The primary form is, as determined by Levy, an oblique rhombic prism; but the only natural joints that have been observed (they are occasionally visible by transmitted light) are parallel with both diagonals of the prism; one of them is easily obtained with brilliant surfaces. Streak white or greyish. According to Children, it contains alumina, lime, magnesia, and a little iron.
Primary.
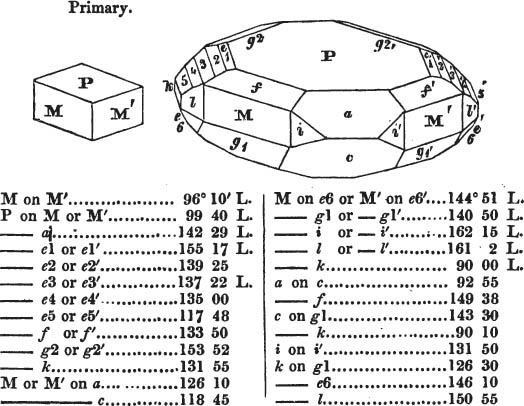
Such of the preceding measurements as have the letter L added to them, were taken by Levy.
It has been found only on Mount Sorel in Dauphiné, accompanying quartz, adularia, crichtonite, and anatase; and has occasionally been brought into this country under the name of Pictite.
HYDRATE OF MAGNESIA.
Native Magnesia, Bruce. Hydrate of Magnesia, A.
Combination of magnesia and water.
| Hoboken. | Unst. | ||
| Magnesia | 70·0 | 69·75 | 66·67 |
| Water | 30·0 Bruce. | 30·25 Fyfe. | 30·39 Stromeyer. |
Stromeyer also observed portions of manganese, iron, and lime.
Sp. Gr. 2·35. H. = 1·0 to 1·5.
It occurs in plates, which have a lamellar structure, and occasionally present indications of flat six-sided prisms. It is
[page] 85
white, occasionally with a tinge of green; semi-transparent, with a somewhat pearly lustre, but becomes opake by exposure; is rather elastic, adheres slightly to the tongue, and is so soft as to yield to the nail. It dissolves entirely in the muriatic, nitric, and dilute sulphuric acids, without effervescence.
This mineral has been found at Hoboken in New Jersey, in veins traversing serpentine; and at Swinaness in Unst, one of the Shetland Isles.
CHRYSOLITE.*
Krysolith, W. Peridot, H. Prismatic Chrysolite, M.
Combination of magnesia, silica, and protoxide of iron.
| Olivine, | Olivine, | |||
| Chrysolite. | Unkel. | Somma. | Meteoric. | |
| Magnesia | 43·5 | 38·5 | 44·24 | 48·42 |
| Silica | 39·0 | 50·0 | 40·08 | 38·48 |
Oxide of iron 19·0 Klap. 12·0 Klap. 15·26 Walm. 11·19 Strom.
Stromeyer noticed also a minute portion of nickel, Klaproth a little lime, and Walmstadt some manganese and alumina.
Sp. Gr. 3·3—3·5. H. = 6·5—7·0.
Chrysolite occurs in angular or somewhat rounded crystalline masses, and in prismatic crystals variously terminated. Their primary form is a right prism with rectangular bases, which may be obtained by cleavages parallel to all its planes, yielding the measurement of 90° every way by the reflective goniometer; the cross fracture is conchoidal with a vitreous lustre. Colour bright yellow, sometimes tinged with green or brown; transparent or translucent; and possesses double refraction. Per se, it is infusible before the blowpipe, but becomes darker; with borax it forms a transparent green glass.
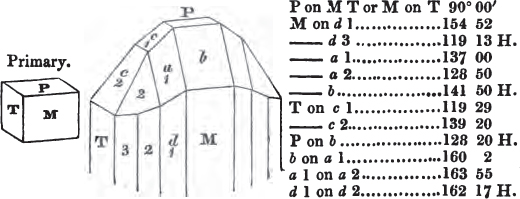
The best specimens of chrysolite are brought from Constantinople and the Levant, but under what circumstances they oc-
* Chrysolite, signifying a valuable stone, or gem.
[page] 86
cur is not authenticated. It is found also occasionally in pale green transparent crystals, among sand, at Expaillie in Auvergne; at Vesuvius; and in the Isle of Bourbon.
Olivine. Olivin, W. Peridot granuliforme, H. Olivine is a variety of chrysolite, differing slightly in respect of analysis, the general form of its crystals, and also in cleavage. It is chiefly found in olive-coloured semi-transparent masses, which, from their being in a state of decomposition, have externally an iridescent and somewhat metallic lustre; fracture imperfect conchoidal; not so hard as chrysolite. It occurs in crystals whose primary form may be considered a right rectangular prism, but they yield to cleavage with regularity only parallel to the terminal plane P of the following figure. Before the blowpipe alone it becomes somewhat brown without fusing; with borax it melts slowly into a diaphanous glass, coloured by iron.
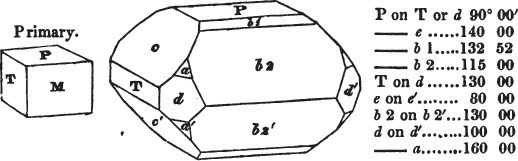
The above figure represents a crystal in Mr Majendie's collection, from the current of lava which flowed into the sea at Torre del Greco.
Olivine is met with abundantly in certain lavas, basalts, and other volcanic rocks; crystals, several inches in diameter, are found in compact greenstone at Unkle, near Bonn on the Rhine; large spheroidal masses in trap-tuff at Kapferstein in Styria; at Habichtswald in Hessia; and at Vesuvius. These, however, are generally granular, disintegrating and falling to pieces on the pressure of the fingers. In small quantity it occurs in many of the basalts of this country.
Meteoric Olivine. The semi-transparent olivine inclosed in the mass of meteoric iron found in Siberia by Pallas, is only peculiar for its straw-yellow colour.
The Hyalosiderite of Walchner, though possessing inferior hardness and specific gravity, and containing a little more iron, is also generally classed with chrysolite. It occurs in small masses, imbedded in brown basaltic amygdaloid, at the Kaiserstuhl in the Brisgau.
The small, uncrystalline, wax- or honey-yellow masses, of which the one is with difficulty, the other more easily fusible by the blowpipe, observed by Saussure in the basalt of Limbourg, and which he denominated Limbelite and Chusite, appear to be decomposed varieties of this species.
[page] 87
LIGURITE*
Leonhard.
This mineral is described as occurring in oblique rhombic prisms of 140° and 40°, sometimes modified, of an apple-green colour, and occasionally speckled externally. Its fragments are uneven and transparent, with a vitreous lustre. Streak greyish white. Sp. Gr. 3·49. It does not become electric either by heat or friction, and exhibits no phosphorescence when placed on live coal.
It occurs in a sort of talcose rock, on the banks of the Stara in the Apennines. According to Leonhard, it is considered as a gem superior to chrysolite in colour, hardness, and transparency.
FORSTERITE.
Levy.
H. about 7·0.
Contains magnesia and silica, according to Children.
Primary form a right rhombic prism, whose faces are inclined to one another at angles of 128° 54′ and 51° 6′.
Occurs in small colourless and brilliant translucent crystals. Cleavage perfect, and easily obtained parallel to o.
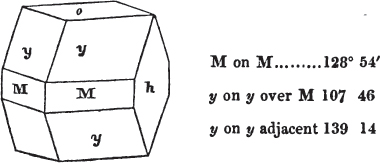
Is associated with pleonaste and olive-green pyroxene at Vesuvius. Its angles pretty nearly correspond with those of chrysoberil; but its cleavage perpendicular to the axis, which is extremely distinct in Forsterite, has not been observed in that mineral.
* Ligurite, after Liguria, the country in which it is found.
[page] 88
CONDRODITE.*
Condrodite, Berzelius. Brucite, Gibbs. Hemi-prismatic Chrysolite, M. Maclureite, Seybert.
| Pargas. | New Jersey. | |
| Magnesia | 54·00 | 54·00 |
| Silica | 38·00 | 32·66 |
| Oxide of iron | 5·10 | 2·33 |
| Alumina | 1·50 | 0·00 |
| Potash | 0·86 | 2·11 |
| Fluoric acid | 0·00 D'Ohsson. | 4·09 Seybert |
Sp. Gr. 3·15 to 3·25. H. = 6·5.
This mineral occurs massive, and in small grains, having occasional, but not very decided appearances of regular external form, and crossed by nearly parallel refts, of which the surfaces have a somewhat pearly lustre. No decided marks of regular internal structure are discernible in them; but the massive of Pargas is divisible into apparently rhombic prisms; and that of New Jersey is described by Cleaveland as occurring in rhombic prisms of 124° and 56°, with dihedral terminations. The colour is wax-yellow, or brown; it is translucent; yields to the knife with difficulty; and by friction acquires a resinous electricity.
Before the blowpipe it is infusible, but loses its colour; with borax it fuses slowly but completely, into a transparent glass, tinged by iron. The brown varieties act slightly on the magnet; not affected by acids.
The largest and most crystalline masses occur at Newton in New Jersey, and Amity and Edenville in New York, accompanying graphite in granular carbonate of lime. It is also found massive, and in grains, associated with pargasite at Pargas in Finland; and at Gulsjö and Aker in Sweden.
HUMITE.†
Bournon.
It occurs in very small crystals, which are of a yellowish or deep reddish-brown colour; and transparent or translucent; with a shining lustre. The crystals are modified in an extraordinary degree; their primary form may be considered as being a right rhombic prism of 60° and 120°, but they yield to cleavage paral-
* Condrodite, from its occurring in grains. Brucite, in honour of the late Professor Bruce of New York. Maclureite, in honour of Mr Maclure.
† Humite, in honour of Sir Abraham Hume.
[page] 89
lel only to its shorter diagonal (i.e. to the plane h of the following figure). Before the blowpipe it becomes opake, but does not fuse; and with borax affords a clear glass.
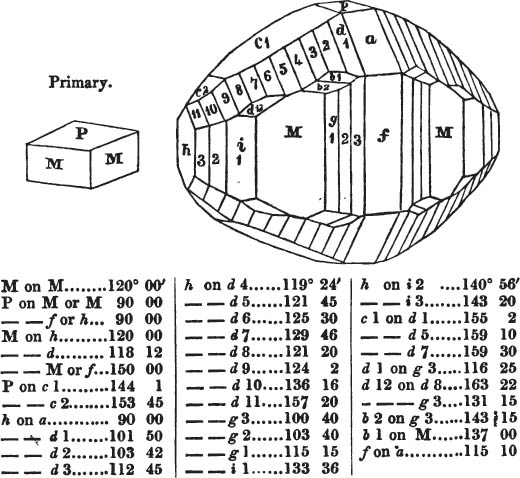
It is found on Monte Somma, with brownish mica, pleonaste, and other volcanic minerals; the crystals, though minute, being extremely distinct.
TAUTOLITE.
Breithaupt.
Sp. Gr. 3·865. H. = 6·5—7·0.
Opake, and of a velvet-black colour, with a vitreous lustre, and grey streak. Cleavage only in traces, and interrupted. Fracture conchoidal, uneven. Very brittle. Before the blowpipe it melts into a black scoria, which acts on the magnet; and with borax forms a clear green glass: with solution of cobalt it presents a blue colour; and, on the whole, appears to be a silicate of the protoxide of iron, combined with a silicate of magnesia.
It occurs in volcanic felspar at the lake of Laach, near Bonn on the Rhine.
[page] 90
SERPENTINE*
Ophite, L.
Has been divided into noble and common serpentine, a distinction which it is not very easy to draw. The term noble applies to such varieties as have an uniform green colour, and are translucent, and fit for cutting; while common serpentine has a more earthy texture, is less impalpable, and often contains admixtures of foreign matter.
| Fahlun. | Pseudomorphous, Snarum. | ||
| Magnesia | 38·68 | 40·64 | 41·66 |
| Silica | 42·50 | 41·95 | 42·97 |
| Alumina | 1·00 | 0·37 | 0·87 |
| Lime | 0·25 | 0·00 | 0·00 |
| Oxide of iron | 1·50 | 2·12 | 2·48 |
| Oxide of manganese | 0·62 | 0·00 | 0·00 |
| Oxide of chrome | 0·25 | 0·00 | 0·00 |
| Water | 15·20 | 11·68 | |
| Carb. acid and bitum. | 0·00 John. | 3·42 Lychn. | 12·02 Hartw. |
Sp. Gr. 2·5 to 2·56. H. = 3·0.
Colour commonly dark green, passing into yellow and grey. Translucent or opake, with a slightly resinous lustre. Fracture conchoidal or splintery; is occasionally somewhat unctuous to the touch, and yields easily to the knife; but it neither adheres to the tongue nor is scratched by the nail. It loses its water, and hardens, on exposure to heat; before the blowpipe the thin edges may be fused into an enamel; with borax it melts slowly into a greenish transparent glass.
Dark-green opake crystals of this substance have been met with in the Fassa valley, Tyrol; their form, however, is generally so indistinct that a few only of the faces can be traced. Crystalline varieties of a blackish-green colour, sometimes of considerable dimensions, occur at Amity, New York, disseminated through limestone with black spinel and ilmenite. At Snarum in Norway it is found in greenish-grey masses, which contain large and perfect pseudomorphous crystals. Noble serpentine occurs at Fahlun and Gulsjö in Sweden, in the Isle of Man, the vicinity of Portsoy in Aberdeenshire, in Corsica, Silesia, Saxony, &c.
*Serpentine and Ophite, from the Latin and Greek, in allusion to the spotted or variegated appearance,—like the skin of a snake,—which it frequently presents.
[page] 91
Common serpentine is frequently traversed by veins of asbestus, and occurs in masses and beds in primitive rocks in the Shetland Isles, at the Lizard in Cornwall, in Piedmont,Saltzburg, and elsewhere on the continent.
SOAP-STONE.
Speckstein, W. Talc Steatite, H.
The soap-stone of Cornwall consists of 45 silica, 9·25 alumina, 24·75 magnesia, 0·75 potash, 1 oxide of iron, and 18 water—Klaproth. It is found massive, and nearly white or of a grey colour,sometimes with a tinge of yellow, and mottled with green or purple; when first raised it may be kneaded like dough, but by exposure loses a part of its moisture, and is then translucent on the edges, yielding to the nail, and possessing an unctuous feel ; hence its name.
It occurs in a vein of serpentine at the Lizard Point in Cornwall, where it sometimes presents the appearance of passing into asbestus. It is used in the manufacture of porcelain, at Swansea in Wales; and is also found near the Cheesewring, at St Cleer in Cornwall.
It is commonly classed with steatite, but is much softer. In the composition of steatite no alumina has been detected, and it is infusible, whereas soap-stone fuses into a white enamel. It sometimes includes veins of asbestus.
| 1. STEATITE.* | Speckstein, W. | Talc Steatite, H. |
| Silica | 59·5 | 50·60 |
| Magnesia | 30·5 | 28·83 |
| Oxide of iron | 2·5 | 2·59 |
| Water | 5·5 Klaproth. | 15·00 Dewey. |
Sp. Gr. 2·65.
Steatite presents various shades of white, grey, yellow, green, and red, and is met with only massive; the distinctly pronounced crystals, which occur imbedded in a massive variety of the same mineral at Gopfersgrünn in Bayreuth, being pseudomorphous of the common variety of quartz, or occasionally of calcareous and pearl spars. It has generally an unctuous feel; yields to the nail, but does not adhere to the tongue; fracture splintery, sometimes slaty; somewhat translucent on the edges; hardens before the blowpipe, and becomes black, but is infusible.
Steatite is found in considerable masses, or in beds or veins,
* From a Greek word, signifying soap, in allusion to its greasy feel.
[page] 92
in some primitive mountains. It is most common in serpentine. At Freyberg in Saxony it occurs in tin veins, accompanied by or mingled with mica, asbestus, quartz, and occasionally native silver, &c. It abounds in the principality of Bayreuth, and is also found in Bohemia, Norway, Sweden, and France; in the western part of Massachusetts, in New Hampshire, Vermont, and Rhode Island; in the Isle of Anglesey; at Portsoy, in Aberdeenshire, in serpentine; in the Isle of Skye, and others of the Hebrides, in wacké; and in Fifeshire, of a sky-blue colour, with limestone.
The white varieties, or those which become so by calcination, are employed in the manufacture of porcelain; others are used for fulling. The Arabs, according to Shaw, use steatite in their baths instead of soap, to soften the skin; and Humboldt states that the Otomaques, a savage race inhabiting the banks of the Oronoko, are almost entirely supported during three months of the year by eating a species of steatite, which they first slightly bake, and then moisten with water.
2. POTSTONE. Topfstein, W. Talc ollaire, H. Pierre ollaire, Br. Serpentine ollaire, Bt. Is a coarse, indistinctly granular variety of indurated talc, having a greenish-grey or leek-green colour, with a glistening or pearly lustre. Contains silica 49·01, magnesia 30·20, alumina 6·08, protoxide of iron 11·40, water 4·2—Variety from Sweden by Thomson.
Potstone is plentifully found at Chiavenna, in the Valteline; at Como, in Lombardy; and, generally speaking, in serpentine countries; in Norway, Sweden, Finland, and Greenland. Its united properties of infusibility, softness, and tenacity, admit of its being readily turned on the lathe; from time immemorial it has been formed into vessels* in the Valais and Grisons, and Pliny describes it as being used in like manner in his time.
NEPHRITE.
Uncleavable Nephrite Spar, Haid. Nephrite, Common Jade, Axe Stone, J. Jade Nephritique, H.
Contains magnesia 31·00, silica 50·50, alumina 10·00, oxide of iron 5·50, oxide of chrome 0·05, water 2·75—Kastner.
Sp. Gr. 2·9—3·0. H. = 7·0.
Nephrite occurs in masses of a leek-green colour, passing into grey and greenish-white; is translucent on the edges; extremely tough; fracture coarse-splintery. Per se before the blowpipe it is infusible, but becomes white; and with borax forms a transparent glass.
* Whence the name of Potstone.
[page] 93
It occurs in the Hartz, in Corsica, in China, and in Egypt; also in New Zealand, and other islands in the Pacific, where it is made into hatchets and instruments of war.
NEMALITE.
Nemalite, Nuttall. Siliceous Hydrate of Magnesia, Thomson.
| Contains Magnesia | 51·72 |
| Silica | 12·57 |
| Peroxide of iron | 5·87 |
| Water | 29·67 Thomson. |
Sp. Gr. 2·35. Scratched by the nail.
Composed of elastic fibres, easily separable, and bearing a striking resemblance to the fibres of amianthus. Colour white, with a slight shade of yellow. Opake. Becomes brown on exposure to red heat, and gives off water. Is soluble with effervescence in nitric acid, with the exception of a little silica.
This mineral was discovered by Mr Nuttall, in serpentine veins, in New Jersey.
MARMOLITE.
Marmolite, Nuttall. Magnesie Hydratée Siliceuse, Levy.
| Magnesia | 46·0 | 41·25 |
| Silica | 36·0 | 41·67 |
| Water | 15·0 | 13·80 |
| Lime | 2·0 | 0·00 |
| Oxide of iron | 0·5 | 1·64 |
| Bitumen and Carbonic acid | 0·00 Nuttall. | 1·37 Lychnell. |
Sp. Gr. 2·41—2·47. Scratched by the knife.
This mineral occurs in grey and green translucent or opake masses, which have a columnar or foliated texture. Lustre pearly. Cleavage in two directions, parallel to the sides of an oblique four-sided prism; one of them obtained with facility.
It decrepitates and hardens before the blowpipe, separating into feathery-like masses, but does not fuse; and in the matrass yields water. With nitric acid it forms a gelatinous paste.
Marmolite occurs associated with hydrate of magnesia in serpentine veins at Hoboken, in New Jersey; and in the Bare Hills near Baltimore, United States.
[page] 94
PICROLITE.
Pikrolith, L.
| Magnesia | 38·80 | 33·44 |
| Silica | 40·04 | 40·98 |
| Water | 9·08 | 12·86 |
| Protoxide of iron | 8·28 | 8·72 |
| Carbonic acid | 4·70 Almroth. | 1·73 Lychnell. |
H. = 3·5—4·0.
Massive or fibrous, with a radiated structure. Colour leek-green, passing into yellow. Translucent on the edges. Streak somewhat shining. It colours glass of borax green; but the colour disappears on cooling. This mineral occurs in irregular veins at the Taberg of Smaland in Sweden, traversing beds of magnetic iron ore, and associated with calc-spar and serpentine. It is mentioned also from Reichenstein in Silesia. It was described and named by Hausmann. (Manual.)
PICROSMINE.
Picrosmine, Haid. Pikrosmin, L.
According to Beudant it is a tri-silicate of magnesia combined with a hydrate of magnesia. Magnesia 33·34, silica 54·88, protoxide of manganese 0·42, protoxide of iron 1·39, water 7·30— Magnus. Sp. Gr. 2·58—2·66. H. = 2·5—3·0.

Primary form a rectangular four-sided prism. Principal cleavage parallel to M. Colour greenish-white, sometimes dark green; nearly opake. Lustre pearly on M; inclining to vitreous on the other faces. Streak white and dull; very sectile. Before the blowpipe it does not melt, but gives out water, becomes first black, then white and opake, and acquires a hardness equal to 5·0. It is soluble in salt of phosphorus, with the exception of a silica skeleton; and when heated with solution of cobalt it assumes a pale red colour.
[page] 95
The only known locality of picrosmine is the iron mine of Engelsburg, near Presnitz in Bohemia, where it is associated with magnetic iron ore. In external appearance it resembles asbestus, but was distinguished by Haidinger, who named it picrosmine, from Πιχζος, bitter, and οσμη, smell, in allusion to the bitter and argillaceous odour which it exhales when moistened.
ZIRCON.
Pyramidal Zircon, M.
Combination of zirconia and silica.
| Zirconite. | Hyacinth. | Jargoon, Ceylon. | |
| Zirconia | 69·0 | 70·0 | 66·0 |
| Silica | 26·5 | 25·0 | 31·0 |
| Oxide of iron | 0·5 Klaproth. | 0·5 Klaproth. | 2·0 Vauq. |
| Sp. Gr. 4·5—4·7. H. = 7·5. | |||
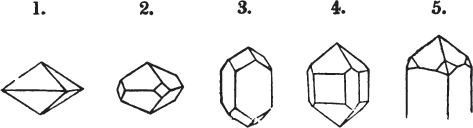
Fig. 1, the primary octahedron. Fig. 2, the same having its lateral solid angles replaced. Fig. 3 is the same as fig. 2; but the replacement of its angles is so considerable as to give to the crystal the form of a quadrangular prism terminated by four-sided pyramids with rhombic planes. Fig. 4; in this, the edges formed by the meeting of two pyramids of the primary octahedron are also replaced. Fig. 5 shows the solid angles formed by the meeting of the prism and pyramid, replaced each by two planes.
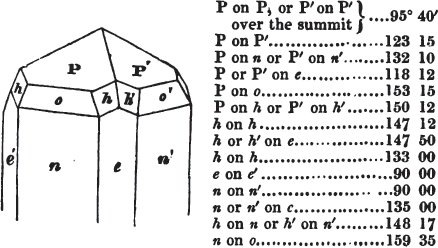
[page] 96
The primary form is an obtuse octahedron, with a square base, which occurs only among the opake brown crystals; its angles, taken by the reflective goniometer, on natural planes, are 84° 20′ and 95° 40′. The crystals of this substance resemble in a remarkable degree those of the oxide of tin, which also have for their primary form a flat octahedron: they are doubly refractive when translucent, are harder than quartz, and their lustre is adamantine. Zircon is infusible, and is not acted upon by acids, but it sometimes loses its colour on exposure to heat.
Zircon is divided into three sub-species—Hyacinth, Jargoon and Zirconite.
1. The Hyacinth presents various shades of red, passing into orange-red; it is transparent or translucent; its structure is lamellar, yielding to cleavage parallel both to P and n of the above figure. Its cross fracture is conchoidal, with a vitreous lustre. Before the blowpipe alone it is infusible, but with borax melts into a diaphanous glass. The hyacinth is commonly found in grains or rolled masses in the beds of rivers. It occurs in the brook Expailly, in Auvergne; at Ohlapian in Transylvania; near Billin in Bohemia; and in the alluvial deposits of Ceylon; occasionally also imbedded in volcanic tuff in Auvergne; at the Laachersee near Bonn; and at Vesuvius.
2. The Jargoon occurs in small transparent or translucent prismatic crystals (fig. 3), of a grey, yellow, or brown colour, having frequently a smoky tinge. It is found in the sands of certain rivers in Ceylon, with spinel, sapphire, and iron sand.
3. The Zirconite* occurs in reddish-brown and nearly opake prismatic crystals (figs. 4 and 5). Of these, magnificent specimens occasionally as large as a walnut are found at Miask in Siberia; at Bunkum in South Carolina; at Kitiksut in Greenland; and in the zircon syenite of Frederickswärn in Norway. In smaller crystals it is found in several granite and gneiss rocks, as at the Saualp in Carinthia; in New York county; at Scalpay in the Isle of Harris; and elsewhere.
The varieties of zircon are cut and polished by the lapidary, but in general are not greatly esteemed: the hyacinth often exhibits a brilliant colour when set as a gem, but it wants dimensions.
* Zirconite, from its containing the earth Zirconia.
[page] 97
OSTRANITE.*
Bretihaupt.
Sp. Gr. 4·32—4·4. H. between 4·0 and 6·0.
Four-sided prism surmounted by a four-sided pyramid.
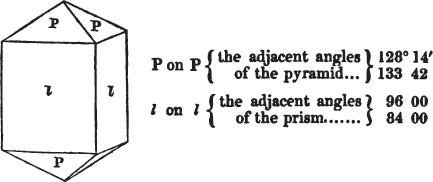
This mineral bears considerable resemblance to zircon, both in form and appearance; its angle however differs. Its colour is clove-brown; lustre vitreous; streak inclining to pale grey; very brittle. It does not fuse before the blowpipe, but its colour becomes paler; with borax it melts, though with difficulty, into a transparent glass. Insoluble in nitric acid.
Locality—Norway, and supposed to belong to the zircon syenite of Frederickswärn. It is a very rare mineral.
EUCLASE.
Prismatic Emerald, M. Euclase, H.
Combination of glucina, silica, and alumina. Glucina 21·78, silica 43·22, alumina 30·56, oxide of iron 2·22, and oxide of tin 0·70—Berzelius.
Sp.Gr.3·06. H. = 7·5.
It occurs in crystals which, when held in one direction, may be termed prismatic; the prism sometimes appearing rectangular, sometimes rhombic, and variously modified and terminated. The principal cleavage is highly perfect, and easily obtained, parallel to the plane P of the following figures: it cleaves also parallel to M and T; which, together with the measurements, and the nature of the modifying planes, prove the primary form to be a right oblique-angled prism. The planes P and T, and
* Ostranite, named by Breithaujjt, from the goddess Ostra.
† Euclase, from the Greek, signifying easily broken; in alluision to this circumstance.
E
[page] 98
the intermediate planes (which at first sight appear only as striæ), are those of the apparent prisms of the crystals, which usually are attached to the matrix at M, or the opposite plane. It is either colourless and nearly transparent, light green of various shades, or bluish-green; fracture conchoidal, with a splendent vitreous lustre. Very fragile; possesses double refraction, and becomes electric by friction or pressure, a property which it retains for many hours. Before the blowpipe it becomes opake, and then melts on the edges into a white enamel; with borax it fuses slowly into a transparent colourless glass. Not affected by acids.
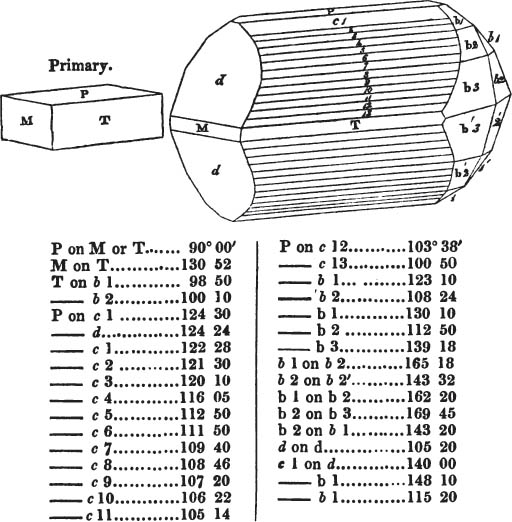
The above figures represent the planes of some crystals in the possession of H. J. Brooke, from which Mr Phillips obtained the accompanying measurements by the reflective goniometer.
Euclase was first found in Peru, in small quantity; and has since been brought from Capao, in the mining district of Villa Ricca in the Brazils. Its matrix is described as chloritic slate, resting on sandstone, but it is principally known in isolated crystals.
[page] 99
BERYL* EMERALD.
Edler Beryl, W. Emeraude, H. Beril Aigue-marine, Bt. Aquamarine. Rhombohedral Emerald, M.
Combination of glucina, silica, and alumina.
| Emerald, Peru. | Beryl, Siberia. | Beryl, Broddbo. | ||
| Glucina | 12·50 | 13·00 | 15·50 | 13·13 |
| Silica | 68·50 | 64·50 | 66·45 | 68·35 |
| Alumina | 15·75 | 16·00 | 16·75 | 17·60 |
| Oxide of chrome | 0·30 | 3·25 | 0·00 | 0·00 |
| Oxide of iron | 1·00 | 0·00 | 0·60 | 0·72 |
| Lime | 0·25 | 1·60 | 0·00 | 0·00 |
| Klaproth. | Vauquelin. | Klaproth. | Berzelius. | |
Berzelius noticed also a small portion of oxide of tantalum.
Sp. Gr.2·76 to 2·73. H. = 7·5—8·0.
The only important difference between emerald and beryl is in their colours; which, since they present an uninterrupted series, is altogether insufficient for a division of the present species. The emerald is emerald-green, which it derives from a small proportion of chrome; all the varieties of other colours, tinged more or less yellow and blue, or altogether colourless, are beryl. Common form the hexahedral prism, which sometimes is deeply striated longitudinally, and terminated by a six-sided pyramid, whose summit is replaced; or the terminal edges and angles of the prisms are replaced by small planes. Readily yields to cleavage parallel to all the planes of its primary form, the hexahedral prism. Transparent, translucent, or opake. Lustre vitreous. Fracture conchoidal and uneven. Transparent varieties become clouded before the blowpipe, and on increasing the heat, assume the appearance of mother-of-pearl; with borax it fuses into a transparent colourless glass.
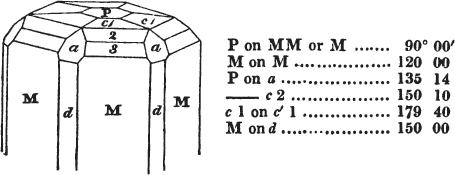
This species occurs principally in veins traversing granite, in implanted crystals, associated with felspar, topaz, tin-ore, &c.;
* Called Beryllos by the Greeks. Aquamarine from the Latin, sea-water, in relation to its colour.
[page] 100
likewise in fractured crystals and rolled masses in secondary depositories. The most splendid crystals of emerald occur in a vein of magnesian limestone, which traverses a hornblende rock at Muso, near Santa Fe de Bogota in Granada; some of these have been found exceeding two inches in length and breadth. Less distinct varieties occur at Mount Zalora in Upper Egypt, the only locality of emerald with which the ancients are believed to have been acquainted; at Cangarjum, in the district of Coimbetoor, in Hindustan; and imbedded in mica slate in the Heubach valley, Pinzgau district, Saltzburg. Such varieties of beryl as are clear, transparent and exhibit brilliant shades of sky-blue, or mountain-green, are denominated by lapidaries aqua-marine, or precious beryl. They are principally from the Brazils, and frequently occur in considerable masses. Of the common beryl, large hexagonal pale-green coloured translucent prisms are met with in the granitic district of Nertschinsk, and in the Uralian and Altai ranges of Siberia; they have been found exceeding a foot in length, and, when divested of their matrix, appear deeply striated longitudinally, The most remarkable, however, in point of size are those from Acworth in New Hampshire, which are described as weighing from two to three hundred pounds, and as measuring four feet in length. A coarse nearly opake variety occurs, both crystallized and in large masses, near Limoges in France; and imbedded in granite at Finbo and Broddbo, near Fahlun in Sweden; and others at Bodenmais and Rabenstein in Bavaria. Beautiful crystals occasionally two or three inches in length, and having a peculiar pale blue colour, occur in granite, associated with topaz, felspar, black quartz, and mica, at the Mourne Mountains, County Down.
GADOLINITE*
Prismatic Gadolinite, M.
Combination of yttria, silica, glucina, and the oxides of cerium and iron.
| Karafvet. | Broddbo. | Ytterby. | ||
| Yttria | 45·00 | 45·00 | 36·54 | 55·5 |
| Glucina | 0·00 | 11·60 | 5·90 | 4·5 |
| Protoxide of cerium | 19·92 | 4·33 | 14·31 | 0·0 |
| Protoxide of iron | 11·43 | 13·59 | 14·41 | 16·5 |
| Silica | 25·80 | 24·33 | 0·45 | 23·0 |
| Berzelius. | Thomson. | Connell. | Ekeberg. |
Sp. Gr. 4·2 to 4·3. H. = 6·5—7·0.
* After Gadolin, its discoverer.
[page] 101
In imperfect oblique rhombic prisms. Colour iron-black, and dull externally, internally black and shining; its primary form appears to be an oblique rhombic prism. Translucent on the edges, or opake. Cleavage imperfect. Fracture conchoidal. Before the blowpipe the Karafvet variety decrepitates, and fuses, when strongly heated, into an opake pearl-grey or reddish glass; that from Ytterby incandesces and loses its colour, but does not fuse. With borax they all melt readily into a globule, more or less tinged with iron. In heated nitric acid it loses its colour and gelatinizes.
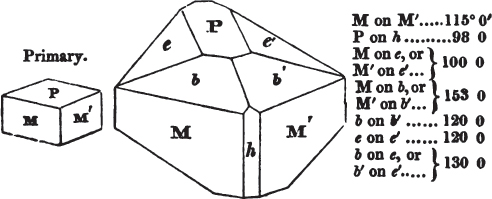
The above figure represents a crystal in the possession of Mr Brooke.
Its principal localities are the quarries of Karafvet and Finbo, near Fahlun in Sweden. Both there, and at Ytterby near Stockholm, it occurs indistinctly crystallized, and in amorphous masses, which are often encircled with a yellow crust, and are imbedded in coarse-grained granite. It has likewise been noticed at Disko in Greenland, and imbedded in granite in Ceylon.
THORITE.
Berzelius.
Thorina 57·91, lime 2·58, oxide of iron 3·40, oxide of manganese 2·39, oxide of uranium 1·58, oxide of lead 0·80, silica 18·98, water 9·50, with minute proportions of magnesia, potash, soda, and alumina—Berzelius. Sp. Gr. 4·63—4·8. Not scratched by the knife.
Massive and compact. Colour black. Streak dark-brown. Fracture vitreous, like that of gadolinite. Before the blowpipe it gives off water, and becomes yellow, but does not fuse; in a glass tube it exhibits traces of fluoric acid; with borax it forms a glass coloured by iron; and in salt of phosphorus fuses, with the exception of its silica.
This species was discovered by M. Esmark, in syenite, near Brevig in Norway.
[page 102]
ALKALINO-EARTHY MINERALS.
THE minerals included under this term generally consist, primarily, of earths in various proportions; they include also some portion of one or more of the alkalies, giving them a very important chemical distinction. Many of them contain iron, and some of them manganese; which in most, if not in all cases, may be considered as accessory rather than as essential ingredients.
MICA.*
Glimmer, W. Mica, H.
Recent optical investigations have pointed out to mineralogists the necessity of separating the varieties of mica which possess only one axis of double refraction, from those which present a double system of rings when viewed through the medium of polarised light.
Rhombohedral Talc Mica, M. Mica, A. Mica Rhomboedrique, ou Mono-Axial, Necker. Exhibits one axis of double refraction;—a lamina placed between two polarising tourmalines presents only one system of coloured rings, traversed by a black cross. Occurs in regular six-sided prisms, which cleave with extreme facility in one direction, viz. perpendicular to their axis. Colour generally dark green or brown; varying between transparent and opake. Lustre pearly, often inclining to metallic on the terminal faces of the prism; streak white or grey; thin laminæ are flexible and very elastic.
The varieties of this species differ also from the following in composition. They generally contain several per cent. of magnesia, and afford no indications of the presence either of fluoric acid, boracic acid, or lithia, which many of the following do.
* Mica, in allusion to its property of shining.
[page] 103
| Black, Siberia. | Siberia. | |
| Potash | 10·0 | 7·55 |
| Silica | 42·5 | 42·50 |
| Alumina | 11·5 | 16·05 |
| Magnesia | 9·0 | 25·97 |
| Oxide of manganese | 2·0 | 0·00 |
| Oxide of iron | 22·0—Klaproth. | 4·93—Rose. |
Before the blowpipe it sometimes fuses into a scoria, but go nerally only becomes white and opake.
To this species belong the dark-coloured micas from Siberia; the deep-brown, transparent, and perfectly formed six-sided prisms, which occur in the ejected debris of Vesuvius; and the black hexagonal prisms from the basalts of the Rhine and the trachytes of Hungary. Its abundance in nature, however, bears no proportion to the universal diffusion of the following.
Hemi-prismatic Talc Mica, M. Talc Mica, A. Mica Prismatique ou Di-Axial, Necker. Exhibits two axes of double refraction;—a lamina placed between two polarising tourmalines presents two systems of coloured rings, each traversed by a single black band. Occurs in oblique rhombic prisms of 60° and 120°, which are easily divisible parallel to their terminal plane (P of the following figures).
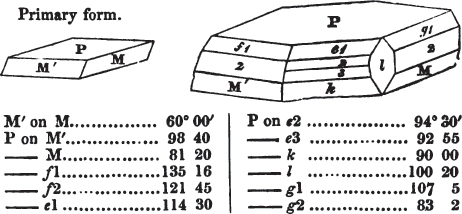
The dark-coloured varieties, which contain most iron, frequently act on the magnet. Before the blowpipe it loses its transparency, but does not fuse, except when lithia is in combination, in which case it melts with facility, and at the moment of fusion tinges the flame of a delicate red hue.
| Zinnwald. | Kimito. | ||
| Potash | 14·50 | 4·90 | 9·22 |
| Silica | 47·00 | 46·23 | 46·36 |
| Alumina | 20·00 | 14·14 | 36·80 |
| Oxide of iron | 15·50 | 17·97 | 4·53 |
| Oxide of manganese | 1·75 | 4·57 | 0·00 |
| Fluoric acid and water | 0·00 | 3·73 | 1·81 |
| Lithia | 0·00 | 4·21 | 0·00 |
| Klaproth. | Gmelin. | Rose. | |
[page] 104
This species is an essential ingredient of many rocks, especially the oldest primitive, as granite, gneiss, mica-slate, &c.; and is often found filling up their fissures, or crystallized in the cavities of the veins which traverse them. It also occurs in sandstones and in schistes. The best-known localities of this mica are Siberia and America, St Gothard in Switzerland, Pargas in Finland, Arendal in Norway, Finbo and Broddbo in Sweden, Zinnwald in Bohemia, Hörlberg in Bavaria, Aberdeenshire, and Cornwall.
According to Haüy, Muscovy glass, which occurs in plates of a yard or more in diameter, in veins of granite and micaceous schiste, in some parts of Russia, may be divided into laminæ no thicker than 1/300000th Part of an inch. It is used for enclosing objects for the solar microscope, and instead of glass in the Russian ships of war, as less liable to be broken by the concussion of the air during the discharge of heavy artillery: an inferior kind, which is found in Pennsylvania, is used there instead of window-glass.
RUBELLANE.
Contains potash and soda 10, silica 45, alumina 10, oxide of iron 20, lime 10, volatile matter 5—Klaproth. Sp. Gr. 2·5—2·7. H. rather below 3·0. In thin laminæ of a reddish-brown colour, which are not flexible. Exfoliates in the flame of a taper.
It occurs with mica and augite at Schima in the Mittelgebirge, Bohemia; and is considered by some mineralogists to be a mica altered by heat.
MARGARITE.*
Rhombohedral Pearl Mica, M. Rhomboidal Pearl Mica, J. Perl-Glimmer, L.
Contains silica 37·00, alumina 40·50, oxide of iron 4·50, lime 8·96, soda 1·24, water 1·00—according to Dumenil; making a loss of 6·80, and rendering a new analysis desirable.
Sp. Gr. 3·0—3·1. H. = 3·5—4·5.
It occurs in thin crystalline laminæ, which intersect each other in every direction. Colour pale pearl-grey passing into reddish-and yellowish-white; translucent; lustre pearly on the terminal faces, vitreous on the others; streak colourless; cleavage highly perfect parallel to the base of a six-sided prism.
Pearl mica is distinguished from the foregoing species by its superior hardness and specific gravity. It is peculiar to primitive rocks, being mixed with and engaged in foliated chlorite at Sterzing in the Tyrol.
* Margarite, from its peculiar pearly lustre.
[page] 105
LEUCITE.*
Leuzit, W. Amphigene, H. Trapezoidal Kouphone Spar, M.
Combination of potash, silica, and alumina.
| Vesuvius. | Vesuvius. | |
| Potash | 21·35 | 21·15 |
| Silica | 53·75 | 56·10 |
| Alumina | 24·63—Klaproth. | 23·10—Arfwedson. |
Sp. Gr. 2·48—2·5. H. = 5·5—6·0.
Leucite occurs in crystals whose planes are twenty-four equal and similar trapeziums, apparently with joints parallel to the rhombic dodecahedron and the cube; the latter of which, being the most simple of the two, has been adopted as the primary form. Leucite is generally of a dirty-white or grey colour, seldom reddish-white, and is occasionally somewhat translucent; its fracture is imperfectly conchoidal, with a vitreous lustre. Under the blowpipe per se it is infusible, even in powder; with borax, fuses slowly into a diaphanous glass; and, with soda, effervesces and forms a transparent blebby glass.
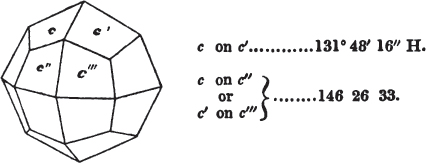
The manner in which this crystal is derived from the cube will be apparent on consulting the crystalline forms of Analcime.
In the vicinity of Rome, at Borghetto some miles to the north, and at Albano and Frescati to the south, some of the older lavas are so thickly studded with this mineral as to appear almost entirely composed of it. Around Vesuvius it occurs in large well-pronounced crystals of the above form; and near Andernach on the Rhine it is equally abundant, though in less remarkable individuals.
This mineral is peculiar in the history of chemical discovery, from being the first in which Klaproth observed the presence of potash.
* Leucite signifies a white substance: Amphigene, of a double origin, in allusion to its being found both in the earlier rocks and in volcanic matter.
E 2
[page] 106
NACRITE.
Erdiger Talc, W. Talc Granuleux, H. Nacrite, Bt. J. Scaly Talc, H.
This mineral is composed of 17·5 potash, 50 silica, 26 alumina, 1·5 lime, 5 oxide of iron, and a small portion of muriatic acid—Vauquelin. Occurs in minute aggregated scales, of a silvery-white or greenish colour, and presenting a glimmering pearly lustre; the mass is friable, very unctuous to the touch, light, adheres to the fingers, and gives out an argillaceous odour when breathed on. It differs from lepidolite and chlorite principally in respect of colour, and by its analysis appears to be allied to mica.
It forms small masses in the cavities of primitive rocks, and in the interstices of crystallized quartz; and occurs in Piedmont; near Freyberg in Saxony; and near Meronitz in Bohemia.
ANDALUSITE*
Andalusit, W. Feldspath Apyre, H. Prismatic Andalusite, M.
Combination of alumina and silica, with lime, potash, and the oxides of iron and manganese.
| Herzogau. | Spain. | Tyrol. | |
| Potash | 0·0 | 8·0 | 2·00 |
| Alumina | 60·5 | 52·0 | 55·75 |
| Silica | 36·5 | 38·0 | 34·00 |
| Oxide of iron | 4·0 | 2·0 | 3·37 |
| Lime | 0·0 | 0·0 | 2·12 |
| Oxide of mangainese | 0·0 Bucholz. | 0·0 Valuq. | 3·62 Brandes. |
Sp. Gr. 3·16. H.= 7·5.
It occurs massive, and in slightly rhombic prisms; it has a lamellar structure, with joints parallel to the sides of a rhombic prism, measuring by the reflective goniometer 88° 40′ and 91° 20′ on the cleavage planes. It has a grey or reddish colour, sometimes purplish-red, and is translucent on the edges or opake. It is infusible before the blowpipe alone; with borax fuses with extreme difficulty, and only when reduced to powder, into a transparent colourless glass. Not affected by acids.
In the Linsenz valley above Inspruck in the Tyrol, where it occurs in very large crystals, this species is accompanied with another which presents the same form, and has hence been taken for grey andalusite. The crystals of this substance are however certainly pseudomorphous, their hardness amounting only to 5·0, while their specific gravity exceeds 3·5.
* Andalusite, from Andalusia in Spain, where it was first found.
[page] 107
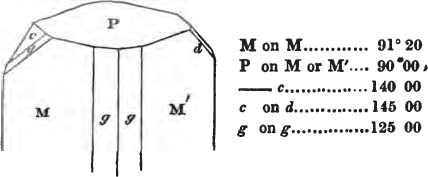
Andalusite belongs to primitive countries. It was first found in Andalusia in Spain; and latterly also near Braunsdorf in Saxony; at Guldenstein in Moravia; in Bavaria; at Forez in France; at Westford in the United States; at Botrifney in Banffshire imbedded in gneiss; and in mica-slate at Killiney, County Dublin. The chiastolite or macle is by some mineralogists united with this species.
BUCHOLZITE*
Brandes.
Combination of alumina and silica.
| Tyrol. | America. | |
| Potash | 1·5 | 0·00 |
| Alumina | 50·0 | 52·92 |
| Silica | 46·0 | 46·40 |
| Oxide of iron | 2·5—Brandes. | traces—Thomson. |
Sp. Gr. 3·19. H. about 6·0.
This mineral is amorphous, spotted white and black, with a glistening lustre, which is waxy, pearly, or vitreous; separating into fibres, especially in the black part, but in the white and grey the texture is often with difficulty perceivable. The cross fracture is occasionally conchoidal. A tendency to a lamellar structure is said to have been observed, the cleavage indicating some analogy with that of felspar. The fragments are mostly wedge-shaped and sharp, and when thin are slightly translucent. It scratches glass, but is scratched by quartz.
It was only known from Fassa-thal in the Tyrol, where it was noticed by its analyst Dr Brandes, until Dr Thomson published his description of the American variety from Chester on the Delaware.
* So named in honour of Bucholz the chemist.
[page] 108
PHILLIPSITE.
Lime-Harmotome, Connell. Staurotypous Kouphone Spar, M.
Contains potash 7·50, silica 48·02, alumina 22·61, lime 6·56, water 16·75—Variety from Marburg, by Gmelin.
Sp. Gr. 2·0—2·2. H. = 4·5.
Has been observed only in macles of the following form.
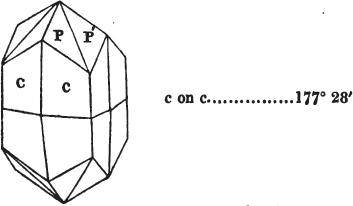
In white translucent or opake crystals, having much the aspect of Harmotome; cleavage imperfect. It occurs with gmelinite in the island Magee, County Antrim, in minute flesh-red coloured crystals, coating cavities of amygdaloid; in large translucent crystals in the same description of rock, at the Giants Causeway in Ireland; forming groups or sheaf-shaped aggregations at Capo di Bove near Rome; at Aci Reale on the eastern coast of Sicily; at Marburg in Hessia; at Lowenstein in Silesia; and among the lavas of Vesuvius. (Manual.)
APOPHYLLITE.*
Fishaugenstein, Albin, W. Apophyllite, H. Ichthyophthalmite. Pyramidal Kouphone Spar, M.
Combination of potash, silica, lime, and water.
| Uton. | Faroe. | Fassa. | Karasrat in Greenland. | Oxahverite, Iceland. | |
| Potash | 5·26 | 5·37 | 5·14 | 5·31 | 4·18 |
| Silica | 52·90 | 52·38 | 59·86 | 51·86 | 50·76 |
| Lime | 25·20 | 24·98 | 25·20 | 25·22 | 22·39 |
| Water | 16·00 | 16·20 | 16·04 | 16·90 | 17·36 |
| Fluoric acid | 00·82 | 0·00 | 0·00 | 0·00 | traces. |
| Berzelius. | Stromeyer. | Turner. |
Sp. Gr. 2·3—2·5. H.= 4·5—5·0.
* Apophyllite, probably from its exfoliating before the blowpipe.
[page] 109
Apophyllite occurs in square prisms whose solid angles are sometimes replaced by triangular planes, which by a deeper replacement assume the form of rhombic planes; the structure is lamellar; cleavage highly perfect parallel to all the planes of its primary form, but most readily perpendicular to its axis; fracture uneven. Colour white or greyish, sometimes with a green or reddish tinge; transparent, translucent, or opake; the lateral planes of the prism have a shining lustre; the terminal are pearly. It becomes feebly electric by friction. It exfoliates before the blowpipe, intumesces, and ultimately fuses into a white blebby glass; with borax it melts readily into a transparent globule; in nitric acid it separates into flakes, and when reduced to powder becomes gelatinous and translucent.
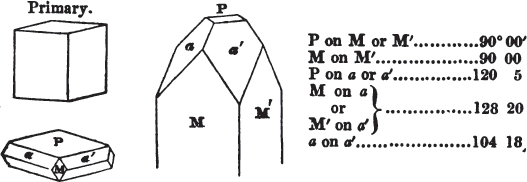
The most splendid crystallized varieties of apophyllite occur coating the cavities of amygdaloid, associated with calcedony, stilbite, chabasie, &c. in Greenland, Iceland, the Faroe Islands, and at Poonah in Hindustan. The peculiar pearly lustre of the crystals is one of the most decided characters of the species, and has obtained for it the denomination of Ichthyophthalmite, or fish-eye stone, from the Greek. At Andreasberg it is found in silver veins traversing grauwacke-slate; in the Bannat associated with wollastonite; and at Oberstein occupying the cavities of agate balls. Foliated apophyllite occurs in the iron mine of Uton in Sweden, and along with analcime in trap at the Seisser Alp, Tyrol; at the former almost transparent, at the latter white and opake. The variety termed oxahverite by Brewster, from the Oxahver springs in Iceland, is of a pale-green colour, indistinctly crystallized, somewhat translucent, and disposed on fossilized wood. Werner's albin rarely presents the terminal faces P; it is white and opake, and occurs associated with natrolite, near Aussig in Bohemia.
The Tesselite of Brewster is that variety particularly accompanying chabasie and mesole, from Nalsoe in the Faroe Islands, which exhibits, upon optical examination, a mosaic-like or tesselated structure.
[page] 110
DYSCLASITE.
Connell.
| Contains Potash | 0·23 |
| Soda | 0·44 |
| Silica | 57·69 |
| Lime | 26·83 |
| Water | 14·71 |
| Oxide of iron | 0·32 |
| Oxide of manganese | 0·22—Connell. |
Sp. Gr. 2·362. H. = 4·0—.5·0.
Occurs in white masses which have an opalescent appearance, and exhibit considerable translucency. Lustre glistening and vitreous. Its texture is imperfectly fibrous, but the fibres in some places diverge with considerable regularity, exhibiting an approach to crystalline structure. It is remarkably tough, and difficultly frangible, so as to require much time and labour to separate a mass into smaller fragments,—from which property its name has been derived. It gives off water at a red heat, and before the blowpipe is per se fusible only on the edges, and without intumescence; in soda it yields with effervescence a semi-transparent glass; with borax, and salt of phosphorus, it presents colourless glasses; and with nitrate of cobalt exhibits no re-action of alumina. When reduced to powder it gelatinizes readily with muriatic acid.
This mineral is found in the Faroe Islands, and was supposed to be a variety of mesotype until distinguished, analysed, and described as above by Mr Connell.
Dysclasite appears to bear considerable analogy with okenite, page 48.
HERSCHELLITE.
Contains potash, silica, and alumina.— Wollaston,
Sp. Gr.2·11. H.=4·5.
Occurs in six-sided prisms, whose lateral faces are streaked horizontally. Colour white. Translucent or opake. Fracture conchoidal. Cleavage easily obtained parallel to the base of the prism.
Herschellite occurs associated with Phillipsite in the cavities of trap, at Aci Reale, near Catania in Sicily. The individuals are sometimes isolated, but generally very closely aggregated, in a manner analogous to that which prehnite frequently presents.
[page] 111
HAUYNE.*
Hauyn, Karsten. Latialite, H.
Combination of potash or soda, silica, alumina, lime, and sulphuric acid.
| Marino. | Lake of Laach. | |
| Potash | 15·45 | 0·00 |
| Soda | 0·00 | 12·24 |
| Silica | 35·48 | 37·00 |
| Alumina | 18·87 | 27·50 |
| Lime | 12·00 | 8·14 |
| Oxide of iron | 1·16 | 1·15 |
| Sulphuric acid | 12·39 | 11·56 |
| Water | 1·20—Gmelin. | 1·50—Bergemann. |
Sp. Gr. 2·68—3·0.
The Haüyne is usually found in grains and massive, but it has been observed in extremely brilliant crystals, in the form of the rhombic dodecahedron. When this mineral is opake, it is of an indigo-blue colour; when translucent, blue or bluish-green; is somewhat harder than quartz, and very brittle; fracture conchoidal, and considerably splendent. Before the blowpipe on charcoal it loses its colour, and fuses slowly into an opake mass; with borax it forms a diaphanous glass, which becomes yellow on cooling, with salt of phosphorus is decomposed with effervescence, leaves a silica skeleton, and becomes opaline on cooling. Is reducible into a white transparent jelly in heated acid.
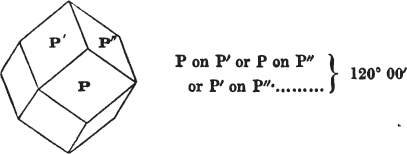
It occurs either disposed in the cavities of volcanic debris, as at Vesuvius, and in the neighbourhood of Rome; or imbedded in lava or pumice, as near Andernach, on the Rhine.
Haüyne is distinguished from lazulite by its vitreous lustre, a character which the latter does not possess.
* Haüyne, in honour of the late celebrated French mineralogist Hatty.
[page] 112
WEISSITE.*
Count Wachtmeister.
Contains potash 4·10, soda 0·68, silica 53·69, alumina 21·78, magnesia 8·99, protoxide of iron 1·43, protoxide of manganese 0·63, oxide of zinc 0·30, water with traces of ammonia 3·20— Wachtmeister. Sp. Gr. 2·80. Scratches glass.
In oblique rhombic prisms, of an ash-grey or brownish colour; translucent; and presenting only feeble traces of cleavage. Lustre pearly or waxy; fracture even or coarse granular. Before the blowpipe, in the matrass, it becomes brown, and yields water slightly acidulous; on charcoal it whitens, fuses on the edges, and becomes surrounded with an areola of zinc fumes; with borax it is slowly soluble into a colourless glass; also with salt of phosphorus, leaving a silica skeleton; with soda it melts into an opake scoria, and on platina foil exhibits the green colour indicative of manganese.
Its locality is the copper mine of Eric Matts at Fahlun in Sweden, where it occurs in chloritic talc.
PEARLSTONE.
Perlstein, W. Lave Vitreuse Perlé, W.
Contains potash 4·50, silica 75·25, alumina 12, lime 4·5, oxide of iron 1·6, and water 4·5—from Hungary.
Sp. Gr. 2·34.
Pearlstone occurs in large coarse angular masses composed of smaller round concretions, which consist of very thin lamellæ. Surface smooth and shining, with a lustre resembling that of pearl.† Colour grey, brown, red, or blackish; is fragile; translucent on the edges, and scarcely hard enough to scratch glass. It almost always gives out an argillaceous odour when breathed on; and before the blowpipe melts into a whitish frothy glass.
At Tokay and elsewhere in Hungary, it is found enclosing round masses of black vitreous obsidian, and is intermixed with the debris of granite, gneiss, and porphyry, and alternating in beds with the latter. It also occurs at Cap de Gat in Spain, of a green or bluish colour; in Iceland, &c.
* In compliment to Professor Weiss of Berlin.
† Whence Pearlstone.
[page] 113
GIESECKITE.*
Giseckite, Stromeyer.
Combination of potash, silica, and alumina, with admixtures of magnesia and the oxides of iron and manganese.
Sp. Gr. 2·78 to 2·85.
Analysis by Stromeyer, potash 6·2, silica 46·27, alumina 33·82, magnesia 1·2, oxide of iron 3·35, oxide of manganese 1·15, water 4·8. This mineral occurs in regular six-sided prisms, externally of a brownish tinge, internally greenish and blackish-green intermixed; it possesses no regular structure, but, on the contrary, being granular, with a waxy lustre, it has rather the appearance of a pseudomorphous steatitic mineral than of a crystalline substance; the crystal is opake, but small fragments are translucent; it yields to the knife, affording a white powder, but scratches common glass, on which the white powder of the mineral is left. Before the blowpipe it is extremely refractory, fusing only on the edges after a long-continued exposure; and becoming at the same time magnetic. It effervesces slightly with nitric acid.
It was brought by Sir C. Giesècké from Akulliarasiarsuk in Greenland, where it occurs imbedded in compact felspar.
PINITE.
Pinit, W. Pinite, H. Micarelle, Kirwan.
Combination of potash, silica, and alumina, with admixture of soda, magnesia, and the oxides of iron and manganese.
| Auvergne. | |
| Potash | 7·89 |
| Soda | 0·59 |
| Silica | 55·96 |
| Alumina | 25·48 |
| Oxide of iron | 5·51 |
| Manganese | 3·76—Gmelin. |
Sp. Gr. 2·78—2·8. H. = 2·0—2·5.
The pinite occurs in six-sided or twelve-sided prisms, of which the lateral, and more rarely the terminal edges, are sometimes replaced, as in the following figure; but it does not appear generally to possess any regular structure; one crystal from the Puy de Dome yielded to mechanical division parallel to the
* In honour of the late Sir C. Giesècké.
[page] 114
terminal planes of the six-sided prism, which is considered to be the primary crystal. It has a brown, blackish-brown, or grey colour, is opake, and almost devoid of lustre, being often somewhat ochreous externally. It yields to the knife easily; and is not affected by acids. Before the blowpipe, on charcoal, it whitens, and fuses on the edges into a white blebby glass, but does not melt; the most ferruginous varieties fuse more readily into a black glass. With borax it yields, after a continued blast, a transparent globule.
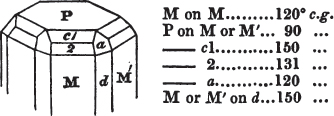
It was first discovered in granite, near Schneeberg in Saxony, in the mine called Pini, whence it was named by Werner. It has since been found in the Puy de Dome in France, in a decomposed porphyritic felspar; at Haddam in Connecticut, in a micaceous rock; at Arendal in Norway, in mica; imbedded in the granite of St Michael's Mount, Cornwall; and in Aberdeenshire.
PYRARGYLLITE.
Nordenskiold.
| Contains Potash | 1·05 |
| Soda | 1·83 |
| Silica | 43·93 |
| Alumina | 28·93 |
| Oxide of iron | 5·30 |
| Magnesia | 2·90 |
| Water | 15·47 |
Sp. Gr. 2·50. H. = 3·5.
Occurs massive; assuming, though rarely, a form analogous to the four-sided prism with bevelled edges; frequently traversed by chlorite. Colour partly black, and in that case shining; partly bluish, and then devoid of lustre. Is entirely soluble in nitric acid. Emits an argillaceous odour when heated.*
It occurs in granite near Helsingfors in Finland.
* Named Pyrargyllite by Nordenskiold, in allusion to this property.
[page] 115
FELSPAR*
Feldspath, W. H.
Combination of potash, silica, and alumina.
| Adularia. | Murchisonite. | ||
| Potash | 14·0 | 14·8 | |
| Silica | 64·0 | 68·6 | |
| Alumina | 20·0 | 16·6 | |
| Lime | 2·0—Vauquelin. | 0·0—Philips. |
| Opdke, white, | Iridescent, | Green, | Flesh-coloured, | |
| Carlsbad. | Frederickswarn. | Siberia. | Lomnitz. | |
| Potash | 11·50 | 12·2 | 13·0 | 12·00 |
| Silica | 64·50 | 63·0 | 62·8 | 66·75 |
| Alumina | 19·75 | 20·0 | 17·0 | 17·50 |
| Lime | a trace. | a trace. | 3·0 | 1·25 |
| Ox. of iron | 1·75 Klap. | 1·3 Klap. | 1·0 Vauq. | 0·75 Rose. |
Sp. Gr. 2·5 to 2·6. H. = 6·0.
Few minerals vary so much in appearance, or present more numerous and complicated crystalline forms, than felspar. It has hence been distinguished into several varieties, the transition from the one to the other of which is however so gradual, that in describing them these distinctions are not very easily made.
1. ADULARIA. Moon stone.† Adular, W. Feldspath nacré, H. Orthoklas, Breithaupt. This variety of felspar is semi-transparent, or translucent; it is greyish, greenish-white, or milk-white, and is frequently iridescent. It occurs both massive and crystallized; the forms of the crystals of Adularia, which consist of two, three, or four individuals, placed either parallel or obliquely to one another, being extremely complicated. Cleavage highly perfect, and easily obtained parallel to P of the following figure. Lustre vitreous, inclining to pearly on the perfect faces of cleavage. When splendent, with a pearly lustre, and exhibiting, especially if cut and polished, a bluish- or greenish-white chatoyant reflection of light, it is termed Moon-stone. The Sun-stone is the same, having exceedingly minute scales of mica interspersed throughout its mass, which being disposed in parallel position, reflect a pinchbeck-brown tint. In these, as well as in the Norwegian Labrador (as it is called), from Frederickswärn,
* From the German Feldspath, field-spar; in allusion perhaps to its being found loose on the surface of some parts of the country.
† Adularia, from Mount Adula, on which it is supposed first to have been found Moon-stone, from the brilliant light reflected by it.
[page] 116
this opalescent appearance is only in one direction, namely, in that which bevels the edge between T and T somewhat obliquely. The variety from this locality is a grey felspar, distinct from the species labradorite which follows, but presenting, like it, some very beautiful hues. Before the blowpipe upon charcoal it becomes glassy, semi-transparent, and white, but fuses only on the edges; with borax it dissolves slowly into a clear globule, and is not acted upon by acids.
It occurs in veins and cavities in granite, gneiss, clay-slate, and limestone, with quartz, amianthus, &c. at St Gothard, where crystals sometimes even a foot in thickness have occurred; in granite in North America; in New York in veins of quartz, traversing limestone; in Britain in the granite of Arran; in veins passing through schiste at Tintagell on the northern coast of Cornwall, &c. The finest specimens of moon-stone occur imbedded in granite in Ceylon.
2. COMMON FELSPAR is mostly opake, or translucent only on the edges; the lustre on the lamellar fragments is vitreous or pearly; the cross fracture uneven and glimmering. It presents white, yellow, blue, green, or red colours; and is either granular or massive, disseminated or crystallized. The crystals yield to cleavage parallel to the planes P M and T of the following figures, affording as primary form a doubly oblique prism, which presents in one direction four angles of 90°; in another, four alternately of 59° 25′ and 120° 35′; in another, four alternately of 67° 15′ and 112° 45′: the latter are obtained with great difficulty, the former with more ease, and the natural joints are generally visible in a direction parallel to the plane P. On charcoal it is fusible with borax into a semi-transparent glass.

[page] 117
Common felspar is a very generally diffused mineral; it is an essential constituent of granite and gneiss, and also frequently occurs in these rocks in veins, and in micaceous and argillaceous schiste. It abounds in primitive and secondary traps, and in most lavas.
The coarser kinds of felspar are also frequently macled, presenting some of the most remarkable hemitrope forms that occur in the mineral kingdom. At Carlsbad and Elbogen in Bohemia, twin crystals occur, exhibiting the union of two individuals which have been turned round to the extent of 180°, and attached to each other laterally. According to their points of junction, these are denominated rights and lefts; for in whatever position one of them is placed, its faces are never parallel or homologous to those of the other. At Carlsbad they occur from two to four inches in length, the rapid decomposition of the surrounding granite strewing the fields with them in vast quantities. They, as well as the varieties from Ekatherineburg in Siberia, and Warmbrunn in Silesia, are opake, have an earthy-brown colour, and are extremely coarse and rough externally. The Land's End granite is also studded with similarly formed crystals, though on a smaller scale; and, what is remarkable, pseudo-crystals of tin have been found in Cornwall assuming precisely similar macles. Large well-defined opake crystals are brought from Elba, and Arendal in Norway. The twins from Baveno in Piedmont, and La Clayette in Auvergne, are well known; as are the beautiful varieties accompanying beryl and topaz in the Mourne Mountains of Ireland. An occasionally crystallized variety of a beautiful apple green colour (Amazon-stone) is met with at the eastern base of the Ural Mountains, near the fortress of Troitzk; and the yellowish-grey and somewhat transparent kind, termed Murchisonite by Levy, is found near Dawlish in Devonshire, and in Arran. (Manual.)
3. GLASSY FELSPAR. Glassiger Feldspath, W. Occurs generally in crystals which have the appearance of being cracked in various directions, and are mostly imbedded. Its name of glassy is derived from its vitreous lustre; it is semi-transparent and translucent, and of a greyish or yellowish-white colour.
It occurs imbedded in trachyte in Bohemia; at Drachenfels near Bonn on the Rhine; in many of the trachytic lavas of the neighbourhood of Naples, and Hungary; and imbedded in pitch-stone in the Isle of Arran.
4. ICE-SPAR.* Eis-spath, W. It occurs in white transparent or translucent flattish crystals, of which the primary form is a right oblique-angled prism, differing but little in its proportions
* Ice-spar, from its possessing a considerable resemblance to ice, both externally and in brittleness.
[page] 118
from a right rhombic prism. It yields to cleavage parallel to all the primary planes—with difficulty parallel to T, more easily to P and M of the following figures. It possesses a shining lustre, and is very brittle. According to Berzelius, under the blowpipe on charcoal it becomes vitreous, semi-transparent, and white, and fuses with difficulty on the edge into a blebby semi-transparent glass; with borax into a diaphanous glass.
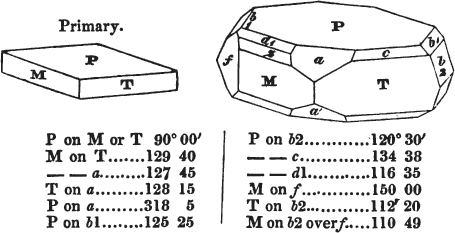
It occurs at Mont Somma near Naples with nepheline, mica, meionite, and hornblende.
5. DECOMPOSED FELSPAR, PORCELAIN-CLAY. Porcellanerde, W. Feldspath decomposé, H. Porcelain-Clay, Kirwan. Sp. Gr. 2·216. It is commonly yellowish-, sometimes reddish-white; occurs massive, and disseminated in certain rocks; and is composed of small particles which possess but slight coherence. It adheres to the tongue, and is soft and meagre to the touch. It often includes crystals of felspar, of quartz, and of mica, and is evidently derived from the decomposition of granitic rocks. Is infusible. A variety from Aue in Saxony yielded silica 52·0, alumina 37·0, and iron 6·33. The Saxon porcelain is made of clay from a bed in granite near Meissen; the Austrian from clay dug near Passau; that of Copenhagen from the produce of Bornholm, an island in the Baltic. The porcelain-clay of China is called Kaolin. In Britain, a large tract of this clay, which includes crystals of felspar, quartz, and mica, exists near St Austle in Cornwall, on the south side of the granite range; it supplies the porcelain manufactories of Worcester.
LATROBITE.
Latrobite, Brooke and Gmelin. Diploite, Breithaupt.
Occurs in crystalline masses, and in oblique rhombic prisms of about 93° 30′ and 86° 30′. Colour pale rose-red; and opake. Lustre vitreous. Cleavage in three directions, intersecting each
[page] 119
other at angles of 98° 30′, 91°, and 93° 30′. Two analyses by Gmelin vielded
| Potash | 6·58 | 6·57 |
| Silica | 44·65 | 41·78 |
| Alumina | 36·81 | 32·83 |
| Lime | 8·29 | 9·79 |
| Oxide of manganese | 3·16 | 5·76 |
| Water | 2·04 | 2·04 |
and a little magnesia.
Before the blowpipe, in the platina forceps, it fuses, intumesces into a white enamel, and with borax yields a globule which is pale amethyst-red in the oxidating flame, and colourless in the reducing one. With salt of phosphorus it fuses into a clear glass, containing a skeleton of silica.
Its only known locality is Amitok Island, near the coast of Labrador, whence it was brought by the Rev. C. J. Latrobe, who found it associated with felspar, mica, and calcareous spar.
AGALMATOLITE.
Bildstein, W. Talc Graphique, H. Steatite Pagodite, Bt.
| China. | |||
| Contains Potash | 7·0 | 0·00 | |
| Silica | 56·0 | 54·50 | |
| Alumina | 29·0 | 34·00 | |
| Lime | 2·0 | 6·25 | |
| Oxide of iron | 1·0 | 0·75 | |
| Water | 5·0—Vauq. | 4·00—Klaproth. |
Sp. Gr. 2·8—2·85. Soft.
It occurs massive, and sometimes presents an imperfectly slaty structure; general colour greenish, or yellowish-green, with veins of blue or brown; rarely also pink or mottled; translucent on the edges, unctuous to the touch, and generally yields to the pressure of the nail. Before the blowpipe on charcoal it whitens, and presents some slight marks of fusion; and with borax affords a colourless glass. It is partly soluble in sulphuric acid, leaving a siliceous residue.
Brongniart has given it the name of steatite pagodite, from its being always brought from China in the form of little grotesque figures* and chimney ornaments, but its different analyses distinguish it sufficiently from steatite, which always contains magnesia, but no potash. The agalmatolite is also found at Nagyag in Transylvania; in Norway; and at Glyder Bach, Caernarvonshire.
* Whence also the name Agalmatolite, from the Greek.
[page] 120
CHLORITE.
Prismatic Talc Mica, M. Chlorite, W. Talc, H.
Sp. Gr. 2·7—2·8. H. = 1·0—1·5.
Crystallized chlorite occurs in flat six-sided prisms. Colour various shades of green,* passing from dark-green into apple-green and greenish-grey; also pure white and yellowish. Semi-transparent, translucent, presenting different colours in different directions. Lustre pearly on the terminal planes, parallel to which the cleavage is highly perfect. Yields to the nail, and when in powder, is unctuous to the touch. Streak corresponding to the colour, generally white or green. Thin laminæ are easily flexible, but not elastic; a character which serves to distinguish this mineral from mica, which is very elastic. Three varieties of this species, the foliated talc, slaty chlorite, and green-earth, have yielded the following results:
| Silica | 62·0 | 29·5 | 52·0 |
| Magnesia | 27·0 | 21·4 | 6·0 |
| Oxide of iron | 3·5 | 23·4 | 23·0 |
| Alumina | 1·5 | 15·6 | 7·0 |
| Water | 6·0 | 7·4 | 4·0 |
| Potash | 0·0 | 0·0 | 7·5 |
| Lime | 0·0 Vauq. | 1·5 Gruner. | 0·0 Vauq. |
presenting considerable discrepancy in their chemical composition. Before the blowpipe some varieties lose their colour, and are difficultly fusible; others (the green-earth in particular) are changed into a black scoria, and, probably from their deficiency in potash and magnesia, will not fuse at all. (Manual.)
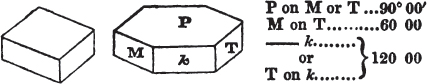
The above figures and measurements are given on the authority of Haüy.
Compact chlorite is amorphous; chlorite-slate possesses a slaty structure; and earthy chlorite consists of slightly coherent scaly particles.
One of the most beautiful dark-green foliated chlorites occurs in the Taberg iron mines of Wermeland in Sweden; the grey variety is found in Aberdeenshire. In Cornwall, where it is known under the title of Peach, some of the more crystalline
* Whence Chlorite, from the Greek, signifying green.
[page] 121
kinds are met with in metallic veins. Apple-green coloured talc, in large foliated masses, occurs in the island of Unst, one of the Shetlands; also in the Greiner Mountain in Saltzburg; and in the Vallais. The same, deposited in stellular concretions, imbedded in quartz, is found in Sweden; and a beautiful massive and translucent white variety at Almorah, in the Himalayah Mountains.
GREEN EARTH. Grünerde, W. Talc Zographique, H. Is met with in small masses, in, or lining the cavities of, amygdaloid; and is of a greyish- or bluish-green colour, passing into blackish-green; it is dull, and yields to the nail; its fracture is generally earthy. It is found wherever amygdaloid occurs; as in Saxony, Bohemia, Monte Baldo near Verona; in the islands of Faroe; and in many parts of Great Britain. When of a good colour it is made use of by painters.
KILLINITE.
| Contains Potash | 5·00 | 6·72 |
| Silica | 52·49 | 49·08 |
| Alumina | 24·50 | 30·60 |
| Oxide of iron | 2·49 | 2·27 |
| Ox. of manganese | 0·75 | 0·00 |
| Water | 5·00—Barker. | 10·00—Thomson. |
Sp. Gr. 2·65 to 2·75. H. = 4·0.
This mineral is of a light green, sometimes tinged brown or yellow, the progress of decomposition resulting from exposure. It occurs massive, with the occasional appearance of prisms, rifted across, and irregularly disposed; one prism afforded, by the common goniometer, angles of 135° and 45°. The structure is lamellar, yielding to mechanical division parallel to the lateral planes of a rhombic prism of 135° and 45°, and its lesser diagonal, but not parallel to its terminal planes; its lustre is glimmering; the cross fracture is fine grained. It is translucent, yields to the knife, and is easily frangible. The coating arising from exposure, yields an argillaceous odour when breathed on. Before the blowpipe it loses its colour and becomes white, intumesces, and fuses into a white enamel.
This mineral was discovered by Dr Taylor in granite veins, near the junction of mica-slate with granite, at Killiney* near Dublin. It is accompanied by spodumene, quartz, felspar, and garnet,—to the first of which it bears considerable resemblance.
* Whence Killinite.
F
[page] 122
COUZERANITE.
Charpentier.
Combination of silica, alumina, lime, magnesia, potash, and soda.
Potash 5·52, soda 3·96, silica 52·37, alumina 24·02, lime 11·85, magnesia 1·40—Dufrénoy.
Sp. Gr. 2·69. H. under 5·0.
Primary form an oblique rhombic prism of 84° and 96°. Occurs in small but highly perfect crystals imbedded in limestone. Colour varying from greyish-black to indigo-blue. Opake, but when in fragments transparent and brilliant; lustre vitreous or resinous; fracture slightly lamellar; not affected by acids; but fusible before the blowpipe into a white enamel.
This mineral was noticed by Charpentier, in the defiles of the valley of Seix in the Pyrenees termed "Des Couzerans."
GLAUCOLITE.
| Contains Potash | 1·27 | 4·57 |
| Soda | 2·96 | 0·00 |
| Silica | 50·58 | 54·58 |
| Alumina | 27·60 | 29·77 |
| Lime | 10·27 | 11·08 |
| Magnesia | 2·96 Bergmann. | 0·00 Bergmann. |
Sp. Gr. 2·72—2·9. H. = 5·0.
Occurs massive, presenting traces of cleavage parallel to the faces of a rhombic prism of 143° 30′ nearly (according to Brooke). Colour lavender-blue, occasionally passing into green. Translucent on the edges; fracture splintery; lustre vitreous. Before the blowpipe it whitens, and fuses only on the edges; but is soluble with effervescence in borax, or salt of phosphorus.
This mineral was first noticed near Lake Baikal in Siberia, imbedded in compact felspar and granular limestone; it has also been found with elaolite at Laurvig in Norway.
LEPIDOLITE.*
Lepidolith, W. Lepidolite, H.
The red variety from Moravia contains potash 9·04, silica 50·35, alumina 28·30, oxide of manganese 1·23, fluoric acid and water 5·20, lithia 4·49—Turner.
Lepidolite is of a pearly grey, or peach-blossom-red colour. It consists of an assemblage of small flexible scales, which are trans-
* From the Greek, signifying a scaly stone.
[page] 123
lucent, and sometimes hexagonal: the mass has a pearly or silvery lustre, yields to the nail, and is somewhat unctuous to the touch. Its specific gravity is 2·85. Before the blowpipe it melts into a spongy semi-translucent white globule.
Lepidolite occurs in granite near Rozena, in Moravia, accompanying the rubellite; also at Perm in Siberia; and associated with petalite at the isle of Uton in Sweden.
MESOTYPE.
Zeolith, W. Var. of Zeolite, J. Peritomous Kouphone Spar, M. Mesotype, H.
Combination of silica, alumina, soda, and water.
| Faroe. | Natrolite, Hohentwiel. | |
| Soda | 17·0 | 16·12 |
| Silica | 49·0 | 47·21 |
| Alumina | 27·0 | 25·60 |
| Water | 9·6 | 8·88 |
| Oxide of iron | 0·0—Smithson. | 1·35—Gehlen. |
Sp. Gr. 2·24—2·5. H. = 5·0—5·5.
This mineral occurs crystallized, fibrous, and pulverulent. The crystallized variety cleaves with facility parallel to the sides of a prism of 91° 20′ and 88° 40′; colour white, yellow, or greyish, and transparent or translucent, with a vitreous lustre; it yields to the knife, but scratches calcareous spar.
It becomes electric by heat, the heated fragments exhibiting a dull blue phosphoric light. Before the blowpipe on charcoal it becomes opake, and then vitrifies without intumescence; with borax, fuses with difficulty into a transparent colourless glass; and is soluble in, and forms a thick jelly with, acids.
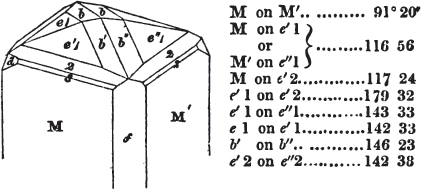
The fibrous variety consists of minute crystals aggregated in a radiating or stellular form; the centre being often compact enough to yield a splintery fracture, while the surrounding part is soft and apparently decomposing: these masses are sometimes globular.
[page] 124
The earthy or pulverulent variety (Mealy Zeolite, J.) occurs in soft, dull, friable masses, having an earthy fracture, and rough meagre feel. It is of a white, greyish, or reddish colour.
The crystalline varieties of this beautiful mineral are principally found forming diverging groups, in the vesicular cavities of amygdaloid in the Faroe Islands. In the trap-rocks of the Giant's Causeway, and some of the Hebrides, it likwise occurs in delicate acicular crystals; associated with analcime at Montecchio Maggiore in the Vicentine; in small silky-like diverging tufts coating cavities of lava in the more ancient portions of Vesuvius; radiated and mamillated at Hauenstein in Bohemia, and elsewhere on the continent.
NATROLITE.* Natrolit, W. H. Prismatic Kouphone Spar, M. It occurs in mamillary masses, which when broken present a fibrous structure; the fibres are diverging, exhibit a pearly lustre, and are white, or of yellowish or reddish-brown colours, disposed in alternate zones around the centre; in the cavities, or on the surface of these may sometimes be observed minute crystals of the same form and measurements as those of mesotype. Its specific gravity is 2·2. Before the blowpipe it affords the same results as mesotype.
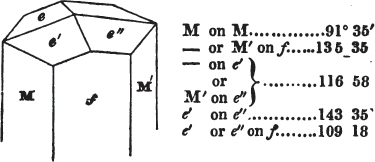
Its principal locality is Hohentwiel in Suabia.
THOMSONITE.†
Orthotomous Kouphone Spar, M.
Combination of silica, alumina, lime, soda, and water.
| Kilpatrick. | |
| Soda | 4·53 |
| Silica | 38·30 |
| Alumina | 30·20 |
| Lime | 13·54 |
| Water | 13·10—Berzelius. |
with traces of magnesia and peroxide of iron.
Sp. Gr. 2·35. H. about 5·0.
This mineral has much resemblance to mesotype or needle-
* Natrolite, from its containing natron.
† Thomsonite, in honour of Dr Thomson.
[page] 125
stone, from which, however, it differs essentially in respect of cleavage and form. It occurs generally in masses having a columnar or radiated structure, in the occasional cavities of which indistinct crystals may be observed. Colourless and translucent, but small fragments are transparent. It possesses considerable lustre, approaching to pearly; and is brittle. Primary form a right prism with square bases; cleavage readily obtained parallel to its sides, affording, by the reflective goniometer, 90° of one plane on the next; but it does not cleave parallel to the terminal planes of the prism. Before the blowpipe it intumesces, and becomes snow-white and opake, but does not melt. When exposed to a red heat, it gives off water, becomes opake, white, and shining like enamel; the edges are rounded, but it does not altogether lose its shape.
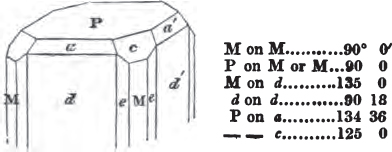
It occurs, imbedded in trap with analcime and prehnite, at Kilpatrick near Dumbarton.
MESOLE.
Flabelliform Kouphone Spar, Haid.
| Sweden. | Faroe. | |
| Soda | 10·19 | 5·63 |
| Silica | 42·17 | 42·60 |
| Alumina | 27·00 | 28·00 |
| Lime | 9·00 | 11·43 |
| Water | 11·77—Hisinger. | 12·70—Berzelius. |
Sp. Gr. 2·35—2·4. H. = 3·5.
In implanted globules, which have a flat columnar or lamellar structure radiating from the centre; colour greyish-white, sometimes yellow; translucent, with a silky or pearly lustre; cleavage perfect parallel to the broad face of the individual; laminæ slightly elastic.
It occurs at Nalsoe in the Faroe Islands, coating the cavities of basalt and amygdaloid, and associated with chabasie, apophyllite, stilbite, and others of the zeolite family; also in Disco Island, Greenland, in large individuals, which have a silvery lustre, a distinctly lamellar composition, and which bear much resemblance to crystallized spermaceti. Skagastrand in the north of Iceland,
[page] 126
and Rostanga in Scania, Sweden, are likewise localities of mesole. It is distinguished from mesotype by its perfect single cleavage and pearly lustre ; from stilbite or heulandite by its superior specific gravity; and from apophyllite by its crest or fan-like aggregations, which never occur in that mineral. When associated with apophyllite or stilbite, it always forms the lowest stratum, immediately adjoining the basalt or amygdaloid, in the cavities of which it is deposited. (Manual.)
NEEDLESTONE. MESOLITE.
Gehlen.
Combination of silica, alumina, lime, soda, and water.
| Iceland. | Faroe. | |
| Soda | 5·4 | 5·40 |
| Silica | 47·0 | 46·80 |
| Alumina | 25·9 | 26·50 |
| Lime | 9·8 | 9·87 |
| Water | 12·3—Gehlen. | 12·30—Berzelius. |
Sp. Gr. 2·26. H. = 5·0—5·5.
It occurs massive, and also in long slender prisms terminated by quadrilateral pyramids. It cleaves parallel to the sides of a slightly rhombic prism, corresponding in measurement with that of mesotype; there is also a remarkable agreement in some of its secondary planes with those of that mineral, of which it would thence appear to be merely a variety. The prisms are translucent or transparent and colourless, or of a greyish colour, externally shining with a somewhat pearly lustre. Specific gravity 2·26. Before the blowpipe, it becomes opake and curls up, and finally melts with the extrication of air-bubbles into a porous and almost opake bead.
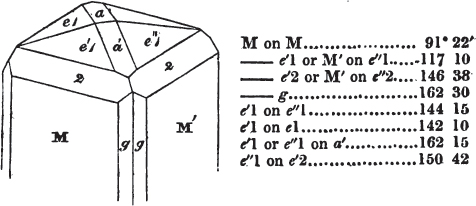
The finest specimens of this mineral are found in the Berufiord, Iceland. In these the crystals often exceed two inches in length, and diverge or interlace in the most beautiful manner. It occurs in colourless transparent radiated masses, also com-
[page] 127
pact and opake, in the trap district of the Vendayah Mountains, Hindostan; in Greenland; in Bohemia; and at Pargas in Finland.
BREVICITE.
Berzelius.
Contains soda 10·32, silica 43·88, alumina 28·39, lime 6·88, magnesia 0·21, and water 9·63.
In transparent prismatic crystals and white striated masses, occupying the cavities of a trachytic rock at Brevig in Norway, the striæ occasionally of a dark-red hue.
GMELINITE.*
Hexahedral Kouphone Spar, Haid. Hydrolite, De Dree.
Combination of silica, alumina, lime, soda, and water.
| Montecchio Maggiore. | Castel. | ||
| Soda | 4·5 | 4·25 | |
| Silica | 50·0 | 50·00 | |
| Alumina | 20·0 | 20·00 | |
| Lime | 4·5 | 4·25 | |
| Water | 21·0 Vauquelin. | 20·00 Vauquelin. |
Sp. Gr. 2·0—2·1. H. = 4·5.
Primary form a rhomboid. Secondary flat six-sided prisms, terminated at both extremities by truncated six-sided pyramids.
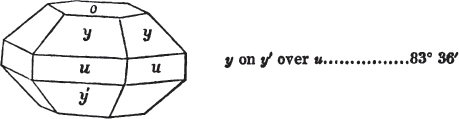
Colour white, passing into flesh-red; translucent; lustre vitreous; streak white; cleavage distinct parallel to the faces of the primary, fracture uneven; surface of the prism striated horizontally. It is soluble in acids. Before the blowpipe in the matrass it gives off water, and is reduced to powder; on charcoal it fuses with intumescence into a white glass. When held in the flame of a candle it separates and flies off in minute scales. Gmelinite occurs coating the cavities of amygdaloidal rocks at Montecchio Maggiore, and Castel in the Vicentine; of a white colour in the Deer Park of Glenarm, County Antrim; and presenting a flesh-red tinge at the Island Magee, near Larne.
* Gmelinite, in compliment to Professor Gmelin of Tubingen.
[page] 128
COMPTONITE.*
Comptonite, Brewster. Comptonitic Kouphone Spar, Haid.
Sp. Gr. 2·35—2·4. H. = 5·0—5·5.
Is found in translucent white crystals, which yield to cleavage parallel to the planes M and T of the following figure; the primary form is a right rectangular prism, of which the bases are not square. Lustre vitreous; streak white; fracture small conchoidal, and uneven; scratches stilbite, but not mesotype. By exposure in powder to the action of nitric acid, it is convertible into a jelly. Before the blowpipe it gives off water, intumesces slightly, becomes opake, and then fuses imperfectly into a vesicular glass; the globule obtained with borax is transparent; that with salt of phosphorus contains a skeleton of silica, and becomes opake on cooling.
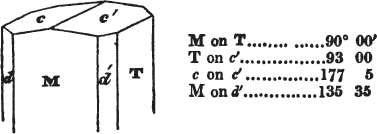
This mineral occurs among the vesicular lava of Vesuvius, associated with mesotype and other species. It has also been noticed in basalt at the Pflaster Kaute, near Eisenach in Hessia; forming a thin coating on the surface of mesotype, and occupying the cavities of graustein at Hauenstein in Bohemia; and associated with analcime and phillipsite at the Cyclopean Isles, Sicily.
LEDERERITE.†
Jackson.
Soda 3·94, silica 49·47, magnesia 21·48, lime 11·48, phosphoric acid 3·48, oxide of iron 0·14, water 8·58—Hayes.
Occurs in extremely brilliant, generally transparent and colourless, six-sided prisms terminated by six-sided pyramids, whose summit is replaced by a plane perpendicular to the axis; a form not uncommon in nepheline, davyne, and apatite. Some crystals are pale red and translucent.
It accompanies mesotype, stilbite, and analcime, in a basaltic rock at Cape Blomidon in Nova Scotia.
* In honour of Lord Compton—the present Earl of Northampton— by whom it was first distinguished.
† Named in compliment to the Austrian minister Von Lederer.
[page] 129
HYPOSTILBITE.
Beudant.
Contains soda 2·41, silica 52·43, alumina 18·32, lime 8·10, water 18·70—Beudant. Sp. Gr. 2·14. Does not scratch glass.
Either in white dull globules, consisting of delicate fibres, or compact; fracture devoid of lustre. Soluble in acid without forming a jelly. Before the blowpipe fuses with difficulty on the edges only, intumesces slightly, and becomes externally rough. Locality, the Faroe Islands, where it occurs associated with stilbite and epistilbite.
EPISTILBITE.
Diplogenic Kouphone Spar, M. Epistilbite, Rose.
Combination of silica, alumina, lime, soda, and water.
| Iceland. | Faroe. | |
| Soda | 1·78 | 1·20 |
| Silica | 58·59 | 58·61 |
| Alumina | 17·52 | 17·03 |
| Lime | 7·56 | 8·21 |
| Water | 14·00—Rose. | 13·80—Beudant. |
Sp, Gr. 2·2—2·25. H. = 4·0—4·5.
Primary form a right rhombic prism of 135° 10′ and 44° 50′.
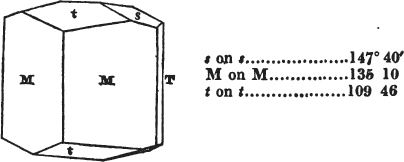
Commonly in macled crystals. Colour white or yellowish; varying from transparent, to translucent only on the edges; lustre vitreous, except on the faces of cleavage, and the corresponding crystalline planes, which are pearly. Cleavage highly perfect parallel to T. Alone before the blowpipe it melts, becomes white, intumesces, and forms a blebby enamel; and with soda fuses into a transparent glass. In concentrated muriatic acid it is dissolved, with the exception of a fine granular residue of silica.
Epistilbite occurs in large distinct crystals, along with others of the zeolite family, in the trap-rocks of Iceland and the Faroe Islands. It was distinguished by Dr Gustav. Rose of Berlin.
F 2
[page] 130
SPHEROSTILBITE.
Beudant.
Consists of soda 0·68, silica 55·91, alumina 16·61, lime 9·03. water 17·84—Beudant. Sp. Gr. 2·31. H. above 3·0.
In globular masses, which present a radiated structure, a pearly lustre, and a brilliant fracture. The fibres are flexible, and the surfaces of the globules may be scratched by the nail. It forms a jelly with acids; and fuses before the blowpipe, with exfoliation and intumescence.
It occurs both in the Faroe Islands and in Iceland.
ERLAMITE.
Breithaupt.
Contains soda 2·61, silica 53·16, alumina 14·03, lime 14·39, magnesia 5·42, oxide of iron 7·14, oxide of manganese 0·64— Gmelin.
Sp. Gr. 3·0—3·1. H. = 5·0.
Occurs massive, occasionally compact, generally in small and fine granular concretions, of a light greenish-grey colour. Lustre feebly shining, or dull. Streak shining, with a resinous lustre, and white; structure distinctly crystalline, but no regular cleavage has been observed; fracture in some specimens foliated, in others splintery. Before the blowpipe it fuses readily into a slightly coloured transparent compact globule; and with horax forms a clear greenish. glass.
Erlamite is described as possessing the aspect of gehlenite. It was discovered by Breithaupt in the Saxon Erzgebirge, forming a part of the oldest gneiss formation. It appears to be a mere mechanical mixture. (Manual).
HUMBOLDTILITE:
Monticelli and Covelli.
Contains soda 4·28, potash 0·38, silica 43·96, alumina 11·20; lime 31·96, magnesia 6·10, protoxide of iron 2·32—Kobell.
Sp. Gr.3·10. H. = 5·0.
In crystals derived from a rectangular prism with a square base; cleavable parallel to the base. Colour greyish-yellow or grey; lustre vitreous, passing into resinous; fracture conchoidal or uneven; between transparent and feebly translucent. Before the blowpipe per se it fuses readily, with a slight intumescence, into a diaphanous glass, which is blebby, and in colour resembles the
[page] 131
mineral, only that it has a more grey or greenish hue. With borax it melts slowly into a colourless glass. Reduced to powder after being calcined, it is quickly soluble in acid, forming a very characteristic jelly.
Humboldtilite occurs at Vesuvius.
LAPIS-LAZULI.*
Lazurstein, W. Lazulite, H. La Pierre d'Azur, Br. Azure-stone, J.
Combination of silica, alumina, lime, oxide of iron, magnesia, soda, and sulphuric acid.
Silica 49·0, alumina 11·0, lime 16·0, soda and potash 8·0, oxide of iron 4·0, magnesia 2·0, sulphuric acid 2·0—Gmelin.
Sp. Gr. 2·95.
This mineral is found massive; also, though rarely, in rhombic dodecahedrons, of an azure blue colour; the texture of the massive is fine grained or compact with a glimmering lustre, and it is hard enough to scratch glass, though it scarcely gives sparks with steel; it is nearly opake; and its blue colour is not uniform, as it frequently encloses iron pyrites, compact felspar, and quartz. On charcoal it fuses with difficulty into a white glass when pure: with salt of phosphorus is soluble with effervescence, the portion melted burning with great brilliancy: with soda is partly soluble into an opake greenish-grey glass, which assumes a red appearance on cooling; and if previously calcined and reduced to powder, loses its colour in acid. The finest specimens are brought from China, Persia, Lake Baikal in Siberia, and Bucharia. Lapis-lazuli is prized by the lapidary, but is chiefly important as affording that beautiful pigment called ultra-marine, so highly valued by painters.
NEPHELINE.
Nephelin, W. H. Sommit, Karsten. Rhombohedral Felspar, M.
Combination of soda, silica, and alumina.
| Katzenbuchel. | Vesuvius. | |
| Soda | 13·36 | 20·46 |
| Silica | 43·36 | 44·11 |
| Alumina | 33·49 | 33·73 |
| Potash | 7·13 | 0·00 |
| Water | 1·39 | 0·00 |
| Lime | 1·90—Gmelin. | 0·00—Arfwedson. |
Sp. Gr. 2·5—2·6. H. = 6·0.
* Lapis-lazuli, azure-stone, from its blue colour.
[page] 132
Occurs in regular six-sided prisms (the form of the primary crystal), of which the terminal edges are sometimes replaced. It may be cleaved, though imperfectly, parallel to all the planes of that solid; fracture conchoidal; colourless or greyish-white, with a shining vitreous lustre; transparent or translucent. Before the blowpipe it is fusible into a blebby colourless glass. With salt of phosphorus is soluble, without effervescence, and leaving a silica skeleton, into a glass which becomes opaline on cooling; and with borax fuses slowly into a transparent colourless globule. A translucent fragment, when immersed in nitric acid, assumes a nebulous appearance (whence the name nepheline, from ΓεΦελη, a cloud); and is finally converted into a jelly. When reduced to powder it gelatinizes in heated muriatic acid.
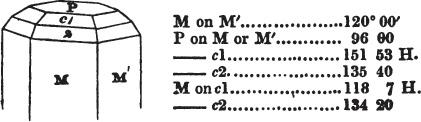
The fine white crystals of this species are as yet almost peculiar to that part of Vesuvius called Monte Somma, where they occur in cavities accompanied by garnet, hornblende, mica, and idocrase; it has been met with in the lava of Capo di Bove, near Rome; and, indistinctly crystallized, engaged in clinkstone at Katzenbuchel near Heidelberg.
ITTNERITE.
Gmelin. Leonhard.
Soda 11·29, potash 1·57, silica 30·17, alumina 28·40, lime 5·24, oxide of iron 0·62, sulphate of lime 4·89, muriate of soda 1·62, water and sulphuretted hydrogen 10·76.
Sp. Gr. 2·3. H. = 5·0—6·0.
Primary form the rhomboidal dodecahedron. Fracture imperfect conchoidal, passing into uneven. Colour bluish-grey or ash-grey; lustre resinous, inclining to vitreous. Before the blowpipe on charcoal it fuses per se with strong effervescence, and the disengagement of sulphurous acid, into an opake blebby glass, which the solution of cobalt colours blue; is imperfectly soluble in salt of phosphorus; with borax melts easily into a transparent colourless globule; and with soda is changed into an opake glass. It dissolves quickly in acids, forming with them a jelly.
This mineral, which bears much analogy with hauyne and sodalite, is found in basalt at Kaiserstuhl in the Brisgau. Beudant considers it a hydrous variety of nepheline.
[page] 133
ELAOLITE.*
Fettstein, W. Pierre Grasse, Levy.
| Contains Silica | 46·50 | 44·19 |
| Alumina | 30·25 | 34·42 |
| Lime | 0·75 | 0·52 |
| Potash | 18·00 | 4·73 |
| Soda | 0·00 | 16·88 |
| Oxide of iron | 1·00 | 1·36 |
| Water | 2·00 Klaproth. | 0·00 Gmelin. |
Sp. Gr. 2·54—2·62. H. = 5·5—6·0.
Primary form a right rhombic prism of about 112° 68′. It occurs massive, of a dark green, bluish-grey, or brick-red colour; translucent; lustre resinous; frequently opalescent when cut; cleavage both parallel and perpendicular to the axis of a four-sided prism; fracture conchoidal. It gelatinizes freely with acids when reduced to powder; and before the blowpipe fuses into a white enamel. It is found in Norway, imbedded in the zircon-syenite of Laurvig, Stavern, and Frederickswarn. The pale blue has a slight opalescence like cats-eye, whence it is occasionally employed for ornamental purposes.
NUTTALITE.
Nuttalite, Brooke. Nuttalit, L.
Contains potash 7·30, silica 37·81, alumina 25·10, lime 18·33, protoxide of iron 7·89, and water 1·50—Thomson.
Sp. Gr. 2·7—2·8. H. between 4·0 and 5·0.
In rectangular four-sided prisms, of a grey colour, imbedded in calcareous spar. Has considerable resemblance to scapolite, but is softer, and more vitreous in the fracture; it also possesses a play of light on the faces of the prism, similar to that of elaolite. Colour white, in some parts yellowish, in others bluish or green; the yellow portions transparent, the blue nearly opake. Streak white; lustre vitreous. Before the blowpipe it melts into a colourless glass, and with borax forms one which is blebby and pale white.
The locality of this mineral is Bolton, Massachusetts, where it occurs in coarse granular limestone, with epidote and titanium ore.
* From the Greek λαor, oil, and λlos, a stone , in allusion to its peculiar resinous or oily-like lustre.
[page] 134
SODALITE.*
Dodecahedral Kouphone Spar, M.
Combination of soda, silica, and alumina, with a small quantity of muriatic acid.
| Greenland. | Vesuvius. | |
| Soda | 25·00 | 26·55 |
| Silica | 36·00 | 35·99 |
| Alumina | 32·00 | 32·59 |
| Muriatic acid | 6·75 | 5·30 |
| Oxide of iron | 0·15 Eckeberg. | 0·00 Arfwedson. |
Sp. Gr. 2·37.
Its colour is white, light green, or bluish-green. It occurs massive, but more often crystallized in rhombic dodecahedrons, parallel to the planes of which it yields to mechanical division: the cross fracture is commonly conchoidal, with a vitreous lustre. It is translucent; and yields with difficulty to the knife. The varieties of this mineral comport themselves differently before the blowpipe on charcoal' that from Vesuvius per se suffers no change, except that its edges become rounded; while the Greenland variety melts, with intumescence and a rapid ebullition, into a colourless globule; with borax both varieties afford a diaphanous glass, fusing however in small quantity and with extreme difficulty.
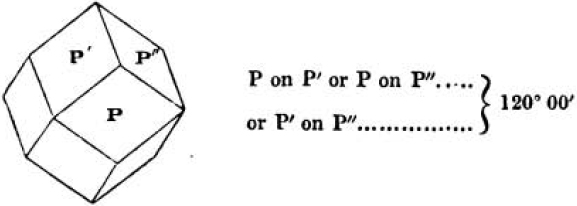
This mineral occurs in white translucent dodecahedral crystals of considerable magnitude, imbedded in or coating the cavities of certain volcanic rocks, with pyroxene, ice-spar &c. at Vesuvius; in the Kangerdluarsukfiord, West Greenland, of a green colour, both massive and crystallized, associated with eudyalite, augite, and arfwedsonite; and massive of a grey colour, imbedded in trap at the Kaiserstuhl in the Brisgau.
The Cancrinite of Hoffman, containing soda 24·47, silica 38·40, alumina 32·04, lime 0·32, loss 4·77, is evidently the same mineral. It is fusible in masses of an azure-blue colour, presenting six pretty distinct cleavages, which form together angles of 120°.
Occurs in the zircon rocks of Miask, near Ilmensee in Siberia.
* Sodalite, from its containing soda.
[page] 135
SPINELLANE.
Spinellane, H. Nosin, Leonhard.
Spinellane bears considerable analogy to sodalite, and by Mohs is arranged along with it. It contains
| Lake of Laach. | ||
| Soda | 19·0 | 16·56 |
| Silica | 43·0 | 38·50 |
| Alumina | 29·5 | 29·25 |
| Lime | 1·5 | 1·14 |
| Oxide of iron | 2·0 | 1·50 |
| Oxide of manganese | 0·0 | 1·00 |
| Sulphuric acid | 1·0 | 8·16 |
| Water | 2·5 Klaproth. | 3·00 Bergmann. |
Sp. Gr. 2·28.
Its crystalline form is the rhomboidal dodecahedron, sometimes elongated, like the sodalite from Vesuvius, into six-sided prisms with triedral terminations. Its colour is generally ash-grey, the crystals being small, though distinctly formed, and translucent: it cleaves with brilliant surfaces parallel to the faces of the dodecahedron. It scratches glass, but is brittle. Under the blowpipe it whitens, but does not melt.
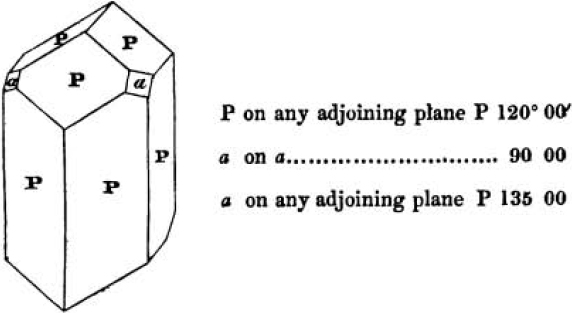
It occurs disposed in the drusy cavities of glassy felspar on the borders of the Lake of Laach, near Andernach on the Rhine, with quartz, hornblende, black mica, and magnetic iron.
[page] 136
PERILINE.
Heterotomous Feldspar, M. Periklin, Breithaupt.
Contains soda 9·99, potash 2·41, silica 69·91, alumina 18·93, lime 0·15, oxide of iron 0·48—from Zöblitz, by Gmelin.
Sp. Gr. 2·54—2·56. H.= 6·0.
Occurs in twin crystals, closely resembling those of albite.
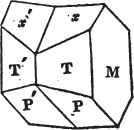
Cleavage perfect parallel to P and T; more so to T than to M, the reverse of which is the case in albite.
It occurs in large distinct crystals at St Gothard in Switzerland; in the Pfundersthal and Schmiernerthal in the Tyrol; upon the Sau-alpe in Carinthia, at Zoblitz in Bohemia, and other places. It is more generally opake than albite or felspar, and its specific gravity is lower.
LABRADORITE.
Labradorstein, W. Feldspath Opalin, H. Polychromatic Felspar, M.
Combination of silica, alumina, lime, soda, and oxide of iron.
| Labrador. | ||
| Silica | 55·75 | 55·00 |
| Alumina | 26·50 | 24·00 |
| Lime | 11·00 | 10·25 |
| Soda | 4·00 | 3·50 |
| Oxide of iron | 1·26 | 5·25 |
| Water | 0·50 Klaproth. | 0·50 Klaproth. |
Sp. Gr. 2·7. H. = 6·0.
Its primary form is an oblique rhombic prism of 119° and 61°. The cleavage parallel to the base is most perfect; lustre internally vitreous, pearly upon the perfect faces of cleavage; translucent when in thin fragments; colour grey, with opaline reflections of a blue, yellow, or brilliant red hue. Before the blowpipe on charcoal it fuses into a compact glass, whose fracture is brilliant; is scarcely affected by salt of phosphorus, unless reduced to powder, when it is decomposed into a skeleton of silica, and a glass which becomes opaline on cooling; and with borax fuses
[page] 137
slowly without effervescence into a diaphanous glass. When in powder it is entirely dissolved and forms a jelly with heated muriatic acid.
Rough indistinctly-formed crystals of considerable size were brought by Giesècké from Greenland; but the beautiful iridescent slabs to which the name of their locality is applied, are found at the island of St Paul on the coast of Labrador, associated with hornblende, hyperstene, and magnetic iron ore. It also occurs in Finland.
ALBITE.
Tetarto-prismatic Feldspar, M. Cleavelandite, Brooke and Levy. Tetartin, Breithaupt.
Combination of silica, alumina, and soda.
| Finbo. | Arendal. | Chesterfield. | |
| Silica | 70·48 | 68·04 | 70·68 |
| Alumina | 18·45 | 20·53 | 19·80 |
| Soda | 10·50 | 9·12 | 9·06 |
| Lime | 0·55 Eggertz. | a trace, Rose. | 0·23 Strom. |
Sp Gr. 2·6—2·68. H. = 6·0.
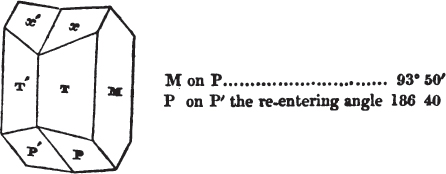
Primary form, an oblique rhombic prism. Generally in flat twin crystals, of which the face M is greatly enlarged. Colour commonly white, sometimes grey, green, or brown, varying from transparent to opake. Lustre pearly upon cleavage planes, vitreous in other directions. Cleavage perfect parallel to M and P, less so to T. Its comportment before the blowpipe resembles that of felspar.
Albite occurs in large transparent colourless crystals, with pearl-spar, in the Tyrol; and at St Gothard in white translucent twins, having a brilliant lustre; at Arendal with epidote and garnet; with eudyalite and hornblende in Greenland; and with tourmaline at Massachusetts; also in Siberia, Norway, Sweden, Bohemia, Oisans in Dauphine, and elsewhere on the continent. In the granite of the Mourne Mountains it is associated with felspar, from which however it may be distinguished by its superior whiteness and translucency. Felspar and albite indeed frequently occur in the same granite, as in that of Pompey's Pillar, and the block
[page] 138
on which the statue of Peter the Great in St Petersburg is placed, the albite presenting a greenish-white colour, while the felspar is flesh-red. The crystals from Baveno are often extremely curious in this respect, the albite being disposed in parallel position upon the faces of the felspar, from which its greater whiteness distinguishes it. Albite, more frequently than felspar, is one of the constituents of syenite and greenstone, as in the rocks around Edinburgh. Romé de l'Isle first distinguished it as a particular species under the name of white schorl; but it is to Dr G. Rose that mineralogists are indebted for their more accurate knowledge of its properties. (Manual.)
ANALCIME.
Kubizit, W. Analcime, H. Hexahedral Kouphone Spar, M.
Combination of silica, alumina, soda, and water.
| Fassa. | Kilpatrick. | |
| Contains Silica | 55·12 | 55·07 |
| Alumina | 22·99 | 22·22 |
| Soda | 13·53 | 13·71 |
| Water | 8·27—Rose. | 8·22—Connell. |
Sp. Gr. 2·2—2·53. H. = 5·5.
Analcime generally occurs in distinct crystals, either colourless and transparent; or white, grey, red, and opake. The cube is the primary form, there being occasional appearances of cleavage parallel to the planes of that solid; fracture imperfect conchoidal; lustre shining, and between pearly and vitreous. It becomes weakly electric* by friction. Alone on charcoal, before the blowpipe, it fuses without intumescence into a diaphanous glass; with borax is very difficultly soluble. It dissolves in acids, and when reduced to powder, forms a jelly with heated muriatic acid.
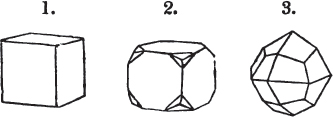
Fig 1, the primary; a cube. Fig 2, the same, of which each solid angle is replaced by three planes, thereby adding twenty-four planes to it. Fig. 3 represents the icositetrahedron, a crystal on which these twenty-four planes are increased to their utmost extent, so that no part of the primary planes is visible, and forming a solid bounded by twenty-four equal and similar trapeziums.
* Whence Analcime, from the Greek, in allusion to the feebleness of this property.
[page] 139
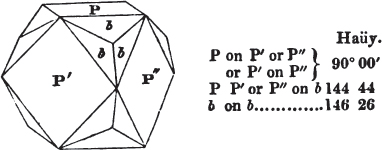
The most perfectly pellucid crystals of this mineral are brought from the Cyclopean Islands, near Catania; they, as well as most of those from the Tyrol, present the above form (the tri-épointé of Haüy); while the only crystallization met with in Dumbartonshire, Glen Farg, and other Scottish localities, is that of fig. 3. These in general are more or less white and opake, occurring in crystals three or four inches in diameter. At the Seisser Alp in the Tyrol, large individuals, extremely similar in appearance, are met with; and in the Faroe Islands, Iceland, several of the Hebrides, the Vicentine, and elsewhere, among the cavities of amygdaloidal, basaltic, and trap rocks, it is of frequent occurrence, associated with prehnite, chabasie, apophyllite, &c.
SARCOLITE.
Octahedral Kouphone Spar, Hoid. Sarcolithe de Thomson, H.
H. about 5·0.
Colour pale flesh-red, or brownish-white; semi-transparent; lustre and fracture vitreous; very brittle.
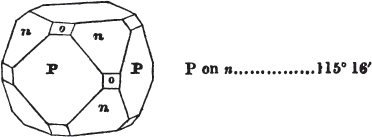
This mineral, from its hardness and vitreous aspect, was classed by Haüy with analcime; but the combination of the octahedron and cube, under which form it occurs, never having been observed in the latter substance, rendered their separation unavoidable. It is found among the anciently ejected debris of Vesuvius, associated with wollastonite, hornblende, and others of the zeolite family; being extremely brittle and full of flaws, it splits and falls to pieces unless carefully handled. It was discovered and named by the late Dr Thompson of Naples, and is designated by Monticelli, analcime carnea; the analysis, however, given both by him and Haüy, refers to Gmelinite. (Manual.)
[page] 140
PITCHSTONE.*
Pechstein, W. Petrosilex Resinite, H.
| Meissen. | Newry. | |
| Soda | 1·75 | 2·85 |
| Silica | 73·00 | 72·80 |
| Alumina | 14·50 | 11·50 |
| Lime | 1·00 | 1·20 |
| Protoxide of iron | 1·00 | 3·03 |
| Water | 8·50 Klaproth. | 8·50 Knox. |
The colours of this mineral are various shades of grey, blue, green, yellow, brown, and black, but they are not lively. Devoid of regular form or cleavage; it occurs massive, the structure sometimes slaty, occasionally curved; has a glistening resino-vitreous lustre, and an imperfectly conchoidal fracture, which is frequently the chief characteristic distinction between pitchstone and obsidian; almost always opake, or only translucent on the edges. Pitchstone is found extensively in the hills around the valley of Tribisch near Meissen in Saxony; also in the Isle of Arran, where it forms veins traversing granite; and in Ireland, near Newry, County Down, in smooth lamellar concretions of a mountain- or leek-green colour. When composed of roundish masses, imbedded in a vesicular matrix, pearl-stone is formed; these consist of concentric coats, and not unfrequently include a grain of obsidian. They form extensive beds in Hungary; also in Iceland, Spain, Mexico, and elsewhere.
PUMICE.
Bimstein, W.
Contains soda and potash 3·0, silica 77·5, alumina 17·5, oxide of iron 1·75.
Pumice is extremely porous, of a fibrous texture, and is harsh to the touch; its colour is grey, tinged with brown or yellow; it has a shining pearly lustre, is translucent on the edges, and very light. It fuses into a dirty-green blebby glass.
Pumice is closely connected with obsidian, being frequently interstratified with it, and at Lipari exhibiting every intervening stage of transition. At this locality it is sometimes compact, and then its lightness and freedom from humidity render it a peculiarly suitable building material; sometimes it is fibrous, its filaments having a peculiarly silky aspect; and frequently it presents the most delicate glassy texture, breaking into a million of
* From the resemblance of some of its varieties to pitch.
[page] 141
atoms on the smallest stroke with the hammer. At the northern extremity of the island of Lipari it forms a hill 800 or 1000 feet in height, which from its peculiar whiteness and scanty herbage is termed Il Campo Bianco. This and the isles of Ponza are the great deposits of the pumice known in commerce, and from these localities it is quarried and exported in large quantities; for though by no means an uncommon mineral in other volcanic countries, as in Hungary, the neighbourhood of Andernach on the Rhine, Teneriffe, Vesuvius, and Ischia, it occurs at these localities in small cinder-like masses, and is neither so massive nor so pure as at Lipari. (Manual)
OBSIDIAN.
Obsidian, W. Lave Vitreuse Obsidienne, H.
| New Spain. | Hecla. | Marekanite, | |
| Soda Potash | 10·0 | 1·6 6·0 | 7·00 |
| Silica | 72·0 | 78·0 | 77·50 |
| Alumina | 12·5 | 10·0 | 11·75 |
| Oxide of iron | 2·0 | 1·0 | 1·25 |
| Lime | 0·0 Descotils. | 1·0 Vauq. | 0·50 Klaproth. |
Sp. Gr. about 2·35. H. = 6·0.
Obsidian occurs in beds, in large masses, and in small grains; colour greenish- or brownish-black, or smoke-brown; possesses internally a shining vitreous lustre; fracture large conchoidal; some varieties are transparent, others nearly opake, or only translucent on the edges; is very brittle. It occasionally much resembles common glass. Iceland and the Lipari Islands are the most celebrated. localities of obsidian; though some remarkable varieties are likewise found in Ascension, Teneriffe, and many of the South Sea Islands, Siberia, and Mexico. The specimens from Iceland are almost opake, exhibiting a brownish tinge only on the thinnest edges, while those from Lipari are more transparent, and of a greyish colour. In Lipari the large continuous tracts of obsidian form the lower strata, while those varieties of a more pumaceous aspect occur invariably at a higher level. The purest, blackest, and most beautiful specimens, however, form imbedded nodules in the pumice at a great height, in masses from two inches to as many feet in diameter. Obsidian is frequently interspersed with small white opake globules, which, being formed in parallel lines, give it a stratified appearance; and some of the Lipari specimens closely resemble certain glass-house slags. A variety presenting a silky and chatoyant lustre is found in New Spain; and another of a transparent bottle-green hue, in detached masses, at Moldantheim in Bohemia. It frequently contains imbedded
[page] 142
crystals and grains of felspar and mica; and certain varieties also include specks of olivine, and traces of other volcanic minerals.
MAREKANITE occurs in the form of grains, of a pearly white, and consisting of thin concentric layers; it is found at Marekan* in the Gulf of Kamtschatka, and it possesses the general characters of obsidian.
SPHÆRULITE.
Sphærulite, J. Sphærulith, L.
Contains potash and soda 3·58, silica 79·12, alumina 12·0, oxide of iron 2·45, magnesia 1·10, water 1·76—Fucinus.
Sp. Gr. 2·4—2·54. H. = 6·5—7·0.
Occurs in roundish or spheroidal imbedded masses, whose surface is sometimes rough, sometimes quite smooth; colour brown, yellow, or grey; opake; no regular cleavage. It is almost infusible before the blowpipe, the edges only becoming covered with a sort of enamel.
This mineral is met with in round nodules imbedded in pitchstone at Spechtshausen, in Saxony; in radiated orbicular masses in ash-grey pearlstone at Glashutte, near Schemnitz in Hungary; in round balls which have a radiated fibrous structure, disposed in soft friable clay, which is evidently a decomposed rock, in the Shetland Islands; and in botryoidal masses of a bright yellow colour in Brittany. It was first distinguished by Breithaupt.
SAUSSURITE.
Prismatic Nephrite Spar, Hoid. Jade Tenace, H.
| Soda | 5·50 | 6·0 |
| Silica | 49·00 | 44·0 |
| Alumina | 24·00 | 30·0 |
| Lime | 10·00 | 4·0 |
| Magnesia | 3·75 | 0·0 |
| Oxide of iron | 6·50 Klaproth. | 12·5 Saussure. |
Sp. Gr. 3·2—3·4. H. = 5·5.
In masses of a greenish-white, mountain-green, or ash-grey colour; lustre pearly, inclining to vitreous on the faces of cleavage; resinous in compound varieties; cleavage in two directions, parallel to faces which meet at an angle of 120° nearly; is translucent on the edges, unctuous to the touch, and extremely tough. Before the blowpipe it fuses with difficulty into a white glass.
* Whence Marekanite.
[page] 143
It was first discovered by Saussure,* in rounded masses, on the edge of the lake of Geneva; it is peculiar to primitive mountains, as in Corsica, in Greenland, at Madras, and elsewhere, constituting with augite and hornblende the rocks called gabbro and euphotide.
SCAPOLITE.†
Scapolith, W. Paranthine, Wernerite, H. Pyramidal Felspar, M.
Combination of silica, alumina, lime, and soda.
| Pargas. | Arendal. | |
| Silica | 43·83 | 45·0 |
| Alumina | 35·43 | 33·0 |
| Lime | 18·96 | 17·0 |
| Soda | 0·00 | 1·5 |
| Water | 1·03 Nordenskiold. | 0·0 Laugier. |
Sp. Gr. 2·5—2·7. H. = 5·0—5·5.
Scapolite occurs in prisms of four or eight sides, sometimes terminated by tetrahedral pyramids; they are often aggregated laterally. The crystals yield to cleavage parallel to the sides, terminal planes, and both diagonals of a square prism. It also occurs massive. Its colours are grey or yellowish; sometimes deep red and opake; or various shades of green. It has a shining or somewhat pearly lustre; is translucent; never transparent; and generally presents a greenish colour, either pale and somewhat translucent, or dark and then the crystals are nearly opake. Before the blowpipe on charcoal, with a strong heat, it fuses, with violent intumescence, into a colourless semi-transparent mass; with borax, with effervescence, into a transparent glass.
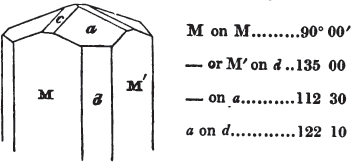
Scapolite occurs in primitive mountains, being met with in the iron mines of Arendal, in gneiss; in the mining district of Wermeland in Sweden; presenting large and beautiful crystals in the parish of Pargas in Finland; at Akudlek in Greenland, &c.
* In honour of whom it is named.
From the prismatic form of its crystals.
[page] 144
The variety termed Wernerite occurs principally at Arendal, in short thick crystals which have a granular composition, and present for the most part darker shades of colour; beautiful specimens are also brought from Greenland. Paranthine, including the more compact varieties, possesses pure white and pale-blue colours, and is met with in the limestone quarries of Gulsjö and Malsjö in Wermeland.
The Bergmannite of Schumacher, from Stavern in Norway, which occurs massive, and of a greyish-white or brick-red colour, is also supposed to be a variety of scapolite.
EKEBERGITE.
Ekebergite, Berzelius. Sodaite, Ekeberg.
Contains soda 5·25, silica 46, alumina 28·75, lime 13·50, protoxide of iron 0·75, water 2·25—Ekeberg.
Sp. Gr. 2·74.
In compact or finely fibrous masses, of a green, greyish, or brownish colour; occasionally in thin laminæ. Transparent; lustre vitreous or resinous; with difficulty acted upon by acids. Before the blowpipe, in the matrass, it yields a little water, without altering its appearance; on charcoal it whitens, loses its transparency, intumesces slightly, and melts into a blebby colourless glass. In borax or salt of phosphorus it fuses with effervescence; and in soda forms with considerable difficulty a greenish glass.
The above description is given by Necker, who however does not mention any locality. It appears to be nearly allied to scapolite.
PEKTOLITE.
Kobell.
Silica 51·30, lime 33·77, soda 8·26, potash 1·57, water 3·89, alumina and oxide of iron 0·90—Kobell.
Sp. Gr. 2·69. H. = 4·0—5·0.
Occurs in spheroidal masses, which have a columnar composition, and consist of delicate divergent fibres radiating from a centre; colour greyish; surface generally dull; opake; lustre pearly on the fracture; small fragments placed in muriatic acid, after several days are converted into a jelly; yields easily a white translucent glass when exposed to the action of the blowpipe.
It bears considerable resemblance to certain fibrous radiated varieties of mesotype. It forms large masses on Monte Baldo in the Southern Tyrol, and at Monzoni in the Fassa-thal.
[page] 145
CHABASIE.*
Schabasit,W. Chabasie, H. Rhombohedral Kouphone Spar, M.
Combination of silica, alumina, lime, and water, with a little soda and potash.
| Gustafsberg. | Faroe. | Kilmalcolm. | |
| Silica | 50·65 | 48·30 | 50·14 |
| Alumina | 17·90 | 19·28 | 17·48 |
| Lime | 9·73 | 8·70 | 8·47 |
| Potash and soda | 1·70 | 2·50 | 2·58 |
| Water | 19·50 Berzel. | 20·00 Arfwed, | 20·83 Connell. |
Sp. Gr. 20—2·1. H. = 4·0—4·5.
This mineral is found crystallized in the form of an obtuse rhomboid of 94° 46′ and 85° 14′ by measurements on the planes of cleavage with the reflective goniometer; it yields to cleavage parallel to the planes of the rhomboid, occasionally with brilliant surfaces. Colour white or greyish, sometimes pale-red superficially; transparent or translucent; scarcely hard enough to scratch glass. Alone it melts easily before the blowpipe into a spongy-like white enamel. Is not acted upon by acids.
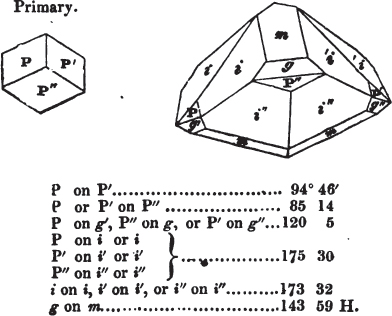
Chabasie is met with in the fissures or cavities of some basaltic rocks, or within geodes of quartz or agate which are disseminated in those rocks. It is thus found in large and very beautiful crystals in the amygdaloids of Faroe, Iceland, and Greenland, often associated with stilbite and green earth. Splendid specimens occur in a kind of greenstone rock (the graustein of Werner)
* From the Greek, signifying a particular species of stone.
G
[page] 146
at Aussig in Bohemia. Smaller but more transparent varieties are met with in the basalt of the Giant's Causeway; disposed on trap and accompanying stilbite at Kilmalcolm in Renfrewshire; in the Isle of Skye, and elsewhere in the west of Scotland; also at Gustafsberg in Sweden; in the agate balls of Oberstein in Deuxponts; and at Husavic in Iceland, in small transparent crystals filling the cavities of the fossil Venus Islandica. It does not occur massive.(Manual,)
LEVYNE.
Macrotypous Kouphone Spar, M. Levyne, Brewster, Journal, II. 332.
Combination of silica, alumina, lime, and water, with a little soda and potash.
| Silica | 46·30 |
| Alumina | 22·47 |
| Lime | 9·72 |
| Soda | 1·55 |
| Potash | 1·26 |
| Oxide of iron | 0·77 |
| Oxide of manganese | 0·19 |
| Water | 19·51—Connell. |
Sp. Gr. 2·0—2·2. H.= 40.
Primary form a rhomboid of 79° 29′. The colour of this species is white. Semi-transparent; lustre vitreous; streak white; cleavage indistinct, parallel to the faces of the primary; fracture imperfect conchoidal; brittle.
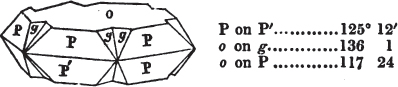
Upon charcoal it intumesces, and with salt of phosphorus yields a transparent globule, which contains a skeleton of silica, and becomes opake on cooling. In the glass tube it gives off a considerable quantity of water, whitens, and becomes opake; but is neither soluble in acids, nor does it gelatinize with them.
Sir David Brewster subjected this mineral to optical examination, and named it in compliment to Mr Levy, who had previously examined its crystallographic properties. It occurs disposed in cavities of trap, associated with acicular and radiated mesotype, at the Little Deer-park of Glenarm, county Antrim; also at Skagastrand in Iceland; at Dalsnypen in Faroe; Godhaven in Disco Island, Greenland; in the Vicentine; and, though rarely, in large reddish coloured opake crystals at Hartfield Moss in Renfrewshire.
[page] 147
TOURMALINE.
Tourmalin, W. Schorl, Br. Rhombohedral Tourmaline, M.
Combination of silica, alumina, oxide of iron, and lime, with small proportions of magnesia, potash, soda, and boracic acid.
Analyses by Gmelin.
| Blacky Rabenstein. | Devonshire. | Karinbricka. | Greenland. | |
| Soda | 1·75 | 2·09 0·00 | 2·53 | 315 |
| Potash | 0·48 | 0·22 | ||
| Silica | 35·48 | 35·20 | 37·65 | 38·79 |
| Alumina | 34·75 | 35·50 | 33·46 | 37·19 |
| Oxide of iron | 17·44 | 17·86 | 9·38 | 5·81 |
| Ox. of manganese | 1·89 | 0·43 | 0·00 | 0·00 |
| Boracic acid | 4·02 | 4·11 | 3·83 | 3·63 |
| Magnesia | 4·68 | 0·70 | 10·98 | 5·86 |
| Lime | 0·00 | 0·55 | 0·25 | 0·00 |
Sp. Gr. 3·0—3·2. H. = 7·0—7·5.
It occurs both in semi-crystalline prisms of irregular form and deeply striated on the surface, and in prisms of six or more sides, variously terminated, the two terminations being generally dissimilar. Sometimes these prisms are extremely short and thick, and at others are acicular or even capillary. Its colour is usually black, dark-green, or brown; the latter being generally translucent in one direction and opake in the other, the black altogether opake; externally the crystals are splendent. The primary form is considered to be an obtuse rhomboid of 133° 50′, and 46° 10′. Cleavage and fracture uneven and imperfect. It is not so hard as quartz. One of its remarkable characters is, that it becomes electric when heated, the termination which presents the greatest number of planes always exhibiting, according to Haüy, the positive or vitreous electricity, while that which consists of the smaller number indicates the negative or resinous. Before the blowpipe the black tourmaline of Bovey intumesces and becomes a black scoriaceous mass; with borax it fuses into a transparent glass.
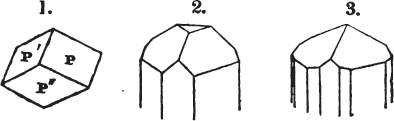
Fig. 1 represents the primary rhomboid, of which the summit is replaced by a triangular plane in fig. 2, as well as each lateral solid angle, by planes which form the ordinary six-sided prism of this mineral; the edges of the prism are modified in fig. 3.
[page] 148
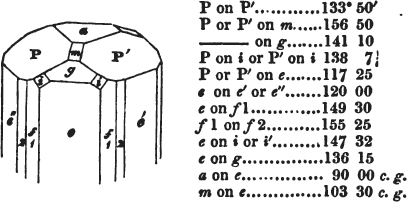
Tourmaline is confined to primitive rocks, such as gneiss, granite, mica-schiste, &c. and the veins which traverse these rocks. The largest and most striking black crystals occur in Greenland; at Hörlberg near Bodenmais in Bavaria; at Karinbricka in Sweden; and near Bovey in Devonshire, coating the cavities of red granite and associated with apatite; small brilliant black crystals, having much the aspect of tin ore, are met with imbedded in white quartz in Norway, at St Just in Cornwall, and in decomposed felspar at Andreasberg in the Hartz. Large curved crystalline prisms occur in granite at Portsoy in Banffshire; also in Norway, Saltzburg, the Tyrol, and Saxony,—the name Schorl, which is applied to this variety, being derived from the village of Schorlan in the latter country.
Tourmaline is also met with in pale yellowish-brown crystals imbedded in talc at Windisch-Kappel in Carinthia; in pale-green translucent: crystals in dolomite at St Gothard; of a dark pistachio-green colour in Brazil; and nearly pure white in Siberia and Switzerland. In Elba the crystals, when transparent, frequently exhibit parallel zones of distinctly different colours, being red at the two extremes, and dark-blue in the centre, or partly grass-green and partly azure-blue, &c. Tourmaline possesses the singular property of exhibiting different colours according as it is viewed parallel or perpendicular to the axis of its crystals, and almost invariably is less transparent in the first of these directions than in the last.
INDICOLITE. Tourmaline d'Uto. Blue tourmaline containing more oxide of iron than oxide of manganese.
| Uton. | Brazil. | |
| Silica | 40·30 | 40·00 |
| Alumina | 40·50 | 39·16 |
| Boracic acid | 1·10 | 4·59 |
| Oxide of iron | 4·85 | 5·96 |
| Oxide of manganese | 1·50 | 214 |
| Lithia | 4·30 | 3·59 |
| Water, &c. | 3·60 Arfwedson. | 1·58 Gmelin. |
In crystals of an indeterminate form and presenting an indigo-blue colour (hence its name). Alone before the blowpipe it
[page] 149
whitens, intumesces slightly, and becomes scoriaceous on the surface, but does not melt. With borax it fuses with effervescence, but more difficultly than rubellite.
Its principal locality is the iron mine of the island of Uton near Stockholm, where it occurs disseminated in a gangue of quartz, steatite, and felspar.
RUBELLITE. Siberite. Tourmaline Apyre, H. Tourmaline rouge. Tourmaline containing more oxide of manganese than oxide of iron.
| Moravia. | Siberia. | |
| Silica | 42·13 | 39·37 |
| Alumina | 36·43 | 44·00 |
| Boracic acid | 5·74 | 4·18 |
| Oxide of manganese | 6·32 | 5·02 |
| Lime | 1·20 | 0·00 |
| Potash | 2·41 | 1·29 |
| Lithia | 2·04 Gmelin. | 2·52 Gmelin. |
The rubellite presents various shades of red, from a slight tinge to a fine pink, and sometimes a violet colour. Its crystals are rarely distinct, being commonly closely aggregated. Alone on charcoal before the blowpipe it turns milk-white, intumesces, splits, vitrifies on the edges, but does not fuse; with borax it forms a transparent glass; on platina, with soda, it exhibits to an intense degree the green colour indicative of manganese. It occurs imbedded in lithomarge near Ekatherineburg in Siberia; accompanying lepidolite at Rozena in Moravia; and in granite with green tourmaline in Massachusetts, U.S. Some of the Siberian specimens exhibit internally a brown or blue colour, surrounded with carmine-red or some other lighter tinge, or internally a red hue bordered with pistachio-green.
MEIONITE.
Meionit, W. H.
Combination of silica, alumina, and lime, with some potash and soda.
| Vesuvius. | Sterzing. | ||
| Potash and soda | 0·82 | 2·4 | 0·89 |
| Silica | 40·53 | 40·8 | 39·92 |
| Alumina | 32·73 | 30·6 | 31·97 |
| Lime | 24·25 | 22·1 | 23·86 |
| Protoxide of iron | 0·18 Strom. | 1·0 Gmelin. | 2·24 Strom. |
Sp. Gr. 2·6—2·8.
It usually occurs in small four or eight-sided prisms, terminated by tetrahedral pyramids, the edges or angles of which are sometimes replaced; the primary form is a right prism with square bases, and it yields to cleavage parallel to the planes
[page] 150
M M of the following figure; in other directions the fracture is flat conchoidal. Its colour is whitish, or greyish-white, with a shining vitreous lustre; and it is translucent or transparent. It scratches glass. Alone before the blowpipe it intumesces and finally melts into a blebby colourless glass; with borax it dissolves slowly and with effervescence into a transparent glass; with salt of phosphorus yields a siliceous residue and a glass which becomes opaline on cooling; and with acids it gelatinizes.
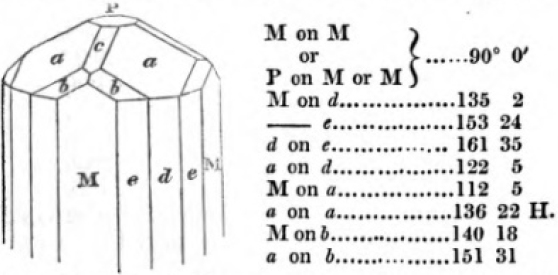
Its principal locality is Vesuvius, where it occurs in distinct crystals imbedded along with other volcanic minerals in the debris of ancient eruptions.
EDINGTONITE.
Hemi-pyramidal Feldspar, Haidinger.
According to Turner, it contains silica 35·09, alumina 27·69, lime 12·68, water 13·32, and, as he supposes, about 10 or 11 per cent, of some alkali.
Sp. Gr. 2·7—2·75. H. = 4·0—4·5,
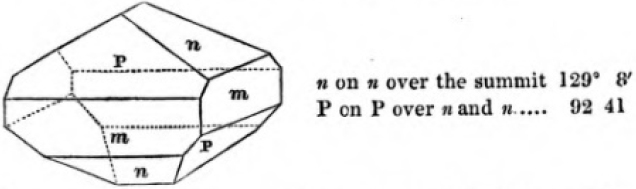
Occurs in small extremely distinct greyish-white translucent crystals, whose form, according to Haidinger, is hemi-pyramidal. Lustre vitreous; streak white; brittle. Cleavage perfect parallel to m. Before the blowpipe it fuses into a colourless mass, though a pretty strong heat is necessary for that purpose. It yields water when exposed to high temperatures, and becomes at the same time opake and white.
Edingtonite was remarked by Haidinger on a specimen of.
[page] 151
Thomsonite from Dumbartonshire, in the collection of Mr Edington of Glasgow. Its peculiar crystalline form, specific gravity, and hardness, are sufficiently characteristic, although from its extreme scarcity a satisfactory analysis has not yet been made.
KROKYDOLITE.*
Krokydolite, Haussman. Blau Eisenstein, Klaproth.
Of this there are two varieties; one like asbestus, the other fibrous.
| Asbestus. | Fibrous. | |
| Soda | 7·03 | 7·11 |
| Silica | 50·81 | 51·64 |
| Protoxide of iron | 33·88 | 34·38 |
| Oxide of mangan. | 0·17 | 0·02 |
| Magnesia | 2·32 | 2·62 |
| Water | 5·58 | 4·01 |
| Lime | 0·02 Stromeyer. | 0·05 Stromeyer. |
Sp. Gr. 3·2—3·39. H. above 4·0.
Colour lavender- or indigo- blue; streak lavender-blue or leek-green. Occurs in fibrous masses which are flexible and elastic like asbestus, and compact; opake; lustre silky; not magnetic; not affected by water or acids; minute fibres are fusible at the flame of a spirit lamp; the mineral itself melts before a strong red heat into a black opake magnetic globule. With borax it forms easily a transparent green-coloured glass.
Its locality is the Orange River in Southern Africa.
ACHMITE.†
Acmite, Akmit, Haid. Achmit, Strom.
Combination of soda, silica, and peroxide of iron.
Soda 10·40, silica 55·25, oxide of iron 31·25, oxide of manganese 1·08, lime 0·72—Berzelius.
Sp. Gr. 3·5. H. = 6·0—6·5.
Primary form an oblique rhombic prism of 86° 57′, and 93° 3′. Opake; when in thin fragments translucent, and exhibiting a yellowish-brown tint; lustre vitreous; streak pale yellowish-grey; cleavage distinct parallel to M, less so parallel to r, l, and s;
* From αζoxus, like wool, on account of its divisibility into minute threads.
† From αxμη, a point, from the form of its crystals.
[page] 152
fracture imperfect conchoidal; surface of r irregularly striated longitudinally.
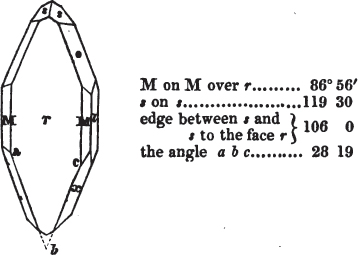
Frequently macled parallel to r. Alone before the blowpipe achmite fuses readily into a brilliant black globule; with borax forms a glass coloured by iron; and when reduced to powder is acted upon by the sulphuric and muriatic acids.
This is a rare mineral, its only known locality being Rundemyr, near Kongsberg, in Norway. It there occurs in crystals sometimes a foot long, imbedded in granite, which, however, from their frangibility, are not easily disengaged entire.
CUMMINGTONITE.
Thomson.
| Contains Soda | 8·44 |
| Silica · | 56·54 |
| Protoxide of iron | 21·67 |
| Protoxide of manganese | 7·80 |
| Loss from heat | 3·18—Thomson. |
Sp. Gr. 3·20. Readily scratched by the knife.
This mineral occurs in fine needles, forming tufts of crystals, which diverge slightly from one another. Colour greyish-white. Lustre silky. Opake, or translucent only on the edges. Alone it does not melt before the blowpipe; with soda it effervesces and fuses into a dark coloured globule; and with borax forms a black glass, indicating the presence of much iron and manganese. It is found at Cummington in Massachusetts in a granitic rock. By most mineralogists it is united with epidote.
[page] 153
EUDYALITE.*
Composed of silica, soda, zirconia, lime, the oxides of iron and manganese, muriatic acid, and water.
| Soda | 13·92 | 13·82 |
| Silica | 52·47 | 53·32 |
| Zirconia | 10·89 | 11·10 |
| Lime | 10·14 | 9·79 |
| Oxide of iron | 6·85 | 6·75 |
| Oxide of manganese | 2·57 | 2·06 |
| Muriatic acid | 1·03 | 1·03 |
| Water | 1·80 Strome | ever. 1·80 Strom lever. |
Sp. Gr. 2·89. H. = 5·0—5·5.
Eudyalite occurs both massive and crystallized, but the crystals are generally small and irregular. The following figure, however, is taken from a very perfect crystal nearly an inch in diameter, which was brought from Greenland by Giesècké.
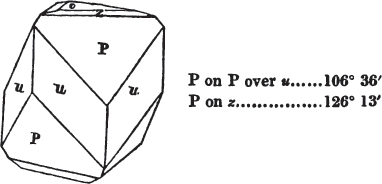
Its colour is red or brownish-red, and generally somewhat translucent; lustre vitreous, cleavage parallel to o very perfect, less so parallel to z; fracture conchoidal or uneven. Before the blowpipe it fuses into a leek-green scoria; and when reduced to powder loses its colour, and gelatinizes with acids. This mineral was distinguished by Stromeyer; its only locality is Kangerdluarsuk in West Greenland, where it occurs either accompanying sodalite and hornblende, or imbedded in compact white felspar.
* Eudyalite from the Greek, in allusion to its ready solubility in acids.
G 2
[page 154]
ACIDS.
OF the acids only two have been found in the concrete state, viz. the sulphuric and boracic acids.
NATIVE SULPHURIC ACID.
This acid has been found in its natural state in the caverns of the volcanic mountain Zaccolino, near Sienna. The concretions are in the form of cauliflowers, depending from the roofs of the grottoes, and adhering to sulphate of lime. Professor Pictet mentions a cavern near Aix in Savoy, from the roof of which this acid mixed with water and a little sulphate of lime is observed to drop. It was also noticed by Dolomieu in the caverns of Etna.
NATIVE BORACIC ACID.
Sassoline, J. Prismatic Boracic Acid, M.
The pure varieties consist of borax 25·83, and oxygen 74·17—Berzelius.
Sp. Gr. 1·48.
In loose scaly particles, or crystalline grains (probably six-sided tables), sometimes aggregated in the form of crusts. Colour greyish or yellowish-white. The latter arising from admixture with sulphur. Lustre pearly; taste acidulous, and slightly bitter. It fuses readily at the flame of a candle, and yields a transparent glassy globule, which becomes opake on cooling if there be any gypsum in union. When dissolved in alcohol it communicates to the flame a fine green tint.
It occurs in a state of perfect purity, or mechanically mixed with a little sulphur, at the island of Volcano, one of the Lipari group; sometimes massive and in incrustations which present a fibrous structure; and frequently pulverulent, and disposed as a loose covering on the surface of the sulphur. It is likewise deposited by some of the lagoni of Tuscany, and at the hot springs of Sasso, a locality which has procured for it the trivial name of Sassoline.
[page 155]
ACIDIFEROUS EARTHY MINERALS.
UNDER this head are comprehended those minerals which chiefly consist of an earth combined with an acid; some of them include variable proportions of oxide of iron, which may be considered only as an incidental ingredient.
SUBSULPHATE OF ALUMINA.
Aluminite, J. Websterite, Levy. Alumine Sous-sulfatée.
Combination of sulphuric acid, alumina, and water.
| Halle. | Newhaven. | Auteuil. | |
| Sulphuric acid | 23·36 | 23·27 | 23·0 |
| Water | 46·33 | 46·76 | 47·0 |
| Alumina | 30·26 | 29·87 | 30·0 |
| Stromeyer. | Dumas. | ||
Sp. Gr. 1·669.
In reniform masses and botryoidal concretions, of a white or yellowish-white colour, occasionally translucent, but more frequently dull and opake, with an earthy fracture; it yields to the nail, is meagre to the touch, and adheres to the tongue. It fuses with difficulty before the blowpipe, but dissolves readily in acids, without effervescence. It imbibes water, but does not in consequence fall to pieces.
It occurs imbedded in ferruginous clay, which rests on the chalk strata on the coast near Newhaven in Sussex; also at Epernay in France, and in plastic clay at Halle on the Saale in Prussia.
[page] 156
SULPHATE OF ALUMINA.
Sulphate of Alumina, Boussingault. Alunogene, Beudant.
| Contains Sulphuric acid | 36·4 | 35·87 |
| Alumina | 160 | 14·64 |
| Water | 46·6 | 46·37 |
| Peroxide of iron | 04 | 0·50 |
| Soda | 0·0—Boussin. | 2·26—Thomson. |
Sp. Gr. 1·66. Very soft.
In crystalline masses and efflorescences. Colour white, occasionally tinged yellow when impure; translucent; lustre silky; taste, and comportment before the blowpipe, similar to alum.
Humboldt observed this mineral in clay-slate at Araya near Cumana; also at Socono, and elsewhere in South America.
WAVELLITE.*
Sub-phosphate of Alumina. Alumine Hydro-phosphatée, H. Lasionite, Devonite, Fuchs. Wavellit, Klaproth. Hydrargillite, Davy.
Combination of phosphoric acid, water, and alumina, with a little lime, the oxides of manganese and iron, and fluoric acid.
| Phosphoric acid | 33·40 | 34·72 |
| Alumina | 35·35 | 36·56 |
| Water | 26·80 | 28·00 |
| Lime | 0·50 | 0·00 |
| Oxide of iron and mangan. | 1·25 | 0·00 |
| Fluoric acid | 2·06 Berzelius. | 0·00 Fuchs. |
Sp. Gr. 2·337. H.= 3·5—4·0.
Primary a right rhombic prism of 122° 15′ and 57° 45′.
It occurs in minute crystals, which usually adhere together and radiate, forming hemispherical or globular concretions from a very small size to that of an inch in diameter. The general form of the crystals is that of a rhombic prism with diedral terminations, but the lateral edges of the prism are sometimes replaced; and they are rarely sufficiently distinct to admit of measurement. It cleaves parallel to M and h, with brilliant surfaces; commonly translucent, sometimes opake, and possessing a silky or vitreous lustre; colour yellowish-white, yellow, greyish, green, or bluish; occasionally of a dingy brown, owing to the progress of decomposition; brittle. It is infusible, but under the blowpipe becomes white, opake, and loses its crystalline form, giving a slight greenish tinge to the flame. It is soluble in heated acid, without effervescence, emitting a vapour which slightly corrodes the glass.
* Wavellite, in honour of Dr Wavel, its discoverer.
[page] 157
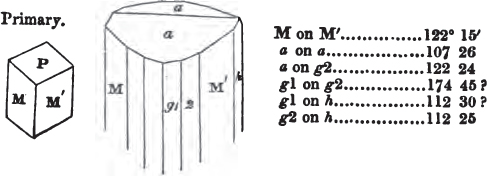
It was first discovered by Dr Wavel in small veins and cavities in argillaceous schiste, near Barnstaple, in Devonshire; it has been since found at Stenna Gwyn, near St Austle in Cornwall, on a decomposing granite; in very highly coloured pistachio-green masses at Clonmell, and near Cork; in white stellated groups on red sandstone at Zbirow, in Bohemia; on brown iron ore at Amberg, in Bavaria (a variety named by Fuchs Lasionite); in the Shaint Isles of Scotland; and near Villa Ricca in Brazil. The Striegisan of Breithaupt, from Striegis in Frankenberg, is evidently a variety of wavellite.
KAKOXENE.*
Steinmann, in Brewster's Journal, v. 163.
Combination of phosphoric acid, water, fluoric acid, alumina, peroxide of iron, silica, and a small quantity of lime.
Phosphoric acid 17·86, alumina 10·01, silica 8·90, peroxide of iron 36·82, lime 0·15, water and fluoric acid 25·95—Steinmann.
Sp. Gr. 3·38.
In extremely minute fibrous crystals, which appear to be irregular six-sided prisms terminated by pyramids of six faces, generally disposed in diverging groups radiating from a point. Colour brownish-yellow of different hues; lustre silky, sometimes adamantine; adheres to the tongue; has an argillaceous odour, and—probably from an accidental admixture with some saline substance—has an astringent taste. In water it partly loses its lustre and becomes brown; and when placed on a red coal it emits a green phosphoric light. Before the blowpipe on charcoal it decrepitates powerfully; with borax is incompletely soluble into a dark bottle-green coloured glass; and with soda fuses with difficulty into a blackish mass.
* From ϰαϰОs, bad, and Ξινοs, a guest, in allusion to the bad influence of its phosphoric acid on the iron extracted from the ore with which it occurs.
[page] 158
Kakoxene occurs disposed on brown iron ore in the iron mines of Hrbeck near Zbirow in Bohemia; and, but for its deeper tint, might be readily mistaken for karpholite, which is found under similar circumstances.
AMBLYGONITE.*
Amblygonite, Leonhard. Amblygonic Augite Spar, Haidinger.
Combination of phosphoric acid, alumina, and lithia.
Phosphoric acid 54·12, alumina 38·96, lithia 6·92—Berzelius.
This mineral occurs massive, and in rhombic prisms of 106° 10′ and 73° 50′, which are rough externally, and present a greenish-white, a mountain or sea-green colour. It cleaves parallel to the sides of the prism with brilliant surfaces; and when reduced to thin laminæ, it varies from translucent to transparent. On charcoal it fuses readily into a clear glass, which becomes opake on cooling; with borax it melts into a transparent colourless glass.
It is found, with tourmaline and topaz, in granite, at Chursdorf near Penig in Saxony; and at Arendal in Norway.
CHILDRENITE.
Childrenite, Levy.
A compound of phosphoric acid, alumina, and iron, according to Wollaston.
H. 4·5—5·0.
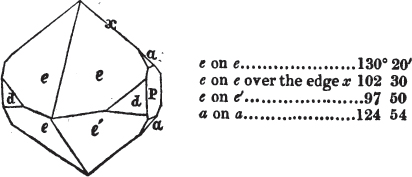
In very minute yellow or brownish-yellow crystals, disposed either singly or in crystalline coats on carbonate of iron or quartz. Cleavage in planes parallel to the axis; lustre vitreous, inclining to resinous; translucent; streak white; fracture uneven. The only known locality of this mineral is the vicinity of Tavistock in Devonshire; it was distinguished by Levy, who named it in compliment to Mr Children of the British Museum. (Manual.)
* From the Greek, in allusion to the obtuse angles of its prism.
[page] 159
AZURITE.
Lazulit, W. H. Azurite, J. Prismatic Azure Spar, M. Klaprothine, Beudant.
Combination of phosphoric acid, alumina, magnesia, and water.
| Krieglach. | Radel-graben. | |
| Phosphoric acid | 43·32 | 41·81 |
| Alumina | 34·50 | 35·73 |
| Magnesia | 13·56 | 9·34 |
| Lime | 0·48 | 0·00 |
| Oxide of iron | 0·80 | 2·64 |
| Silica | 6·50 | 2·10 |
| Water | 0·50—Brandes. | 6·06—Fuchs. |
Sp. Gr. 3·0—3·1. H. = 5·0—6·0.
Primary form a right rhombic prism of 121° 30′.
Lazulite rarely occurs crystallized, being more often granular, or in small fragments, exhibiting various shades of azure-blue. It is slightly translucent; brittle, yet nearly as hard as quartz; the fracture is lamellar, and its cleavage is parallel with the planes of the prism, though indistinct. Before the blowpipe it intumesces a little, and assumes a glassy appearance where the heat has been highest, but does not melt. With borax it yields a clear colourless globule.
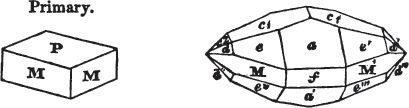
The second of the above figures represents a superb crystal in the possession of H. J. Brooke, Esq.
| M on M' | 121° | 30′ |
| — e or M′ on e′ | 138 | 45 |
| M on d | 140 | 30 |
| a on a′ | 91 | 30 |
| — cl or cl′ | 129 | 10 |
| a on e or e′ or a′ on e″ or e‴ | 158 | 10 |
| cl on cl′ | 120 | 40 |
| cl on c2′ or cl′ on c2′ | 150 | 00 |
| e on d or e′ on d′ | 162 | 36 |
| cl on e. or cl′ on e′ | 139 | 25 |
| cl on d | 141 | 20 |
It occurs in a gangue of quartz, near Vorau in Styria; and in narrow veins traversing clay-slate in the torrent beds of Schlamming and Radel-graben near Werfen in Saltzburg.
[page] 160
CARBONATE OF LIME.
The numerous varieties comprehended under the term carbonate of lime, differ greatly in external character. Scarcely more can be said of them in the general, than that they all readily yield to the knife, and that their specific gravity is below 3·0.
1. CALCAREOUS SPAR. Kalkspath, W. Chaux carbonatée, H. Spath calcaire, Br. Calc Spar, J.
Calcareous spar is pure carbonate of lime, consisting of Iceland.
| Lime | 56·15 | 55·50 | 56·33 |
| Carbonic acid | 43·70 Strom. | 44·00 Phillips. | 43·05 Biot. |
Sp. Gr. 2·5—2·8. H. = 3·0.
Its most prevalent colour is white; it is frequently transparent, and is then strongly doubly refractive. Occurs crystallized in upwards of 800 varieties of form, all originating from an obtuse rhomboid of 105° 5′ and 74° 55′; this rhomboid may readily be obtained by cleavage, and may itself occasionally be cleaved parallel to a plain passing through the greater diagonals in one direction; the brilliant surfaces of the primary are well adapted to the use. of the reflective goniometer. It often occurs both chemically and mechanically united with minute proportions of iron, magnesia, alumina, carbon, bitumen, &c.; cross fracture occasionally conchoidal, but not easily obtained. It effervesces violently with acids. The Iceland variety, which is considered to be the purest form of carbonate of lime, is transparent, and doubly refractive in a high degree, hence its familiar appellation of Iceland spar, or doubly refracting spar. Some varieties of calcareous spar give a yellow phosphorescent light when laid on a hot coal or struck in the dark; as that accompanying garnet in Wermeland; laumonite in Brittany, &c. Alone, on charcoal, before the blowpipe, it becomes caustic by heat, and shines with peculiar brightness as soon as all the carbonic acid is expelled. Does not yield water in the matrass, but with the fluxes comports itself like arragonite.
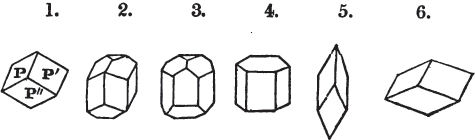
Fig. 1, the primary, an obtuse rhomboid. Fig. 2, the same, of which the lateral edges and terminal solid angles are replaced by planes. Fig. 3, in this both the lateral and terminal solid angles are replaced. Both this and the former figure tend, by the extension of the modifying planes, to the production of the six-sided prism (fig. 4), on which no portion of
[page] 161
the primary planes is visible. Fig. 5, an acute rhomboid. Fig. 6, a rhomboid more obtuse than the primary.
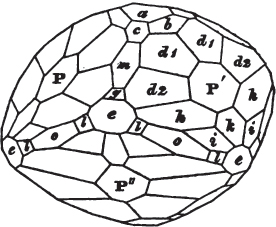
Bournon described fifty-six modifications of the rhomboid of carbonate of lime, and other mineralogists have greatly increased the number. It would be perhaps impossible to represent the whole of these with any tolerable accuracy on one figure; the above figure therefore is intended only to point out the fact, that the several modifications are referable to three great classes, viz. prisms, acute rhomboids, and obtuse rhomboids. Thus the planes a and e e e, replacing the solid angles, tend, by their extension, to produce a six-sided prism represented by the small fig. 4; the plane a with the planes o o o replacing the lateral edges, likewise tend to produce a regular six-sided prism, while a in conjunction with the planes llllll tend to a twelve-sided prism. Thus also of rhomboids, the plane b situated on the primary plane, and c on the edge, tend to two rhomboids much more obtuse than the primary, or than that which would be consequent on the extension of the planes m, while g and k would produce very acute rhomboids. The planes d1 d1, of which six are visible on the figure, would produce very obtuse dodecahedrons; the planes d2 d2, less obtuse; while the consequence of the extension of the planes h h would be acute dodecahedrons, and of the planes i i still more acute. But of rhomboids, both acute and obtuse, there is an almost endless variety, all actually differing by admeasurement.
It occurs in veins in almost every kind of rock, from the oldest to the newest alluvial strata, and accompanies or constitutes the gangue of a great variety of minerals. It is so generally distributed, that any enumeration of its localities would be impossible. Among those most distinguished may be mentioned Andreasberg in the Hartz, where the six-sided prisms have been found in great beauty; Alston Moor in Cumberland, which affords the flat rhombic crystals; and Derbyshire, whence the pale-yellow transparent pyramids, sometimes of very large dimensions, are obtained. The transparent variety from Iceland is not found in distinct crystals, although the surfaces of the masses indicate crystallization, and are often implanted with stilbite and heulandite.
2. SCHIEFER SPAR.* Schiefer-spath, W. Chaux carbonatée nacrée, H. Slate-spar, J. This variety occurs massive, and in
* Schiefer or slate-spar, in allusion to its slaty structure.
[page] 162
extremely thin tabular plates intersecting each other in various directions, but without any determinate crystalline form. Its colour is usually white, with a shining and more or. less pearly lustre; it is translucent, yields easily to the knife, and often possesses a greasy feel. Specific gravity about 2·5. It is infusible; but is soluble with effervescence in acids. It is an almost pure carbonate of lime.
It occurs in metalliferous beds in Norway; in Glen Tilt, Perthshire; in Assynt, Sutherlandshire; and in the county of Wicklow, in Ireland.
4. AGARIC MINERAL.* ROCK MILK. Berg milch, W. Chaux carbonatée spongieuse, H. Is of a white colour, or yellowish or greyish-white; and is soft, dull, meagre to the touch, soils the fingers, is very tender, opake, and so light as to float for a short time on water. It is nearly pure carbonate of lime.
It is found in beds and crevices of calcareous rocks in Switzerland, where it is employed for white-washing the houses; also near Ratisbon; at Sunderland in Durham; and in Oxfordshire.
5. APHRITE.† EARTH-FOAM. Schaumerde, W. Chaux carbonatée nacrée lamellaire, H. Ecume de Terre, Br. This variety is found sometimes solid, more often in a friable state; it consists of white scales of a shining pearly or pseudo-metallic lustre. It is opake, very soft to the touch, and is nearly pure carbonate of lime. It is usually found in calcareous rocks in veins or cavities; and differs from schiefer-spar principally in being less coherent. It occurs in Hessia, and abundantly at Eisleben in Thuringia, in mountains consisting of stratified limestone.
6. STALACTITIC CARBONATE OF LIME. Kalk-sinter, W. Chaux carbonatée concretionnée, H. Calc sinter, J. Occurs mamillated, or in long straight pendulous masses or tubes, coating the interior of caves and fissures. The fracture is either lamellar or fibrous, the fibres diverging from the centre; the cleavage always that of the perfect rhomb; with a pearly or silky lustre; prevalent colour yellowish-white.
Stalactites are sometimes of prodigious dimensions, of which the grotto of Antiparos in the Archipelago, the extensive caves of Adelsberg in Carniola, and that of Auxelle in France, are striking instances. The most remarkable in Britain are to be found in the cavern of Castleton, and other caves in Derbyshire, and Macallister Cave in the Isle of Skye.
Stalactites are now continually forming. They are deposited from water loaded with particles of carbonated lime, in the hollows and caverns of mountains; the water, finding its way into these through crevices in the roof, becomes exposed to the air,
* Described by Pliny under the name of Agaricon;—resembling fungus.
† Aphrite, from the Greek;—a foam-like substance.
[page] 163
evaporation ensues, and thus the calcareous particles are caused to precipitate. Some caverns have been entirely filled with calcareous stalactite, so that it is occasionally obtained in large masses; in this state it is called Alabaster, and is used in statuary and in the formation of vases; its name being derived from Alabastron, an Egyptian village between the Nile and the Red Sea, which was the principal locality known in ancient times.
7. GRANULAR LIMESTONE. Kalkstein, W. Chaux carbonatée saccharoïde, H. Granular limestone is massive, and consists of small grains or minute crystals, presenting a lamellar structure and brilliant lustre; but as these grains intersect each other in every direction, the lustre of the mass is only glimmering. It is of various colours; white, grey, yellow, bluish, reddish, greenish, &c. and is sometimes veined or spotted; fracture splintery, occasionally slaty, in consequence of containing parallel layers of mica; somewhat translucent, and brittle.
Granular limestone is found in many, if not in most primitive countries; it sometimes forms entire mountains, but more often occurs in beds. It is considered to be of contemporaneous formation with gneiss, porphyry, argillaceous and micaceous schiste, with which it frequently alternates. In the Alps and the Pyrenees examples of this are of frequent occurrence.
The whitest and more esteemed primitive limestone was termed by the French mineralogists Chaux carbonatée saccharoïde, from its likeness to sugar when in small masses. From its important uses in the arts, it is commonly called Statuary marble. The most celebrated statuary marbles of ancient times were found in the islands of Paros, Naxos, and Tenos, in the Archipelago. Parian marble is white, large grained, and considerably translucent. The Pentelicon, taken from quarries on a mountain called Pentelicus, near Athens, is traversed by greenish or greyish veins, which are commonly micaceous. The marble of Carrara has a finer grain and closer texture, and is that now usually employed by statuaries; the quarries of this marble are on the eastern coast of the Gulf of Genoa, and are worked on the face of a mountain to the height of about 800 feet.
The name Lucullite, as applied to black marble, arose from the quantity of that colour which Lucullus imported into Rome, from an island in the Nile. That from Kilkenny in Ireland encloses shells of a whitish colour, which, when the marble is cut and polished, present segments of circles; this is much used for chimney-pieces and ornaments.
The Verd antique consists of carbonate of lime imbedded in green serpentine: its geological situation is not known.
The Lumachelli marble exhibits beautiful iridescent colours, which are sometimes prismatic internally, but more commonly of various shades of red or orange, whence it has also obtained
[page] 164
the name of Fire marble. It occurs at Bleyberg in Carinthia in beds forming the roof of a lead mine. Its colours are attributed to the shells of a variety of nautilus.
The Cotham, Ruin, or Landscape marble, found near Bristol, exhibits, when cut and polished, the appearance of a landscape or ruins; it is of common occurrence in the Val d'Arno near Florence.
8. ANTHRACONITE, Swinestone, Stinkstone,* which emits a strong fetid odour when scraped, owing, it is believed, to the presence of sulphuretted hydrogen, is found columnar, granular, and compact, and of various shades of grey, brown, and black. The harder and more compact varieties, which receive a good polish, are used in ornamental architecture.
In Dalmatia a variety of limestone occurs so bituminous that it may be cut like soap, and is employed in the construction of houses; when finished, the walls are set fire to; the bitumen burns out, and the stone becomes white; the roof is then put on, and the house afterwards completed.
9. OOLITE† is always found massive, and in beds. The globular particles are sometimes composed of concentric lamellæ, and usually adhere by means of a calcareous cement; it is soft when first quarried, but hardens by exposure to the air. Its colour is whitish, yellowish-white, or ash-grey, depending, as is believed, on the quantity and quality of the argillaceous matter with which it is usually combined. It is an impure carbonate of lime, and will not burn into quicklime. The Portland and Bath stones are varieties of this, and in many parts of the south of England its properties are well known as a building material.
10. PISOLITE or Pea-stone,‡ differs considerably from oolite. It is generally white, brown, or reddish, and is composed of round or spheroidal masses, from the size of a pea to that of a hazel nut, imbedded in a calcareous cement. These masses always consist of concentric lamellæ, in the midst of which is commonly found a grain of sand. It is opake, soft, and brittle. At Klagenfurt in Carinthia, and at Carlsbad in Bohemia, it occurs in great quantities, the mineral waters in the vicinity of the latter rising from beds of pisolite.
11. CHALK. Kreide, W. Craie, H. Is a massive opake carbonate of lime, of a white, greyish, or yellow colour, having an earthy fracture and a low specific gravity. It varies much in hardness, but is generally soft to the touch, and adheres to the tongue. It composes a large portion of the newest secondary
* From the strongly fetid odour it gives out when rubbed.
† Oolite, or Roe-stone; so denominated from the resemblance between the little round masses of which it is composed, and the roe of a fish.
‡ Pisolite or Pea-stone, from the similarity of its spherical masses to the pea.
[page] 165
rocks in the south of England, and contains abundance of marine as well as terrestrial organic remains. Its uses are well known, in furnishing lime for manure and cement, in polishing metals and glass, as a marking material, and in painting and white-washing.
12. MARL is a mixture of limestone and clay, possessing an earthy fracture, a greater or less degree of compactness, and a yellow or reddish-grey colour. It falls to pieces on exposure to the air, and is then plastic in water; it is partially soluble in acids, with violent effervescence. It occurs in considerable quantity in Thuringia, and is produced by the decomposition of shells in bogs and standing water.
13. TUFA, Kalk Tuff, W. is the most impure, the most irregular, and the most porous of all the varieties of carbonate of lime, being an alluvial deposit from calcareous springs. Immense formations of this substance have taken place near Terni, Tivoli, and other places in Italy; also in some parts of Germany. From its generally occurring in a soft state, and its possessing the property of hardening on exposure to air and moisture, tufa makes a useful building material in the construction of bridges and docks. It varies in respect of hardness, is opake, rough, light, cellular, and often incrusts other substances, as vegetable stems, leaves, &c.
ARRAGONITE.*
Arragon, W. Arragonite, H. Prismatic Lime Haloide, M.
Combination of carbonic acid and lime, with generally a small proportion of carbonate of strontites and water.
| Arragon. | Waltsh. | |
| Carbonate of lime | 94·82 | 99·29 |
| Carbonate of strontites | 4·08 | 0·51 |
| Water | 0·98 | 0·15 |
| Stromeyer. |
Sp. Gr. 2·6—3·0. H. = 3·5—4·0.
This mineral occurs massive, the texture being generally fibrous, with a silky lustre; in the form of small branches consisting of fibrous crystals which diverge from a centre,—a variety known under the denomination of Flos-ferri; also in crystals which at first sight appear to be regular six-sided prisms, but on close inspection present a longitudinal crevice down each lateral face, and somewhat similar appearances converging in the centre of the terminal planes; these, in fact, are macles consisting of three simple crystals which cross each other
* Arragonite, from its having been first found in the province of Arragon in Spain.
[page] 166
at particular angles. It cleaves parallel to the lateral planes of a right rhombic prism of 116° 5′ and 63° 55′,—the primary form. Most prevalent colour white, though sometimes tinged yellow, green, and blue. The crystals are internally shining or vitreous; they are translucent—the small ones sometimes colourless and transparent; yield to the knife and are brittle, but scratch calcareous spar easily. They refract doubly in particular directions. Thin fragments of transparent crystals decrepitate in the flame of a candle; other varieties lose their translucency and become friable. With borax it dissolves and forms a transparent glass, which crystallizes on cooling; but in soda it is insoluble. It presents a yellowish-red phosphorescent light upon hot iron; and is soluble in the nitric and muriatic acids, during which process carbonic acid is disengaged; paper dipped into a mixture of this solution and alcohol burns with a purple flame.
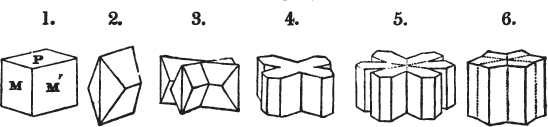
Fig. 1, the primary form, a right rhombic prism, which in fig. 2 is modified by planes replacing the four acute angles, so as to cause the plane P of fig. 1 to disappear. In fig. 3, two crystals of the same form as fig. 2 cross each other. Fig. 4 represents two crystals crossing each other, of which the planes M M and P of fig. 1 appear, but the acute edges of that figure are replaced by planes parallel to the axis of the prism. In fig. 5, three similar crystals cross each other; these do not often occur so distinct, but usually as represented by fig. 6, in which they are more closely united in the general form of a six-sided prism. The dotted lines represent the cracks observable down each face, arising from the contact of the planes forming the diedral terminations of the several crystals of which fig. 5 is composed; and from the same cause the six lateral planes of this apparently six sided crystal are not flat, but each presents a slightly re-entering angle.
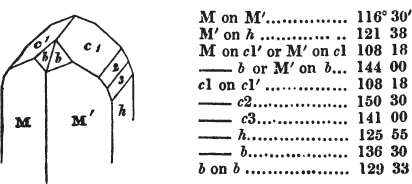
This mineral is named from its locality, the province of Arragon, in Spain, where it was first found in large detached twin crystals, disseminated in a ferruginous clay, accompanied by
[page] 167
sulphate of lime, the most transparent and best-defined prisms however occur near Bilin in Bohemia, in a vein traversing basalt; while the branching or coralloidal varieties, to which the name of Flos ferri has been given, occur in beds of iron ore, and are particularly beautiful in the Styrian mines of Eisenerz, where they appear stalactitically disposed on the roofs and sides of considerable cavities. Radiated and acicular minute white crystals have been found in the recent lavas of Vesuvius. The massive, silky, and fibrous variety, termed satin spar, occurs at Dufton, in thin veins, traversing shale, generally accompanied by iron-pyrites; it is susceptible of a fine polish, and is employed in the manufacture of ornaments. Stalactitic specimens of a snowy whiteness have been met with at Lead hills; also in Buckinghamshire; in Devonshire; and in Dirk Hatterick's Cave, on the coast of Galloway.
Arragonite may with facility be distinguished from calcareous spar by exposing it to heat, before which it at once flies into powder, while the calcareous spar placed alongside of it remains unchanged, and even retains its transparency. Its cleavage in a longitudinal direction should also be a sufficient characteristic— the faces of cleavage in calc spar, however small the individuals, being always inclined.(Manual.)
BITTER SPAR.
Chaux Carbonatée Magnesifère Primitive, H. Bitterspath, W. Rbomb or Dolomite Spar, J. Macrotypous Lime Haloide, M. in part Brown spar.
Combination of carbonate of lime and carbonate of magnesia, the latter occasionally replaced by a small proportion of carbonate of iron.
| Halle in | Taberg in | Miemite, | |
| Tyrol. | Sweden. | Tuscany. | |
| Carbonate of lime | 68·0 | 73·0 | 53·0 |
| Carbonate of magnesia | 25·5 | 25·0 | 42·5 |
| Carbonate of iron | 1·0 | 0·0 | 3·0 |
| Manganesian oxide of iron | 0·0 | 2·2 | 0·0 |
| Water | 2·0 | 0·0 | 0·0 |
| Klaproth. | Klaproth. |
Sp. Gr. 2·85—2·9. H. = 3·5—4·0.
Bitter spar is usually found in the form of its primary crystal, an obtuse rhomboid, so nearly allied to that of carbonate of lime that it was considered the same until Wollaston discovered the difference by means of the reflective goniometer. Its angles are
[page] 168
106° 15′ and 73° 45′. Colour greyish or yellow, with a somewhat pearly lustre; harder than calcareous spar, semi-transparent, and very brittle. It cleaves readily into rhomboids of the same form as the crystals. Before the blowpipe, bitter spar is not distinguishable from calcareous spar; it however is more slowly soluble in acids, and produces only a very slight effervescence.
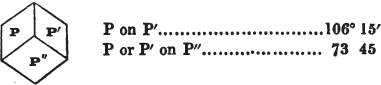
The finest and most transparent crystals occur at Traversalla in Piedmont, at St Gothard, and near Gap in France; it is also found in the Tyrol and Salzburg; and at Taberg in Sweden, with asbestus, talc, and chlorite.
The following substances are considered varieties of bitter spar.
1. MIEMITE occurs crystallized, but more often massive. Internally it is splendent and pearly; its fracture is foliated and curved; colour greenish-white or green; translucent; and brittle. Sp. Gr. 2·8. It is found imbedded in gypsum at Miemo* in Tuscany.
2. PEARL SPAR.† Chaux carbonatée ferro-manganesif è re, H. This is the most common variety of bitter spar. It occurs in obtuse rhombs, with curvilinear faces; generally presents a shining pearly lustre; is translucent, yields to the knife, but is harder than calcareous spar; colour white, grey, or yellowish. Specific gravity about 2·5.
It occurs abundantly in the lead mines of the north of England; in those of Derbyshire; in that of Beeralston in Devonshire; in several mines in Cornwall; at Schemnitz in Hungary; at Kapnik in Transylvania; at Clausthal in the Hartz; at Freyberg in Saxony; and in many other places on the continent.
3. DOLOMITE.‡ Magnesian limestone. Occurs massive, and has sometimes a slaty texture; it consists of fine crystalline grains, which are lamellar; is generally white, occasionally with a tinge of yellow or grey; is translucent on the edges, and, when struck, frequently emits a phosphorescent light, which is visible in the dark. It greatly resembles primitive limestone, but is readily distinguished by its feeble effervescence in acid.
* Whence Miemite.
† From its pearly lustre.
‡ Dolomite, in honour of the geologist Dolomieu.
[page] 169
It occurs in the Pyrenees, Saxony, France, Sweden, in Iona one of the Hebrides, and, though in a more impure state, in many counties of England—Somersetshire, Yorkshire, Nottinghamshire, &c. At Building Hill near Sunderland it forms globular earthy-like concretions; and in the same vicinity is found in slaty masses, which, when split into thin pieces, are very flexible,—a quality supposed to depend on the water it contains, as it is nearly lost when the mineral dries.
Gurhofian is of a snow-white colour, and very compact; the fragments, which are sharp, are translucent on the edges; fracture flat conchoidal. In many respects it may be mistaken for semi-opal. It occurs in veins traversing serpentine between Gurhof* and Aggsbach in Lower Austria.
The mortar obtained from this species is esteemed for cement, being less subject to decay, owing to its absorbing less carbonic acid from the atmosphere than that of common limestone. But for agricultural purposes it is of inferior value; for when laid on particular soils it tends rather to injure than to improve vegetation, which is wholly destroyed when the quantity is large; this effect is owing to the magnesia it contains. The cathedral of Milan, and the Minster and city walls of York, are built of magnesian limestone; the white marble of Paros, and that of Iona in the Hebrides, belong to this species; it therefore often admits, as well as limestone, of being cut and polished, and is described as being particularly durable.
ANKERITE.
Paratomous Lime Haloide, M. Rohe Wand and Wandstein of Styrian Miners.
Carbonic acid with oxide of iron 35·31, lime 50·11, magnesia 11·85, oxide of manganese 3·08—Schrotter.
Sp. Gr. 2·95—31. H. = 3·5—4·0.
Primary form a rhomb of 106° 12′. In crystalline masses of a white colour, though sometimes tinged yellow and brown, from an admixture of iron. Cleavage perfect parallel to the faces of the rhomb; lustre vitreous; slightly translucent; streak white; fracture uneven; and the surface generally striated. Before the blowpipe per se it becomes black, and acts on the magnet, but does not fuse; with borax it melts into a pearl. In nitric acid it is soluble with a brisk effervescence; and on exposure to the atmosphere its surface becomes darker. This species occurs at the Rathhausberg in the Gastein valley, Saltzburg; and in considerable quantity at the Styrian mines of Eisenerz, where it is
* Whence Gurhofian.
H
[page] 170
prized both as an iron ore, and as a flux in the process of smelting; it is also met with at Golrath and the Neider Alp in Styria. It was distinguished by Mohs, who named it in compliment to Professor Anker of the Johannæum in Gratz. (Manual.)
PLUMBO-CALCITE.
Johnstone.
Carbonate of lime 92·2, carbonate of lead 7·8—Johnstone.
Form and cleavage the same as the primary rhomb of calcareous spar; massive. When heated the carbonic acid is driven off, and the specimen assumes a reddish colour. Before the blowpipe it yields with soda a white enamel, but no reduced lead appears. Occurs among the old workings at Wanlockhead in Dumfries-shire.
APATITE*
Phosphate of Lime. Apatit, W. Rhombohedral Fluor Haloide, M. Chaux Phosphatée, H.
Combination of the phosphoric, fluoric, and muriatic acids, with lime.
| St Gothard. | Cap de Gates. | Greiner. | Asparagus Stone. | |
| Phosphoric acid | 44·32 | 44·27 | 44·35 | 44·25 |
| Fluoric acid | 0·00 | |||
| Lime | 55·66 | 55·30 | 55·57 | 44·75 |
| Muriatic acid | 0·02 | 0·43 | 0·07 | 0·00 |
| Rose. | Rose. | Rose. | Klaproth. |
Some massive varieties also contain silica, carbonic acid, and oxide of iron.
Sp. Gr. 3·1—3·3. H. = 5·0.
Phosphate of lime is found massive (Phosphorite); and crystallized in six-sided prisms, terminated by one or more planes (Apatite), or the prism is terminated by a six-sided pyramid, and the lateral edges are sometimes replaced; it yields, though with some difficulty, to mechanical division parallel to all the planes of the regular hexahedral prism, which therefore is considered the primary form; fracture more or less conchoidal, with a vitreous lustre; translucent, rarely transparent; white, yellowish-white, wine-yellow, green, blue or bluish-green, and red,—these co-
* Named by Werner, from αϰαταω, to deceive; in allusion to its being readily mistaken for certain other minerals.
[page] 171
lours sometimes intermixed in the same crystal. In a very high temperature, the edges and angles are rounded off, but it does not fuse without addition; with borax it forms a clear globule, and in salt of phosphorus dissolves in great quantity, affording a transparent glass, which when nearly saturated becomes opake on cooling, and presents crystalline faces. Soluble without effervescence in the nitric and muriatic acids; and when thrown in powder on live coal, emits a yellow phosphorescent
Fig. 1, the primary—a six-sided prism. Fig. 2, the same, of which the terminal edges are replaced; tending to a six-sided pyramid, which is perfect in fig. 3, the lateral edges of the prism being replaced. Fig. 4, in this the lateral and terminal edges of the prism are all replaced by planes, and its solid angles by small six-sided faces.
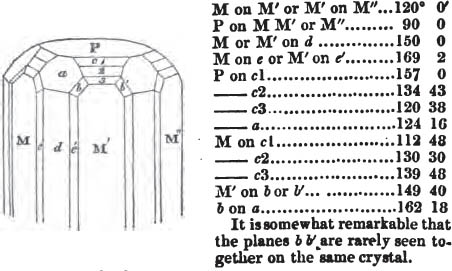
Ehrenfriedersdorf in Saxony, and Schlackenwald in Bohemia, are well-known continental localities of this mineral. The crystals from St Gothard in Switzerland are remarkable for their whiteness and transparency, and the regularity of their complex forms; those from Arendal in Norway (Moroxite) are opake, and of a greenish-blue colour; while the asparagus-stone or spargelstein, from the Zillerthal in the Tyrol, is translucent, of a wine-yellow hue, and imbedded in green talc. In the St Lawrence county, United States, apatite occurs abundantly in well-defined sea-green coloured crystals, occasionally four to six inches in length, imbedded in granular limestone; and in the British Museum there is one very remarkable crystal, which is said to be from the vicinity of St Petersburg. Beautiful crystals have also been
[page] 172
discovered at Caldbeck-fell in Cumberland; in Cornwall in tin veins in the granite of St Michael's Mount, with topaz, &c.; with axinite on the cliffs of Botallack near the Land's End; and near Bovey Tracey in Devonshire, in large greyish-white translucent prisms, with crystallized tourmaline, in a quarry of red granite.
The massive variety of apatite is usually distinguished under the appellation of phosphorite. It presents a granular texture, and a yellowish or reddish-white colour; is nearly opake; and becomes phosphorescent on the application of heat. It is found at Schlackenwald in Bohemia, and abundantly near Lagrofan in Estremadura in Spain, in beds alternating with limestone and quartz.
HERDERITE.*
Prismatic Fluor Haloide, Haidinger.
Primary form a right rhombic prism of 115° 9′ and 64° 51'.
Sp. Gr. 2·9—3·1. H. = 5·0.
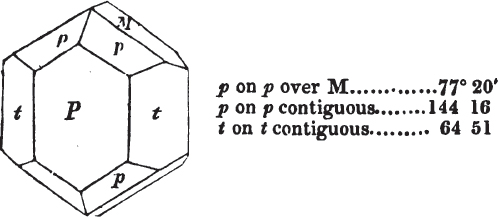
Cleavage interrupted parallel to M. Fracture small conchoidal. Colour several shades of yellowish- and greenish-white; very translucent; with a vitreous or somewhat resinous lustre. Streak white; surface of M smooth, and delicately striated parallel to its edges of combination with p.
Herderite much resembles asparagus-stone, but was distinguished by Haidinger. It occurs imbedded in fluor, in the tin mines of Ehrenfriedersdorf in Saxony; and is a very rare species.
FLUOR SPAR.†
Fluorine, Beudant. Fluate of Lime. Fluss, W. Chaux fluatée, H. Octahedral Fluor Haloide, M.
Combination of fluoric acid and lime.
| Lime | 67·75 | 72·14 |
| Fluoric acid | 32·25—Klanroth. | 27·86—Berzelins. |
Sp. Gr. 3·0 to 3·3. H. = 4·0.
* In compliment to Baron von Herder, the director of the Saxon mines at Freyberg.
† From the Latin fluo, to flow—in allusion to its important use as a flux to the metallic ores.
[page] 173
Fluor occurs crystallized, nodular, compact, and earthy; the first has a perfectly lamellar structure, and may be cleaved with facility into the tetrahedron, acute rhomboid, and regular octahedron, the latter of which has been adopted as its primary form: occasionally however the edges of a cube of fluor may be displaced mechanically, affording an apparent cleavage parallel to the planes of the rhombic dodecahedron; but this is only deceptive, for the planes so produced are irregular, and therefore unlike those parallel to the planes of the octahedron, and may be termed planes of composition. It occurs in the form of the octahedron and its varieties; as the cube, dodecahedron with rhombic planes, &c. Fluor is found perfectly limpid and transparent; also white, grey, and exhibiting various shades of blue, green, red, yellow, and purple: when pounded and placed on live coal it emits a phosphorescent light, blue, green, purple, or yellow; when thrown in mass into the fire, it decrepitates and flies. Is acted upon by acids, and particularly by heated sulphuric acid, which decomposes it, and disengages fluoric acid in vapours. Alone on charcoal it fuses by much heat into an opake white globule; with borax, and salt of phosphorus it forms a transparent glass, which when saturated to a certain extent becomes opake.
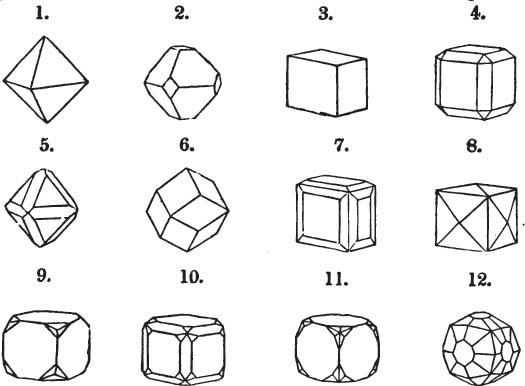
Fig. 1, primary; the regular octahedron. Fig. 2, the same, having all its solid angles replaced by square planes, which are enlarged and complete in fig. 3, the cube. Fig. 4, the cube, of which the edges and angles are replaced. Fig. 5, the octahedron, with its edges replaced by six-sided planes; which in fig. 6 are complete and of a rhombic shape, forming the rhombic dodecahedron. Fig. 7 the cube with its edges bevelled, or replaced by two planes; these planes are complete in fig. 8, forming a solid
[page] 174
bounded by twenty-four triangular planes. Fig. 9, the cube, having each solid angle replaced by three planes, which in fig. 10 are seen in combination with planes replacing the edges of the cube. Fig. 11, the cube, of which each solid angle is replaced by six planes; these are enlarged and nearly complete in fig. 12: representing a crystal bounded by fifty-four planes.
One crystal (from Devonshire) in Mr Phillips's possession exhibited all the faces represented on the annexed figure except b1 3 and 4, and c3.
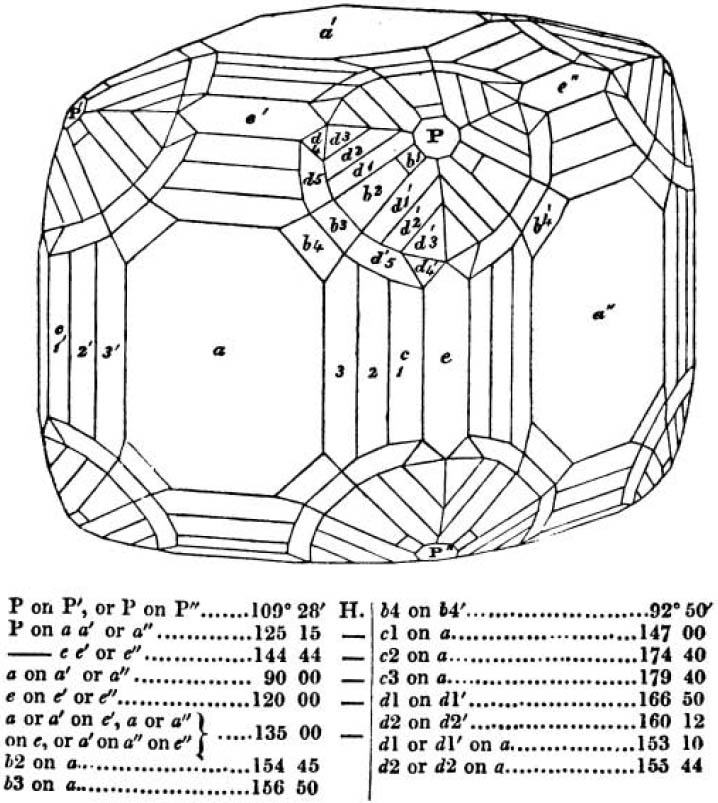
No part of the world has hitherto produced finer specimens of this species than the counties of Cumberland, Derby, and Cornwall. Those varieties from the lead mines of Alston Moor and Derbyshire usually assume the form of the cube, and present the finest shades of blue and green, varying in colour as the light by which they are examined is reflected or transmitted; some beautiful octahedral forms occur at Beeralston in Devonshire, while the neighbouring county of Cornwall has afforded an endless
[page] 175
variety of crystallizations. Dark-blue cubical crystals have been noticed in porphyritic greenstone near Gourock in Renfrewshire, also though sparingly in Aberdeenshire; but fluor is on the whole a rare production either of Scotland or Ireland.
Crystallized fluor is found at Mont Blanc and St Gothard—on the latter in octahedrons of a rose-red colour; in Saxony, the Bannat, and other countries, it occurs in veins in primitive mountains, exhibiting various modifications and great varieties of colour; and at Zinnwald in Bohemia, accompanies oxide of tin, mica, apatite, and quartz.
Near Castleton in Derbyshire fluor is found in detached masses, whose structure is divergent, and their colours, as grey, yellow, blue, brown, are generally disposed in concentric bands: of this variety, called blue John by the miner, beautiful vases, obelisks, and other ornaments, are made.
Compact fluor is harder than common fluor, and has sometimes a granular texture; in general it is translucent only on the edges; when placed on live coal, it mostly gives out a green light, but some specimens exhibit various shades of green, blue, violet, and red. It occurs at Stolberg in the Hartz, where, when first raised, it presents a fine sky-blue colour, but shortly, on exposure, becomes perfectly white; also in Norway, Sweden, and Cornwall.
Earthy fluor exhibits a friable texture. It is found in Saxony and Norway; in Durham, with partially decomposed galena; and in a granular pulverulent state, in the Beeralstone lead mine, Devonshire.
The name Chlorophane has been applied to those varieties which, when exposed to heat, exhibit the phenomenon of phosphorescence in peculiarly bright-green colours. One presenting an imperfectly lamellar structure, and of a pale-violet colour, from Nertschinsk in Siberia, possesses this property to a remarkable degree; and in some of the Cornish specimens it is also easily detected. It does not fly in the fire, but gives out a phosphorescent light of a most beautiful emerald green,* which, if not exposed to too high a temperature, it will exhibit repeatedly.
The circumstance of its fluoric acid being disengaged when treated with sulphuric acid, renders fluor spar a useful medium for executing etchings on glass, which are readily obtained by exposing a plate partially coated with wax to the action of this acid as it is evolved in a gaseous state; that portion of the glass covered by the wax of course remains entire, while whatever has been laid bare will shortly be found to have been acted upon to a considerable depth, and thus figures of any description may be produced on glass without much difficulty.
* Whence Chlorophane, signifying brilliant green.
[page] 176
ANHYDRITE.
Muriacite, Wurfelspath, W. Chaux Sulphatée Anhydre, H. Prismatic Gypsum Haloide, M.
Combination of sulphuric acid and lime.
| Sulz. | Eisleben. | Vulpino. | Bohemia. | |
| Sulphuric acid | 57·0 | 56·28 | 58·00 | 56·0 |
| Lime | 42·0 | 41·48 | 41·70 | 39·0 |
| Water | 0·0 | 0·75 | 0·07 | 0·0 |
| Baryta | 0·0 | 0·00 | 0·00 | 2·0 |
| Silica | 0·2 | 0·00 | 0·09 | 0·2 |
| Klaproth. | Rose. | Stromeyer. | Beudant. |
Sp. Gr. 2·5—2·9. H. = 3·0—3·5.
Anhydrite occurs crystallized in the form of a rectangular prism, of which the lateral edges are sometimes, though rarely, replaced. It readily yields to cleavage parallel with the planes of the prism, but with more difficulty in one direction than in the other two. It is white, violet, bluish, or reddish; is translucent, sometimes transparent; with a splendent, pearly lustre. It possesses double refraction. In the matrass it yields no water, and it does not exfoliate like gypsum before the blowpipe, but becomes glazed over with a white friable enamel; with borax it fuses with effervescence into a transparent glass, which on cooling is yellowish-brown, and which, if the proportion of the assay be considerable, becomes brown and opake; with fluor spar it forms a transparent globule while hot, which alters into an opake white enamel when cold.
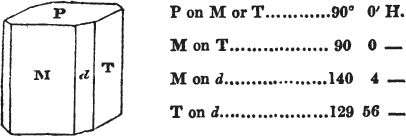
It is found in the salt mines of Hall in the Tyrol, and Bex in Switzerland; also in cleavable masses of a brick-red colour imbedded with gypsum and polyhalite, in beds of rock salt at Aussee in Upper Austria.
Compact Anhydrite or Vulpinite occurs massive, contorted, and reniform. It is found in the salt mines of Upper Austria and Salzburg; at Sulz on the Neckar in Wirtemberg, and at Bleiberg in Carinthia. The contorted variety termed pierre des trippes (from its resemblance to the convolutions of the intestines) occurs in clay, in the salt mines of Wielitzka and Bochnia in Poland; while the variety which takes a fine polish, and is known by artists as the marmo bardiglio di Bergamo, occurs with limestone at Vulpino in Italy.
GYPSUM.*
[page] 177
Sulphate of Lime. Selenite. Gyps, W. Chaux Sulphatée, H. Prismatoidal Gypsum Haloide, M.
Combination of sulphuric acid, lime, and water.
| Sulphuric acid | 46·0 |
| Lime | 33·0 |
| Water | 21·0—Bucholz. |
Sp. Gr. 3·26 to 2·4. H. = 1·5—2·0.
Of gypsum there are several varieties. It occurs crystallized; fibrous; having a granular texture; compact; and earthy.
Selenite† occurs generally in flattish crystals. The primary form is a right oblique-angled prism, of which the bases are oblique-angled parallelograms of 113° 8′ and 66° 52′; it cleaves with ease and brilliancy parallel only with the terminal planes P of the following figures, but cleavages parallel to the lateral planes may be attained from the finely divided laminæ; lustre shining, sometimes pearly; more or less transparent, and so soft as to yield to the nail.
The preceding figures are given only with a view to elucidate the manner in which the modifying planes a b c d of the following figure are situated on the angles or edges of the primary.
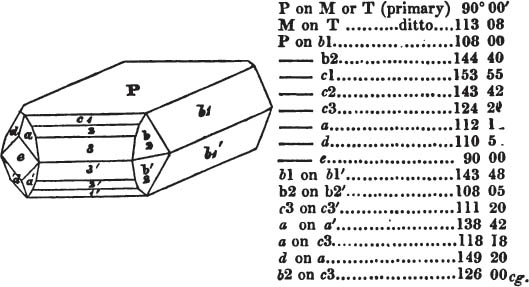
* Gypse is said to have been the term given by the ancients to calcined sulphate of lime: that mineral in its natural state is now termed gypsum: it contains water.
† Selenite, from the Greek; in allusion to the brilliancy with which it reflects the moon.
H2
[page] 178
It presents various shades of white, yellow, grey, brown, red, and violet. Reduced to thin laminæ, it is flexible but not elastic. Gives off water in the matrass; and before the blowpipe in the platina forceps exfoliates and fuses with difficulty into a white enamel.
Selenite is most commonly met with disseminated in argillaceous deposits; not often in veins. The finest and most transparent crystals occur in the salt mines of Bex in Switzerland, at Hall in the Tyrol, in the sulphur mines of Sicily, near Oçana in Spain, and in detached and very symmetrical individuals in clay at Shotover Hill in Oxfordshire. Large lenticular crystals, and the scaly varieties, are found at Montmartre near Paris; and in the Ohio county, and other parts of the United States, it is met with abundantly.
Fibrous Gypsum occurs in extremely delicate and easily separated fibres; also massive, of which the fibres are either straight or curved. It has a glistening or pearly lustre, and presents various shades of white, grey, yellow, and red; it is generally translucent. It occurs in Derbyshire in long slender fibres, and particularly at Matlock, where it is found in masses of great brilliancy and remarkable lustre.
Granular Gypsum generally occurs massive, being composed of an aggregation of small crystalline laminæ. It has a shining pearly lustre; is translucent; and very soft. Its colours resemble those of selenite. It occurs in beds in primitive and secondary rocks. At Luneburg it is the matrix of the boracite. Large quarries of a pure white variety exist near Cavalese in the Southern Tyrol; also at Vizille, near Grenoble in France. In Cheshire and Derbyshire it forms beds in marl.
Compact Gypsum occurs only massive; its fracture is compact, or slightly splintery; it is dull, or possesses a glimmering lustre; is soft and translucent on the edges. Its colours are much the same as those of selenite, but it is often party-coloured; either spotted or veined.
It occurs in England, at Ferrybridge in Yorkshire, in Nottinghamshire, and in Derbyshire. Near Sienna in Tuscany it is obtained extremely pure and compact; and is employed by the architect for columns and other ornaments, being more easily worked than marble; it also admits of being turned on the lathe into cups, basons, vases, and other similar articles.
Earthy Gypsum occurs in loose earthy particles or scales, which are dull, or possess a glimmering lustre, in beds, enclosed within the strata of secondary formations of gypsum, in Saxony, Salzburg, and Norway.
The most decided characteristic of crystallized gypsum is the flexibility of its laminæ, into which it may be separated to almost any degree of thinness; the massive varieties are at once
[page] 179
distinguished from limestone by their inferior hardness, being readily scratched by the nail, and yielding a white powder.
NITRATE OF LIME.
Kalk Salpeter, L. Chaux Nitratée, H. Nitrate de Chaux, Beudant.
| Consists of Nitric acid | 66·2 |
| Lime | 33·8—WenzeL |
Primary form a rhomboid, or a regular six-sided prism.
It occurs in fibrous efflorescences often united in the form of silken tufts, or pulverulent; is very deliquescent, and soluble in water. On burning coals it melts slowly, with slight detonation, and, as it dries, loses its acid; the residue does not after wards attract moisture from the air, and is phosphorescent; taste bitter and disagreeable.
It is found in silky efflorescences on old walls, in caverns, or on calcareous rocks, in the neighbourhood of decayed vegetable matter; and in some mineral waters.
DATHOLITE.*
Borate of Lime. Datholit, W. Chaux Boratée Siliceuse, H. Chaux Datolit, Bt. Prismatic Dystome Spar, M. Esmarkite, Hausmann. Humboldtite, Levy.
Combination of silica, boracic acid, lime, and water.
| Arendal. | Andreasberg. | Botryolite. | ||
| Boracic acid | 24·0 | 21·67 | 21·26 | 13·5 |
| Silica | 36·5 | 36·66 | 37·36 | 36·0 |
| Lime | 35·6 | 34·00 | 35·67 | 39·5 |
| Water | 4·0 | 5·50 | 5·71 | 6·5 |
| Klaproth. | Vauquelin. | Stromeyer. | Klaproth. | |
Sp. Gr. 2·9 to 3·3. H. = 5·0—5·5.
Datholite occurs massive, and crystallized in rhombic prisms of which the lateral edges and the solid angles are commonly replaced by planes; colour greyish, or greenish-white; translucent; fracture imperfectly conchoidal, with a somewhat vitreous lustre. The primary form is a right rhombic prism of about 103° 40′ and 76° 20′ from the measurement of its natural planes by the reflective goniometer. When exposed to the flame of a candle it becomes opake, and crumbles down between the fingers; before the blowpipe it intumesces into a white mass, and then melts into a transparent or pale rose-coloured globule. It dissolves readily in, and gelatinizes with, nitric acid.
* Datholite, from the Greek, signifying turbid; in allusion to the want of transparency in the mineral.
[page] 180
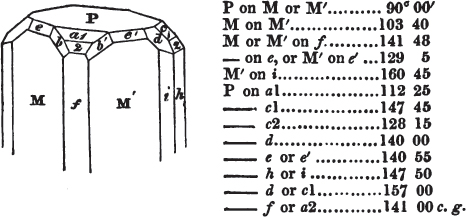
Interesting crystallizations of datholite occur in greenstone in New Jersey, United States; at Arendal in Norway; at Andreasberg in the Hartz; in the island of Uton in Sweden; and in the valley of Glen Farg in Perthshire. The humboldtite is found in agate balls at the Seisser Alp in the Tyrol.
Botryolite occurs in mamillary concretions formed of concentric layers, having a splintery or fibrous texture; it is brittle, translucent on the edges, and externally of a pearl- or yellowish-grey colour; internally white, greyish, and red in concentric circles. It also occurs in small botryoidal* masses, which are white and have an earthy texture. Before the blowpipe it melts into a white glass. Its locality is Arendal in Norway, where it forms in a bed in gneiss, accompanied by schorl, magnetic iron ore, and iron pyrites.
PHARMACOLITE.†
Arseniate of Lime. Arsenikblüthe, W. Chaux Arseniatée, H. Hemiprismatic Gypsum Haloide, Haid.
Combination of arsenic acid, lime, and water.
| Wittichen. | Andreasberg. | |
| Arsenic acid | 50·54 | 45·68 |
| Lime | 25·00 | 27·28 |
| Water | 24·46—Klaproth. | 23·86—John. |
Sp. Gr. 2·64—2·8. H. = 2·0—2·5.
The pharmacolite is found in minute fibrous or acicular crystals, which commonly are aggregated into botryoidal or globular masses, having a glimmering or silky lustre; more rarely in distinct crystals. Cleavage parallel to P, highly perfect and easily obtained. Its colour is white or greyish-white; but the surface is often tinged of a red or violet colour by arseniate of
* Whence botryolite, from the Greek; in allusion to the resemblance in form to grapes.
† Pharmacolite, from the Greek; in allusion to its containing poison.
[page] 181
cobalt. Before the blowpipe it is almost entirely volatilized, with a dense white arsenical vapour. In nitric acid it dissolves readily without effervescence.
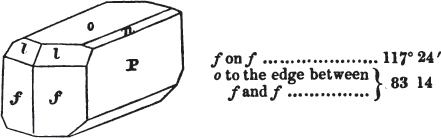
Pharmacolite occurs at Andreasberg in the Hartz; at Riegelsdorf, and Glucksbrunn in Thuringia; at St Marie-aux-Mines in the Vosges, in minute silky white crystals; and at Wittichen, near Furstenberg in Germany, disseminated on granite, in a vein containing cobalt, barytes, and sulphate of lime. Clear transparent crystals of pharmacolite, very distinctly pronounced, and fully a line in diameter, were at one period found in the Grand Duchy of Baden, probably at Badenweiler, and of these some fine specimens are preserved in the palace at Carlsruhe.
The Picro-pharmacolite of Stromeyer, from Riegelsdorf in Hessia, contains about three per cent. of magnesia, but in other respects corresponds with this mineral.
HAIDINGERITE.
Diatomous Gypsum Haloide.
Arseniate of lime 85·68, water 14·32—Turner.
Sp. Gr. 2·84. H. = 2·0—2·5.
Primary, a rectangular four-sided prism; colour white and transparent, with a vitreous lustre, and white streak. Cleavage highly perfect and easily obtained parallel to d. Readily soluble in acid.
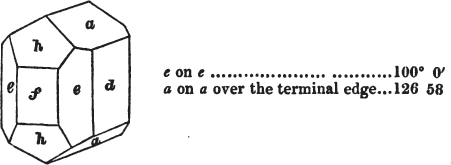
This very rare mineral was distinguished by Haidinger from the pharmacolite of Baden, which it accompanies, and with which it used to be confounded. Its form and lustre are distinct, and it contains one half less water than pharmacolite.
[page] 182
TUNGSTATE OF LIME.
Pyramidal Scheelium Baryte, M. Tungsten* Schwerstein, W. Scheelin Calcaire, H. Scheelite, Necker.
Combination of tungstic acid and lime.
| Sweden. | Huntingtown. | |
| Tungstic acid | 80·42 | 76·05 |
| Lime | 19·40 | 19·36 |
| Oxide of iron | 0·00 | 1·03 |
| Silica | 0·00—Berzelius. | 2·54—Bowen. |
Sp. Gr. 6·0—6·1. H. = 4·0—4·5.
Tungsten has a greyish and yellowish-white colour, and occurs both crystallized and amorphous; the crystals present the form of a four-sided pyramid, approaching nearly to the octahedron. Two of the lateral angles are often replaced by the faces of another pyramid considerably more acute (fig. 1); the angles formed by the meeting of a plane of the upper with the adjoining plane of the lower pyramid being, according to the measurements annexed to the following figure, 128° 40′. It yields to cleavage parallel to the faces of both pyramids (figs. 1 & 3), with a somewhat shining lustre; it is translucent generally only on the edges. Before the blowpipe it crackles and becomes opake, but does not melt, except that the thinnest edges are converted, at a high temperature, into a semi-transparent vitrified mass: with borax it yields a white glass; and with salt of phosphorus melts in the oxidating flame into a transparent colourless globule —and in the reducing, into a green one, which becomes of a fine blue colour on cooling. When pulverized and thrown into heated nitric acid, it assumes a yellow colour, but does not dissolve. Fragments dropped upon live coal exhibit a phosphorescent light.
This mineral, when massive, considerably resembles carbonate and sulphate of lead, and also barytes. It may be distinguished from the two first by its not effervescing in acids; from the last by the yellow colour which it assumes when placed in nitric acid.
* Tungsten, German; a heavy stone.
[page] 183
Fig. 1, the primary; an acute four-sided pyramid. Fig. 2 represents the usual form in which this substance occurs; its larger faces arise from the deep replacement of the pyramidal edges of fig. 1, by planes which are parallel therewith; those of the primary crystal being thus reduced to small triangles. The replacing planes of fig. 2 are complete in fig. 3, forming a pyramid which is less acute than the primary.
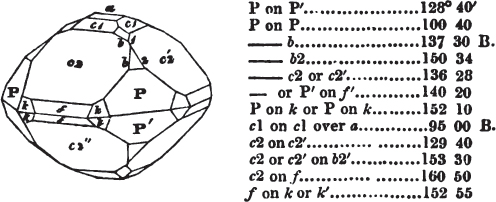
The above figure (with the exception of the upper planes, which are given on the authority of Bournon) was taken from a crystal in the possession of Mr Sowerby.
This mineral occurs both crystalline and amorphous, particularly in the repositories of tin ore at Schlackenwald and Zinnwald in Bohemia, and in Monro County, United States; the crystals from these localities are occasionally of large dimensions; the most symmetrical, however, are found associated with apatite, molybdena, and wolfram, in quartz, at Caldbeckfell in Cumberland. Sweden, Dauphiné, and Cornwall, are other localities of this species.
CARBONATE OF MAGNESIA.
| Styria. | Salem. | |
| Magnesia | 48·0 | 47·88 |
| Carbonic acid | 49·0 | 51·82 |
| Water | 3·0—Klaproth. | 0·00—Stromeyer. |
Sp. Gr. 2·8.
Magnetite occurs massive, amorphous, and reniform; one variety, from Salem in the Carnatic, presents occasionally slight indications of crystallization. The fracture is splintery or flat conchoidal; it is nearly opake, dull, and yields to the nail externally, but internally is slightly harder than calcareous spar; is somewhat meagre to the touch, and adheres to the tongue. It is of a grey or yellowish colour, with spots and dendritic delineations of blackish-brown.
It occurs in serpentine, with bronzite, at Gulsen in Upper Styria; at Hrubschitz in Moravia; at Baldissero and Castellamonte in Italy; at Vallecas in Spain; at Baumgarten in Silesia; and in the Bare Hills near Baltimore, North America.
[page] 184
Earthy Carbonate of Magnesia. Meerschaum, W. Ecume de Mer, Br. Meerschaum is of a white or yellowish colour: opake and dull; it has an earthy fracture, yields easily to the nail, and adheres to the tongue; sometimes it is so light as to swim on water, and occasionally is very porous. It consists of 45·42 magnesia, 47 carbonic acid, 4·5 silica, 2 water, 0·5 alumina, with traces of manganese and lime.—Tromsdorff.
It occurs in the isles of Samos and Negropont in the Archipelago, in mass, or disseminated, or in beds; and at Kiltschik in Natolia. It is soft when first dug, and in that state is made into pipes, but hardens on exposure to the air. It is also met with in Carinthia, Moravia, and Spain; and is mentioned as occurring in veins in the serpentine of Cornwall.
In the Turkish dominions Meerschaum is employed as fuller's earth; and it is well known as the material used in the manufacture of Turkish pipes.
BREUNNERITE.
Breunnerite,* A. Carbonate of Magnesia and Iron. Brachytypous Lime Haloide, M. Spath Magnesien, Necker. Giobertite, Beudant.
Carbonate of magnesia, with occasional admixtures of protoxide of iron and manganese.
Analyses by Stromeyer.
| Zillerthal. | Hall in Tyrol. | ||
| Carbonic acid | 48·94 | 49·67 | 49·93 |
| Magnesia | 41·06 | 42·10 | 43·44 |
| Protoxide of iron | 8·57 | 6·47 | 4·98 |
| Ox. of manganese | 0·43 | 0·62 | 1·52 |
Sp. Gr. 3·0—3·2. H. = 4·0—4·5.
Primary form a rhomb of 107° 22′.† Occurs in single, yellowish or brown, translucent crystals; lustre vitreous, sometimes inclining to pearly; cleavage perfect parallel to the faces of the rhomb; fracture flat conchoidal. Soluble without effervescence in nitric acid. The best-known localities of this mineral are the Rothen-Kopf and Greiner Mountains in the Zillerthal, Tyrol, where it occurs imbedded in chlorite slate, and associated with crystals of bitter spar, from which, however, it may be distinguished by its colour,—the breunnerite being brown or yellow, whilst the other is white and translucent. Under similar circumstances it is met with imbedded in green foliated talc on the ilsand of Unst in Shetland. (Manual.)
By Necker and Beudant this species and the preceding are united.
* In honour of Count Breunner of Austria.
† According to Brooke it is 107° 30′.
[page] 185
CONITE.
Conite, Friesleben, J.
Sp. Gr. 3·0. Scratches glass.
Amorphous, massive, and in crusts. Colour flesh-red, externally coated with iron ochre. Devoid of lustre. Opake. Brittle. Fracture sometimes fine grained or imperfectly conchoidal. Consists of carbonate of magnesia 67·5, carbonate of lime 28·0, oxide of iron 3·5, water 1·0—John.
It occurs in Iceland, on the Meissner in Hessia, and in Saxony.
SULPHATE OF MAGNESIA.
Epsomite, Beudant. Naturlicher Bittersalz, W. Magnesie Sulphatée, H. Sel d'Epsom Natif, Br. Prismatic Epsom Salt, M.
Combination of sulphuric acid, magnesia, and water.
| Catalonia. | ||
| Sulphuric acid | 32·57 | 32·53 |
| Water | 51·43 | 51·43 |
| Magnesia | 16·00—Berzelius. | 16·04—Gav-Lussac. |
Sp. Gr. 1·66 to 1·75.
Primary form a rhombic prism of 90° 30 and 89° 3C'. It occurs in crystalline fibres, rarely pulverulent; colour white or grey, transparent or opake; very brittle; its taste bitter and saline. Soluble in less than double its weight of cold water.
This salt forms the principal ingredient of several mineral waters, and is a product of the decomposition of certain rocks, upon the surface of which it appears in efflorescences. In the former state it is obtained at Epsom in Surrey,—hence its name; and in the latter it occurs in the old coal wastes or alum mines of Hurlet near Paisley; in the quicksilver mines of Idria; on gypsum in the quarries of Montmartre near Paris; and on the surface of the soil in many parts of Spain, and in Chili. It occasionally exhibits a fine fibrous texture.
NITRATE OF MAGNESIA.
Nitro-Magnesite, Sheperd. Magnesie Nitratée, Necker.
Contains nitric acid 72·0, magnesia 28·0—Wenzel.
Colour white; is usually met with in a deliquescent state, mixed with nitre and nitrate of lime, on old walls and in limestone caves.
[page] 186
WAGNERITE.
Hemi-prismatic Fluor Haloide, Haid. Magnesie Phosphatée of the French Wagnerit, Fuchs. Pleuroklas, Breithaupt. Phosphorsaurer Talk, Leonhard.
Combination of phosphoric acid, magnesia, the oxides of iron and manganese, and fluoric acid.
| Phosphoric acid | 41·73 |
| Magnesia | 46·66 |
| Oxide of iron | 5·00 |
| Oxide of manganese | 0·50 |
| Fluoric acid | 6·50—Fuchs. |
Sp. Gr. 3·11. H. = 5·0—5·5.
Primary form an oblique rhombic prism of 95°25' and 84° 35′, whose base is inclined to its planes at an angle of 109° 20′. In crystals extremely complicated. Colour yellow of different shades. often inclining to grey. Translucent. Streak white. Lustre vitreous. Most of the planes of the prism are deeply striated. Fracture uneven and splintery. Before the blowpipe per se it fuses with difficulty into a dark greenish-grey glass; with borax and salt of phosphorus, however, it is readily and entirely dissolved. From its powder digested in the nitric or sulphuric acids, fluoric acid fumes ere given off.
It occurs in the valley of Holgraben, near Werfen in Saltzburg, in irregular veins of quartz, traversing clay-slate; but it is an extremely rare mineral.
BORACITE.
Borate of Magnesia. Boracit, W. Magnesie Boratée, H. Tetrahedral Boracite, M.
Combination of boracic acid and magnesia, occasionally mixed with lime and a little silica.
| Luneburg. | Segeberg. | Schildstein. | |
| Boracic acid | 69·7 | 54·55 | 64·14 |
| Magnesia | 30·3 | 30·68 | 31·11 |
| Silica | 0·0 | 2·27 | 0·00 |
| Oxide of iron | 0·0 Arfwedson. | 0·57 Pfaff. | 1·50 Dumenil. |
Sp. Gr. 2·56—3·0. H. = 7·0.
It occurs only crystallized in the general form of the cube, of which the edges are replaced, and the diagonally opposed solid angles dissimilarly modified; the cube is considered by Haüy its primary form; fracture uneven, or imperfectly conchoidal, with a glistening lustre; more or less translucent; is hard enough to give sparks with the steel, and is of a yellowish-, greyish-, or greenish-white. The opake white crystals are not so hard, and contain a
[page] 187
proportion of carbonate of lime. Without addition on charcoal it intumesces, imparts a green tinge to the flame of the blowpipe, and fuses into an opake white glass. It is remarkable that the diagonally opposed solid angles, on the application of heat, become, the one positive electric, the other negative.
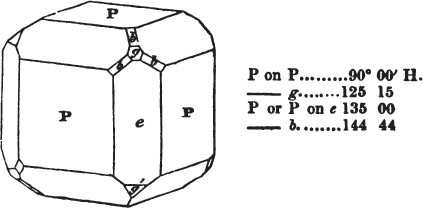
It is found only in gypsum at the Kalkberg near Luneburg, and at Segeberg near Kiel in Holstein, in small but very perfect isolated crystals. It is unknown massive.
HYDRO-BORACITE.
Hess.
Boracic acid 49·92, lime 13·29, magnesia 10·43, water 26·33— Hess.
Sp. Gr. 1·9.
Slightly soluble in water, and readily in acids, the saturated solution yielding crystallized boracic acid on cooling. Resembles in appearance a worm-eaten wood, and is riddled with small holes, which are filled with a mixture of clay and salt.
WITHERITE.*
Carbonate of Barytes.† Witherite, W. Baryte Carbonatée, H. Diprismatic Hal-baryte, M. Rhomboidal Baryte, J.
Combination of carbonic acid and baryta.
| England. | Styria. | ||
| Carbonic acid | 20·66 | 22·5 | 22·0 |
| Baryta | 79·00 | 77·1 | 78·0 |
| Water | 0·33—Buicholz. | 0·0—Beudant. | 00·0—Klap. |
Sp. Gr. 4·3. H. = 3·0—3·5.
* Witherite, after Dr Withering, its discoverer.
† Barytes, from the Greek, signifying heavy; in allusion to the great specific gravity of the earth.
[page] 188
It occurs massive, stalactitic, and crystallized; the structure of the massive is fibrous; the crystals in their general form resemble the common variety of quartz, namely, a six-sided prism terminated by six-sided pyramids; but by the assistance of the reflective goniometer it is found that the measurements are not those of a regular six-sided prism, being on the lateral planes (M on M') only 118° 30′; hence these crystals may be considered as macles, analogous to the artificial crystals of sulphate of potash, their primary form probably being a right rectangular prism. Occasionally a re-entering angle is observable on the alternate planes of the prism, as in the following figure, in which case the crystal is a macle in a double sense. Internally translucent, with a glistening lustre; externally the small crystals are shining, the larger opake; generally white, sometimes greyish or greenish. Exposed in the platina forceps to the blowpipe, it melts readily and with a brilliant light into a white enamel: it is soluble slowly and with feeble effervescence in dilute muriatic or nitric acid.
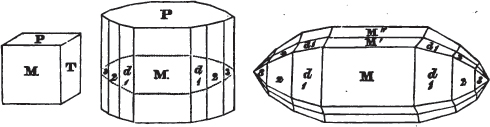
The first figure represents the probable primary form, namely, a right rectangular prism, of which, in the second figure, the lateral edges are replaced (the plane T totally disappearing) by the planes d1, 2, and 3, and the dotted lines include one, of several similar portions, contributing to form the macled crystal on the right of it.
| M on M′ | 118° | 30′ |
| — —d1 or d1′ | 145 | 30 |
| — — d2 or d2′ | 126 | 16 |
| — — d3 or d3′ | 110 | 30 |
| M′ on M″ (re-entering angle)., | 175 | 30 |
It was first noticed by Dr Withering, at Anglesark in Lancashire, in a vein, with sulphuret of lead and some of the ores of zinc, in globular concretions having a radiated structure. It occurs abundantly in the lead veins of the north of England, generally in botryoidal and reniform concretions, but of late years also in large transparent crystals. It has likewise been found in Styria, in Salzburg, Sicily, and the Altai Mountains in Siberia, but nowhere so abundantly as in England.
[page] 189
BARYTO CALCITE.
Hemi prismatic Hal baryte, M. Baryto-calcite, Brooke.
Combination of carbonic acid, baryta, and lime.
Carbonate of baryta 65·9, carbonate of lime 33·6—Children.
Sp. Gr. 3·6—3·7. H. = 4·0.
Primary form an oblique rhombic prism of 106° 54′ and 73° 6′.

Cleavage perfect and easily obtained parallel to the faces M and P. Occurs both crystallized and massive; and of a white, yellow, or greyish colour. Transparent or translucent, with a vitreous or resinous lustre, and white streak. Before the blowpipe per se it does not fuse; but with borax in the oxidating flame affords a diaphanous globule of a light amethystine tinge, which becomes colourless in the reducing flame. It effervesces briskly in nitric or muriatic acid.
Alston Moor in Cumberland is the only known locality of baryto-calcite. In the lead mines there, it is met with in considerable quantity, and occasionally in crystals which exceed an inch in length; but the larger crystals often suffer decomposition, and are converted into a white mealy-like mass resembling barytes. It was first described by Brooke.
BARYTES.
Sulphate of Barytes, Heavy Spar. Schwerspath, W. Baryte Sulphatée Crystallisée, H. Lamellar Heavy Spar, J. Prismatic Hal Baryte, M. Barytine, Beudant.
Combination of sulphuric acid and baryta.
| Sulphuric acid | 34·37 | 33·0 |
| Baryta | 65·63—Beudant. | 67·0—Klaproth. |
Sp. Gr. 4·41 to 4·67. H. = 3·0—3·5.
It occurs both massive and crystallized, with a lamellar structure, which in the massive is sometimes curved; the crystals are
[page] 190
divisible into the form of a right rhombic prism, which therefore is the primary crystal; its angles by the reflective goniometer, from fractured surfaces, being 101°42' and 78° 18′: the lustre of the fragments is shining. It occurs transparent and opake; white, yellow, red, grey, and blue; it possesses double refraction when held in a particular direction. It decrepitates briskly before the blowpipe, and is difficultly fusible, but eventually melts into a hard white enamel which is not affected by acid.
Fig. 1, the primary—a right prism with rhombic bases. Fig. 2, the same; of which the obtuse edges are replaced by planes parallel with those edges. Fig. 3, the same; of which each obtuse solid angle is replaced by a triangular plane. In fig. 4, each acute edge of the prism is replaced by a quadrangular plane. In fig. 5, all the acute solid angles are replaced by triangular planes, which, in fig. 6, are so greatly increased as to give the crystal a prismatic form: in this figure the triangular planes of fig. 3 are also visible. In fig. 7, the triangular planes of fig. 5 are so greatly increased as entirely to replace the primary terminal plane, and to reduce the primary lateral planes to small triangles.
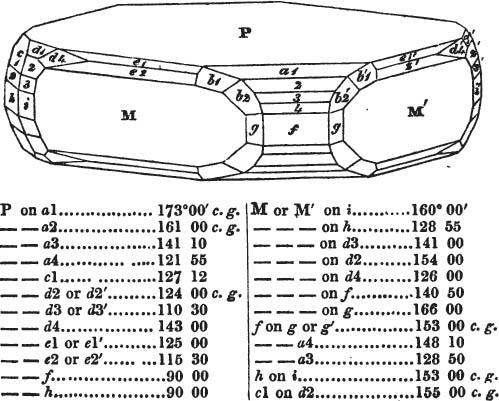
[page] 191
Barytes is a very widely diffused species, and is also one which presents great variety of crystalline form. In the size and beauty of its specimens, the most noted locality is Dufton in Cumberland, where perfect crystals exceeding half a cwt. have occasionally been met with. Many elegant forms, though on a smaller scale, occur at Przibram and Mies in Bohemia. Crystals of large dimensions, and exhibiting splendid colours, are met with at Felsobanya and Cremnitz in Hungary; while at Roya and Raure in Auvergne the form represented by fig. 7 is of general occurrence. The deposits of this species in America, particularly the uncrystallized varieties, are very numerous.
The following have been described as sub-species:—
Columnar Heavy Spar, J. Stangenspath,W. Which occurs in rhombic prisms, generally ill defined, and aggregated laterally into columns. It is white or greenish, with a shining pearly lustre, and translucent; structure lamellar. It occurs near Freyberg in Saxony.
Bolognian Stone. Radiated Barytes. Baryte sulfatée radiée, H. Occurs in roundish masses, composed apparently of minute fibrous crystals radiating from the centre. Internally it is shining or glistening, and of a grey or yellowish-grey colour; it is translucent on the edges, and the fragments are wedge-shaped and soft. It is remarkably phosphorescent when heated, and retains that property for some time even after cooling. It occurs imbedded in marl at Monte Paterno, near Bologna.*
Cawk.† Occurs massive, with a coarse earthy fracture, and is opake, rarely translucent on the edges. It is white, grey, yellow, or reddish, and is glimmering or dull, soft, and brittle. Specific gravity 4·81. It occurs in Bohemia, Saxony, the Hartz, and particularly in Staffordshire, and the lead mines of Derbyshire.
HEPATITE.‡ Baryte sulfatée fetide. H. Applies to such varieties as on being rubbed or heated emit a fetid, sulphureous, or hepatic odour, and are generally of a yellow or brown colour. It consists of 85·2 sulphate of baryta, 6 sulphate of lime, I alumina, 5 oxide of iron, and 0·5 carbon—Klaproth. It occurs at Andrarum, and Kongsberg in Norway; at Lublin in Galicia; and in Albemarle county, North America.
Barytes is one of the most common accompaniments of metallic minerals in veins, and, when associated with ores of iron, possesses a deleterious influence on the process of smelting. The pure white varieties are ground and used as a pigment, either alone or mixed with white lead; but it is otherwise of no great value.
* Whence Bolognian Stone.
† The name of Cawk is said to have been given to this substance from its resemblance to chalk.
‡ From the Greek, signifying of a liver colour.
[page] 192
STRONTITES.
Carbonate of Strontian. Strontianite.* Strontian, W. Strontiane Carbonatée, H. Peritomous Hal Baryte, M. Strontites, A.
Combination of carbonic acid and strontia.
| Braunsdorf. | Strontian. | |
| Carbonic acid | 29·94. | 30·31 |
| Strontia | 67·51 | 65·60 |
| Lime | 1·28 Stromeyer. | 3·47 Stromeyer. |
with minute proportions of oxide of manganese and water.
Sp. Gr. 3·6 to 3·8. H. = 3·5.
It occurs massive, fibrous, stellated, and regularly crystallized in the form of a hexahedral prism modified on the edges, or terminated by a pyramid. The primary crystal is a right rhombic prism of 117° 32′ and 62° 28′, by measurements on planes produced by cleavage, to which the crystals readily yield parallel to the lateral faces of the prism. The structure of the massive is fibrous, sometimes divergent, with a shining pearly lustre; it is translucent, yields easily to the knife, and is brittle. Colour grey, green, or brown. It is infusible before the blowpipe, except on the surface, but becomes white and opake, and tinges the flame of a dark purplish-red. It is soluble with effervescence in muriatic or nitric acid; and paper dipped into the solution, and then dried, burns with a red flame.
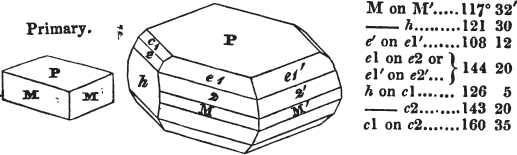
This mineral was described and its properties first determined by Dr Hope; it was discovered at Strontian in Argyleshire, in veins traversing gneiss, and accompanied by galena, barytes, calc-spar, and pyrites, in massive, stellated, fibrous, and diverging groups, but rarely presenting more than mere traces of crystallization. The finest asparagus green, as well as dark-brown fibrous varieties, are from this locality. In Yorkshire it occurs in acute snow-white pyramids; and at Braunsdorf in Saxony in brilliant white and brown hexagonal prisms. The most splendid crystals of strontites, however, have been found at Leogang in Saltzburg; but they are very rare.
* From its having been first found at Strontian in Scotland.
[page] 193
BARYSTRONTIANITE.
Stromnite, Traill.
Contains carbonate of strontia 68·6, sulphate of baryta 27·5, carbonate of lime 2·6, oxide of iron 0·1, loss 1·2—Trail.
Sp. Gr. 3·7.
Occurs massive, of a greyish-white colour externally, but approaching to yellowish-white internally on the fresh fracture; lustre weakly shining and pearly; translucent on the edges; brittle and soft. It effervesces with acids, but does not melt before the blowpipe.
This mineral was distinguished and described by Dr Traill, who found it in veins with galena and barytes in a kind of clayslate at Stromness* in Orkney. It appears to be very rare.
CELESTINE.
Sulphate of Strontia. Celestin, W. Prismatoidal Hal Baryte, M. Strontiane Sulphatée, H.
Combination of sulphuric acid and strontia.
Sulphuric acid 43·64, strontia 56·36—Beudant; even the purest varieties, however, are mixed with small portions of foreign matter.
Sp. Gr. 3·6—4·0. H.= 3·0—3·5.
This mineral is white, grey, yellow, or reddish; also of a delicate blue colour;† it occurs massive, fibrous, stellated, and crystallized; the primary form is a right rhombic prism of 104° and 76°, by measurements with the reflective goniometer, from the planes produced by cleavage; the transparent crystals are pretty readily divisible into that form. It possesses a shining lustre; is translucent, transparent, or opake; and is brittle. Before the blowpipe it decrepitates, and melts into a white, opake, friable enamel.
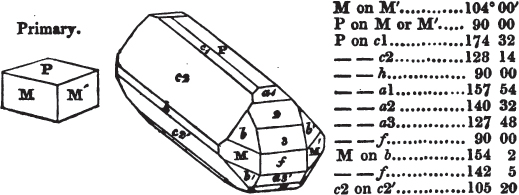
* Whence Stromnite; Barystrontianite, from its containing both baryta and strontia.
† Sometimes approaching to sky-blue, whence Celestine.
[page] 194
The sulphur mines of Sicily have long been celebrated for their magnificent groups of this substance; it there occurs in prismatic crystals, often beautifully transparent, aggregated, and either disposed on, or accompanied by, sulphur and gypsum. Tabular crystals, several inches in diameter, and of a bluish tinge, have been found at Strontian Island in Lake Erie; while numerous interesting forms on a smaller scale, occur at Bex in Switzerland, at Conil in Spain, and in the Vicentine. It is met with in straight fibrous concretions of a blue colour, imbedded in clay at Dornberg near Jena, and at Frankstown in Pennsylvania; in radiated scopiform groups, opake and of a bluish tinge, in red clay at Aust Ferry near Bristol; crystalline and massive in magnesian-limestone, near Knaresborough in Yorkshire; radiated and fibrous at Norton in Hanover (a variety which, according to Turner, contains twenty per cent. of sulphate of baryta); and in earthy nodules, cracked and hollow, at Monte Martre near Paris.
PHOSPHATE OF YTTRIA.
Phosphorsaure Yttererde, L. Xenotime, Beud. Phosphyttria, Berzel. Yttria Phosphatée, Necker.
Combination of phosphoric acid and yttria—mixed with traces of fluoric acid, and a little sub-phosphate of iron.
Phosphoric acid and fluoric acid 33·49, yttria 62·58, sub-phosphate of iron 3·93—Berzelius.
Sp. Gr. 4·14—4·55. H. = 4·5—5·0.
Primary form, a rectangular prism, with a square base.
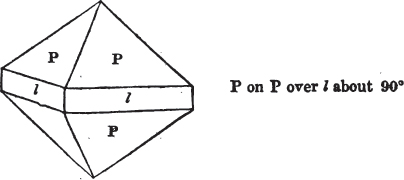
Colour yellowish-brown; with a resinous lustre; and pale brown streak. Cleavage perfect parallel to l; fracture uneven and splintery; nearly opake. Before the blowpipe it does not in the matrass yield water. On charcoal per se it is infusible; with borax it affords a colourless bead, which becomes milky on cooling; with salt of phosphorus is difficultly soluble into a colour less glass; and with boracic acid and iron wire yields phosphuret of iron. Insoluble in acids.
This mineral occurs in crystalline masses imbedded in granite at Lindenaes in Norway, where it was first noticed by Mr Tank of Friederickshall.
[page] 195
ACIDIFEROUS ALKALINE MINERALS.
UNDER this head are included such minerals as consist chiefly of an alkali united with an acid; but several of them are very impure in their native state.
NITRATE OF POTASH.
Nitre, Naturlischer Salpeter, W. Potasse Nitratée, H. Prismatic Nitre Salt, M.
Contains potash 46·46, nitric acid 53·54.
Sp. Gr. 1·9—2·0.
Primary form a rhombic prism of about 60° and 120°. Occurs in crusts, and in capillary crystals, of which the forms are not discernible; it is whitish or yellow; is translucent or transparent; brittle; saline, and cooling to the taste: it deflagrates when placed on a hot coal, and detonates with combustible substances.
It occurs on or near the surface of the earth, on old walls, &c. In Hungary, Persia, Arabia, Egypt, and in many of the plains of Spain, it is found in considerable quantities. It is also common in India, especially on a large plain near Agra in Bengal. The mountainous regions of Kentucky, which are calcareous and full of caverns, afford it to the inhabitants of North America. In South America, the plains bordering the sea near Lima are covered with it. It is not however produced naturally to an extent sufficient for its multiplied uses; and is therefore principally procured artificially from the decomposition of animal and vegetable substances.
Nitre is employed in medicine, the arts, and in metallurgy for assisting the processes of oxidating and smelting; but its principal, if not its chief use, is in the manufacture of gunpowder, for which that imported from Egypt is most esteemed, as it contains the least calcareous matter. Gunpowder consists of seventy-six parts of nitre, nine of sulphur, and fifteen of light charcoal.
[page] 196
SULPHATE OF POTASH.
Prismatoidal Glauber Salt, M. Aphthitalite, Shep. Potasse Sulphatée, N.
Sp. Gr. 1·731. H. = 2·5—8·0.
Massive; mamillary, apparently formed in successive layers. Colour white or yellow, with certain bluish or greenish stains. Lustre vitreous; translucent; taste saline and bitter; cleavage and fracture indistinct. Consists of sulphate of potash, with a trace of sulphate and muriate of copper.
The artificial crystals present right rhombic prisms, having their acute angles replaced, so as to form dihedral summits; they consist of sulphuric acid 45·93, and potash 54·07; they decrepitate when heated, and fuse at an increased temperature.
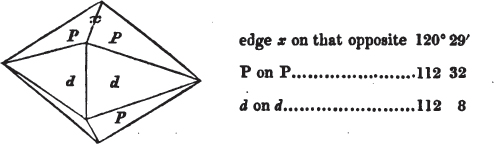
The natural salt has only been met with in a state of sublimation surrounding the fumaroles of volcanoes, and particularly at Vesuvius.
CARBONATE OF SODA.
Natron,* Beudant. Naturlisches Mineralalkali, W. Soude Carbonatée, H. Prismatic Natron Salt, M.
Combination of soda, carbonic acid, and water.
Analyses by Beudant.
| Hungary. | Egypt. | Vesuvius. | |
| Soda | 50·2 | 43·8 | 46·7 |
| Carbonic acid | 35·1 | 80·9 | 32·3 |
| Water | 14·7 | 13·5 | 14·0 |
| Sulphate of soda | 0·0 | 7·3 | 0·0 |
Sp. Gr. 1·5. H. = 1·0—1·5.
It is found crystallized, massive, fibrous, and sometimes radiated, in crusts, and efflorescent. When fresh, the massive is compact or granular, of a glistening lustre, and translucent, but on exposure it becomes opake. Colour grey or yellowish-white;
* Natron; from the desert of Natron, where it is said to have been anciently collected.
[page] 197
taste urinous and saline. It effervesces with acids, is very soluble in water, and melts readily before the blowpipe.
The natural crystals of this species are rarely found distinct. Being a salt which loses its water on exposure to a dry atmosphere, it occurs most frequently in the state of efflorescent powder on the surface of the earth, at the sides of lakes, or in natural caverns. In the plain of Debretzin in Hungary, it appears during the heat of summer in saline efflorescences like heaps of snow; also in Bohemia, and Italy. It is likewise met with either dissolved in the water of certain hot springs, as those of Carlsbad in Bohemia and Rykum in Iceland, or in some lakes, as the soda lakes of Egypt.
The natron both of Egypt and Hungary is imported in pulverulent masses of a dirty-grey colour. Its chief employment is in the manufacture of soap, but it enters also into the composition of glass, and is used in dyeing, bleaching, &c.
TRONA.
Urao, Beudant. Sesqui-carbonate of Soda of the Chemists. Hemi-prismatic Natron Salt, M.
Combination of soda, carbonic acid, and water.
| Crystallized, Barbary. | Fibrous. | Columbia. | |
| Soda | 37·43 | 38·62 | 41·22 |
| Carbonic acid | 39·27 | 40·13 | 39·00 |
| Water | 23·28 | 21·24 | 18·80 |
Sp.Gr.2·112. H. = 2·5—2·75.
Primary an oblique rhombic prism of 132° 30′ and 47° 30′.
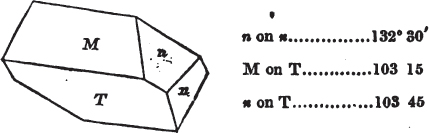
Cleavage perfect and easily obtained parallel to M. Surface of n and M smooth, of T generally striated horizontally. Seldom in distinct crystals. Colour white, inclining to yellowish-grey when impure. Transparent when in minute crystals, translucent in large masses; streak white; taste pungent and alkaline; fracture uneven; rather brittle. Soluble in water, though less so than natron. This substance is distinguished from the preceding
[page] 198
not only in crystalline form, but in superior specific gravity and hardness; in being more difficultly soluble in water and in its taste being less intensely alkaline; neither does it deliquesce as natron does, and it may be preserved for any length of time unchanged in a dry atmosphere.
It occurs in the province of Sukena in Egypt, forming a thin stratum in muriate of soda; also in Barbary, and in Maracaibo and Columbia in South America. Trona is its African, Urao its American name.
SULPHATE OF SODA.
Glauber Salt. Naturlisches Glaubersalz, W. Soude Sulphatée, H. Prismatic Glauber Salt, M. Exantholose, Beudant.
| Vesuvius. | Hildesheim. | |
| Contains Soda | 35·0 | 33·4 |
| Sulphuric acid | 44·8 | 42·5 |
| Water | 20·2 Beudant. | 18·8 Beudant. |
Sp. Gr. 1·47.
Primary form an oblique rhombic prism of 99° 36′ and 80° 24′.
Sulphate of soda is found in efflorescences of a yellow or greyish-white colour, or in an earthy form, but is more commonly dissolved in certain mineral waters; translucent or opake; lustre vitreous on the fresh fracture, dull on the surface; extremely efflorescent, and falling spontaneously into powder. It is cooling, bitter and saline to the taste, and is usually met with in the neighbourhood of rock-salt or brine springs. Before the blowpipe in the matrass it melts in its water of composition.
Sulphate of soda is found in the salt mines of Upper Austria, Hungary, and Switzerland; near Madrid in efflorescences at the bottom of a ravine; at Grenoble in France; in the workings of old mines, and sometimes on old walls, in the same manner as nitre. It is an ingredient of the hot springs of Carlsbad, Eger, and Sedlitz in Bohemia. When purified of the iron with which it is usually tinged in the native state, or when prepared artificially, it is used in medicine under the name of Glauber's Salt.
NITRATE OF SODA.
Zootinsalz, Breithaupt. Soude Nitratée, Necker.
| Consists of Soda | 45·03 | 37·2 |
| Nitric acid | 54·97 | 62·8 Gmelin. |
Sp. Gr. 2·1. H. = 1·5—2·0.
Primary form an obtuse rhomboid of about 106° and 74°; cleavage parallel to the faces of the primary. This salt appears occasionally
[page] 199
as an efflorescence, sometimes crystallized, more often intermixed with clay and sand; to the taste it is cool and bitter; it is deliquescent; and when exposed on heated charcoal it melts and deflagrates. It is described as occurring in immense quantity in the district of Tarapaca in Peru, near the frontiers of Chili. It there forms a bed several feet thick, which in many places appears on the surface, and occupies an extent of more than forty leagues.
BORATE OF SODA.
Boraxsaures Natron, L. Soude Boratée, H. Prismatic Borax Salt, M. Borax. Tincal.
| Contains Soda | 14·5 |
| Boracic acid | 37·0 |
| Water | 47·0—Klanroth. |
Sp. Gr. 1·74. H. = 2·0—2·5.
Tincal occurs in prismatic crystals, variously terminated, and yielding to mechanical division parallel to the lateral planes of the primary form—an oblique rhombic prism of 86° 30′ and 93°30′—and both its diagonals. The crystals are whitish, occasionally possess a tinge of blue or of green, and vary from translucent or nearly transparent, to opake. Taste feebly alkaline; soft, and brittle. Before the blowpipe it intumesces violently, and then fuses into a transparent globule.
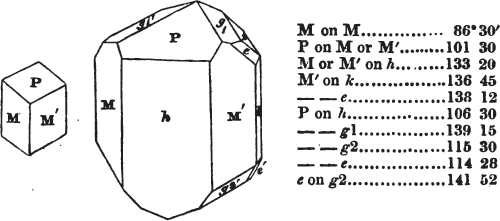
Tincal is chiefly brought from Thibet, where it is found on the surface of the soil in the vicinity and at the bottom of certain lakes. It is mentioned likewise from the province of Potosi in Peru. The borax in its crude state is called tincal, and is brought to Europe in the form of a brownish-grey impure salt, or in detached crystals resembling the above figure.
It is employed as a flux in several metallurgical processes, and in the manufacture of solder.
[page] 200
MURIATE OF SODA.
Hexahedral Rock Salt, M. Steinsalz, Leonhard. Sal Mare, Beudant. Sel Gemme, Necker.
Rock-salt consists of muriatic acid and soda, in the proportion of 46·71 to 53·29; it, however, always contains some impurities. The Cheshire salt yielded to Henry, muriate of soda 98·32, sulphate of lime 0·65, muriate of magnesia 0·02, muriate of lime 0·01, undissolved matter 1·00. Sp. Gr. 2·3. H. = 2·0. It occurs in beds or masses; sometimes crystallized in the form of the cube, which is that of its primary crystal, and into which, when pure, it may readily be cleaved; lustre vitreous; translucent or transparent; when pure, colourless or white; but when with any foreign admixture, reddish-brown, brick-red, violet-blue, and green. It yields with facility to the knife; and when scratched with the nail receives an impression, but yields no powder. It attracts moisture, but remains unaltered in a dry atmosphere. It has sometimes, though rarely, a fibrous texture.
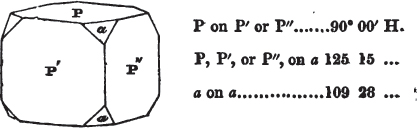
Muriate of soda is one of the most abundant substances in nature. Not only is it found in large beds and masses, but also in the waters of certain springs and lakes, and in those of every sea. It forms about one-thirtieth part of the waters of the ocean.
Rock-salt is commonly disposed in thick beds, either superficially, as in Africa, or at a very great depth, as in Poland; sometimes also at a high level, as in the Cordilleras of America, and in Savoy, where it occurs at an elevation equal to that of perpetual snow.
Its principal European deposits are the salt-mines of Wieliczka in Poland, where perfect cubes are frequently met with; the Saltzkammergut in Upper Austria, Hallein in Saltzburg, and Hall in the Tyrol, in which it is accompanied with, and imbedded in clay, gypsum, and other extraneous matter; and Northwich in Cheshire, where it occasionally presents pure, transparent, and highly cleavable specimens. All these deposits afford extensive supplies for culinary and other economic purposes, though generally in a state so far from pure as to render the process of solution and subsequent evaporation indispensable.
[page] 201
SULPHATE OF AMMONIA.
Mascagnin, Karsten. Ammoniaque Sulphatée, H.
Contains ammonia 22·80, sulphuric acid 53·29, water 23·91— Gmelin.
Sulphate of ammonia has an acrid, bitter taste. Its colour is greyish or yellow, and it generally occurs stalactitic, pulverulent, or in mealy efflorescences; translucent or opake; attracts moisture from the atmosphere, and is entirely volatile at a high temperature. It is found in the fissures of the earth, and among the lavas of Etna and Vesuvius; in the Solfatara; and in the lagune near Sienna in Tuscany.
MURIATE OF AMMONIA.
Sal Ammoniac. Naturlicher Salmiak, W. Ammoniaque Muriatée, H. Octahedral Ammoniac Salt, M.
When pure, it consists of ammonia 32·06, muriatic acid 51·16, water 16·78. Two varieties yielded to Klaproth,
| Vesuvius. | Bucharia. | |
| Muriate of ammonia | 99·5 | 97·5 |
| Sulphate of ammonia | 0·5 | 2·5 |
Sp. Gr. 1·45 to 1·5. H. = 1·5—2·0.
Primary form the regular octahedron. It occurs massive; with a fibrous texture; plumose; in crusts; and in octahedral crystals of a minute size. Colour, when pure, white, grey, or yellow; generally pungent and saline to the taste; transparent or opake; externally dull or glistening; internally shining and vitreous. This salt is readily soluble in water, but does not attract moisture on exposure to the air. It is completely volatile at a high temperature, rising in white fumes; and emits, when triturated with lime, a pungent ammoniacal odour.
It is principally found in the neighbourhood of volcanoes, sublimed among other volatile substances, in the cracks and fissures of lava. It thus occurs at Etna and Vesuvius, in the island of Volcano, and at the Solfatara near Naples. Small quantities have been noticed in the vicinity of ignited coal seams, as at St Etienne in France, in Scotland, and Newcastle. A variety presenting a greyish-white colour, and conchoidal fracture, is mentioned as occurring with sulphur, in rocks of indurated clay or clay-slate in Bucharia. Though extensively employed in dyeing and in medicine, this salt is of trifling importance as found in nature, from its scarcity.
I 2
[page] 202
ACIDFEROUS ALKALINO-EARTHY MINERALS.
THE mineral substances included under this head are few in number.
ALUM*
Naturlischer Alaun, W. Alumine Sulfatée Alkaline, H. Octahedral Alum Salt, M. Alaun, L.
Combination of sulphate of alumina, sulphate of potash, and water; and contains alumina 10·8, potash 10·1, sulphuric acid 33·7, water 45·4—Gmelin.
Sp. Gr. 1·75.
Form the octahedron, though it chiefly occurs in fibrous masses, or as an efflorescence on argillaceous minerals, as alum-slate, alum-stone, &c. Colour white or greyish; lustre vitreous, transparent or translucent; to the taste sweetish, styptic, and acidulous. When artificially prepared, it crystallizes in the octahedron, which is its primary form, and in some of its varieties. It is soluble in about twenty times its weight of cold, and little more than its own weight of boiling water. On exposure to heat it melts in its water of crystallization, froths up in a remarkable manner, and is converted into a spongiform mass of anhydrous alum.
It is found on the alum-slate rocks near Christiania in Norway: in strata of brown coal in Bohemia; on the lavas of Stromboli, Nevis, and other volcanoes; in bituminous shale and slate-clay at Hurlet near Paisley; and near Whitby in Yorkshire.
It is used in dyeing, in medicine, in the manufacture of paper and leather, and for the prevention of putrefaction.
* Alum, from its base consisting of alumina.
[page] 203
ALUM-STONE.
Alunite, Necker. Alaunstein, W. Rhombohedral Alum Haloide, M.
Combination of sulphuric acid, alumina, potash. and water.
| Crystallized, Tolfa. | Montione. | |
| Sulphuric acid | 35·49 | 35·6 |
| Alumina | 39·65 | 40·0 |
| Potash | 10·00 | 13·8 |
| Water | 14·83—Cordier. | 10·6—Descotils. |
Sp. (Gr. 2·7—2·75. H. = 5·0.
The colour of this mineral is usually greyish-white, occasionally red; and it occurs both massive and crystallized, the crystals generally occupying the cavities of the mass; they are minute, shining, and sometimes brownish externally; their form is an obtuse rhomboid of 92° 50′ and 87° 10′; but the rhomboid is variously modified, one or more of the solid angles being generally replaced. Cleavage distinct perpendicular to the axis. The massive is translucent and easily frangible, being frequently mixed mechanically with silica, and appearing cellular and porous. It decrepitates under the blowpipe, but does not fuse per se. With borax it forms a transparent colourless globule, but is not affected by soda; when pounded it is soluble in sulphuric acid.
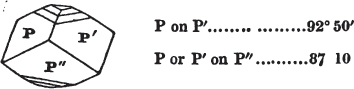
It occurs well crystallized in a secondary rock at Tolfa, near Civita Vecchia, in the Roman States; decomposed and friable at the Isle of Nevis, and in the crater of Volcano; and so compact and hard at Beregh in Hungary, as to be there employed in the formation of millstones.
POLYHALLITE.*
Stromeyer.
| Sulphate of lime combined with water | 28·45 |
| Anhydrous sulphate of lime | 22·42 |
| Anhydrous sulphate of magnesia | 20·03 |
| Sulphate of potash | 27·70 |
| Muriate of soda | 0·19 |
| Red oxide of iron | 0·34—Stromeyer. |
Sp. Gr. 2·77. H. = 2·5—3·0.
* From the Greek ϰολας, αλς, signifying a stone of many salts; in allusion to its composition.
[page] 204
In masses, which are either compact, or present a fibrous texture, the fibres being parallel and mostly curved Of a brick- or flesh-red colour, and somewhat translucent; brittle; has a resinous or pearly lustre; taste bitter and astringent, but very faint. It is slightly acted upon by exposure to a moist atmosphere, but its solubility in water is very inconsiderable. In the flame of a candle it immediately forms an opake brownish-coloured mass; and melts instantaneously under the blowpipe.
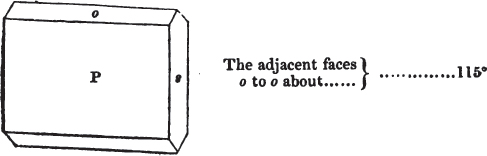
It is found at Ischel and Aussee, in Upper Austria, in beds of rock-salt, and at Hall in the Tyrol. The mineral noticed by Leonhard under the name of Bloedit (after the Dresden mineralogist Bloede) as occurring at Ischel with the polyhallite, and described as being soft, of a close fibrous texture, of a colour between fleshand brick-red, as transparent and brilliant, but losing both these characters by exposure,—is probably only an impure variety of this species. (Manual.)
CRYOLITE.*
Kryolith,W. Alumine Fluatée Alkaline, H. Prismatic Cryone Haloide, M.
Combination of fluoric acid, soda, and alumina.
| Alumina | 24·0 | 24·40 |
| Soda | 36·0 | 31·35 |
| Fluoric acid | 40·0—Vauquelin. | 44·25—Berzelius. |
Sp. Gr.2·96. H. = 2·5—30.
It occurs massive; white, but, when associated with iron, yellow or brown; structure perfectly lamellar, parallel to all the planes of a rectangular prism; translucent, but by immersion in water it becomes transparent, and admits more readily of cleavage when retained in that fluid for some time. It is very fusible, dissolving even in the flame of a taper. Before the blowpipe, on charcoal, it melts into a transparent globule while hot, but which becomes opake on cooling.
The only known locality of this mineral is Arksut-fiord in West Greenland, where it occurs in veins in gneiss, accompanied with carbonate of iron, pyrites, galena, quartz, and felspar.
* Cryolite, from the Greek, in allusion to its easy fusibility,—to its melting before the blowpipe like ice.
[page] 205
GLAUBERITE*
Glauberite, Bt. H. Henri-Prismatic Brythine Salt, M. Brogniartin, L.
Combination of sulphuric acid, lime, and soda, without water.
| Villa Rubia. | |
| Sulphate of lime | 49·0 |
| Sulphate of soda | 51·0—Brogniart. |
Sp. Gr. 2·75 to 2·85. H. = 2·5—3·0.
It occurs massive; also crystallized in the form of oblique and extremely flat rhombic prisms, the crystals being for the most part constituted only of the planes P, e, and e′ of the following figure; but they yield readily to mechanical division, parallel to the faces P, M, and M', the primary form being an oblique rhombic prism, whose terminal planes incline from one acute edge of the prism to the other, and whose lateral angles are alternately 83°20′ and 96° 40′. Colour pale yellow or grey; translucent, rarely transparent; taste slightly saline; lustre vitreous; fracture conchoidal: when immersed in water, it becomes opake and is partly soluble. Before the blowpipe it decrepitates, and then melts into a white enamel.
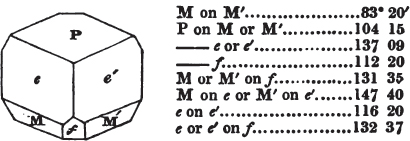
It is found at Villa Rubia, ten leagues south of Madrid, in Spain, imbedded in rock-salt; also at Aussee in Upper Austria. Its optical properties, according to Sir David Brewster, are very peculiar—having one axis of double refraction for violet, and two axes for red light.
REUSSITE.
Sulphate of soda 66·04, sulphate of magnesia 31·35, muriate of magnesia 2·19, sulphate of lime 0·42—Reuss.
Occurs in mealy efflorescences, flat six-sided prisms, and acicular crystals. Colour white, shining; fracture conchoidal.
This substance forms superficial efflorescences in the vicinity of Sedlitz and Seidschutz in Bohemia.
* From its containing a very large proportion of Glauber's salt, or sulphate of soda.
[page] 206
SODA-ALUM.
Thomson.
Sulphuric acid 38·5, alumina 12·0, soda 7·5, water 42·0, with a little silica, lime, iron, and manganese—Thomson.
Sp. Gr. 1·88. H. about 2·0.
Occurs in white fibrous masses, the outer fibres opake from decomposition, internally transparent, and exhibiting a glossy or silky aspect. Resembles alum in taste, but is more soluble in water.
Is found in irregular nodules resembling fibrous gypsum, imbedded in soft blue slate, at St Juan in South America; and Beudant mentions it as also occurring in the solfataras of Milo in the Archipelago.
GAYLUSSITE.*
Cordier. Boussingault.
Combination of carbonic acid, soda, lime, and water.
| Lagunilla. | |
| Contains Carbonic acid | 28·66 |
| Soda | 20·44 |
| Lime | 17·70 |
| Water | 32·20 |
| Alumina | 1·00—Boussinerault, |
Sp. Gr. 1·92—1·95. H. 20—30.
Occurs in detached lengthened prisms, and aggregated crystals disseminated in clay; the less perfect of them might be mistaken for selenite, the more perfect and smooth have rather the aspect of calcareous spar. Primary, an oblique rhombic prism of 109° 30′ and 70° 30′, according to Beudant; of 111° 10′ and 68° 50′, according to Leonhard. Surfaces striated; limpid and colourless, or of a dirty white; transparent, with marked double refraction; or translucent; fracture conchoidal, with a vitreous lustre; very brittle; easily reduced to a grey powder, and soluble with brisk effervescence in nitric acid. When reduced to powder, is, to a trifling extent, soluble in water. In the matrass it decrepitates slightly, gives off water, and becomes opake, decrepitation continuing until it has acquired a red heat. Before the blowpipe it fuses rapidly into an opake globule, which has a distinctly alkaline taste.
This mineral is found abundantly at Lagunilla near Merida, in
* In honour of the celebrated French chemist Gay-Lussac.
[page] 207
Maracaibo, disseminated at the bottom of a lake in a bed of clay, covering trona, which the natives term urao, in contradistinction to the gaylussite, the latter, from its generally elongated form, being denominated clavos or nails, (manual.)
NATIVE CARBONATE OF LIME AND SODA.
Barrnel.
| Contains Carbonate of lime | 70·0 |
| Carbonate of soda | 14·0 |
| Water | 9·7 |
| Peroxide of iron | 1·0 |
| Matrix | 5·0 |
Sp. Gr. 2·92. H. = 3·0—3·5.
Primary form a rhomb, not differing materially from that of calc-spar. Cleavage in three directions parallel to the faces of the primary. Lustre vitreous; structure laminated; fragments perfectly transparent; possesses double refraction. Before the blowpipe it decrepitates a little, becomes brown, and eventually is reduced to lime. Entirely soluble, and with effervescence, in nitric acid.
Locality unknown.
SULPHATE OF ALUMINA AND AMMONIA.
Ammonalum, Necker and Beudant.
Combination of sulphuric acid, alumina, ammonia, and water.
| Tschermig. | ||
| Sulphate of alumina | 37·0 | 36·68 |
| Sulphate of ammonia | 18·0 | 12·47 |
| Sulphate of magnesia | 0·0 | 0·33 |
| Water | 45·0 Lampadius. | 43·39 Stromeyer. |
Primary form the cube or the regular octahedron. White; taste bitter. Before the blowpipe in the matrass it yields water, intumesces, and forms a sublimation of the sulphate of ammonia, which is soluble in water. The dried mass becomes blue with solution of cobalt.
Occurs in small fibrous masses in the lignite of Tschermig in Bohemia.
[page] 208
NATIVE METALS
AND
METALLIFEROUS MINERALS.
INCLUDING such metals as are found nearly pure in the native. state; or variously combined with other substances, forming metalliferous ores; as, with other metals, with sulphur, with oxygen; also in the state of oxides, minerallized by acids; beginning with the oldest and most universally diffused of the metals—Iron.
NATIVE IRON.
Gediegen Eisen, W. Fer Natif, H. Octahedral Iron, M.
| Agram. | Siberia. | Mexico. | Atacama. | |
| Iron | 96·5 | 98·5 | 96·75 | 93·40 |
| Nickel | 3·5 | 1·5 | 3·25 | 6·62 |
| Cobalt | 0·0 | 0·0 | 0·00 | 0·53 |
| Klaproth. | Turner. |
Sp. Gr. 7·44 to 7·8. H. = 4·5.
Primary form the regular octahedron. Colour pale steel-grey; lustre metallic; acts powerfully on the magnet; is soluble in all the acids.
Native iron has been noticed under three different forms.
1. At Kamsdorf in Saxony, disseminated through a mass of brown oxide of iron mingled with spathose iron and sulphate of barytes; in this Klaproth found about 6 per cent. of lead, and 1·5 of copper. It is not however considered a natural production; but, like the native steel from La Bouiche in France, appears to be of secondary formation. The American mineralogists, however, describe a variety from Canaan in Connecticut, which forms a vein about two inches thick in mica-slate.
2. Native volcanic iron. Fer natif volcanique, H. Was discovered in a ravine formed by torrents across the lava and scoriæ of the mountain of Graveneire, in Auvergne.
3. Native meteoric iron. Fer natif meteorique, H. This occurs in irregular isolated masses, sometimes of very considerable size, in different parts of the globe: but the only piece described
[page] 209
as having been seen to fall from the atmosphere is that of Hraschina, near Agram in Croatia. The mass found in Siberia by Professor Pallas exhibited a vesicular structure, and contained crystals and grains of chrysolite; that discovered by Don Rubin de Celis, in the district of Chaco-Gualamba in South America, weighed about fifteen tons.
Many masses are scattered over the continent of North America, as in Louisiana, and still farther north in the countries inhabited by the Esquimaux; several specimens also occur in Africa, as in the Senegal river, and near the Cape of Good Hope. With the exception of the Siberian variety mentioned by Pallas, and a mass lately noticed in the Atacama desert of Peru, both of which had a vesicular appearance and contained straw-yellow coloured olivine, native irons have uniformly presented a solid structure.
Iron has also been found entering into the composition of those stony masses termed meteoric stones or aerolites, which have been seen to fell from the atmosphere in various parts of the world, and even in our own country. These, however, may with more propriety be looked upon as mixed minerals or rocks, than as distinct species.
IRON PYRITES*
Schwefelkies, W. Fer Sulphuré, H. Bt. Hexahedral Iron Pyrites, M. Pyrite Martiale, Br.
combination of iron and sulphur.
| Iron | 47·85 | 45·74, |
| Sulphur | 52·15—Hatchett. | 54·26—Berzelius. |
Sp. Gr. 4·75 to 5·0. H. = 6·0—6·5.
Colour brass-yellow, sometimes approaching to bronze-yellow, occasionally to steel-grey; often brown, owing to decomposition; lustre metallic; streak brownish- or greenish-black. It occurs disseminated in rocks, veins, and beds, investing other minerals, and sometimes enclosed in them; also amorphous, mamillated, globular, cellular, stalactitical, pseudomorphous, capillary, and crystallized in the cube and octahedron, and in forms common to them both as primary crystals. It yields to cleavage parallel to all the planes of the cube and regular octahedron, affording surfaces sufficiently brilliant for the use of the reflective goniometer, but with the greatest ease and brilliancy parallel to those of the cube; its fracture is granular or uneven, sometimes approaching to conchoidal; it is brittle, but does not yield to the knife, which serves at once to distinguish it from copper pyrites,
* Pyrites, from the Greek, in allusion to its giving sparks when struck.
[page] 210
which it readily scratches. In the flame of a candle it becomes red; the sulphur being driven off, and an oxide of iron, which is magnetic, remaining. After a lengthened exposure to the reducing flame of the blowpipe, it forms an uneven and crystalline black mass.
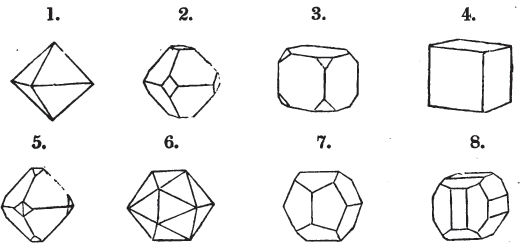
Fig. 1,the primary; 2, the same, its solid angles replaced; the 3d more deeply, passing into the cube; 4, the cube; 5, the primary, each solid angle replaced by two planes; which are increased in fig. 6, complete in fig. 7, forming the pentagonal dodecahedron, and connected with the planes of the cube in fig. 8.
The above figure and the following measurements are given on the authority of Haüy.
[page] 211

Iron pyrites is universally diffused, and is found in most descriptions of rock. Cubes of gigantic dimensions have occurred in some of the Cornish mines; very perfect octahedrons, also of large size, are found at Persberg in Sweden; and crystals, forming pentagonal dodecahedrons of three or four inches in diameter, in Elba. Alston Moor, Derbyshire, and the mining districts of Cornwall, afford it in great profusion, and under various forms, sometimes crystallized, frequently coating fluor spar, galena, and other minerals; and very brilliant crystals, remarkable for the variety of their facets, occur at Traversella in Piedmont.
Hepatic Pyrites. Fer sulfuré epigène. It occurs in most of the forms assumed by common iron pyrites. Externally it presents a liver-brown colour (hence its name); internally is steelgrey, with more or less lustre. Its colours appear to arise from the progress of decomposition, which often proceeds so far as to divest the substance of internal metallic lustre, without altering its form. It occurs in veins in primitive rocks.
Arsenical Iron Pyrites. Fer sulfuré arsenicale, H. Is of a paler yellow colour than common iron pyrites, passing, according to the proportion of arsenic it contains, into steel-grey. When struck with the hammer, and also before the blowpipe, it yields arsenical as well as sulphureous vapours. Sometimes it is magnetic. It occurs with common pyrites, and arsenical cobalt, in Sweden and in Cornwall.
Auriferous Iron Pyrites. Fer sulphuré aurifère, H. Occurs in grains of a deep yellow colour; also crystallized in cubes, of which all the planes are deeply striated, and sometimes partially decomposed on the surface. The gold it contains is but small, and exists, according to Bergmann, enclosed in the pyrites in small angular grains, and therefore in a state of simple mixture.
It is found abundantly in the gold mines of Beresoff in Siberia, and in Brazil in detached crystals.
[page] 212
WHITE IRON PYRITES.
Cockscomb Pyrites, A. Sparkies, Kamkies, Strahlkies, W. Fer Sulfuré Blanc, H. Prismatic Iron Pyrites, M.
Combination similar to that of the preceding; its strong tendency, however, to decompose on exposure, and the efflorescences it affords of the sulphate of iron, appear to indicate some difference in the composition.
| Iron | 45·07 | 45·56 |
| Sulphur | 53·35—Berzelius. | 54·34—Hatchett. |
with minute proportions of silica and manganese.
Sp. Gr. 4·69 to 4·84.
Colour nearly tin-white, hence its name, occasionally tarnished with a tinge of yellow or grey. It occurs stalactitical, reniform, botryoidal; and in crystals which assume the form of modified, rhombic prisms. The variety termed spear-pyrites is found only in very flat crystals, having at first sight the appearance of dodecahedrons with triangular planes, but which are macles, consisting of similar portions of five crystals, connected as in the last of the three following figures. Cockscomb pyrites, or kamkies, is of the same description, the individuals being aggregated in such a manner as frequently to represent the crest or comb of a cock. Primary form a right rhombic prism of about 106° and 74°; parallel to the planes of which it yields to cleavage.
It occurs detached and imbedded in plastic clay at Littmitz and Altsattel, near Carlsbad in Bohemia; in Derbyshire accompanying galena and fluor spar; and in the western part of Cornwall in extremely delicate stalactitic concretions.
[page] 213
MAGNETIC IRON PYRITES.
Magnetkies, W. Fer Sulfuré Ferrifère, Fer Sulfuré Magnetique, H. Rhombohedral Iron Pyrites, M.
| Cornwall. | Uton. | Pyrenees. | ||
| Iron | 63·5 | 59·85 | 56·37 | 60·32 |
| Sulphur | 36·5 | 40·15 | 43·63 | 38·78 |
| Hatchett. | Stromeyer. | Stromeyer. | Rose. |
Sp. Gr. 4·4—4·7. H. = 3·5—4·5.
Colour bronze-yellow, reddish, or brownish; subject to speedy tarnish on exposure to the air; lustre metallic. Bournon describes it as occurring in irregular six-sided prisms variously modified; the cleavage being parallel to the terminal planes of the prism, but it is rarely found crystallized. The massive varieties frequently exhibit a lamellar structure, yielding to cleavage parallel to all the planes of a regular six-sided prism; fracture uneven passing into imperfect conchoidal. Acts on the magnet. Before the blowpipe it affords similar results to the preceding species; and is soluble in dilute sulphuric acid.

The crystalline varieties of this species have been noticed at Kongsberg in Norway, and at Andreasberg in the Hartz. The cleavable varieties are principally from Bodenmais in Bavaria, where they are associated with iolite; and the granular, compact, and massive, from Cornwall, Appin in Argyleshire, Saxony and Silesia.
ARSENICAL IRON.
Mispickel. Fer arsenical, H. Bt. Prismatic Arsenical Pyrites, M. Arsenik-kies of the Germans.
Combination of sulphuret of iron and arseniuret of iron.
| Freyberg. | |||
| Iron | 34·94 | 36·04 | 36·4 |
| Arsenic | 43·42 | 42·88 | 48·1 |
| Sulphur | 20·13 | 21·08 | 15·4 |
| Chevreul. | Stromeyer. | Thomson. |
Sp. Gr. 5·7 to 6·2. H. = 5·5—6·0.
It is nearly of a tin-white colour, sometimes with a tinge of yellow. It occurs massive, acicular, and crystallized in the form of a right rhombic prism, parallel to whose planes it may be cleaved (to the lateral planes most readily), affording, by the
[page] 214
planes so produced, angles of 111° 12′ and 68° 48′; this prism is considered the primary form of mispickel; fracture uneven, with a metallic lustre. It gives fire with steel, and the sparks are attended with a little train of white smoke, having an alliaceous odour. Before the blowpipe on charcoal it gives out copious arsenical vapours, and forms a globule of nearly pure sulphuret of iron, which attracts the magnetic needle.
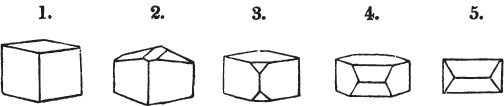
Fig. 1, the primary; a right rhombic prism. Fig. 2, the same, of which each terminal plane is in part replaced by two faces slightly inclining on the acute angles of the prism. Fig. 3 exhibits the primary prism modified by triangular planes replacing each obtuse solid angle: these planes are further advanced in fig. 4, and still further in fig. 5; having totally replaced the terminal faces, and reduced the lateral planes to triangles, meeting two and two at the acute edges of the prism.
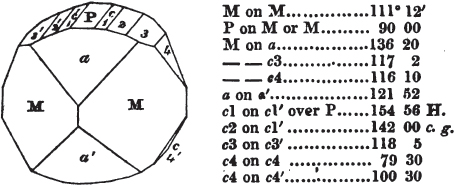
It chiefly occurs in the veins and beds of primitive mountains, accompanying ores of silver, lead, and tin. It is found abundantly at Freyberg, Munzig, and other mining districts of Saxony; at Andreasberg in the Hartz; at Joachimsthal in Bohemia; and at Wheal Maudlin and many others of the Cornish mines.
Argentiferous Arsenical Iron. Weisserz, W. Fer arsenical argentifère, H. It is whiter than pure arsenical iron, being of a somewhat silvery-white: but agrees with it in all other characters, except that it contains from 1 to 15 per cent of silver. That of Andreasberg consists of 44 iron, 35 arsenic, 13 silver, and 4 antimony—Klaproth.
At Freyberg and Braunsdorf in Saxony, it is worked for the silver it contains.
According to Brogniart, arsenical iron sometimes contains gold, and even cobalt in small proportion, without any sensible alteration of external character.
[page] 215
OXYDULATED IRON.
Magneteisenstein, W. Fer Oxydulé, H. Bt. Octahedral Iron Ore, M. Magnetic Iron Ore, J. A. Siderite Aimant, Necker.
Consists entirely of iron and oxygen, in the proportion, according to Berzelius, of two atoms peroxide to one protoxide; contains 28-21 per cent of oxygen.
Occurs earthy, compact, lamelliform, and crystallized in the regular octahedron, which is considered to be its primary form; structure imperfectly lamellar parallel to the planes of the octahedron; fracture uneven, or conchoidal with a splendent lustre; colour iron-black, with a shining or glimmering metallic lustre; streak black. It is highly magnetic, with polarity, especially the massive (Native Loadstone), and attracts iron filings. Before the blowpipe it becomes brown, and loses its influence on the magnet, but does not fuse. Colours glass of borax in the oxydating flame deep red, which becomes dingy yellow and impure on cooling; and in the reducing flame bottle-green. Soluble in heated muriatic, but not in nitric acid.
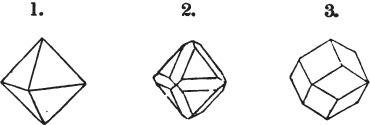
Fig. 1, the primary; the regular octahedron. Fig. 2, the same, of which all the edges are replaced by planes: this replacement has proceeded further, and the planes are complete, in fig. 3, the rhombic dodecahedron.
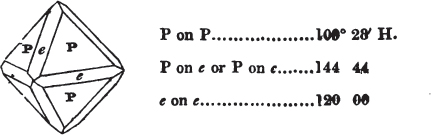
Oxydulated iron is most common in primitive countries, generally forming beds and large irregular masses; and accompanied by hornblende, granular limestone, and garnet; occasionally also by blende, pyrites, fluor, tin, and galena. The extensive beds of Arendal in Norway, and almost all the celebrated iron mines of Sweden, consist of massive magnetic iron. Dannemora, Gellivara, and the Taberg (a mountain of considerable dimensions), are entirely formed of it, and immense quantities of iron are annually obtained from these localities. It is plentifully found also
[page] 216
in Corsica, Savoy, Saxony, Bohemia, Silesia, Russia, and the East Indies; in North America, in beds in granitic mountains, with little interruption, from Canada to New York, and in many other districts.
In Scotland, it occurs in serpentine in Unst, one of the Shetland Isles. In England, in the parishes of St Roach and St Stephens, and at Treliswell near Penryn in Cornwall. The most interesting crystallized varieties occur imbedded in chlorite-slate at Fahlun in Sweden, at Normark in Wermeland, at Traversella in Piedmont, and among the ejected masses of Mount Vesuvius.
Siberia, Elba, Sweden, and the Hartz, yield the most powerful natural magnets; these usually form either compact or earthy amorphous masses, and are unknown crystallized.
Titaniferous Oxydulated Iron. Fer Titané, Cordier. Siderite Titanique, Necker. Titaneisen, Leonhard. A combination of the protoxide of iron with the oxide of titanium; in very different proportions, however. One variety from Auvergne yielded iron 82, titanium 13, and manganese 5; while in others the proportion of titanium is much larger, and it is therefore impossible to draw any accurate line of distinction between this variety and the menaccanite.
SPECULAR* IRON.
Eisenglanz, W. Fer Oligiste,* H. Fer Speculaire, Br. Iron Glance, J. Fer Oxydé, Berz. Eisenoxyd, L. Rhombohedral Iron Ore, M.
It is the pure peroxide of iron, in the proportion, according to Beudant, of iron 69·34, to oxygen 30·66.
Sp. Gr. 5·0—5·3. H.= 5·5—6·5.
It is considerably magnetic, especially the highly crystalline, but does not, like oxydulated iron, attract iron filings. It occurs lamellar, and crystallized in many forms which are derived from a slightly acute rhomboid of 86° 10′ and 93° 50′, the structure of the crystallized being lamellar, and reducible into the form of its primary crystal; fracture uneven, passing into conchoidal; colour deep steel-grey, with a brilliant and often iridescent tarnish externally; internally it possesses a shining lustre; opake in large fragments, but the edges of thin laminæ present a blood-red colour by transmitted light. Streak cherryred, or reddish-brown; occasionally acts feebly on the magnet; is infusible without addition, but with borax forms a green or yellow glass, like pure oxide of iron.
* Specular, from its brilliancy: Oligiste, from the Greek, in allusion to its containing only a small portion of metal.
[page] 217
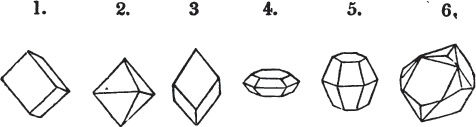
Fig. 1, the primary; a slightly acute rhomboid. Fig. 2, an octahedron, arising from the replacement of the upper and lower acute angles of the primary Fig. 3, an acute rhomhoid, of which only the upper plane, and that parallel with it, correspond with the planes of the primary. The upper and lower lateral faces of fig. 4 belong alternately to the primary. In fig. 5 none of the primary planes are visible. Of fig. 6 all the larger planes belong to the primary, and the small triangular faces result from the replacement of its lateral and lower angles by two planes, and the upper acute angle by three planes. Sometimes the edges and angles are so rounded that the crystals assume a lenticular form.
The crystals of this substance, however, and especially those from Elba, most commonly occur in the general form of the second of the two following figures.
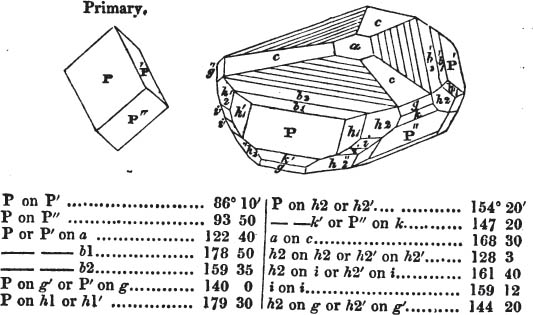
It occurs in transition and primitive rocks, both in beds and veins, and is accompanied by oxydulated iron, &c.
The mines of this substance in the Isle of Elba are of great extent, and are said to have been worked upwards of 3000 years; the surfaces of the splendid crystals from this locality frequently present the most magnificent tarnish-colours. It is also met with in Saxony; in Bohemia in beds of mica-slate; in specimens consisting of large crystalline plates, grouped together in the form of rosettes, accompanying adularia at St Gothard; at Arendal in
K.
[page] 218
Norway; at Langbanshyttan in Sweden; in South America; and in Siberia. In England, it occurs finely crystallized in one or two of the Cornish mines. Very resplendent crystalline plates, sometimes of considerable dimensions, and intersecting each other at various angles, are formed by sublimation in the fissures of lava at Stromboli and Lipari; likewise, though in smaller individuals, at Etna, Vesuvius, and in Auvergne.
A micaceous variety, consisting of minute shining scales, either loose or slightly cohering, which appears by reflected light of an iron-black, sometimes tinged red, and by transmitted light, blood-red—occurs at Tavistock in Devonshire, and near Dunkeld in Perthshire.
RED HÆMATITE *
Red Iron Ore. Red Iron-stone, J. Rother Eisenstein, W.
| Contains Peroxide of iron | 94·0 |
| Silica | 2·0 |
| Lime | 1·0 |
| Water | 3·0—D'Aubuission. |
The more compact hæmatites sometimes slightly affect the magnet, rendering it probable that they contain a small portion of the protoxide of iron. None of them are blood-red by transmitted light; and they never assume acrystalline form.
The fibrous variety (Rother Glaskopf, W. Fer oligiste concretionné, H.) has externally a bluish or iron-grey colour, and presents either a metallic lustre, or is red and without lustre; internally it is red or brownish-red. It occurs in botryoidal masses, or in stalactites, formed of concentric coats, and having a fibrous or radiated structure.
It occurs abundantly in Saxony, Bohemia, the Palatinate, Silesia, and the Hartz; also near Ulverstone in Lancashire, and in smaller quantities in many parts of England and Scotland. It affords excellent iron both cast and malleable. When ground to fine powder it is employed in the polishing of metals.
The scaly variety (Rother Eisenrahm, W.) occurs in slightly cohering scales or particles of a red colour with a tinge of brown, and opake; the lustre is somewhat metallic. It is unctuous to the touch, and stains the fingers. It accompanies the preceding, but is principally known from Cattas Altas in the Brazils.
* Hæmatite, from the Greek, in allusion to its blood-red colour. Now, however, the original meaning of the term is so far lost sight of, that we have brown and even black hæmatite.
[page] 219
FRANKLINITE.*
Franklinite, Berthier. Dodecahedral Iron Ore, M. Zinc Oxydé Ferrifère, H. Siderite Zincifère, Necker.
Combination of the peroxide of iron, with the oxide of zinc, and red oxide of manganese.
| New Jersey. | |||
| Peroxide of iron | 66·0 | 66·10 | 68·86 |
| Oxide of zinc | 17·0 | 17·43 | 10·81 |
| Red oxide of manganese | 16·0 | 14·96 | 18·17 |
| Berthier. | Thomson. | Abich. | |
Sp. Gr. 5·0—5·1. H. = 6·0—6·5.
This mineral bears much resemblance to oxidulated iron. It occurs in grains or in granular masses composed of imperfect crystals, occasionally exhibiting the planes of the octahedron, and those replacing its edges, but bounded more often by the irregular faces produced by contact; the structure is lamellar, parallel to the planes of the regular octahedron; brittle; fracture conchoidal; streak deep reddish brown—distinguishing it from oxidulated iron, the streak of which is black. Acts slightly on the magnet; soluble without effervescence in heated muriatic acid. At a high temperature the zinc is driven off, and a hard compound of iron and manganese remains. Before the blowpipe with borax it forms a green glass, which when completely saturated becomes red, and on cooling assumes a greenish-brown colour, and remains transparent; with salt of phosphorus it yields a yellowish-grey glass, and with soda is insoluble.
The measurements by the reflective goniometer prove the regular octahedron to be its primary form.
It occurs in Sussex County, New Jersey, accompanying the red oxide of zinc, and is frequently imbedded in calcareous spar, and associated with quartz, yellowish-green garnet, and other substances. It is also mentioned as accompanying ores of zinc, in amorphous masses, at the mines of Altenberg near Aix-la-Chapelle.
* Franklinite, in honour of the celebrated Franklin.
[page] 220
HYDROUS OXIDE OF IRON.
Prismatic Iron Ore, M. (in part). Brown Iron Ore. Onegite. Pyrrhosiderite, Limonite, Beudant. Fer Hydro-oxidé, Bournon.
Peroxide of iron combined with water.
| Hæmatitic. | Compact. | |
| Peroxide of iron | 82·0 | 84·0 |
| Water | 140 | 11·0 |
| Oxide of manganese | 20 | 20 |
| Silica | 1·0 | 2·0 |
| D'Aubuisson. | ||
Sp. Gr. 3·50. H. = 5·0—5·5.
This mineral presents considerable diversity of external appearance. It is found both crystallized and massive. The crystals are small; externally black, and very brilliant; internally blackish-brown. Streak yellowish-brown; brittle; and opake. It also occurs in extremely tender stalactites, of which the fibres radiate from the centre; and compact. The primary form appears to be a right rectangular prism, the only cleavage being parallel to the plane M. Before the blowpipe in the matrass it gives off water, the remainder being red oxide of iron; with borax it forms a yellowish-green glass. Occasionally it acts on the magnet, and does so always after exposure to heat. When rubbed upon paper it leaves a black mark like manganese, for which it may occasionally be mistaken.
The second figure represents the crystals from the vicinity of Bristol.
It occurs at Clifton near Bristol in quartzose geodes, in the form of mamillary masses, and often enclosed in quartz crystals; on compact hard iron-stone at Botallack, and in highly brilliant crystals near Lostwithiel, in Cornwall; also disposed in groups at Lake Onega in Siberia; and in France.
[page] 221
GOETHITE.*
Rubin-Glimmer, Hanssman. Goethite, Lenz. Lepidokrokite, Ullmann.
Combination of the oxide of iron, and water.
| England. | Hollerterzug. | |
| Peroxide of iron | 89·2 | 88·00 |
| Oxide of manganese | traces | 0·50 |
| Water | 10·8 | 10·75 |
| Silica | 0·0—Beudant | 0·50—Brandes. |
Colour brownish red, by reflexion yellowish, and of a brilliant red when transparent and viewed in strong light. Streak orange-red; lustre metallic adamantine. Primary form, either a right rhomboidal or rectangular prism. Occurs in minute laminæ or tables modified on their edges by oblique facets.
The principal locality of this species is the Hollerterzug, in the Westerwald, Germany. It is not a common mineral.
BROWN HÆEMATITE.
Fibrous Brown Iron Ore. Brauner Glaskopf, W. Fer Oxidé Hæmatite, H.
Sp. Gr. 3·7—4·0.
Is of a clove or blackish-brown colour; externally is often steel-grey and splendent. It is more finely fibrous than the red hæmatite, sometimes with a silky lustre, and often radiated; in the other direction it is generally concentric lamellar, the colours being disposed in bands of brown of various shades. It occurs in mamillary and botryoidal masses, in stalactites and tubes. It is brittle.
It forms beds in limestone and other secondary rocks in most European countries; affording materials for extensive iron works in Bohemia, Styria, &c. Sweden and Lapland, which abound in magnetic iron, contain but small quantities of the brown or red hæmatites.
In Scotland it forms veins in sandstone at Cumberhead in Lanarkshire; at Sandloge in the Shetland Isles; and in Hoy, one of the Orkneys. In Cornwall it occurs at Botallack near the Land's End, and in Tin Croft mine near Redruth. It affords a very malleable and much harder iron than the red hæmatite and excellent steel.
Compact Brown Iron Ore. Dichter brauneisenstein, W. It'
* In honour of the celebrated German poet Goëthe.
[page] 222
occurs massive and cellular, sometimes with an iridescent tarnish superficially.
Scaly and Ochrey Brown Iron are varieties of the same species more or less decomposed, presenting either slightly cohering scales and particles, or having an earthy consistence which is meagre to the touch, and soils the fingers. Bog iron ore, the morasterz, sumpferz, wiesenerz of the Germans, is of recent formation; it arises from the decomposition of certain rocks over which water passes, and is deposited by it in low and marshy situations. It frequently contains traces of phosphoric acid, and forms considerable repositories in Germany, Poland, and Russia. The variety termed bohnenerz or pea-ore, consists of concentric globuliform concretions, imbedded either in friable or compact brown hæmatite; at St Stephens in Styria this kind of ore yields about 33 per cent. of iron. Brown iron ore not unfrequently assumes the form of other minerals; at Huttenberg in Carinthia, for instance, it has evidently taken the place of sparry iron; and at Beresof in Siberia occupies large cubical pseudo-crystals of iron pyrites. (Manual.)
As an ore it is not inferior in value to any of the preceding; and the pig-iron obtained from smelting its purer varieties with charcoal, may be easily converted into steel. That yielded by bog-iron is what is termed cold short; it therefore cannot be used in the manufacture of wire, and seldom even of sheet or plate iron, though it is well adapted for casting.
STILPNOSIDERITE.*
Ullmann.
| Contains Oxide of iron | 80·50 | 80·25 |
| Water | 16·00 | 15·00 |
| Silica | 2·25—Ullmann. | 3·75—Vauquelin. |
Sp. Gr. 3·6—3·65. H. = 4·5.
In botryoidal groups, massive, and dendritic; of a black or brownish-black colour, with a splendent lustre both externally and internally; fracture conchoidal; opake; brittle. Streak yellowish-brown. It becomes black before the blowpipe, but does not fuse; and tinges borax dark olive-green, though it is not melted itself.
It occurs at Rashau and Schiebenberg in Saxony, in Thuringia, Nassau, and the Hartz, frequently associated with brown hæmatite, to which it appears to be nearly allied.
* From the Greek, signifying a shining ore of iron.
[page] 223
Rhombohedral Melane Mica, M. Cronstedit, L.Sideroschisolite.
| Contains Oxide of iron | 58·85 |
| Silica | 22·45 |
| Oxide of manganese | 2·89 |
| Magnesia | 5·08 |
| Water | 10·70—Steinmann. |
Sp. Gr. 3·3—3·35. H. = 2·5.
Massive and crystallized; the massive consisting of black and opake fibres, having a brilliant lustre; the crystallized occasionally in separate six-sided prisms, more often however the prisms adhere laterally. In thin laminæ, somewhat elastic; streak dark leek-green; cleavage distinct perpendicular to the axis. Before the blowpipe it intumesces slightly, but does not melt. With borax it affords with difficulty a hard, black, and opake enamel. When reduced to powder it gelatinizes in concentrated muriatic acid.
It is found near Przibram in Bohemia with carbonate of iron; in diverging groups at Wheal Maudlin in Cornwall; and associated with quartz and magnetic pyrites at the mines of Conghonas do Campo in Brazil.
PINGUITE.
Leonhard,
Contains oxide of iron 35·60, silica 36·90, alumina 1·80, magnesia 0·45, oxide of manganese 0·14, water 25·10—Kersten.
Sp. Gr. 2·315. H. under 20.
Pinguite occurs in masses of a siskin- or oil-green colour; with a slightly resinous lustre; and conchoidal or uneven fracture; feels greasy; does not adhere to the tongue, and emits a feeble argillaceous odour when struck. Streak lighter than the mineral. Extremely soft, resembling newly made soap.
In the matrass it yields much water. Before the blowpipe per se it becomes black, but only fuses on the edges. With borax it melts easily, exhibiting the presence of iron; as also with salt of phosphorus, in which a skeleton of silica remains.
Occurs in a vein of barytes at Wolkenstein in the Erzgebirge, and has been severally described by Breithaupt, Freisleben, and Beckmann.
* In honour of Cronstedt, the Swedish mineralogist.
[page] 224
ANHYDROUS SILICATE OF IRON.
Thomson.
Consists of protoxide of iron 68·60, silica 29·60, and protoxide of manganese 1·85—Thomson.
Sp. Gr. 3·884. H. = 4·0.
Occurs in foliated opake masses of a dark-brown colour; cleaves with facility into four-sided prisms; is brittle; acts on the magnet. It emits ammoniacal vapours when heated in a glass tube, and loses about one-fiftieth in weight; before the blowpipe it is infusible, but in the reducing flame it acquires a metallic lustre, and assumes the appearance of magnetic iron. In heated muriatic acid it dissolves without effervescing, leaving a residue of silica in flakes. It is found at Sclav-corrach, one of the Mourne Mountains, in Ireland.
CHLOROPAL.
Sp. Gr. 1·7—2·0. H. = 3·0—4·0.
Of this there are two varieties; the one massive and compact, the other earthy. Colour pistachio-green; opake, or feebly translucent on the edges; fracture conchoidal and splintery; it does not phosphoresce. It consists, according to Brandes, of
| Conchoidal. | Earthy. | |
| Oxide of iron | 35·3 | 32·0 |
| Silica | 46·0 | 45·0 |
| Magnesia | 2·0 | 0·0 |
| Alumina | 1·0 | 0·7 |
| Water | 18·0 | 20·0 |
| Manganese | a trace | 2·0 |
It occurs associated with opal at Unghwar in Hungary, and appears to be closely allied to green iron-earth.
CHAMOISITE.
Kobell.
Contains oxide of iron 60·5, silica 14·3, alumina 7·8, water 17·4—Berthier.
Sp. Gr. 3·0—3·4. Pretty hard.
Occurs massive, of a greenish-grey or black colour, and has a granular earthy fracture; magnetic. Is soluble in acids, with the exception of its silica, and gives off water when heated in the matrass.
Is found in the calcareous deposit of Mount Chamoison in the Valais; whence it is advantageously extracted as an ore of iron.
[page] 225
SIDEROSCHISOLITE.
Wernekinck.
Contains protoxide of iron 70·16, silica 16·30, alumina 4·10, water 7·30—Wernekinck.
Sp. Gr. 3·0. H. = 2·0—3·0.
Primary form a rhomboid, presenting only a single cleavage, which is perpendicular to the axis. This mineral occurs in small six-sided prisms of a black colour. Its streak is green; its lustre brilliant; it becomes magnetic and black from exposure to heat. Before the blowpipe is readily fusible into a black magnetic glass; and is soluble in acids.
It occurs at Conghonas do Campo in the Brazils.
HISINGERITE.
Berzelius.
Protoxide of iron 47·80, silica 27·50, alumina 5·50, oxide of manganese 0·77, water 11·75—Hisinger.
Sp. Gr. 3·04.
Occurs in masses which are cleavable in one direction only, and possess a foliated structure. Black; dull; with an earthy fracture; streak greyish-green; capable of being cut with the knife. Before the blowpipe at a gentle heat becomes magnetic; at a more elevated temperature fuses into an opake black dull globule; and with borax forms a yellowish glass.
It is found in the cavities of calcareous spar, in the parish of Suarta in Sudermanland, Sweden; but it is not common,
YENITE.*
Lievrite, J. Diprismatic Iron Ore, M. Ilvait, Haus. Fer Calcareo-Siliceux, H.
Combination of silica, protoxide of iron, and lime.
| Protoxide of iron | 55·0 | 52·54 |
| Silica | 28·0 | 29·28 |
| Lime | 12·0 | 13·78 |
| Oxide of manganese | 3·0 | 1·59 |
| Alumina | 0·6 | 0·61 |
| Water | 00—Descotils. | 1·27—Stromeyer. |
Sp. Gr. 3·8—4·06. H. = 5·5—6·0.
* Lievrite, in honour of Le Lievre, its discoverer; Yenite, in commemoration of the battle of Jena.
[page] 226
Its colour is brown, or brownish-black; sometimes dull externally, but the crystals have often a metallic lustre; opake. Primary form a right rhombic prism of 111° 30′ It occurs amorphous, acicular, and also crystallized, generally in the form of a rhombic prism terminated by a pyramid with several modifications; cleavage not very distinct, parallel to a plane passing through its longer diagonal. Streak black, inclining to green or brown; fracture uneven; the faces of the prism deeply striated longitudinally. On charcoal it fuses into a black globule, which attracts the magnet if not heated to redness; and with borax melts readily into a dark green and almost opake glass. It is soluble in, and forms a jelly with, heated muriatic acid.
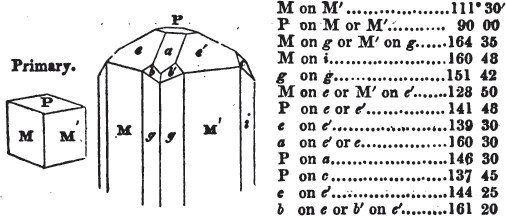
It is principally found in Elba, at Rio la Marina, and Cape Calmite, where it occurs both in solitary crystals of considerable dimensions, and imbedded in compact augite. It has also been noticed in Siberia; Silesia; at Fossum in Norway; and at Cumberland, Rhode Island, United States, in long slender crystals, traversing quartz.
PITCHY IRON-ORE.*
Eisensinter. Eisenpecherz, W. Fer Oxydé Resinite, H. Pittizite, Levy. Sideritine, Beudant.
| Saxony. | Freyberg. | ||
| Oxide of iron | 33·46 | 62·4 | 40·45 |
| Arsenic acid | 26·06 | 0·0 | 30·25 |
| Sulphuric acid | 10·75 | 15·9 | 0·00 |
| Protox. of mangan. | 0·57 | 0·0 | 0·00 |
| Water | 28·48 | 21·7 | 28·50 |
| Stromever | Berzelius. | Kersten. |
Sp. Gr. 2·2—2·4. H. = 2·5.
* From its more or less perfect resemblance to pitch.
[page] 227
Colour blackish-brown, or reddish-black. It occurs in small masses, reniform, and stalactitic, having much the aspect of resin; fracture flat conchoidal, with a vitreous lustre; translucent on the edges, and yields to the knife; streak olive-green or lemon-yellow.
Before the blowpipe per se it instantly becomes opake and cracks, some varieties emitting a strong arsenical odour, during which they are partly volatilized; at an increased temperature it fuses into a black enamel, and on charcoal becomes magnetic; in the matrass it gives off water profusely.
It occurs in several old mines near Freyberg and Schneeberg in Saxony, in the district of Pless in Upper Silesia, in Brittany, and in Chili. It is supposed to be produced from the decomposition of iron pyrites.
PYROSMALITE.*
Pyrosmalith, Karsten. For Muriaté, H. Pyrosmalite, or Native Muriate of Iron, J. Pyroxène Ferro-Manganesien, Beudant.
| Contains Protoxide of iron | 21·81 |
| Muriate of iron | 1409 |
| Protoxide of manganese | 2114 |
| Silica | 35·85 |
| Lime | 1·21 |
| Water and loss | 5·89—Hisinger. |
Sp. Gr. 2·95 to 3·10. H. = 4·0—4·5.
In six-sided prisms of a liver-brown or pistachio-green colour, of which the terminal edges are sometimes replaced. Cleavage distinct and easily obtained perpendicular to the axis. External lustre shining, that of the terminal planes pearly; structure lamellar, translucent on the edges, and brittle. On charcoal before the blowpipe, with a gentle heat, it becomes reddish brown, and gives out a weak acid odour; in a strong fire it fuses readily into a globule presenting a brilliant smooth surface and iron-black colour, which is attractable by the magnet. With glass of borax it melts readily, exhibiting the colours characteristic of iron; and is soluble in muriatic acid, leaving a small residuum of silica.
It occurs, both crystallized and massive, with magnetic iron, calcareous spar, and hornblende, in Bjelke Gruvan, one of the iron mines of Nordmark near Philipstadt in Sweden.
* Pyrosmalite, or Pyrodmalite, from the Greek πυζ, and fire, and οδμη, small,—emitting an odour when heated.
[page] 228
SPATHOSE IRON.
Carbonate of Iron. Brown Spar.* Brachytypous Parachrose Baryte, M. Spath-Eisenstein, W. Fer Oxydé Carbonaté, H. Fer Spathique, Br.
Combination of carbonic acid, and protoxide of iron, of which occasionally a small portion is replaced by protoxide of manganese, magnesia, and lime.
| England, six-sided prisms, | Baigony, lamellar. | Sphærosiderite. | Hartz. | |
| Protoxide of iron | 59·97 | 53·0 | 63·75 | 57·50 |
| Carbonic acid | 38·72 | 41·0 | 34·00 | 36·00 |
| Oxide of mangan. | 0·39 | 0·6 | 0·75 | 3·30 |
| Lime | 0·92 | 0·0 | 0·00 | 1·25 |
| Magnesia | 0·00 | 5·4 | 0·52 | 0·00 |
| Beudant. | Berthier. | Klaproth. | Klaproth. |
Sp. Gr. 3·6—3·8. H. = 3·5—4·5.
Colour various shades of yellow, passing on exposure into brown, and brownish-black; transparent, translucent, or opake. Occurs in obtuse rhomboids (whose faces are occasionally curvilinear); in acute rhomboids, sometimes perfect, or having the terminal angles replaced; in six-sided prisms; in octahedrons; and in lenticular crystals; also striated and massive. Externally it is shining. Structure lamellar, with a brilliant or pearly lustre; yields readily to cleavage parallel to all the planes of an obtuse rhomboid of 107° and 73°. Affects the magnetic needle. Before the blowpipe blackens and becomes more magnetic, but does not melt; colours borax bottle-green in the reducing flame, and yellow in the oxidating; and is with difficulty soluble in acids, unless previously reduced to powder.
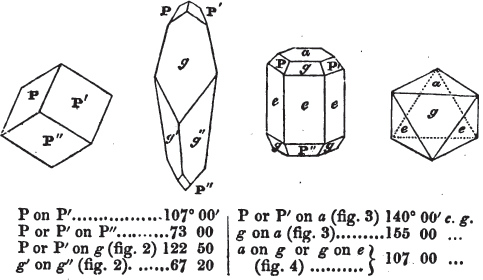
* Spathose iron, from its presenting rather a sparry than a metallic substance; brown spar, from its prevailing colour.
[page] 229
It occurs abundantly in some countries, and particularly in Styria and Carinthia, where it forms coherent tracts, which extend along the chain of the Alps into Austria and Saltzburg. On these the great iron manufactories of Eisenerz and Vordernberg are situated, so celebrated for the fine steel they produce. Magnificent crystals of spathose-iron occur at Harzgerode in the Hartz, in veins traversing grauwacke; at Freyberg in silver veins; and presenting many interesting varieties in the mining districts of Alston-Moor, Cornwall, and Devonshire.
A Fibrous Carbonate of Iron occurs in the veins of Tin Croft mine in Cornwall, in tabular masses of half an inch or less in thickness, striated in a direction perpendicular to the surfaces of the mass, and of a brown colour. Sphærosiderite is the name applied to a spheroidal and radiated variety from Hanau, where it is met with occupying hollows in greenstone without any indication of crystalline form.
Columnar Clay Iron-stone. Its colours are the same as the amorphous. It occurs in angular pieces composed of columnar concretions like starch, often closely aggregated, and cohering slightly; or the interior is found to be columnar, the interstices being in some cases filled either with bitumen or calcareous spar. It is dull, soft, brittle, and when of a reddish-brown colour is magnetic. It occurs in Bohemia, the Upper Palatinate, and Saarbruck; in Scotland in the Isle of Arran; in England in the Wednesbury coal deposit in Staffordshire, where the columns are coated with pyrites, and their interstices partially filled with carbonate of lime, sometimes with blende and galena.
Lenticular Clay Iron-stone. Fer oxydé brun granuleux, Bt. It occurs in small granular or lenticular concretions, which are separate or aggregated into masses. Its colour is reddish, or yellowish-brown, or greyish-black, with a pseudo-metallic lustre. Is brittle, and easily broken. It occurs in Franconia, Bavaria, Salzburg, Switzerland, France, and the Netherlands.
PHOSPHATE OF IRON.
Vivianite, W. Fer Phosphaté, H. Blue Iron Ore, J. Prismatic Iron Mica, M. Dicromatic Euclas Haloide, Haid.
Combination of phosphoric acid, protoxide of iron, and water.
| Cornwall. | Bodenmais. | Earthy Phosphate. | |
| Protoxide of iron | 41·23 | 41·0 | 32·0 |
| Phosphoric acid | 31·18 | 26·4 | 47·5 |
| Water | 27·49 | 31·0 | 20·0 |
| Stromeyer. | Vogel. | Klaproth. |
Sp. Gr. 2·66. H. = 2·0.
[page] 230
Vivianite occurs crystallized in the form of a right obliqueangled prism, which is that of its primary crystal. It cleaves readily, and only parallel to the plane P of the following figures; the crystals are prismatic, and of considerable length, being attached to the matrix at the face M or the opposite plane, in which case the faces P T and c form the prismatic planes of the crystal. Colour varying from pale green to indigo-blue; by transmitted light green at right angles to the axis, and of a pale blue colour parallel to it. Transparent or translucent, with a partly metallic, partly vitreous lustre; streak almost white, but on exposure to the air soon changes into indigo-blue. The powder produced by crushing the mineral in a dry state, is liver-brown. The crystals are often very small, aggregated, and divergent; those of Cornwall are flexible, but not elastic, while the crystallized variety of New Jersey is extremely brittle. Before the blowpipe on charcoal it intumesces, reddens, and fuses into a steel-grey coloured globule with metallic lustre. Soluble without effervescence in dilute sulphuric and nitric acids.
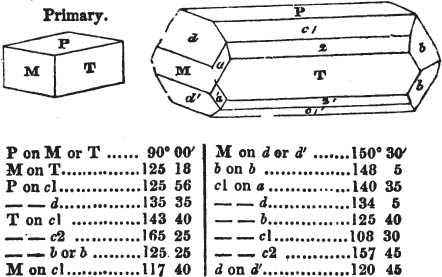
It occurs with iron- and magnetic-pyrites in gneiss at Bodenmais in Bavaria; in the gold mines of Vöröspatak in Transylvania; in the Isle of France; and in Brazil.
In England it is met with finely crystallized near St Agnes in Cornwall, accompanied by magnetic iron-pyrites and spathose iron; in Derbyshire in crystals of the same form and colour, in the decomposed shale of that country.
The Anglarite of Kobell is a fibrous and compact variety of phosphate of iron. Its colour is grey, inclining to blue; and it is translucent. Before the blowpipe it melts into a black globule, and in the matrass yields water.
It occurs at Anglar, in the Haute Vienne, France.
[page] 231
Earthy Phosphate of lron. Blaue Eisenerde, W. Fer phosphaté terreux, H. Blue Iron Earth, J. The colour of this variety on its first exposure is grey, yellow, or greenish-white, or with a very slight tinge of blue; afterwards it becomes blue of different degrees of intensity. It occurs massive, disseminated in or coating other substances; and is sometimes loose, occasionally cohering, and with an earthy fracture. It is dull, meagre to the touch, soils the fingers slightly, and is light. Before the blowpipe it becomes reddish-brown, and then melts into a brownish-black slag, attractable by the magnet.
It occurs in clay, and mud, more or less intermingled with animal matter, from; which the phosphoric acid is conjectured to have proceeded.
It is found in solid masses in the argillaceous deposits of New Jersey, occasionally with bog iron-ore; also in Styria, Carinthia, and in Greenland. The friable varieties have been met with in forming excavations in the river mud of the Isle of Dogs; in the same deposit at Toxteth near Liverpool. On the surface of peat-mosses in several of the Shetland Isles; at Ballagh in the Isle of Man, accompanying animal matter, particularly the bones of the elk and deer; and elsewhere.
It is sometimes employed as a pigment
HETEPOSITE.
Kobell.
Combination of phosphoric acid, protoxide of iron, protoxide of manganese, and water.
| Phosphoric acid | 41·77 | 48·0 |
| Oxide of iron | 34·89 | 35·5 |
| Red oxide of manganese | 17·57 | 16·5 |
| Silica | 0·20 | 0·0 |
| Loss by heat | 4·40 Dufrésnoy. | 0·0 Vauquelin. |
Massive; having a lamellar structure, and a greenish-grey or blue colour; lustre resinous, like that of apatite. Primary form an oblique rhombic prism of 100° and 80°, and 101° and 79°, obtained by cleavage. After long exposure to the atmosphere its colour becomes violet, and its lustre is changed into semi-metallic. Is soluble in acids, with the exception of its silica; and before the blowpipe fuses into a brown enamel, which has a semi-metallic lustre.
It occurs at Hureaux, in the Haute Vienne, and was noticed and described by M. Dufrésnoy.
[page] 232
KARPHOSIDERITE.
Breithaupt.
Consists of phosphoric acid, oxide of iron, and water. Sp. Gr. 2·5. H. = 4·0—4·5.
Occurs in reniform masses of a straw-yellow colour; lustre resinous; fracture uneven; feels greasy. Before the blowpipe, in the open tube, it gives off water, accompanied by fumes which redden turmeric paper; alone on charcoal it becomes black; and at a high temperature melts into a globule, which is powerfully magnetic. Is soluble with facility in borax, and with salt of phosphorus forms a black scoria.
This mineral was distinguished by Breithaupt, who named it in allusion to its straw-yellow colour. Its locality is Labrador.
SULPHATE OF IRON.
Green Vitriol. Melantérie, Beudank Eisen-Vitriol,W. Fer Sulphaté, H. Hemi-Prismatic Vitriol Salt, M.
Sulphuric acid 28·8, protoxide of iron 25·7, water 45·4— Berzelius.
Sp. Gr. 1·84—1·9. H. = 2·0.
Primary form an oblong rhombic prism of 99° 23′ and 80° 27′.
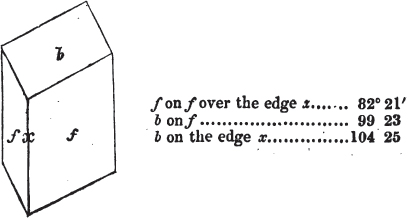
It presents various shades of green, sometimes emerald-green; but more frequently, owing to exposure, is externally of a yellow or yellowish-brown colour. It occurs massive, pulverulent, and in stalactites; and crystallizes in the form of a right oblique-angled prism; sometimes modified ; cleavage perfect parallel to b, less so to f; translucent; lustre vitreous; taste extremely astringent; fracture conchoidal. It is readily soluble in double its weight of water, and the solution turns black on the ad-
[page] 233
dition of tincture of galls. Before the blowpipe on charcoal it becomes magnetic, and colours glass of borax green. Exposed to the air, it soon appears covered with a yellow powder, which is the persulphate of iron.
This species in its native state is rare; in most cases it is produced by the decomposition of other minerals, particularly iron pyrites. It is also found dissolved in the waters of several mines. It occurs in the Rammelsberg mine near Goslar in the Hartz, at Schwartzenberg in Saxony, and at Schemnitz in Hungary; also in aluminous shale, at Hurlet near Paisley; and in New England, where it forms crusts upon the surfaces of such mica-slate rocks as happen to abound in iron pyrites. It is used in dyeing, in making ink, Prussian blue, and sulphuric acid.
BOTRYOGENE.
Native Red Iron Vitriol of Fahlun, Haidinger. Rother Eisen-Vitriol, Leonhard. Neoplase, Beudant.
Bisulphate of the peroxide of iron, combined with bisulphate of the protoxide of iron, and water.
Sulphuric acid 32·55, peroxide of iron 23·86, protoxide of iron 10·71, water 32·85.
Sp. Gr. 2·039. H. = 2·25—2·5.
Primary form an oblique rhombic prism of 119° 66′ and 60° 4′.
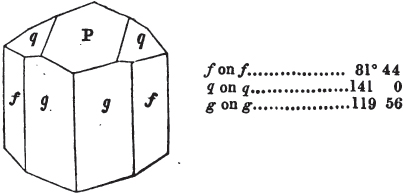
Occurs in small crystals which are usually aggregated in reniform and botryoidal masses, consisting of globules with a crystalline surface; sometimes like a bunch of grapes, hence its name, from βονζυς. Faces of f and g striated parallel to the axis, and less perfectly formed than the inclined planes; cleavage parallel to g. Colour deep hyacinth-red, passing, when massive, into ochre-yellow, the colour of its streak; translucent; lustre vitreous; taste slightly astringent. When exposed to a moist atmosphere it becomes covered with a dirty yellow powder, but re-
[page] 234
mains unchanged when dry. Before the blowpipe it intumesces and gives off water, leaving a reddish-yellow earth; with salt of phosphorus yields a red glass, which becomes colourless on cooling. Boiling water dissolves only a part of it. It occurs in the great copper mine of Fahlun in Sweden, in the level called Melorumsort, forming a coating on gypsum or pyrites, along with Epsom salt, green vitriol, &c. (Manual.)
MISY.
Hausmann.
The persulphate of iron with excess of base, according to Berzelius. Occurs in pulverulent opake masses of a sulphur- or lemon-yellow colour. It is found principally at Goslar in the Hartz, but is also met with accompanying botryogene at Fahlun in Sweden. Misy is a name applied by Pliny to some artificial compound obtained in the process of making vitriol in the Island of Cyprus, and was given by Hausmann to this substance.
The atrament stone is another curious compound of sulphuric acid and iron; it is a mixture of the sulphate and peroxide of iron; is compact, ponderous, and of a dark brick-red colour; and occurs with the present species in the deserted part of the copper mines of Goslar. (Manual)
ARSENIATE OF IRON.
Wurfelerz, W. Fer Arseniaté, H. Cube-Ore, J. Pharmakosiderit, Haus. Hexahedral Lirocone Malachite, M.
Combination of arsenic acid, protoxide and peroxide of iron, and water, mixed with phosphoric acid and oxide of copper in very minute proportions.
| Peroxide of iron | 39·20 | 40·56 | 45·5 |
| Arsenic acid | 37·82 | 38·00 | 31·0 |
| Phosphoric acid | 2·53 | 0·70 | 0·0 |
| Oxide of copper | 0·65 | 0·60 | 9·0 |
| Water | 18·61 | 19·57 | 10·5 |
| Insoluble matter | 1·76 | 0·35 | 4·0 |
| Berzelius. | Berzelius. | Chenevix. |
Sp. Gr. 2·9—3·0. H. = 2·5.
Various shades between light- and bottle-green, and yellowish and brownish-green; it rarely occurs massive, mostly crystallized in cubes, either perfect or having the alternate angles replaced by one or by three planes, very rarely all the edges and angles are replaced. The small planes b, b, on the largest of the following figures, appear at first only as striæ, apparently indicating
[page] 235
the tetrahedron as the primary form; but the crystals yield to cleavage parallel to the planes of the cube, though not with sufficient brilliancy for the use of the reflective goniometer. The cross fracture is uneven or imperfectly conchoidal, with a shining vitreous lustre. It varies from transparent to opake; sometimes ochreous externally, from partial decomposition; and is brittle. Streak pale olive-green. Before the blowpipe on charcoal it emits arsenical vapours, and fuses into a grey scoria which exhibits metallic brilliancy and is attractable by the magnet. With the fluxes, after the disengagement of arsenical fumes, it forms bottle-green coloured globules. On exposure to heat it becomes electric, and is soluble in concentrated acid.
Fig. 1, the cube. Fig. 2, the same, of which the alternate solid angles are replaced by triangular planes. Fig. 3, in which they are replaced by four planes; these are sometimes rounded, and appear but as one plane. Fig. 4, the cube, of which both the edges and angles are replaced.
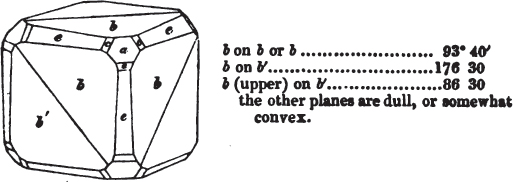
Its principal localities are the mines of Huel Gorland, Huel Unity, and Carharack in Cornwall, where it occurs associated with various ores of copper. On the continent it has been found at St Leonard in France, and at Schneeberg and Schwartsenberg in Saxony, but it is a rare mineral out of England.
OXALATE OF IRON.
far Oxalaté, Levy. Oxalate de Fer. Humboldtine, Rivero. Hum boldtite, Necker and Beudant.
Combination of oxalic acid and protoxide of iron.
| Oxalic acid | 46·14 |
| Protoxide of iron | 53·86—Rivero. |
[page] 236
Sp. Gr. 2·13 (Leonhard), 1·3 (Beudant). H. about 2·0.
It occurs in small flattish masses of a bright yellow colour. and crystalline, but the crystals are not determinable; opake, devoid of lustre, and having an uneven earthy fracture. It acquires resinous electricity by friction; it decomposes easily on live coal, giving out a vegetable odour, the residue passing by degrees from yellow to black, and finally to red. It is insoluble in boiling water and alcohol, but dissolves without effervescence in nitric acid, imparting to it a yellowish tint.
It is found at Koloseruk, near Bilin in Bohemia, imbedded in moor-coal or friable lignite, and is supposed by its analyst to result from the decomposition of succulent plants.
TUNGSTATE OF IRON.
Wolfram, W. Scheelin Ferruginé, H. Bt. Prismatic Scheelium Ore, M. Combination of the oxides of tungsten, iron, and manganese.
| Oxide of iron | 18·00 | 18·32 |
| Tungstic acid | 67·00 | 78·77 |
| Oxide of manganese | 6·25 | 6·22 |
| Silica | 1·50—Berzelius. | 1·25—Delhuyar. |
Sp. Gr. 7·11—7·33. H. = 5·0—5·5.
Colour brownish-black; is found both massive and crystallized. Structure lamellar; cleavage perfect parallel to a plane which bevels the lateral edge between r and r; streak dark reddish-brown; lustre brilliant, often metallic; opake; and is brittle. Before the blowpipe it decrepitates, and fuses under a strong heat into a black and somewhat scoriaceous globule; it is readily soluble in borax, and does not act on the magnet.
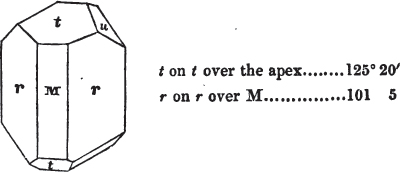
Wolfram is peculiar to primitive rocks; and is a common associate of tin ore, particularly in the mines of Saxony, Bohemia, and Cornwall. In the former countries it is found in large well-defined crystals; in the latter in such abundance as frequently to impede the working of the tin ore. At Wheal Maudlin in Cornwall it has occurred in pseudomorphous crystals, assuming the precise form of tungsten. Greenland, Siberia, Limoges in France, and the island of Rona in the Hebrides, are other localities of this species.
[page] 237
HAUSMANNITE.
Hausmannite, Haid. Pyramidal Manganese Ore, M. Black Manganese. Manganese Oxidé Hydraté, H. Blättricher Schwarz Braunsteinerz, Hausmann. Foliated Black Manganese Ore, J.
Anhydrous red oxide of manganese, mixed with a small proportion of the peroxide.
| Red oxide of manganese | 98·09 |
| Oxygen | 0·22 |
| Water | 0·43 |
| Baryta | 0·11 |
| Silica | 0·34—Turner. |
Sp. Gr. 4·72—4·8. H. = 5·0—5·5.
It occurs massive, and crystallized in four-sided pyramids, which yield to cleavage parallel to their base, which is square. Colour iron-black, opake, very hard, and affords a dark reddish or chesnut-brown powder. Lustre imperfect metallic. On charcoal in a strong heat it fuses on the edges; with borax readily forms a deep violet-blue or almost black globule; and with soda produces a green coloured scoria. Is insoluble in muriatic acid, but is decomposed by heated sulphuric acid.
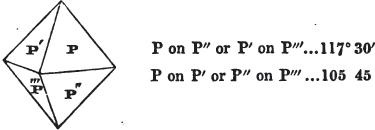
Hausmannite is found in veins of porphyry, along with other ores of manganese, at Œhrenstock near Ilmenau in Thuringia; at Ihlefeld in the Hartz; and at Lebanon in Pennsylvania, United States. The most distinctly crystallized specimens are met with at Framont in Alsatia; but it is on the whole not a common species.
BRAUNITE.*
Brachytypous Manganese Ore, M. Brachytypous Manganerz, L.
It is an anhydrous deutoxide of manganese. The variety from Elgersburg yielded to Turner, protoxide of manganese 86·94, oxygen 9·85, water 0·95, and baryta 2·26.
Sp. Gr. 4·8—4·9. H. = 6·0—6·5.
* Named by Turner and Haidinger, in compliment to their mutual friend Mr Braun of Gotha.
[page] 238
Primary form, an octahedron with a square base whose faces are inclined at angles of 109° 58′, and 108° 39′. Secondary form the same, occasionally truncated. Occurs both crystalline and massive, frequently fibrous and divergent, of a dark brownish-black colour, with an imperfect metallic lustre; streak black or slightly brownish; cleavage distinct parallel to the faces of the primary; fracture uneven; brittle. It is soluble in muriatic acid, leaving a trace of siliceous matter. Before the blowpipe on charcoal it is per se infusible, but assumes in the reducing flame a reddish colour. With borax it melts with a slight effervescence.
This species of manganese forms veins in porphyry at Œhrenstock near Ilmenau, at Elgersburg, Friedrichsroda, and elsewhere in Thuringia; also with red epidote at St Marcel in Piedmont. Besides its superior hardness to other orės of manganese, the direction of its cleavage parallel to the faces of the pyramid sufficiently distinguishes this species from hausmannite, in which the cleavage always takes place parallel to the base.
PYROLUSITE.*
Prismatic Manganese Ore, M.
It is an anhydrous peroxide of manganese, according to Turner, and contains
| Cretnich. | ||||
| Red oxide | 84·05 | 85·62 | 83·44 | 82·3 |
| Oxygen | 11·78 | 11·60 | 11·43 | 11·5 |
| Water | M2 | 1·56 | 0·75 | 1·2 |
| Baryta | 0·5S | 0·55 | 2·31 | 0·0 |
| Silica | 0·51 | 0·66 | 0·88 | 40 |
| Turner. | Gmelin. | Berthier. | ||
Sp. Gr. 4·6—4·9. H. = 2·0—2·5.
Primary form a right rhombic prism. Colour iron-black, sometimes bluish; opake; lustre metallic; streak black; cleavage parallel to M, ν, and Ω.
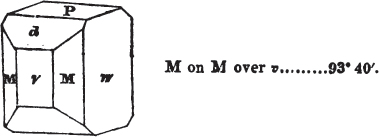
* Pyrolusite, from ϰνς, fire, and λονΩ, I wash, in allusion to its valuable property of discharging the brown and green tints in glass.
[page] 239
Before the blowpipe, at a powerful heat, in the reducing flame, it becomes brownish-red, but does not fuse; is soluble with brisk effervescence in borax, colouring the globule of an amethystine tinge, but yields no water when heated in the matrass.
In an economical point of view, pyrolusite is the ore of manganese properly so called, and is extensively worked in many countries, particularly at Ilmenau, Friedrichsroda, Elgersburg, and other places in Thuringia. The mines of Ehrensdorf, near Maehrisch-Triebau in Moravia, afford annually many hundred tons of this ore; and in Cornwall, Devonshire, Saxony, France, Hungary, and other countries, it is of more or less frequent occurrence. In all these localities it is associated with psilomelane, from which, however, it is easily distinguished by its greatly inferior hardness; indeed it is generally so soft as, even in crystalline specimens, to soil the finger when handled. At first sight it may be confounded with certain crystallized varieties of antimony; but its dark steel-grey colour is sufficiently characteristic; and if not, the blowpipe will distinguish it, pyrolusite being perfectly infusible, while antimony yields even to the flame of a candle. (Manual.)
GREY OXIDE OF MANGANESE.
Grau Braunsteinerz. W. Manganese Oxidé Metalloide, H. Prismatoidal Manganese Ore, M. Manganite, Haid. Prismatic Manganese Ore, J. Hydrated Deutoxide of Manganese, Turner.
| Ihlefeld. | Undenaes. | ||
| Red oxide of mangan. | 86·85 | 87·1 | 86·41 |
| Oxygen | 3·05 | 3·4 | 3·51 |
| Water | 10·10 | 9·5 | 10·08 |
| Turner. | Gmelin. | Arfwedson. | |
Sp. Gr. 4·31—4·4. H. = 4·0—4·2.
Colour steel-grey, passing into iron-black; occurs in prismatic crystals, which are occasionally modified; cleaves readily and with brilliant surfaces parallel to the lateral planes of a rhombic prism of 100° and 80°* (the primary form), and both its diagonals. It occurs also in acicular crystals longitudinally striated, either diverging, or confusedly intersecting each other; also massive, with a fibrous structure, or having a granular or earthy texture; lustre imperfect metallic; opake, except in the thinnest fragments, which exhibit a feeble translucence; brittle; marks strongly when rubbed, giving a dark reddish-brown, and in the massive varieties a black, streak.
* According to Necker, 99° 41′ and 80° 19′.
[page] 240
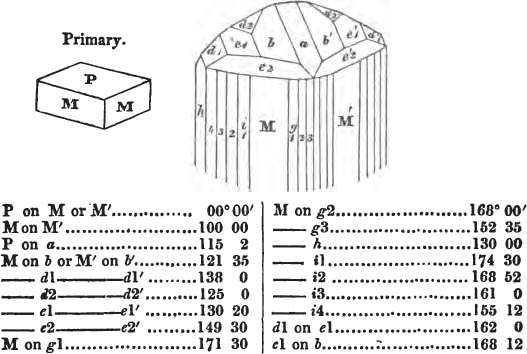
Before the blowpipe it yields water in the matrass, Per se it is infusible, but assumes a reddish tinge in the oxidating flame. With borax it affords a violet-blue coloured globule. It is insoluble in nitric acid, but in muriatic it gives off chlorine, and dissolves without residue; when exposed to a powerful heat, oxygen is disengaged.
This is the purest and most beautifully crystallized ore of manganese. It occurs both in primitive and secondary countries, in veins, beds, and irregular masses. Its principal locality is Ihlefeld in the Hartz, where it is associated with calcareous spar and barytes, in veins traversing porphyry. It occurs also, though less abundantly, in Bohemia, Alsatia, Saxony, Aberdeenshire, Cornwall, and at Undenaes in West Gothland. It is distinguished from pyrolusite by its superior hardness and characteristic brown streak, which sometimes appears black until a portion has been abraded.
PSILOMELANE.*
Uncleavable Mangarese Ore, M. Compact and Fibrous Manganese Ore, or Black Hematite, J. Black Iron Ore. Schwartz Eisenstein. Schwartzer Glaskopf, W. Dichtes Schwartz Manganerz, L. Manganese Oxidé Hydraté Concretionné. Manganese Oxydé Non-Barytifère, H.
Though this ore has been placed by mineralogists among the
* Psilomelane, from ΨςλОς, smooth or naked, and μςλας, black, in al. lusion to its smooth or botryoidal form, and black colour.
[page] 241
oxides of iron, under the names of black hæmatite and black iron ore, pure fragments of it do not contain a trace of that metal.
| England. | Romanèche. | |
| Red oxide of manganese | 69·795 | 70·3 |
| Oxygen | 7·364 | 7·2 |
| Baryta | 16·365 | 16·5 |
| Water | 6·216 | 4·0 |
| Silica | 0·260—Turner. | 2·0—Berthier. |
Sp. Gr. 4·0—4·15. H.= 5·0—6·0.
Crystalline form unknown; massive and botryoidal; colour black, passing into dark steel-grey; lustre imperfect metallic; opake; streak brownish-black, and shining ; cleavage and fracture not observable. Before the blowpipe it colours glass of borax violet-blue, like other ores of manganese; and is completely soluble in muriatic acid, with the exception of a small quantity of silica.
This species is frequently associated with pyrolusite, sometimes even alternating with it in layers of different thickness; and occurs in botryoidal and stalactitic-shaped masses in Devonshire and Cornwall; at Ihlefeid in the Harts; in the district of Siegen in Hessia; and at several places in Saxony, Silesia, and Bayreuth. The Romanèche variety possesses a somewhat higher specific gravity.
WAD.
Farthy Manganese. Black Wad.
| Contains Oxide of manganese | 68·0 |
| Oxide of iron | 6·5 |
| Water | 17·5 |
| Carbon | 1·0 |
| Baryta and sdica | 9·0 |
Sp. Gr. 3·7, though apparently very light when taken in the hand.
It occurs of various shades of brown, blackish-brown, and grey, sometimes approaching to steel-grey. It is commonly dull, but the grey possesses a glimmering lustre. Occurs massive, botryoidal and amorphous, sometimes pulverulent;. or in froth-like coatings on other minerals. The massive commonly yields to the nail and soils the fingers. From its giving off water abundantly on exposure to heat in the matrass, it is considered by Berzelius as a hydrate of manganese.
It occurs principally at the manganese pits of Upton Pyne, Devonshire; in Cornwall, the Hartz, and in Piedmont.
L
[page] 242
CUPREOUS MANGANESE.
Kupfer Mangan of the Germans. Manganese Hydraté Cuprifère, Necker.
Hydrate of the oxide of manganese, mixed with oxide of copper and gypsum.
| Oxide of manganese | 74·10 |
| Oxide of copper | 4·80 |
| Water | 20·10 |
| Gypsum | 1·05 |
| Silica | 0·30—Kersten. |
Sp. Gr. 3·15—3 o25. H.about 1·5.
Is found massive, in small reniform and botryoidal opake groups of a bluish-black colour; lustre resinous; streak corresponding to the colour; not brittle. Before the blowpipe it becomes brown, but is infusible; to borax or salt of phosphorus it communicates the amethystine and green colours characteristic of manganese; with a mixture of soda and borax, grains of reduced copper are obtained. This very rare mineral was distinguished by Breithaupt and Lampadius. It occurs in the tin mines of Schlaggenwald in Bohemia.
HELVINE.*
Helvine, W. and H. Tetrahedral Garnet, M.
Combination of silica, glucina, alumina, and the protoxides of iron and manganese.
| Schwartzenberg. | |
| Silica. | 35·27 |
| Alumina | 1·45 |
| Glucina | 8·02 |
| Protoxide of manganese | 29·35 |
| Protoxide of iron | 7·99 |
| Sulphuret of manganese | 14·00—Gmelin. |
Sp. Gr. 3·1—3·3. Ho = 6·0—6·5.
Primary form the regular tetrahedron. Occurs in small tetrahedrons, whose solid angles are replaced; of a pale wax-yellow colour, inclining to brown or siskin-green; translucent on the edges; lustre vitreous, inclining to resinous; and streak white. Before the blowpipe, on charcoal, in the reducing flame, it fuses into an opake globule of nearly the same colour as the mineral; with borax it melts slowly into a diaphanous glass, which remains yellow when cold, if the dissolution be not complete;
* From the Greek, signifying sun-yellow; in allusion to its colour.
[page] 243
and which, when it is complete, becomes colourless in the reducing, and of a deep amethystine tinge in the oxidating flame.
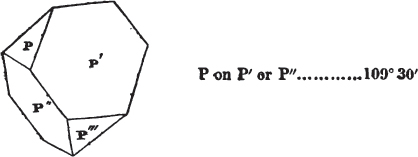
Schwartzenberg in Saxony, where it occurs in beds of gneiss, accompanied with garnet, quartz, fluor, and calc spar, and Hortekulle, near Modum in Norway, are the best known localities of helvine.
SILICIFEROUS OXIDE OF MANGANESE.
Manganese Oxidé Silicifere, H. Silicate of Manganese, A. Manganese Spar, J. Manganspath, W.
| Langbanshyttan. | ||
| Oxide of manganese | 52·60 | 49·04 |
| Silica | 39·60 | 48·00 |
| Oxide of iron | 4·60 | a trace |
| Lime and magnesia | 1·50 | 3·34 |
| Water | 2·75—Berzelius | 0·00—Rose. |
Sp. Gr. 3·5—3·7. H. = 5·0—5·5.
It occurs massive; of a pale rose-red colour. The fracture is even or flat conchoidal; it is translucent on the edges, and is very hard; lustre intermediate between pearly and resinous; cleavage apparent in two directions perpendicular to each other, exhibiting as the primary form a doubly oblique prism; that parallel to P highly perfect

[page] 244
Alone before the blowpipe on charcoal it becomes dark brown, and fuses into a reddish-brown or black globule. With borax it forms a violet-coloured glass. Reduced to powder and treated with muriatic acid, it is partly dissolved; the insoluble remainder assuming a white colour.
It occurs at Hamburgh, New Jersey, with magnetic iron and garnet; at Langbanshyttan in Wermeland, Sweden, in beds of iron ore; at Ekatherineburg in Siberia; at Elbingerode in the Hartz; in Devonshire on Black Down, near Tavistock, associated with grey oxide of manganese; and in Cornwall, near Calliagton, in a manganese quarry. It is cut and polished by the lapidary, and employed for inlaid work.
The substances 'described by Leonhard under the names of allagite, photizite, rhodonite, and corneous manganese, all from the vicinity of Rubeland in the Hartz, are evidently compact varieties of this species under different states of oxidation, and in more or less perfect conditions of purity.
| Allagite. | Rhodonite. | Photizite. | Corneous Manganese. | |
| Oxide of manganese | 75·0 | 49·87 | 46·13 | 54·58 |
| Silica | 16·0 | 39·00 | 39·00 | 34·00 |
| Carbonic acid | 7·5 | 4·00 | 11·00 | 8·00 |
| Alumina | 0·0 | 0·12 | 0·25 | 0·00 |
| Water | 0·0 | 6·00 | 3·00 | 2·00 |
| Oxide of iron | 0·0 | 0·25 | 0·50 | 0·50 |
| Du Menil. | Brandes. | Brandes. | ||
The rhodonite has frequently a fibrous texture, and, as well as the photizite and corneous manganese, presents various red, green, and grey colours, which become darker on exposure to the air.
HYDROSILICATE OF MANGANESE.
Opsimose, Beudant. Schwarzer Mangan-Kiesel.
Occurs compact, of a black colour and metallic appearance; streak brownish-yellow. Gives off water in the matrass, and becomes grey. Is fusible into a green glass in the reducing flame, and forms a black one in the oxidating. Upon platina foil it communicates a green colour to soda. Is acted upon by acids.
Locality, Claperude in Dalecarlia, Sweden.
[page] 245
KNEBELITE.*
Lenz.
| Contains Protoxide of manganese | 35·0 |
| Protoxide of iron | 32·0 |
| Silica | 32·5—Dobereiner. |
| Sp. Gr. 3·714. | |
Colour grey, spotted with dirty white, brownish-red, brown, and green; it is massive, but the surface is cellular and uneven; and both internally and externally it is glistening; fracture imperfectly conchoidal; is opake, hard, brittle, and difficultly frangible. No locality is given.
BUSTAMITE.
Bregniart.
| Contains | Silica | 48·90 |
| Protoxide of manganese | 36·06 | |
| Lime | 14·57 | |
| Protoxide of iron | 0·81—Dumas. | |
| Sp. Gr. 3·1—3·3. | H. about 7·0. |
Occurs in irregularly disposed prismatic crystals, having a somewhat fibrous structure, and a pale grey, greenish, or reddish colour; almost opake. It occurs, associated with iron pyrites, at Real de Minas in Mexico.
SULPHURET OF MANGANESE.
Mangan Blende, Breit. Hexahedral Glance Blende, M. Prismatic Manganese Blende, J. Schwartzerz, Haus. Manganèse Sulfuré, H. Manganglanz, Leonhard. Alabandine, Beudant.
Combination of sulphur and manganese.
| Protoxide of manganese | 85·0 | 82·0 |
| Sulphur | 15·0 | 11·0 |
| Carbonic acid | 0·0—Vauquelin. | 5·0—Klaproth. |
Spp. Gr. 3·95—4·05. H.= 3·5—40.
Primary form the cube, or a rectangular prism nearly corresponding to it. Cleavage parallel to its faces, distinct; traces parallel to its edges.
* After Major Von Knebel, who presented the mineral to Dobereiner.
[page] 246
Colour brownish-black, but, when fresh fractured, of a dark steel-grey; it occurs massive, sometimes botryoidal, with an imperfect metallic lustre; fracture commonly fine grained; streak dark green; opake. Before the blowpipe it fuses with difficulty, and only on the thinnest edges, forming a brownish scoria; reduced to powder it is dissolved when thrown into acid, emitting at same time fumes of sulphuretted hydrogen.
It occurs in the gold mines of Nagyag in Transylvania, with tellurium, blende, copper pyrites, and other ores of manganese; also in Mexico.
CARBONATE OF MANGANESE.
Manganese Oxydé Carbonatée, H. Macrotypous Parachrose Baryte, M. Red Manganese, A. Rothbraunsteinerz, Haid. Kohlensaures Mangan, L. Rother Braunstein, W. Diallogite, Beudant.
Carbonate of manganese, more or less mixed with the carbonates of iron and lime.
| Buchenberg. | Freyberg. | Nagyag | |
| Oxide of manganese | 54·60 | 52·6 | 56·0 |
| Carbonic acid | 33·75 | 38·7 | 38·6 |
| Oxide of iron | 1·87 | 4·5 | 0·0 |
| Silica | 4·37 | 0·0 | 0·0 |
| Lime | 2·50 | 5·0 | 5·4 |
| Du Menil. | Berthier. | Berthier. |
Sp. Gr. 3·3—3·6. H. = 3·5.
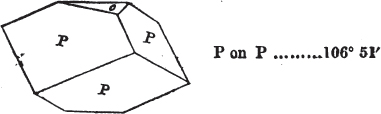
Primary form a rhomb of 106° 51′.* Cleavage parallel to the faces of the rhomb. Surface of o deeply streaked parallel to its edges of combination with P; this produces lenticular crystals, and when the surface of P is curved, those peculiar saddle-Shaped lenses so common in this species are formed. It also occurs massive. Colour rose-red, and translucent; possesses a lamellar structure; scarcely scratches glass, and yields to the knife; fracture splintery; lustre vitreous, inclining to pearly.
Before the blowpipe its colour is changed into brown or black, and it decrepitates strongly, but is infusible without addition.
* 103° and 77°, according to Beudant.
[page] 247
It is readily soluble in, and colours borax or salt of phosphorus violet-blue in the oxidating flame, becoming, however, colourless in the reducing flame; it effervesces rather briskly in nitric acid. On exposure to the air it becomes browner in colour; and the bright rose-red varieties lose their hue from the action of light.
This species generally occurs in metalliferous veins accompanying various ores of silver and lead, both massive, and in botryoidal concretions coating cavities. The mines of Freyberg in Saxony, and those of Kapnik, Offenbanya, and Nagyag in Tran. sylvania, are its principal localities. It is apt to be confounded with manganese spar, though its very inferior degree of hardness is sufficiently characteristic. (Manual)
PELOKONITE.
Richter.
A mixture of hydrous oxide of manganese, hydrous oxide of iron, oxide of copper, and silica—Kersten.
Sp. Gr, 2·5—2·57.
Uncrystallized; colour blackish blue; streak liver-brown, with a low degree of lustre, and conchoidal fracture.
It occurs at Remolinos in Chili, associated with malachite and chrysocolla, and derives its name from Πελος, brown, and ϰονις, powder, in allusion to the colour of its streak, from which last property it may be distinguished from cupreous manganese.
HURAULITE.
Combination of phosphoric acid, oxide of iron, oxide of manganese, and water.
| Phosphoric acid | 36·60 | 32·8 |
| Oxide of iron | 11·10 | |
| Oxide of manganese | 32·85 | 47·2 |
| Water | 15·00 Dufrésnoy. | 200 Vauquelin. |
Sp. Gr. 2·27. H. above 3·0
Primary form an oblique rhombic prism of 117° 30′; in minute translucent crystals of a reddish-yellow colour; fracture conchoidal, with a vitreous lustre; heated in the mantrass, it yields water. Alone it fuses readily before the blowpipe, affording a black button, which has a metallic lustre.
Described by Dufrésnoy, who named it from its locality, the Commune des Huréaux in the Haute Vienne.
[page] 248
PHOSPHATE OF MANGANESE.
Phosphormangan, W. Manganèse Phosphatèe Ferrifére, H. Phosphate of Iron and Manganese, A. Manganese Phosphatée, Bt. Triplit, Haid.
Combination of phosphoric acid with the protoxides of iron and manganese.
| Limoges. | |
| Protoxide of manganese | 32·6 |
| Protoxide of iron | 31·9 |
| Phosphoric acid | 32·8 |
| Phosphate of lime | 3·2—Berzelius. |
Sp. Gr. 3·4—3·8 H. = 50.
Occurs in compact cleavable masses of a brownish-black colour; structure lamellar, with a brilliant and somewhat adamantine lustre; cleavage in three directions perpendicular to each other, one of them less distinct than the others, the primary form (thus indicated) being a rectangular prism; fracture flat conchoidal; opake in the mass, but thin fragments are semitransparent; streak yellowish-grey. Alone on charcoal it fuses very easily with brisk intumescence into a black metallic-like globule, which is magnetic; with borax is readily soluble into a glass, which appears of an amethystine colour in the oxidating flame, bottle-green in the reducing; with soda it is insoluble on charcoal, but on platina leaf exhibits a green colour; and with boracic acid melts, and forms, with iron wire, phosphuret of iron. Is slowly soluble with effervescence in nitric or muriatic acid.
It occurs in large-grained granite, near Limoges in France, associated with the beryl of that locality; also at Washington in Connecticut, and at Sterling in Massachusetts.
The Dufrénite of Brogniart, or phosphate de fer manganesien vert of Beudant, contains phosphoric acid 24·8, protoxide of iron 51·0, water 15·0, peroxide of manganese 9·0. Specific gravity 3·227. Is of an olive- or dull-green colour; in small radiated masses; slightly translucent; and extremely fusible, melting even on exposure to the candle. Occurs at Anglar near Limoges.
[page] 249
SULPHURET OF MOLYBDENA.*
Wasserblei, W. Molybdène Sulfuré, H. Molybdena, J. Rhombohedral Molybdena Glance, M. Molybdenite, Beudant.
Combination of molybdena 60, and sulphur 40.
Sp. Gr. 4·4—4·7. H. = 1·0—1·5.
Colour nearly that of fresh cut metallic lead. It occurs massive, with a lamellar structure; and in very flat hexahedral tables, which are readily divisible parallel with their terminal planes. Lustre metallic; opake; sectile; highly flexible, but not elastic; and unctuous to the touch. On paper it leaves traces of a metallic-grey colour, and on pottery or porcelain a greenish streak. Before the blowpipe it gives out sulphureous fumes, which are deposited on the charcoal, but neither fuses nor is reduced. It is soluble with effervescence in nitric acid, but leaves a grey residue; and it deflagrates with nitre.
Molybdena occurs in hexagonal plates in Greenland; and in flat, indistinct, six-sided prisms at Arendal in Norway, and Numedahl in Sweden. Generally it is met with in foliated masses, either imbedded in or disseminated through granite, gneiss, syenite, and other primitive rocks; in Bohemia and Saxony with tin; in Silesia, in granite; at Chessy in France, in syenite; in grey granite near Mont Blanc; abundantly in gneiss at Hoddam, Saybrook, and Shutesbury in the United States; also in Peru. Many of the Cornish mines produce it in considerable quantity; and in the granite of Caldbeck Fell, in Cumberland, it occurs associated with tungsten, wolfram, and apatite. This species is readily distinguished from graphite by its streak and lustre; by its specific gravity, which is much higher; and by its comportment before the blowpipe, which is totally dissimilar.
OXIDE OF MOLYBDENA.
Molybdic Ochre, Shepard. Molybdena Ocker, Karsten.
Combination of molybdena and oxygen.
Colour yellow of various shades, occasionally greenish; massive; composition impalpable, pulverulent, friable and dull. According to Berzelius, its characters before the blowpipe resemble those of pure molybdic acid; but treated with soda it sinks into the charcoal, leaving a residuum of protoxide of iron on the surface. With salt of phosphorus it affords a green glass.
It is found in very minute quantities, incrusting the sulphuret of molybdena, at Numedahl in Sweden, and in other localities of that species.
* Molybdena, from the Greek, in allusion to its resemblance in colour to lead.
L2
[page] 250
OXIDE OF TIN.
Zinnstein, W. Etain Oxydé, H. Tinstone, J. Pyramidal Tin Ore, M-Stannolite, Necker. Cassitérite, Beudant,
Oxide of tin, sometimes accidentally mixed with small quantities of oxide of iron, oxide of manganese, and tantalite.
| Cornwall. | Finbo. | |
| Oxide of tin | 99·00 | 93·6 |
| Oxide of iron | 0·25 | 1·4 |
| Oxide of manganese | 0·00 | 0·8 |
| Oxide of tantalum | 0·00 | 2·4 |
| Silica | 0·75—Klaproth. | 0·0—Berzelius. |
Sp. Gr. 6·4—6·9. H. = 6·0—70.
Common form a quadrangular prism terminated by four-sided pyramids (fig. 4). The primary is an obtuse octahedron with a square base; the angle over the apex being 112° 10′; and of a plane of one pyramid on the adjoining plane of the other, 67° 50′. It occurs almost transparent, and either colourless or of a yellowish tint; hair-brown or reddish-brown, and translucent; most commonly deep brown passing into black, and opake; bright-yellow and red colours are produced artificially by exposure to heat. Rarely occurs massive, mostly in crystals coating cavities in veins, or disseminated; also fibrous and granular. Cleavage with some difficulty parallel to the planes of the primary octahedron, viz with the narrow planes, which appear to replace the pyramidal edges of fig. 3. Externally the crystals are splendent; fracture uneven or imperfectly conchoidal, with a more or less shining resinous lustre; structure lamellar; streak greyish white. It gives sparks with the steel, and is brittle. When heated it decrepitates strongly; but is reducible when exposed on charcoal to the continued action of the blowpipe. It is insoluble in acids.
Fig. 1, the primary, an obtuse octahedron; but no crystal has been found of this form: the nearest approach to it is fig. 2, which is prismatic, the planes of the prism arising from the replacement of the lateral solid angles of the primary octahedron by six-sided planes (see Zircon, fig. I, 2, 3). In fig. 3, the angles formed by the meeting of the terminal (primary) planes with the lateral planes, are each replaced by triangular faces; which are complete in fig. 4 (the form of the common crystal), on which no part of the primary is visible. In fig. 5, each solid angle formed by the meeting of the lateral and terminal planes of a crystal similar to fig. 4, is replaced by two triangular planes; which in fig. 6 are considerably enlarged, and assume another form.
[page] 251
Macles.
The large figure on the left exhibits a combined view of all the planes hitherto observed on the crystals of oxide of tin; f1 and f1′ are those of the common pyramid; e and e′ those of the commonly observed prism.
The uppermost of the figures on the right represents the most common macle of the oxide of tin, consisting of equal portions of two similar crystals, the lower of which is turned and attached to the upper; the common plane of their attachment being parallel to the plane P' of the upper half. The lines down the planes e of both halves represent the striæ always observable on the prismatic planes of the crystals, and are here represented in order to show the connexion between this and the lower of the two macles, which for the sake of illustration is placed in a different point of view; it will be observed that the lower made Consists only of portions of the prisms of crystals attached together, if we except the small planes, which are parts of P' and f1: only three, or at most four, of these sections are usually to be seen thus attached.

Tin belongs almost exclusively to primitive mountains, and is found in veins traversing granite, gneiss, and mica-slate.
On the European continent it is met with abundantly, on both
[page] 252
the Bohemian and Saxon sides of the Erzgebirge, particularly at Zinnwald and Schlackenwald, where it frequently occurs in macled crystals of considerable magnitude, weighing several pounds. It is also found in Gallicia in Spain; in the granite hill of Puy les Vignes, Haut Vienne, in France; in Greenland, Sweden, and the United States; in Asia on the east coast of Sumatra, in the island of Banca, and on the peninsula of Malacca; in Mexico; among alluvial deposits in Chili, &c.
The chief repository, however, of this ore is in Cornwall, where it occurs in veins traversing granite and schist, accompanied by chlorite, apatite, fluor, topaz, schorl, arsenical pyrites, wolfram, and blende; also disseminated in granite, as in that of St Michael's Mount. Generally speaking, the Çornish varieties are not of large size, though extremely perfect in form and symmetry, nor do they so often occur macled as those of Bohemia. The tin mines of this country are well known to have been worked at a period anterior even to the Romans, and ore to the value of about L.300,000 is still annually obtained from those of Cornwall.
Fibrous Oxide of Tin. Wood Tin. Etain oxydé concretionné, H. Contains from 5 to 9 per cent. of iron. This occurs in reniform, globular, and botryoidal masses, or in wedge-shaped pieces, which have arisen from their partial destruction; the surfaces are generally water-worn. It exhibits various shades of brown, which sometimes appear in concentric bands, giving it a ligneous appearance; structure divergingly fibrous in one direction, concentric lamellar in the other; lustre glimmering or silky.
It occurs in some of the principal stream works of Cornwall, frequently in masses weighing several pounds. The variety which has received the name of toad's eye wood tin consists of minute spherical masses, of a silky lustre, and of alternate hair-brown and yellowish-white colours disposed concentrically; the fibres radiating from a centre. Its usual matrix is a quartz rock, which abounds in the neighbourhood of Tregurthy Moor in Cornwall.
Stream Tin, as its name indicates, is the alluvial debris of tin veins separated from the deposit of gravel by washing; it consists of detached fragments, which occur in many of the low grounds or marshy places of Cornwall; it is sometimes accompanied by grains of native gold; and frequently associated with animal and vegetable remains, such as deer-horns, hazel-nuts, &c. It is a valuable ore of tin.
Tantaliferous Oxide of Tin, from Finbo in Sweden, occurs in small grains, imbedded in quartz. It is black, with a shade of red or reddish-grey; lustre metallic; fracture uneven; opake, and hard enough to scratch glass. Sp. Gr. 6·55. Does not alter before the blowpipe. It consists of oxide of tin 63·6, oxide of tantalum 2·4, oxide of iron 1·4, oxide of manganese 0·8. Another variety affords upwards of 12 per cent. of oxide of tantalum.—Berzelius.
[page] 253
SULPHURET OF TIN.
Tin Pyrites. Zinnlies, W. Etaine Sulfuré, H. Hexahedral Copper Glance, M.
Combination of sulphuret of tin and sulphuret of copper.
Tin 34·0, copper 36·0, iron 2·0, sulphur 25·0—Klaproth.
Sp. Gr. 4·35—4·76. H. = 4·0.
Massive; of a steel-grey colour when pure, often yellowish from an admixture of copper pyrites; and these colours are sometimes intermingled in the same specimen, imparting to the mass somewhat the aspect of bell-metal, whence bell-metal ore; fracture granular and uneven, passing into flat conchoidal, with a shining metallic lustre; opake and brittle. Before the blowpipe sulphur is driven off, after which it fuses readily into a black scoria. It is soluble in nitro-muriatic acid, with the exception of the sulphur, which is precipitated.
It occurs only in Cornwall, and that principally at Huel Rock in the parish of St Agnes, accompanying blende, pyrites, and other minerals.
OXIDE OF TUNGSTEN.
Tungstic Ochre, Shepard.
Soft. Sp. Gr. 6·0.
This mineral varies in colour from orange- or chrome-yellow to yellowish-grey. It occurs massive; composition impalpable; earthy, and pulverulent; is inodorous; tasteless; assumes a greenish hue under the blowpipe; is insoluble in acids, but by digestion in nitric acid the powder, which is greyish, assumes a brilliant yellow colour, and would probably afford a fine pigment. It is readily soluble in warm liquid ammonia; and is precipitated white by acids, the precipitate, by standing, re-acquiring a yellow colour.
It occurs in quartz, associated with wolfram and tungsten, in minute veins or thin coatings, at Lane's mine, in Monroe county, United States.
ANATASE*
Pyramidal Titanium Ore, M. Oktaedrite, W. Titane Anatase, H. Bt. Octahedrite, J.
It is the pure oxide of titanium.
Sp. Gr. 3·85. H. = 5·5—6·0.
* Anatase, from the Greek, signifying elevated, in allusion to the height of the pyramids of the octahedral crystals; Octahedrite, from its occurring in octahedrons.
[page] 254
This mineral presents various shades of brown, indigo-blue, or steel-grey; by transmitted light it is greenish-yellow. It occurs in small crystals, having the general form of an acute octahedron with equal and similar isosceles triangular faces, which is the form of the primary crystal; the crystals exhibit the planes of several modifications; structure lamellar; cleavage both parallel to the faces of the octahedron, and perpendicular to the axis; lustre of the fragments splendent and adamantine; varies from semi-transparent to opake; streak white; and is brittle. When heated, it exhibits a reddish-yellow phosphorescent light; and acquires resinous electricity by friction. Before the blowpipe it is infusible without addition; with soda it forms a dull yellow globule, which becomes white on cooling.
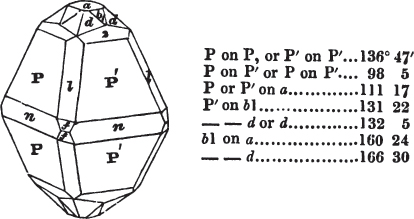
The principal locality of anatase is Oisans in Dauphiné, where it occurs in veins in granite and mica-slate, accompanying felspar, axinite, rock-crystal, chlorite, &c. Is also found disposed in mica-slate, in the Grisons; at Tavatsch in the Tyrol; and in Brazil.
RUTILE.
Peritomous Titanium Ore, M. Rutil,W. Titane Oxydé, H. Rutile,Br. Titane Rutile, Bt.
The oxide of titanium, sometimes mixed with oxide of iron or chrome.
Sp. Gr. 4·24—4·4. H. = 6·0—6·5.
Usual colour reddish-brown, in which case it is opake; also in prismatic crystals terminated by pyramids, of a blood-red colour, and then translucent or transparent. It occurs in four- or eight-sided prisms, either single or geniculated, and commonly striated longitudinally; also in minute reticulated crystals. The structure is lamellar; the primary a right prism with square bases; cleavage perfect parallel to M, interrupted parallel to d; the frag-
[page] 255
ments possess metallic adamantine lustre; cross fracture imperfectly conchoidal or uneven; streak very pale brown; brittle; acquires resinous electricity by friction. Before the blowpipe it is infusible per se, but with borax it forms a transparent reddish-yellow glass, which when long in the reducing flame assumes an amethystine colour.
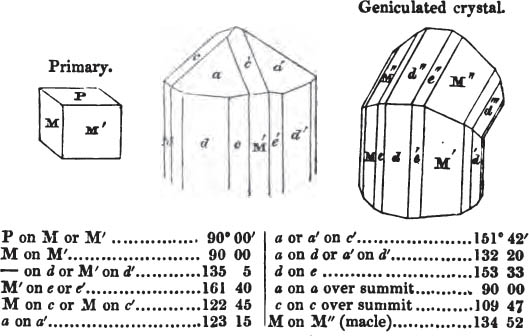
Rutile is most frequently met with in lengthened prismatic crystals imbedded in quartz, as at Crianlarich in Perthshire, Rosenau in Hungary, St Gothard, and in Brazil. When the quartz is limpid, and is cut and polished to shew the rutile, its slender needle-shaped or hair-like crystals appear of a blood-red hue by transmitted light. The reticulated variety occurs at St Gothard disposed in red translucent flat prisms on crystals of fer-oligiste. At St Yrieix in France, and in Castile, it forms remarkable geniculated twin crystals, which are often of large size.
Nigrin,* W. Titane oxydé ferrifère, H. Titan nigrin, Bt. Oxide of titanium containing about 14 per cent. of iron, Of a brownish-black colour, and generally in loose angular or rounded masses; structure lamellar; cross fracture flat and imperfectly conchoidal; lustre shining; streak pale-brown, and in most of its characters precisely similar to rutile.
It is found in alluvium in Ceylon with iron sand, hyacinth, &c.; and at Ohlapian in Transylvania with gold, almandine, rutile, and fragments of granite and other primitive rocks in yellow sand.
* From its black colour.
[page] 256
MENACCANITE. ISERINE.
Is a titanitic iron, containing,
| Menaccanite. | Iserine. | |
| Oxide of titanium | 45·25 | 48·0 |
| Oxide of iron | 51·00 | 48·0 |
| Oxide of manganese | 0·25 | 0·0 |
| Oxide of uranium | 0·00 | 4·0 |
| Silica | 3·50—Klaproth. | 0·0—Thomson. |
It occurs in the form of black sand, which slightly affects the magnetic needle, and was first noticed in the bed of a rivulet near Menaccan, in Cornwall; and near the rise of the stream Iser* in the Riesengebirge of Silesia.
Different titanitic iron sands necessarily vary considerably in their proportions of titanium and iron; the analyses which are published of them are consequently little to be depended on.
BROOKITE.†
Prismatic Titanium Ore, Haidinger. Brookite, Levy.
Consists of oxide of titanium, with some traces of iron and manganese.
H. = 5·5—6·0.
Primary form a right rhombic prism of 100° and 80′.
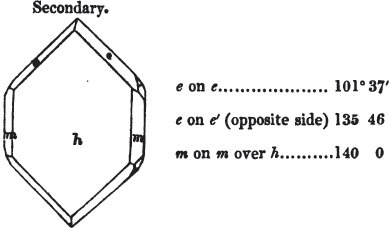
In crystals of a hair-brown colour, passing into deep orange-yellow, more or less translucent; streak yellowish-white; lustre brilliant, metallic-adamantine. Insoluble and indecomposible in boiling muriatic acid, even when reduced to powder. Alone on charcoal it is infusible, but it is entirely soluble and forms a brownish-yellow glass with salt of phosphorus.
This species occurs with anatase and crichtonite at Bourg
* Whence Menaccanite and Iserine.
† In compliment to the talented crystallographer, Mr Brooke.
[page] 257
d'Oisans in Dauphiné, on the Tête-noire in Savoy, and in large distinct crystals on Snowdon in Wales. It is however a rare mineral.
CRICHTONITE*
Fer Oxyduté Titané, H. Crichtonite, Bournon.
Sp. Gr. 4·0. H. = 4·5.
It occurs in small crystals in the form of acute rhomboids, having the summits replaced, and being otherwise variously modified by secondary planes; the only cleavage is at right angles to the axis of the rhomboid, i.e. parallel to the plane a. Colour bluish-black, opake, and of a brilliant metallic lustre; the cross fracture conchoidal and shining; streak deep black. Infusible before the blowpipe, but with salt of phosphorus affords a glass which becomes red on cooling. It does not affect the magnet. Crichtonite is classed by Berzelius with menaccanite.
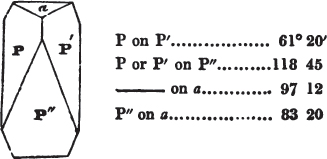
It occurs accompanying anatase and on rock crystal at St Christophe near Oisans in Dauphiné.
ILMENITE-†
Axotomous Iron Ore, M. Ilmenit, L. Kibdelophan, Beudant.
Combination of the oxides of titanium and iron.
| Miask. | Gastein. | |
| Oxide of titanium | 46·67 | 59·00 |
| Peroxide of iron | 11·71 | 4·25 |
| Protoxide of iron | 35·87 | 36·00 |
| Protoxide of manganese | 2·39 | 1·65 |
| Magnesia | 0·60 | 0·00 |
| Lime | 0·25 | 0·00 |
| Oxide of chrome | 0·38 | 0·00 |
| Silica | 2·80—Mosander. | 0·00—Kobell. |
Sp. Gr. 4·4—4·8. H. = 5·0—5·2.
Occurs, though rarely, in irregular opake crystals of a dark iron black colour; generally massive. Primary form an acute
* In honour of Dr Crichton.
† From its locality, the lake Ilmen in Siberia.
[page] 258
rhomboid of 85° 59′ and 94° 1′. Cleavage perfect parallel to 0 ; lustre imperfect metallic: streak black; fracture conchoidal; slightly affects the magnet. Before the blowpipe on charcoal it is infusible, but with fluxes comports itself like oxide of iron.
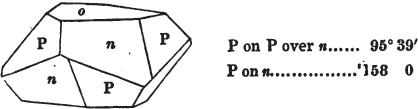
This species occurs imbedded in serpentine, and associated with apatite and sparry iron, at Inglisberg near Hoff, in the Gastein Valley, Saltzburg. It is also met with massive and compact at Eggersund in Norway, and imperfectly crystallized at Ilmensee and Ekatherineburg in Siberia.
MOHSITE*
Mohsite, Levy. Uncleavable Iron Ore, Shepard.
Scratches glass easily.
Primary form a rhomboid of 73° 45′. Occurs in twin crystals, flattened in a direction perpendicular to the axis, presenting the aspect of small flat tables, nearly circular, with alternate re-entering and salient angles on their edges. Cleavage not observable; colour iron-black; opake; with a perfect metallic lustre; brittle; does not act on the magnet.
This species was noticed by Levy on a specimen which was understood to be from Dauphiné.
SPHENE.†
Hemi-Prismatic Titanium Ore, M. Sphen, Karsten. Titane Siliceo- Calcaire, H.
Combination of oxide of titanium, silica, and lime.
| Passau. | Felberthal. | St Gothard. | |
| Oxide of titanium | 33·0 | 46·0 | 33·3 |
| Silica | 35·0 | 26·0 | 28·0 |
| Lime | 33·0 | 16·0 | 32·2 |
| Water | 0·0 | 1·0 | 0·0 |
| Klaproth. | Klaproth. | Cordier. |
Sp. Gr. 3·49—3·6. H. = 5·0—5·8.
Colour grey, yellow, hyacinth-red, and brown, also various shades of green; streak greyish white; occurs amorphous, and
* Named by Levy in compliment to Professor Mohs.
† Sphene, from the Greek, probably in allusion to its crystals being somewhat wedge-shaped.
[page] 259
in crystals differing greatly in form; the primary is an oblique rhombic prism, of which the lateral angles are alternately about 133° 30′ and 46° 30′; cleavage parallel to the faces of this prism, but not easily observed; fracture imperfect conchoidal; translucent on the edges; lustre adamantine, sometimes inclining to resinous. Before the blowpipe the yellow varieties do not change their colour; all the rest become yellow; they slightly intumesce, and fuse on the edges into a dark-coloured enamel, and with borax afford a yellowish-green diaphanous glass. With salt of phosphorus it fuses slowly into a globule, which, after a long blast, becomes opaline on cooling; in the reducing flame this globule assumes the amethystine tinge characteristic of titanium. Is soluble in heated muriatic or nitric acid, leaving a siliceous residue; the fragments, when exposed to heat, exhibit a brilliant white phosphorescence.
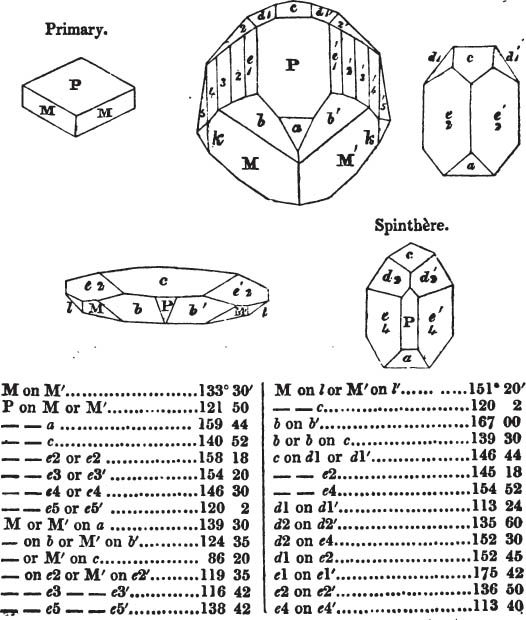
[page] 260
It occurs chiefly in primitive rocks.
The brown and almost entirely opake varieties of this species occur with augite in beds of iron ore at Arendal in Norway; in a granitic rock at Sartut in Greenland; and with scapolite and tremolite in the limestone quarry of Malsjö in Wermeland, Sweden. The light-coloured and frequently transparent crystals, on the other hand, are met with of considerable magnitude, frequently macled in the most fantastic manner, and associated with felspar and chlorite, at Graubinden in the Grisons; on mica slate at St Gothard; in distinctly pronounced brownish crystals disposed on chlorite at the Val Maggia in Piedmont; of a yellowish-grey colour accompanying the rock crystals of Mont Blanc, and elsewhere among the Alps. Small individuals occur in certain syenites, as at Strontian in Argyleshire, and Criffle in Galloway. More rarely it appears among volcanic rocks, as at the Laacher See, and Andernach on the Rhine. (Manual.)
The Spinthère of Haüy, found at Isère in France on crystals of carbonate of lime, is only a variety of sphene.
PYROCHLORE.
Octahedral Titanium Ore, M. Pyrochlore, Wōhler.
Combination of lime, titanic acid, protoxide of manganese, and the oxides of iron, uranium, cerium, and tin.
| Titanic acid | 62·75 |
| Lime | 12·85 |
| Protoxide of manganese | 2·75 |
| Oxide of iron | 2·16 |
| Oxide of uranium | 5·18 |
| Oxide of cerium | 6·80 |
| Oxide of tin | 0·61 |
| Water | 4·20 |
| Fluoric acid and magnesia | traces—Wöhler. |
Sp. Gr. 4·2—4·25. H. = 5·0.
Primary form the regular octahedron, without distinct cleavages. Occurs in well-pronounced octahedrons of a dark reddish-brown colour, which are slightly translucent on the edges, or opake; streak pale brown; fracture conchoidal, with a resinous or vitreous lustre, and, when fresh, of a black colour. Before the blowpipe, in the matrass, it yields water, and becomes greenish-yellow; per se on charcoal it fuses with great difficulty into a blackish-brown scoria; with borax it effervesces, and is completely soluble into a transparent globule, which appears reddish-yellow in the oxidating flame, but becomes opake on flaming, while in the reducing flame it is deep red, but changes into a bluish-grey enamel on flaming; with salt of phosphorus it w
[page] 261
also entirely soluble into a globule, which is yellow in the oxidating flame, but becomes grass-green on cooling, and which again is superseded by a deep-red colour when exposed to the reducing flame.
Pyrochlore was discovered by Otto von Tank, and received its name from Berzelius, in allusion to its property of turning yellow when exposed to the blowpipe. It bears considerable resemblance to zircon, with which it occurs imbedded in syenite, at Frederickswärn and Laurvig in Norway. It has also been brought from Greenland, but is by no means a mineral of frequent occurrence. (Manual.)
AESCHYNITE.
Combination of zirconia, titanic acid, oxide of cerium, lime, and the oxides of tin and iron.
| Titanic acid | 56·0 |
| Zirconia | 20·0 |
| Oxide of cerium | 15·0 |
| Lime | 3·8 |
| Oxide of iron | 2·6 |
| Oxide of tin | 0·5—Hartwall. |
Sp. Gr.5·14—5·5. H. = 5·0—6·0.
Primary form an oblique rhombic prism (according to Brooke), of about 127° and 53°; secondary form, the primary terminated by four-sided pyramids. Colour dark black, inclining to brownish-yellow when translucent; lustre resinous; streak dark grey or black; fracture imperfectly conchoidal; and translucent only on the edges, and when in thin fragments. Before the blowpipe it yields in the matrass some water; on charcoal it intumesces, and becomes yellow; with borax fuses readily into a dark yellow glass, and with salt of phosphorus forms a transparent colourless bead, but with soda is insoluble.
This mineral occurs in the Ilmen range near Miask in Siberia, imbedded in felspar, and associated with mica and crystals of zircon.
POLYMIGNITE.*
Berzelius.
Combination of the oxide of titanium, zirconia, yttria, the oxides of cerium, iron, manganese, lime, &c.
* From Πολνς, much, and μςΓνοΩ, I mix; in allusion to its numerous constituents.
[page] 262
| Contains Oxide of titanium | 46·30 |
| Zirconia | 14·14 |
| Yttria | 11·50 |
| Oxide of iron | 12·20 |
| Lime | 4·20 |
| Oxide of manganese | 2·70 |
| Oxide of cerium | 5·00 |
with traces of magnesia, potash, silica, and oxide of tin—Berzelius.
Sp. Gr. 4·77—4·85. H. = 6·5.
Primary form, according to Rose, a rhomboidal octahedron, whose dihedral angles are 136° 28′, 116° 22′, and 80° 16′. Occurs in long thin prismatic crystals, the edges of which are commonly replaced. Colour black; opake; lustre imperfect metallic, but brilliant; streak dark-brown; traces of cleavage parallel to T and M; fracture perfect conchoidal, presenting, like the surface, a brilliáncy almost metallic. Surfaces of the crystals deeply striated longitudinally. Per se it is infusible, and remains unaltered; with borax is soluble with facility into a glass coloured yellow by iron, and with salt of phosphorus fuses into a reddish glass.
Polymignite occurs in crystals sometimes exceeding an inch in length, imbedded in the syenite of Stavern and Frederickswärn in Norway.
CERITE.
Rhombohedral Cerium* Ore, M. Cerit, Hisinger. Cerium Oxidé Silicifère, H. Cerinstein, W. Cererit, L.
| Oxide of cerium | 68·59 | 67·0 |
| Silica | 18·00 | 1·70 |
| Oxide of iron | 2·00 | 2·0 |
| Lime | 1·25 | 2·0 |
| Water and carbonic acid | 9·60 Hisinger. | 12·0 Vauquelin. |
Sp. Gr. 4·9—5·0. H. = 5·5.
* Cerium, after the planet Ceres.
[page] 263
Colour rose-red or clove-brown, passing into grey; streak whitish-grey; it occurs massive; the fracture splintery and more or less shining; opake, or slightly translucent on the edges; scratches glass; gives sparks with the steel, and is hard and difficultly frangible. Before the blowpipe on charcoal it splits, but does not fuse; with borax it melts slowly, and forms in the oxidating flame an orange-yellow coloured globule, which becomes nearly colourless on cooling; and in the reducing flame assumes a feeble tint of iron. With salt of phosphorus, in the oxidating flame, it presents a deep-red glass, which becomes as limpid as water on cooling; and which, in the reducing flame, is colourless.
It is found only in the copper mine of Bastnaes near Riddarhyttan in Sweden, where it forms a bed in gneiss, accompanying copper, molybdena, bismuth, mica, and hornblende.
TITANIFEROUS CERITE. Laugier describes a variety under this name from the Coromandel coast, of a blackish-brown colour, with a vitreous conchoidal fracture. H. equal to that of gadolinite. It contains oxide of cerium 36·0, oxide of iron 19·0, lime 8·0, alumina 6·0, water 11·0, oxide of manganese 1·8, silica 19·0, oxide of titanium 8·0. These quantities exceed 100 by 9·55 parts, an excess occasioned by the protoxide of cerium in the mineral becoming peroxide in the analysis. It intumesces when heated, and is acted upon both by acids and alkalies.
SILICATE OF CERIUM.
Wollaston.
In regular hexagonal prisms of a pale yellowish-brown colour; cleavage parallel to the axis of the prism; translucent.
Occurs with emerald in magnesian carbonate of lime at Santa Fé de Bogota in Peru.
ALLANITE.
Cerine, Hisinger and Berzelius. Cerium Oxydé Siliceux Noir, H. Anorthitic Melane Ore, Haid. Prismatic Cerium Ore, J.
Combination of silica, lime, alumina, with a mixture of the protoxides of cerium and iron.
[page] 264
| Greenland. | Riddarhyttan. | ||
| Silica | 35·4 | 33·02 | 30·17 |
| Protoxide of cerium | 39·9 | 21·60 | 28·19 |
| Oxide of iron | 25·4 | 15·10 | 20·72 |
| Alumina | 4·1 | 15·23 | 11·31 |
| Lime | 9·2 | 11·08 | 9·12 |
| Water | 0·0 | 3·00 | 0·00 |
| Oxide of copper | 0·0 | 0·00 | 0·87 |
| Thomson. | Stromeyer. | Hisinger. | |
Sp. Gr. 3·5—4·0. H. = 6·0.
Colour brownish-black; streak greenish-grey. It generally occurs massive, and rarely crystallized in oblique four-sided prisms, variously terminated; fracture uneven, passing into small conchoidal, with an imperfect metallic lustre; opake, the thinnest fragments sometimes slightly translucent and of a yellowish-brown. Before the blowpipe on charcoal it fuses readily with effervescence into a black and shining glass; with borax forms a black opake globule, which in the oxidating flame appears blood-red while hot, but becomes deep-yellow on cooling. Gelatinizes readily in nitric acid.
Allanite is easily distinguished from gadolinite and orthite, by its difference in specific gravity and hardness; thin fragments of the gadolinite also are translucent on the edges and of a fine-green colour; allanite is commonly opake, rarely translucent, and of a yellowish-brown.
Allanite occurs imbedded in granite at Alluk, near the southern extremity of East Greenland, where it was discovered by Professor Giesècké, but was first noticed and recognised as a distinct species by the late Thomas Allan, Esq. of Edinburgh, in compliment to whom it was named.
The cerine of Berzelius is found associated with cerite, at Bastnaes near Riddarhyttan in Sweden.
* The drawing and measurements of this mineral, given by Mr Phillips in his last edition, evidently belong to another species. Those now substituted were taken by Haidinger from crystals brought from Greenland by Giesècké, and have since been authenticated by Rose in regard to the minute crystallization of the Swedish variety.—A.
[page] 265
TORRELITE.
Renwick.
Consists of peroxide of cerium 12·32, silica 32·60, protoxide of iron 21·00, alumina 3·68, lime 24·08, water 3·50—Renwick. It is infusible per se, but forms with borax a glass which is green while hot, but which becomes colourless on cooling. It effervesces with acid. Colour dull vermilion-red; streak rose-red; fracture granular; affects the magnet slightly.
Beudant considers torrelite a variety of red manganese, not an ore of cerium. It occurs in Sussex County, New Jersey.
ORTHITE.
Combination and mixture of silica, alumina, the protoxides of iron and cerium, lime, yttria, oxide of manganese, and a small quantity of water.
| Finbo. | Gottliebsgang. | |
| Protoxide of cerium | 17·39 | 19·44 |
| Silica | 36·25 | 32·00 |
| Lime | 4·89 | 7·84 |
| Alumina | 14·00 | 14·80 |
| Protoxide of iron | 11·42 | 12·44 |
| Oxide of manganese | 1·36 | 3·40 |
| Yttria | 3·80 | 3·44 |
| Water | 8·70 Berzel. | 5·36 Berzel. |
Orthite occurs either massive or in long thin acicular crystals. Primary form undetermined. Colour ash-grey,inclining to black; opake; lustre vitreous; streak brownish-grey; fracture conchoidal. It resembles gadolinite, but differs in fusibility. Alone on charcoal before the blowpipe it intumesces, and becomes yellowish-brown. In heated acid it gelatinizes, and fuses into a black blebby glass; with borax dissolves readily into a globule, which, when hot, is red; when cold, yellow.
Orthite occurs in acicular diverging dark-brown coloured prisms sometimes exceeding a foot in length, imbedded in quartz, at Finbo near Fahlun in Sweden; also in black vitreous masses, disseminated through granite, at Skeppsholm, one of the islands of Stockholm. It has likewise been met with at Lindenaes in Norway, and was brought from Greenland by Giesècké. (Manual.)
PYRORTHITE.
Protoxide of cerium 13·92, silica 10·43, lime 1·81, alumina 3·59, protoxide of iron 6·08, protoxide of manganese 1·39, yttria 4·87, water 26·50, carbon 31·41—Berzelius.
Sp. Gr. 2·19. H. = 2·5.
M
[page] 266
Massive; composition columnar; fracture conchoidal, splintery; earthy; internal lustre resinous, externally dull; colour brownish-black; becoming yellowish-brown by decomposition; streak brownish-black; opake. If gently heated on one side it takes fire, and burns without either flame or smoke; after which it becomes white, and melts into a black enamel. With borax it forms a transparent blood-red glass when hot, which changes into yellow on cooling; and in heated acids is soluble, with the exception of a black powder; in the matrass it yields much water.
It accompanies gadolinite in granite at Kararfvet near Fahlun in Sweden; and, except that it is devoid of lustre, bears much resemblance to the orthite from the same locality.
CARBONATE OF CERIUM.
Carbo-cerine, Beudattt. Carbonate of Cerium, Berzelius.
Contains oxide of cerium 75·7, carbonic acid 10·8, water 13·5 —Berzelius.
Occurs in thin four-sided crystalline plates of a greyish-white colour. Does not alter its appearance, though it loses 19 per cent. of its weight, when exposed to a slight red heat. It forms coatings on the cerite from Bastnaes in Sweden, and is probably derived from the decomposition of that mineral. It is extremely rare. (Manual.)
YTTROCERITE*
Fluate of Yttria and Cerium, Berzelius. Cerium Oxydé Yttrifère, Beudant.
| Consists of Oxide of cerium | 18·22 | 13·15 |
| Lime | 47·63 | 47·77 |
| Fluoric acid | 25·05 | 24·45 |
| Yttria | 9·11 | 14·66 |
| Berzelius. | ||
Sp. Gr. 3·4—3·5. H. between 4·5 and 7·0.
The colour of this mineral is violet, or greyish-red, often mingled in the same specimen. It occurs in amorphous masses, varying from a thin crust to half a pound in weight, and presenting occasional traces of cleavage parallel to the sides of an oblique rhombic priam, whose lateral planes incline under angles of about 108° 30′. It is opake; lustre glistening. Before the blowpipe, per se, it loses its colour and becomes white, but does not fuse; but on the addition of gypsum it melts readily into an opake globule. Is soluble with residue in boiling muriatic acid.
It has been found only at Finbo near Fahlun in Sweden, disseminated in quartz, and associated with albite and pyrophysalite.
* From its consisting chiefly of yttria and cerium.
[page] 267
NEUTRAL FLUATE OF CERIUM.
Deuto-fluate of Cerium. Flucerine, Beudant.
Combination of fluoric acid and oxide of cerium.
| Broddbo. | |
| Oxide of cerium | 82·64 |
| Yttria | 1·12 |
| Fluoric acid | 16·24—Berzelius. |
Sp. Gr. 4·7. H. about 4 0.
Occurs in six-sided prisms, in plates, and in amorphous masses, of a reddish or wax-yellow colour. Dull; translucent in extremely thin fragments; and having an uneven fracture. Before the blowpipe on charcoal it does not fuse, but merely becomes slightly brown; with borax and salt of phosphorus it gives in the oxidating flame a red or orange coloured globule, which becomes pale on cooling; in the reducing flame it loses its colour entirely. Heated in a tube it corrodes the glass.
This mineral has hitherto only been found at Finbo and Broddbo near Fahlun in Sweden, in very small quantities; it occurs imbedded in granite, and associated with pyrophysalite, orthite, &c.
SUB-FLUATE OF CERIUM, or Fluate of Cerium with excess of base—Berzelius. Basi-cerine—Beudant. This mineral differs from the above in containing rather a larger proportion of the oxide of cerium—in the absence of yttria—and in the presence of a variable quantity of water. It possesses considerable resemblance to porcelain-jasper. Colour yellow; with occasional slight traces of crystallization. Before the blowpipe on charcoal it is infusible, becomes black at an incipient redness, but on cooling assumes successively dark-brown, red, and orange tints.
It occurs in minute quantities, accompanying allanite, at Bastnaes near Riddarhyttan in Sweden.
DOUBLE FLUATE OF CERIUM AND YTTRIA.
This is an earthy mineral found at Finbo in Sweden; it is much more common than the preceding, but its size seldom exceedsjthat of a pea. It is usually of a pale-red colour, but occurs deep-red, yellow, and even white. It is very soft, and may easily be scratched by the nail. According to Berzelius, it is a mechanical mixture of the fluate of yttria, with fluate of cerium, and silica. It presents nearly the same re-actions as the Neutral Fluate.
[page] 268
PITCH-BLENDE.
Pech-blende, Pecherz, W. Uiane Oxydulé, H. Uranpecherz, L. Uncleavable Uranium Ore, M.
| Protoxide of uranium | 86·5 | 84·52 |
| Protoxide of iron | 2·5 | 8·24 |
| Silica | 5·0 | 2·02 |
| Sulphuret of lead | 6·0 | 4·20 |
| Oxide of cobalt | 0·0—Klaproth. | 1·42—Pfaff. |
Sp. Gr. 6·5—7·5. H. = 5·5.
Colour greyish-black, brownish-black, and iron-black; and occurs globular, reniform, massive, disseminated, and pulverulent; form and crystalline structure unknown; fracture uneven, or small conchoidal; lustre dull or imperfect metallic; in the former case frequently presenting iridescent colours superficially; it is opake; very brittle, but sometimes scratches glass. Per se it is infusible before the blowpipe; but with borax it yields a deep yellow coloured glass, which becomes of a dirty green in the reducing flame, and when saturated to a certain point turns black on flaming. If reduced to powder it is slowly soluble in nitric acid, with effervescence and a disengagement of nitrous gas. Does not act on the magnet.
It occurs in botryoidal masses, accompanying various ores of silver and lead, at Marienberg, Schneeberg, and Johanngeorgen-stadt in Saxony; at Joachimsthal and Przibram in Bohemia; at Rezbanya in Hungary; and with uranite in several of the Cornish mines. It is a valuable ore to the porcelain painter, yielding a fine orange colour in the enamelling fire, and a black one in that in which the porcelain itself is baked. (Manual.)
URANITE.
Phosphate of Uranium. Uran Glimmer, W. Urane Oxidé, H. Urane Micacé, Br. Uran-Mica, J. Pyramidal Euchlore Mica, M.
Combination of phosphoric acid, lime, oxide of uranium, and water, with traces of fluoric acid and ammonia, and a mechanical admixture of silica, barytes, magnesia, and the oxides of iron and manganese.
| Autun. | |
| Oxide of uranium | 59·37 |
| Phosphoric acid | 14·63 |
| Lime | 5·66 |
| Magnesia and oxide of manganese | 01·9 |
| Silica and oxide of iron | 2·85 |
| Barytes | 1·51 |
| Water | 14·90 |
| Fluoric acid and ammonia | traces—Berzelius. |
Sp. Gr. 3·12.
[page] 269
Colour lemon-yellow, the same crystal is sometimes yellow at one end, green at the other; it becomes brownish and dull by decomposition. It is found crystallized in quadrangular prisms, in four-, six-, and eight-sided tables, rarely in acute and obtuse octahedrons; the structure is lamellar, and it is mechanically divisible parallel to all, and with remarkable ease to the terminal planes (P) of a right square prism—the form of the primary crystal. Transparent, translucent, or opake; the lustre shining, pearly upon the face P; it yields easily to the knife, and is brittle. It decrepitates violently on charcoal before the blowpipe, and loses about 33 per cent. by ignition; with borax and salt of phosphorus the glass obtained in the reducing flame remains green after cooling; with soda the assay yields no metallic particles. Its solution in nitric acid is yellow, and in ammonia white.
Fig. 1, the primary; a right square prism. Fig. 2, a tabular crystal of a quadrangular form; these occur of various lengths. Fig. 3; in this the solid angles of the preceding are replaced, producing a six-sided tabular crystal; these are commonly very long. Fig. 4; in this all the terminal edges of the quadrangular prism are replaced, tending to produce an octahedron, which sometimes occurs. In fig. 5, the solid angles of the prism (fig. 1) are replaced by triangular planes. In fig. 6, all the edges and solid angles of the quadrangular prism are replaced.
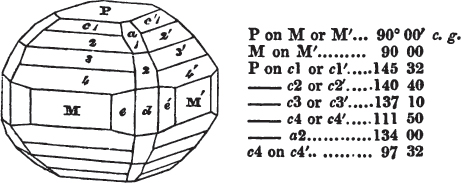
It occurs in veins in granite at St Symphorien near Autun, at St Yrieux near Limoges in France, and in several places in Saxony.
The Uran Ochre appears to be the same substance in a friable state. It presents a brilliant orange-yellow colour, is extremely soft, and adheres to the finger; it is frequently met with in minute flocculent masses, coating pitch-blende from Joachimsthal. (Manual.)
[page] 270
CHALKOLITE.
Uran-Ghjnmer (in part), W. Pyramidal Euchlore Mica (in part), M. Chalkolite, Beudant. Grüner Uranerz.
Combination of phosphoric acid, the oxides of uranium and of copper, and water.
| Cornwall. | ||
| Oxide of uranium | 60·25 | 60·0 |
| Phosphoric acid | 15·56 | 16·0 |
| Oxide of copper | 8·44 | 9·0 |
| Water | 15·05—Berzelius. | 15·0—Phillips. |
Sp. Gr. 3·33. H. = 2·0—2·5.
This species corresponds with the preceding in crystalline form, and in all other points except in the following.—Its colour is green; its solution in ammonia is blue; before the blowpipe with borax and salt of phosphorus the glass obtained in the reducing flame becomes, on cooling, red and opake. With soda the chalkolite of Cornwall is reduced into white metallic grains.
Beautiful varieties of this species have been found in Cornwall, particularly in the veins of Tin Croft mine, and Huel Buller near Redruth, with red copper and arseniate of iron in Huel Gorland and Huel Unity, and in Gunnislake mine near Callington. This species might sometimes be confounded with green mica, but the laminæ of mica are flexible and elastic, while those of uranium are brittle, and do not bend; mica, moreover, is not soluble in nitric acid.
CARBONATE OF URANIUM.
Uran-Bloom, Uran-Bliithe, Zippe. Uraconise, Beudant.
In crystalline flakes of a small size, devoid of distinct form. Colour bright yellow, between lemon- and sulphur-yellow; opake, with little lustre. When slightly heated before the blowpipe, its colour becomes orange-yellow. It is soluble with effervescence in acid, yielding a yellow solution, which affords a brown precipitate with prussiate of potash, thus demonstrating it to be a carbonate of uranium.
Professor Zippe of Prague distinguished this substance from uran-ochre, and the yellow oxide of uranium, chiefly from its want of lustre and more brilliant colour. It occurs in silver veins at Joachimsthal in Bohemia, with pitch-blende, uran-ochre, and pharmacolite; and apparently is derived from the decomposition of the pitch-blende, on which it commonly forms a coating.
[page] 271
JOHANNITE*
Urane Sulfaté, Necker. Johannite, Haidinger. Uran Vitriol, John. Sulfate Vert d'Urane, Beudant.
Hydrous sulphate of uranium mixed with sulphate of copper.
Sp. Gr.3·19. H. = 2·0—2·5.
Primary form an oblique rhombic prism. Secondary, according to Haidinger,
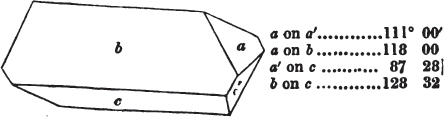
In very minute crystals. Colour deep grass-green; translucent; lustre vitreous; streak pale siskin-green; taste slightly bitter; cleavage traces parallel to a, and to a face which bevels the edge between b and c. Fracture imperfect conchoidal. Partially soluble in water. Heated in the matrass it yields much moisture, leaving a dark blackish-brown mass. Fused upon charcoal with soda, and then laid on a piece of silver and moistened, it blackens the metallic surface. In the reducing flame with soda, a bead of copper is obtained. With borax it forms a fine green glass, as well in the oxidating as in the reducing flame; in the latter it becomes red and opake on cooling, exhibiting the presence of oxide of copper. With salt of phosphorus only green colours are produced, that of the oxidating flame having rather the appearance of copper, the reducing more of uranium. It therefore contains water, sulphuric acid, and the oxides of copper and uranium, but in what proportion has not been determined.
The sulphate of uranium occurs in extremely small crystals at Joachimsthal in Bohemia. It is a species as beautiful as it is rare, having only been observed in one mine, and that in the year 1809. (Manual.)
* Named by Haidinger in compliment to his Imperial Highness the Archduke John of Austria.
[page] 272
TANTALITE.
Columbite,*Hatchelt. Tantalit, Karsten. Tantale Oxidé Ferro-man ganésifère, H. Tantalite, J. A. Prismatic Tantalum Ore, M.
Combination of the oxide of tantalum with the oxides of iron and manganese.
| Kimito. | Broddbo. | Bodenmais. | America. | ||
| Oxide of tantalum | 83·2 | 68·22 | 75·0 | 75·0 | 80·0 |
| Oxide of iron | 7·2 | 9·58 | 20·0 | 17·5 | 15·0 |
| Oxide of mangan. | 7·4 | 7·15 | 4·0 | 1·1 | 0·0 |
| Oxide of tin | 0·6 | 8·26 | 0·5 | 1·0 | 0·0 |
| Tungstic acid | 0·0 | 6·19 | 0·0 | 0·0 | 0·0 |
| Lime | trace | 1·19 | 0·0 | 0·0 | 5·0 |
| Berzelius. | Borkowsky. | Vogel. | Wollas. | ||
Sp. Gr. 6·3—6·8. H. = 6·0.
Colour greyish or brownish-black; it occurs in single crystals, and in small crystalline masses; the crystals are mostly incomplete, but possess the general form of quadrangular prisms, striated longitudinally, shining externally, and variously modified. The primary form appears to be a right rectangular prism. Cleavage parallel to M and T, rather distinct; streak brownish-black. It is opake, scratches glass, and gives sparks with the steel. Alone before the blowpipe none of the varieties of tantalite suffer any change; with borax, however, those of Kimito and Finbo, which contain large proportions of tantalum, dissolve slowly but perfectly, communicating to it a faint green colour; those, on the contrary, which contain less tantalum, fuse readily into a black or extremely dark-green and almost opake glass. In heated sulphuric acid it is partly soluble.
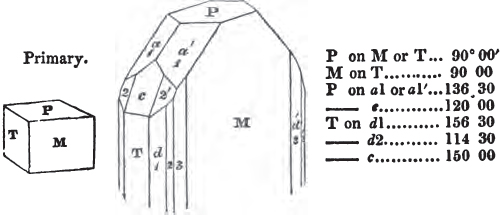
This figure represents a crystal in the possession of Mr Brooke.
* Columbite, from its having been first discovered in America; whence Columbium, the designation of the pure metal.
[page] 273
The most crystalline varieties of this species have occurred at Bodenmais in Bavaria, associated with beryl. It was however first noticed in America, at Haddam in Connecticut, and in the tourmaline deposit of Chesterfield, Massachusetts; and is still obtained in granite at Kimito in Finland, at Finbo near Fahlun, and at Rabenstein near Zwiesel in Bohemia.
YTTROTANTALITE.*
Yttrotantal, Karsten. Tantale Oxydé Yttrifère, H. Yttrotantalite, J.
Of the yttrotantalite, Berzelius has described three varieties, all differing considerably in composition. It is evidently an impure mineral, and having never been observed in a distinct crystalline form, he considers it with propriety to be a mechanical mixture of tantalum, yttria, uranium, and lime, and occasionally of tunstic acid.
| Dark. | Black. | Yellow | |
| Oxide of tantalum | 52·82 | 57·00 | 59·50 |
| Yttria | 38·52 | 20·25 | 24·90 |
| Lime | 3·26 | 6·25 | 3·29 |
| Tungstic acid with tin | 2·59 | 825 | 1·25 |
| Oxide of iron | 0·55 | 3·50 | 2·72 |
| Oxide of uranium | Ml | 050 | 8·23 |
| Berzelius. |
The dark yttrotantalite occurs in amorphous masses, has a lustre intermediate between vitreous and resinous, and when in thin fragments appears translucent and slightly yellow. Sp. Gr. not exceeding 5·0.
The yellow yttrotantalite forms laminæ in the fissures of felspar. Its colour is yellowish-brown, it has a white streak, and is opake. Sp. Gr. 5·8 to 5·9.
In the black variety, extremely indistinct traces of crystallization have been observed. It is black and opake, with an imperfect metallic lustre, and grey streak. Sp. Gr. 5·3 to 5·5.
None of these varieties are acted upon by acids, nor are fusible per se before the blowpipe, although they decrepitate and acquire a lighter colour; but they exhibit very different results when fused with re-agents.
They are all found in Sweden—at Ytterby, in red felspar; and at Broddbo and Finbo near Fahlun, imbedded in quartz and albite, and associated with garnet, mica, and pyrophysalite.
* From its consisting chiefly of yttria and tantalum.
M 2
[page] 274
FERGUSONITE*
Haidinger.
Combination of the oxide of tantalum, yttria, zirconia, and the oxides of cerium, tin, uranium, and iron.
| Oxide of tantalum | 47·75 |
| Yttria | 41·91 |
| Zirconia | 302 |
| Oxide of cerium | 4·68 |
| Oxide of tin | 1·00 |
| Oxide of uranium | 0·95 |
| Oxide of iron | 0·34—Hartwall. |
Sp. Gr. 5·8—5·9. H. = 5·5—6·0.
In pyramidal crystals of a brownish-black colour, which are opake except when in thin splinters, and are externally dull.
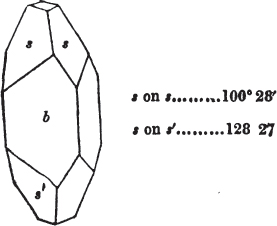
Fracture perfect conchoidal, with a brilliant vitreous lustre; streak very pale brown; traces of cleavage parallel to the faces s. Before the blowpipe per se it is infusible, but loses its colour and becomes pale greenish-yellow; is difficultly soluble with borax into a yellow glass; previous to complete solution with salt of phosphorus, the glass assumes in the reducing flame a slightly rose-red tinge; with soda is decomposed without melting, and leaves a reddish scoria.
This rare species was found by Giesècké, disseminated in quartz, at Kikertaursak, near Cape Farewell, in Greenland.
* In compliment to Robert Ferguson, Esq. of Raith, M. P.
[page] 275
OXIDE OF CHROME.*
Chrome-Ochre. Chrome Oxydé.
Contains chrome 70·11, oxygen 29·89.
Occurs in a pulverulent state, and of a green colour more or less intense, at Ecouchets in Burgundy. Before the blowpipe it is infusible, but changes to a lighter green. With borax it forms a fine green-coloured globule. It is mentioned as occurring in the Isle of Unst in Shetland, and in serpentine rocks in Savoy and Piedmont.
CHROMATE OF IRON.
Siderite Chromifère, N. Eisenchrom, L. Octahedral Chrome Ore, M. Chromeisenstein, W. Fer Chromaté, H. Bt.
Combination of peroxide of iron and oxide of chrome with alumina.
| Siberia. | Krieglach. | Chester. | St Domingo. | |
| Oxide of chrome | 530 | 55·5 | 51·36 | 36·0 |
| Oxide of iron | 340 | 33·9 | 3514 | 37·0 |
| Alumina | 110 | 6·0 | 9·72 | 21·5 |
| Silica | 1·0 | 20 | 2·00 | 5·0 |
| Laugier. | Klaproth. | Seybert. | Berthier. |
Sp. Gr. 4·3—4·6, H. = 5·5.
Occurs massive, disseminated in grains, and crystallized in the octahedron, which is its primary form; cleavage parallel to all the planes of that figure; colour iron-black or brownish-black; the massive has sometimes, though rarely, a perfectly lamellar structure, the fracture being commonly imperfect conchoidal and uneven, with a shining and somewhat metallic lustre; occasionally magnetic; has a brown streak, and is opake. Insoluble in nitric acid, and infusible before the blowpipe without addition; but with borax or salt of phosphorus it melts slowly, though completely, and on cooling exhibits the fine green of the oxide of chrome, which becomes still more intense on the addition of tin.
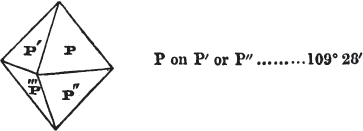
* Meaning a colouring substance; probably in allusion to its preparation as a pigment.
[page] 276
The chromate of iron forms irregular veins in serpentine at Gassin in the Department du Var; near Nantes; in the Gulsen Mountains near Kraubat in Styria; in the Uralian Mountains of Siberia; in the Bare Hills near Baltimore; and at Hoboken in New Jersey, where it occasionally presents octahedral crystals; in the Shetland Isles of Unst and Fetlar; and near Portsoy in Banffshire.
Its large proportion of chrome renders this a highly valuable ore. It is employed as a pigment,—yielding, in combination with the oxides of other metals, green, yellow, and red colours, which are used in oil-painting, and colouring porcelain.
NATIVE BISMUTH.
Octahedral Bismuth, M. Gediegen Wismuth, W. Bismuth Natif, H.
The pure metal, occasionally mixed with a small quantity of arsenic.
Sp. Gr. 9·6—9·8. H. = 2·0—2·5.
Its colour is silver-white tinged with red, presenting generally an external tarnish; it occurs feathery, reticulated, amorphous, and crystallized in the form of the regular octahedron, which is its primary form; structure lamellar, with joints parallel to the planes of the octahedron, and probably also in other directions; lustre metallic; soft, sectile, and not very frangible. When cold, brittle; but, on being heated, may be hammered into plates. Fuses readily at the comparatively low temperature of 476°. It is soluble in nitric acid, but the solution yields a white precipitate if diluted. After fusion it crystallizes, on slow cooling, in regular cubes. On friction it presents resinous electricity. When placed on live coal, or exposed to the candle, it melts; before the blowpipe it is volatilized in the form of white vapour, which forms a yellow coating on the charcoal, emitting at same time an arsenical odour, from an accidental admixture of arsenic.
Bismuth chiefly occurs in the veins of primitive mountains, accompanying various ores of silver, cobalt, lead, and zinc.
Its principal localities are Johanngeorgenstadt and Schneeberg in Saxony, Joachimsthal in Bohemia; Modum in Norway; Transylvania, Suabia, France, and Sweden.
In Cornwall it occurs in feathery masses, with arsenical cobalt, at Huel Sparnon, near Redruth; and in Herland mine near St Ives. At Schneeberg it forms arborescent delineations disseminated in brown jasper, which appear very distinctly when the mass is cut into slabs and polished.
Its great fusibility renders bismuth a useful compound in the formation of several metallic alloys, as in the fabrication of printers' types, pewter, and solder.
[page] 277
SULPHURET OF BISMUTH.
Wismuthglanz, W. Bismuth Sulfuré, H. Bt. Bismuth Glance, J. Prismatic Bismuth Glance, M. Bismuthine, Beudant.
Combination of sulphur and bismuth.
| Ridderhyttan. | |
| Bismuth | 80·98 |
| Sulphur | 18·72—Rose. |
Sp. Gr. 6·5. H. = 20—2·5.
Colour between tin-white and lead-grey, but is sometimes yellowish-white, with a metallic lustre; it occurs in acicular prisms, and in minute crystals deeply striated longitudinally, in cavities; also massive, in which case it presents sometimes a toliated structure like galena, sometimes a fibrous one like antimony; cleavage parallel to the planes P and f of the following figure, and at right angles to the latter; the principal one parallel to f. It is soft and brittle. It melts in the flame of a candle; and before the blowpipe is for the most part volatilized with a sulphureous odour, emitting numerous small drops in an incandescent state, covering the charcoal with a yellow areola, and leaving a residue which is reducible with difficulty to the metallic state. In nitric acid it is readily soluble, the solution yielding a white precipitate when farther diluted.

The lines parallel to the plane f represent the striæ constantly observed on the crystals, but which in reality are a series of planes.
P on f…………… 91° 30′–Necker.
Its localities are pretty much the same as those of native bismuth, but it occurs in small quantities. At Caldbeckfell in Cumberland it accompanies molybdena and apatite, in foliated masses; in Cornwall it is found in small brilliant tin-white crystals in Huel Sparnon near Redruth, and of a yellowish-white colour disseminated in jasper, at Botallack near the Land's End; massive, imbedded in limestone, at Johanngeorgenstadt; foliated and granular at Magurka in Hungary; with cerite at Bastnaes in Sweden; and elsewhere.
[page] 278
CUPREOUS BISMUTH.
Cupriferous Sulphuret of Bismuth. Kupferwismutherz, W. Bismuth Sulphuré Cuprifère, Necker. Cuivre Sulfuré Bismutifère, Berzelius.
Combination of sulphuret of copper and sulphuret of bismuth. Sulphur 12·58, bismuth 47·24, copper 34·66—Klaproth.
It is of a lead-grey, steel-grey, or tin-white colour, speedily acquiring a yellowish or reddish tarnish from exposure; and occurs very indistinctly crystallized, massive, disseminated, and acicular; fracture small grained and uneven; sectile; lustre metallic; streak white. Partly soluble in nitric acid, the sulphur being left.
It occurs in certain mines near Wittichen in Furstenberg, in veins traversing granite, with barytes, native bismuth, and copper pyrites.
NEEDLE ORE.
Acicular Bismuth Glance, J. Plumbo-Cupriferous Sulphuret of Bismuth. Nadelerz, W. Bismuth Sulfuré Plumbo-Cuprifère, H.
Combination of sulphuret of bismuth, sulphuret of lead, and sulphuret of copper, with a small quantity of tellurium.
| Ekatherineburg. | ||
| Bismuth | 43·20 | 36·45 |
| Sulphur | 11·58 | 16·61 |
| Lead | 24·32 | 3605 |
| Copper | 12·10 | 10·59 |
| Nickel | 1·58 | 0·00 |
| Tellurium | 1·32—John. | 0·00—Frick. |
Sp. Gr. 6·1—6·15. H. = 2·0—2·5.
Occurs in imbedded acicular four- or six-sided prisms, indistinctly terminated, and striated longitudinally; structure lamellar; cleavage parallel to the axis of the prism; the cross fracture small grained and uneven, with a shining metallic lustre. Colour, when first broken, steel-grey or blackish lead-grey, soon acquiring a yellowish tarnish. Before the blowpipe it partly volatilizes and deposits on the charcoal a yellow powder, after which there remains a red globule, enclosing a grain of metallic lead. Soluble with brisk effervescence, and the disengagement of red fumes in nitric acid, which it colours green; ammonia precipitates the copper of this solution.
It has only been found near Ekatherineburg in Siberia, imbedded in quartz, and accompanying galena and gold.
[page] 279
OXIDE OF BISMUTH.
Bismuth Ochre. Wismuthocher, W. Bismuth Oxydé, H. Bt. Oxygen 10·13, bismuth 89·87.
Sp. Gr. 4·36.
Colour straw-yellow or yellowish-grey; occurs massive and disseminated; the structure sometimes lamellar, with a shining lustre; sometimes fine-grained OP earthy, and dull; it is opake, soft, and often friable. On charcoal, before the blowpipe, it is easily reduced to the metallic state. In nitric acid it is soluble, the solution throwing down a white precipitate on the addition of water.
It has been found in small quantities, upon the ores of bismuth, cobalt, and nickel, at Schneeberg and Johanngeorgenstadt in Saxony, at Joachimsthal in Bohemia, and with plumbo-cupriferous sulphuret of bismuth and native gold at Beresof in Siberia.
BISMUTH BLENDE,
Bismuth-Blende, Breithaupt. Arsenical Bismuth. Arsenik-Wismuth, W. Kiesel-Wismuth, L.
| Schneeberg. | |
| Contains Oxide of bismuth | 69·38 |
| Silica | 22·23 |
| Oxide of iron | 2·40 |
| Oxide of manganese | 0·30 |
| Phosphoric acid | 3·31 |
| Water, &c. | 1·01—Kersten. |
Sp. Gr. 5·9—6·0. H. = 3·5—4·0.
In minute crystals, presenting the rare form of the trigonal dodecahedron, or in implanted globular masses. Colour dark. hair-brown or wax-yellow; streak yellowish-grey; semi-transparent or opake; lustre resinous or adamantine; fracture uneven; cleavage parallel to the faces of the dodecahedron, imperfect; rather brittle. Decrepitates briskly before the blowpipe, emits an arsenical odour, and is ultimately converted into a glass which effervesces with borax.
This species accompanies cobaltand native bismuth, at Schneeberg in Saxony; its general appearance is that of implanted globules, which rarely exceed the size of a pin-head, and are of a dark-brown colour.
[page] 280
TELLURIC BISMUTH.
Molybdena Silver, J. and A. Molybdan Silber, Werner. Tellur-Wismuth, Leonhard. Bornine, Beudant. Rhombohedral Bismuth Glance, M.
Composed of tellurium, bismuth, and selenium. Tellurium 29·74, bismuth 61·15, sulphur with traces of selenium 2·33, silver 2·07 Wehrle.
Sp. Gr. 7·2—8·0. Soft.
Of a light steel-grey inclining to lead-grey, with a metallic lustre; occurs in crystalline masses, or six-sided prisms, which are divisible into thin laminæ parallel to the terminal planes, but not so easily as mica; elastic, and when reduced to powder, is of an iron-black. Before the blowpipe on charcoal it melts on the first impression of the point of the flame, into small globules, which become of a yellow colour, and somewhat tarnished, disengaging at the same time vapours of selenium. When pulverized, it is soluble in nitric acid, with the exception of the sulphur.
It occurs with brown spar and iron-flint at Pilsen, and near Schernowitz on the Gran in Hungary; but it is an extremely rare mineral.
NATIVE ARSENIC.
Gediegen Arsenic, W. Arsenic Natif, H. Rhombohedral Arsenic, M.
| Joachimsthal. | ||
| Consists of Arsenic | 96·0 | 970 |
| Antimony | 30 | 20 |
| Iron and water | 1·0—John. | 1·0—John. |
Sp. Gr. 5·75. H. = 3·5.
When fresh broken, it presents a lead-grey colour, inclining to tin-white, but is generally greyish-black, becoming dull on exposure; it occurs reniform, botryoidal, and in flat mammillary masses; is not found crystallized, although indications of a rhomb of 114° 26′ and 65° 34′ have been noticed; cleavage occasionally observable perpendicular to the axis of this rhomb; fracture fine-grained and uneven, occasionally with a slight appearance of fibrous structure; it yields to the knife, and is easily frangible. Before the blowpipe it fuses readily: burns with a bluish flame and a dense white arsenical vapour; and is, when pure, entirely volatilized. Acquires resinous electricity from friction.
Arsenic occurs chiefly in the veins of primitive rocks, accompanying ores of siver, cobalt, and copper. It is common in the Saxon silver mines of Freyberg, Annaberg, and Schneeberg; also at Joachimsthal in Bohemia, at Andreasberg in the Hartz, at Kapnick in Transylvania, at Orawitza in the Bannat, at Zmeoff in Siberia in large masses, at Wittichen in Suabia, and at St
[page] 281
Marie aux Mines in Alsace. It is at once distinguished by the facility with which it volatilizes, as well as by the odour and copious white fumes it emits when exposed to the blowpipe, or thrown upon ignited charcoal. This odour is also distinctly perceptible when the specimen is struck with a hammer. The effects of arsenic as a violent poison are well known; it is notwithstanding made use of in several pharmaceutical preparations, and is variously employed in metallurgical processes. (Manual.)
OXIDE OF ARSENIC.
Octahedral Arsenic Acid, M. Arsenikblüthe, Karsten. Arsenic Oxidé, H. Acide Arsenieux, Beudant.
The oxide of arsenic. Arsenic 75·81, oxygen 24·19.
Sp. Gr. 3·6—3·71. H. = 1·5.
Colour snow-white, sometimes tinged accidentally reddish, yellowish, or greenish. It occurs earthy, capillary, investing other substances, in stalactites, and also in tabular and prismatic crystals. Cleavage octahedral; semi-transparent or opake; lustre vitreous; fracture conchoidal; taste astringent. Soluble in hot water. Exposed to a high temperature it is volatilized without any odour, but when heated on charcoal before the blowpipe the acid is decomposed, and the strong garlic smell which characterizes metallic arsenic is emitted.
It occurs at Andreasberg in the Hartz, with the ores of silver, arsenic, and lead, from the decomposition of some of which it probably arises; also at Joachimsthal in Bohemia; and at Bieber in Hanau. It resembles the pharmacolite, and is often confounded with it; but that substance is not soluble in water, which the oxide of arsenic is.
In some of the Hartz furnaces it has been obtained by sublimation, and in that case it presents large distinct octahedral crystals.
SULPHURET OF ARSENIC.
Of this substance there are two varieties.
1. REALGAR.
Hemi-prismatic Sulphur, M. RotherRauschgelb, W. Realgar Rouge, Brochant. Arsenic Sulfuré Rouge, H.
| Bi-sulphuret of arsenic. | ||
| Arsenic | 69·57 | 690 |
| Sulphur | 30·43—Laugier. | 31·0—Klaproth. |
| Sp. Gr. 3·3—3·6. |
[page] 282
Of a brilliant red colour, passing into scarlet, sometimes with a tinge of orange; translucent, rarely transparent. It occurs massive, disseminated, investing, acicular, and crystallized; the crystals usually assume the prismatic form, and are externally very brilliant. It cleaves indistinctly parallel to all the planes of an oblique rhombic prism, whose lateral planes are 74° 15′ and 105° 45′ by the reflective goniometer, the terminal on a lateral plane being about 104° 6′; the declination of the terminal plane is from one acute angle of the prism to its opposite. Fracture conchoidal, with a splendent vitreous lustre; streak orange-yellow or aurora-red; yields to the pressure of the nail. It becomes electric by friction, acquiring the resinous or negative electricity; before the blowpipe, alone, on charcoal, it burns with a pale yellow flame. Loses its colour in nitric acid.
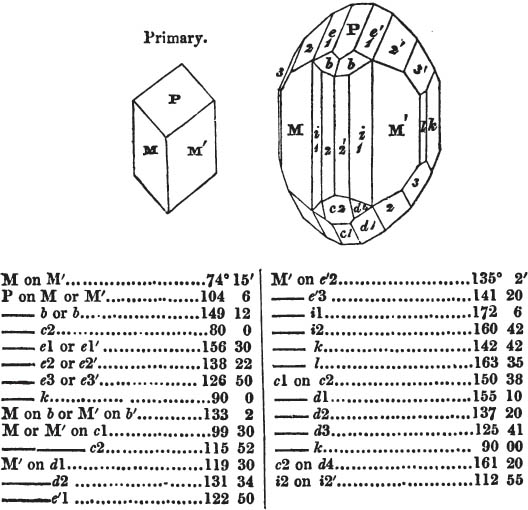
Felsobanya in Upper Hungary, and Kapnik and Nagyag in Transylvania, are the most noted localities of this beautiful mineral; it also occurs at Andreasberg in the Hartz; in dolomite
[page] 283
on St Gothard; in Bohemia; in Saxony; and in minute crystals in the vicinity of active volcanoes, as Vesuvius, the Solfatara, &c.
2. ORPIMENT.
Prismatoidal Sulphur, M. Gelbes Rauschgelb, W. Arsenic Sulfuré Jaune, H.
Orpiment is a sulphuret of arsenic, containing three atoms of sulphur to one of arsenic. It is a tri-sulphuret, while the preceding sub-species is a bi-sulphuret.
| Arsenic | 620 | 61·86 |
| Sulphur | 38·0—Klaproth. | 38·14—Laugier. |
Sp. Gr. 3·45.
Colour bright lemon-yellow, passing into gold yellow.* Occurs disseminated, reniform, in stalactites, investing, and also, though rarely, in minute crystals. The primary form appears to be a right rhombic prism of 100° and 80°, but the crystals yield to cleavage parallel only to the greater diagonal of the prism; namely, parallel to the plane f of the following figure. Semi-transparent, or translucent only on the edges; lustre metallic; pearly upon the perfect faces of cleavage, the rest resinous; streak yellow, somewhat paler than the colour; sectile, thin laminæ flexible, but not elastic Before the blowpipe its comportment corresponds with that of realgar; it burns, however, with a bluish-coloured flame.
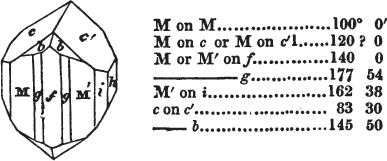
Yellow orpiment has been found in small crystals imbedded in blue clay at Tajowa, near Neusohl in Lower Hungary. Most frequently, however, it forms foliated and fibrous masses, and in that state is met with at Kapnik in Transylvania, at Moldawa in the Bannat, and Felsobanya in Upper Hungary, where it accompanies realgar and native arsenic in metalliferous veins.
* Whence orpiment, from the Greek, signifying gold yellow.
[page] 284
ARSENICAL PYRITES.
Axotomous Arsenical Pyrites, M. Prismatic Arsenical Pyrites, J. Arsenical Pyrites, A. Arsenik Eisen, L.
| Reichenstein. | Schladming. | |
| Contains Arsenic | 65·99 | 60·41 |
| Sulphur | 1·94 | 5·20 |
| Iron | 28·06 | 13·49 |
| Nickel | 0·00 | 13·37 |
| Cobalt | 0·00 Hoffmann. | 5·10 Hoffmann. |
Sp. Gr. 7·1—7·4. H. = 5·0—5·5.
Primary form a right rhombic prism of 122° 26′ and 57° 34′. Seldom occurs crystallized, generally in masses of a silver-white or steel-grey colour; lustre metallic; streak greyish-black; cleavage distinct perpendicular to the axis; fracture uneven; brittle.
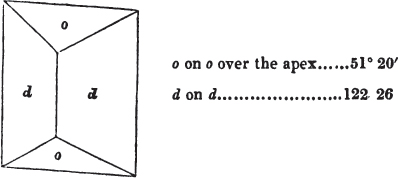
This mineral is found associated with copper nickel at Schiadming in Styria; with serpentine at Reichenstein in Silesia; and in a bed of sparry iron, along with bismuth and scorodite, at Löling near Huttenberg in Carinthia. It occurs, however, only in small quantities, and is a rare species.
BRIGHT WHITE COBALT.
Cobaltine, Beudant. Glanz Kobalt, W. Cobalt Gris, H. Cobalt Eclatant, Br. White Cobalt, A. Hexahedral Cobalt Pyrites, M.
Combination in nearly equal volumes of the sulphuret and the arseniuret of cobalt.
| Skutterud. | Tunaberg. | Tunaberg. | |
| Cobalt | 33·10 | 3666 | 4400 |
| Arsenic | 43·47 | 49·00 | 5500 |
| Sulphur | 20·08 | 6·66 | 0·50 |
| Iron | 3·23 | 5·66 | 0·00 |
| Stromeyer. | Tessaert. | Klaproth. | |
| Sp. Gr. 6·23. | H. = 5·5. |
[page] 285
Colour silver- or yellowish-white, with a tinge of red. It occurs in the cube and its varieties—its crystalline forms resembling those of iron pyrites; the planes of the cube are generally striated, those of the modifications smooth; structure lamellar, yielding readily to cleavage parallel with all the planes of the cube, which therefore is the primary form; fracture fine grained; streak greyish-black; it also occurs arborescent, stalactitic, botryoidal, and amorphous; it yields with difficulty to the knife, and is not very frangible. Before the blowpipe on charcoal it disengages copious arsenical fumes, and, after being roasted for some time, melts into a metallic globule of a dull black externally, which attracts the magnet, but which is not malleable; it tinges borax of a deep-blue colour; and effervesces in heated nitric acid.
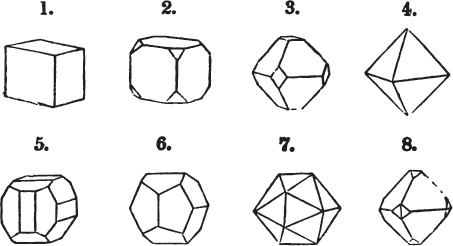
Fig. 1, the primary; a cube. Fig. 2, the same, of which the solid angles are replaced by triangular planes; which in fig. 3 are so greatly increased as to reduce the primary planes to small squares; and are complete in fig. 4, the regular octahedron. Fig. 5, the cube; of which each edge is replaced by an irregularly six-sided plane, alternately placed in different directions. In fig. 6, these planes are complete, forming the pentagonal dodecahedron. In fig. 7, they are in connection with the planes of the octahedron, which are increased in fig. 8; reducing the irregularly six-sided planes of fig. 5 to small triangles.
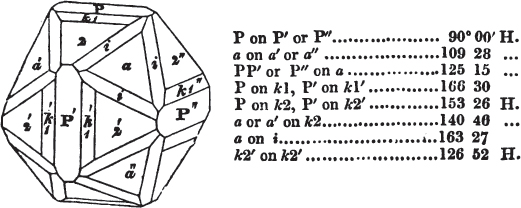
[page] 286
At Tunaberg and Hokensbo in Sweden, this species is met with in large resplendent, distinctly-pronounced crystals, which are generally combinations of the cube and pentagonal dodecahedron, as in fig. 5. It also occurs abundantly in mica-slate at Wehna in Sweden, and at Modum and Skutterud in Norway; less so at Querbach in Silesia, and in the vicinity of St Just in Cornwall. From the following species it may be distinguished by its inferior specific gravity and reddish hue, also by its lamellar structure, its more distinct cleavage, and by its requiring considerably greater heat to drive off the arsenic.
The cobalt or smalt of commerce is chiefly obtained from it.
TIN-WHITE COBALT.
Octahedral Cobalt Pyrites, M. Grauer Spieskobold, W. Cobalt Arsenical, Necker. Tin-White Cobalt, L. Grey Cobalt, A.
Union of cobalt and arsenic, in which the latter preponderates; and sometimes containing accidentally, small quantities of copper and iron.
| Riechelsdorf. | Schneeberg. | |
| Cobalt | 20·31 | 2800 |
| Arsenic | 74·21 | 65·75 |
| Iron | 3·42 | 0·00 |
| Copper | 015 | 6·25 |
| Sulphur | 0·88—Stromeyer. | 0·00—John. |
Sp. Gr. 6·4—7·7. H. = 5·5.
Colour tin-white, inclining, when massive, to steel-grey. It occurs in cubes, octahedrons, and in crystals which form the passage of the one into the other (see Bright White Cobalt, fig. 1, 2, 3, 4); but it is somewhat remarkable that the crystals of this variety differ from the preceding, in exhibiting only the regular planes of modification belonging to the cube. The crystals are often cracked or rent in various directions, and their planes are commonly more or less convex. Cleavage parallel to the faces both of the octahedron and cube. It also occurs arborescent, reticulated, botryoidal, stalactitic, and amorphous. The fracture is fine-grained and uneven, with a glistening metallic lustre; it yields with difficulty to the knife, and is brittle and hard. Before the blowpipe on charcoal it gives out a copious arsenical vapour on the first impression of the heat; it fuses, however, only partially, and that with difficulty; to borax and other fluxes it imparts a deep blue colour; and in nitric acid affords a pink solution.
[page] 287
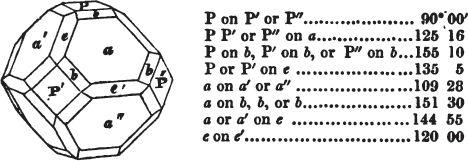
It occurs chiefly in primitive rocks, accompanying ores of silver, bismuth, and copper, as at Freyberg, Annaberg, and particularly at Schneeberg, in Saxony; at Joachimsthal in Bohemia; and at Huel Sparnon in Cornwall. At Riechelsdorf in Hessia its veins are included in cupriferous shale; and the reticulated variety from Joachimsthal is frequently imbedded in calcareous spar.
BISMUTH COBALT ORE.
Kersten.
Consists of arsenic 77·96, cobalt 9·88, iron 4·77, bismuth 3·88, copper 1·30, nickel 1·11, sulphur 1·01—Kersten.
Occurs massive, with a radiated and porous-like structure. Colour intermediate between lead-grey and steel-grey, with a glistening or glimmering metallic lustre; streak dull, same colour as the mineral. Before the blowpipe it emits copious fumes of arsenious acid, and deposits on the charcoal a yellow crust, the assay at the same time assuming a brown colour. When well roasted, it communicates to borax a smalt blue colour.
This mineral has hitherto only been found at Schneeberg in Saxony.
SULPHURET OF COBALT.
Koboldine, Beudant. Schwefel Kobalt, Berzelius. Isometric Cobalt Pyrites, M. Cobalt Sulfuré, Lucas. Cobalt-Kies, L. Cobalt Pyriteux, Necker.
Sulphuret of cobalt, mixed with the sulphurets of iron and copper.
| Ridderhyttan. | Mussen. | |
| Cobalt | 43·20 | 43·86 |
| Copper | 14·40 | 410 |
| Iron | 3·53 | 5·34 |
| Sulphur | 38·50—Hisinger. | 41·00—Wernekinck. |
[page] 288
Sp. Gr. 6·3—6·4. H.= 5·5.
Colour steel-grey, or whitish with a tinge of yellow; massive, with an uneven fracture, presenting a granular surface; and botryoidal. Lustre metallic; cleavage parallel to the faces of the cube, imperfect; fracture uneven or imperfect conchoidal. On charcoal alone before the blowpipe it fuses after roasting into a grey metallic globule, from which it is difficult to drive off the last portions of sulphur; with the fluxes the effects of the cobalt predominate so much that it is impossible to distinguish those of iron or copper. Soluble in nitric acid, with the disengagement of nitron gas, leaving a whitish residue. Neither in the analysis nor before the blowpipe does it exhibit the slightest indication of arsenic.
It is found at Bastnaes near Riddarhyttan in Sweden, in gneiss, associated with copper pyrites and hornblende; and at Mussen in Prussia, with barytes and carbonate of iron.
EARTHY COBALT.
Black Cobalt Ochre, A. Oxide of Cobalt. Erdkobold, W. Cobalt Oxydé Noir, H. Cobalt Ochre, J.
Consists of oxide of cobalt, rendered more or less impure by an admixture of arsenic, iron, or manganese, with about 23 per cent. of water, according to Dobereiner.
Sp. Gr. 2·10—2·42. Soft.
Colour various shades of brown, bluish-black, and black. Occurs massive, mammillary, botryoidal, investing, and pulverulent; the fracture of the massive is earthy, and it is dull, but acquires a polish by friction; yields easily to the knife. Before the blowpipe on charcoal it exhales a slight arsenical odour, but does not fuse; with borax it forms a deep cobalt-blue coloured globule.
It occurs in sandstone, with yellow copper, at Alderley Edge, in Cheshire; at Nertschinsk in Siberia; and at Riechelsdorf in Hessia; at Saalfeld in Thuringia, associated with several species of cobalt pyrites; in the Tyrol, Bohemia, Saxony, and elsewhere. In Ireland, of a blue colour, investing fissures in slate-clay in the peninsula of Howth near Dublin. The brilliancy which its surface attains when streaked with, or rubbed against a hard body, is perfectly characteristic.
[page] 289
COBALT-BLOOM.
Bother Erdkobold, W. Cobalt Arseniaté, H. Red Cobalt Ochre, J. Prismatic Cobalt Mica, M. Diatomous Euclas Haloide, Haid. Arseniate of Cobalt. Kobalt Blüthe, Haus. Erythrine, Beudant.
Combination of arsenic acid, oxide of cobalt, and water.
| Riegelsdorf. | |
| Oxide of cobalt | 39·0 |
| Arsenic acid | 37·0 |
| Water | 22·0—Bucholz. |
Sp. Gr. 2·9—3·1. H. = 2·0—2·5.
Colour crimson and peach-blossom red, sometimes whitish, or greyish-white, or green. Is found in small botryoidal masses, and short acicular diverging crystals, modified on the edges, whose form is a right oblique-angled prism. The crystals which possess most nearly the characters of regular form are translucent and shining, the other varieties are glimmering or dull, and nearly opake; it is soft, light, and flexible; translucent; the red tints very brilliant by strong transmitted light; lustre pearly, on some faces inclining to vitreous; streak corresponding to the colour, though a little paler. When crushed in a dry state, the powder possesses a lavender-blue tinge, which is not the case if moistened. Cleavage perfect, in the direction of the prism. Before the blowpipe on charcoal it fumes abundantly, emitting an arsenical odour, and melts in the reducing flame into a bead of arseniuret of cobalt. With borax and other fluxes it yields a fine blue-coloured glass; and is soluble in nitric acid, to which it communicates a red tinge.
It occurs in primitive and secondary rocks, with other ores of cobalt; either in micaceous scales radiating from a centre, as at Schneeberg in Saxony; in minute aggregated crystals, as at Saalfeld in Thuringia, and Riegelsdorf in Hessia; or coating other minerals in the state of a peach-blossom red powder. This last is met with in Dauphiné, in Cornwall, at the lead mine of Tyne Bottom near Alston in Cumberland, and in many other places. A perfectly green variety occurs at Platten in Bohemia; and sometimes red and green tinges appear on the same crystal.
N
[page] 290
ROSELITE.
Levy.
Contains oxide of cobalt, arsenic acid, water, lime, and magnesia, according to Children.
H. = 3·0.
Lustre vitreous; translucent; streak white; cleavage distinct, and brilliant parallel to P. Before the blowpipe it gives off water and blackens. It imparts a blue colour to borax and salt of phosphorus; and is entirely soluble in muriatic acid. This species resembles the last in colour, though quite distinct in crystalline form.
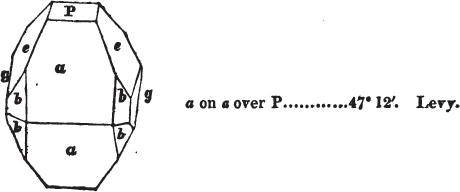
This extremely rare mineral occurs in small deep rose-red coloured twin crystals, associated with cobalt bloom, at Schneeberg in Saxony. It was noticed by Levy, who named it in compliment to Dr Gustavus Rose of Berlin. (Manual.)
SULPHATE OF COBALT.
Red Vitriol. Kobalt Vitriol, Koppe. Rhodhalose, Beudant.
It is a hydrous sulphate of cobalt.
| Bieber. | ||
| Sulphuric acid | 30·2 | 19·74 |
| Oxide of cobalt | 28·7 | 38·71 |
| Oxide of iron | 0·9 | 0·00 |
| Water | 41·2—Beudant. | 41·55—Koppe. |
Primitive form an oblique rhombic prism of 97° 35′ and 82° 25', whose base is inclined to its lateral planes at about 108° and 82° (Beudant). Colour rose- or flesh-red. Occurs investing other minerals, in small friable masses, and in stalactites; the masses are semi-transparent and crystalline; lustre pearly; streak yellow; taste styptic and bitter. Before the blowpipe in the matrass it gives off water, and assumes a brighter colour; with borax it forms a blue glass. It is soluble in water.
It occurs in the mining heaps of Bieber near Hanau, with lamellar heavy spar, earthy and grey cobalt; also at Leogang in Saltzburg.
[page] 291
SULPHURET OF NICKEL.
Schwefel-Nickel, L. Haarkies, W. Capillary Pyrites, H. Nickel Sulfuré, Levy.
| Analyses by Arfwedson. | ||
| Nickel | 64·76 | 65·35 |
| Sulphur | 35·24 | 34·26 |
with traces of cobalt and arsenic
Sp. Gr. 6·45. H. about 4.
It occurs in capillary and sometimes diverging filaments of a yellowish colour, inclining to steel-grey. Primary form the cube; flexible; opake, with a metallic lustre; not magnetic. Before the blowpipe on charcoal, with a good heat, it fuses into a globule which is metallic, malleable, and magnetic, and consists wholly of nickel; but in the open tube it exhales the odour of sulphureous acid. With nitric acid it forms a greenish solution.
It is found at Johanngeorgenstadt in Saxony, at Joachimsthal in Bohemia, at Andreasberg in the Hartz, in Cornwall and other places, in thin capillary filaments, filling the cavities, and dispersed among the crystals, of other minerals.
ANTIMONIAL NICKEL.
Nickeliferous Grey Antimony, J. and A. Eutomous Cobalt Pyrites, M. Nickelspiesglaserz, Haussman. Nickel Arsenical Autimonifère, Antimoine Sulfuré Nickelière, Antimon-nickel, Beudant.
Combination of nickel, sulphur, and antimony, sometimes with arsenic.
| Siegen. | |||
| Nickel | 27·36 | 26·10 | 25·25 |
| Sulphur | 15·98 | 16·40 | 15·25 |
| Antimony | 55·76 | 47·56 | 47·75 |
| Arsenic | 0·00 | 9·94 | 11·75 |
| Rose. | Ull man. | Klaproth. |
Sp. Gr. 6·45—6·5. H. = 5·0—5·5.
Primary form the cube. In masses which have a granular composition, and possess a steel-grey or silver-white colour; lustre metallic; cleavage perfect parallel to the faces of the cube; brittle. Before the blowpipe it is partly volatilized, disengaging vapours of antimony, and sometimes of arsenic, and ultimately melts into a metallic globule, which communicates a blue colour to glass of borax. It is acted upon by nitric acid, forming an immediate precipitate, and colouring the solution green.
It occurs in several of the mines near Freussberg in the principality of Nassau, with sparry-iron, galena, and copper pyrites.
[page] 292
ARSENICAL NICKEL.
Kupfernickel, W. Nickel Arsenical, H. Prismatic Nickel Pyrites, M. Copper Nickel, J.
| An union of nickel and arsenic. | ||
| Riegelsdorf. | ||
| Nickel | 44·20 | 48·90 |
| Arsenic | 54·72 | 46·42 |
| Iron | 0·33 | 0·34 |
| Lead | 0·32 | 0·56 |
| Sulphur | 0·40—Stromeyer. | 0·80—Pfaff. |
Sp. Gr. 6·6—7·6. H.= 5·0—5·5.
Of a copper or yellowish-red colour, but acquiring a grey or blackish tarnish by exposure. It occurs reticulated, dendritic, and botryoidal, but more commonly massive; never crystallized; streak pale brownish-black. The fracture is imperfectly conchoidal, or fine-grained and uneven, with a glistening or shining metallic lustre; it yields to the knife with difficulty, and is brittle. Before the blowpipe it gives out an arsenical vapour, and then fuses, though not very easily, into a white metallic globule. After roasting, it usually colours glass of borax blue, indicating the presence of a certain quantity of cobalt. In nitric acid it assumes a green coating, and in nitro-muriatic acid is dissolved.
It usually accompanies the ores of cobalt, silver, and copper; and is found in veins of primitive rocks at Schneeberg and Annaberg, Johanngeorgenstadt and Freyberg in Saxony; at Schladming in Styria; Joachimsthal in Bohemia; at Allemont in France, and the Bannat; in transition rocks in the Hartz; at Saalfeld in Thuringia; at Riegelsdorf in Hessia; and, though less frequently, in Cornwall.
NICKEL OCHRE.
Nickel Blüthe, Nickel Ocher, W. Nickel Oxidé, H. Bt. Nickel Ar. seniaté, Berthier.
Combination of arsenic acid, oxide of nickel, and water.
| Arsenic acid | 36·97 | 36·8 |
| Oxide of nickel | 37·35 | 36·2 |
| Water | 24·32 | 24·5 |
| Oxide of cobalt | 0·00 | 2·5 |
with a little iron and sulphuric acid—Stromeyer.
This substance is found adhering to or coating arsenical nickel, and is considered to be derived from its decomposition. It is sometimes compact, of a fine apple-green colour; and generally filamentous or friable. By calcination it assumes a yellowish hue,
[page] 293
and loses somewhat less than a fourth part of its weight of water, without emitting any odour. It dissolves readily and completely in acids, without effervescence, the solution becoming violet on the addition of ammonia; and before the blowpipe on charcoal exhales a strong arsenical smell; fusing in the interior flame into a globule of arseniferous nickel.
It is found adhering to arsenical nickel at Allemont and other places.
PIMELITE.
Pimelit, W. Br. Nickel Oxydé, Bt. Pimelite, J.
Oxide of nickel 15·62, silica 35·00, alumina 5·10, water 37·91, magnesia 2·25, lime 0·40—Klaproth.
Of an apple-green or yellowish-green colour; occurs investing other minerals, and massive; is earthy and dull; opake, and devoid of lustre; soft, and greasy to the feel. It is infusible before the blowpipe, but loses part of its weight, and assumes a dark-grey hue. With borax it forms a violet-coloured globule, in which the nickel is reduced.
It occurs at Kosemütz, and Glassendorf in Silesia, in veins traversing serpentine, and associated with chrysoprase, of which nickel is supposed to be the colouring matter.
NATIVE SILVER.
Gediegen Silber, W. Argent Natif, H. Br. Hexahedral Silver, M.
Pure silver, with occasional minute admixture of copper, arsenic, antimony, and iron, which renders it less malleable than the fused metal.
Sp. Gr. 10·47. H. = 2·5.
Colour pure white, with a shining metallic lustre, but generally tarnished externally of a greyish-black, probably owing to the presence of sulphur. Occurs crystallized in the cube and regular octahedron; but as it does not possess a lamellar structure, either of these solids may be considered as the primary form; in the following figure the cube is assumed, as being the most simple. It also occurs capillary, ramose, and reticulated, but a close inspection will discover, by the assistance of a microscope, that these varieties consist of a congeries of elongated crystals, or of minute cubes or octahedrons closely aggregated, and disposed perpendicularly in straight rows. Flexible, ductile, and malleable; acquires vitreous electricity when isolated and rubbed. It is fusible before the blowpipe into a globule, which is not altered by continuing the heat, although, on cooling, it presents a crystalline form, in which the faces of the octahedron, cube, and
[page] 294
dodecahedron may be distinguished. It is soluble in cold nitric, and in heated sulphuric acid.
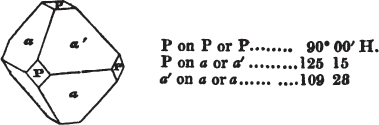
Virgin or native silver generally occurs in veins of calcareous spar or quartz, traversing gneiss, slate, and other primitive rocks; occasionally also in selenite and clay. Magnificent specimens, presenting crystals half an inch in diameter, used to be found in the mines of Kongsberg in Norway; Freyberg, Schneeberg, and Johanngeorgenstadt are its principal Saxon localities; Przibram, Joachimsthal, and Ratiborzitz, its chief Bohemian ones. It is also met with in smaller quantities at Andreasberg in the Hartz, in Swabia, Hungary, at Allemont in Dauphiné, and in some of the Cornish mines. The most celebrated localities, however, of native silver, are those of South America, and particularly the mines of Peru. The employment of silver in coinage, and in the manufacture of plate and articles of luxury, is well known; but it is not from this ore alone that the fused metal is obtained.
Auriferous Native Silver. Guldisches gediegen silber, W. Argent natif aurifère, Br.
| Gold | 64·0 | 74·0 | 76·41 | 28·0 |
| Silver | 34·0 | 26·0 | 32·12 | 72·0 |
| Klaproth. | Boussingault. | Rose. | Fordyce. |
Sp. Gr. 14·0—17·0.
Of a colour between silver-white and brass-yellow; disseminated, capillary, and crystallized in cubes.
It occurs in veins at Kongsberg in Norway, at Rauris in Salzburg, and at Schlangenberg in Siberia.
ANTIMONIAL SILVER.
Prismatic Antimony, M. Antimon-silber, Leonhard. Spiesglassilber, W. Argent Antimonial, H. Br.
| Union of silver and antimony | ||
| Wolfach. | Andreasberg. | |
| Silver | 84·76 | 78·0 |
| Antimony | 16·24—Klaproth. | 22·0—Vauquelin. |
Sp. Gr. 9·44—9·8.
Colour between silver-white and tin-white, often externally tarnished yellow or reddish. Generally occurs massive or in grains, but has been observed also indistinctly crystallized. Pri-
[page] 295
mary form an obtuse rhomboid of 109° 28′ and 70° 32′— Necker. The faces somewhat convex, and deeply striated longitudinally. Structure lamellar, with a shining metallic lustre, and the cross fracture flat conchoidal; easily frangible; soft; and possessing a slight degree of malleability. Before the blowpipe on charcoal it melts into a grey metallic globule, which is not malleable, the antimony being at the same time driven off in white vapour; on continuing the blast a bead of pure silver is produced. In nitric acid it becomes soon covered with a bluish coating, which is the oxide of antimony.
It occurs with native arsenic and other ores of silver, in granite, at Wittichen and Altwolfach in Baden; in clay-slate at Andreasberg in the Hartz; also at Casalla near Guadalcanal in Spain; in Salzburg; and at Allemont in France. It is a rare mineral.
Arsenical Antimonial Silver. Arsenical Silver, A. Argent antimonial ferro-arsenifère, H. Argent arsenical, Br.
Antimonial silver, mixed with arsenic and iron.
A specimen from Andreasberg yielded to Klaproth silver 12·75, antimony 4·00, iron 44·25, arsenic 35·00.
Sp. Gr. 9·4. H. = 4·0.
Nearly of the same colour as native silver, but commonly tarnished yellow or blackish; it occurs mammillated, or in small globular and reniform masses, sometimes investing other substances; structure lamellar, with a shining or glimmering metallic lustre. It is harder than antimonial silver, but is sectile, brittle, easily frangible, and heavy. Before the blowpipe the arsenic and antimony are volatilized, emitting at same time a powerful alliaceous odour, and leaving a globule of impure silver.
Its localities and associations are nearly the same as those of antimonial silver; it is best known at Andreasberg in the Hartz, where it accompanies native arsenic.
TELLURIC SILVER.
Tellur-silber, Rose. Argent Telluré, Necker.
| Contains Silver | 62·42 | 62·32 |
| Tellurium | 36·96 | 36·89 |
| Iron | 0·24—Rose. | 0·50—Rose. |
Sp. Gr. 8·41—8·56.
Uncrystallized; in coarse grained masses; colour between steel-grey and lead-grey; lustre metallic; soft, and partially malleable. Before the blowpipe on charcoal it fuses into a black mass, which on cooling appears covered with numerous minute specks of metallic silver; in the matrass it fuses, and colours the glass yellow; and is soluble in nitric acid, especially if heated. From the silver mines of Savodinski in the Altai Mountains, Siberia.
[page] 296
SULPHURET OF SILVER.
Vitreous Silver. Glaserz, W. Argent Sulfuré, H. Bt. Argent Vitreuse, Br. Hexahedral Silver Glance, M. Silver Glance, J.
Combination of silver and sulphur.
| Freyberg. | ||
| Silver | 85·0 | 87·05 |
| Sulphur | 15·0—Klaproth. | 12·96—Berzelius. |
Sp. Gr. 6·9L—7·2. H. = 2·0—2·5.
Of a dark lead-grey colour, with occasionally a superficial iridescent tarnish. Primary form the cube; also found in octahedrons and rhombic dodecahedrons, parallel to the faces of which traces of cleavage are sometimes observable; fracture, fine grained and uneven, sometimes small and flat conchoidal, with a more or less shining metallic lustre; malleable and sectile, yielding readily to the knife. In the flame of a taper, or before the blowpipe, it intumesces, the sulphur flies off, and on continuing the blast a bead of pure silver remains. Soluble in dilute nitric acid; and, when isolated and rubbed, acquires resinous electricity.
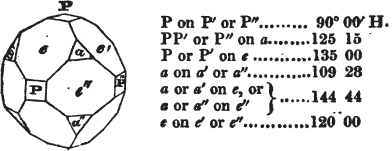
This species occurs both crystallized and massive, assuming also various reticulated, filiform, arborescent, and capillary shapes. It is subject to tarnish from exposure, loses its lustre, and becomes covered with a black earthy-like coating. It occurs in gneiss at Freyberg in Saxony, accompanying other ores of silver; in mica-slate at Joachimsthal in Bohemia; in greywacke in the Hartz; occasionally in Cornwall; but in great abundance only in Mexico, most of the silver obtained at the celebrated mines of Guanaxuato in that country being extracted from this ore.
Black Sulphures of Silver. Silberschwartze, W.; Earthy silver glance J. Is a decomposed and almost friable variety of the preceding. It is dark lead-grey, inclining to black, and without lustre, or only feebly glimmering; it occurs massive and pulverulent, sometimes investing other ores of silver, and filling up cavities in them; fracture uneven; is more or less sectile; and gives a shining metallic streak. Before the blowpipe it is converted. into a slaggy mass, containing globules of impure silver. It occurs in the veins of primitive mountains with other ores, as at Cremnitz and Schemnitz in Hungary; at Chalanches near Allemont in Dauphiné; at Kongsberg in Norway; and at Schlangenberg in Siberia.
[page] 297
FLEXIBLE SULPHURET OF SILVER.
Argent Sulfuré Flexible, Bournon. Biegsamer Silberglanz, L.
Consists, according to Wollaston, of silver, sulphur, and a little iron,
Externally of a dark colour, approaching to black; occurs both massive and in small tabular crystals, which appear to be right oblique-angled prisms, whose lateral planes are alternately 125° and 55°. Flexible when in thin laminæ, and readily separable into them. Cleavage parallel with the terminal planes; very soft, yielding readily to the knife; lustre metallic, but less brilliant than that of sulphuret of silver.
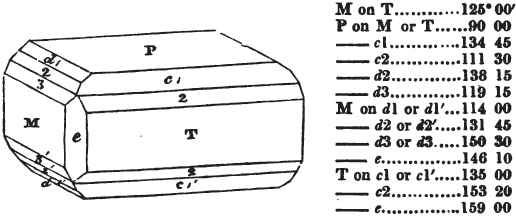
This rare mineral has hitherto been met with only in Hungary, and at Freyberg in Saxony, and even at these localities in very small quantity. The crystal figured is from the Himmelsfurst mine at Freyberg.
STERNBERGITE*
Haidinger.
| Contains Silver | 33·2 | 32·9 |
| Iron | 36·0 | 32·8 |
| Sulphur | 30·0—Zippe. | 34·3—Berzelius. |
Sp. Gr. 4·2—4·25. H. = 1·0—1·5.
Primary form a rhombic octahedron of 118°, 84° 28′, and 128° 49′.

Occurs generally in implanted crystals, attached to the matrix laterally so as to form rose-like aggregations; sometimes they are macled. Cleavage perfect parallel to a. Colour pinchbeck-brown, with an occasional superficial violet-blue tarnish on the faces f; high
[page] 298
* In honour of Count Caspar Sternberg of Prague.
N 2
degrees of metallic lustre on the broad faces a, not so bright on the others; streak black; flexible in thin laminæ; and, after being bent, may be smoothed down again with the nail, like tinfoil. Before the blowpipe it burns per se with a blue flame, emits powerful sulphureous vapours, and fuses into a globule, which is generally hollow, has a crystalline surface, and is covered with metallic silver. This globule acts powerfully on the magnet, and communicates to fluxes the ordinary colours produced by iron. Borax readily removes the iron, and leaves a button of metallic silver. In the matrass it loses its lustre, and becomes dark-grey, and friable. It leaves traces on paper like graphite, which may be removed by caoutchouc.
Sternbergite occurs with other ores of silver, particularly the red and brittle silvers, at Joachimsthal in Bohemia; but it is a very rare species.
BRITTLE SULPHURET OF SILVER.
Spröd.Glaserz, W. Argent Antimonié Sulfuré Noir, H. Prismatic Melane Glance, M. Brittle Silver Glance, J. Schwarz Gültigerz, L.
| Freyberg. | ||
| Contains Silver | 66·5 | 68·54 |
| Sulphur | 12·0 | 16·42 |
| Antimony | 10·0 | 14·68 |
| Iron | 5·0 | 0·00 |
| Copper | 0·5—Klaproth. | 0·64—Rose. |
Sp. Gr. 5·9—6·4. H. = 20—30.
Colour dark lead or bluish-grey, passing into iron-black; when pulverised, dark-grey or brownish. Primary form a right rhomic prism of 107° 47' and 72° 13′.
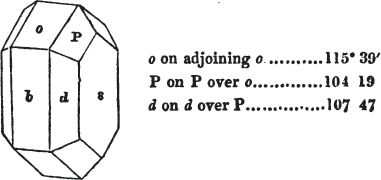
Crystals most frequently macled; cleavage in the directions of o and P; the structure sometimes distinctly lamellar, but the fracture commonly conchoidal, with a shining metallic lustre; soft, and brittle. Before the blowpipe it melts, the sulphur, antimony, and arsenic fly off, and there remains a dark-coloured metallic globule, which may be reduced on continuing the blast, or adding soda. Soluble in dilute nitric acid.
[page] 299
It occurs principally in veins traversing primitive rocks, and associated with other ores of silver, as at Schneeberg and Freyberg in Saxony; Przibram and Ratieborzitz in Bohemia; Cremnitz and Schemnitz in Hungary; in Siberia; and in Mexico. It is the compact and massive variety of this species to which the name of Schwarz-gültigerz particularly applies; that termed Weiss-gültigerz, on the other hand, is merely a mechanical mixture of this species, galena, and grey antimony. The richer it is in silver, the nearer it approaches the brittle sulphuret, while, in the contrary case, it resembles compact galena or antimony; evidently therefore it cannot be considered a distinct species.
SULPHURET OF SILVER AND ANTIMONY.
Peritomous Antimony Glance, M. Schwefel-Silber und Antimon, L. Argent Sulfuré Antimonifère et Cuprifère, Levy.
Consists chiefly of antimony, sulphur, silver, and copper.
Sp. Gr. 5·5—5·6. H. = 2·0—2·5.
This rare mineral occurs in small crystals, sometimes irregularly associated, more often separate; externally they are shining and splendent, of a colour approaching to silver-white; and deeply striated longitudinally; the striæ however are for the most part only a series of planes modifying the obtuse edges of the prism, as in the following figure. Cleavage perfect parallel to M; extremely brittle, yielding readily to mechanical division parallel to the planes of a right rhombic prism of 100° and 80°, and probably also in other directions. Before the blowpipe it emits copious white vapours, accompanied by a slight sulphureous odour, a small white bead, apparently of silver, remaining.
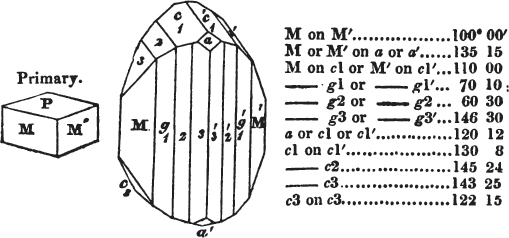
It occurs in the Himmelsfurst mine at Freyberg in Saxony, accompanying sulphuret of silver, blende, carbonate of iron, and galena; occasionally also at Kapnik in Transylvania.
[page] 300
POLYBASITE.
Kobell.
| Mexico. | Freyberg. | |
| Silver | 64·29 | 65·50 |
| Sulphur | 17·04 | 19 40 |
| Antimony | 5·09 | 0·00 |
| Arsenic | 3·74 | 3·30 |
| Copper | 9·93 | 3·75 |
| Iron | 0·06—Rose. | 5·46—Brandes. |
Sp. Gr. 6·214. H. = 2·0—3·0.
Primary form a rhomboid. Occurs in tabular-shaped six-sided prisms. The planes of the prism are striated parallel to its base, and the terminal faces parallel to the sides of an inscribed equilateral triangle, indicative of the rhomboid being its primary form. Colour iron-black; opake; lustre metallic; streak black; cleavage not observable; fracture uneven; susceptible of being cut with a knife. From Guarisamay in Mexico.
RED SILVER.
Ruby Silver. Rhombohedral Ruby Blende, M. Rothgültigerz, W. Argent Antimonié Sulfuré, H. Argent Rouge, Br. Bt. Silber-blende, Breithaupt.
This species is subdivided into,
1. The sulphuret of silver and antimony (Argyrythrose, Beudant. Antimon silber-blende, Breithaupt. Argent rouge antimonié Necker.)
2. The sulphuret of silver and arsenic (Proustite, Beudant. Arsen silber-blende, Breithaupt. Argent rouge arsenié, Necker.)
The former refers to the dark-red variety, which contains
| Andreasberg. | |
| Silver | 58·94 |
| Antimony | 22·84 |
| Sulphur | 16·61—Bonsdorff. |
The latter to the light-red variety, of which the following analyses have been made.
| Jachimsthal. | |||
| Silver | 64·67 | Sulphuret of silver | 74·35 |
| Arsenic | 15·09 | Sulphuret of arsenic | 25·00 |
| Sulphur | 19·51 | Proust. | |
| Antimony | 0·69—Rose. |
These two varieties also differ considerably in specific gravity, the light-red seldom exceeding 5·4—5·6, while that of the dark-red amounts to 5·8—5·9. They however correspond so entirely in their crystalline form, and, with the above exception, so perfect an analogy exists in all their physical characters, that mineralogists have not ventured to distinguish them farther.
[page] 301
It occurs crystallized in a great variety of forms, also dendritic, massive, and micaceous; structure lamellar; mechanically divisible into an obtuse rhomboid of 108° 30′ and 71° 30°,—the primary form,—but its extreme brittleness renders this difficult.
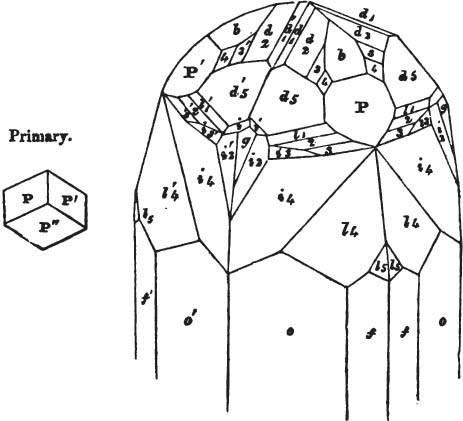
The above figure represents a crystal in the possession of H. J. Brooke, Esq. This crystal, perhaps one of the most complex that has been observed, tends to confirm the observation already annexed to the rhomboidal figure accompanying the notice of calcareous spar, viz. that the modifications to which the rhomboid is liable are almost endless. The planes b of the above figure tend, by their extension, to produce a rhomboid more obtuse than the primary, the planes g to acute rhomboids: the planes 41,2,3,4, and 5, to the production of obtuse dodecahedrons; all the planes i and l to acute dodecahedrons; o o and ff to regular six-sided prisms.
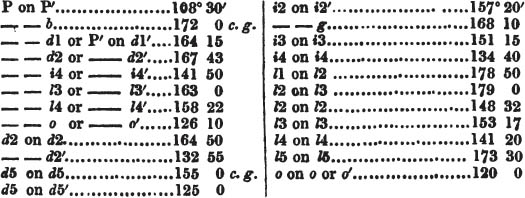
[page] 302
It varies by reflected light from lead-grey to iron-black, by transmitted light from brilliant to dark red; semi-transparent or opake; lustre imperfect-metallic in dark-coloured varieties, adamantine in such as are light; streak different shades of cochineal-red, according to the colour; cross fracture conchoidal, with a shining lustre; sectile, yielding readily to the knife. Before the blowpipe on charcoal it first decrepitates, fuses, emits fumes of sulphur and antimony, and ultimately leaves a globule of silver. Soluble without effervescence in nitric acid.
This very beautiful mineral is confined to a small number of localities, though in some of them it is of pretty frequent occurrence. The light-red varieties, which exhibit by transmitted light the most splendid cochineal hues, are met with principally in the Saxon and Bohemian mining districts of the Erzgebirge, particularly at Marienberg, Annaberg, and Johanngeorgenstadt, in Saxony, and at Joachimsthal in Bohemia, usually associated with other ores of silver, galena, blende, pyrites, and arsenic; while the dark-red ones occur chiefly with calcareous spar, native arsenic, and galena, at Andreasberg in the Hartz. Freyberg in Saxony, Schemnitz and Nagybanya in Hungary, Guadalcanal in Spain, Kongsberg in Norway, and St Marie aux Mines in France, are also well-known localities of this species.
It was formerly found at Huel Duchy in Cornwall; and from the produce of some of the Mexican mines, vast quantities of the precious metal have been obtained. Red silver, from its colour, may sometimes be mistaken for red orpiment; but the yellow streak of the latter is sufficiently characteristic, and its specific gravity is also lower. Cinnabar volatilizes before the blowpipe, whilst red silver forms a metallic globule. As an ore, it has been observed that the dark yield a larger proportion of silver than the light varieties, but both of them are highly valuable to the smelter.
MIARGYRITE.
Hemi-Prismatic Ruby-Blende, M. Miargyrite, Rose.
Combination of silver, antimony, and sulphur, in proportions different from those of the preceding species.
| Braunsdorff. | |
| Silver | 36·40 |
| Antimony | 39·14 |
| Sulphur | 21·95 |
| Copper | 1·06 |
| Iron | 0·62—Rose. |
Sp. Gr. 5·2—5·4.
Primary form an oblique rhombic prism of 93° 56′, and 86° 4′, and whose base is inclined to its axis at an angle of 101° 6′.
[page] 303
Imperfectly cleavable in the direction of the larger diagonal of the base. Colour iron-black; opake, except when viewed by transmitted light, in which case thin fragments present a deep blood-red hue; lustre intermediate between metallic and adamantine; streak dark cherry-red; fracture imperfect conchoidal; surfaces of the crystals deeply striated; very sectile. Before the blowpipe alone on charcoal it fuses with abundant white vapours, which have occasionally a slight alliaceous odour, leaving a globule of silver. It is soluble in nitric acid, with an immediate white antimonial precipitate.
This very rare mineral used to be comprised among the varieties of dark-red silver, until distinguished by reason of its form, and described by Mohs. It occurs with argentiferous arsenical pyrites, in one of the mines of Braunsdorff near Freyberg in Saxony.
SULPHURET OF SILVER AND COPPER.
Argent et Cuivre Sulfuré, Bournon. Silberkupferglanz, Stromeyer. Stromeyerine, Beudant. Argentiferous Copper Glance, J. Argentiferous Sulphuret of Copper, A. Cuivre Sulfuré Argentifère, Levy.
Sulphuret of copper mixed with silver.
| Schlangenberg. | |
| Contains Copper | 30·83 |
| Silver | 52·87 |
| Sulphur | 15·96 |
| Iron | 0·34—Stromeyer. |
Sp. Gr. 6·25. H. = 3·0—4·0.
Crystalline form unknown. Occurs compact; colour steel-grey, with a metallic lustre; the surface produced by fracture being brilliant, granular, and partially conchoidal; very brittle, and readily fusible before the blowpipe, emitting sulphuric acid fumes, and forming a grey globule with a metallic lustre. With the fluxes it exhibits the re-action of copper, and on the cupola yields a large globule of silver.
It occurs associated with copper pyrites, calcareous spar, and hornblende, at Schlangenberg near Colivan in Siberia. It is a very rare mineral.
BISMUTHIC SILVER.
Wismuth Silbererz, Selb. Bismuthic Silver, J. A. Bismuth Sulfuré Plumbo-Argentifère, Levy.
Consists of bismuth 27·0, lead 33·0, silver 15·0, iron 4·3, copper 0·9, sulphur 16·3—Klaproth.
[page] 304
Of a light lead-grey colour, but subject to tarnish on exposure. It occurs disseminated or in amorphous masses, rarely acicular; fracture fine-grained and uneven, with a glistening metallic lustre; it is soft, sectile, and somewhat brittle; opake. Before the blowpipe it fuses readily into a silver button, at same time covering the charcoal with an areola of the oxides of lead and bismuth.
It accompanies pyrites and galena at Schapbach in the valley of Kinzig, Baden.
SELENIURET OF SILVER.
Selen-silber. Seleniure d'Argent, Beudant.
Contains seleniuret of silver 89·71, seleniuret of lead 6·79— Rose.
Sp. Gr. 8·0.
Occurs in very thin veins traversing seleniuret of lead, at Tilkerode in the Hartz, from which mineral it is distinguished by its being of a darker hue. Possesses three cleavages perpendicular to one another. Before the blowpipe with borax and soda, it yields a metallic button of silver mixed with lead.
SELENIURET OF SILVER AND COPPER.
Eukairite,*Berzelius. Selen Kupfersilber, L. Cuivre Selenié Argental, H.
Compound of seleniuret of silver and copper. Copper 23·05, selenium 26·00, silver 38·93, earthy matter 8·90, carbonic acid and loss 3·12—Berzelius.
Colour shining lead-grey, texture granular; massive; disposed in thin superficial black metallic films, staining the calcareous spar in which it is contained. Before the blowpipe it exhales a strong odour of selenium, and on charcoal fuses readily into a grey metallic globule, which is not malleable. To borax or salt of phosphorus it imparts a green colour in the oxidating flame, becomes colourless in the reducing one, and on hardening appears opake and of a cinnabar-red hue. Is soluble in heated nitric acid, and when cold water is added to the solution it forms a white precipitate.
This extremely rare mineral was discovered and analyzed by Berzelius. It occurs in a copper mine at Skrickerum in Smaland, Sweden, with carbonate of lime, serpentine, seleniuret of copper, and copper pyrites.
* Eukairite, from the Greek, signifying opportune; in allusion to its discovery just as Berzelius had completed his examination of selenium.
[page] 305
IODIC SILVER.
Iodure d'Argent, Necker. Iod-Silber, Leonhard.
H. about 1·0.
Occurs massive, in thin plates of a greyish-white or silver-white colour, which change to lavender-blue on exposure to the air. Transparent or translucent; lustre resinous, passing into adamantine; malleable and flexible in thin laminæ; streak semi-metallic. Soluble in heated muriatic acid, which it colours reddish-brown, disengaging, after a short time, violet-coloured vapours. Before the blowpipe on charcoal it instantly melts, and produces a smoke which tinges the flame of a beautiful violet hue, globules of silver at the same time appearing on the charcoal.
It is found at Albarradon near Mazapil in Mexico, and forms thin veins in steatite.
CARBONATE OF SILVER.
Argent Carbonaté, H. Grey Silver Ore, J.
Consists of silver 72·5, carbonic acid 12, oxide of antimony and a trace of copper 15·5—Selb.
It is of a greyish colour, passing into iron black; it occurs massive and disseminated; the fracture is fine-grained and somewhat uneven, with a glistening metallic lustre; it is soft, brittle, and heavy. It is almost instantaneously reduced before the blowpipe; and effervesces with nitrous acid.
This species was observed many years ago by M. Selb in veins traversing granite in a mine at Altwolfach in the Black Forest, accompanying native silver, sulphuret of silver, and barytes; but not having again occurred, its properties are indistinctly defined.
MURIATE OF SILVER.
Horn Silver. Hornerz, W. Argent Muriaté, H. Bt. La Mine Corne, Br. Corneous Silver, J. Hexahedral Pearl Kerate M. Chlorsilber, Berz., Chloride of Silver, A.
| Combination of Chlorine and silver. | ||
| Saxony. | Peru. | |
| Silver | 67·75 | 76·0 |
| Chlorine | 21·50 | 24·0 |
| Oxide of iron | 6·00 | 0·0 |
| Alumina | 1·75 | 0·0 |
| Sulphuric acid | 0·25—Klaproth. | 0·0—Klaproth. |
| Sp. Gr. 4·75—5·55. | ||
[page] 306
Primary form the cube. Of a pearl-grey, greenish, or reddish-blue, but commonly tarnished externally of a brown colour; it occurs massive, also investing other substances, and crystallized in small cubes and acicular prisms; feebly translucent or opake, with a glistening or waxy lustre; yields to the pressure of the nail, and is malleable and sectile; cleavage none; fracture conchoidal. It is fusible in the flame of a candle. Before the blowpipe on charcoal it is reducible to a metallic globule, emitting at same time vapours of muriatic acid; when rubbed with a piece of moistened zinc, the surface becomes covered with a thin film of metallic silver. Insoluble in nitric acid.
It occurs in veins chiefly in primitive rocks, with some of the other ores of silver. The largest masses, and particularly those of a green colour, are brought from Peru and Mexico. It used to be found in considerable quantities in the Saxon mining districts of Freyberg and Johanngeorgenstadt, but it is now very scarce. It also occurs in Siberia, in Cornwall, and at Huelgoet in Brittany.
Buttermilk Silver. Buttermilch silber, W. Earthy corneous silver, J.
Silver 24·64, muriatic acid 8·28, alumina 67·08—Klaproth.
This is considered an earthy variety of the above species. It is commonly found massive, and investing other substances; is opake, and dull, with an earthy fracture, and is soft, sectile, and heavy.
It occurs only at Andreasberg in the Hartz, in veins traversing transition rocks.
GANSEKOTHIG-ERZ.*
An arseniate of silver and iron.
H. = 2·0—3·0.
In irregularly mammillated translucent masses of a yellow or pale-green colour. Shining, with white streak, resinous lustre, and conchoidal fracture; sometimes earthy, and mixed with cobalt. Before the blowpipe it emits copious arsenical fumes, and fuses into a blackish scoria; when the heat is continued, on charcoal, it melts, diminishes in bulk, and yields a button of silver, but the slag contains metallic iron, which strongly affects the magnet.
It occurs principally at the mines of Clausthal in the Hartz, where, when obtained in sufficient quantity, it is highly prized as an ore of silver. It is also met with in Cornwall, and at Allemont in Dauphiné. (Manual.)
* Or goosedung-ore, in allusion to its peculiar colour.
[page] 307
NATIVE COPPER.
Octahedral Copper, M. Gediegen Kupfer, W. Cuivre Natif, H.
Consists of 99·8 pure copper, with a trace of gold and iron— John.
Sp. Gr. 8·5—8·9. H. = 2·5—3·0.
Colour reddish-yellow, frequently with a tinge of brown; often tarnished externally blackish. Occurs crystallized in the cube and octahedron, the former of which is adopted as its primary form; often in macles; also capillary, dendritic, in thin plates filling crevices, and massive; no regular structure: tough, malleable, flexible, and sectile. Before the blowpipe it fuses into a bead of apparently pure copper. Soluble in nitric acid, which it colours green; and in ammonia, to which it gives a fine blue tinge. Isolated and rubbed, it acquires vitreous electricity. Fusible at 27° Wedgewood.
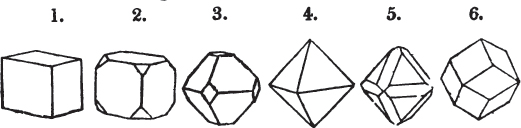
Fig. 1, a cube. Fig. 2, the same, of which the solid angles are replaced by triangular planes, which in fig. 3 are so greatly enlarged as to have become six-sided, reducing the planes of the cube to small quadrangles. The triangular planes of fig. 2 are complete in fig. 4;—the regular octahedron. Fig. 5, an octahedron of which the edges are replaced, forming a passage of that solid into the rhombic dodecahedron, fig. 6, in which the planes replacing the edges of fig. 5 are complete.
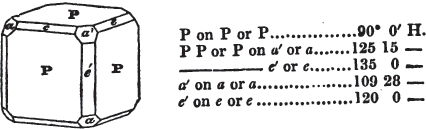
The most splendid crystalline varieties of native copper are those of Siberia, and the island of Nalsoe in Faroe, where it accompanies fibrous mesotype in amygdaloidal trap. Some very singular crystallizations, produced by the elongation of the simple individual, occur at Moldawa in the Bannat, at Chessy in France, Herrengrund in Hungary, and elsewhere. Cornwall, however, is certainly the greatest depository of native copper; and many of the mines near Redruth, the Consolidated Mines, Wheal Buller, and some others, afford it in considerable quantities. Its crystals are rarely regular, their faces being disproportionately en-
[page] 308
larged, and they are generally grouped in branches formed by the union of these crystals in rows, in a manner analogous to the crystalline structure of native silver. (Manual.)
SULPHURET OF COPPER.
Vitreous Copper. Kupfer Glanz, W. Cuivre Sulfuré, H. Bt. Cuivre Vitreux, Br. Copper Glance, J. Prismatic Copper Glance, M.,
| Combination of copper and sulphur. | |||
| Siberia. | Rothenburg. | Cornwall. | |
| Copper | 78·50 | 76·5 | 84·0 |
| Sulphur | 18·50 | 22·0 | 12·0 |
| Iron | 2·25 | 0·5 | 4·0 |
| Klaproth. | Klaproth. | Chenevix. | |
Sp. Gr. 5·69—5·8. H. = 2·5—3·0.
Colour lead- or iron-grey, often tarnished black, and occasionally iridescent. Primary form the cube; occurs crystallized in regular six-sided prisms, mostly modified on the terminal edges, sometimes on the lateral; and in acute and obtuse double six-sided pyramids with triangular planes; also massive, and occasionally in pseudomorphous crystals; structure perfectly lamellar; all the solid angles of the prism may be removed by the knife, producing a double six-sided pyramid with brilliant planes, the incidence of an upper on the adjacent plane of the lower pyramid being about 147° 30′. Fracture often conchoidal, with a vitreous lustre; the massive varies greatly in respect of hardness and colour; it is sometimes sectile and soft. Before the blowpipe, on charcoal, it disengages the odour of sulphureous acid; in the oxidating flame it fuses readily; in the reducing it emits sparks; and when the sulphur is wholly driven off, it yields a bead of copper. In ammonia it forms a blue solution. In heated nitric acid the copper is dissolved, and the solution assumes a green colour, but the sulphur remains.
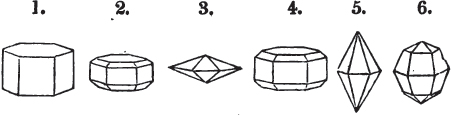
Fig. 1, the most simple of its forms; a six-sided prism. Fig 2, the same, of which the terminal edges are replaced by planes tending to obtuse six-sided pyramids; which are complete in fig. 3, a fiat six-sided pyramid. Fig. 4, a six-sided prism of which the terminal edges are replaced by planes tending to acute six-sided pyramids; which are complete in fig. 5. Fig. 6 represents a crystal consisting of portions of the planes of the acute pyramid, fig. 5, terminated by the obtuse pyramidal planes of fig. 3.
[page] 309
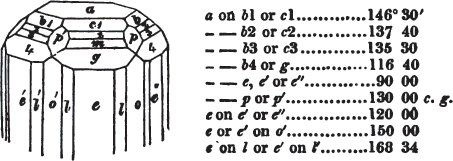
The sulphuret is met with in veins and beds accompanying other ores of copper, and is highly prized by the miner. The crystallized varieties occur abundantly, and almost exclusively, in the mines of Cornwall, and particularly in those near Redruth; while the more compact and massive are found also in Siberia, Hessia, Saxony, and the Bannat.
The argent en epis, or cuivre spiciforme of Haüy, from Frankenberg in Hessia, is supposed to be vegetable matter impregnated with black sulphuret of copper.
Vitreous copper is readily distinguished from either bournonite or fahlerz by its comportment before the blowpipe, and the green solution it produces with nitric acid; and from red silver ore by the colour of its streak, which resembles that of the mineral, while red silver presents a fine cochineal-red. (Manual.)
Variegated Vitreous Copper. Cuivre sulfuré hépatique, H. Colour that of tempered steel, violet-blue, greenish, and yellow. It seems to arise from an intimate mixture of the vitreous and yellow copper; both of which generally appear distinctly in the same specimen.
It occurs in most of the mines of Cornwall in which vitreous copper is found.
KUPFERINDIG.
Contains copper 64·77, sulphur 32·64, lead 1·05, iron 0·46— Walchner.
Sp. Gr. 3·8—3·82. H. about 2·0.
Occurs in spheroidal masses, presenting superficial indications of crystallization. Colour indigo-blue, or darker; opake, with a faintly resinous lustre, and a lead-grey shining streak. Sectile. Before the blowpipe it burns, prior to becoming red-hot, with a blue flame, and melts into a globule, which is strongly agitated, and emits sparks; finally, it yields a button of copper.
It occurs at Sangerhausen in Thuringia, imbedded in grauwacke, and is a rare mineral. (Manual.)
[page] 310
BI-SULPHURET OF COPPER.
Covelline,*Beudant. Bi-Sulphuret of Copper, Covelli.
Contains copper 66, sulphur 32.
In black or greenish-blue incrustations having the appearance of spiders' webs, deposited around the fumaroles of the crater of Vesuvius, and supposed to be derived from the action of sulphuretted hydrogen on the sulphate and muriate of copper. Is soluble in nitric acid, with the disengagement of nitrous gas.
PURPLE COPPER.
Buntkupfererz, W. Cuivre Pyriteux Hépatique, H. Variegated Copper, J. Octahedral Copper Pyrites, M.
| Eillarney. | Norway. | Silesia. | |
| Sulphur | 23·75 | 19·0 | 19·0 |
| Copper | 61·07 | 69·5 | 58·0 |
| Iron | 14·00 | 7·5 | 18·0 |
| Silica | 0·50 | 0·0 | 0·0 |
| Oxygen | 0·00 | 4·0 | 5·0 |
| Klaproth. | Phillips. | Klaproth. |
Sp. Gr. 5·0. H. = 3·0.
It occurs both massive and crystallized; colour of the massive between copper-red and tombac-brown; in the crystallized, the latter colour prevails, with an iridescent tarnish, generally of blue, sometimes yellow. The general form of the crystals is that of the cube, of which the solid angles are replaced, and the faces are mostly curvilinear; lustre metallic; streak pale-greyish-black, and slightly shining; not perfectly lamellar, but manifestly yields to mechanical division parallel to the planes of the regular octahedron; in other directions the fracture is imperfect conchoidal; it is soft, easily frangible, and slightly sectile. Before the blowpipe it blackens and becomes red on cooling, but at an increased temperature it is fusible into a globule, which acts powerfully on the magnet; and with soda is reduced, forming a copper bead. Soluble in nitric acid.
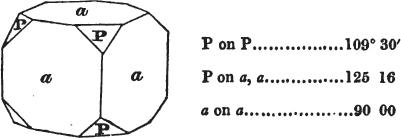
* In compliment to its discoverer, the late Sig. Covelli of Naples.
[page] 311
Crystalline varieties of buntkupfererz are almost peculiar to Cornwall, and that principally to the mines of Cook's Kitchen, Tin Croft, and Dolcoath near Redruth, where it is associated with vitreous copper and yellow copper ore. It occurs massive and compact, however, with green carbonate of copper, at Arendal in Norway, in Siberia, Hessia, Silesia, and the Bannat; also at Ross Island in Killarney, Ireland; and in cupriferous shale in Thuringia.
GREY COPPER.
Tetrahedral Copper Glance, M. Fahlerz, W. Br. Cuivre Gris, H. Panabase, Beudant.
A combination of sulphur, copper, iron, silver, and antimony or arsenic; the proportions of which, however, have not been satisfactorily ascertained.
| Fahlerz. | Gwennap, | Tennantite. | |
| Freyberg. | Cornwall. | Cornwall. | |
| Copper | 48·0 | 48·4 | 45·32 |
| Arsenic | 140 | 11·5 | 11·84 |
| Antimony | 00 | 0·0 | 0·00 |
| Iron | 25·5 | 14·2 | 926 |
| Sulphur | 10·0 | 21·8 | 28·74 |
| Silver | 0·5 | 00 | 0·00 |
| Zinc | 00 | 00 | 0·00 |
| Silica | 0·0. Klaproth | 5·0 Hemming. | 0·00 Phillips. |
| Arsenical and Antimonial | |||
| Grey Copper. St Marie aux Mines. | Schwarzerz. | Clausthal. | |
| Copper | 40·60 | 40·25 | 34·48 |
| Arsenic | 10·19 | 0·75 | 0·00 |
| Antimony | 12·46 | 23·00 | 28·24 |
| Iron | 4·66 | 13·50 | 2·27 |
| Sulphur | 26·83 | 18·50 | 24·73 |
| Silver | 0·60 | 0·30 | 4·97 |
| Zinc | 3·69 | 0·00 | 5·55 |
| Silica | 0·00 Rose. | 0·00 Klaproth. | 0·00 Rose. |
Sp. Gr. 4·4—5·2. H. = 3·0—4·0.
The marked diversity in their chemical composition would seem to demand a separation of some of the varieties of the present species. Many of them, indeed, are readily distinguishable at first sight; but there are others which present such intermediate stages, as to render all attempts at reducing the differences to fixed limits impossible. Of a steel-grey or iron-black colour; it occurs crystallized in the tetrahedron, which is considered its primary form; also massive and disseminated; cleavage octahedral, imperfect; streak same colour as the mineral, sometimes inclining to brown; fracture uneven or imperfect conchoidal, with
[page] 312
a shining or glistening metallic lustre; brittle. Before the blow-pipe it disengages vapours of an arsenical or antimonial odour. With soda, after considerable roasting, it yields a grain of metallic copper; and with borax presents the deep-green tinge characteristic of iron. It colours nitric acid green; and its powder, placed in that acid, soon becomes grey.
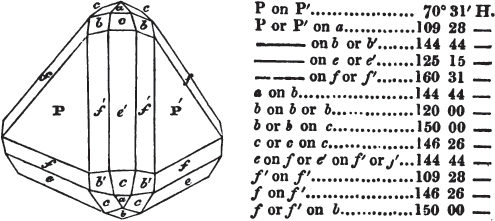
The above figure and measurements are given on the authority of Haüy.
The largest known crystals of Fahlerz occur in some of the Cornish mines near St Austle, generally in tetrahedrons, with dull rough surfaces. At Andreasberg in the Hartz, Cremnitz in Hungary, Freyberg in Saxony, Kapnik in Transylvania, and Dillenburg in Nassau, it not only presents more complicated crystallizations, but a greatly brighter and more brilliant aspect.
Tennantite.* This mineral usually occurs crystallized in the form of the rhombic dodecahedron, either perfect or variously modified; also in the cube and regular octahedron, of which the edges and angles are replaced; often very splendent; sometimes lead-grey, with but little lustre; occasionally approaching to iron-black and dull; fracture imperfectly lamellar and uneven; cleavage parallel to the planes of the dodecahedron; primary form the octahedron, as is indicated by occasional striæ on the planes of the dodecahedral crystals parallel with their longer diagonal. Somewhat harder than the preceding; and is brittle. Its powder is reddish-grey. Before the blowpipe on charcoal, it first burns with a blue flame and slight decrepitation, emits copious arsenical vapours, and ultimately fuses, leaving a greyish-black scoria, which affects the magnetic needle. After fusion it yields with soda a bead of copper. Soluble in nitric acid.
* In honour of the late excellent chemist, Tennant.
[page] 313
Fig. 1, the regular octahedron, of which the solid angles are replaced. Fig. 2; in this the edges also are replaced: the same planes appear on fig. 3. Fig. 4, the rhombic dodecahedron. Fig. 5, the same, having its edges replaced.
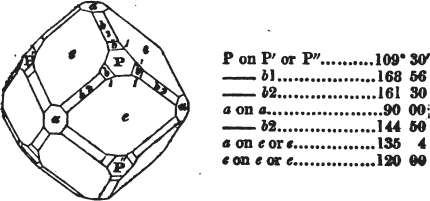
Tennantite is a variety exclusively Cornish. It usually occurs in small but very splendent crystals, investing other ores of copper, in veins which traverse granite and clay-slate, in the mines near Redruth and St Day. The rhombic dodecahedron (fig. 4), the cube with its edges replaced (fig. 3), and the octahedron and dodecahedron in various combinations, are its most frequent forms; but it has not been met with massive. (Manual.)
Antimonial Grey Copper. Schwartzerz, W. Cuivre gris antimonié, Bt. This mineral rarely occurs crystallized; its colour is dark lead-grey, approaching to iron-black, both externally and internally; no appearance of regular structure; fracture conchoidal, and surface glistening; not very brittle.
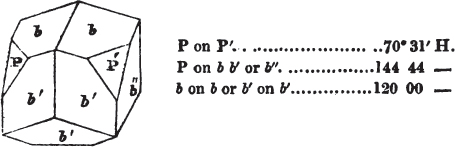
Represents a crystal from Schwatz in the Tyrol, the principal locality of this variety; it, however, is also met with at Kapnik in Transylvania, at Clausthal in the Hartz, and in Siberia; frequently imbedded in red manganese.
O
[page] 314
Arsenical and Antimonial Grey Copper. Occurs in iron-grey coloured crystals, having a very brilliant metallic lustre, principally at St Marie aux Mines. Its essential characters correspond with those of the following variety. Before the blowpipe it gives off an arsenical odour, and in nitric acid is soluble, with the exception of a precipitate of antimony.
Arsenical Copper Pyrites, White Copper. Weiss kupfererz, W. Colour between silver-white and pale brass-yellow, with a glistening metallic lustre, but soon tarnishes by exposure. It occurs massive and disseminated; the fracture fine-grained, uneven; yields easily to the knife, and is brittle. Specific gravity 4·5. It contains 40 per cent. copper, the remainder iron, arsenic, and sulphur—Henckel. Before the blowpipe it yields a white arsenical vapour, and melts into a greyish-black slag.
It accompanies other ores of copper at Huel Gorland, and elsewhere in Cornwall.
Platiniferous Grey Copper, A grey-coloured variety, agreeing generally with fahlerz in external character, consisting, according to Vauquelin, of copper, lead, antimony, iron, silver, platina, and sulphur; was formerly found at Guadalcanal in Estremadura in Spain, where it occurs with ores of silver and arsenic.
COPPER PYRITES.
Pyramidal Copper Pyrites, M. Kupferkies, W. Cuivre Pyriteux, H. Chalkopyrite, Beudant. Pyrite Cuivreuse, Br.
Combination of the sulphurets of copper and iron.
| Baygory. | Ramberg. | Furstemberg. | Cornwall. | ||
| Copper | 30·2 | 30·5 | 34·40 | 33·12 | 30·00 |
| Iron | 32·3 | 33·0 | 30·47 | 30·00 | 32·20 |
| Sulphur | 37·0 | 35·0 | 35·87 | 36·52 | 35·16 |
| Silica | 0·5 | 1·5 | 0·27 | 0·39 | 0·00 |
| Guenyveau. | Rose. | Rose. | Phillips. | ||
Sp. Gr. 4·16—4·3. H. = 3·5—40.
Colour brass-yellow, but externally subject to tarnish, and often iridescent; the crystals present the general form of the tetrahedron, having the solid angles always replaced; the structure is perfectly lamellar, affording brilliant surfaces parallel to the planes of a somewhat acute octahedron with a square base; so that the tetrahedron, as is shown in the following figures, is in fact only the remarkable consequence of an alternate enlargement of the planes l, modifying the edges of the primary octahedron; fracture conchoidal and splendent; lustre metallic; streak greenish-black, and somewhat shining; brittle. It also occurs stalactitic, botryoidal, mammillated, and amorphous, the
[page] 315
latter being often variegated; the structure of the botryoidal is granular; these are harder than the crystallized varieties; brittle. Copper pyrites yields to the knife, and may hence be distinguished from iron pyrites, which it often greatly resembles; its colour is generally also of a deeper yellow than that of iron pyrites. It fuses on charcoal before the blowpipe, emits a sulphureous vapour, and melts into a brittle black globule, which finally attracts the magnet; with borax, in small proportion, it yields a copper bead; and with soda, after the sulphur is entirely roasted off, separate globules of iron and copper may be obtained. In dilute nitric acid it forms a green solution, a portion of sulphur remaining undissolved.
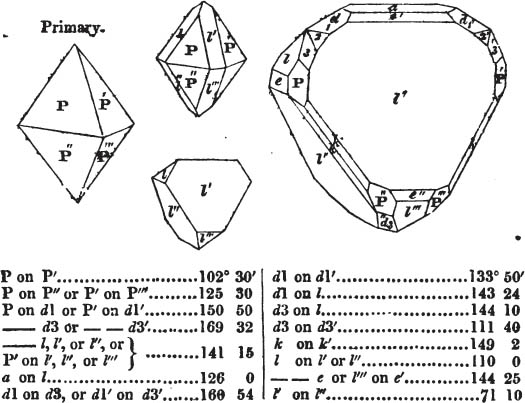
This is the most abundant variety of copper; nearly one third of the ore obtained by metallurgical processes being extracted from it, and in Great Britain yielding more metallic copper than all the other ores of copper together. In Cornwall it occurs associated with tin, forming veins in killas, and accompanying buntkupfererz, galena, grey copper, and blende. The great repository of copper at Fahlun in Sweden consists of extensive masses of this species, which are surrounded by a coating of serpentine, and imbedded in gneiss. At Rammelsberg near Goslar in the Hartz it forms a bed in grauwacke-slate, along with iron pyrites, galena, blende, and minute portions of silver and gold. Well defined crystals are found in the Kurprinz mine at Freyberg in Saxony, and many others in differ-
[page] 316
ent continental districts, the Bannat, Hungary, Thuringia, &c.; also in Siberia, Japan, and America. (Manual.)
SELENIURET OF COPPER.
Seleniure de Cuivre, Berzelius. Cuivre Selenié, H. Berzeline, Beudani. Selen Cuprite, Shepard.
Union of copper and selenium. Copper 64, selenium 40— Berzelius.
In masses, having an impalpable composition, and of a silver-white colour; streak shining; lustre metallic; soft, and admits of being smoothed down and polished, assuming then the colour of tin. Isolated and rubbed, it acquires resinous electricity. Before the blowpipe it fuses into a grey globule, which is slightly malleable, emitting at the same time powerful fumes of selenium; in the open tube yields selenium, which sublimes in the form of a red powder. With soda, after a lengthened roasting, it affords a grain of copper. Is decomposed by nitric acid, and the solution deposits metallic copper on a plate of iron. The decomposition which this substance undergoes from exposure to the air gives it a black colour; and it is therefore generally found in the form of black dendritic delineations, or in minute seams traversing calcareous spar, at the copper mine of Skrickerum in Smaland, Sweden.
RED OXIDE OF COPPER.
Oxydulated Copper. Rothkupfererz, W. Cuivre Oxydulé, H. Cuivre Oxidé Rouge, Br. Octahedral Copper Ore, M. Zigueline, Beudant.
The protoxide of copper.
| Cornwall | Siberia. | |
| Copper | 88·5 | 91·0 |
| Oxygen | 11·5—Chenevix. | 9·0—Klaproth. |
Sp. Gr. 5·6—6·1. H. = 3·5·4·0.
The colour of this mineral is red of various shades, by transmitted light sometimes crimson-red. It occurs crystallized in the form of the octahedron and its modifications, which are very numerous. The crystals are externally splendent, occasionally iridescent; or superficially of a lead-grey colour, with a metallic lustre; and sometimes nearly black and dull. Structure lamellar; cleavage much interrupted, and not easily obtained, parallel to the faces of the regular octahedron, which therefore is considered its primary form; fracture uneven, more often conchoidal, with a splendent and somewhat adaman-
[page] 317
tine lustre; transparent or translucent; yields easily to the knife; and is brittle. Streak several shades of brownish-red, and shining. Before the blowpipe it is reducible on charcoal to the metallic state, and with borax fuses readily into a fine-green glass. Soluble with effervescence in nitric acid, disengaging nitrous gas, and colouring the solution green; it may thus be distinguished from red silver ore, which does not effervesce in nitric acid, and from cinnabar, which does not dissolve in it.
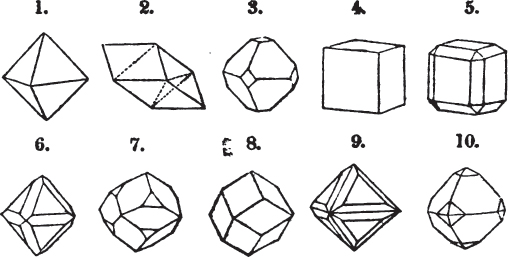
Fig. 1, the primary; the regular octahedron. Fig. 2, an acute rhomboid, arising, as will be perceived by the dotted lines, from an increase of crystalline laminæ on two opposite and parallel planes of the octahedron, the laminæ progressively diminishing to a point. Fig. 3, an octahedron, of which the solid angles are replaced by quadrangular planes; these planes meet and arc complete in fig. 4, forming the cube. Fig. 5, the cube, having its edges and solid angles replaced. Fig. 6, an octahedron whose edges are replaced by six-sided planes; which, in fig. 7, are increased; and in fig. 8 are complete, forming the rhombic dodecahedron. Fig. 9, an octahedron, of which each edge is bevelled by two planes. Fig. 10, an octahedron, of which each solid angle is replaced by four triangular planes, forming an obtuse pyramid on each. The varieties arising from combinations of the planes exhibited in the above figures are very numerous.
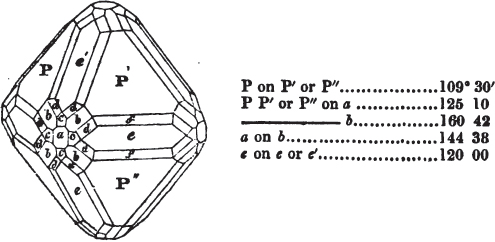
[page] 318
Particularly translucent varieties of this species, presenting numerous modifications of the octahedron, occur with native copper and quartz in Huel Gorland, and other mines in Cornwall. Isolated crystals, sometimes an inch in diameter, are met with inrbedded in lithomarge, at Chessy, near Lyons, generally coated or intimately mixed with the green and blue carbonates; and many splendid specimens are brought from Moldawa in the Bannat, and Ekatherineburg in Siberia. (Manual.)
Capillary Red Oxide of Copper. This differs from the preceding only in consisting of extremely slender transparent or translucent crystals; they are chiefly quadrangular prisms or elongated octahedrons, which appear reticulated or variously aggregated, sometimes even, fibrous or flocculent.
It is found in most of the mines of Cornwall in which the crystallized variety occurs; and at Rheinbreitbach on the Rhine, where it presents bright scarlet colours and a silky lustre.
Ferruginous Red Oxide of Copper, Ziegelerz, or Tile Ore, applies to the earthy varieties. Externally it is of a brick-red or reddish-brown colour; internally sometimes of a dark metallic grey, and then nearly compact and hard; more commonly the fracture is earthy. Yields to the knife, sometimes to the nail, and is opake. Before the blowpipe it blackens, but does not fuse. Consists of red oxide of copper and iron.
It is found, but not plentifully, with the red oxide, in some of the Cornish mines; also in the Bannat, at Camsdorf and Saalfeld in Thuringia, and elsewhere. It is a valuable ore of copper.
BLACK COPPER.
Kupferschwarze, W. Copper Black, J. and A. Melaconise,. Beudant. Cuivre Oxidé Neir.
Contains copper 79·83, and oxygen 20·17.
Colour brownish-black, or black. Never occurs crystallized, rarely massive, mostly disseminated in or investing other ores of copper; commonly friable, soils the fingers, and is heavy. It is fusible before the blowpipe into a black slag, yielding globules of copper in the reducing flame; and is acted upon by nitric acid, without the disengagement of gas.
It occurs in most of the Cornish mines, on the surface of and associated with the botryoidal varieties of copper pyrites, with crystallized and massive red oxide of copper and vitreous copper, and may not improperly be considered as resulting from the decomposition of these ores, which are frequently found passing into black copper. It has likewise been observed at Chessy near Lyons, in Siberia, Peru, and many other places, but only in small quantities.
[page] 319
BLUE CARBONATE OF COPPER.
Kupferlazur, W. Cuivre Carbonate Bleu, H. Azure Copper Ore, J. Prismatic Azure Malachite, M. Azurite, Beudant.
Combination of carbonic acid, copper, and water.
| Chessy. | Bannat. | Siberia. | |
| Deutoxide of copper | 69·08 | 69·08 | 70·0 |
| Carbonic acid | 26·46 | 25·72 | 24·0 |
| Water | 5·46 | 5·20 | 6·0 |
| Phillips. | Klaproth. |
Sp. Gr. 3·5—3·77. H. = 3·0—4·0.
Colour azure- or Berlin-blue, sometimes with a tinge of black. It occurs crystallized in a great variety of forms; structure lamellar; cleavage perfect parallel to the planes MM, and both diagonals of an oblique rhombic prism of 98° 50′ and 81° 10′, which is the primary form; the plane P is usually striated in the direction Indicated by the lines on the largest of the following figures; fracture conchoidal, with a vitreous lustre; translucent or opake, the most complex crystals possessing the greatest degree of translucency; yields easily to the knife.
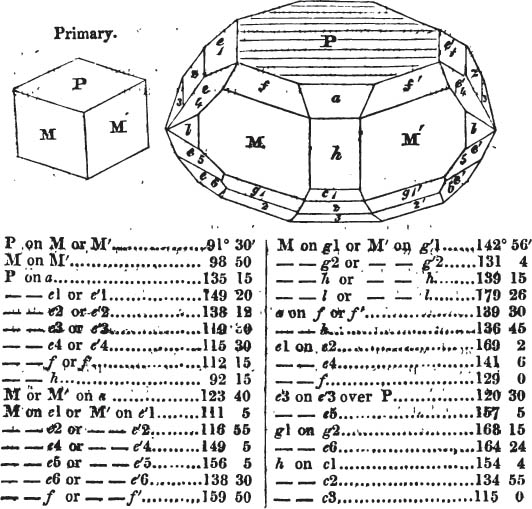
[page] 320
Before the blowpipe it blackens, decrepitates, and ultimately fuses; with borax on charcoal it effervesces, and colours the flux green. It is soluble with effervescence in nitric acid.
It occurs in the veins of primitive and secondary mountains, chiefly with the green carbonate and red oxide of copper. Chessy near Lyons is the principal locality of this beautiful mineral; it is there met with in considerable abundance, and under a great variety of crystalline form. Very fine crystals occur in Siberia; while those from Moldawa in the Bannat, though of smaller size, are often extremely distinct. Huel Buller, near Redruth in Cornwall, has also afforded some fine crystallized varieties; and at Alston-Moor and Wanlockhead small quantities are occasionally met with. It is found massive, and of a smalt-blue colour, in Cornwall; compact and earthy in Siberia and Thuringia; and in amorphous rounded concretions, sometimes of considerable dimensions, at Chessy. When obtained in sufficient quantity it is a valuable ore of copper.
GREEN CARBONATE OF COPPER.
Hemi-Prismatic Habroneme Malachite, M. Green Carbonated Copper. Fibrous Malachite.* Malachit, W. B. Cuivre Carbonaté Vert, H.
Combination of carbonic acid, deutoxide of copper, and water.
| Siberia. | Chessy. | ||
| Deutoxide of copper | 71·7 | 70·10 | 72·2 |
| Carbonic acid | 20·5 | 21·25 | 18·5 |
| Water | 7·8 | 8·65 | 9·3 |
| Klaproth. | Vauquelin. | Phillips. | |
Sp. Gr. 3·5—4·0. H. = 3·5—4·0.
Colour various shades of green. Occurs in slender fibres, which sometimes are fasciculated, sometimes stellated; in the cavities, however, extremely minute and transparent crystals may occasionally be observed, which in reality are macles, as shown by the following figures; of these, the primary is a right oblique-angled prism, yielding to cleavage readily parallel to the planes P and M, with difficulty parallel to T; transparent or translucent, sometimes only on the edges; lustre adamantine, inclining to vitreous; streak green, rather paler than the colour; brittle. Before the blowpipe it decrepitates, and fuses in part into a black scoria; with borax it readily affords a bead of copper, and colours
* Malachite, from the Greek; Marsh Mallow; the colour of both being green.
[page] 321
the flux green. In the matrass it yields water; and is entirely soluble in nitric acid.
* Malachite, from the Greek; Marsh Mallow; the colour of both being green.
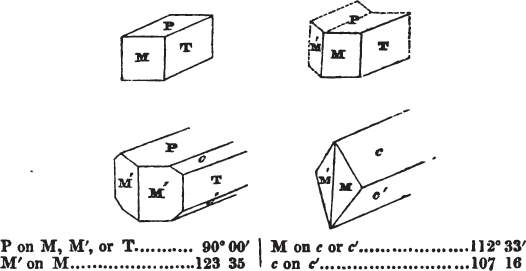
The fibrous and massive, into which this species has been divided, is not a distinction of any consequence, as they run so insensibly into one another, that it is frequently difficult to ascertain to which of them a specimen should be referred. The crystallized variety is extremely rare, having been observed only in minute transparent twins, coating the cavities of the more fibrous kinds. Exteriorly it assumes globular, reniform, botryoidal, and stalactitic shapes, and occurs in the same repositories as the last species. Splendid specimens of the fibrous variety are found in Siberia, at Chessy in France, in the old mine at Sandlodge in Shetland, and disseminated in iron ore at Moldawa in the Bannat. Compact malachite is chiefly known from Schwatz in the Tyrol; though in small quantities, it also occurs accompanying the blue carbonate in Cornwall, Wales, Ireland, and many other places. The green carbonate is a valuable ore of copper, and, from its variegated appearance, and the brilliant polish of which it is susceptible, is prized by the lapidary for ornamental purposes. Such varieties as are sufficiently compact are cut into vases, snuffboxes, &c.; and in St Petersburg it is formed into tables, and other magnificent articles of luxury; for this purpose, as the malachite rarely occurs in slabs exceeding a foot square, the pieces are united so as to render the concentric lines of the stone continuous, and thus massive tablets of six or seven feet in length are formed of apparently one piece of this beautiful substance. Some varieties are used as pigments, and in the preparation of the sulphate of copper. (manual.)
O 2
[page] 322
CHRYSOCOLLA.
Uncleavable Staphyline Malachite, M. Eisenscbüssig Kupfergrün, W. Cuivre Carbonaté Terreux, H. Chrysocolle, Br. Copper Green, J.
| Siberia. | Siberia. | ||
| Oxide of copper | 50·0 | 49·63 | 40·00 |
| Silica | 26·0 | 28·37 | 86·54 |
| Water | 17·0 | 17·60 | 20·20 |
| Carbonic acid | 7·0 | 3·00 | 0·00 |
| Sulphate of lime | 0·0 | 1·50 | 0·00 |
| Iron | 0·0. | 0·00 | 1·00 |
| Klproth. | John* | Kobell. |
Sp. Gr. 2·0—2·2.
Colour emerald- and pistachio-green, passing into sky-blue; and inclining to brown when impure. It occurs botryoidal, stalactitic, reniform, massive, and investing other ores of copper; fracture earthy or conchoidal; translucent or opake; is shining or dull; it varies in hardness from almost friable to that of quartz. Before the blowpipe on charcoal it blackens in the exterior flame, and reddens in the reducing, but does not fuse; with borax it forms a green glassy globule, and is partly reduced. If pure, it is soluble with effervescence in nitric acid, and leaves a residue of silica.
This substance differs much in appearance; in the same specimen it sometimes bears at one end the character of an earthy decomposed felspar, passing by insensible degrees towards the other, into brittle translucent green chrysocolla.
It is found in veins in primitive and secondary mountains, with other ores of copper, as in Cornwall; at Somerville in New Jersey; at Falkenstein and Schwatz in the Tyrol in limestone; in the Bannat, Hungary, Siberia, and Mexico.
DIOPTASE.*
Rhombohedral Emerald Malachite, M. Emerald Copper. Achirite.† Kupferschmaragd, W. Cuivre Dioptase, H. Bt.
Combination of oxide of copper. silica, and water.
| Oxide of copper | 55·0 | 45·45 | 48·89 |
| Silica | 33·0 | 43·18 | 36·60 |
| Water | 12·0 | 11·36 | 12·29 |
| Protoxide of iron | 0·0 | 0·00 | 2·00 |
| . | Lowitz. | Vauquelin. | Hess. |
Sp. Gr. 3·2—3·4. H. = 5·0.
* From the Greek, in allusion to the possibility of seeing—by transmitted light—the natural joints.
† Achirite—from Achir Maimed, the name of the merchant by whom it was first introduced into Europe.
[page] 323
Colour fine emerald-green; it occurs in crystals, having the general form of elongated rhombic dodecahedrons; structure lamellar; cleavage perfect parallel to the planes of its primary crystal, an obtuse rhomboid of 126° 17′ and 53° 43′; fracture flat conchoidal; translucent; with a shining lustre; scratches glass feebly; and is brittle. Before the blowpipe in the matrass yields water and blackens; on charcoal becomes black in the oxidating flame, and red in the reducing, but does not melt; it fuses however with glass of borax, imparting to the globule a green tinge, and is ultimately reduced. Insoluble in nitric acid, even when heated, but is dissolved without effervescence in muriatic.
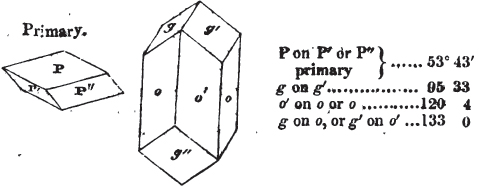
It occurs in the Kirghese steppes of Siberia, disposed on quartz, and always crystallized in well-defined elongated dodecahedrons, or in modifications thereof.
SULPHATE OF COPPER.
Blue Vitriol. Cyanose, Beudant. Tetarto-Prismatic Vitriol Salt, M. Vitriol Bleu. Cuivre Sulfaté, H. Kupfer Vitriol, W.
Hydrous sulphate of copper.
| Mexico. | |||
| Oxide of copper | 31·80 | 32·13 | 66·2 |
| Sulphuric acid | 32·14 | 31·57 | 16·6 |
| Water | 36·06 | 36·30 | 17·2 |
| Berzelius. | Berthier. |
Sp. Gr. 2·213. H. = 2·5.
Colour deep sky-blue, sometimes passing into bluish-green. Occurs massive, stalactitic, and pulverulent; lustre vitreous; translucent; cleavage imperfect; fracture conchoidal; taste nauseous, and metallic. When artificially prepared, it crystallizes. It is readily soluble in water, and affords a blue solution, a polished surface of iron dipped into which becomes coated with a film of metallic copper.
[page] 324
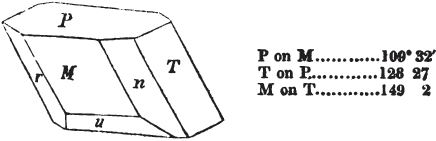
The above figure refers to the artificial crystals, for in its natural state the sulphate of copper has not yet occurred distinctly crystallized. It owes its existence principally to the decomposition of copper pyrites, and is found dissolved in water issuing from mines, from which it deposits itself spontaneously. Its principal localities are the Rammelsberg mine near Goslar in the Hartz, Fahlun in Sweden, Neusohl in Hungary, Pary's mine in Anglesea, Cornwall, and Wicklow. Before being used in the arts it requires purification, and is then employed in printing cotton and linen, dyeing, &c.
BROCHANTITE.*
Combination of sulphuric acid, oxide of copper, and water.
| Oxide of copper Sulphuric acid | 66·93 |
| 17·43. | |
| Water | 11·91 |
| Oxide of lead | 1·04 |
| Oxide of tin | 3·14—Magnus. |
Sp. Gr. 3·78—3·87. H. = 3·5—4·0.
Primary form a right rhomboidal prism of 117° and 63°.
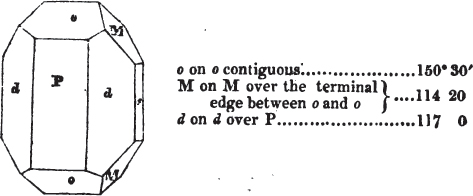
* In honour of Mons. Brothant, the well-known French mineralogist.
[page] 325
'Traces of cleavage parallel to M. Brochantite occurs in small well-defined transparent crystals of an emerald-green colour, and having a vitreous lustre. Is soluble in acids, but does not dissolve in water. Before the blowpipe in the matrass it yields water, exhaling the odour of sulphureous acid; on charcoal per se it is reduced into a non-malleable grain of copper; and with soda fuses into a metallic globule.
This mineral occurs associated with malachite and native copper at Ekatherineburg in Siberia. It was described by Levy.
The Königine of Levy, or Kœnigite of Beudant, is generally supposed to be nearly allied to Brochantite. Its primary form is a rhomboidal prism of about 105° and 75°. It cleaves with facility parallel to the base of the prism. Hardness between 2·0 and 3·0; colour emerald- or blackish-green; transparent. It occurs at Werchoturi in Siberia.
KUPFERSAMMTERZ.
Velvet Blue Copper, J. Cuivre Velouté, Levy.
A compound of oxide of copper, sulphuric acid, silica, and zinc—Brooke.
It consists of short delicate fibres of a smalt-blue colour, frequently grouped in spherical globules, which are produced by the divergement of the capillary crystals from a centre. Translucent; lustre pearly. When dissolved in nitric acid, a skeleton remains, which is not soluble in any acid.
It occurs principally at Moldawa in the Bannat, coating the cavities of an earthy oxide of iron; but, from its extreme rarity, its characters have not been satisfactorily ascertained.
MURIATE OF COPPER.
Prismatoidal Habroneme Malachite, M. Atacamite. Salzkupfererz, W. Salzsaures Kupfer, L. Cuivre Muriaté,H. Bt.
| Peru. | Chili. | ||
| Oxide of copper Muriatic acid | 70·5 | 76·5 | 73·0 |
| 11·4 | 10·6 | 10·1 | |
| Water | 18·1 | 12·7 | 16·9 |
| Proust. | Proust. | Klaproth. | |
Sp. Gr. 4·0—4·3. H. = 3·0—3·5.
[page] 326
Colour various shades of green; by transmitted light, sometimes of an emerald-green. It occurs in minute crystals, of which the primary form is a right rhombic prism of about 100° and 80° by the common goniometer, on the planes M M′ produced by cleavage,* which however have never been observed, although represented in the following figure, to show their position. In some of the crystals the planes a2 a2 ′, and c, c′, of the following figure, prevail to the exclusion of the rest, converting them into the octahedron with a rectangular base. The faces produced by cleavage parallel to the plane P are very brilliant and easily obtained; those parallel to M and M′ are less so. It is translucent or nearly transparent, soft, and brittle. Streak apple-green; lustre vitreous. It tinges the flame of the blowpipe bright green and blue, muriatic acid arises in vapours, and a bead of copper remains on the charcoal. Is soluble without effervescence in nitric acid, and communicates instantaneously to ammonia a fine blue colour.
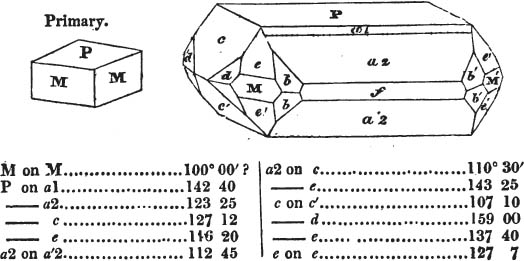
It is found at Remolinos in Chili on brown iron-stone, sometimes with ruby copper and carbonate of copper; in Peru with some of the ores of silver; and in the form of green sand in the river Lipas, in the Atacama desert (whence Atacamite), which separates Chili from Peru. It also occurs in the iron mines of Schwartzenberg in Saxony; and on the lavas of Vesuvius, where it is probably formed by the union of muriatic acid and carbonate of copper, both of which are well known to be deposited by sublimation at that volcano.
* According to Mohs and Beudant, the angles of this prism are 112° 45′ and 67° 15′.
[page] 327
PHOSPHATE OF COPPER.
Phosphorkupfrrerz, W. Cuivre Phosphaté, H. Br. Bt. Di-prismatic Olive Malachite, M. Octaedrisches Phosphorsaurer Kupfer, Leonkard. Phosphorkupfer von Libethen, Haid. Libethenite, N. Aphérèse, Beud.
Combination of phosphoric acid, oxide of copper, and water.
Phosphoric acid 28·7, oxide of copper 63·9, water 7·4—Berthier.
Sp. Gr. 3·6—3·8.
It occurs crystallized, and in radiated masses; externally the crystals are greenish or blackish-green, approaching nearly to black, and are considerably splendent, but their surfaces are uneven, and not adapted to the use of the reflective goniometer; translucent on the edges, or opake; by transmitted fight their fragments are olive-green, occasionally with a tinge of yellow; lustre resinous; streak dark olive-green. When radiated, the colour is bluish-green and black intermixed, the exterior being often nearly block. The crystals are frequently prismatic, but occasionally the prism is so short as to reduce them to the general figure of an octahedron. They possess a distinct cleavage parallel to the plane P of the following figure; less perfect cleavages may also be obtained parallel to the edges x x, thus reducing the crystals to a right rhombic prism of about 110° and 70°, which may be considered as the primary form. On charcoal it fuses into a brownish globule, which by the continued action of the blowpipe acquires a reddish-grey metallic lustre; in the centre is a small bead of metallic copper. With borax and salt of phosphorus it presents in the oxidating flame a green glass, which becomes, in the reducing, colourless while hot, and of a cinnabar- or ruby-red when cold. Soluble without effervescence in nitric acid, to which, as well as to ammonia, it imparts a sky-blue colour.
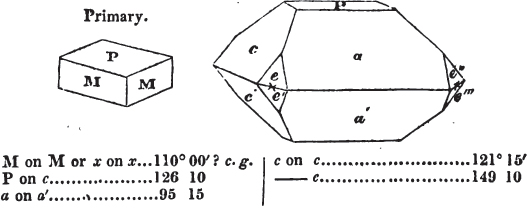
It is found in quartzose cavities associated with copper pyrites at Libethen near Neusohl in Hungary; also in small quantity in Cornwall, both crystallized and fibrous, in Gunnis Lake mine, on the banks of the Tamar.
[page] 328
HYDROUS PHOSPHATE OF COPPER.
Prismatic Habroneme* Malachite, M. Pseudomalachit, Haus. Phosphorkupfer von Rheinbreitbach, Haid. Hydrous Phosphate of Copper, A. Ypoleime, Beudant.
Combination of phosphoric acid, oxide of copper, and water, in different proportions from the preceding.
Analysis by the Rev. F. Lunn, confirmed by M. Arfwedson.
| Phosphoric acid | 21·687 |
| Oxide of copper | 62·847 |
| Water | 15·454 |
Sp. Gr. 4·2—4·3. H. = 5·0.
It occurs both massive and crystallized. The colour of the massive approaches to emerald-green, striated with black or blackish-green; and it appears to consist of minute crystals often diverging or radiated. The more determinate crystals are generally dull and of a blackish-green colour externally, sometimes black and splendent; by transmitted light they are emerald-green; translucent generally on the edges only, and possessing a vitreous or adamantine lustre. Streak a little paler than the colour. By exposure to red heat in a close crucible, it becomes dark olive-green, and the powder increases considerably in bulk. Before the blowpipe on charcoal it fuses into a reddish-black slag, and by the addition of carbonate of soda is reduced to a bead of pure copper. Soluble without effervescence in nitric acid, particularly if heated. Primary form an oblique rhombic prism of 141° 5 ° and 38° 56°.
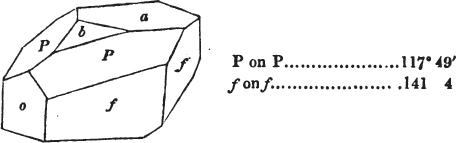
The hydrous phosphate of copper is found at Rheinbreitbach near Bonn on the Rhine, in veins traversing grauwacke-slate, and accompanied by quartz and ores of copper. It generally presents itself either aggregated in extremely minute individuals, or mammillated and compact—its crystalline form therefore is not easily determined.
* From αβζòs, delicate, and Γημα, the thread or fibre.
[page] 329
ARSENIATE OF COPPER.
There are several varieties of arseniate of copper, which do not materially differ in their chemical characters, although readily distinguishable by external form.
1. OCTAHEDRAL ARSENIATE.
Prismatic Lirocone Malachite, M. Liroconite, Beudant. Linsenerz, W. Cuivre Arseniaté Primitif, H. Linsen Kupfer, Haus. Lenticular Copper Ore, J. Lenticular Arseniate of Copper, A.
Combination of arsenic acid, oxide of copper, and water.
| Cornwall. | ||
| Oxide of copper | 49·0 | 35·19 |
| Arsenic acid | 14·0 | 20·79 |
| Water | 35·0 | 22·24 |
| Alumina | 0·0 | 8·03 |
| Oxide of iron | 0·0 | 3·41 |
| Phosphoric acid | 0·0 | 3·61 |
| Silica | 0·0 Chenevix. | 4·04 Wachtmeister. |
Sp. Gr. 2·88—2·92. H. = 2·0—2·5.
Colour sky-blue, smalt-blue, and occasionally deep grass- or verdigris-green; translucent; cleavage imperfect parallel to all the planes of a flat octahedron; streak corresponding to the colour, but paler; in the matrass it yields much water. Before the blowpipe on charcoal, fuses imperfectly, emits arsenical fumes, and is converted into a black friable scoria; and by subsequent fusion with borax affords a bead of copper. Soluble without effervescence in nitric acid. Crystallized in obtuse rectangular four-sided prisms, whose faces are inclined at angles of 60° 40′ and 72° 22'.
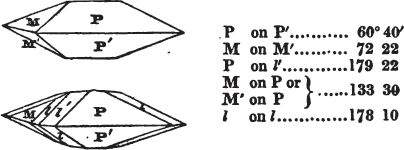
It occurs in veins passing through the adjoining mines of Huel Muttrell, Huel Gorland, and Huel Unity in Cornwall, associated with the following varieties; also with red oxide of copper, copper-pyrites, arseniate of iron, and the martial arseniate of copper. It is likewise met with at Herrengrund in Hungary in minute crystals.
[page] 330
2. BHOMBOIDAL ARSENIATE.
Rhombohedral Euclore Mica, M. Hexahedral Arseniate, Bournon. Cuivre Arseniaté Lamélliforme, H. Prismatic Copper Mica, J. Kupferglimmer, W. and L. Cypromica, Necker.
Combination of arsenic acid, oxide of copper, and water.
| Oxide of copper Arsenic acid | 39·0 | 58·0 |
| 43·0 | 21·0 | |
| Water | 17·0—Vauquelin. | 21·0—Chenevix. |
Sp. Gr, 2·5—2·6. H. = 2·0.
Colour emerald- or grass-green. In six-sided tabular crystals, of which the lateral planes are trapeziums, inclining alternately in contrary directions, being sections of an acute rhomboid of about 110° 30′ and 69° 30′; and it yields to cleavage parallel to all the planes of the rhomboid, but with perfect ease and brilliancy only at right angles to its axis, i.e. parallel to the tabular planes a; these tables are often applied to each other laterally, forming rosettes, which may be separated into laminæ like mica. Streak rather paler than the colou. Lustre pearly parallel to a, vitreous parallel to P. Transparent or translucent. Before the blowpipe it decrepitates, emits arsenical fumes, and passes first into a spongy scoria, after which it melts into a black slightly vitreous globule; with borax it affords a green glass, which includes grains of metallic copper. Soluble in nitric acid.
The dotted lines in the first of the following figures exhibit the portion of the tabular crystal in the primary rhomboid.
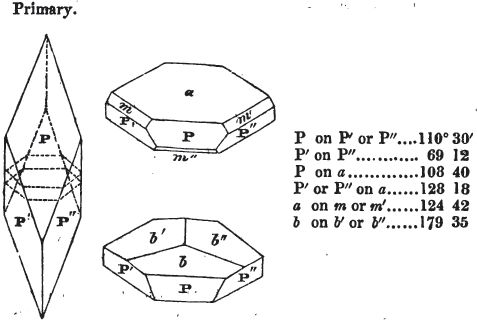
This species is as yet peculiar to the mining districts of Cornwall: it occurs accompanying the preceding in the mines near Redruth; also at Gunnis Lake on the banks of the Tamar.
[page] 331
3. OBLIQUE PRISMATIC ARSENIATE.
Diatomous Habroneme Malachite, M. Axotomous Habroneme Malachite, Haid. Radiated Acicular Olivenite, J. Strahlerz, A. Cuivre Arsenaté en Prisme Rhomboidal Oblique, Levy. Aphanèse, Necker.
Combination of arsenic acid, oxide of copper, and water.
| Cornwall. | |
| Oxide of copper | 54·0 |
| Arsenic acid | 30·0 |
| Water | 16·0—Chenevix. |
Sp. Gr. 4·1—4·28. H. = 2·5—3·0.
Externally bluish-black passing into deep black, with a shining lustre; internally greenish-blue. Occurs, though rarely, in extremely minute oblique rhombic prisms, whose lateral planes meet alternately at angles of about 56° and 124°, and of which the oblique terminal plane declines from one acute angle to the other; they are frequently fasciculated in a somewhat radiating position, so that only the terminal planes of the following figures are distinctly visible; it also occurs in curved lamellar concretions. The minute crystals are often transparent and of a beautiful blue or greenish-blue colour by transmitted light; the larger crystals are sometimes black and opake, but, on being scratched by the knife, appear internally of a blue colour. Streak verdigris green. Before the blowpipe in the matrass yields water; on charcoal emits arsenical vapours, and fuses into a bead, which on cooling crystallizes in small rhombic plates of a brown colour.
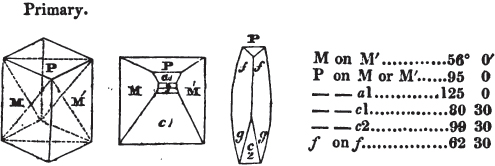
The dotted lines on the primary show that from the replacement of its acute angles arise the planes el of the second figure.
This species is only known in Cornwall, where it accompanies the preceding and other ores of malachite. The crystals present a very dark-blue colour and brilliant lustre, but are rarely recognisable, being aggregated in diverging groups, or disposed in extremely minute individuals in the cavities of quartz.
[page] 332
4. RIGHT PRISMATIC ARSENIATE.
Prismatic Olive Malachite, M. Cuivre Arseniaté Octaèdre Aigu, H. Acicular Olivenite, J. Acicular Arseniate of Copper, A. Olivenerz, W. Olivenkupfer, Haus. Olivenit, L. Beudant.
Combination of arsenic acid, and oxide of copper.
| Wood Arseniate. | ||||
| Oxide of copper | 60·0 | 50·62 | 56·63 | 50·0 |
| Arsenic acid | 39·7 | 45·00 | 36·71 | 29·0 |
| Water | 0·0 | 3·50 | 3·50 | 21·0 |
| Phosphoric acid | 0·0 | 0·00 | 3·36 | 00·0 |
| Chenevix. | Klaproth. | Kobell. | Chenevix. |
Sp. Gr. 4·2—4·6, H. = 3·0—3·5.
Colour olive-green, pistachio-green, and blackish-green, passing into liver-brown and wood-brown; the fibrous variety siskin-green. Occurs in prismatic crystals, which are divisible parallel to the planes of a right rhombic prism of about 110° 50′ and 69° 10′ (M M ′). Streak olive-green or brown. Lustre between vitreous and resinous. Fracture conchoidal and uneven. Sometimes however the planes c c prevail to the exclusion of P, and the prism is then so short as to give it the general form of an octahedron with a rectangular base; the face a is extremely rare; the crystals are usually attached to the matrix at M M′. It also occurs capillary. Before the blowpipe, yields no water in the matrass; on charcoal emits an arsenical odour, fuses with a kind of deflagration, and is reduced, forming a white metallic globule, which during cooling becomes covered with a red coating. Is soluble in nitric acid, and colours ammonia blue.
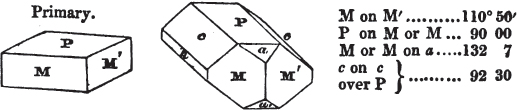
Capillary or Amiantiform Arseniate. This variety presents the same colours, and occurs in minute crystals, occasionally exhibiting the planes c, c of the preceding figure; generally they are indeterminate, the capillary prisms being distinct for a part of their length, and terminated by extremely minute fibres of a greenish-white colour and silky lustre.
Hæmatitic or Wood Arseniate. Cuivre arseniaté mamelonné fibreux, H. It occurs of various shades of brown, green, and yellow, often whitish or yellowish; and is found investing some
[page] 333
of the preceding varieties, also mammillated; the structure is finely and divergingly fibrous, generally with a silky lustre. It sometimes possesses considerable hardness; frequently adheres to the fingers. Before the blowpipe it yields a hard black cellular scoria.
This species occurs in Huel Gorland, and Huel Unity near St Day; also in Tin Croft mine near Redruth, and elsewhere in Cornwall. It has likewise been observed at Alston Moor in Cumberland, but not in such splendid specimens as in Cornwall.
EUCHROITE.*
Prismatic Emerald Malachite, M. Euchroite, Breithaupt.
Combination of arsenic acid, oxide of copper, and water.
| Libethen. | |
| Contains Oxide of copper Arsenic acid | 47·85 |
| 33·02 | |
| Water | 18·80—Turner. |
Sp. Gr. 3·38—3·41. H. = 3·5—4·0.
Primary form a right rhombic prism of 117° 20′; cleavage indistinct; fracture uneven; colour bright emerald-green; transparent or translucent, with vitreous lustre, and considerable double refraction. Streak pale apple-green. In the matrass it yields water, changes its colour, and becomes friable. When heated upon charcoal to a certain point, it is reduced in an instant with a kind of deflagration, leaving a globule of malleable copper, with white metallic particles disposed through it, which are crystallized on continuing the blast. It dissolves readily in nitric acid without effervescence.
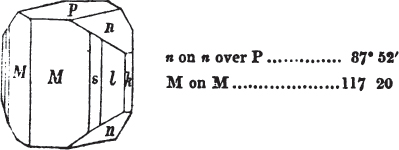
This beautiful mineral occurs in crystals of considerable dimensions at Libethen in Hungary in quartzose mica-slate. It is very rare.
* From Eνϰζοα (pulcher color).
[page] 334
KUPFERSCHAUM.*
Prismatic Euclore Mica, M. Zinc Hydraté Cuprifère, Levy, Kupferschaum, W.
Oxide of copper 43·88, arsenic acid 25·01, water 17·46, carbonate of lime (probably matrix) 13·65—Kobell.
Sp. Gr. 3·0—3·2. H. = 1·0—1·5.
Primary form a right rhombic prism. Occurs in rhomboidal plates, which present perfect cleavage parallel to the faces of the rhomb; generally in small aggregated and diverging fibrous groups of a pale apple-green or verdigris-green colour, inclining to sky-blue, and translucent; lustre pearly on the faces of the rhomb; streak same as the colour, but paler; thin laminæ are flexible. Entirely soluble in heated acids. Before the blowpipe fuses readily in the platina forceps into a blebby copper-red coloured scoria; upon charcoal, it intumesces, disengages an alliaceous odour, and melts into a green scoria, containing numerous grains of metallic copper; with borax it readily forms a green limpid glass, and with soda is reduced.
Is found disposed in the cavities of calamine, associated with barytes, calc-spar, or quartz, in the Bannat, at Libethen in Hungary, Nerzschinsk in Siberia, Schwatz in the Tyrol, Saalfeld in Thuringia, and at Matlock in Derbyshire.
ERINITE.†
Dystomic Habroneme Malachite, M.
Oxide of copper 59·44, arsenic acid 33·78, alumina 1·77, water 5·01—Turner.
Sp. Gr. 4·0—4·1. H. = 4·5—5·0.
Form unknown; cleavage indistinct. In mammillated crystalline groups, consisting of concentric coats with rough surfaces, and exhibiting a fibrous structure. Colour brilliant emerald-green, slightly inclining to grass-green; streak the same, but a little paler; lustre none; faintly translucent on the edges; fracture uneven, or imperfect conchoidal.
This species was distinguished by Haidinger; it occurs, though not abundantly, with arseniate of copper, in the County Limerick.
* The cupriferous calamine of Mr Phillipss last edition,—it having been considered by Brooke as a combination of the hydrate of zinc and copper.
† Erinite, in reference to its locality, as well as to its characteristic emerald-green colour.
[page] 335
SCORODITE.*
Cuprous Arseniate of Iron, Bournon. Cuivre Arseniaté Ferrifère, H. Martial Arseniate of Copper, J. and A. Prismatic Fluor Haloide, M.
Combination of arsenic acid, protoxide of iron, oxide of copper, and water.
| Cornwall. | |
| Oxide of copper Arsenic acid | 22·5 |
| 33·5 | |
| Protoxide of iron | 27·5 |
| Water | 200 |
| Matrix | 3·0—Chenevix. |
Sp. Gr. 3·1 —3·2, H.- 8·5—4·0.
Colour pale Leek-green or liver-brown. Occurs in transparent or translucent prismatic crystals, terminated by four-sided pyramids; primary form a right rhombic prism of 120° 10′ and 59° 50′, by measurements taken with the reflective goniometer from natural planes; cleavage imperfect parallel to the planes M, M of the prism, and to its lesser diagonal; they are not often single, and usually are small, and grouped in a globular form; lustre of the crystal adamantine; streak pale greenish-grey or white. Before the blowpipe on charcoal it emits abundant fumes of arsenic, and fuses in the reducing flame into a reddish-brown magnetic scoria. With the fluxes it exhibits the bottle-green colour characteristic of iron; and is soluble in nitric and muriatic acid.
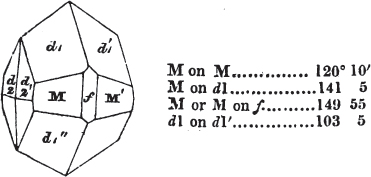
The brown-coloured variety of this species occurs at Schwartzenberg in Saxony; while the fine leek green crystals are found in certain of the Cornish mines, coating cavities of ferruginous quartz. Beautiful specimens have been brought from Brazil, and occasionally also from Löling, near Huttenberg in Carinthia.
* From the Greek, in allusion to its emitting an alliaceous odour under the blowpipe.
[page] 336
NATIVE GOLD.
Hexahedral Gold, M. Gediegen Gold, W. Or Natif, H. Br. Bt.
Commonly alloyed with copper, silver, and iron, in minute proportions.
Sp. Gr. 17—19. H. = 2·5—3·0.
Native gold is bright yellow of various shades. It occurs crystallized in the cube and regular octahedron and several of their varieties, but does not possess a lamellar structure; the cube has been adopted as the primary form, as being the most simple. It is also found capillary, ramified, and in grains; occasionally in masses weighing several pounds. Soft, inelastic, flexible, and malleable. Fusible at 32° Wedgewood; but is soluble only in nitre-muriatic acid. By friction it acquires resinous electricity.
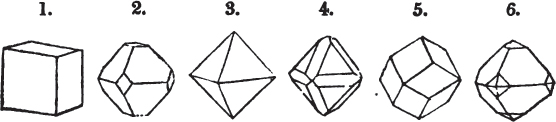
Fig. 1, the cube. Fig. 2, the same, of which the solid angles are replaced by planes; which are complete in fig. 3, forming the regular octahedron. Fig. 4, an octahedron, with its edges replaced by six-sided planes; which are complete in fig . 5, the rhombic dodecahedron. Fig. 6, an octahedron, whose solid angles are replaced by four triangular planes, forming on each an obtuse quadrangular pyramid.
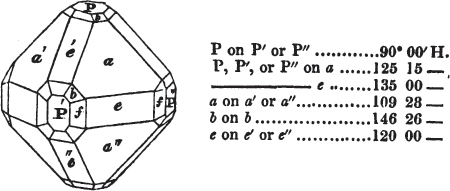
It is found in veins or beds in primitive mountains, in nodules, plates, and small crystals, coating the cavities or interspersed through the mass.
It occurs in granite in Salzburg, and at La Gardette in France; in gneiss and mica-slate in Mexico and the Tyrol; in hornblende rock at Edelfors in Sweden; and at Schlangenberg in Siberia.
[page] 337
But gold is more common in alluvial deposits, and in the sands of rivers, whither it has been conveyed from the decomposition of the rocks in which it originally existed. This is particularly the case at Matto-Grosso, Villa Rica, and other places in Brazil, Mexico, and Peru, where it is sometimes met with in masses of several pounds weight. In Siberia, too, it occurs in a similar alluvium or sand, in the country eastward of the Ural Mountains, where masses of eight, ten, or sixteen pounds have occasionally been discovered. In Transylvania a considerable quantity of gold is obtained from stream works, as at Ohlapian near Hermanstadt. In the Wicklow Mountains of Ireland, and at Leadhills in Scotland, it occurs in alluvial soil; and in many districts of Germany it appears under similar circumstances. The mines of Hungary and Transylvania, Cremnitz, Schemnitz, Posing, Botza, Magurka, Nagyag, Offenbanya, and Boitza, are all worked for this metal, and occasionally afford the most splendid specimens; and in Saltzburg, and thence along the chain of the Alps as far as La Gardette near Allemont in France, there are numerous other establishments of a similar description. The Russian and Siberian mines have of late years afforded considerable quantities of gold; and to the United States it promises to be a product of some importance. (manual.)
Gold is frequently combined with other metalliferous minerals, in various proportions; particularly native tellurium, and occasionally iron pyrites, which thence are termed auriferous.
Argentiferous Gold. Electrum. Is distinguished by its silver-white colour, although, as all gold contains a proportion of silver, no definite separation can be made between this variety and the above.
| Contains Gold | 64 | 74·41 |
| Silver | 36—Klaproth. | 23·12—Rose. |
Sp. Gr. 14·0—17·0.
Before the blowpipe it fuses into a more or less pale-yellow globule. It occurs at Schlangenberg in Siberia in tabular crystals and imperfect cubes; also at Kongsberg in Norway; in Transylvania; and in other mining districts.
Beudant considers that not less than 88,100 marks of gold are annually produced from the different quarters of the globe, of which South America alone supplies 70,000, Africa 7000, Siberia 3000, Hungary and Transylvania 5100, &c. Its uses are well known; it is the most ductile and flexible of all metals, and is at the same time very soft. The electric shock converts it into a purple oxide. Its colour when melted is bluish-green, the same as is exhibited by light transmitted through gold leaf.
P
[page] 338
NATIVE PLATINA *
Gediegen Platin, W. Platine Natif Ferrifère, H. Hexahēdral Platiua, M.
Is never met with pure, being alloyed with about 20 per cent. of other substances, particularly iron; also rhodium, iridium, osmium, and palladium,—four metals which were unknown till discovered in platina.
| Ural, | Columbia, | |
| Small grains, | Large grain*, | |
| Platina | 78·94 | 84·30 |
| Rhodium | 0·86 | 3·40 |
| Palladium | 0·28 | 1·06 |
| Iridium | 4·97 | 1·46 |
| Osmium | 1·96 | 1·03 |
| Iron | 11·04 | 5·31 |
| Copper | 0·70—Berzelius | 0·74—Berzelius. |
Sp. Gr. 16·0—20·0. H. = 4·0—4·5.
Colour perfect steel-grey. Primary form the cube. Occurs in irregular masses or grains, rarely exhibiting traces of crystallization; cleavage none; lustre metallic; streak unchanged and shining; ductile, and malleable. It requires a much higher degree of heat than can be produced by the common blowpipe, to cause fusion; but at the oxy-hydrogen flame it melts like lead. It slightly affects the magnet, in proportion to the amount of iron which it contains; and is soluble only in nitro-muriatic acid.
The original repositories of native platina are not known, it having hitherto been found only in pebbles and grains, associated with zircon and other gems, gold, and magnetic iron, in certain alluvial deposits. It has principally been obtained from the provinces of Choco and Barbacoas in South America; also from Matto-Grosso in Brazil; St Domingo; and Siberia. Latterly platina has occurred in such abundance at Joetsk in the Perm government of Siberia, that the Russians have converted it into a medium of exchange, by coining it into ducats of ten roubles.
The refractory properties of this metal, its freedom from rust or tarnish, and its not being acted upon by most chemical re-agents, render it extremely valuable in the construction of philosophical and chemical apparatus. It is used also for covering other metals, for painting on porcelain, &c.
* From a Spanish word signifying silver; in allusion doubtless to the colour of platina.
[page] 339
NATIVE PALLADIUM*
Octahedral Palladium, Haidinger.
It consists of palladium, alloyed by minute portions of platina and iridium.
Sp. Gr. 11·5—12·5. H. above 4·5.
Primary form the octahedron. Occurs in grains apparently composed of diverging fibres; in other respects these grains differ little in external character from those of platina, amongst which they are found. Cleavage none; lustre metallic; colour steel-grey; ductile, and very malleable. Yields a red solution with nitric acid; and is soluble in muriatic, but not in sulphuric acid, unless heated. Before the blowpipe per se it is infusible, but on the addition of sulphur it melts with ease; and when the heat is continued the sulphur is deposited, and a globule of malleable palladium remains. Isolated and rubbed, it acquires resinous electricity.
It is found intermixed with native platina in Brazil and Siberia; and, except that its texture appears more fibrous, it bears much resemblance to that substance.
NATIVE IRIDIUM.
Breihaupt.
Sp. Gr. 23·55. In minute grains, occasionally accompanying platina, at Nische-Tagilsk in Siberia.
ALLOY OF IRIDIUM† AND OSMIUM.
Wollaston.
Iridosmine, Necker. Rhombohedral Iridium, M. Iridium Osmié, H.
Berzelius maintains that there are three different combinations of iridium and osmium, in which 1 atom of the former is united with 1, 3, and 4 atoms of the latter.
| Iridium | 72·9 | 46·77 |
| Osmium | 24·5 | 49·34 |
| Rhodium | 0·0 | 3·15 |
| Iron | 2·6—Thomson. | 0·74—Berzelius. |
Sp. Gr. 18·25—19·5. H. above 4·5.
Primary form the rhomb. This natural alloy is rarely found crystallized; generally in small, irregular, and flattened grains, which have a shining metallic lustre, but are of a somewhat paler steel-grey colour than native platina, and are harder and
* Palladium; from the planet Pallas.
†Iridium; Iris, a rainbow; its solutions are variegated.
[page] 340
heavier; they possess a lamellar structure parallel to the terminal planes of the crystals, and are brittle. Is not soluble in nitro-muriatic or any other acid; and is infusible before the blowpipe, both alone and with fluxes. Fused with nitre, it emits a peculiar chlorine-like odour, and becomes black; but recovers its original colour and lustre by heating on charcoal.

It occurs along with platina, in the province of Choco in South America, and in the Ural Mountains of Siberia.
NATIVE TELLURIUM.*
Hexahedral Tellurium, J. Gediegen Sylvan, W. Gediegen Tellur, Haus. Tellure Natif Auro-Ferrifère, H.
Tellurium 92·55, iron 7·20, gold 0·25—Klaproth.
Sp. Gr. 6·1—6·2. H. = 2·0—2·5.
Colour tin-white, passing into lead-grey; with a shining metallic lustre. Primary form the rhomb; secondary, an hexagonal prism, with the terminal edges replaced by single planes; cleavage parallel to the faces of the primary, but, from the minuteness of the crystal, indistinct. Easily frangible.
It also occurs in crystalline grains, either aggregated, solitary, or disseminated; yields to the knife, and is brittle. Exposed to the blowpipe, it melts readily, burns with a greenish flame, and is almost entirely volatilized in a dense white vapour; it at the same time emits a pungent odour like that of horse-radish, which, however, is derived from a minute proportion of selenium in combination. Is soluble in muriatic acid.
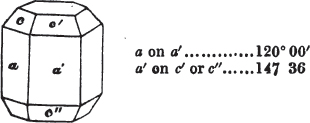
It has only been found in the mine of Maria Loretto, at Facebay near Zalathna in Transylvania, where it occurs in veins in sandstone, with iron pyrites and quartz. It is a scarce mineral.
* From the Latin, Tellus, the earth.
[page] 341
GRAPHIC TELLURIUM.
Graphic Gold. Schrifterz, W. Tellure Natif Auro-Argentifère, H. Silvanite, Necker. Sylvane Graphique, Brochant. Prismatic Antimony Glance, M.
Union of tellurium, gold, and silver.
| Tellurium | 60·0 | 61·35 | 52·0 |
| Gold | 30·0 | 28·36 | 24·0 |
| Silver | 10·0 | 10·29 | 11·3 |
| Lead | 0·0 | 0·00 | 1·5 |
| Klaproth. | Klaproth. | Berzelius. |
Berzelius also remarked small proportions of copper, iron, antimony, sulphur, and arsenic.
Sp. Gr. 5·7. H. = 1·5—2·0.
Of a steel-grey colour, approaching to tin-white, and is generally splendent, but sometimes slightly tarnished externally. Primary a right rhombic prism; the crystals are commonly modified on the edges and angles, are extremely indistinct, and generally minute. Cleavage perfect parallel to M; fracture uneven; yields easily to the knife, and is brittle. Before the blowpipe it fuses into a dark-grey metallic globule, covers the charcoal with white fumes, which at the reducing flame disappear, and, after a continued blast, is converted into a brilliant and malleable bead. Soluble in nitric acid.
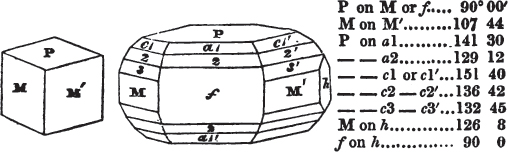
The German name of shrifterz was applied to this species by Werner, in allusion to the peculiar disposition of its crystals, which are frequently arranged in rows more or less resembling graphic delineations. Several different crystalline forms have been noticed, but the individuals being small, and in this manner disseminated and engaged, they have not yet been satisfactorily described. It occurs accompanying gold in narrow veins, which traverse porphyry, at Offenbanya in Transylvania; also at Nagyag in the same country. Its large proportion of gold renders this a highly valuable ore. (Manual.)
[page] 342
YELLOW TELLURIUM.
Weiss Sylvanerz, W. Weiss Tullur. Gelberz, L. Mullerine, Necker-Tellure Aurifère and Plombifère, H.
Union of tellurium, gold, and lead.
| Tellurium | 44·75 |
| Gold | 26·75 |
| Lead | 19·50 |
| Silver | 8·50 |
| Sulphur | 0·50—Klaproth. |
Sp. Gr. 8·9—10·67. Soft.
Silver-white, passing into brass-yellow. Primary a right rhombic prism of 105° 30′, and 74° 30′. Occurs in small but well-defined crystals; possesses a bright metallic lustre; and is somewhat sectile. Before the blowpipe it covers the charcoal with oxide of lead, melts into a white metallic globule, and emits a pungent odour. Soluble in nitric acid, leaving a yellow metallic residue.
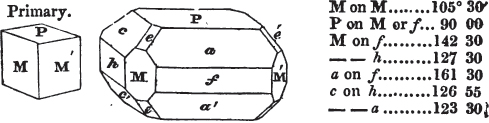
This very rare mineral occurs at Nagyag in Transylvania, in irregular veins in porphyry, with gold, native arsenic, sulphuret of manganese, and black tellurium; also in the Altai Mountains, Siberia.
BLACK TELLURIUM.
Nagyagererz, W. Tellure Natif Auro-Plombifère, H. Pyramidal Tellurium Glance, M. Blattertellur, Haus. Foliated Tellurium, A. Telluro-Galène, Necker. Elasmose, Beudant.
Combination of tellurium, lead, gold, and sulphur.
| Nagyag. | Nagyag. | Nagyag. | |
| Tellurium | 32·2 | 26·40 | 13·0 |
| Lead | 54·0 | 46·00 | 63·1 |
| Gold | 9·0 | 7·50 | 6·7 |
| Silver | 0·5 | 0·00 | 0·0 |
| Copper | 1·3 | 1·00 | 1·0 |
| Sulphur | 3·0 | 2·50 | 11·7 |
| Antimony | 0·0 | 0·00 | 4·5 |
| Klaproth. | Brandes. | Berthier. | |
| Sp. Gr. 7·0—7·2. | H. = 1·0—1·5. |
[page] 343
Colour between iron-black and dark lead-grey; is found crystallized in small and nearly tabular crystals, of which the primary form is a right square prism; cleavage perfect parallel with P; lustre metallic; yields easily to the knife, is sectile, and in thin laminæ highly flexible, though not elastic On paper it leaves slightly black traces; and, when isolated and rubbed, acquires resinous electricity. Before the blowpipe it melts, emitting a dense vapour, which partly concretes on the charcoal in the form of reddish-brown powder; and yields a malleable metallic globule. With borax it affords a bead of gold containing a little silver; and in nitro-muriatic acid is soluble without much difficulty, leaving a white residue.
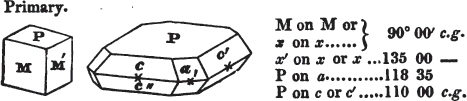
Occurs in foliated masses and crystalline plates, associated with gold, blende, and red manganese, at Nagyag in Translyvania, and accompanying antimony ores at Offenbanya in the same country.
NATIVE ANTIMONY.
Gediegen Spiesglas, W. Antimoine Natif, H. Br. Bt. Rhombohedral Antimony, M.
Contains about 98 per cent. of antimony, with minute proportions of silver, iron, arsenic, &c.
Sp. Gr. 6·5—6·8. H. = 3·0—3·5.
Of a tin-white colour, but by exposure becomes tarnished yellow. In nature it occurs reniform and amorphous, and in distinctly lamellar concretions, but has not been observed crystallized. The crystals, however, produced by fusion are readily recognised, and, being the identical substance, may be assumed as the same. The primary form of these is an obtuse rhomboid of 117° 15′ and 62° 45'. It possesses a highly perfect cleavage, with a splendent metallic lustre, parallel to o, and another, though with a minor degree of lustre, parallel to P.
[page] 344
Isolated and rubbed, it acquires resinous electricity. It yields to the knife, is somewhat sectile, and easily frangible. Before the blowpipe it fuses readily, and, by continuing the heat, may be entirely volatilized in the form of a grey vapour; but if the fused mass be allowed to cool slowly, it becomes covered with brilliant white acicular crystals. When alloyed with a small proportion of arsenic, the vapour has the odour of garlic. Is soluble in nitric acid, leaving a whitish deposit.
It occurs in veins traversing gneiss in Dauphiné, with the ores of antimony and cobalt; at Andreasberg in the Hartz; at Allemont near Grenoble in France; at Sahlberg in Sweden, in reniform masses disseminated in calcareous spar; also in Mexico; and in Connecticut with sulphuret of antimony. An arsenical variety is found at Allemont. It is frequently associated with antimonial silver, from which it may be distinguished by its comportment before the blowpipe; and is generally accompanied by antimonial ochre, which appears to be produced by its decomposition. From its property of hardening the softer metals, antimony is employed as an alloy, particularly with lead and tin; and in several pharmaceutical preparations.
BERTHIERITE.*
Haidinger.
| Contains Antimony | 52·0 |
| Sulphur | 30·3 |
| Iron | 16·0 |
| Zinc | 0·3—Berthier. |
Does not occur crystallized, but is found in masses confusedly lamellar, or composed of indistinct elongated prisms; cleavage parallel to the axis of the prism; colour dark steel-grey, inclining to pinchbeck-brown; lustre metallic. It fuses readily before the blowpipe, emits vapours of antimony, and forms a black slag which acts on the magnet. With fluxes it presents the indications of iron. It is soluble quickly in muriatic acid, with disengagement of sulphuretted hydrogen.
This species is found at Chazelles in Auvergne, associated with quartz, calcareous spar, and iron pyrites; when fused it yields antimony of such inferior quality that it is useless as an ore.
* Named by Haidinger in compliment to its discoverer, Professor Berthier of Paris.
[page] 345
SULPHURET OF ANTIMONY.
Prismatoidal Antimony Glance, M. Antimoine Gris, Brockant. Stilbine, Beudant. Grey Antimony. Grau Spiesglaserz, W. Antimoine Sulfuré, H.
Combination of sulphur and antimony.
| Antimony | 73·77 | 75·0 | 74·0 |
| Sulphur | 26·23 | 25·0 | 26·0 |
| Thomson. | Proust. | Bergmann. |
Sp. Gr. 4·3—4·6. H. = 2·0.
The massive presents a long columnar composition; and the fibrous variety occasionally exhibits a plumose, woolly, or feltlike appearance: this last is the federerz of German mineralogists—the antimoine sulfuré capillaire of Haüy. Colour light lead-grey, sometimes dull externally, often iridescent. Primary form a right rhombic prism of about 88° 30′ and 91° 30′. It occurs massive, disseminated, and crystallized in rhombic prisms, variously modified and terminated; the crystals are sometimes closely aggregated laterally; it yields readily to cleavage at right angles to the plane h of the following figure, with brilliant surfaces. Brittle; yielding to the pressure of the nail; soils paper black when rubbed on it; and emits on friction a sulphureous odour. In the flame of a candle it melts, even when in considerable masses; before the blowpipe it is absorbed by the charcoal, and gives off at the same time a sulphureous odour and white fumes.
It occurs principally in veins, which in some places, as at Wolfsthal in Hungary, are almost entirely composed of grey antimony. Felsobanya, Schemnitz, and Cremnitz in Hungary, are, however, the most celebrated localities of this species; being frequently found in these mines in distinct diverging prisms several inches in length, associated with and penetrating crystals of barytes and other minerals. It occurs also fibrous and laminated in Dumfriesshire; massive in Cornwall; and com-
[page] 346
pact particularly at Magurka in Hungary. The federerz is almost peculiar to Freybergand Braunsdorf in Saxony, and Stolberg in the Hartz.
P 2
This is the principal ore of antimony employed for commercial purposes, and it is prepared by simple fusion. It is used in the formation of several alloys, in the fabrication of types, and in medicine.
JAMESONITE.*
Azotomous Antimony Glance, M. Jamesonite, Haidinger.
| Cornwall. | ||
| Contains Antimony | 34·40 | 34·90 |
| Lead | 40·75 | 38·71 |
| Iron | 2·30 | 2·65 |
| Sulphur | 22·15—Rose | 22·53—Rose. |
and a little zinc and copper.
Sp. Gr. 5·5—5·8. H. = 2·0—2·5.
Primary form a right rhombic prism, whose lateral faces are inclined to one another at angles of 101° 20′ and 78° 40′; cleavage perfect perpendicular to the axis of the prism, less so parallel to it; colour steel-grey; lustre metallic; streak unchanged; sectile.
Jamesonite, like the preceding species, occurs both in acicular diverging crystals, and in fibrous masses of considerable dimensions. Its perfect cleavage perpendicular to the axis of the prism is sufficiently characteristic. It occurs principally in Cornwall, associated with quartz, and minute crystals of bournonite; occasionally also in Siberia; and disseminated in calcareous spar in Hungary.
PLAGIONITE.†
Rose.
Combination of antimony 37·94, lead 40·52, and sulphur 21·53.
Occurs crystallized in oblique four-sided prisms, occupying the drusy cavities of the matrix at Wolfsberg in the Hartz. Berzelius doubts this combination, although it appears to be extremely similar to that of the preceding species.
* In honour of Professor Jameson of Edinburgh.
† From the Greek, πλαγιος, oblique, from the form of its crystals.
[page] 347
ZINKENITE.
Rose.
| Wolfsberg. | |
| Contains Antimony | 44·11 |
| Lead | 31·97 |
| Sulphur | 22·58 |
| Copper | 0·42—Rose. |
Sp. Gr. 5·3—5·35. H. = 3·0—3·5.
Occurs in regular six-sided prisms, terminated by flat six-sided pyramids; the faces of the prism inclined to one another at an angle of 120°, those of the pyramid to the corresponding faces of the prism at 102° 42'. The faces of the prism are usually striated deeply in a longitudinal direction, those of the pyramid, though not furrowed, are uneven. Colour steel-grey; lustre bright metallic; streak corresponding to the colour; fracture uneven; no traces of cleavage. It is soluble in nitric acid, yielding an immediate precipitate of white antimony. When heated alone on charcoal it decrepitates briskly, and melts as readily as grey antimony; small metallic globules are formed, which are entirely volatile on the blast being continued, while the charcoal is covered with a white coating of oxide of lead. With soda it yields globules of metallic lead.
Zinkenite occurs in the antimony mine of Wolfsberg near Stolberg in the Hartz, and was named by its original discoverer, Dr Gustavus Rose, in honour of his friend M. Zinken, the director of the Anhalt mines. It much resembles both grey antimony and bournonite in colour and fracture, but may be distinguished from them by its superior hardness and specific gravity. Its crystals are aggregated in groups, which present a columnar composition, and occur on a massive variety of the same species in quartz. Their length often exceeds half an inch, their breadth two or three lines; but frequently they are extremely thin, and form fibrous masses. (manual.)
RED ANTIMONY.
Rothspiesglaserz, W. Antimoine Oxydé Sulfué, H. Antimoine Rouge, Br. Antimon Blende, L. Prismatic Purple Blende, M.
Combination of the protoxide and sulphuret of antimony.
| Braunsdorf. | ||
| Antimony | 74·45 | 67·5 |
| Oxygen | 4·27 | 10·8 |
| Sulphur | 20·47—Rose. | 19·7—Klaproth. |
Sp Gr. 4·5—4·6. H. = 1·0—1·5.
Primary form an oblique rhombic prism, whose base, ac-
[page] 348
cording to Mohs, is inclined to its axis at an angle of 101° 19′. Secondary form, the primary having its edges replaced. Cleavage highly perfect parallel to both sides of the primary prism. Surface striated longitudinally; lustre adamantine; feebly translucent, streak brownish-red; fuses easily on charcoal, by which it is absorbed, and is at last entirely volatilized. When immersed in nitric acid, it becomes covered with a white coating. The capillary variety, in which the individuals are so interlaced as to present flakes resembling tinder, is distinguished by the German mineralogists, under the name of Zundererz or Tinder Ore,
By reflected light of a cherry-red, by transmitted light of a crimson colour, but commonly tarnished externally with a brownish or bluish tinge, or is iridescent. It forms very fine diverging or interlaced acicular crystals; has a shining lustre, is translucent, and brittle.
Red antimony occurs in veins with quartz, accompanying grey and white antimony, at Malazka near Posing in Hungary; at Braunsdorf near Freyberg in Saxony; and at Allemont in Dauphiné. The principal localities of tinder ore, are Clausthal and Andreasberg in the Hartz.
OXIDE OF ANTIMONY.
White Antimony. Weiss Spiesglaserz, W. Antimoine Oxydé, H. Antimoine Blanc, Br. Prismatic Antimony Baryte, M. Prismatic White Antimony, J. Spiessglanzweiss, Haus.
Combination of oxygen and antimony. When pure it consists of antimony 84·32, oxygen 15·68—Berzelius.
Sp. Gr. 5·5—5·6. H. = 2·5—3·0.
Colour snow-white, yellow, or grey, sometimes peach-blossom red. Primary form a right rhombic prism of 137° 43' and 42° 17'. Generally in tabular and acicular crystals, in diverging groups; more rarely massive. Principal cleavage highly perfect parallel to the lesser diagonal of the prism; lustre between pearly and adamantine; translucent; streak white. It melts very easily before the blowpipe, and is volatilized in the form of a white vapour. With borax it forms a glass which appears yellowish while hot, but becomes almost colourless on cooling. Soluble in nitromuriatic acid.
Beautiful varieties of aggregated tabular crystals occur with other ores of antimony at Przibram in Bohemia; the acicular variety is found at Braunsdorf in Saxony, Malazka in Hungary, and at Allemont in Dauphiné.
[page] 349
ANTIMONIAL OCHRE.
Spiessglanz Ochre, W. Antimoine Oxydé Terreux, H. Antimonoker, L. Stibiconise, Beudant,
Combination of oxygen, antimony, and water.
Sp. Gr. 3·7—3·8.
Occurs in earthy masses of a yellow, grey, or brownish colour. Dull; soft and friable; streak grey or yellowish-white. Upon charcoal it does not fuse, but forms a slight antimonial sublimation; and yields water in the matrass. With borax or salt of phosphorus it comports itself like oxide of antimony; with soda is reduced.
This substance is found associated with the sulphuret and other ores of antimony at Bruck in Rhenish Prussia; in Nassau; in the Erzgebirge of Saxony; and in Gallicia in Spain, where prisms of the sulphuret are frequently observed partly changed into antimonial ochre.
ANTIMONPHYLLITE.
Breithaupt.
Sp. Gr. 4·025. H. = 1·0—1·5.
Crystallized in thin unequiangular six-sided prisms, of a greyish-white colour; lustre pearly, inclining to adamantine; translucent; sectile; and, when in thin laminæ, flexible like talc. Contains oxide of antimony, a copious precipitate of which is thrown down from its solution in muriatic acid by water. Specimens of this mineral are preserved in the collections of Dresden and Halle, but their locality is unknown.
NATIVE LEAD.
Lead is described as occurring in the metallic state, in small masses, in the lavas of the island of Madeira and other volcanic districts, forming the native lead of some mineralogists. But it is still a very problematical mineral.
SULPHURET OF LEAD.
Galena. Bleiglanz,W. Plomb Sulfuré H. Galène,Bt. Lead Glance, J. Hexabedral Lead Glance, M.
Bi-sulphuret of lead.
| Durham. | |||
| Contains Lead | 79·6 | 83·00 | 85·13 |
| Sulphur | 13·4 | 16·41 | 13·02 |
| Silver | 7·0 | traces | 0·00 |
| Beudant. | Westrumb. | Thomson. |
Sp. Gr. 7·4—7·6. H. = 2·7.
[page] 350
Silver is very frequently found mixed with galena, and in extremely variable proportions; its presence, however, which can only be ascertained by cupellation, does not influence either the physical or external characters of the species in any way. Externally of a lead-grey colour, occasionally blackish-grey; sometimes irisated superficially. Primary form the cube. It occurs crystallized in the cube and regular octahedron, and in some of their varieties; structure lamellar; cleavage parallel with the planes of the cube, highly perfect and easily obtained; the fractured surfaces possess a brilliant metallic lustre. It also occurs in amorphous masses, possessing a straight or curved lamellar structure; frequently granular, consisting of small crystalline plates irregularly disposed in regard to one another; and sometimes almost compact, yielding a flat conchoidal fracture, and presenting little lustre. A beautiful iridescent tarnish is frequently observable, which is confined however (as in some other minerals) to the secondary forms; the faces of the octahedron appearing iridescent, while those of the cube are not.
Before the blowpipe it first decrepitates, but when heated with precaution it melts, and yields, after the sulphur has been driven off, a globule of metallic lead. It is partly soluble in nitric acid, and leaves a white residue.
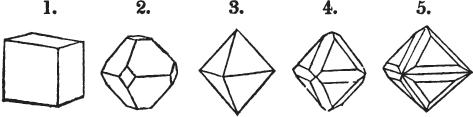
Fig. 1, the primary; a cube. Fig. 2, the same, of which the solid angles are replaced by triangular planes, forming the passage into the regular octahedron, fig. 3, in which these planes are complete. Fig. 4, the octahedron, having the edges replaced. Fig. 5: in this each edge of the octahedron is bevelled, or replaced by two planes.
Galena is a mineral of very frequent occurrence, forming veins and beds both in primary and secondary rocks. Veins in gneiss
[page] 351
are its repositories at Freyberg in Saxony; veins in primitive limestone at Sala in Sweden; and veins in clay-slate at Clausthal and Neudorf in the Hartz, Przibram in Bohemia, and elsewhere. The grauwacke at Leadhills and the killas of Cornwall are equally interspersed with veins of galena; and the rich repositories of Derbyshire, Cumberland, and the northern districts of England, as well as those of Bleiberg, and the neighbouring localities in Carinthia, are contained in transition or mountain limestone. The general forms of its crystals are the cube and octahedron, with various intervening modifications. Individuals of very large dimensions have been obtained at Dufton and Alston Moor in Cumberland; at Pfaffenberg near Neudorf in the Hartz; in Transylvania and Saxony. Leadhills is its principal Scottish locality, though it has been noticed also in large octahedral crystals near Inverkeithing in Fifeshire, at East Calder, the Isle of Isla, and elsewhere. It is associated in the English localities with calcareous and fluor spars, with blende, calamine, barytes, witherite, and pearl spar; in Greenland with cryolite and sparry iron.
The compact variety chiefly occurs at Freyberg in Saxony, in the Hartz, Carinthia, and at Leadhills. (Manual.)
American localities of galena are extremely numerous, although there are few valuable mining deposits of this species in the western continent.
Specular Galena. Plomb sulfuré speculaire, H. consists of an extremely thin coating of lead on quartz or some other substance, and exhibits an appearance of polish, and a lustre, from which the name of Slickenside, or looking-glass lead ore, has been derived. It is found principally in the mines of Derbyshire.
Blue Lead. Blau Bleierz, W. Plomb sulfuré prismatique epigène, H. Plomb bleu, Br. Plomb noir, Bt. This is evidently pseudomorphous of phosphate of lead. It occurs massive, likewise in six-sided prisms of a colour between lead-grey and indigo-blue, which sometimes are narrower near the terminations than across the middle, and which are superficially dull and rough; the fracture is even, or flat conchoidal, with a glimmering metallic lustre; it is soft, somewhat sectile, and easily frangible. Specific gravity 5·4. It has been found at Zschoppau in Saxony; at Huelgöet near Poullaouen in France, accompanying carbonates of lead and copper; and in the mine of Huel Hope in Cornwall. The prisms internally consist of fibrous galena, occasionally mixed with a translucent substance, of a rich brown colour by transmitted light, and greatly resembling some varieties of phosphate of lead; or they consist almost wholly of this substance, the surface only appearing to have passed into the sulphuret; other specimens consist of remarkably compact galena, and they all bear the external appearance of the ordinary sulphuret of lead.
[page] 352
Galena is distinguished from plumbago by its weight, and by its not affording distinct traces on paper; from sulphuret of molybdena also by its structure, which is never foliated; and from the brilliant metallic varieties of blende, by the surfaces of its crystals resuming their lustre instantly when breathed upon, while those of blende remain dull for some time.
The Sulphuret of Lead and Antimony, and the Sulphuret of Lead, Antimony, and Silver, may be classed with this species; the difference in their chemical composition being insufficient to distinguish them otherwise than as varieties.
Supersulphuret of Lead is earthy, of a bluish-grey colour, and so highly inflammable as to take fire and burn on being held in the flame of a candle. It occurs in the Dufton lead mines.
The lead mines of Great Britain produce annually from 45,000 to 48,000 tons of smelted lead, which is principally obtained from the sulphuret.
BOURNONITE.*
Triple Sulphuret. Endellione, Bournon. Schwarz Spiessglaserz, W. Spiesglanzbleierz, Klapr. Bleifahlerz, Haus. Plombe Sulfuré Antimonitere (in part), H. Diprismatic Copper Glance, M.
Combination of sulphuret of lead, sulphuret of copper, and sulphuret or antimony.
| Clausthal. | Hartz. | Pfaffenberg. | Cornwall | ||
| Lead | 42·50 | 34·50 | 40·84 | 41·0 | 42·62 |
| Sulphur | 18·00 | 13·50 | 20·31 | 20·0 | 17·00 |
| Antimony | 19·75 | 16·00 | 26·28 | 25·0 | 24·23. |
| Copper | 11·75 | 16·25 | 12·65 | 13·0 | 12·80 |
| Iron | 5·00 | 13·75 | 0·00 | 0·0 | 1·20 |
| Silver | 0·00 | 2·25 | 0·00 | 0·0 | 0·00 |
| Klaproth. | Rose. | Smithson. | Hatchett. |
Sp. Gr. 5·79—5·83. H. = 2·5—3·0.
Colour approaching to steel-grey, with a shining lustre; but occasionally the crystals appear of a dull lead-grey, with a tinge of black. Primary form a right rectangular prism. It occurs crystallized in this form, variously modified; structure lamellar, affording cleavage planes parallel to the lateral faces of the primary and both its diagonals; fracture uneven or flat conchoidal, with a brilliant metallic lustre; it is very brittle, and yields to the pressure of the nail. Before the blowpipe it decrepitates, then melts, emitting a white sulphureous vapour, after which
[page] 353
* Bournonite, in honour of the Comte de Bournon, who first described this mineral, and who gave it the name of Endellione, from the parish in Cornwall in which it was found.
there remains a crust of sulphuret of lead, enclosing a globule of copper. Readily soluble in heated nitric acid.
Fig. 1, a rectangular prism, of which the lateral edges are replaced, converting the crystal into an eight-sided prism. In fig. 2, two opposite edges of each terminal plane are replaced by planes inclining on the terminal planes, so as to reduce them greatly. Fig. 3, a macle, in which two crystals similar to fig. 2, but elongated, cross each ether.
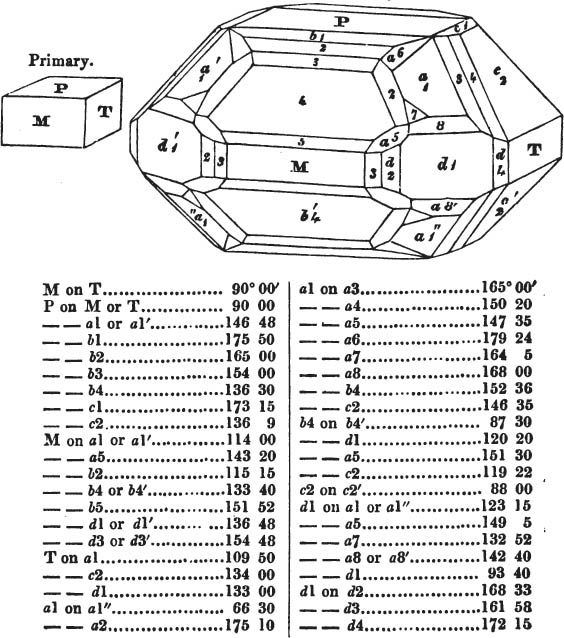
[page] 354
The most magnificent crystals of bournonite are found in the mines of Neudorf in the Hartz, where they occasionally exceed an inch in diameter. It occurs accompanying quartz, fahlerz, and phosphorescent blende, at Kapnik in Transylvania, in compressed crystals, which, from their peculiar macled arrangement, produce the variety termed in German radelerz or wheel-ore; also with pearl spar and quartz, at a mine near Servos in Piedmont; at Braunsdorf and Gersdorf in Saxony; at Clausthal and Andreasberg in the Hartz; in some of the gold mines of Hungary and Transylvania; in Peru; and in Cornwall.
PRISMATOIDAL COPPER GLANCE.
Prismatoidal Copper Glance, M. Cuivre Sulfuré Prismatoīde, Necker.
Contains lead 29·90, sulphur 8·60, antimony 16·65, arsenic 6·04, copper 17·35, iron 1·40—Schrotter.
Sp. Gr. 5·7—5·8. H. = 2·0—3·0.
Primary form a right rhombic prism, cleavable parallel to the axis in the direction of the small diagonal of the base. It is generally somewhat decomposed, externally coated with oxide of iron, and, when fresh fractured, presents a blackish lead-grey colour. It occurs with carbonate of iron at St Gertraud, near Wolfsberg in Carinthia.
NATIVE MINIUM.
Native Minium, Smithson. Plombe Oxydé Rouge, H.
Colour aurora-red. It occurs amorphous and pulverulent, but when closely examined exhibits a crystalline structure. Before the blowpipe on charcoal it is first converted into litharge, and then into metallic lead. It is supposed to be an oxide of lead, and to arise from the decomposition of galena, in veins of which it commonly occurs.
It is found in Grassington Moor in Craven, and at Grasshill Chapel, in Weirdale, Yorkshire. On the continent, near Badenweiler; and in Siberia.
SELENIURET OF LEAD.
Selenblei. Plomb Seleniuré, Levy. Clausthalie, Beudant.
| Lead | 70·98 | 63·92 |
| Selenium | 28·11 | 31·42 |
| Cobalt | 0·83—Turner. | 3·14—Rose. |
Sp. Gr. 8·2—8·8, Haidinger; 6·7—6·8, Silliman.
Crystalline form unknown. Colour lead-grey inclining to
[page] 355
bluish; lustre metallic; cleavage indistinct; fracture granular and shining. Bears considerable resemblance to fine granular galena. Before the blowpipe on charcoal it is quickly decomposed, and affords, besides the usual phenomena arising from the presence of lead, the odour of decayed horse-radish, a brownish matter being at the same time deposited on the charcoal; heated over the spirit-lamp in a glass tube closed at one extremity, the selenium almost instantly sublimes, and forms a red ring within the tube, at the open extremity of which its peculiar odour is very perceptible. It is a rare substance, occurring only in the massive state in veins of hematite, near Clausthal, and Tilkerode in the Hartz, sometimes with particles of native gold.
At the latter locality the following compounds have likewise been met with.
1. SELENIURET OF LEAD AND COPPER.
| Lead | 59·67 | 47·43 |
| Copper | 7·86 | 15·45 |
| Selenium | 29·76 | 34·26 |
| Iron | 0·77 | 0·00 |
| Silver | 0·00—Rose. | 1·29—Rose. |
Sp. Gr. 7·0.
Occurs in amorphous masses of a lead-grey colour. Is ductile and sectile. Fuses readily before the blowpipe, yielding oxide of lead, and reddish metallic grains. Acted upon by nitric acid.
2. SELENIURET OF LEAD AND COBALT.
| Lead | 63·92 | 70·98 |
| Cobalt | 3·14 | 0·83 |
| Selenium | 31·42 | 28·11 |
| Iron | 0·45—Rose. | 0·00—Stromeyer. |
Sp. Gr. 7·697.
Has much the aspect of seleniuret of lead. Gives off in the closed tube a sublimation of selenium, and exhibits with the fluxes the re-action of cobalt, by colouring them blue.
3. SELENIURET OF LEAD AND MERCURY.
| Lead | 55·84 | 27·33 |
| Mercury | 16·94 | 44·69 |
| Selenium | 24·97—Rose. | 27·98—Rose. |
Sp. Gr. 7·8—7·87.
Exhibits a very distinct cubical cleavage. In the matrass yields a crystalline sublimation of the seleniuret of mercury.
[page] 356
PLOMBGOMME.
Hydrous Aluminate of Lead, Smithson, Plomb Hydro-Alumineux, H.
Combination of the oxide of lead, alumina, and water.
| Huelgoet. | |
| Oxide of lead | 40·14 |
| Alumina | 37·00 |
| Water | 18·80 |
| Sulphuric acid | 0·20 |
| Lime, oxides of iron and manganese | 1·80 |
| Silica | 0·60—Berzelius. |
Sp. Gr. 6·425. H. = 4·0—5·0.
This mineral is of a yellow colour, sometimes tinged with brown. It occurs in small reniform masses, composed of many concentric spherical layers, which are externally splendent, often resembling mammillated chalcedony, sometimes possessing a degree of pearly lustre on their inner surfaces, and occasionally irisated. The concentric layers, when broken across, are without splendour, and rarely present slight appearances of a radiated texture, but are without any regular crystalline structure. Fracture conchoidal; translucent. When suddenly heated it decrepitates violently; but when approached with caution it becomes white and opake, although it does not fuse. With borax it forms a colourless transparent glass, but without reducing the lead, which, however, is effected on the addition of soda. It acquires negative electricity by friction.
It is found only at Huelgoet near Poullaouen in Brittany, associated in clay-slate with galena, blende, and iron pyrites. There are certain varieties of mammillated blende to which it bears much resemblance.
CARBONATE OF LEAD.
Diprismatic Lead Baryte, M. Weiss Bleierz, W. Plombe Carbonaté, H. Ceruse, Beudant. Kohlensaures Blei, L. White Lead Ore, J.
Combination of carbonic acid, and protoxide of lead.
| Leadhills. | Zellerfeld. | Nertschinsk. | |
| Carbonic acid | 16·0 | 16·0 | 15·5 |
| Protoxide of lead | 82·0 | 81·2 | 84·5 |
| Lime | 0·0 | 0·9 | 0·0 |
| Oxide of iron | 0·0 | 0·3 | 0·0 |
| Klaproth. | Westrumb. | John. |
Sp. Gr. 6·3—6·6. H. = 3·0—3·5.
Primary form a right rhombic prism. Either colourless or white, passing into grey and greyish-black; tinged also green
[page] 357
and blue by admixture with ores of copper. It occurs in tabular crystals, in six-sided prisms variously terminated, and in other macled crystals of different forms. It cleaves parallel to the planes P, M, and M′ of the following figures, but not distinctly, being frequently interrupted by conchoidal fracture; the lustre of the planes produced by cleavage is somewhat adamantine; the fracture small conchoidal, with a resinous lustre; transparent or translucent; when transparent it is doubly refractive in a high degree; very brittle. It also occurs massive. Its powder thrown upon live coal emits a phosphorescent light. Before the blowpipe it decrepitates, becomes yellow, then red, and is immediately reduced to the metallic state, the charcoal being covered with the yellow fumes of lead; with the fluxes it forms a diaphanous glass. It effervesces in dilute muriatic acid, especially if warm.
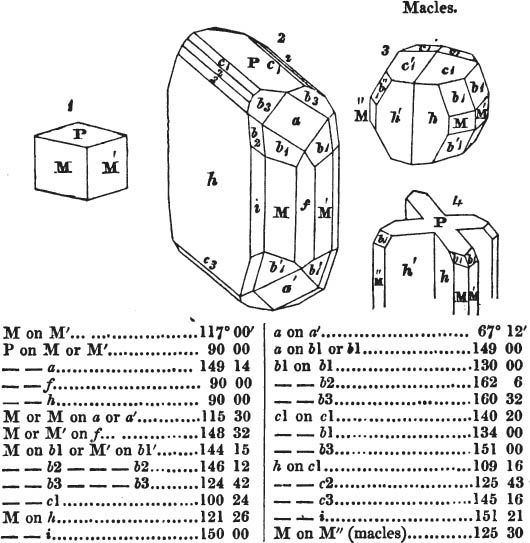
There are few substances whose crystallizations are more complex than the carbonate of lead. The circumstance, too, of its crystals being usually macled, in general small, and the number of their facets very numerous, accounts for its having long puzzled mineralogists.
[page] 358
Leadhills and Wanlockhead are well known as the Scotch localities of this mineral; it there occurs with other ores of lead, particularly the phosphate, sulphate, sulphato-tri-carbonate, and cupreous-sulphate, accompanying galena in transition-slate. Very beautiful crystals are found in the mining districts of Saxony, particularly at Johanngeorgenstadt; at Nertschinsk and Beresof in Siberia, near Bonn on the Rhine, at Clausthal in the Hartz, at Tarnowitz in Silesia, at Bleiberg in Carinthia, and at Mies and Przibram in Bohemia. In England it has also been met with at Alston Moor, at Keswick, and in Cornwall, where, particularly at the mine of St Minvers, it occurs in snow-white and easily frangible acicular crystals, so delicate as almost to preclude the possibility of transport. (Manual.)
Earthy Carbonate of Lead. Bleierde, W. Plomb carbonate terreux, H. Indurated and friable earthy lead-ore, J. Colour grey, occasionally tinged green, yellow, or red, also reddish-brown; massive, disseminated and pulverulent; commonly dull and opake, sometimes friable, soft, and heavy. It occurs in several European countries, commonly associated with the preceding.
SULPHATO-CARBONATE OF LEAD.
Prismatoidal Lead Baryte, Haid. Schwefel und Kohlensaures Blei of the Germans, Lanarkite, Beudant. Dyoxylite, Shepard.
Combination of carbonic acid, sulphuric acid, and oxide of lead.
Carbonate of lead 46·9, sulphate of lead 53·1—Brooke.
Sp. Gr. 6·8—7·0. H. = 2·5.
Colour greenish-white, pale yellow, or grey. Primary form a right rhombic prism of 59° 15′ and 120° 45′. The crystals are seldom distinct, always minute and aggregated lengthways, presenting a character approaching to fibrous. Cleavage perfect and easily obtained parallel to a plane which replaces the acute lateral edges of the primary; the laminæ resulting from cleavage are flexible, like gypsum; lustre adamantine; streak white; translucent.
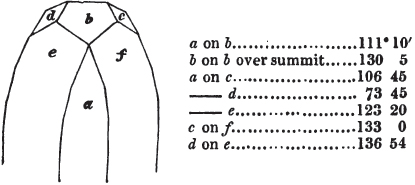
[page] 359
It is soluble in nitric acid without perceptibly effervescing, leaving a residue of the sulphate of lead; and before the blowpipe on charcoal fuses into a globule which is white when cold, and is nearly reduced to metallic lead.
It occurs among other species of lead ore at Leadhills in Scotland.
SULPHATO-TRI-CARBONATE OF LEAD.
Axotomous Lead Baryte, M. Rhombohedrischer Schwefel Kohlensaures Blei, Leonhard. Leadhillite, Beudant.
Combination of carbonic acid, sulphuric acid, and oxide of lead.
| Carbonate of lead | 72·5 | 72·7 | 71·1 |
| Sulphate of lead | 27·5 | 27·3 | 30·0 |
| Brooke. | Stromeyer. | Berzelius. |
Sp. Gr. 6·2—6·4. H. = 2·5.
Colour white, passing into pale yellow, green, or grey. The primary form of the crystals of this species used to be considered an acute rhomb of 72° 30′ and 107° 30′, but the optical investigations of Sir David Brewster, who ascertained their two axes of double refraction, and the crystallographic researches of Haidinger, determined it to be an oblique rhombic prism of 120° 20′ and 59° 40′. The crystals seldom exceed an inch in diameter, generally they are much smaller, and when macled, as is not unfrequently the case, they present forms which are with difficulty determinable. Cleavage perfect and easily obtained parallel to a; translucent; streak white; lustre resinous, inclining to adamantine; pearly on the face a, which is one of the most distinguishing characteristics of the species. Before the blowpipe it intumesces and becomes yellow, but re-assumes its white colour on cooling. It effervesces briskly in nitric acid, leaving a white residue of sulphate of lead.

This substance also occurs with other ores of lead at Lead-hills, Scotland.
[page] 360
CUPREOUS SULPHATO CARBONATE OF LEAD.
Paratomous Lead Baryte, Haid. Schwefel und Kohlensaures Blei und Kupfer of the Germans. Calcedonite, Beudant. Cupreous Sulphato-Carbonate of Lead, Brooke.
Combination of carbonic acid, sulphuric acid, oxide of lead, and oxide of copper. Carbonate of lead 32·8, carbonate of copper 11·4, sulphate of lead 55·8—Brooke.
Sp. Gr. 6·4. H. = 2·5—3·0.
Colour bright verdigris-green, or bluish. Primary form a right rhombic prism of 95° and 85′, parallel to the planes of which it cleaves indistinctly. Sometimes its crystals are large and well-defined, at others it appears in small tufts radiating from their common point of attachment. Translucent; streak greenish-white; lustre resinous; rather brittle. Before the blowpipe on charcoal it is reduced. Soluble with feeble effervescence in nitric acid, leaving a residue of sulphate of lead.
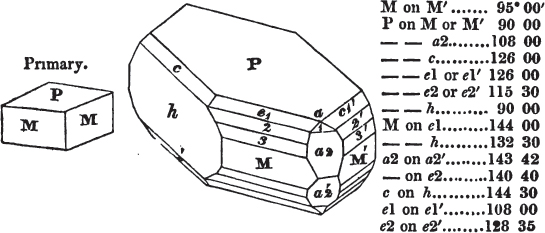
The above figure and measurements by Brooke.
It is found, with the two preceding varieties of lead-ore, at Leadhills in Scotland.
MURIATE OF LEAD.
Berzelite, Levy. Peritomous Lead Baryte, M. Saltsaures Blei von Mendip of the Germans.
Composition not exactly determined, it being difficult to ascertain whether the carbonate of lead, which is in small proportions in this species, is combined or only mixed with it. Berzelius supposes it to be a combination of one atom of chloride with two atoms of the oxide of lead, mixed with carbonate of lead.
[page] 361
| Mendip. | |
| Chloride of lead | 34·63 |
| Oxide of lead | 55·82 |
| Carbonate of lead | 7·55 |
| Silica | 1·46 |
| Water | 0·54—Berzelius. |
Sp. Gr. 7·0—7·1. H. = 2·5—3·0.
Occurs in crystalline masses having a fibrous and radiated columnar structure. Primary form a right rhombic prism of 102° 27′ and 77° 33′, parallel to all the faces of which it cleaves with facility. Colour white, with a yellow or reddish tinge; feebly translucent or opake; and presenting a pearly lustre on the faces of cleavage. Fracture conchoidal or uneven. Before the blowpipe on charcoal it is reduced, and emits fumes of muriatic acid; and in a mixture of salt of phosphorus and peroxide of copper the flame assumes an intense blue colour. Is soluble with slight effervescence in dilute nitric acid.
Churchill in the Mendip Hills of Somersetshire is the principal locality of this rare species; it is there found disposed on earthy black manganese. It is said to occur as a product of sublimation upon the lava of Vesuvius; but from that locality the specimens are so indistinct as to render its identity doubtful.
COTUNNITE.
Cotunnia, Monticelli and Covelli. Cotunnite, Kobell.
| Contains Lead | 74·52 |
| Muriatic acid | 25·48—Berzelius. |
Sp. Gr. 1·897. Slightly scratched by the nail.
In extremely minute acicular crystals of a white colour. Lustre adamantine, occasionally silky, or pearly. Fuses with facility before the blowpipe, colouring the flame blue, and emitting a white smoke, which is condensed on the charcoal; with soda globules of reduced lead are formed; in the matrass it fuses and is sublimated; and in about twenty-seven times its weight of cold water is entirely dissolved.
This substance was observed by Monticelli and Covelli in the crater of Vesuvius, after the eruption of 1822; it was accompanied with muriate of soda, muriate and sulphate of copper, and other salts. It is named in compliment to one of the medical men of Naples. (Manual.)
Q
[page] 362
MURIO-CARBONATE OF LEAD.
Hornblei, W. Plomb Corné, Br. Plomb Muriaté, Bt. Corneous Lead-Ore, J. Blei-Hornerz, Leonhard. Plomb Murio-Carbonaté, Levy. Brachytypous Lead Baryte, M. Kerasine, Beudant.
This mineral appears to be muriate of lead mechanically mixed with carbonate of lead.
| Matlock. | |
| Oxide of lead | 85·5 |
| Muriatic acid | 8·5 |
| Carbonic acid | 6·0—Klaproth. |
Sp. Gr. 6·0—6·1. H. = 3·0.
Colour white, greyish, or yellow. Primary form a rectangular four-sided prism; in which it also occurs either perfect, or having the lateral and also the terminal edges replaced, cleaves parallel to all the faces of the prism; structure lamellar; fracture conchoidal, with a splendent adamantine lustre; transparent or translucent; sectile, and easily frangible. Before the blowpipe on charcoal it fuses into a transparent globule, which becomes pale-yellow on cooling. With salt of phosphorus mixed with deut-oxide of copper it colours the flame green or bluish-green.
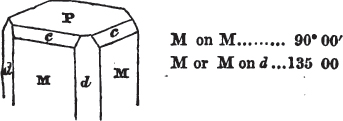
The above from a crystal in the British Museum, by Brooke.
The finest crystals of this species have been obtained in Cromford Level, near Matlock in Derbyshire, with carbonate and sulphuret of lead and fluor. Of these some splendid specimens, exceeding an inch in length, are preserved in the British Museum. It is described also as occurring at Badenweiller in Germany; and crystallized and of a green colour on galena in Southampton, Massachusetts.
PHOSPHATE OF LEAD.
Rhombohedral Lead Baryte (in part), M. Phosphorsaures Blei, Leonhard. Pyromorphite, Beudant. Grün Bleierz, W. Plomb Phosphaté, H.
Combination of protoxide of lead, phosphoric acid, and muriatic acid.
| Huelgoet. | Zschopau. | |
| Oxide of lead | 78·58 | 82·29 |
| Phosphoric acid | 19·73 | 15·73 |
| Muriatic acid | 1·65—Klaproth. | 1·98—Wöhler. |
Sp. Gr. 6·9—7·0. H. = 3·5—4·0.
[page] 363
It is of various shades of green, yellowish-green, yellow, ash-grey, and brown. Primary form the regular six-sided prism, in which it also occurs crystallized, generally, however, modified on the edges; traces of cleavage are visible parallel to all the faces of the prism c c′ c″ replacing its terminal edges, thereby affording cleavages parallel to the planes of a six-sided pyramid; it also occurs botryoidal, reniform, and massive, and often barrel-shaped, or contracted at the ends of the prisms. Fracture imperfect conchoidal and dull. Surface of M always striated horizontally; P rough and often indented; streak white or yellow; semi-transparent to translucent on the edges; lustre resinous; easily frangible, but less so than sulphate or carbonate of lead. Before the blowpipe on charcoal it melts in the outer flame into a globule, which crystallizes on cooling, and becomes brown; in the reducing flame the globule appears bluish, is luminous while hot, and on cooling crystallizes with large facets of a lighter colour, approaching the aspect of mother-of-pearl. It is acted upon by nitric acid.
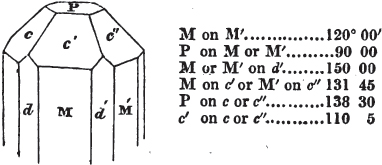
It occurs with galena in primitive and secondary rocks. Finely crystallized specimens are found at Zschopau and other places in Saxony; at Przibram and Mies in Bohemia; at Badenweiller in Baden; in Cornwall; at the Leadhills in Scotland; and in Siberia. The brown varieties occur principally at Poullaouen and Huelgoet in Brittany, at Wanlockhead in Scotland, and at Bleistadt in Bohemia.
POLYSPHARITE.
Breithaupt.
Sp. Gr. 5·83—5·89.
In roundish masses, having internally a radiated structure; colour brown or yellow; lustre greasy; fracture conchoidal. It scratches mica, but is scratched by fluor spar. Contains oxide of lead, phosphoric acid, and magnesia. From the mines of Freyberg in Saxony, where it accompanies blende, galena, quartz, and iron pyrites. (Manual.)
[page] 364
ARSENIATE OF LEAD.
Plomb Arseniaté, H. Rhombohedral Lead Baryte, M. Mimetèse Beudant. Arseniksaures Blei, Leonhard.
Combination of arsenic acid, muriatic acid, and oxide of lead.
| Johanngeorgenstadt. | Cornwall. | ||
| Oxide of lead | 75·59 | 77·50 | 69·76 |
| Phosphoric acid | 1·32 | 7·50 | 0·00 |
| Arsenic acid | 21·20 | 12·50 | 26·40 |
| Muriatic acid | 1·89 | 1·50 | 1·58 |
| Wöhler. | Rose. | Gregor. | |
Sp. Gr. 6·9—7·3. H. = 3·5—4·0.
Colour various shades of yellow, passing into hyacinth- and aurora-red—frequently very brilliant. Primary form the regular six-sided prism, in which it likewise occurs either perfect or having the terminal edges replaced; also mammillated, reniform, and compact. The structure of the crystals is lamellar, yielding indistinctly to cleavage parallel to the planes of the prism; it is translucent, rarely transparent; external lustre of the crystal resinous; easily frangible; fracture imperfect conchoidal, or uneven. Before the blowpipe on charcoal it fuses with difficulty, emits arsenical vapours, and is reduced to globules of metallic lead.

The largest crystals of this species have been found at Johanngeorgenstadt in Saxony; but at that locality they are now rare. Latterly it has occurred in beautiful translucent yellow crystals, disposed on quartz at Huel Alfred in Cornwall; and at Caldbeck Fell in Cumberland, aggregated in opake, orange-yellow coloured individuals, which consist each of three hexagonal prisms curved towards their terminations in a manner often beautifully symmetrical. The varieties from Leadhills are more remarkable for the richness of their colours than the beauty of their crystalline forms, being generally aggregated, grouped in rosettes, forming superficial coatings. and otherwise indistinctly defined. The orange phosphate from this locality has been ascertained by the Rev. W. Vernon to contain about one per cent. of the chromate of lead, to which admixture he attributes the splendid tinges of that variety. (Manual.)
[page] 365
Mammillated arseniate of lead occurs at Huelgoet; the reniform and orbicular varieties are met with at the Puy de Dome in Auvergne, and in the Grand Duchy of Baden; while the filamentous or capillary kind is found at St Prix in the department of the Saone in France.
The Hedyphan of Breithaupt, described as a white, shining, massive mineral, having a specific gravity equal to 5·404, and containing oxide of lead 52·95, muriatic acid 2·03, arsenic acid 22·78, phosphoric acid 6·20, and lime 14·03—Kersten, from Langbanshyttan in Sweden, is evidently a variety of this species.
SULPHATE OF LEAD.
Blei Vitriol, W. Plomb Sulfaté, H. Bt. Vitriol de Plomb Natif, Br. Prismatic Lead Baryte, M. Anglesite, Beudant.
Combination of sulphuric acid and protoxide of lead.
| Zellerfeld. | Anglesea. | Wanlockhead. | |
| Protoxide of lead | 72·47 | 71·0 | 70·50 |
| Sulphuric acid | 26·09 | 24·8 | 25·75 |
| Water | 0·12 | 2·0 | 2·25 |
| Silica | 0·51 | 0·0 | 0·00 |
| Stromeyer. | Klaproth. | Klaproth. |
Sp. Gr. 6·23—6·31. H. = 3·0.
Colour white, grey, or yellowish; frequently tinged blue or green by the oxide of copper. It occurs crystallized in rhombic prisms with diedral terminations, but the crystals, when the prism is short, assume the general form of an octahedron; the structure is perfectly lamellar; it cleaves parallel only to the planes of a right rhombic prism of 103° 42′ and 76° 18′, which therefore is the form of its primary crystal.
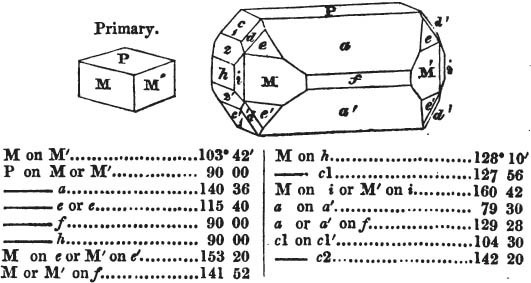
When reduced to thin laminæ it is often colourless and transparent, with a splendent lustre; fracture conchoidal and resin-
[page] 366
ous; brittle, and yields to the nail. It also occurs massive. Before the blowpipe it decrepitates, then melts; fuses in the oxidating flame into a transparent globule, which becomes milky on hardening; and in the reducing flame effervesces, and is soon reduced to the metallic state.
The finest specimens of this species are found at the mines of Wanlockhead and Leadhills in Dumfries-shire, often in tabular-shaped crystals some inches in diameter. Pary's Mine in Anglesea, and Mellanoweth in Cornwall, are its principal English localities; while on the Continent it is best known at Clausthal and Zellerfeld in the Hartz, and at Badenweiller in the Brisgau. Small but extremely perfect transparent crystals have been brought from Fondon in Granada; while the massive and compact varieties are chiefly from Siberia, Andalusia, and Alston Moor.
Many of the ores of lead are unquestionably derived from the decomposition of galena, and none more distinctly so than the sulphate, which not only contains the same ingredients, but is frequently met with at Leadhills, either occupying the cavities of cubical crystals, or disposed on a surface of galena, which has all the appearance of having been acted upon by acids. (Manual.)
CUPREOUS SULPHATE OF LEAD.
Diplogenic Lead Baryte, Haidinger. Schwefelsaures Blei-und-Kupfer, L. Sulfate de Plomb Cuivreuz, Beudant. Cupreous Sulphate of Lead, Brooke.
Combination or mixture of sulphate of lead, oxide of copper, and water.
| Sulphate of lead | 74·4 |
| Oxide of copper | 18·0 |
| Water | 4·7—Brooke. |
Sp. Gr. 5·3—5·4. H. = 2·5—3·0.
Of a deep azure-blue colour, greatly resembling that of the brightest and more translucent varieties of blue carbonate of copper. Primary form a right oblique-angled prism; cleavage very perfect parallel to M, less so to T; translucent; lustre vitreous or adamantine; streak pale blue.
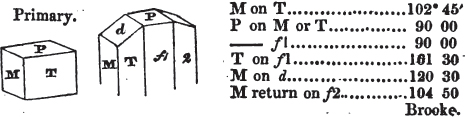
Leadhills in Scotland is the only well-ascertained locality of this very rare species, although it is mentioned also from Linares in Spain.
[page] 367
MOLYBDATE OF LEAD.
Bieigelb, Haus. Gelb-Bleierz, W. Plomb Molybdaté, H. Bt. Plomb Jaune, Br. Pyramidal Lead Baryte, M. Melinose, Beudant.
. Combination of molybdic acid and oxide of lead.
| Oxide of lead | 60·86 | 64·42 |
| Molybdie acid | 39·14—Berzelius. | 34·25—Klaproth. |
Sp. Gr. 6·69—6·76. H. = 3·0.
Colour generally orange or wax-yellow, passing into grey or brown, rarely aurora-red. Primary form, the octahedron with a square base. It occurs crystallized in flat and in acute four-sided pyramids variously modified, and in tabular crystals; structure perfectly lamellar; yields to cleavage parallel to the planes of the primary, and also to the common base of the two pyramids; fracture uneven, passing into small conchoidal, with a glistening resinous lustre; translucent, soft, and brittle. It rarely occurs massive.
Fig. 1, an octahedron, exhibiting only the planes of c1 of the following figure, and much flatter than the primary. Fig. 2, the same, of which the summits, and edges of the common base of the pyramids, are replaced by planes; these planes are increased and complete in fig. 3, producing a crystal nearly in the proportions of the cube. Fig. 4, an octahedron, of which all the solid angles and the edges of the pyramids are replaced. Fig. 5, a tabular crystal arising from the deep replacement of the summits of a crystal similar to fig. 2, combined with the planes of fig. 4, which replace the lateral solid angles. Fig. 6, a quadrangular prism (fig. 3) terminated by acute pyramids.
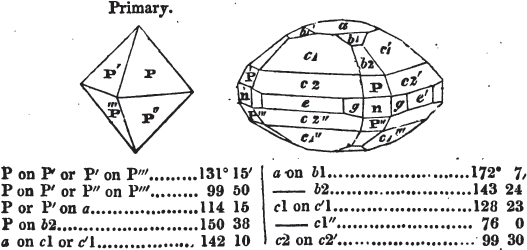
Before the blowpipe it decrepitates; on charcoal it fuses into a dark-grey mass, in which globules of reduced lead are visible;
[page] 368
with a little borax it forms a brownish globule, and with a larger quantity a blue or greenish-blue glass. Slowly and with difficulty soluble in nitric acid, leaving a residue.
At Schwarzenbach, Bleiberg, and Windisch-Kappel in Carinthia, the molybdate of lead occurs in beds and veins of limestone, along with other ores of lead. It is also met with at Rezbanya in Hungary, and at Moldawa in the Bannat, where its crystals bear at first sight much resemblance, particularly in colour, to the chromate.
CHROMATE OF LEAD.
Hemi-Prismatic Lead Baryte, M. Prismatic Lead Spar, or Red Lead Spar, J. Rothbleierz, W. Kallochrom, Haus. Chromsaures Blei, Leonhard. Plomb Chromaté Rouge, H. Crocoise, Beudant.
Combination of chromic acid and lead.
| Oxide of lead | 68·5 | 63·96 |
| Chromic acid | 31·5—Berzelius. | 34·40—Thenard. |
Sp. Gr. 5·95—6·6. H. = 2·5.
Colour deep-red or hyacinth-red. Primary form an oblique rhombic prism. Occurs in very distinct crystals; also massive. Cleavage parallel to M, perfect; translucent, sometimes only on the edges; lustre adamantine; streak orange-yellow. Before the blowpipe it becomes black and decrepitates, if quickly heated; it may be fused, however, into a black slag, containing globules of metallic lead. It colours glass of borax green; and is soluble without effervescence in nitric acid, forming with it a yellow solution.
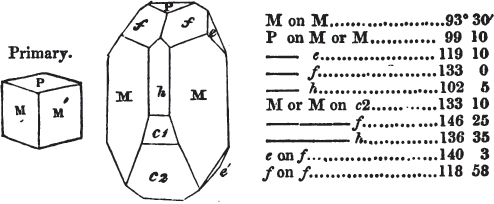
Of this rare and beautiful mineral there are only a few known localities, the principal of which is Siberia; it occurs near Beresof, in narrow veins traversing decomposed gneiss, and associated with gold, iron-pyrites, galena, quartz, and vauquelinite. In Brazil, at Conghonas do Campo, it occurs in equally splendid crystals, though more sparingly, in decomposed granite.
[page] 369
MELANOCHROITE.*
Herrman.
Sp. Gr. 5·75.
Contains oxide of lead 79·69, chromic acid 23·31.
This mineral occurs, along with the preceding, at Beresof in Siberia. Its form is that of a rhombic prism, having two faces enlarged, so as to impart to it a tabular shape. Colour hyacinthor orange-red; lustre resinous; nearly opake. Before the blowpipe it does not decrepitate, but retains its shape until it fuses; and on cooling assumes a crystalline structure. Its matrix is calcareous, and it is associated with galena, vauquelinite, and quartz.
VAUQUELINITE.†
Chromate of Lead and Copper. Hemi-Prismatic Olive Malachite, Haid.
Combination of chromic acid, oxide of lead, and oxide of copper. Chromic acid 28·33, oxide of lead 60·87, oxide of copper 10·80—Berzelius.
Sp. Gr. 5·8. H. = 2·5—3·0.
Primary form supposed to be an oblique rhombic prism. This substance occurs in mammillated masses, or minute and generally macled crystals, aggregated irregularly, and constituting a thin crust, occasionally with a tendency to the form of stalactites, which sometimes are hollow, sometimes include the chromate of lead of a dingy orange colour. The crystals are black, occasionally with a tinge of green, and, when viewed under the microscope, often appear splendent; or they are without lustre, and brown. Streak siskin-green or brownish. Fracture uneven; faintly translucent or opake. Before the blowpipe on charcoal it intumesces slightly, and fuses into a dark-grey globule of metallic brilliancy, surrounded by small beads of reduced lead; but the globule suffers no change. Partly soluble in nitric acid.
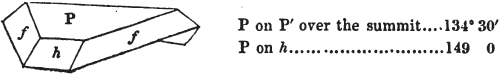
It occurs in Siberia on quartz accompanying chromate of lead; also at Pont Gibaud in the Puy de Dome; and, it is said, in Brazil, along with the chromate of lead from that country.
* From μsλαΓxϱos, dark colour.
† In honour of the celebrated French chemist, Vauquelin.
[page] 370
TUNGSTATE OF LEAD.
Scheelsaures Blei, L. Plomb Tungstaté, Levy. Schéelitine, Beudant.
Combination of tungstic acid and oxide of lead.
| Oxide of lead | 48·28 | 48·0 |
| Tungstic acid | 51·72—Lampadius. | 52·0—Levy. |
Sp.Gr. 8·1. H. = 3·0.
Primary form a rectangular four sided prism, having one single distinct cleavage parallel to its base. Occurs in aggregated indistinctly pronounced four-sided prisms, whose terminal edges are replaced by octahedral planes. Colour yellowish-grey; faintly translucent; lustre resinous; fracture conchoidal and shining. Before the blowpipe it melts, and gives off vapours of lead, leaving a dark-coloured metallic-like crystalline globule; when sufficiently roasted to drive off the lead, it yields, with borax, a yellow bead, which becomes transparent and deep-red on cooling; and with salt of phosphorus, at a certain degree of saturation, affords a blue one in the reducing flame.
Tungstate of lead occurs at Zinnwald in Bohemia, associated with quartz and mica. Levy mentions it as accompanying the molybdate of lead at Bleiberg in Carinthia.
VANADIATE OF LEAD.
Vanadinsaures Blei, Leonhard.
| Zimapan. | |
| Contains Vanadiate of lead | 74·00 |
| Chloride of lead | 25·33 |
| Hydrous oxide of iron | 0·67—Berzelius. |
Sp. Gr. 6·99—7·23. Scratched by the knife.
Occurs, though rarely, in indistinct hexagonal prisms, generally in globules. Colour varying from straw-yellow to reddish-brown; opake, and dull. The fractured surfaces present a resinous lustre; streak white; fracture conchoidal; brittle. Before the blowpipe, in the forceps, it fuses, and on cooling retains its yellow colour; if kept some time in fusion, however, it is changed into a steel-grey porous mass, which upon charcoal yields immediately globules of lead. Per se on charcoal it fuses readily, exhales the odour of arsenic, is reduced, and leaves, after heating in the inner flame, a steel-grey very fusible slag, which exhibits the re-actions of chromium. It forms green solutions with the sulphuric and muriatic acids; and a beautiful yellow solution with nitric acid.
This mineral was first noticed at Zimapan in Mexico, by Del Rio. Rose also observed it at Beresof near Ekatherineburg in
[page] 371
Siberia, associated with phosphate of lead; and latterly it has occurred in considerable quantity among some of the old workings at Wanlockhead, in Dumfries-shire, where at first, from the resemblance it bears to that species, it was mistaken for arseniate of lead. It is there found in small globular masses sprinkled over calamine, or in thin coatings on the surface of that mineral. Isolated and perfect crystals are rare, but occasionally the larger globules exhibit traces of six-sided prisms. (Manual.)
SULPHURET OF ZINC.
Blende,* W. Zinc Sulfuré, H. Bt. Dodecahedral Garnet Blende, M.
The sulphuret of zinc, mixed with variable proportions of the proto-sulphuret of iron; in some varieties also from two to three per cent. of proto-sulphuret of cadmium.
| England. | Pyrenees. | Var. | |
| Zinc | 61·5 | 63·0 | 50·2 |
| Sulphur | 33·0 | 33·6 | 30·2 |
| Iron | 4·0 | 3·4 | 10·8 |
| Berthier. | Berthier. | Berthier. |
Sp. Gr. 4·0—4·2. H. = 3·5—4·0.
Colour brown, yellow, blackish-brown, red, and black, rarely green. Primary form the rhombic dodecahedron. It occurs crystallized and amorphous; the forms of its crystals are very numerous; structure perfectly lamellar, and mechanically divisible with facility into the dodecahedron, octahedron, obtuse rhomboid, acute rhomboid, and irregular tetrahedron; the lustre of the fragments is splendent, sometimes adamantine; it is translucent or opake, yields to the knife, is moderately brittle, and easily frangible in the direction of the laminæ. Streak varying with the colour, from white to reddish-brown. When strongly heated in the oxidating flame of the blowpipe, it emits vapours of zinc, which deposit on the charcoal; but it is infusible, even with the addition of borax. It gives out an hepatic odour when pulverized and digested in sulphuric acid. Some varieties are highly phosphorescent when rubbed or struck with the steel.
Though the forms and colours of blende are extremely various, the perfect cleavage which it presents parallel to the faces of the dodecahedron is highly characteristic. It may be distinguished also from those varieties of galena, garnet, and tin, which it occasionally resembles, by the facility with which it yields to the knife.
* From the German, signifying glistening; in allusion to its shining crystals.
[page] 372
Fig. l,the primary; a rhombic dodecahedron. Fig. 2, the same, of which eight of the solid angles are replaced by as many triangular planes; which, in fig. 3, are increased greatly, forming the passage of the rhombic dodecahedron into the regular octahedron, fig. 4. Fig. 5 is an octahedron, which has received an increase of crystalline laminæ progressively di. minishing in size, on opposite faces; this crystal forms the passage of the octahedron into the tetrahedron, fig. 6, in which the triangular planes of fig. 5 have received a still further increase of laminæ. Fig. 7, a regular octahedron, of which the six solid angles are replaced by quadrangular planes, which are increased and complete in fig. 8, the cube. Fig. 9, a crystal in the general form of the rhombic dodecahedron (fig. 1), but modified in part with the small equilateral triangular planes of fig. 2, and of which the edges are alternately replaced by isosceles triangular planes inclining on the solid angles.
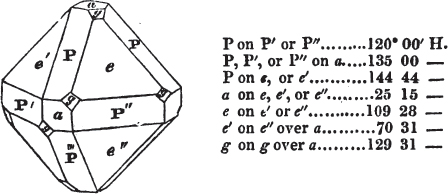
Blende (the black-Jack of English miners) is a mineral of very frequent occurrence, being met with in beds and veins accompanying most of the ores of silver and lead. It is found not only crystallized as above, and in macles, but massive, fibrous, and botryoidal. The dark-coloured crystalline varieties are principally from Derbyshire, Cumberland, and Cornwall, though many splendid specimens are also brought from Transylvania, Hungary, and the Hartz. A transparent bright-yellow variety accompanies bournonite and fahlerz at Kapnik; a still more brilliant one of an oil-green colour occurs at Schemnitz; while
[page] 373
Sahla in Sweden, Ratieborzitz in Bohemia, and several of the Saxon localities, are celebrated for the splendid brown and black crystals which they afford. (Manual.)
Cadmiferous Blende, The splendent fibrous blende of Przibram possesses a lustre very nearly metallic, especially after fresh fracture; its structure is radiated, the fibres are shining, and of a brown colour, and it contains a small proportion of cadmium. A white fibrous variety in botryoidal concretions also occurs in Fowey consolidated mines; the massive in many other Cornish localities.
Though this ore is frequently found in large quantities, the difficulty of reducing it, and the limited extent to which it can consequently be applied, render it a production of little importance; it is however in some instances employed as an ore of zinc.
RED OXIDE OF ZINC.
Prismatic Zinc Ore, M. Zink Oxyd, L. Zinc Oxydé Manganesifère, B.
Oxide of zinc, usually mixed with red oxide of manganese.
| New Jersey. | ||
| Oxide of zinc | 92·0 | 88·0 |
| Oxide of iron and manganese | 8·0 | 12·0 |
| Bruce.: | Berthier. |
Sp. Gr. 5·4—5·5. H. = 4·0—4·5.
Colour aurora- or vermilion-red, inclining to yellow. Primary form a right rhombic prism of about 125° and 55deg;. It occurs massive, disseminated, and micaceous; the structure is lamellar; principal cleavage parallel to the terminal plane of the prism; translucent when reduced to thin laminæ, or opake; with an adamantine or shining lustre; but on exposure it becomes dull and covered by a pearly crust; streak orange-yellow; fracture conchoidal; brittle, and easily scratched by the knife. It is infusible before the blowpipe without addition, covering the charcoal with zinc fumes when exposed to the reducing flame; but with borax melts into a transparent yellow bead, and with salt of phosphorus forms a colourless one. It is soluble without effervescence in nitric acid, and is supposed to derive its red colour from the manganese it contains.
It occurs massive, and in considerable quantities, mixed with calc-spar and franklinite, at the Franklin and Stirling mines near Sparta in New Jersey. Mitscherlich has described some minute six-sided prisms, formed artificially in the iron furnaces of Konigshü in Silesia, which he believes to belong to this species.
[page] 374
SILICEOUS OXIDE OF ZINC.
Electric Calamine. Prismatic Zinc Baryte, M. Prismatic Calamine, J. Galmei (in part), W. Zinc Oxydé Silicifère, H.
Combination of oxide of zinc, silica, and water.
| Limbourg. | Altenberg. | Brisgau. | |
| Oxide of zinc | 66·83 | 66·87 | 64·5 |
| Silica | 24·89 | 26·23 | 25·5 |
| Water | 7·46 | 7·40 | 10·0 |
| Berzelius. | Berzelius. | Berth ier. |
Sp. Gr. 3·3—3·6. H. = 5·0 when crystallized, the massive varieties being less.
Most prevalent colour white, occasionally blue, green, yellow, or brown. Primary form a right rhombic prism. It occurs crystallized, stalactitic, mammillated, botryoidal, and massive. The crystalline forms are numerous; the crystals are mostly disposed in radiated groups; cleavage perfect parallel to M; fracture uneven; streak white; lustre vitreous; varies from transparent to opake; yields to the knife, but is much harder than the carbonate of zinc. When gently heated it is strongly electric; some varieties become so by friction. Before the blowpipe it slightly decrepitates, loses its transparency, intumesces, and emits a green phosphorescent light; it is infusible without addition, but is soluble with borax into a clear glassy globule, which becomes opake on cooling. Reduced to powder it dissolves in heated sulphuric and muriatic acid, and the solution gelatinizes on cooling.
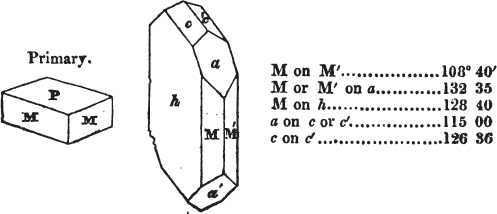
This species and the following are frequently found associated in veins, with blende, iron, and lead. Considerable quantities occur at Bleiberg and Raibel in Carinthia, often in extremely delicate crystals. Several beautiful varieties are met with near Freiburg in the Brisgau, at Rezbanya in Hungary, Tarnowitz in Silesia, and Altenberg near Aix-la-Chapelle. Concentric botryoidal groups occur in the Mendip Hills, and at Wanlockhead in Dumfries-shire; and numerous pseudomorphic crystals, as-
[page] 375
suming different forms of the carbonate of lime, are found in Derbyshire, and at Schemnitz in Hungary.
CARBONATE OF ZINC.
Galmei (in part), W. Zinc Carbonaté, H. Calamine.* Rhombohedral Zinc Baryte, M. Zinc Spath, L. Rhombohedral Calamine, J.
It is the carbonate of zinc.
| Somerset. | Derby. | Altai. | |
| Oxide of zinc | 64·8 | 65·2 | 62·5 |
| Carbonic acid | 35·2 | 34·8 | 36·0 |
| Smithson. | John. | ||
Sp. Gr. 4·2—4·5. H. = 5·0.
Calamine is found crystallized, compact, pseudomorphic, earthy, and cupriferous. Colour commonly greyish or yellowish, but also occurs of various shades of green and brown. Primary form an obtuse rhomb of 107° 40′. It is found in obtuse and acute rhombs, and in long quadrilateral tables which sometimes are modified; structure perfectly lamellar, yielding to cleavage parallel to all the primary planes; the external lustre of the crystals is between vitreous and pearly; translucent or opake, and yields easily to the knife. It dissolves with effervescence in nitric or muriatic acid, but it does not, like the silicate of zinc, form a jelly with them. Before the blowpipe it is infusible, but loses its transparency, the carbonic acid is driven off, and the residue acts like pure oxide of zinc. With salt of phosphorus it fuses into a transparent glass, which becomes, in the reducing fire, clouded on flaming, and forms a white enamel when cold. It is negatively electrified by friction.
Most of the localities of the foregoing species are also common to this. A dark-brown coloured variety, and another of a beautiful bright green, are found in Siberia. Jefferson County in the United States; Dognatzka and the Bannat in Hungary; Reibel and Bleiberg in Carinthia; Tarnowitz in Silesia; Aix-la-Chapelle; Mendip in Somersetshire; Matlock in Derbyshire Wanlockhead and the Lead Hills in Scotland; all produce it in considerable abundance. A compact, fibrous, semi-transparent variety, of a pale-yellow colour, disposed in concentric laminæ, also occurs at Alston Moor in Cumberland.
This species, however, does not so often occur crystallized as the siliceous oxide, being more generally stalactitic, reniform, mammillated, cellular, and amorphous; sometimes imperfectly fibrous; and frequently assuming the aspect of calcedony.
* From the Latin, calamus, a reed; when in fusion, it adheres to the base of the furnace, in the form of reeds.
[page] 376
WILLELMINE.
Willelmine, Levy and Beudant. Willemit, Leonhard.
Sp. Gr. 4·18.
An anhydrous silicate of zinc,—being composed of silica, oxide of zinc, and a small quantity of oxide of iron. Crystallized in regular six-sided prisms, terminated by rhombic faces inclined to one another at an angle of about 128°. Primary form an obtuse rhomboid of 128°. Colour white, yellow, red, or reddish-brown. Cleavable in one direction, perpendicular to the axis.
It occurs in the calamine deposits of the Vielle Montagne, near Aix-la-Chapelle.
SULPHATE OF ZINC.
Prismatic Vitriol Salt, M. Gallizinite, Beudant. Zink Vitriol, Kars. Zinc Sulphaté, H. White Vitriol, A.
The hydrous sulphate of zinc.
| Scnemnitz. | ||
| Oxide of zinc | 28·5 | 27·5 |
| Sulphuric acid | 29·8 | 20·0 |
| Oxide of manganese | 0·7 | 0·5 |
| Oxide of iron | 0·4. | 0·0 |
| Water | 40·8—Beudant. | 50·0—Klaproth. |
Sp. Gr. 2·036. H. = 2·0—2·5.
Greyish-, yellowish-, reddish-, or greenish-white. Primary form a right rhombic prism of 90° 42′.* It seldom occurs distinctly crystallized, generally massive, stalactitic, botryoidal, reniform, and investing; cleavage perfect parallel to the face o; fracture conchoidal; lustre vitreous; streak white; transparent or translucent; with a nauseous metallic taste. Before the blowpipe it is fusible with ebullition, giving off its sulphuric acid, and covering the charcoal with a white coating. It is readily soluble in water.
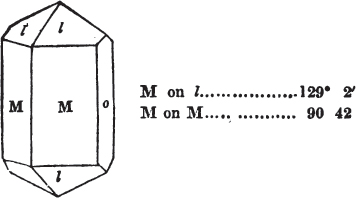
It occurs principally in the deserted galleries of old mines, frequently with blende, and is supposed to arise from the decomposition of that mineral. It is found at Rammelsberg near Goslar in the Hartz, in Austria, Hungary, at Fahlun in Sweden, and at Holywell in Flintshire; but it is a rare species.
* 91° 7′ and 88° 53′ according to Beudant.
[page] 377
HOPEITE*
Brewster, Trans. Royal Soc. Edin. x. 107.
" A compound of some of the stronger acids,—as phosphoric or boracic—zinc, an earthy base, a little cadmium, and a great deal of water."
Sp. Gr. 2·46—2·76. H. = 2·5—3·0.
Primary a right rhombic prism of 98° 26′ and 81° 34′ Cleavage perfect parallel to l. Colour greyish-white; transparent or translucent; lustre vitreous, inclining to pearly on the face e; streak white. Surface of p deeply striated longitudinally; rest of the faces smooth. Entirely soluble without effervescence in the muriatic and nitric acids, but is acted upon very slowly by sulphuric. Neither phosphorescent nor electric by heat. Yields water in the matrass, and melts before the blowpipe into a clear colourless globule, which tinges the flame green.
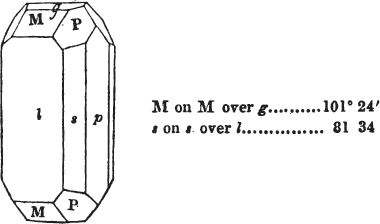
This is a very rare substance; it was noticed by Sir David Brewster; and has hitherto been found only in the calamine mines of Altenberg near Aix-la-Chapelle.
NATIVE QUICKSILVER.
Mercury. Gediegen Quecksilber, W. Mercure Natif, H. Bt.
Fluid mercury is the pure metal as produced by nature; it presents no determinate form, but occurs in small globules disseminated through its matrix.
It is of a silver-white colour, with a splendent metallic lustre. Specific gravity 13·6. It volatilizes entirely before the blowpipe at less than a red heat; becomes solid at a temperature of — 40°; and is easily soluble in nitric acid.
* In honour of Dr Hope, Regius Professor of Chemistry in the University of Edinburgh.
[page] 378
It occurs in most of the mines producing the ores of quicksilver, particularly those of Idria in Carniola, Almaden in Spain, and the Palatinate. At Idria it is found in a kind of slate-clay, which forms the upper portion of the mines; and from this source is obtained by means of washing. Mercury is used in various chemical and pharmaceutical preparations; in the amalgamation of gold and silver ores, for which purpose vast quantities are annually exported from Europe to the South American continent; in the formation of artificial cinnabar, and fulminating powder for percussion guns; in silvering mirrors; making thermometers and barometers; and for many other purposes.
NATIVE AMALGAM.
Naturlisches Amalgam, W. Mercure Argental, H. Bt. Dodecahedral Mercury, M. Amalgame, Necker.
Union of mercury and silver.
| Mercury | 65·2 | 72·5 |
| Silver | 34·7—Klaproth. | 27·5—Cordier. |
Sp, Gr. 10·0—14·1. H. = 1·0—3·5.
Silver-white, or grayish. Primary form, rhombic dodecahedron. It occurs in a semi-fluid state; also massive; and occasionally forms large and very perfect crystals, with numerous modifications of the rhombic dodecahedron; but no distinct cleavage has been observed; has a flat conchoidal fracture; is soft, cracks when cut, and acquires vitreous electricity from friction when isolated. Before the blowpipe the mercury is volatilized, and a bead of silver remains. It whitens the surface of copper when rubbed warm upon it. Soluble in nitric acid.
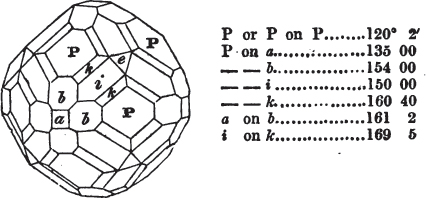
It is found principally at Rosenau in Hungary, and at Moschellandsberg in Deux Ponts, accompanied by quicksilver and cinnabar, in ferruginous and argillaceous veins, and is described as occurring at those points where veins of silver and mercury traverse one another.
[page] 379
SULPHURET OF MERCURY.
Peritomous Ruby Blende, M. Quecksilber-Lebererz. Zinnober, W. Mercure Sulfuré, H. Bt. Cinnabar, A.*
Combination of mercury and sulphur.
| Japan. | Idria. | |
| Mercury | 84·50 | 85·00 |
| Sulphur | 14·75—Klap. | 14·25—Klap. |
Sp. Gr. 6·7—8·2. ·H. = 2·0—2·5.
It varies in colour from carmine, through cochineal red, to lead-grey; in this last case it is opake, and has a metallic lustre; when it is red it is more or less translucent, and exhibits an adamantine lustre. Primary form an acute rhomboid of 71° 48′ and 108° 12′, in which it also occurs crystallized; but the crystals are mostly modified by secondary planes; also massive, fibrous, and pulverulent. Structure lamellar, in the massive sometimes curved, with a shining lustre; cleavage highly perfect parallel to P, the primary rhomboid; streak bright scarlet. Before the blowpipe it melts, and is volatilized with a blue flame and sulphureous odour. On being sublimated it crystallizes in columnar masses. It is soluble in nitro-muriatic acid.
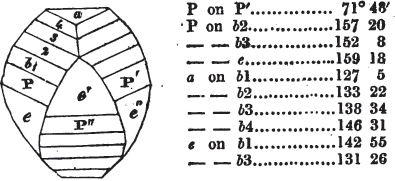
Figure and measurements on the authority of Haüy.
The Lebererz or Hepatic Cinnabar is dark-red, sometimes nearly iron-grey; it occurs both compact and slaty; and, being generally mixed with impurities, such as bituminous matter or clay, affords a brownish streak, and is always opake.
The best-defined crystals of cinnabar are found in the coal formation of Moschellandsberg and Wolfstein in the Palatinate, though it also occurs in beds traversing gneiss at Reichenau in Upper Carinthia, in grauwacke at Dumbrawa in Transylvania, and in limestone at Neumarktel in Carniola. Well-crystallized specimens are mentioned also from Japan, Mexico, and Brazil. The principal repositories of this ore, however, are Almaden in
[page] 380
Spain, and Idria in Carniola, where it occurs almost exclusively massive, and whence it is obtained in large quantities as an ore of mercury. The variety termed coralinerz, from the latter locality, consists of curved lamellar concretions, which present the form and apparent structure of fossilized shells.
* From the Greek, signifying a red-coloured grain.
Cinnabar is the most abundant and most important ore of mercury, which is obtained from it in a metallic state by sublimation. Vermilion is, in fact, pure cinnabar, being a compound of mercury and sulphur, in nearly the same proportions as in this species. (Manual.)
MURIATE OF MERCURY.
Horn Quicksilver. Quecksilber Hornerz, W. Mercure Muriaté, H. Chlorquecksilber, Bern. Pyramidal Pearl Kerate, M. Calomel, Beudant.
Chloride of mercury. Chlore 14·89, mercury 85·11.
Sp. Gr. 6·48—6·5. H. = 1·5—2·0.
Colour greyish-white, grey, yellowish and greenish-grey; sometimes occurs crystallized in distinct quadrangular prisms terminated by pyramids; also in tubercular crusts; sometimes fibrous; rarely compact; is occasionally translucent, with an adamantine or vitreous lustre; and is sectile; fracture conchoidal or uneven. Before the blowpipe on charcoal it is totally volatilized if pure; in the matrass it affords a white sublimation, and mixed with soda forms numerous globules of mercury.

The preceding figure and measurements are on the authority of Brooke.
The principal locality of this rare mineral is Moschellandsberg in Deux Ponts, where the crystals are often large and well defined, coating the cavities of a ferruginous gangue, and associated with cinnabar; but it is also met with in the quicksilver mines of Idria in Carniola, at Almaden in Spain, and at Horzowitz in Bohemia.
[page] 381
IODIC MERCURY.
Iodure de Mercure, or Mercure Ioduré, Necker. Iod-Quecksilber, Del Rio and Leonhard.
In spots of a fine lemon-yellow colour, in the variegated sandstone of Casas Viegas, Mexico. Exposed to the air, as well as in ammonia, it changes to black. It resembles the artificial protiodide of mercury. (Shepard.)
[page] 382
COMBUSTIBLE MINERALS;
Those of which the base is sulphur or carbon. It includes substances of the most opposite external characters; both the hardest and the softest in nature.
SULPHUR.
Natürlicher Schwefel, W. Soufre, H. Prismatic Sulphur, M.
Sp. Gr. 1·9—2·1. H. = 1·5—2·5.
Colour when pure, citron-yellow; from accidental admixture sometimes red, brown, yellowish-grey, and even green. Primary a four-sided pyramid, with equal and similar scalene triangular planes, and of which the common base is rhombic. It occurs massive, disseminated, investing other minerals, and crystallized in the form of an acute four-sided pyramid, either perfect or variously modified; cleavage imperfect and interrupted; fracture conchoidal, uneven in the impure varieties; lustre shining and resinous, varying from transparent to translucent on the edges; very brittle. It burns readily with a lambent blue, or white flame, according to the low or high degree of temperature, emitting at the same time a pungent smell of sulphureous acid, and fuses into a brown liquid. It acquires resinous electricity by friction.
Fig. 1, the primary pyramid. Fig. 2. the same elongated. Fig. 3, the same, having its summits replaced. Fig. 4: in this two opposite solid angles of the octahedron are replaced by rhombic planes. Fig. 5: in this the edges of the base of the pyramid are deeply replaced by quadrangular planes. In fig. 6, the summits of the primary are replaced by four triangular planes, forming a low pyramid on each.
[page] 383
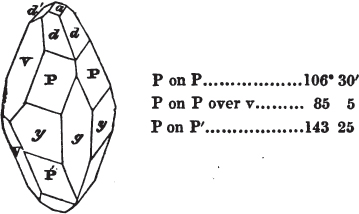
Sulphur occurs principally in two kinds of repositories,—either in gypsum and salt rocks, as in the valleys of Noto and Mazzara in Sicily, at Conil near Cadiz in Spain, and at Cracow in Poland; or in the vicinity of active and extinct volcanoes. In the latter case it is the result of sublimation, forming either crystals in the clefts and cavities of the rock, or crusts, stalactites, and loose efflorescent coatings. In small quantities it is likewise met with in certain metalliferous veins, as in Savoy, Switzerland, Hanover, &c. In Iceland it is deposited by hot springs, and many of the natural medicinal waters both of this and other countries hold it largely in solution. In Sicily crystals of sulphur two or three inches in diameter are occasionally met with; and by much the larger proportion of what is used for commercial purposes is obtained from that island; at Radoboy, near Crapina in Croatia, it occurs in imbedded spheroidal masses, of a brown tinge, which is owing to bitumen; and in the dark-reddish coloured sulphur of the Lipari Islands, Stromeyer detected selenium. (Manual.)
DIAMOND.*
Octahedral Diamond, M. Diamond, W. and H.
Pure carbon.
Sp. Gr. 3·55. H.= 10·0.
Diamonds are either colourless, or of a yellowish, bluish, yellowish-green, clove-brown, or rose-red tinge. Primary form the regular octahedron; always found in detached crystals, the varieties of form in which are numerous; the faces often convex, giving its crystals a spherical appearance; frequently macled; structure perfectly lamellar, yielding readily to cleavage parallel to the planes of the octahedron; lustre brilliant adamantine;
* Perhaps from adamut (Pliny), signifying unconquerable.
[page] 384
fracture conchoidal; varies from transparent to nearly opake. At a heat less than the melting point of silver (viz. at 14° Wedgewood) it gradually dissipates, burns, and, combining with nearly the same quantity of oxygen, forms the same volume of carbonic acid as charcoal, whence it appears to consist of pure carbon. It is not acted upon by acids or alkalies; possesses vitreous electricity when rubbed; and, after exposure to the solar rays, presents in the dark a distinct phosphorescence.
For transitions of fig. 1, 2, 3, 4, 5, see Red Oxide of Copper, p. 317.
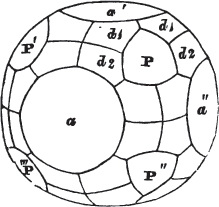
The crystal represented by the annexed figure was selected, as exhibiting, with more than usual beauty and precision, the planes of the primary octahedron P′, P″, P‴, and P'", and those of the cube a, a′, and a″, which are generally flat and brilliant. The numerous faces d1 and d2 are uniformly convex; each of these faces is in reality a series of planes, as is manifest on other crystals, but in no instance sufficiently perfect for the use of the reflective goniometer. In the last of the small preceding figures, these faces are shown, to the exclusion of the planes of the octahedron and cube. The true angles of these solids are;
P on P′ or P″………109° 28′
P on a, a′, or a″ ………125 15
a on a′ or a″ ………90 00
The rocks hitherto considered as the matrix of the diamond are secondary ones, as several kinds of sandstone, consisting of aggregated quartz pebbles; it is also found in strata of iron-shot sand and clay, and in the alluvium of plains and rivers.
Hindostan and Brazil are the principal localities of diamond, and in both these countries it is confined to the tropics. In India, where it has for ages been an article of commerce, it is met with in the district between Golconda and Masulipatam, near Parma in Bundelcund, where some of the most magnificent specimens have been found; and extensively on the Mahanuddy, and in the vicinity of Ellore. Up to the commencement of last century, diamonds were wholly derived from India and Borneo. In Brazil, the district of Minas Geraes comprehends, so far as
[page] 385
is known, the whole diamond grounds of the American continent. The largest and most magnificent specimens have hitherto been brought from the East. The Pitt or Regent Diamond, for instance, one of the crown jewels of France, weighing 136 carats, was found in the Golconda district; that of the emperor of Russia, the weight of which is 193 carats, is said to have once formed the eye of an Indian idol, and is doubtless from the same vicinity. The Rajah of Mattan possesses one of 367 carats, which was found in Borneo; and that of the Great Mogul is said in its rough state to have weighed not less than 800 carats. In Brazil, although of fine water, they rarely exceed twenty carats.
PLUMBAGO*
Rhomboidal Graphite, J. Graphit, W. Fer Carburé, H. Rhombohedral Graphite Mica, M.
Combination of a considerable quantity of carbon, with a small proportion of iron.
| Cornwall. | |||
| Carbon | 91·9 | 96·0 | 92·0 |
| Iron | 9·1 | 4·0 | 8·0 |
| Bertholet. | Saussure. | Vauquelin. |
Sp. Gr. 2·08—2·45. H. = 1·0—2·0.
Colour iron or steel-grey. Primary form a regular six-sided prism. It occurs in kidney-shaped masses, or disseminated in rocks; also, though rarely, crystallized in regular six-sided prisms, of which the summits are striated parallel to three of their edges; cleavage perfect perpendicular to the axis. It has a glistening* metallic lustre, a granular and uneven fracture; is unctuous to the touch; sectile, and the thin laminæ very flexible; not very brittle; streak lead-coloured and shining. Before the blowpipe it becomes yellow or brown after long-continued heat, but is infusible; and is not affected by the addition of any reagent.
It belongs chiefly to primitive rocks, and to the coal formation.
The purest and most esteemed plumbago is found at Borrowdale in Cumberland, where it occurs in rocks consisting chiefly of grauwacke, and whence it is obtained in considerable quantity for the manufacture of pencils. It occurs crystallized at Pargas in Finland, in Greenland, and in the United States; in scales like mica at Arendal in Norway; forming irregular masses imbedded with particles of garnet in gneiss, at Strathferran near Beauly in Aberdeenshire; passing into a kind of columnar
[page] 386
coal at Craigman in Ayrshire; at Passau in Austria; in Ceylon; and many other places. Besides being extensively used in the fabrication of pencils, graphite is employed in the manufacture of crucibles, particularly those required for the purposes of the mint, as they sustain intense heat, and are esteemed for their tenacity and expansibility. It is also used to diminish friction, and to protect iron from oxidation. (Manual.)
* Plumbago, from its drawing like lead (its streak being of the same colour). Graphite, from the Greek, to draw; in allusion to its use.
R
ANTHRACITE.*
Glanzkohle, W. Blind Coal. Anthracite, H. Bt. Blende Charbonneuse, Br. Non-bituminous Mineral Coal, M. Glance Coal, J. Kohlenblende, L.
Combination of carbon in considerable quantity, with a small proportion of silica.
| Tarantaise. | ||
| Carbon | 68·0 | 72·05 |
| Silica | 30·0 | 13·19 |
| Oxide of iron | 2·0 | 3·47 |
| Alumina | 0·0—Vauquelin. | 3·29—Dolomieu. |
Sp. Gr. 1·4—1·8. H. = 2·0—2·5.
Primary form a rhombic prism of 120°.
Of this substance there are three varieties. Massive Anthracite is of an iron-black colour, often superficially tarnished, and occasionally with a splendent metallic lustre; fracture conchoidal and shining; is light and brittle. It burns without flame or odour, leaving a whitish ash. It occurs at the Meissner, in Hesse.
Slaty Anthracite has a brownish-black colour. The structure is imperfectly slaty in one direction, with a somewhat metallic lustre; fracture flat conchoidal; easily frangible, somewhat sectile, and brittle. Vast deposits of anthracite exist in the United States, the most celebrated of which is the anthracite region, as it is called, of the Susquehanna in Pennsylvania; it is between sixty and seventy miles long, and about five broad, constituting a trough or elongated basin, through which the Susquehanna river, and Lackawanna creek flow.
In England it is found in the coal-formation near Walsal in Staffordshire (Stone Coal); in Wales, in the southern parts of Brecknockshire, Carmarthenshire, and Pembrokeshire (Welsh Culm); in the same situation near Cumnock and Kilmarnock in Ayrshire, and many other parts of Scotland (Blind Coal); and at Kilkenny in Ireland (Kilkenny Coal).
* Anthracite, from the Greek; consisting of carbon.
[page] 387
Columnar Anthracite occurs in short prismatic concretions, either straight or curved; of an iron-black colour, with a shining metallic lustre, and occasionally tarnished externally. It is opake, soft, light, and brittle. It burns without flame or smoke. It is principally found at the Meissner, in Hessia, forming the upper portion of a bed of brown coal, which is covered by basalt; at Craigman in Ayrshire, also, and in some of the Newcastle pits, it occurs in contact with dykes of greenstone, at the former frequently passing into plumbago.
These varieties being more difficultly inflammable than bituminous coal, are principally used in lime-kilns, malt-kilns, iron founderies, and such like; for when ignited in considerable quantity they burn with a strong and durable heat; and indeed much of the difficulty of kindling may be overcome by the addition of some charcoal, and the judicious application of a current of air. In the northern states of America, where common coal is little known, this forms the principal fuel of the maritime cities, and is there applied to nearly every purpose of raising temperature.
Closely allied to the present species is the Mineral Carbon or Mineral Charcoal, which occurs in thin layers, and fibrous distinct concretions of a delicate silky black colour, in most of the coal fields of Great Britain; at Voitsberg in Styria; Disko Island, Greenland, and elsewhere.
MINERAL OIL.
Under this term are comprehended two substances,-Naphtha and Petroleum; both of which are liquid, highly inflammable, and lighter than water.
1. NAPHTHA.*
Bitume Liquide Blanchatre, H. Le Napthe, Br. Bitume Napthe, Bt.
| Carbon | 82·2 | 87·60 |
| Hydrogen | 14·8—Thomson. | 12·78—Saussure. |
It is nearly colourless, sometimes yellow, and transparent; it burns with a white flame, much smoke, gives out a penetrating odour, and leaves no residuum. It dissolves resins, but is not itself soluble either in alcohol or ether.
It is found in large quantity in Persia, and in the Birman empire. At Rangoon there are said to be upwards of 500 naphtha wells, which yield annually about 412,000 hhds. It is of essential use in the manufacture of varnish, and is preferred to oil in
[page] 388
the formation of oil paint, from its property of drying with great rapidity. Like alcohol, it is employed for removing spots of grease from woollen and other stuffs, but it is difficult to destroy the disagreeable odour which it emits. Near the Caspian, and elsewhere in Persia, it is used instead of oil for lamps.
* From the Greek, signifying to take fire.
2. PETROLEUM.*
Bitume Liquide Noiratre, H. Petrol, Br. Bitume Petrole, Bt.
Petroleum, at the usual temperature, is rather thicker than common tar, has a strong disagreeable bituminous odour, and is of a blackish or reddish-brown colour. It is very combustible, emitting during ignition a thick black smoke, and leaving a little residue in the form of black coal.
It is found in many countries, principally in those producing coal. At several places in France. In England, at Ormskirk in Lancashire, and at Coal Port, near Coalbrookdale. In Scotland, at St Catherine's Well near Edinburgh, and in the Isle of Pomona one of the Hebrides. It occurs also in Bavaria, Switzerland, and in Italy near Parma. At the latter place the petroleum gives out so powerful an odour that the workmen cannot long endure it at the bottom of the petroleum wells without danger of fainting. It is found in many other parts of Europe, and in America.
When naphtha is exposed to the air and light, it becomes brown, thickens, and seems to pass into petroleum; and when petroleum is distilled, an oil is obtained similar to naphtha. When petroleum is exposed to the air, it thickens and passes into a kind of bitumen. Considerable alliance is thus proved to exist between mineral oil and bitumen.
BITUMEN.
Of bitumen there are three varieties.
1. EARTHY BITUMEN.
Erdiges Erdpech, W. Bitume Glutineux, H. La Poix Minerale Terreuse, Br. Earthy Mineral Pitch, J. Malthe, Beudant.
It is blackish-brown, and dull; fracture earthy and uneven; soft enough to take an impression of the nail; sectile, and possesses a strong bituminous odour. It burns with clear brisk flame, emits a powerful smell, and deposits much soot. It consists of
[page] 389
inflammable matter, mingled with a considerable proportion of earthy substances.
* Front two Greek words, signifying rock or mineral oil.
It is found in Persia, between Schiraz and Bender-congo; at the coal mines of Hurlet near Paisley, enclosing crystals of calcareous spar; in East Lothian, and other places. It is occasionally used as a pitch, and in the fabrication of certain varnishes.
2. ELASTIC BITUMEN.
Elastisches Erdpech, W. Bitume Elastique, H. La Poix Minerale Elastique, H. Elastic Mineral Pitch, J. Elaterite, Beudant.
Elastic bitumen is of various shades of brown; it is soft, yields easily to pressure, is flexible, elastic, possesses a strongly bituminous odour, and is about the weight of water. It burns readily with large flame and much smoke, but melts by a gentle heat, and is thereby converted into a substance resembling petroleum, or asphalt, according to its previous consistence. It takes up the traces of a pencil, in the same manner as caoutchouc or India rubber, whence its name of Mineral Caoutchouc. It consists, according to Henry, of
| England. | France. | |
| Carbon | 52·250 | 58·260 |
| Hydrogen | 7·496 | 4·890 |
| Oxygen | 40·100 | 36·746 |
| Nitrogen | 0·154 | 0·104 |
It occurs principally in the Odin mine, near Castleton in Derbyshire, in a secondary limestone.
3. COMPACT BITUMEN. ASPHALT.
Schlackiges Erdpech, W. Bitume Solide, H. La Poix Minerale Scoriacée, Br. Bitume Asphalte, Bt. Slaggy Mineral Pitch, J.
Varies from brownish-black to black; occurs massive, with a conchoidal fracture, and shining resinous lustre; is opake, and very brittle. Specific gravity 1—1·6. When rubbed, it gives out a bituminous odour. By combustion, it leaves a small quantity of ashes. It consists chiefly of bituminous oil, hydrogen gas, and charcoal, but the latter is in greater proportion than in elastic bitumen. Like the elastic variety, it is often soft when found, but soon hardens.
This is much the most common variety of bitumen. It is found in the Palatinate; in France; at Neuchatel in Switzerland; in large strata in Avlona in Albania; and in masses on the shores or floating on the surface of the Asphaltic lake in Judea, called the Dead Sea. It abounds in the islands of Barbadoes and Trinidad; in the latter it forms with sand a lake three miles in circumference, called the Pitch Lake, the thickness of which is unknown. A gentle heat renders it ductile, and when mixed with
[page] 390
grease or common pitch it is used for paying the bottoms of ships. The ancients employed bitumen in the construction of their buildings, and the Egyptians used it for embalming.
COAL.
Schiefferkohle, Blätterkohle, Grobkohle, W. Houille, H. Slate-Coal, Foliated Coal, Coarse Coal, J. Bituminous Mineral Coal, M.
Sp. Gr. 1·2—1·5. H. = 1·0—2·3.
It is of a black colour, frequently with an iridescent tarnish. It occurs massive; the structure in one direction is slaty, sometimes it is so in two directions; the fragments vary in shape from nearly the proportions of the cube, to those of a rhombic prism greatly resembling that of mica; it sometimes contains thin parallel layers of mineral carbon; fracture small and imperfectly conchoidal, frequently with a brilliant semi-metallic lustre. It burns with a bright flame and much smoke; but this coal commonly contains some proportion of earthy ingredients.
Analyses of several varieties of coal by Dr Thomson. Newcastle, or caking coal, carbon 75·28, hydrogen 4·18, azote 15·96, oxygen 4·58. Splint, or light-burn hard coal, from Glasgow, used for making coke and smelting iron, carbon 75, hydrogen 6·25, azote 6·25, oxygen 12·5. Cherry, or soft coal, from one of the Glasgow upper beds (of the same kind with the Staffordshire), carbon 74·45, hydrogen 12·4, azote 10·22, oxygen 2·93. Cannel coal, carbon 64·72, hydrogen 21·56, azote 10·72. Dr Thomson inclines to the opinion that coal is a direct combination of these elements, and not a compound of bitumen, &c. as has been supposed.
It occurs in many countries of the European continent, and is the common coal of the most extensive British collieries.
1. Cannel Coal. Kennel Kohle, W. Houille, H. Of a greyish-black colour, and occurs massive; fracture large and flat conchoidal, with a glimmering resinous lustre; brittle. Specific gravity 1·2. It burns with a bright flame, but at the same time decrepitates and flies into angular fragments. It is common in the upper beds of our coal deposits, as near Wigan in Lancashire, at Clee Hill in Shropshire, and Newcastle; and in Scotland, at Gilmerton near Edinburgh, and Muirkirk in Clydesdale. The name Cannel is supposed to be derived from the word candle, because in some places it is used as a substitute. In Scotland it is termed Parrot coal. As it receives a polish, it is occasionally made into snuff-boxes, ink-stands, &c.
2. Jet. Pechkohle, W. Jayet, H. Pitch Coal, J. Jet is generally of a velvet-black; it occurs in elongated reniform masses, and sometimes in the shape of branches, with a regular woody
[page] 391
structure; this structure is visible internally only by transmitted light and in specimens cut extremely thin; it has then a brown translucent appearance. It presents a brilliant resinous lustre, and a perfect conchoidal fracture; is soft and brittle, and little heavier than water; burns with a greenish flame and strong bituminous smell, leaving a yellowish ash. Jet occurs principally in marly, schistose, or sandy beds, in several places in France, where it is sometimes found enclosing amber; near Wittemberg in Prussia; and in detached fragments, in the amber mines on the coasts of the Baltic. In England it occurs in aluminous shale, at Whitby in Yorkshire. It is worked into various trinkets, chiefly worn as part of the mourning habit; but when not sufficiently fine and hard for that purpose, it is used as fuel.
3. Brown Coal. Braunkohle, Lignite, W. This substance is perhaps principally characterized by its odour when in a state of combustion, which resembles that of peat; the flame is weak; it appears to have but little analogy with common coal. It occurs massive, and brown of various shades, and brownish-black (Moor coal); the fracture is earthy, or fibrous, and in the latter case it generally possesses more or less of the structure of wood (Wood coal); but it is frequently sufficiently compact to afford a more or less perfect conchoidal fracture, with a somewhat resinous lustre, and is nearly black. It yields to the knife, occasionally to the pressure of the nail; 200 grains of the Bovey brown coal afforded on distillation 60 grains of water, acidulous and bituminous; 21 grains of thick brown oily bitumen; 90 of charcoal; and 29 of mixed gases, hydrogen, carbonated hydrogen, and carbonic acid.
The earthy and fibrous varieties occur together in Thuringia, in the circles of Saale and Leipsic, and at the Meissner in Hessia, forming beds 20 to 40 feet thick, and several square miles in extent; also in France, Silesia, Bavaria, and other European countries. In England, the fibrous and compact kinds (Bovey coal) are found near Bovey Tracey in Devonshire, forming beds of various thickness, interposed between brownish clay; small veins of coal are found in the clay, together with retinasphalt. The fibrous variety also occurs at the mouth of the Ouse in Sussex; abundantly in the Faroe Isles, particularly Suderoe; and in the county of Antrim imbedded in trap.
DYSODILE.
Dysodile, Cordier. Houille Papyracée, Lucas. Merda di Diavolo des Siciliens.
It occurs in masses of a greenish-grey or yellow colour, and either compact or laminated, sometimes both. It is extremely
[page] 392
fragile, emits an argillaceous odour when breathed on, and is of the specific gravity of 1·146. It burns with a considerable flame and smoke, and an almost insupportably fetid odour, with a crackling noise, leaving a residue of nearly half its weight, unaltered in form. Macerated in water, it becomes translucent, and its laminæ acquire flexibility.
It occurs at Melili, near Syracuse, in a bed in secondary limestone.
AMBER.
Bernstein, W. Succin, H. Yellow Mineral Resin, M.
| Contains Carbon | 80·59 | 70·68 |
| Hydrogen | 7·31 | 11·62 |
| Oxygen | 6·73—Drapier. | 7·77—Ure. |
Sp. Gr. 1·0—1·1. H. = 2·0—2·5.
In irregular nodules, masses, or grains, generally of a yellow or yellowish-white colour; sometimes reddish-brown. It is brittle, and yields easily to the knife; is occasionally transparent, always translucent; fracture more or less perfectly conchoidal, with a vitreous or resinous lustre. Resinous electricity easily produced by friction; this property gave rise to the science of electricity, which was so called from Hλςχτζον, the Greek name for amber.
It yields by distillation an acid called the succinic acid, and leaves an extremely black, shining residue, which is employed as the basis of the finest black varnishes. It burns with a yellow flame, emits an agreeable odour, and leaves a light, shining, black coal. Is soluble in alcohol. The experiments of Sir David Brewster on the optical properties of amber, leave no doubt of the origin of this substance being derived from the vegetable kingdom, as the traces of regular structure indicated by its action on polarized light are not the effect of the ordinary laws of crystallization by which mellite has been formed, but are produced by the same causes which influence the mechanical condition of gum arabic and other gums, which are known to be formed by the successive deposition and induration of vegetable fluids.
The largest specimens of amber occur on the Prussian coast, where it is disengaged by the action of the waves, and cast ashore. It also occurs occasionally presenting very peculiar tinges of blue, on the Sicilian coast near Catania; imbedded in brown coal at Hasen Island in Greenland; in Poland, France, Italy, and many other countries; and occasionally in the beds of gravel in the neighbourhood of London, and on the coasts of Norfolk and Suffolk. Of those insects which have been originally enclosed in amber, some have evidently struggled hard for their liberty, and even left their limbs behind them in the at-
[page] 393
tempt; it being no unusual thing to find in a mass of amber which contains a stout beetle, the animal wanting one or perhaps two of its legs, and those limbs left in different places nearer that part of the mass from which it set out. This also may account for the common accident of finding legs or wings of flies without the rest of their bodies in pieces of amber; the insects having, when entangled in the yet soft and viscid matter, escaped at the expense of leaving those limbs behind them. Most if not all of these insects are unknown at the present day. (Manual.)
Amber is used in the fabrication of ornaments by the Turks, as mouth-pieces for their pipes, &c.; and considerable value is attached to large transparent specimens. The common varieties are used for making varnish.
HATCHETINE.*
Mountain Tallow, Mineral Adipocere, Conybeare.
This singular mineral varies in colour from yellowish-white to wax and greenish-yellow. It occurs either flaky, like spermaceti, or sub-granular, like bees′ wax. When flaky it has a slightly glistening and pearly lustre, and a considerable degree of translucency; when sub-granular it is dull and opake. It is of about the hardness of soft tallow, and possesses neither odour nor elasticity; but is so fusible as to melt in water heated below 170°; and is very light. Like elastic bitumen, it is readily soluble in ether; and both solutions, by spontaneous evaporation, leave a viscid oily matter in separate drops,, but that from Hatchetine is still inodorous, while the one from elastic bitumen retains strongly the peculiar odour of that substance. Hatchetine distilled over the naked flame of a spirit-lamp assumes the bituminous smell, and yields a butyraceous substance of a greenish-yellow colour, a coaly matter remaining in the retort; at a lower heat it affords a light oil.
It occurs filling small contemporaneous veins, lined with calcareous spar and small quartz crystals, in iron-stone at Merthyr Tydvil in South Wales.
SCHERERITE.
Schererit, Stromeyer. Naturliche Naphthaline, Schcrer.
Combination of carbon and hydrogen. Carbon 73·0, hydrogen 24·0—Macaire.
* So named in honour of the eminent chemist Chas. Hatchett, Esq. F.R.S.
R 2
[page] 394
Exists in loosely aggregated, whitish, feebly shining, pearly, crystalline grains, and foliæ, which generally occur in nests. It is rather heavier than water, does not feel greasy, is very friable, and has no taste. It melts readily into a colourless liquid at a temperature of 112°, and in that state resembles a fatty oil, and penetrates paper in the same manner; the spots, however, thus produced disappear when the paper is heated. On cooling, the melted mineral crystallizes in four-sided acicular crystals. When exposed to fire, it inflames and burns completely away, with a feeble aromatic smell. Is insoluble in water, but melts easily in alcohol, ether, and concentrated sulphuric acid. Occurs in beds of lignite at Ussnack near St Gall, and at Bagh in the Wester-wald, Switzerland; and was named after its discoverer, Captain Scherer.
OZOKERITE.*
Glocker.
Sp. Gr. 0·955. Soft.
Colour yellowish-brown; translucent; has a slight odour of bitumen, and softens by the heat of the hand, so that it may be kneaded like wax. Fuses readily, and emits a stronger bituminous odour whilst doing so; burns with a clear bright flame, and leaves no residue. Not affected by acids or water, and only slightly by heated alcohol. Is soluble in ether or oil of turpentine, with a yellow colour.
Occurs in considerable masses at Slanik in the Buchau district of Moldavia, where it has been long used by the peasants for fuel.
MELLITE.
Pyramidal Mellichrone Resin, M. Honeystone, J. Honigstein, W. Mellite, H.
Combination of mellitic acid, alumina, and water.
| Mellitic acid | 46·0 | 41·4 |
| Alumina | 16·0 | 14·5 |
| Water | 380—Klanroth. | 44·1—Wöhler. |
Sp. Gr. 1·58—1·66.
Colour honey-yellow, reddish, or brown. Primary form the octahedron with square base, in which it also occurs with the summits of the octahedron truncated. Cleavage parallel to the planes
* From οζειν, smelling, and xspos, wax.
[page] 395
of the primary, but difficultly obtained fracture conchoidal; transparent or translucent; lustre resinous, inclining to vitreous. It is slightly resino-electric by friction; before the blowpipe it becomes of an opake-white with black spots, and is at length reduced to ashes; when burnt in the open air, neither smoke nor flame is observable, and it eventually acquires the colour and consistence of chalk. Soluble in nitric acid.
The mellite is a rare mineral, having hitherto only been found at Artem in Thuringia. It occurs in bituminous wood, and earthy coal, and is generally accompanied by sulphur.
RETINASPHALT.
Retinite, J. Retinasphalt, Hatchett.
Contains, according to the researches of Hatchett,
| Resin soluble in alcohol | 55 |
| Insoluble bituminous matter | 41 |
| Earthy substances | 5 |
Sp. Gr. 1·1—1·2. H. = 1·0—2·0.
It occurs in irregular opake masses of a pale brownish-yellow colour, having a glistening lustre and imperfect conchoidal fracture. It is brittle and soft; when placed on hot iron it melts, smokes, and burns with a bright flame, emitting a fragrant odour. Partly soluble in alcohol, with an unctuous residue. Though this species exhibits characters somewhat different from those of bitumen, it yet appears to be more nearly allied to that than to any other substance. The variety from Bovey Tracey in Devonshire, where it is found accompanying brown coal, has a dry earthy texture; while that from Wolchow in Moravia is hard and resinous.
FOSSIL COPAL.
Fossil Copal, Highgate Resin.
Fossil copal or Highgate resin was found in considerable quantity in the bed of blue clay of which Highgate Hill, near
[page] 396
London, in great measure consists. It is in irregular pieces of a light-yellowish and dirty-brown colour, somewhat translucent, and with a resinous lustre; it is brittle, yields easily to the knife, and is but little heavier than water, its specific gravity being only 1·046. It gives out a resinous aromatic odour when heated, and melts into a limpid fluid; when applied to the candle it takes fire and burns with a clear yellow flame and abundance of smoke, as is the case with other resins; before the blowpipe it burns away without leaving any perceptible ash.
It has been found in considerable abundance at Wolchow in Moravia.
[page] 397
APPENDIX:
CONSISTING PRINCIPALLY OF MINERALS OF WHICH NO AUTHENTIC ANALYSIS HAS HITHERTO BEEN PUBLISHED, OR RESPECTING WHICH FULLER INFORMATION IS REQUIRED BEFORE THEIR PLACE IN THE GENERAL ARRANGEMENT CAN WITH ACCURACY BE DETERMINED.
ARSENICAL ANTIMONY.
Arsenik-Spiesglanz, L.
Sp. Gr. 6·2. H. = 2·0—4·0.
In kidney-shaped masses. Colour tin-white. Occasionally splendent, sometimes dull. Before the blowpipe it melts, and at the same time emits considerable fumes of arsenic and antimony.
This species was noticed by Zippe at Przibram in Bohemia, where it occurs in metallic veins, associated with blende, antimony, sparry iron, &c.
ARSENIURET OF MANGANESE.— Kane.
Contains manganese 45·50, arsenic 51·80, oxide of iron 2·70—Kane.
Sp. Gr. 5·55.
Occurs massive and botryoidal; of a greyish-white colour. Composition granular. Fracture uneven. When exposed to the atmosphere it becomes coated with a black powder. Before the blowpipe it burns with a blue flame, attended by a white smoke, and the odour of garlic. Is soluble in nitric acid.
Locality, Saxony.
ATELESTITE.— Shepard.
Heavy. H. about 3·0.
Crystalline, in structure resembling sphene. Colour pure sulphur-yellow. Lustre between resinous and adamantine; transparent or translucent. Before the blowpipe affords indications of bismuth.
Locality, Schneeberg in Saxony.
[page] 398
BATRACHITE.— Shepard.
Sp. Gr. 3·038. H. = 5·0.
Massive, exhibiting traces of a rhombic prism of 115°. Composition impalpable. Cleavage parallel to the sides and shorter diagonal of the prism, but mostly indistinct. Fracture small conchoidal. Colour light greenish-grey, to almost white. Streak white. Lustre resinous or vitreous, more inclined to the latter. Before the blowpipe per se it is infusible, without any perceptible change of colour to the flame. Heated in the matrass it affords a little moisture. It is slowly soluble in salt of phosphorus, leaving a silica residue; with soda it fuses with difficulty into a dark-coloured pearl. From these and other experiments it has been inferred by Merlet to be a silicate of magnesia.
Is found at Rizoni, a mountain in Southern Tyrol.
BERZELINE.— Necker.
H. about 5·0.
In extremely minute white octahedral crystals, whose surface is dull. Slightly translucent; and having a vitreous lustre on the fracture. Very brittle; but devoid of distinct cleavage. Before the blowpipe in the forceps it is fusible, though with difficulty, into a pale glass. Forms with heated muriatic acid a greenish coloured jelly.
From Galloro near La Riccia in the Roman States, where it accompanies crystals of black garnet and pinchbeck-brown mica, in the drusy cavities of an augitic rock.
BEUDANTITE—Levy.
H. = 4·0—4·5:
Occurs in small closely aggregated crystals, being slightly obtuse rhombs (viz. 92° 30′) with the summits truncated. Colour black. Translucent in thin fragments, and of a deep-brown colour by transmitted light. Lustre resinous. Streak greenish-grey. Cleavage easily effected perpendicular to the axis of the rhomb. The only substances Dr Wollaston could detect in it were the oxides of lead and iron.
Beudantite is found associated with brown iron ore at Horhausen, in the district of Nassau, on the Rhine.
[page] 399
BIOTINE.
Biotina, Monticelli Biotin, Leonhard.
Sp. Gr. 3·11. Scratches glass.
Colour white or yellowish. Transparent and limpid. Lustre brilliant. Fracture vitreous, inclining to conchoidal.Presents double refraction. Is not affected by the blowpipe, and is only partly soluble in nitric acid.
Sig. Monticelli noticed this mineral among the volcanic debris of Vesuvius, and named it in honour of M. Biot. It is easily distinguished from other species with which it is associated, by the superior brilliancy of its lustre.
BREISLAKITE.
Occurs in delicate capillary prismatic crystals of a reddish or chestnut-brown colour, coating the cavities of certain lavas. Its fibres are flexible, its lustre semi-metallic. It contains silica, alumina, iron, and a considerable proportion of copper. Before the blowpipe per se it fuses readily into a brilliant and magnetic black scoria; with borax forms a green glass, which becomes colourless in cooling; and with salt of phosphorus a green globule, which reddens in the reducing flame. It accompanies nepheline, pyroxene, and other Vesuvian minerals; and is met with both at that locality, and at Capo di Bove near Rome. (Manual.)
CHELMSFORDITE.— Cleaveland.
Sp. Gr. 2·4.
In rectangular prisms, occasionally modified; also amorphous. Presents one imperfect cleavage. Contains 75 per cent. of silica.
It occurs in Chelmsford in the United States, associated with quartz, mica, and apatite.
CHLOROPHÆITE.*—Dr M Culloch.
This mineral, when newly broken, is of a green colour, varying from the fine transparent yellow green of olivin, which it
[page] 400
sometimes resembles, to the dull muddy green of steatite, to which it then bears an equal similitude. After a few hours' exposure it turns darker, and shortly becomes black. The fracture is generally conchoidal. It is so soft as to be scratched by a quill, and is brittle. Specific gravity 2·020. Before the blowpipe it remains unchanged, neither cracking nor sensibly altering its colour nor translucency. Consists principally of silica and iron, with a little alumina.
* Chlorophæite, from the Greek, in allusion to its appearing of a green colour (when newly broken).
It is found imbedded in the amygdaloid of Scuirmore in the Isle of Rum, the base being either a basalt or a black indurated clay-stone. It also occurs in Fifeshire, in Iceland, and at Gill and Southbury in the United States. The nodules are generally round, and vary from the size of a radish seed to that of a pea and upwards; sometimes they are hollow.
CHONIKRITE.—Kobell.
Hydro-silicate of alumina, magnesia, and lime.
| Contains Silica | 35·69 |
| Alumina | 17·12 |
| Magnesia | 22·50 |
| Lime | 12·00 |
| Protoxide of iron | 1·46 |
| Water | 9·00—Kobell. |
H. between 2·0 and 4·0.
Occurs massive. Composition impalpable. Fracture uneven and imperfectly conchoidal. Lustre glimmering or dull. Colour white, with shades of yellow and grey. Translucent often only on the edges. It melts readily before the blowpipe into a greyish glass, and with borax fuses slowly into a globule coloured by iron. It is easily decomposed by concentrated muriatic acid.
Occurs in rounded masses at Elba.
COBALTIC GALENA.
Cobaltic Galena, or Cobaltic Lead Glance, J. and M. Cobaltbleierz, Haus.
Sp. Gr. 8·44. Soft and sectile.
Contains lead 62·89, arsenic 22·47, sulphur 0·47, iron 2·11, cobalt 0·94, arsenical pyrites 1·44 (the loss of 9·76 being attributed to intermixed calcareous spar)—Du Menil. In minute moss-like groups of crystals, or cleavable masses. Colour leadgrey, inclining to blue; opake; lustre metallic and shining. Soils a little. Splits into fragments before the blowpipe, and communicates a smalt-blue colour to glass of borax.
It occurs in a vein of clay-slate with brown spar, traversing grauwacke, at Clausthal in the Hartz.
[page] 401
DERMATINE.— Breithaupt.
Sp. Gr. 2·136. H. about 2·0.
In reniform masses, rarely globular, and in thin coatings or crusts. Colour dark olive-green or liver-brown; with low resinous lustre. Translucent on the edges. Fracture conchoidal. Feels greasy, and does not attach itself to the moist lip. Streak yellow, inclining to grey. Emits an argillaceous odour when breathed upon. Splits and becomes somewhat friable before the blowpipe, assuming at the same time a darker hue.
From the serpen time quarry near Waldheim in Saxony.
GREEN IRON EARTH.
Griine Eisenerde, W. Hypochlorite, Schüler.
Contains oxide of bismuth 13·03, silica 50·24, alumina 14·65, oxide of iron 10·54, phosphoric acid, with traces of manganese, 9·62—Schüler.
Occurs in reniform, botryoidal, and globular masses; structure impalpable; colour siskin-green, passing into black and yellow; lustre resinous and dull; streak yellowish-grey; brittle; becomes brown and black before the blowpipe, but does not melt; nor is it soluble in nitric acid. Is found at Schneeberg in Saxony, and in the county of Sayn in Germany.
HERRERITE.— Shepard.
Contains carbonic acid 31·86, tellurium 55·58, peroxide of nickel 12·32—Herrera.
Sp. Gr. 4·3. H. = 4·0—4·5.
Occurs in reniform masses. Cleavage in three directions, affording rhomboidal fragments, whose angles are incapable of measurement on account of the curvatures of the faces. Lustre vitreous to pearly, and shining on fresh surfaces. Colour pistachio-, emerald-, and grass-green. Streak yellowish-grey. Translucent. Brittle. Before the blowpipe on charcoal it at first becomes grey, and afterwards emits a white smoke, which adheres to tthe charcoal; in the reducing flame it becomes of a beautiful grass-green. Heated in an open tube, it gives off abundant white fumes, which adhere to the glass; and on examining these with a microscope they appear to consist of numerous white transparent globules.
It occurs at Albarradon in Mexico, traversing transition limestone in a metallic vein, consisting chiefly of ores of lead, silver, muriate of silver, and iodic silver. When decomposed it appears earthy and dull, with a somewhat fibrous structure.
[page] 402
MARCELIKE.—Beudant.
Silicate of Manganese from Piedmont— Berzelius.
Sp. Gr. 3·8. Scarcely scratches glass.
A silicate of the deutoxide of manganese without water.
| Silica | 15·17 | 26·00 |
| Oxide of manganese | 75·80 | 67·23 |
| Oxide of iron | 4·14 | 1·23 |
| Alumina | 2·80 | 3·00 |
| Lime | 0·00 | 1·40 |
| Magnesia | 0·00—Berzelius. | 1·40—Berthier. |
Occurs crystallized in octahedrons, with a square base. Colour greyish-black, with a slightly metallic or vitreous lustre; yields no water when calcined; is fusible before the blowpipe, without alteration of colour, imparting to soda a distinct re-action of the oxide of manganese. Is acted upon by muriatic acid, with disengagement of chlorine, leaving a gelatinous residue.
Marceline forms considerable repositories in mica-schist, in the valley of Saint Marcel in Piedmont.
MICROLITE.—Shepard.
Sp. Gr. 4·75—50. H. = 5·0—5·5.
Primary form, the regular octahedron. Secondary, the same having all its edges bevelled. Occurs in minute crystals. Cleavage imperfect, parallel with the primary faces. Colour straw-yellow, passing into dark reddish-brown. Transparent, to translucent on the edges. Lustre resinous. Fracture conchoidal, passing into uneven. Surface of the primary faces dull. Streak white, except where the colour of the mineral is brown; it then resembles the colour. Alone, before the blowpipe, it remains unaltered. Is slowly soluble in borax, communicating to it a yellow colour, which grows paler on cooling; but remains transparent unless subjected to flaming, when it becomes nebulous, and presents, on cooling, a pale yellow enamel. It is not readily acted upon by soda, and is insoluble in nitric acid. The chief ingredient of this substance is supposed to be the oxide of cerium. It is found at Chesterfield in Massachusetts, imbedded in albite, along with the green and red tourmalines from that locality.
MONAZITE.—Breithaupt.
Sp. Gr. 4·924. H. = 5·0.
Primary form, a doubly oblique prism; colour hyacinth- or brick-red; translucent on the edges; lustre vitreous; streak white. Heated to redness in a glass tube, it suffers no change. In
[page] 403
the strongest heat of the blowpipe it only melts upon the edges, where it becomes greenish-yellow. Treated with soda or borax in the reducing flame, it dissolves with effervescence into a light-yellowish opake mass. Dissolved in salt of phosphorus, the globule is yellow while warm, but on cooling becomes yellowish-green and opake. From these and other experiments, it has been inferred that monazite is a compound of the oxide of uranium, with some one or more of the earths.
It is found in the mountains about Ilmensee in Siberia; and has been described also under the name of Mengite.
MONTICELLITE.—Brooke.
H. = 5·0—6·0.
In small imbedded crystals, having the general aspect of quartz. Colour yellowish; sometimes nearly transparent, and colourless.
Occurs at Vesuvius imbedded in a crystalline carbonate of lime, along with particles of black mica and minute crystals of pyroxene. Its name was proposed by Brooke, in honour of the celebrated Neapolitan mineralogist Monticelli.
MYSORINE.— Beudant.
Contains deutoxide of copper 60·75, peroxide of iron 19·50, carbonic acid 16·70, silica 2·10—Thomson.
Sp. Gr. 2·62.
Qccurs massive, having an impalpable composition. When pure its colour is brownish-black, but it is frequently tinged green and red from admixture with carbonate of copper and oxide of iron. Fracture small conchoidal. Is soluble in acids when pure, but if mixed with foreign matter the solution deposits a red precipitate. This rare substance was noticed by Dr Heyne, near the eastern frontier of Mysore in Hindustan, where it is mentioned as forming beds in the older rocks.
NEOCTESE.*—Beudannt.
Hydrated arseniate of iron.
Contains arsenic acid 50·78, peroxide of iron 34·85, water 15·55—Berzelius.
This substance occurs crystallized, though in ill-pronounced individuals. Its primary form is supposed to be a right square prism. Its colour is green, but when heated it gives off its moisture and becomes yellow. To the fluxes it imparts the colour of iron, emitting at the same time a powerful alliaceous odour.
It occurs at St Antonio Perreira, near Villa Ricca, in Brazil,
* From xos, new, and xτηois, acquisition,
[page] 404
imbedded in or mixed with compact hydrate of iron; and appears to be closely allied to arseniate of iron. Page 234.
NONTRONITE— Berthier.
Contains silica 44·0, peroxide of iron 29·0, alumina 3·6, magnesia 2·1, water 18·7, clay 1·2—Berthier.
This substance resembles clay. It is of a pale straw, or canary-yellow colour, with a greenish tinge. Opake. Unctuous to the touch; and exhaling an odour when breathed upon. Acquires a fine polish and resinous lustre from the friction of softer bodies. Is not reduced to powder, but becomes lumpy under the pestle; and does not affect the magnet. When immersed in water it disengages numerous air bubbles, becomes translucent on the edges, and increases in weight. When slightly heated, it gives off water, and assumes the colour of red oxide of iron; and when calcined becomes sensibly magnetic.
Nontronite was noticed by Berthier in small kidney-shaped masses among manganese in the arrondissement of Nontron in France.
OSMELITE— Breithaupt.
Sp. Gr. 2·79—2·83. H. = 40—5·0.
Massive; in thin prismatic concretions, scopiformly or stellu-larly arranged. Colour greyish-white inclining to smoke-grey. Translucent. Lustre not great, between pearly and vitreous. Feels greasy, and when breathed upon emits an argillaceous odour; hence its name, from tffiTj, smell. Cleavage in one direction. In the mouth it feels as if about to dissolve, although no change takes place.
Occurs superimposed on calcareous spar, mixed with datho-lite, in trachyte at Niederkirchen, near Wolfstein, on the Rhine.
PHENAKITE.— Nordenskiold.
Silicate of Glucina.
Contains silica 55·14, glucina 44·47, alumina and magnesia 0·39—Hartwall.
Sp. Gr. 2·969. H. above 6·0.
Primary form a rhomboid of 115° 25'.
[page] 405
Cleavage parallel to n. Colour bright wine-yellow, inclining to red or white. Varying from transparent to opake. Lustre vitreous. Not affected by acids, and with difficulty fusible before the blowpipe.
Occurs associated with beryl in the Perm district of Siberia. At first sight it is described as being readily mistaken for quartz; and is hence named from psvag, the deceiver.
POONAHLITE.— Brooke.
H. = 5·0—5·5.
In slender rhombic prisms of 92° 20°
This species at first sight much resembles needlestone, with which it also nearly corresponds in hardness. Its crystals, however, traverse the matrix instead of forming groups in the cavities, and have not been observed with natural terminations. It occurs along with the fine apophyllites from Poonah in Hindustan.
PROTHEITE.— Ure.
Heavy. Scratches glass.
In rectangular prisms, the faces being striated longitudinally. Colour olive-green or white. Nearly opake in large specimens, translucent in smaller. Lustre vitreous, inclining to adamantine. Is infusible before the blowpipe, and becomes electric by friction.
Has lately been discovered in the Zillerthal in the Tyrol.
PYROSKLERITE.— Kobell.
Sp. Gr. 2·71. H. = 2·5—3·5.
Silicate of alumina and magnesia.
Contains silica 37·03, alumina 13·50, magnesia 31·62, protoxide of iron 3·52, green oxide of chrome 1·43, water 11·00—Ko-bell.
Massive. Cleavage parallel with the faces of a rhombic prism, Fracture uneven and splintery. Lustre on the cleavage faces feebly pearly, in other directions dull. Colour apple-green or light greenish-grey. Before the blowpipe it fuses with difficulty into a greyish glass. With borax it slowly yields a glass coloured by chrome. It is decomposed when in the state of powder by concentrated sulphuric acid.
It occurs at Elba with chonikrite; and seems to be closely re: lated to picrolite.
[page] 406
SOMERVILLITE.
H. under 60.
Colour pale dull yellow. Lustre vitreous. Cleavage perfect, parallel to P.
It decrepitates before the blowpipe, fusing per se into a grey coloured globule, and with borax into a transparent one.
Somervillite occurs among the ancient scoriæ of Vesuvius, associated with black mica and other minerals. The determination of this species is due to Brooke, who named it in compliment to Dr Somerville, from whom he obtained the specimens. It may be distinguished from idocrase by its comportment before the blowpipe, as the latter does not decrepitate; and when it fuses, which it does with greater difficulty, it yields globules of a greenish tinge.
STEINMANNITE.
Sp. Gr. 6·833. H. = 2·5.
Primary form the cube. Secondary form the regular octahedron.
Cleavage parallel to the cube, imperfect and scarcely visible. Fracture uneven. Surface of the crystals smooth. Lustre metallic. Colour pure lead grey. Botryoidal; massive. Composition fine granular; in some varieties a curved lamellar composition is visible. Composition also compact, sometimes porous. When heated before the blowpipe on charcoal, it decrepitates with violence. Its powder, heated, emits the odour of sulphurous acid, and a metallic globule remains, as in the case of galena, but which finally yields a distinct button of silver. It appears to consist of lead, antimony, silver, and sulphur.
It is found at Przibram with quartz, blende, and iron pyrites.
STILPNOMELAN— Glocker.
Sp. Gr. 3·27—3·4. H. = 30—4·0.
In crystalline, lamellar, and fibrous masses, of a black or greenish colour; lustre vitreous; cleavage in one direction; streak olive-green to liver-brown. Insoluble in acids; fusible before the blowpipe into a bluish-black scoria.
Localities, Obergrund and Zinkmantel in Silesia.
[page] 407
SYLVYNE.— Beudant.
Muriate of Potash. Chlorure de Potassium.
Consists of chlorine 47·46, potassium 52·54.
Soluble, with the taste of common salt. Crystallizes in the form of the cube, and cleaves parallel to the faces of that solid. When in solution the addition of muriate of platina produces a yellow precipitate. Treated with sulphuric acid, it leaves, after evaporation, acicular crystals, which do not effloresce in the air.
It is found in small quantity, mixed with salt, in the mines of Hallein and Berchtesgaden, in Saltzburg, where it was first noticed by M. Vogel.
TEPHROITE.— Leonhard.
Sp. Gr. 4·116. H. = 5·0—6·0.
Massive and compact. Colour ash-grey, tarnishing black. Lustre adamantine. Streak somewhat paler than the mineral. Cleavage perfect in several directions; two of them forming together a right angle. Fracture imperfect conchoidal, or uneven. Forms a black slag before the blowpipe.
Occurs with franklinite and red zinc, at Sparta in the United States.
THENARDITE.— Necker.
Sp. Gr. 2·73.
Anhydrous sulphate of soda, mixed with a minute proportion of the sub-carbonate of soda.
| Sulphate of soda | 99·78 |
| Sub-carbonate of soda | 0·22—Casaseca. |
Primary form a right rhombic prism of about 125° and 55°.
Occurs in rhombic octahedrons, simple, or modified on the summit, which are grouped one upon another.
Cleavage parallel to the faces of the prism; most distinct parallel to its base. Colour white or reddish; transparent or translucent. Superficially efflorescent. Before the blowpipe, in the matrass, yields no water. Soluble in water.
This substance occurs in crystalline coatings at the bottom of certain lakes, at a place called Les Salines Espartines, five leagues from Madrid, and two and a half from Aranjuez; where it is collected for the fabrication of artificial sub-carbonate of soda.
[page] 408
UWAROWITE.*— Hess.
H. above 6·0.
In extremely small rhomboidal dodecahedrons, of an emeraldgreen colour; transparent In the matrass affords no water, and does not alter its colour. Before the blowpipe is infusible and unalterable without addition; and colours glass of borax chromegreen. By Hess this is considered a chrome-coloured garnet, but from that species it is distinguished by its infusibility before the blowpipe, by its not decrepitating, and not altering either colour or appearance.
It occurs at Bissersk in the Ural Mountains.
ZURLITE.†— Monticelli.
Sp. Gr. 3·27. H. about 6·0.
Occurs in rectangular four-sided prisms, lengthened in the direction of their axis, and having occasionally their lateral edges replaced. Colour asparagus-green, inclining to grey. Opake. Lustre resinous. Cleavage indistinct. Fracture conchoidal. Surface of the crystals rough, frequently covered with a white coating. It is infusible before the blowpipe, but yields with borax a black glass. Nitric acid dissolves it, partly with effervescence, and the solution becomes yellow. Zurlite is a Vesuvian mineral; it is generally found in large distinct crystals, associated with calc spar and other species.
* Named after the President of the Academy at St Petersburg, M. Uwarow.
† In compliment to the Neapolitan minister, Sig. Zurlo.
[page 409]
INDEX.
| Page. | |
| Abrazite | 49 |
| Acanticonite | 30 |
| Achirite | 322 |
| Achmite | 151 |
| Acicular arseniate of copper | 332 |
| bismuth glance | 278 |
| olivenite | 332 |
| Acide arsenieuz | 281 |
| Actynolite | 58 |
| Adamantine-spar | 66 |
| Adhesive slate | 36 |
| Adipocere mineral | 393 |
| Adularia | 115 |
| .dEschynite | 261 |
| Agalmatolite | 119 |
| Agaric mineral | 162 |
| Agate | 10 |
| Arabandine | 245 |
| Alabaster | 163 |
| Alalite | 51 |
| Alaun | 202 |
| Alaunstein | 203 |
| Albin | 108 |
| Albite | 137 |
| Allagite | 244 |
| Allanite | 263 |
| Allochroite | 17 |
| Allophane | 71 |
| Alloy of iridium and osmium | 339 |
| Almandine | 14 |
| ruby- | 82 |
| Alum | 202 |
| Alumine hydraté silicifère | 70 |
| fluatée alkaline | 204 |
| hydro-phosphatée | 156 |
| sous-sulfatee | 155 |
| sulphatée alkaline | 202 |
| Aluminite | 155 |
| Alumocalcite | 13 |
| Alumstone | 203 |
| Alunite | 203 |
| Alunogene | 156 |
| Amalgam | 378 |
| Amazon stone | 117 |
| Amber | 39 |
| Amblygonic augite spar | 158 |
| Amblygonite | 158 |
| Amethyst | 5 |
| Amianthus | 59 |
| Ammonalum | 207 |
| Ammoniaque muriatée | 201 |
| sulphatée | 201 |
| Amphibole | 54 |
| Amphigène | 105 |
| Amphodelite | 34 |
| Analcime | 138 |
| Anatase | 253 |
| Andalusite | 106 |
| Anglarite | 230 |
| Anglesite Anhydrite | 365 176 |
| Anhydrous silicate of iron | 224 |
| Ankerite | 169 |
| Anorthite | 35 |
| Anorthitic melane ore | 263 |
| Anorthotomous feldspar | 35 |
| Anthophyllite | 33 |
| Anthracite | 386 |
| Anthraconite | 164 |
| Antimonoker | 349 |
| Antimonphyllite | 349 |
| Antimoine blanc | 348 |
| gris | 345 |
| natif | 343 |
| oxydé | 348 |
| oxydé sulfuré | 347 |
| terreux | 349 |
| rouge | 347 |
| sulfuré | 345 |
| Antimon blende | 347 |
| nickel | 291 |
| silber | 294 |
| silber blende | 300 |
| Antimonial grey copper | 313 |
S
[page] 410
| Antiraonial nickel | 291 |
| ochre | 349 |
| silver | 294 |
| Antimoine sulfuré niekelifère | 291 |
| Antimonphyllite | 349 |
| Apatite | 170 |
| Aphanèse | 331 |
| Aphérèse | 327 |
| Aphrite | 162 |
| Aphthitalite | 196 |
| Aplome | 16 |
| Apophyllite | 108 |
| Aquamarine | 99 |
| Arendalite | 30 |
| Arfwedsonite | 61 |
| Argent antimonial | 294 |
| antimonié sulfuré | 300 |
| antimonié sulfuré noix | 298 |
| carbonaté | 305 |
| muriaté | 305 |
| natif | 293 |
| rouge | 300 |
| sulfuré | 296 |
| antimonifère et cup-rifère | 299 |
| flexible | 297 |
| telluré | 295 |
| vitreuse | 296 |
| Argentiferous copper glance | 303 |
| sulphuret of copper | 303 |
| gold | 337 |
| Argile | 35 |
| smectique | 37 |
| Argyrythrose | 300 |
| Arragonite | 165 |
| Arseniate of cobalt | 289 |
| of copper | 329 |
| of iron | 234 |
| of lead | 364 |
| of lime | 180 |
| Arsenic natif | 280 |
| oxydé | 281 |
| sulfuré jaune | 283 |
| sulfuré rouge | 281 |
| Arsenical antimonial silver | 295 |
| antimony | 397 |
| bismuth | 279 |
| copper pyrites | 314 |
| iron | 213 |
| nickel | 292 |
| pyrites | 284 |
| silver | 295 |
| Arsenik kies | 213 |
| wismuth | 279 |
| Arsenikbluthe | 180, 281 |
| Arsenikeisen | 284 |
| Arseniksaures blei | 364 |
| Arsenik-spiesglanz | 397 |
| Arseniuret of manganese | 397 |
| Arsen-silber Mende | 300 |
| Asbestus | 59 |
| Asparagus stone | 171 |
| Asphalt | 389 |
| Atacamite | 325 |
| Atelestite | 397 |
| Augite | 49 |
| Auriferous native silver | 294 |
| Automalite | 76 |
| Avanturine | 4 |
| Axe stone | 92 |
| Axinite | 39 |
| Axotomous antimony glance | 346 |
| arsenical pyrites | 264 |
| augite spar | 53 |
| habroneme malachite | 331 |
| iron ore | 257 |
| leadbaryte | 359 |
| stone | 131 |
| triphane spar | 22 |
| Azure copper ore | 319 |
| Azurite | 159, 319 |
| Babingtonite | 53 |
| Baikalite | 52 |
| Balas ruby | 62 |
| Barystrontianite | 193 |
| Baryte carbonatée | 187 |
| sulphatée | 189 |
| Barytes | 189 |
| Barytine | 189 |
| Baryto-calcite | 189 |
| Batrachite | 398 |
| Berg crystal | 2 |
| Bergmannite | 144 |
| Bergmehl | 39 |
| Berg-milch | 162 |
| Bern-stein | 392 |
| Berthierite | 344 |
| Beryl | 99 |
| Berzeline 316. | ,398 |
| Berzelite 45 | ,360 |
| Beudantite | 398 |
| Biegsamer silber glanz Bildstein | 297 119 |
| Bimstein | 140 |
| Biotine | ·99 |
| Bismuth blende | 279 |
| cobalt ore | 287 |
| glanz | 277 |
| natif | 276 |
[page] 411
| Bismuth ochre | 279 |
| oxydé | 279 |
| sulfuré | 303, 277 |
| cuprifère | 278 |
| plumbo-cuprifère | 278 |
| Bismuthine | 277 |
| Bismuthic silver | 303 |
| Bi-sulphuret of copper | 310 |
| Bitter spar | 167 |
| Bitume asphalte | 389 |
| elastique | 389 |
| glutineux | 388 |
| napthe | 387 |
| petrole | 388 |
| solide | 389 |
| Bitumen | -388 |
| Bituminous coal | 390 |
| Black chalk | 39 |
| cobalt ochre | 288 |
| copper | 318 |
| hematite | 240 |
| iron ore | 240 |
| manganese | 237 |
| tellurium | 342 |
| wad | 241 |
| Blatter-zeolith | 25 |
| teliur | 342 |
| Blattricher schwarz braun-steinerz | 237 |
| Blau-eisenstein | 151 |
| Bleierde | 398 |
| Bleifahlerz | 352 |
| Bleigelb | 367 |
| Bleiglanz | 349 |
| Blei-hornerz | 362 |
| Blei-vitriol | 365 |
| Blende | 371 |
| charbonneuse | 386 |
| Blind coal | 386 |
| Bloedite | 204 |
| Bloodstone | 10 |
| Blue carbonate of copper | 319 |
| iron ore | 229 |
| lead | 351 |
| vitriol | 323 |
| Bole | 38 |
| Bolognian stone | 191 |
| Boracite | 186 |
| Borate of lime | 179 |
| of magnesia | 186 |
| of soda | 199 |
| Borax | 199 |
| Boraxsaures natron | 199 |
| Bornine | 280 |
| Botryogene | 233 |
| Botryolite | 180 |
| Bournonite | 352 |
| Brachy typous lead baryte | 362 |
| lime haloide | 184 |
| manganese ore | 237 |
| parachrose baryte | 228 |
| Braunite | 237 |
| Brauner glaskopf | 221 |
| Bretslakite | 399 |
| Breunnerite | 184 |
| Brevicite | 127 |
| Brewsterite | 45 |
| Brewsteritic kouphone spar | 45 |
| Bright white cobalt | 284 |
| Brittle sulphuret of silver | 298 |
| silver glance | 298 |
| Brochantite | 324 |
| Brogniartin | 205 |
| Bronzite | 62 |
| Brookite | 256 |
| Brown iron ore | 220 |
| hæmatite | 221 |
| spar | 228 |
| Brucite | 88 |
| Bucklandite | 54 |
| Bucholzite | 107 |
| Buntkupfererz Bustamite | 310 245 |
| Buttermilk silver | 306 |
| Cacholong | 10 |
| Cadmiferous blende | 373 |
| Calaite | 69 |
| Calamine | 375 |
| Calamite | 57 |
| Calc spar | 160 |
| Calcareous spar | 160 |
| Calcedony | 9 |
| Calcedonite | 360 |
| Calomel | 380 |
| Candite | 82 |
| Cannel coal | 390 |
| Capillary pyrites | 291 |
| Carbo-cerine | 266 |
| Carbonate of barytes | 187 |
| of cerium. | 266 |
| of copper | 320 |
| of iron | 228 |
| of lead | 356 |
| of lime | 160 |
| of lime and soda | 307 |
| of magnesia | 183 |
| of magnesia and iron | 184 |
| of manganese | 246 |
| of silver | ;*os |
[page] 412
| Carbonate of soda | 196 |
| of strontian | 192 |
| of uranium | 270 |
| of zinc | 375 |
| Carinthin | 55 |
| Carnelian | 10 |
| Cassitéite | 250 |
| Cat's eye | 5 |
| Cawk | 191 |
| Celestine | 193 |
| Cererit | 262 |
| Cerinstein | 262 |
| Cerine | 263 |
| Cerite | 262 |
| Cerium oxydé yttrifère | 266 |
| silicifère | 262 |
| siliceux noir | 263 |
| Cerolite | 40 |
| Ceruse | 356 |
| Ceylanite | 82 |
| Chabasie | 145 |
| Chalk | 164 |
| Chalkolite | 270 |
| Chalkopyrite | 314 |
| Chamoisite | 224 |
| Chaux arseniatée | 180 |
| boratés siliceuse | 179 |
| carbonatée | 160 |
| magnesifère | 167 |
| datolit | 179 |
| fluatée | 172 |
| nitratée | 179 |
| phosphatée | 170 |
| sulphatée | 177 |
| anhydre | 176 |
| Chalmsfordite | 399 |
| Chiastolite | 41 |
| Childrenite | 158 |
| Chlorquecksilber | 380 |
| Chloride of silver | 305 |
| Chlorite | 120 |
| Chloropal | 224 |
| Chlorophæite | 399 |
| Chiorophane | 176 |
| Chlorsilber | 305 |
| Chonikrite | 400 |
| Christianite | 35 |
| Chromate of iron | 368 |
| of lead | 275 |
| of lead and copper | 369 |
| Chromeisenstein | 275 |
| Chrome ochre | 275 |
| Chromsaures blei | 368 |
| Chrysoberyl | 80 |
| Chrysocolla | 322 |
| Chrysolite | 85 |
| Chrysoprase | 10 |
| Chusile | 86 |
| Cimolite | 38 |
| Cinnabar | 379 |
| Cinnamon stone | 19 |
| Clausthalie | 354 |
| Clays | 35 |
| Cleavelandite | 137 |
| Coal | 390 |
| Cobalt arseniaté | 282 |
| arsenical | 286 |
| eclatant | 284 |
| gris | 284 |
| ochre | 289 |
| oxydé noir | 288 |
| pyriteux | 287 |
| sulfuré | 287 |
| vitriol | 290 |
| Cobalt-bleierz | 400 |
| Cobalt-bloom | 289 |
| Cobalt kies | 287 |
| Cobaltic galena | 400 |
| Cobaltine | 284 |
| Coccolite | 53 |
| Cockscomb pyrites | 212 |
| Colophonite | 17 |
| Columbite | 272 |
| Compact bitumen | 389 |
| Common jade | 92 |
| Comptonite | 128 |
| Comptonitic kouphone spar | 128 |
| Conite | 185 |
| Condrodite | 88 |
| Copper, Black | 318 |
| glance | 308 |
| Green | 322 |
| mica | 330 |
| nickel | 294 |
| pyrites | 314 |
| Cordierite | 42 |
| Corindon | 67 |
| Corneous lead ore | 362 |
| manganese | 244 |
| silver | 305 |
| Corundum | 64 |
| Cotunnite | 961 |
| Couzeranite | 122 |
| Covelline | 3lO |
| Craie | 164 |
| Crichtonite | 257 |
| Crocoise | 368 |
| Cronstedtite | 223 |
| Cross-stone | 43 |
| Cryolite | 204 |
[page] 413
| Cube ore | 234 |
| Cuivre arseniaté ferrifère | 335 |
| lameliiforme | 330 |
| enprisme rhomboidal oblique | 331 |
| octaedre aigu | 332 |
| primitif | 329 |
| carbonaté bleu | 319 |
| terreux | 322 |
| vert | 329 |
| dloptase | 322 |
| gris | 311 |
| muriaté | 325 |
| natif | 307 |
| oxidé noir | 318 |
| rouge | 316 |
| oxydulé | 316 |
| phosphaté | 827 |
| pyriteux | 314 |
| hepatique | 318 |
| selenié argental | 304 |
| sulfuré | 308 |
| sulfaté | 323 |
| argentifère | 303 |
| bismutifère | 278 |
| prismatoide | 354 |
| velouté | 325 |
| vitreux | 308 |
| Cummingtonite | 152 |
| Cupreous arseniate of copper | 335 |
| bismuth | 278 |
| manganese | 242 |
| sulphate of lead | 366 |
| sulphato-carbonate of lead | 360 |
| Cupriferous sulphuret of bismuth | 278 |
| Cyanose | 323 |
| Cymophane | 80 |
| Cyprine | 21 |
| Cypro-mica | 330 |
| Datolit | 179 |
| Davina | 26 |
| Davyne | 26 |
| Davytic kouphone spar | 26 |
| Datholite | 179 |
| Dermatine | 401 |
| Deuto-fluate of cerium | 267 |
| Devonite | 156 |
| Diallage | 34 |
| chatoyante | 62 |
| Diallogite | 246 |
| Diamond | 383 |
| Diaspore | 68 |
| Diatomous kouphone spar | 27 |
| Diatomous euclas haloide | 289 |
| gypsum haloide | 181 |
| habroneme malachite | 331 |
| schiller spar | 62 |
| Dichroite | 42 |
| Dicromatic euclas haloide | 229 |
| Dtopside | 51 |
| Dioptase | 322 |
| Diplogenic kouphone spar | 129 |
| lead baryte | 366 |
| Diploite | 118 |
| Diprismatic copper glance | 352 |
| iron ore | 225 |
| hal baryte | 187 |
| lead baryte | 356 |
| olive malachite | 327 |
| Dipyre | 26 |
| Ditthène | 73 |
| Dodecahedral garnet | 13 |
| corundum | 81 |
| garnet blende | 391 |
| iron ore | 219 |
| kouphone spar | 134 |
| mercury | 378 |
| Dolomite | 167 |
| Double fluate of cerium yttria and | 267 |
| Dyoxylite | 358 |
| Dysclasite | 110 |
| Dysodile | 391 |
| Dystomic augite spar | 54 |
| habroneme malachite | 334 |
| Earth foam | 162 |
| Earthy carbonate of lead | 358 |
| bitumen | 388 |
| cobalt | 288 |
| manganese | 241 |
| mineral pitch | 388 |
| Ecume de mer | 184 |
| Edingtonite | 150 |
| Egeran | 21 |
| Eisen-vitriol | 232 |
| Eisenchrom | 275 |
| Eisenglanz | 216 |
| Eisenkiesel | 5 |
| Eisenoxyd | 216 |
| Eisenpecherz | 226 |
| Eisenschüssig kupfergrün | 322 |
| Eisensinter | 226 |
| Ekebergite | 144 |
| Elaolite | 133 |
| Elasmose | 342 |
| Elastic bitumen | 389 |
| mineral pitch | 389 |
[page] 414
| Elaterite | 389 |
| Electric calamine | 374 |
| Electrum | 337 |
| Emerald | 99 |
| copper | 322 |
| Emeraude | 99 |
| Emeril | 67 |
| Emery | 67 |
| Endellione | 352 |
| Epidote | 29 |
| Epistilbite | 129 |
| Epsom safe | 186 |
| Epsomite | 185 |
| Erdpech | 388 |
| Erdiger talc | 106 |
| Erdkobold | 288 |
| Erinite | 334 |
| Erlamite | 130 |
| Erythrine | 289 |
| Esmarkite | 179 |
| Essonite | 19 |
| Etain oxydé | 250 |
| suifuré | 253 |
| Euehroite | 333 |
| Euclase | 97 |
| Eudyalite | 153 |
| Eukairite | 30 |
| Euklastic disthene spar | 68 |
| Eutomous cobalt pyrites | 291 |
| Exanthoiose | 198 |
| Fahlerz | 311 |
| Fahlunite | 40 |
| Fassaite | 51 |
| Felspar | 115 |
| Feidspath apyre | 106 |
| opalin | 136 |
| Fer arseniaté | 234 |
| arsenical | 213 |
| calcareo-siliceux | 225 |
| carburé | 385 |
| chromaté | 275 |
| hydro-oxidé | 220 |
| muriaté | 227 |
| natif | 208 |
| oligiste | 216 |
| oxalaté | 235 |
| oxydé | 216 |
| carbonaté | 228 |
| hæmatite | 221 |
| resinite | 226 |
| oxydulé | 215 |
| titané | 257 |
| phosphaté | 229 |
| spathique | 228 |
| Fer speculaire | 216 |
| sulphaté | 232 |
| suifuré | 209 |
| blanc | 212 |
| ferrifère | 213 |
| magnetique | 213 |
| titané | 216 |
| Fergusonite | 274 |
| Fettstein | 133 |
| Feueratein | 8 |
| Fibrolite | 72 |
| Fibrous brown iron ore | 221 |
| malachite | 320 |
| Fiorite | 6 |
| Fishaugenstein | 108 |
| Flabeluform kouphone spar | 125 |
| Flexible sulphuret of silver | 297 |
| Flint | 8 |
| Float stone | 5 |
| Fluate of lime | 172 |
| of yttria and cerium | 168 |
| Flucerine | 267 |
| Fluellite | 78 |
| Fluor spar | 172 |
| Fluorine | 172 |
| Fluss | 172 |
| Foliated black manganese ore | 237 |
| tellurium | 342 |
| zeolite | 25 |
| Forsterite | 87 |
| Fossil copal | 395 |
| Franklinite | 219 |
| Fuller's earth | 37 |
| Gadolinite | 100 |
| Gahnite | 76 |
| Galena | 349 |
| Gallizinite | 376 |
| Galmei | 374, 375 |
| Ganse-köthig-erz | 308 |
| Garnet | 13 |
| Gay-lussite | 206 |
| Gediegen arsenic | 208 |
| eisen | 208 |
| gold | 338 |
| kupfer | 307 |
| platin | 338 |
| quecksilber | 377 |
| silber | 293 |
| spiesglas | 343 |
| sylvan | 340 |
| tellur | 340 |
| wismuth | 276 |
| Gehlenite | 22 |
| Gelb-bleierz | 367 |
[page] 415
| Gelberz | 342 |
| Gelbes rauschgelb | 283 |
| Gibbsite | 68 |
| Giésèckite | 113 |
| Giobertite | 184 |
| Girasol | 7 |
| Gismondine | 49 |
| Glance coal | 386 |
| Glanz kobalt | 284 |
| Glanzkohle | 386 |
| Glaserz | 296 |
| Glauber salt | 198 |
| Glauberite | 205 |
| Glaucolite | 122 |
| Glimmer | 102 |
| Gmelinite | 127 |
| Goethite | 221 |
| Grammatite | 56 |
| Granular limestone | 163 |
| Graphic gold | 341 |
| tellurium | 341 |
| Graphite | 385 |
| Grau braunsteinerz | 239 |
| spiesglaserz | 345 |
| Grauer spieskobold | 286 |
| Green carbonate of copper | 320 |
| earth | 121 |
| iron earth | 401 |
| vitriol | 232 |
| Grenat | 14 |
| Grenatit | 74 |
| Grey antimony | 345 |
| cobalt | 286 |
| copper | 311 |
| oxide of manganese | 239 |
| silver ore | 305 |
| Grossular | 15 |
| Grün bleierz | 362 |
| Grüne eisenerde | 401 |
| Grüner uranerz | 270 |
| Grünerde | 121 |
| Gurhofian | 169 |
| Gyps | 177 |
| Gypsum | 177 |
| Haarkies | 291 |
| Haidingerite | 181 |
| Halloysite | 72 |
| Hard fahlunite | 43 |
| Harmotome | 43 |
| Hatchetine | 393 |
| Hausmannite | 237 |
| Hauyne | 111 |
| Heavy spar | 189 |
| Hedenbergite | 55 |
| Hedyphan | 365 |
| Heliotrope | 10 |
| Helvine | 242 |
| Hematitic copper | 332 |
| Hemi-prismatic augite spar | 54 |
| brythine salt | 205 |
| chrysolite | 88 |
| fluor haloide | 186 |
| gypsum haloide | 180 |
| habroneme malachite | 320 |
| hal baryte | 189 |
| kouphone spar | 25 |
| lead baryte | 368 |
| natron salt | 197 |
| olive malachite | 369 |
| ruby blende | 302 |
| schiller spar | 62 |
| sulphur | 281 |
| talc mica | 105 |
| titanium ore | 258 |
| vitriol salt | 232 |
| Hemi-pyramidal feldspar | 150 |
| Hepatic cinnabar | 379 |
| Hepatite | 191 |
| Herderite | 172 |
| Herrerite | 401 |
| Herschelite | 110 |
| Hétéposite | 231 |
| Heterotomous feldspar | 136 |
| Heulandite | 25 |
| Hexahedral cobalt pyrites | 284 |
| copper glance | 253 |
| glance blende | 245 |
| gold | 336 |
| iron pyrites | 209 |
| kouphone spar | 127, 138 |
| lead glance | 349 |
| lirocone malachite | 234 |
| pearl kerate | 305 |
| platina | 338 |
| rock salt | 200 |
| silver | 293 |
| silver glance | 296 |
| tellurium | 340 |
| Highgate resin | 395 |
| Hisingerite | 225 |
| Holspath | 41 |
| Honey-stone | 394 |
| Hopeite | 377 |
| Horn silver | 305 |
| quicksilver | 380 |
| Hornblei | 362 |
| Hornblende | 54 |
| Hornerz | 305 |
| Hornstone | 12 |
[page] 416
| Houille | 390 |
| papyracée | 301 |
| Humboldtilite | 130 |
| Humboldtine | 235 |
| Humboldtite | 179 |
| Humite | 88 |
| Huraulite | 247 |
| Hyacinth | 96 |
| Hyalite | 6 |
| Hyalosiderite | 86 |
| Hydrargillite | 156 |
| Hydrate of manganese | 84 |
| Hydrated deutoxide of manganese | 239 |
| Hydro-boracite | 187 |
| Hydrolite | 127 |
| Hydrophane | 8 |
| Hydrosilicate of manganese | 244 |
| Hydrous aluminate of lead | 356 |
| oxide of iron | 220 |
| phosphate of copper | 328 |
| Hypersthene | 61 |
| Hypochlorite | 401 |
| Hypostilbite | 129 |
| Ice spar | 117 |
| Ichthyophthalmite | 108 |
| Idocrase | 20 |
| Ilmenite | 257 |
| Ilvait | 225 |
| Indianite | 32 |
| Indicolite | 148 |
| Iod-quecksilber | 381 |
| Iod-silber | 305 |
| Iodic mercury | 381 |
| silver | 305 |
| Iodure d'argent | 305 |
| Iolite | 42 |
| Iridium | 339 |
| osmié | 339 |
| Iridosmine | 339 |
| Iron flint | 5 |
| glance | 216 |
| pyrites | 209 |
| Iserine | 256 |
| Isometric cobalt pyrites | 287 |
| Isopyre | 32 |
| Isopyric quartz | 32 |
| Rtnerite | 132 |
| Jade nephritique | 92 |
| tenace | 142 |
| Jamesonite | 346 |
| Jargoon | 96 |
| Jeffersonite | 46 |
| Jet | 390 |
| Johannite | 371 |
| Kakoxene | 157 |
| Kalk-sal peter | 179 |
| Kalk sinter | 162 |
| Kalk-tuff | 106 |
| Kalkstein | 163 |
| Kallochrom | 368 |
| Kaneelstein | 19 |
| Kaolin | 118 |
| Karpholite | 13 |
| Karphosiderite | 232 |
| Kerasine | 362 |
| Kerolite | 40 |
| Kibdeiophan | 257 |
| Kiesel-wismuth | 279 |
| Kieselsinter | 6 |
| Killinite | 121 |
| Kimmeridge-coal | 36 |
| Klaprothine | 159 |
| Knebelite | 245 |
| Kobalt bliithe | 289 |
| Koboldine | 287 |
| Kohlenblende | 386 |
| Kohlensaures blei | 356 |
| mangan | 246 |
| Kokkolith | 53 |
| Kollyrite | 70 |
| Königine | 326 |
| Königite | 325 |
| Kreide | 164 |
| Kreutzstein | 43 |
| Krokydolite | 151 |
| Krjrolith | 204 |
| Krysoberyll | 85 |
| Krysolith | 85 |
| Kubizit | 138 |
| Kupfer glanz | 308 |
| mangan | 242 |
| Kupfer-glimmer | 330 |
| Kupfer.schmaragd | 322 |
| Kupfer-sammterz | 326 |
| Kupfer-vitriol | 323 |
| Kupferindig | 309 |
| Kupferkies | 314 |
| Kupferlazur | 319 |
| Kupfernickel | 292 |
| Kupferschaum | 334 |
| Kupferschwarze | 318 |
| Kupferwismutherz | 278 |
| Kyanite | 73 |
| Labradorische hornblende | 61 |
| Labradorite | 136 |
[page] 417
| Labradorstein | 136 |
| Lamellar heavy spar | 189 |
| Lanarkite | 358 |
| Lapis-lazuli | 131 |
| Lasionite | 156 |
| Latialite | 111 |
| Latrobite | 118 |
| Laumonite | 27 |
| Lave vitreuse perté | 112 |
| Lazulite | 131 |
| Lazurstein | 130 |
| Lead glance | 349 |
| Leadhiilite | 359 |
| Lebererz | 379 |
| Ledererite | 128 |
| Leelite | 12 |
| Lemnian eanh | 38 |
| Lenticular arseniate of copper | 329 |
| Lenzinite | 70 |
| Lepidokrokite | 221 |
| Lepidolite | 122 |
| Leucite | 105 |
| Levyne | 146 |
| Libethenite | 327 |
| Lievrite | 225 |
| Ligurite | 87 |
| Limbelite | 86 |
| Lime harmotome | 108 |
| Limonite | 220 |
| Linsenerz | 329 |
| Linsenkupfer | 329 |
| Liroconite | 329 |
| Lithomarge | 37 |
| Lomonit | 27 |
| Macle | 41 |
| Maclureite | 88 |
| Macrotypous kouphone spar | 146 |
| lime haloide | 167 |
| parachrose baryte | 246 |
| Magnesian limestone | 168 |
| Magnesie boratée | 186 |
| hydratée siliceuse | 93 |
| nitratée | 186 |
| phosphatée | 186 |
| sulphatée | 185 |
| Magnesite | 183 |
| Magneteisenstein | 215 |
| Magnetic iron ore | 215 |
| pyrites | 213 |
| Magnetkies | 213 |
| Malachite | 320 |
| Malacolithe | 52 |
| Malthe | 388 |
| Manganblende | 245 |
| Manganese hydraté cuprifère | 242 |
| oxidé carbonatée | 246 |
| oxidé hydraté | 237 |
| oxidé hydraté concretionné | 240 |
| oxidé metalloide | 239 |
| phosphatée | 248 |
| silicifère | 243 |
| spar | 243 |
| sulfuré | 246 |
| Manganesian epidote | 30 |
| garnet | 16 |
| Manganglanz | 245 |
| Manganite | 239 |
| Manganspath | 243 |
| Marble | 163 |
| Marceline | 402 |
| Marekanite | 142 |
| Margarite | 104 |
| Marl | 165 |
| Marmolite | 93 |
| Martial arseniate of copper | 336 |
| Mascagnin | 201 |
| Meerschaum | 184 |
| Meionite | 149 |
| Melaconise | 318 |
| Meianite | 17 |
| Melanochroite | 369 |
| Melanterie | 232 |
| Melinose | 367 |
| Meililite | 48 |
| Meilite | 394 |
| Menaccanite | 256 |
| Menelite | 8 |
| Mengite | 403 |
| Mercure argental | 378 |
| ioduré | 381 |
| muriaté | 380 |
| natif | 377 |
| sulfuré | 379 |
| Mercury | 377 |
| Mesole | 125 |
| Mesolite | 122 |
| Mesotype | 123 |
| Miargyrite | 302 |
| Mica | 102 |
| Micarelle | 113 |
| Microlite | 402 |
| Miemite | 168 |
| Mimetèse | 364 |
| Mineral charcoal | 387 |
| oil | 387 |
| pitch | 388 |
| Minium | 354 |
| Mispickel | 213 |
| Misy | 234 |
[page] 418
| Mocha stone | 11 |
| Mohsite | 258 |
| Molybdate of lead | 367 |
| silver | 280 |
| Molybdena | 249 |
| Molybdène sulfuré | 249 |
| Molybdenite | 249 |
| Molybdic ochre | 249 |
| Monazite | 402 |
| Monticellite | 403 |
| Moon stone | 115 |
| Moroxite | 171 |
| Mountain cork | 60 |
| leather | 60 |
| meal | 39 |
| wood | 60 |
| Mullerine | 342 |
| Muller's glass | 6 |
| Murchisonite | 115 |
| Muriacite | 176 |
| Muriate of ammonia | 201 |
| of copper | 325 |
| of lead | 360 |
| of mercury | 380 |
| of silver | 305 |
| of soda | 200 |
| Murio-carbonate of lead | 362 |
| Mussite | 51 |
| Mysorine | 403 |
| Nacrite | 106 |
| Nagyagererz | 342 |
| Naphtha | 387 |
| Naphthaline | 393 |
| Native amalgam | 378 |
| antimony | 343 |
| arsenic | 280 |
| bismuth | 276 |
| boracic acid | 154 |
| carbonate of lime and soda | 207 |
| copper | 307 |
| gold | 336 |
| iridium | 339 |
| iron | 208 |
| lead | 349 |
| magnesia | 84 |
| mercury | 377 |
| minium | 354 |
| palladium | 339 |
| platina | 338 |
| quicksilver | 377 |
| red iron vitriol | 233 |
| silver | 293 |
| sulphuric acid | 154 |
| tellurium | 340 |
| Natrolite | 124 |
| Natron | 196 |
| Naturliche naphthaline | 393 |
| Naturlischer alaun | 202 |
| bitter salz | 185 |
| salmiak | 201 |
| salpeter | 195 |
| schwefel | 382 |
| Naturlisches amalgam | 378 |
| glauber salz | 198 |
| mineral alkali | 198 |
| Needle ore | 278 |
| stone | 126 |
| Nemalite | 93 |
| Neoctèse | 403 |
| Neoplase | 233 |
| Nepheline | 131 |
| Nephrite | 92 |
| Neutral fluate of cerium | 267 |
| Nickel arseniaté | 292 |
| arsenical | 292 |
| arsenical antimonifère | 291 |
| blüthe | 292 |
| ochre | 292 |
| oxydé | 293 |
| spies glaserz | 291 |
| sulfuré | 291 |
| Nickeliferous grey antimony | 291 |
| Nigrin | 255 |
| Nitrate de chauz | 179 |
| of lime | 179 |
| of magnesia | 185 |
| of potash | 195 |
| of soda | 198 |
| Nitre | 195 |
| Nitro-magnesite | 185 |
| Nontronite | 404 |
| Non-bituminous mineral coal | 386 |
| Nosin | 135 |
| Nuttalite | 133 |
| Oblioue prism, arsen. of copper | 331 |
| Obsidian | 141 |
| Octahedral alum salt | 202 |
| ammoniac salt | 201 |
| arseniate of copper | 329 |
| arsenic acid | 281 |
| bismuth | 276 |
| chrome ore | 275 |
| cobalt pyrites | 286 |
| copper * | 307 |
| copper ore | 316 |
| copper pyrites | 310 |
| corundum | 76 |
| diamond | 383 |
[page] 419
| Octahedral fluor haloide | 172 |
| iron | 208 |
| iron ore | 215 |
| kouphone spar | 139 |
| palladium | 339 |
| titanium ore | 260 |
| Oetahedrite | 253 |
| Odontalite | 69 |
| Okenite | 48 |
| Ollven kupfer | 332 |
| Olivenerz | 332 |
| Olivenit | 332 |
| Olivine | 86 |
| Onegite | 220 |
| Onyx | 9 |
| Oolite | 164 |
| Opal | 6 |
| Ophite | 90 |
| Opsimose | 244 |
| Or natif | 336 |
| Orpiment | 283 |
| Orthite | 265 |
| Orthoklas | 115 |
| Orthotomous kouphone spar | 124 |
| Osmelite | 404 |
| Ostranite | 97 |
| Ozahverite | 109 |
| Oxalate of iron | 235 |
| Oxide of antimony | 348 |
| · of arsenic | 281 |
| of bismuth | 279 |
| of chrome | 275 |
| of cobalt | 288 |
| of molybdena | 249 |
| of tin | 250 |
| of tungsten | 253 |
| Oxydulated iron | 215 |
| copper | 316 |
| Ozokerite | 394 |
| Palladium | 339 |
| Panabase | 311 |
| Paranthine | 143 |
| Paratomous augite spar | 49 |
| kouphone spar. | 43 |
| lead baryte | 360 |
| lime haloide | 169 |
| pargasite | 56 |
| Paulite | 61 |
| Pearl-glimmer | 104 |
| Pearlsinter | 6 |
| Pearlspar | 168 |
| Pearlstone | 112 |
| Peastone | 164 |
| Pech-blende | 268 |
| Pechkohle | 390 |
| Pecherz | 268 |
| Pectolite | 144 |
| Peliom | 42 |
| Pelokonite | 247 |
| Pericline | 136 |
| Peridot | 85 |
| Peritomous antimony glance | 299 |
| hal-baryte | 192 |
| kouphone spar | 123 |
| lead baryte | 360 |
| ruby blende | 379 |
| titanium ore | 254 |
| Petalite | 45 |
| Petroleum | 388 |
| Petrosilex resinite | 140 |
| Pechstein | 140 |
| Pharmacolite | 180 |
| Pharmacosiderite | 234 |
| Phenakite | 404 |
| Phillipsite | 108 |
| Phosphate of copper | 327 |
| of iron | 229 |
| of iron and manganese | 248 |
| of lead | 362 |
| of lime | 170 |
| of mangan | 248 |
| of uranium | 268 |
| of yttria | 194 |
| Phosphorite | 170 |
| Phosphorkupfer von Libethen | 327 |
| von Rheinbreitbach | 328 |
| Phosphorkupfererz | 327 |
| Phosphormangan | 248 |
| Phosphorsaure yttererde | 194 |
| Phosphorsaures-blei | 362 |
| talc | 186 |
| Phosphyttria | 194 |
| Photizite | 244 |
| Physalite | 79 |
| Piorolite | 94 |
| Picro-pharmacolite | 181 |
| Picrosmine | 94 |
| Pictite | 84 |
| Pierre d'azur | 131 |
| cruciforme | 43 |
| grasse | 433 |
| Pimelite | 293 |
| Pinguite | 223 |
| Pimte | 113 |
| Pipe-clay | 39 |
| Pisolite | 164 |
| Pistacit | 29 |
| Pitich.blende | 268 |
| Pitchstone | 140 |
[page] 420
| Pitchy iron-ore | 226 |
| Pittizite | 226 |
| Plagionite | 346 |
| Plasma | 9 |
| Piatina | 333 |
| Platiniferous grey copper | 314 |
| Pleonaste | 82 |
| Pleuroklas | 186 |
| Plomb arseniaté | 364 |
| carbonaté | 356 |
| chromaté rouge | 368 |
| corné | 362 |
| hydro-alumineux | 356 |
| jaune | 367 |
| molybdaté | 367 |
| muriaté | 362 |
| oxydé rouge | 354 |
| phosphaté | 362 |
| seleniuré | 354 |
| sulphaté | 385 |
| sulfuré | 349 |
| sulfuré antimonifère | 352 |
| tungstaté | 370 |
| Plombgomme | 356 |
| Plumbago | 385 |
| Plumbo-argentifère | 303 |
| Plumbo-calcite | 170 |
| Plumbo-cupriferous sulphuret of bismuth | 378 |
| Polishing slate | 36 |
| Polybasite | 300 |
| Polychromatic felspar | 136 |
| Polyhallite | 203 |
| Polymignite | 261 |
| Polyspharite | 363 |
| Polystomous augite spar | 46 |
| Poonahlite | 405 |
| Porcelain clay | 118 |
| jasper | 12 |
| Pot-stone | 02 |
| Potasse nitratée | 195 |
| sulpha tée | 196 |
| Potter's clay | 39 |
| Prase | 4 |
| Prehnite | 22 |
| Prismatic andalusite | 106 |
| antimony | 294 |
| antimony baryte | 348 |
| antimony. glance | 341 |
| arsenical pyrites | 213 |
| augite spar | 47 |
| axinite | 30 |
| azure malachite | 319 |
| azure spar | 159 |
| Prismatic bismuth glance | 277 |
| blende | 245 |
| boracic acid | 154 |
| borax salt | 199 |
| calamine | 374 |
| cerium ore | 263 |
| chrysolite | 85 |
| cobalt mica | 289 |
| copper glance | 308 |
| copper mica | 330 |
| corundum | 89 |
| cryone haloide | 204 |
| disthene spar | 73 |
| dystoine spar | 179 |
| emerald | 97 |
| emerald malachite | 333 |
| epsom salt | 185 |
| euclore mica | 334 |
| fluor haloide | 172, 335 |
| gadolinite | 100 |
| glauber salt | 198 |
| gypsum haloide | 176 |
| babroneme malachite | 328 |
| hal baryte | 189 |
| iron mica | 229 |
| iron ore | 220 |
| iron pyrites | 212 |
| kouphone spar | 124 |
| lead baryte | 365 |
| lime haloide | 165 |
| lirocone malachite | 329 |
| manganese ore | 238 |
| melane glance | 298 |
| natron salt | 196 |
| nephrite spar | 142 |
| nickel pyrites | 292 |
| nitre salt | 195 |
| olive malachite | 332 |
| petaline spar | 45 |
| purple blende | 347 |
| pyramidal garnet | 20 |
| pyrgom | 51 |
| quartz | 42 |
| scheelium ore | 236 |
| schiller spar | 33 |
| sulphur | 382 |
| talc mica | 120 |
| tantalum ore | 272 |
| titanium ore | 256 |
| topaz | 77 |
| triphane spar | 46 |
| vitriol salt | 376 |
| white antimony | 348 |
| zinc baryte | 371 |
[page] 421
| Prismatoidal zinc ore | 373 |
| antimony glance | 345 |
| Prismatic augite spar | 29 |
| copper glance | 254 |
| garnet | 74 |
| glauber salt | 196 |
| gypsum haloide | 177 |
| habroneme malachite | 325 |
| hal baryte | 193 |
| kouphone spar | 24 |
| lead baryte | 358 |
| manganese ore | 239 |
| schiller spar | 61 |
| sulphur | 283 |
| Protheite | 405 |
| Proustite | 300 |
| Pseudo-malachite | 328 |
| Psilomelane | 240 |
| Pumice | 140 |
| Purple copper | 310 |
| Pyrnite | 79 |
| Pyrallolite | 58 |
| Pyramidal copper pyrites | 314 |
| euchlore mica | 268 |
| felspar | 143 |
| kouphone spar | 108 |
| lead baryte | 367 |
| manganese ore | 237 |
| mellichrone resin | 394 |
| pearl kerate | 380 |
| scheelium baryte | 182 |
| tellurium glance | 342 |
| tin ore | 250 |
| titanium ore | 253 |
| zircon | 95 |
| Pyrargylite | 114 |
| Pyreneite | 15 |
| Pyrite cuivreuse | 314 |
| Pyrite martiale | 209 |
| PyTochlore | 260 |
| Pyrolusite | 238 |
| Pyromorphite | 362 |
| Pyrope | 18 |
| Pyrophylite | 40 |
| Pyrophysalite | 79 |
| Pyrorthite | 265 |
| Pyrosklerite | 405 |
| Pyrosmalite | 227 |
| Pyroxene | 49 |
| ferro-manganesien | 227 |
| Pyrrhosiderite | 220 |
| Quartz | 1 |
| Quecksilber lebererz | 379 |
| hornerz | 380 |
| Quicksilver | 377 |
| Radiated acicular olivenite | 331 |
| Realgar | 281 |
| rouge | 281 |
| Red antimony | 347 |
| cobalt ochre | 289 |
| copper ore | 316 |
| hæmatite | 218 |
| iron ore | 218 |
| lead spar | 368 |
| manganese | 246 |
| oxide of zinc | 373 |
| of copper | 316 |
| silver | 300 |
| vitriol | 290 |
| zinc ore | 373 |
| Retinite | 395 |
| Retinasphalt | 395 |
| Reussite | 205 |
| Rhætizite | 73 |
| Rhodonite | 244 |
| Rhodhalose | 290 |
| Rhomb spar | 167 |
| Rhombohedral alum haloide | 203 |
| antimony | 343 |
| arsenic | 280 |
| bismuth glance | 280 |
| calamine | 375 |
| cerium ore | 262 |
| corundum | 64 |
| emerald | 99 |
| emerald malachite | 322 |
| euclore mica | 330 |
| felspar | 131 |
| fluor haloide | 170 |
| graphite mica | 385 |
| iridium | 339 |
| iron ore | 216 |
| iron pyrites | 213 |
| kouphone spar | 145 |
| lead baryte | 362, 364 |
| melane mica | 223 |
| molybdena glance | 249 |
| pearl mica | 104 |
| quartz | 1 |
| ruby blende | 300 |
| talc mica | 102 |
| tourmaline | 147 |
| zinc baryte | 375 |
| Rhombohedrischer schwefel kohlensaures blei | 359 |
| Rhomboidal arseniate of copper | 330 |
| baryte | 187 |
| graphite | 385 |
[page] 422
| Right prismatic arseniate of copper | 332 |
| Rock milk | 162 |
| Rohe wand | 169 |
| Romanzovite | 19 |
| Roselite | 290 |
| Rothbleierz | 368 |
| Rothbraunsteinerz | 246 |
| Rother braunstein | 246 |
| eisenstein | 218 |
| eisen-vitriol | 233 |
| erdkobold | 289 |
| rauschgelb | 281 |
| Rothgültigerz | 300 |
| Rothkupfererz | 316 |
| Rothspiesglaserz | 347 |
| Rottenstone | 36 |
| Rubellane | 104 |
| Rubellite | 149 |
| Rubinglimmer | 221 |
| Rubicelle | 82 |
| Ruby silver | 300 |
| Rutile | 254 |
| Sahlite | 52 |
| Sal mare | 200 |
| Sal-ammoniac | 201 |
| Saltsaures blei von Mendip | 360 |
| Salzkupfererz | 325 |
| Salzsaures kupfer | 325 |
| Saphirine | 82 |
| Sappare | 73 |
| Sapphire | 64 |
| Sarcolife | 139 |
| Sassoline | 154 |
| Satin spar | 167 |
| Saussurite | 142 |
| Scaly talc | 106 |
| Scapolite | 143 |
| Scarbroite | 71 |
| Schaalstein | 47 |
| Schabasit | 145 |
| Scheelin | 182 |
| ferruginé | 236 |
| Scheelite | 182 |
| Scheelitine | 370 |
| Scheelsaures blei | 370 |
| Schererite | 393 |
| Schiefer spar | 161 |
| Schieferthon | 36 |
| Schiller spar | 62 |
| Schillerstein | 62 |
| Schiste à polir | 36 |
| Schmelzstein | 26 |
| Schmiergel | 67 |
| Schorl | 147 |
| Schrifterz | 341 |
| Schwartz eisenstein | 240 |
| Schwartzer glaskopf | 240 |
| Schwartzerz | 245, 313 |
| Schwarz gültigerz | 298 |
| spiessglaserz | 352 |
| Schwarzer mangan-kiesel | 244 |
| Schwefel und kohlensaures blei | 358 |
| und kohlensaures blei und kupfer | 360 |
| silber und antimon | 299 |
| Schwefel-kobalt | 287 |
| Schwefel-nickel | 291 |
| Schwefelkies | 209 |
| Schwefelsaures blei und kupfer | 366 |
| Schwerspath | 189 |
| Schwerstein | 182 |
| Schwimmstein | 5 |
| Scorodite | 335 |
| Scorza | 30 |
| Sel-d'epsom natif | 185 |
| Sel-gemme | 200 |
| Selen cuprite | 316 |
| kupfersilber | 304 |
| silber | 304 |
| Selenblei | 354 |
| Selenite | 177 |
| Seleniure d'argent | 304 |
| de cuivre | 316 |
| Seleniuret of copper | 316 |
| of lead | 354 |
| of lead and cobalt | 355 |
| of lead and copper | 355 |
| of lead and mercury | 355 |
| of palladium | 304 |
| of silver | 304 |
| Semi-opal | 7 |
| Serpentine | 90 |
| Sesqui-carbonate of soda | 197 |
| Severite | 70 |
| Shale | 36 |
| Siberite | 149 |
| Siderite aimant | 215 |
| chromifère | 275 |
| zincifère | 219 |
| Sideritine | 226 |
| Sideroschisolite | 225 |
| Silber-blende | 300 |
| Silber-kupfer-glanz | 303 |
| Silicate of cerium | 263 |
| of iron | 224 |
| of manganese | 243 |
| Silice fluateé alumineuse | 77 |
| Siliceous hydrate of magnesia | 93 |
[page] 423
| Slliceous oxide of zinc | 374 |
| sinter | 6 |
| Siliciferous hydrate of alumina | 70 |
| oxide of manganese | 243 |
| Sillimanite | 73 |
| Stiver glance | 296 |
| Slate-clay | 36 |
| spar | 161 |
| Slickenside | 351 |
| Smaragdite | 34 |
| Soapstone | 91 |
| Soda alum | 206 |
| Sodaite | 144 |
| Sodalite | 134 |
| Somervillite | 406 |
| Sommit | 131 |
| Sordawalite | 43 |
| Soude boratée | 109 |
| carbonatée | 196 |
| nitratée | 198 |
| sulphatée | 198 |
| Soufre | 382 |
| Spargelstein | 171 |
| Sparkles | 212 |
| Spath calcaire | 160 |
| eisenstein | 228 |
| en tables | 47 |
| magnesien | 184 |
| Spatbose iron | 228 |
| Speckstein | 91 |
| Specular iron | 216 |
| Spessartine | 16 |
| Sphserulite | 142 |
| Sphserosiderite | 229 |
| Spbene | 285 |
| Spherostilbite | 130 |
| Spiesglanzweiss | 348 |
| Spiesglassilber | 294 |
| Spiessglanz ocbre | 349 |
| Spiezglanzbleierz | 152 |
| Spinel | 81 |
| Spinelle zincifère | 76 |
| Spinellane | 135 |
| Spodumene | 46 |
| Spröd-glaserz | 298 |
| Stalactitic carbonate of lime | 162 |
| Stangen spath | 191 |
| Stannolite | 250 |
| Staurolite | 74 |
| Staurotypous kouphone spar | 108 |
| Steatite | 91 |
| pagodite | 119 |
| Steinheilite | 42 |
| Steinmannite | 406 |
| Steinmark | 37 |
| Steinsalz | 200 |
| Sternbergite | 297 |
| Stibiconise | 349 |
| Stilbine | 345 |
| Stilbite | 24 |
| Stilpnomelan | 406 |
| Stilpnosiderite | 222 |
| Stinkstone | 164 |
| Strahl-zeolith | 24 |
| Strahlerz | 331 |
| Strahlkies | 212 |
| Strahltstein | 58 |
| Strohstein | 13 |
| Stromeyerine | 303 |
| Stromnite | 193 |
| Strontiane sulphatés | 193 |
| carbonatée | 192 |
| Strontianite | 192 |
| Strontites | 192 |
| Sab-fluate of cerium | 267 |
| Sub-phosphate of alumina | 156 |
| Sub-sulphate of alumina | 155 |
| Succin | 392 |
| Succinite | 19 |
| Sulfate vert d'urane | 271 |
| de plomb cuivreux | 366 |
| Sulphate of alumina and ammonia | 207 |
| of alumine | 156 |
| of ammonia | 201 |
| ofbarytes | 189 |
| of cobalt | 290 |
| of copper | 323 |
| of iron | 232 |
| of lead | 365 |
| of lime | 177 |
| of magnesia | 185 |
| of potash | 196 |
| of soda | 198 |
| of strontia | 193 |
| of zinc | 376 |
| Sulphato-carbonate of lead | 358 |
| Sulphato-tri-carbonate of lead | 359 |
| Sulphur | 382 |
| Sulphuret of antimony | 345 |
| of arsenic | 281 |
| of bismuth | 277 |
| of cobalt | 287 |
| of copper | 308 |
| of lead | 349 |
| of lead and antimony | 352 |
| of nickel | 291 |
| of manganese | 245 |
| of mercury | 379 |
| of molybdena | 249 |
[page] 424
| Sulphuret of silver | 296 |
| of silver and antimony | 299 |
| of silver and copper | 303 |
| of tin | 253 |
| of zinc | 371 |
| Super-sulphuret of lead | 253 |
| Swinestone | 164 |
| Sylvane graphique | 341 |
| Sylvanite | 314 |
| Sylvyne | 407 |
| Tabular spar | 47 |
| Tachylite | 32 |
| Talc | 129 |
| granuleux | 106 |
| graphique | 119 |
| ollaire | 92 |
| steatite | 91 |
| Tantale oxydé yttrifère | 273 |
| Tantalite | 272 |
| Tautolite | 89 |
| Tellur aurifère et plombifère | 342 |
| natif auro-argentifère | 341 |
| natif auro-ferrifère | 340 |
| natif auro-plombifère | 342 |
| silber | 295 |
| wismuth | 239 |
| Telluric bismuth | 280 |
| silver | 295 |
| Telluro-galene | 342 |
| Tennantite | 311 |
| Tephroite | 407 |
| Tesselite | 109 |
| Tetartin | 137 |
| Tetarto-prismatic feldspar | 137 |
| vitriol salt | 323 |
| Tetrahedral boracite | 186 |
| copper glance | 311 |
| Tballite | 29 |
| Thenardite | 407 |
| Thomsonite | 124 |
| Thon | 35 |
| Thorite | 101 |
| Thulite | 63 |
| Thumerstone | 30 |
| Tile ore | 318 |
| Tinder ore | 348 |
| Tin ore | 250 |
| Tin pyrites | 253 |
| Tin stone | 250 |
| Tin-white cobalt | 286 |
| Tincal | 199 |
| Titane anatase | 253 |
| oxydé | 254 |
| siliceo-calcaire | 258 |
| Titaneisen | 216 |
| Titaniferous cerite | 263 |
| Titanitic iron | 216, 256 |
| Topaz | 77 |
| Topazolite | 18 |
| Topfstein | 92 |
| Torrelite | 265 |
| Tourmaline | 147 |
| Trapezoidal kouphone spar | 105 |
| Tremolite | 56 |
| Tricklasite | 40 |
| Tripel | 38 |
| Triphane | 46 |
| Triplite | 248 |
| Triple sulphuret | 352 |
| Tripoli | 38 |
| Trona | 197 |
| Tufa | 165 |
| Tungstate of iron | 236 |
| of lead | 370 |
| of lime | 182 |
| Tungsten | 182 |
| Tungstic ochre | 253 |
| Turnerite | 83 |
| Turquoise | 69 |
| TJncleavable azure spar | 69 |
| iron ore | 258 |
| nephrite spar | 92 |
| manganese ore | 240 |
| quartz | 6 |
| staphyline malachite | 322 |
| uranium ore | 268 |
| Uran glimmer | 268, 270 |
| mica | 268 |
| ochre | 269 |
| vitriol | 271 |
| Uran-bloom | 270 |
| blüthe | 270 |
| Urane micacé | 268 |
| oxydé | 268 |
| oxydulé | 268 |
| sulfaté | 271 |
| Uranite | 268 |
| Uranpecherz | 268 |
| Urao | 197 |
| Uwarowite | 408 |
| Yanadiate of lead | 370 |
| Yanadinsaures blei | 370 |
| Variegated copper | 310 |
| Vauquelinite | 369 |
| Velvet blue copper | 325 |
| Vesuvian | 20 |
[page] 425
| Vitreous copper | 308 |
| silver | 296 |
| Vitriol bleu | 323 |
| de plomb natif | 365 |
| Vivianite | 229 |
| Vulpinite | |
| Wad | 241 |
| Wagnerite | 186 |
| Wandstein | 169 |
| Wasserblei | 249 |
| Wavellite | 156 |
| Websterite | 155 |
| Weiss bleierz | 356 |
| spiesglaserz | 348 |
| sylvanerz | 342 |
| tellur | 342 |
| Weissite | 112 |
| Wernerite | 143 |
| Willelmine | 376 |
| Wismuth glance | 177 |
| ochre | 279 |
| silbererz | 303 |
| Withamite | 63 |
| Witherite | 187 |
| White antimony | 348 |
| cobalt | 284 |
| copper | 314 |
| iron pyrites | 202 |
| lead ore | 356 |
| vitriol | 376 |
| Wolchonskoit | |
| Wolfram | 236 |
| Wollastonite | 47 |
| Wood opal | 7 |
| tin | 252 |
| arseniate of copper | 332 |
| Worthite | 72 |
| Wurfelspath | 176 |
| Wurfelerz | 324 |
| Xanthite | 33 |
| Xenotime | 194 |
| Yellow mineral resin | 392 |
| tellurium | 342 |
| Yenite | 225 |
| Ypoleime | 328 |
| Yttria phosphatée | 194 |
| Yttro-cerite | 266 |
| tantalite | 273 |
| Zeagonite | 49 |
| Zeolite | 123 |
| Zeylanite | 82 |
| Ziegelerz | 318 |
| Zigueline | 316 |
| Zinc carbonaté | 375 |
| hydraté cuprifère | 334 |
| oxyd | 373 |
| oxidé ferrifère | 219 |
| oxydé silicifère | 374 |
| spath | 375 |
| sulfuré | 371 |
| sulfaté | 376 |
| vitriol | 376 |
| Zinkenite | 347 |
| Zinnkies | 253 |
| Zinnober | 379 |
| Zinnstein | 250 |
| Zircon | 95 |
| Zirconite | 96 |
| Zoisite | 28 |
| Zootinsalz | 198 |
| Zundererz | 348 |
| Zurlite | 408 |
FINIS.
Printed by THOMAS ALLAN & Co.
265 High Street, Edinburgh.