[page i]
REPORT
OF THE
FIRST AND SECOND MEETINGS
OF THE
BRITISH ASSOCIATION
FOR THE
ADVANCEMENT OF SCIENCE;
AT YORK IN 1831, AND AT OXFORD IN 1832:
INCLUDING ITS
PROCEEDINGS, RECOMMENDATIONS, AND TRANSACTIONS.
LONDON:
JOHN MURRAY, ALBEMARLE STREET.
1833.
[page ii]
PRINTED BY RICHARD TAYLOR,
RED LION COURT, FLEET STREET.
PREFACE
[page iii]
THE plan of the British Association for the Advancement of Science, and the principles on which it was founded, are given in the first pages of this volume, which contain a reprint of the Report of the Meeting at York in September 1831: the second part of the volume presents a specimen of the results furnished by the Meeting at Oxford in June 1832.
The contents of the present publication will show distinctly the path which the Association is pursuing, and the difference between its objects and those proposed by any other scientific Societies or Meetings at home or abroad.
It will be observed that the Papers here printed in detail consist chiefly of reviews of the progress of various branches of science, drawn up expressly at the request of the Association and by the recommendation of its Committees. The want of better information respecting the recent advances and actual state of our knowledge has long been felt in every department of inquiry, and the influence which the Association has been able to exercise, in procuring the supply of this desideratum, may be judged of from the declaration of the Professor of Astronomy at Cambridge, who stated at the late Meeting that no inducement but that of such a solicitation as he had received, could have impelled him to undertake the task which, in the following pages, he has fulfilled. The ability and industry which have thus been enlisted in rendering a laborious and responsible service to science, prove the efficacy of a system of public invitation in giving incitement and direction to the energies of individuals, and show the existence of a public spirit entirely in accordance with the designs of the institution.
In publishing these reviews or reports, the responsibility which the Association takes upon itself must be understood
A 2
[page] iv
to be limited to the selection of the subject and of the reporter, leaving the author accountable for his own opinions, and exercising only that general censorship which an Editor is entitled to claim.
The Association also holds itself liable to print in detail those researches on particular points of inquiry which it has requested individuals or Societies to undertake. A few experiments on the magnetic intensity of the earth, and on the quantity of rain which falls at different heights in the atmosphere, are all that will be found under this head in the present volume. It is to be hoped that hereafter they may hold a more prominent place in these Transactions, and that that part of the designs of the Association may be diligently prosecuted, which aims at promoting in a direct manner the investigation of such questions as, in the existing state of science, especially require to be solved in order to open the way to the application of abstract reasoning and the deduction of general laws.
The rest of the Transactions printed in this Report consist of notices or abstracts of the miscellaneous papers which were read at the Meeting, arranged under general heads. It may probably be found necessary in future, that these contributions should not only be printed, but delivered in and read to the Meetings in the same abbreviated form; the business of the session would thus be brought within compass, and the subsequent trouble and delay which it costs to collect the abstracts for publication would be saved. To prevent delay however, and to enable the Officers of the Association to publish the Transactions of the Meeting soon after it has been held, it is of still greater moment that those who draw up the REPORTS on the state of science, which are printed at length, should put the finishing hand to their labours previous to the Meeting, and bring them ready prepared for the press.
The discussions on questions of science which occurred in the Sectional Committees were not the least interesting of the proceedings: but of these it has scarcely been attempted to offer any account; they are a part of the spirit
[page] v
of the Meetings which it is not possible to embody in a Report. The speeches also, which were delivered on various occasions, have only been given so far as they were materially connected with the order of the proceedings, or might serve to exemplify the principles on which the Meeting was conducted. It was a gratification indeed, of no common kind, to listen to the sentiments of so many men of varied talents and high reputation collected together from every part of the United Kingdom; but the record of those sentiments which could have been here presented would have been cold and imperfect, and in such a publication as this would have appeared also redundant and misplaced.
A supply of information not less copious and valuable than that which is now laid before the public, is in preparation to enrich the next volume of these Reports. The printing of an Account of the recent Additions to our Knowledge of the Phœnomena of Sound, which was delivered at Oxford by the REV. MR. WILLIS, has been deferred, to allow the author leisure to prepare it for publication. The postponed Report on the Advances which have been recently made in the Integral and Differential Calculus, by the REV. MR. PEACOCK; that on the principal Questions debated in the Philosophy of Botany, by PROFESSOR LINDLEY; and that on the Question of the Permanence of the relative Level of the Sea and Land, by MR. STEVENSON, are promised for the next Meeting; and in addition, Reports have been undertaken on the following subjects:—
On the State of our Knowledge respecting the Magnetism of the Earth, by MR. CHRISTIE;
On the present State of the Analytical Theory of Hydrostatics and Hydrodynamics, by the REV. MR. CHALLIS;
On the State of our Knowledge of Hydraulics considered as a Branch of Engineering, by MR. GEORGE RENNIE;
On the State of our Knowledge of the Strength of Materials, by MR. BARLOW;
On the State of our Knowledge respecting Mineral Veins, by MR. JOHN TAYLOR;
[page] vi
On the State of Physiological Knowledge, by the REV. PROFESSOR CLARK;
On the State of Zoological Knowledge, by MR. VIGORS.
There is also reason to hope that the ensuing Meeting will be favoured with the communication of some results of researches which have been undertaken in compliance with the Recommendations contained in this volume. It is highly desirable that the attention of the Members of the Association should be particularly directed to these Recommendations of its Committees, and that the suggestions offered by them in 1831 as well as in 1832 should be attended to (See page 48, &c. and page 115, &c.)
The time fixed for the Association to assemble at Cambridge, is Monday the 24th of June 1833.
POSTSCRIPT.
BY the direction of the First Meeting of the Association, a request was made to the chief Secretary of the Government in India, Mr. Swinton, to form a Corresponding Committee at Calcutta, with the aid of Sir Edward Ryan, Major Benson, Mr. Herbert, Mr. Prinsep, and Dr. Christie. An answer has recently arrived from Mr. Swinton, announcing that he has received the Report of the Association, and will have the greatest pleasure in becoming a Member of the Calcutta Committee, in concert with the gentlemen whose names had been conjoined with his, to such of whom as are resident in Calcutta he had communicated the invitation. Sir Edward Ryan, President of the Asiatic Society, has accepted the office of President of the Committee. A further communication is promised respecting "the means which the Committee possess of following up the objects to which their attention has been particularly directed, and the steps which have been taken for inviting the cooperation of the lovers of science in the other Indian Presidencies."
YORK, April 9, 1833.
[page vii]
CONTENTS.
| Page | |
| Objects and Rules of the Association | ix |
FIRST REPORT—1831.
| Preface | 9 |
| Proceedings of the General Meeting | 15 |
| Proceedings of the General Committee | 45 |
| Recommendations of the Committees | 48 |
| Transactions | 56 |
SECOND REPORT—1832.
| Proceedings of the General Meeting | 95 |
| Proceedings of the General Committee | 111 |
| Recommendations of the Committees | 115 |
TRANSACTIONS:—
| Report on the Progress of Astronomy during the present Century. By G. B. AIRY, M.A., F.R. Ast. Soc., F.G.S., Fellow of the American Academy of Arts and Sciences; late Fellow of Trinity College, Cambridge; and Plumian Professor of Astronomy and Experimental Philosophy in the University of Cambridge. | 125 |
| Report on the Tides. By J. W. LUBBOCK, V.P. & Treas. R.S. | 189 |
| Report upon the Recent Progress and Present State of Meteorology. By JAMES D. FORBES, Esq. F.R.S.L. & E. F.G.S. Member of the Royal Geographical Society, of the Society of Arts for Scotland, and Honorary Member of the Yorkshire Philosophical Society. | |
| Introduction, Discoveries on Heat; Systematic Works on Meteorology. Constitution of the Atmosphere. Temperature, Thermometers; Atmospheric Temperature; Climatology; Decrease with Height; Proper Temperature of the Globe. Atmospheric Pressure, Barometers; Periodical Variations; Accidental Variations; Variation with Height. Humidity, Hygrometers; Distribution of Vapour in the Atmosphere. Atmospheric Phœnomena and Precipitations, Winds; Rain; Atmospherical Electricity, Hail; Aurora Borealis, its Influence on the Magnetic Needle. | 196 |
[page] viii
| Report on the present State of our Knowledge of the Science of Radiant Heat. By the Rev. BADEN POWELL, M.A. F.R.S., Savilian Professor of Geometry in the University of Oxford. | 259 |
| Report on Thermo-electricity. By the Rev. JAMES CUMMING, F.R.S., Professor of Chemistry in the University of Cambridge | 301 |
| Report on the recent Progress of Optics. By Sir DAVID BREWSTER, LL.D. F.R.S. &c. | 308 |
| Report on the Recent Progress and Present State of Mineralogy. By W. WHEWELL, M.A., Fellow and Tutor of Trinity College, and late Professor of Mineralogy in the University of Cambridge. | 322 |
| Report on the Progress, Actual State, and Ulterior Prospects of Geological Science. By the Rev. W. D. CONYBEARE, F.R.S. V.P.G.S. Corr. Memb. Institute of France, &c. &c. &c. | 365 |
| Report on the Recent Progress and Present State of Chemical Science. By JAMES F. W. JOHNSTON, A.M. &c. | 414 |
| Remarks on the Application of Philological and Physical Researches to the History of the Human Species. By J. C. PRICHARD, M.D. F.R.S. | 530 |
TRANSACTIONS OF THE SECTIONS:—
| Mathematics | 545 |
| Optics | 547 |
| Acoustics | 556 |
| Magnetism.—Electricity | 557 |
| Chemistry | 566 |
| Meteorology | 574 |
| Geography.—Geology | 576 |
| Zoology.—Anatomy.—Physiology | 587 |
| Botany | 595 |
| Arts | 597 |
| Miscellaneous | 602 |
| Index | 603 |
| List of Members. | 607 |
[page] ix
OBJECTS OF THE ASSOCIATION.
THE ASSOCIATION contemplates no interference with the ground occupied by other Institutions. Its objects are,—To give a stronger impulse and a more systematic direction to scientific inquiry,—to promote the intercourse of those who cultivate Science in different parts of the British Empire, with one another, and with foreign philosophers,—to obtain a more general attention to the objects of Science, and a removal of any disadvantages of a public kind, which impede its progress.
RULES.
MEMBERS.
All Persons who have attended the first Meeting shall be entitled to become Members of the Association, upon subscribing an obligation to conform to its Rules.
The Fellows and Members of Chartered Societies in the British Empire shall be entitled, in like manner, to become Members of the Association.
The Office-Bearers, and Members of the Councils or Managing Committees, of Philosophical Institutions shall be entitled, in like manner, to become Members of the Association.
All Members of a Philosophical Institution, recommended by its Council or Managing Committee, shall be entitled, in like manner, to become Members of the Association.
Persons not belonging to such Institutions shall be eligible, upon recommendation of the General Committee, to become Members of the Association.
SUBSCRIPTIONS.
The amount of the Annual Subscription shall be One Pound, to be paid in advance upon admission; and the amount of the composition in lieu thereof, Five Pounds.
MEETINGS.
The Association shall meet annually, for one week, or longer. The place of each Meeting shall be appointed by the General Committee at the previous Meeting; and the Arrangements for it shall be entrusted to the Officers of the Association.
GENERAL COMMITTEE.
The General Committee shall sit during the time of the Meeting, or longer, to transact the Business of the Association. It shall consist of all Members present, who have communicated any scientific Paper to a Philosophical Society, which Paper has been printed in its Transactions, or with its concurrence.
Members of Philosophical Institutions, being Members of this Association, who may be sent as Deputies to any Meeting of the Association, shall be members of the General Committee for that Meeting.
[page] x
COMMITTEES OF SCIENCES.
The General Committee shall appoint, at each Meeting, Committees, consisting severally of the Members most conversant with the several branches of Science, to advise together for the advancement thereof.
The Committees shall report what subjects of investigation they would particularly recommend to be prosecuted during the ensuing year, and brought under consideration at the next Meeting. They shall engage their own Members, or others, to undertake such investigations; and where the object admits of being assisted by the exertions of scientific bodies, they shall state the particulars in which it might be desirable for the General Committee to solicit the co-operation of such bodies.
The Committees shall procure Reports on the state and progress of particular Sciences, to be drawn up from time to time by competent persons, for the information of the Annual Meetings.
LOCAL COMMITTEES.
Local Committees shall be appointed, where necessary, by the General Committee, or by the Officers of the Association, to assist in promoting its objects.
Committees shall have the power of adding to their numbers those Members of the Association whose assistance they may desire.
OFFICERS. A President, two Vice-Presidents, two or more Secretaries, and a Treasurer, shall be annually appointed by the General Committee.
COUNCIL.
In the intervals of the Meetings the affairs of the Association shall be managed by a Council, appointed by the General Committee.
PAPERS AND COMMUNICATIONS.
The General Committee shall appoint, at each Meeting, a Sub-Committee, to examine the papers which have been read, and the register of communications; to report what ought to be published, and to recommend the manner of publication. The Author of any paper or communication shall be at liberty to reserve his right of property therein.
ACCOUNTS.
The Accounts of the Association shall be audited, annually, by Auditors appointed by the Meeting.
TREASURER.
JOHN TAYLOR, Esq. 14 Chatham Place, London.
LOCAL TREASURERS.
DR. DAUBENY, Oxford.
PROP. FORBES, Edinburgh.
JONATHAN GRAY, Esq. York.
PROF. HENSLOW, Cambridge.
WILLIAM HUTTON, Esq. New-castle-on-Tyne.
REV. THOMAS LUBY, Dublin.
DR. PRICHARD, Bristol.
GEORGE PARSONS, Esq Birming ham.
REV. J. J. TAYLER, Manchester.
H. WOOLCOMBE, Esq. Plymouth.
[page 7]
FIRST REPORT
OF
THE BRITISH ASSOCIATION
FOR
THE ADVANCEMENT OF SCIENCE.
[page 8]
[page 9]
PREFACE TO THE FIRST REPORT.
IN giving to the public a Report of the Proceedings of the BRITISH ASSOCIATION FOR THE ADVANCEMENT OF SCIENCE, it has been considered an important object to add to the account of the past Meeting a distinct view of what is to be expected from the next, and to announce the result of the applications which have been made to individuals, requesting them, in the name of the Association, to undertake the reports and researches recommended by its Committees in different branches of science.
The success of these applications will appear from the following statement.
[It has not been thought necessary to reprint this statement, as the Recommendations are contained in the First Report, and the effect of the applications now appears in the Second.]
It will be observed that the object to which the Committees have in general paid the first attention has been, to procure Reports on the state and desiderata of the several branches of science, preliminary to measures which may be hereafter adopted to advance them. To the investigation, however, of a few points of prominent interest and importance they have at once proceeded to invite attention; and of these there are some which it is highly desirable should receive the consideration of experimenters and observers who cannot be individually solicited to take a share in them. Such is the examination of those first data of chemistry, (Recommendations, p. 53), which, lying at the very foundation of the science, are proposed to be settled by the common consent of experienced chemists, and to which it is hoped that every one possessing the necessary means
[page] 10
and habits of accurate experiment will lend his assistance; such, also, are those meteorological and botanical researches, (Recommendations, p. 50, 55), which, belonging to a lower order of facts, are open to a much wider class of observers, and are capable of being extended through all parts of the country by the exertions of individuals, and still more effectually by those of Societies.
The nature and value of the aid which Provincial Societies might render to science through the system of the British Association, and the advantages which they may themselves derive from it, have been lately adverted to by the Council of the Yorkshire Philosophical Society in the following manner*.
"The object of this system is not only to give connection to the efforts of insulated inquirers, but to link Societies themselves together in unity of purpose, and in a common participation and division of labour. There are many important questions in philosophy, and some whole departments of science, the data of which are geographically distributed, and require to be collected by local observations extended over a whole country; and this is true not only of those facts on which single sciences are founded, but of many which are of more enlarged application. Thus, for instance, were the elevation above the sea of all the low levels, and chief heights and eminences, of a country ascertained so generally, that every observer of nature might have a station within his reach from which he could fix the relative position in this respect of whatever might be the object of his research,—of how many questions, in how many sciences, would these facts contribute to the solution? Again, supposing it to be ascertained also, at these stations, what is the temperature of the air, and of the water,—as it falls from the sky, and as it is held in the reservoirs of the earth,—these are data of the same kind, interesting not only to meteorological science, but to the philosophy of organized and animated existence. Yet, extensive as might be the importance of such facts, and simple as are the processes for ascertaining them, and numerous as are the individuals capable of contributing to their in-
* Report of the Council of the Yorkshire Philosophical Society for 1831—32.
[page] 11
vestigation, how little, nevertheless, even of this elementary work has yet been accomplished, either by insulated observers, or by those who are associated together for the express purpose of advancing the sciences to which it is of such essential interest.
None of our Societies has ever pretended to collect observations of this kind on a regular system, nor to form a national catalogue of the scattered particulars of any one science, accurately detailed; and yet the great value which would attach to such collections of facts, when reduced and analysed, must often have occurred to the enlightened conductors of such institutions; but that which has prevented any single Society from venturing on the undertaking, has been the impracticability of carrying it on over so extensive a territory as an entire kingdom. There is a method, however, by which these important objects might be achieved. Were there in every county one or more provincial Societies, having some members competent to superintend, and others ready to execute, the observations within definite limits, and were these Societies willing to work together on a common plan, the natural history of the country, and all the geographical data of philosophy included within it, might easily be collected in a manner far more perfect than has ever yet been attempted.
With a just sense, therefore, of the consequence to science of combining the Philosophical Societies dispersed through the provinces of the empire in a general cooperative union, the British Association has not only invited them to join its Meetings, but has given to those whom they may specially depute to represent them, the privilege of becoming members of the Committee by which its affairs are conducted.
It appears to the Council that in availing themselves of the bond of connection thus offered, Societies will not only contribute most essentially to the success of this extensive plan, but will add greatly to their own efficiency. When individuals meet for scientific objects, the effect of the general effort, emulation, and example, is to produce a spirit of exertion which gives to such meetings their principal value. And if Societies shall concur in thus meeting each other, in proposing certain
[page] 12
common objects, in communicating from year to year the means which they are employing and the progress which they are making,—it seems impossible that this should be done in the presence of an assembly concentrating a great part of the scientific talent of the nation, without kindling an increased ardour of emulous activity; it seems impossible that the deputies of any Society should attend such meetings without bringing back into its bosom an enlargement of views, and communicating to its members new lights of knowledge, new motives for inquiry, and new encouragement to perseverance.
The actual assembling of one of the meetings at the place in which any Society is established, has a tendency to produce the same effect in a still more powerful degree, and the Council does not hesitate to state that this institution has received a sensible impulse in all these respects, from the visit with which it has recently been honoured. The plan indeed on which it was first founded, and on which it has been since conducted, was in the spirit of the design which may now be contemplated for the whole kingdom. Its especial aim has been to collect information respecting its own County, and the end to which it aspires has been described in a former Report to be the execution of such a History of Yorkshire as the Natural Philosopher and the Antiquary may be contented to possess. But how greatly will the importance of this object be heightened when it is incorporated into a national system, and when all the results of our inquiries become part of the materials of a far more extensive analysis. It could not but be felt before by a provincial Society, that, in executing the task which it had undertaken, advice and consultation were wanted. With how much more confidence may it proceed when it has the advantage of consulting with the Committee of this great national Association. In comparing the views which it entertains, and the methods which it employs, with those that may be offered to its consideration, how largely may it profit by such a commerce, without sacrificing any portion of its real dignity or independence."
Should views like those which are here expressed be generally adopted, should the Societies established in different
[page] 13
districts be disposed to combine their exertions through the medium of this Association, for the purpose of carrying a general system of observations into effect, each Society would then become a centre of instruction to its own neighbourhood, from which correct means and methods of investigation might be derived. Thus, for instance, a large proportion of the philosophical instruments at present in use are so imperfectly constructed, and so discordant in their indications, as to be of little service to science; but if Societies will send to the next Meeting of the Association the Thermometer or portable Barometer which they employ, in order that they may be examined, and that any error which may be found in them may be rectified or estimated, the instruments will thenceforward not only speak the same language among themselves, but will become standards with which in every part of the kingdom those of insulated observers may be compared.
The principles which have been already noticed as having regulated the choice of some of the subjects of investigation recommended in the present Report, are important to be borne in mind, at the ensuing Meeting, by those who may take a share in proposing matter of inquiry or discussion. To come to a common understanding on unsettled questions of general interest, to fix the data on which important points of theory hinge, to collect and connect extensive series of observations; these appear to be the objects which peculiarly belong to the Association, and which should therefore be chiefly, if not exclusively, contemplated. It is also very material that those who propose any subject of inquiry should have considered it well in a practical point of view. It is not enough to put forth general recommendations of inquiries without making specific arrangements for their being actually undertaken. The Committee which met for the first time at York laboured under a disadvantage in this respect, from not knowing on what auxiliaries to reckon. Much was in consequence left to subsequent correspondence with the members of the different Sub-committees, which, had it been possible, ought to have been settled at the Meeting itself.
These deficiencies, however, have been so far surmounted,
b
[page] 14
that a highly valuable store of appropriate scientific communications, as has been seen, is already provided for the approaching Meeting; and in this respect also it will possess a great advantage over the last. The Transactions, of which an account is given in the Report, were miscellaneous contributions not expressly designed for the use of this Institution, and in consequence they occupy but a small space in the present publication. It is a principle of the Association to claim no right of property in the papers which it receives; and, with the exception of one Essay, which, by leave of the accomplished writer, has been printed at length, the remainder of this part of the Report consists of abstracts or notices of memoirs which will be communicated to the public through other channels. A few interspersed memoranda of the occasional discussions which followed the reading of the papers, have been inserted, chiefly to illustrate the plan of proceeding which was pursued at the meeting.
It only remains to be added, that the time which has been fixed upon as that on which it will be most convenient for the Association to assemble at Oxford, is the 18th day of June, 1832.
YORK, February, 1832.
[page 15]
FIRST REPORT.
PROCEEDINGS
OF
THE GENERAL MEETING.
1831.
ON the morning of September the 27th, 1831, the Theatre of the Yorkshire Museum was filled by an assemblage of more than three hundred persons*, including many distinguished members of learned and scientific bodies in different parts of the united kingdoms, who were collected together in consequence of a general invitation to the friends of science, which had been issued by the Yorkshire Philosophical Society. At half past twelve o'clock, on the motion of Dr. Brewster, Viscount Milton, the President of the Society, was called to the Chair, and addressed the Meeting nearly in the following words:
"Gentlemen,
You have been kind enough to call me to the Chair of this Meeting, which is indeed one of the most important description; and I only regret, that you have not turned your eyes towards a person, whose acquirements would render him more qualified to fulfil the duties imposed on him. But I trust that, although I may be in some respects deficient, at least I am not deficient in an anxious desire to promote those objects which have been in the view of the authors of the Philosophical Society established in this city, and also those which will be brought under the consideration of the Meeting now assembled. It must undoubtedly be highly satisfactory to the Members of the Society who have taken an active part in making the arrangements for the purpose, to see that we are honoured with the attendance of persons from all parts of the kingdom, who testify,
* The number of Tickets issued, was three hundred and fifty-three.
[page] 16
by coming from so great a distance, their desire to cooperate with the movers of this Meeting, and to carry its objects into effect. Similar Meetings, it is well known, have taken place on the continent of Europe, which have been attended by the most beneficial effects, and I trust that the same effects will attend those that we are now commencing here. In our insular and insulated country, we have few opportunities of communicating with the cultivators of science in other parts of the world. It is the more necessary, therefore, to adopt means for opening new channels of communication with them, and at the same time of promoting a greater degree of scientific intercourse among ourselves. Nor do I see any reason to doubt the successful issue of this undertaking. When I consider what the Yorkshire Philosophical Society has accomplished,—when I view the establishments it has founded, and when I recollect, that it has not existed for more than eight or ten years; having owed its origin, I believe, to the curious discoveries which were made at Kirkdale,—when, I say, we can trace the progress of a body now so considerable, to so inconsiderable a source,— may we not entertain a confident hope, that the Meetings thus auspiciously begun, will rapidly advance to still greater importance, and become the source of incalculable advantage to science hereafter? In addition to other more direct benefits, I hope they will be the means of impressing on the Government of this country the conviction, that the love of scientific pursuits, and the means of pursuing them, are not confined to the metropolis; and I hope that when the Government is fully impressed with the knowledge of the great desire entertained to promote science in every part of the empire, they will see the necessity of affording it due encouragement, and of giving every proper stimulus to its advancement. Perhaps the most effectual method of promoting science is by removing the obstacles which oppose its progress; though I am aware of the fact, that there are some investigations which require to be carried on upon so great a scale, as to be beyond the reach of individual enterprise: and to these, undoubtedly, the energies of Government should be directed. We all know, that the laws of this country,—I mean in particular the fiscal laws of this country,—offer numerous obstacles to scientific improvements. I will name only one instance. In the science of optics very serious obstacles are found to result from the regulations relative to the manufacture of glass. I mention only this; but it must occur to many of the persons present, that there are various other instances, in which the laws interfere materially with the progress of science. With regard to the more direct advantages which we have a right to anticipate from these Meetings, I have no
[page] 17
doubt, that, if they shall be extended to different parts of the country, and held in well-selected places, this result will be obtained: the men of science, now scattered over the empire, will be enabled to meet each other, and mutually communicate their ideas; they will state the advances which have been made in their own respective spheres of action, and also what the deficiencies may be. Thus not only will an extraordinary impulse be given, but the individuals and the Societies taking part in the Meetings will learn what parts of science they can cultivate with the greatest utility, and will give their researches the most advantageous direction. Such, Gentlemen, are a few of the benefits which, it appears to me, will be derived from Meetings of this description; and if they shall be extensively held, and shall be found thus pregnant with important consequences, sure I am that it will redound to the honour of this Society to have been the first to set the example."
Lord Milton concluded by expressing the sense which he entertained of the services which, his friend and predecessor in the office of President, Mr. W. V. Harcourt, had rendered to the Institution, within whose walls they were assembled.
The Rev. William Vernon Harcourt, Vice-President of the Yorkshire Philosophical Society, and Chairman of the Committee of Management, then addressed the Meeting:
"Gentlemen,
I am desired by the Council of the Yorkshire Philosophical Society to submit to your consideration a plan, which they beg leave to propose for the conduct of this Meeting, and for the establishment of a system, on which similar Meetings may continue to be conducted hereafter.
The Meeting, Gentlemen, owes its origin to some distinguished cultivators of science* here present, who were of opinion that great advantage might be expected from an Association for scientific intercourse in these kingdoms, formed upon the model of that which has subsisted in Germany for several years,—an Association which appears to have answered the hopes of its founders, as well in approximating men of science to each other, and promoting among them friendly feelings and an instructive interchange of ideas, as in giving to their union a collective efficacy, and bringing their aims and views more prominently into public notice.
* The Meeting was proposed by Dr. Brewster to the Yorkshire Philosophical Society in a letter to one of the Secretaries (Mr. Phillips). The proposal was approved and encouraged by the Society, and it received the most zealous and effective support from Mr. Robison, Mr. Forbes and Mr. Johnston in Edinburgh, and from Mr. Murchison in London.
B
[page] 18
Fully concurring in the utility of such objects, our Society cordially embraced the proposal which was made to us, that the first Meeting should be held in these apartments—happy if the accommodation which we have to offer could be made serviceable to a purpose of so much public interest, and not insensible, Gentlemen, to the honour and advantage which the presence of so distinguished an assembly would confer upon our own Institution.
In conformity also with the express desire of the promoters of the Meeting, we undertook to make all the arrangements for it, and to prepare the plan of a permanent Association. I will request the Secretary of the Committee of Management to state, in the first place, what arrangements were made, and will afterwards proceed to give an account of the plan which I have to offer to your consideration."
Mr. Phillips, Secretary of the Society and of the Committee of Management, made the following statement:
"The Committee, Gentlemen, being of opinion that the invitations to this preliminary. Meeting should be co-extensive with whatever desire there might be in the country to promote the objects of Science, drew up in the first instance a circular letter inviting the attendance of all persons interested in scientific pursuits, which, in case any one who is here present should not have received it, it may be proper for me to read:—
Sir,
The Council of the Yorkshire Philosophical Society having received intimation from men of scientific eminence in various parts of the kingdom, of a general wish that a Meeting of the Friends of Science should be held at York during the last week in September next, we are directed to announce that the Society has offered the use of its apartments for the accommodation of the Meeting, and that arrangements will be made for the personal convenience of those who may attend it. It will greatly facilitate these arrangements, if all who intend to come to the Meeting, would signify their intention as early as possible to the Secretaries.
The apartments, which the Yorkshire Philosophical Society has to offer for the use of the Meeting, consist of a Theatre, which affords seats for about three hundred persons, five rooms containing the Museum of Natural History, a Library, Laboratory, and Council Room.
All persons interested in scientific pursuits are admissible to the Meeting.
WILLIAM VERNON HARCOURT, VICE-PRESIDENT.
JOHN PHILLIPS, SECRETARY.
Yorkshire Museum, York,
July 12, 1831.
[page] 19
Copies of this circular letter were sent to the Presidents and Secretaries of all the Scientific Institutions in England, metropolitan and provincial, which were known to the Committee, with a request that the invitation might be communicated to any members of those Institutions who might be disposed to accept it. The number of Societies in London thus addressed was THIRTEEN; the number in other parts of England was TWENTY-SIX, NINE of these being in the County of York.
The letter was sent individually to the more distant Members of our own Society, and to persons, whether belonging to any Society or not, who were known to be active cultivators and promoters of science. One hundred and eighty-nine copies were issued on the latter account. In this list, and even in the list of Societies, it is more than possible that the Committee may have been guilty of some omissions, which they hope, however, will be pardoned, when the number of letters sent out is considered, amounting, in the whole, to more than four hundred. One hundred copies were also transmitted for similar distribution to Societies and individuals, by the correspondents of the Committee in Scotland and Ireland; and two or three eminent foreigners were in like manner individually invited, though the Committee did not deem it prudent to extend invitations abroad, till it should be seen what reception the plan of the Association might meet with at home. Lastly, to ensure more general publicity, advertisements of the Meeting were inserted in the Philosophical Magazine for the months of August and September, an announcement of it having before appeared in the Edinburgh Journal of Science."
Mr. Phillips then proceeded to read the answers which had been received to these invitations from persons who had been prevented, by unavoidable engagements, from being present at the Meeting:—answers, which, whilst they excited a deep regret for the absence of the distinguished writers, showed what valuable support the Association might justly count upon receiving from them hereafter. He stated, "that in several instances deputations had been appointed by provincial Institutions to attend the Meeting, and that gentlemen were present, who had come for that purpose from London, Edinburgh, and Dublin, from Newcastle, Manchester, Liverpool, and Birmingham, and even from Bath and Bristol. The great distance of the Plymouth Institution had prevented any of its members from being present; but the official letter received from that body was a gratifying proof of the general interest felt in these proceedings, and of the benefit to be expected from a migratory Association, which might another year be as conveniently at-
B 2
[page] 20
ended by the Southern, as on this occasion by the Northern Societies.
"When the time appointed for the Meeting drew nearer, the Committee of Management put into circulation another notice, specifying more particularly the nature of the regulations which they proposed to adopt. The second circular notice was as follows:—
GENERAL SCIENTIFIC MEETING AT YORK.
It is requested that persons proposing to attend the Meeting will give notice of their intention to the Secretaries of the Yorkshire Philosophical Society.
Models of Inventions, Specimens of Natural and Artificial Products, to be exhibited at the Meeting, Instruments or Drawings to illustrate any communication, and Materials for Experiments, will be received by the Secretaries, and may be transmitted to them previous to the Meeting.
It is also desirable that Memoirs intended to be read, or a short statement of their contents should be sent beforehand, in order to their being registered; and that any Memoir which may be too detailed to admit of being read at length, should be accompanied by an abstract of its principal contents.
On Monday, the 26th inst. the Managing Committee will receive, at the Museum, the names of Persons intending to be present; and will deliver Tickets for the Morning and Evening Meetings, and Dinners, and references for Lodgings. The Committee will think it right to pay regard to œconomy, as well as convenience, in these arrangements*.
The Apartments of the Society will be opened on Monday Evening; and the first Morning Meeting for scientific purposes will be on Tuesday, the 27th, at Twelve o'clock.
Yorkshire Museum,
Sept. 7, 1831.
To this account of the regulations of the Committee, it only remained to be added, that the number of scientific papers to be brought forward, was so considerable, as to demand the employment of the evenings as well as the mornings of the week, and the Committee recommended that the communications of the least abstract nature should be allotted to the evening Meet-
* On Tuesday a public dinner was provided at Twelve Shillings a Ticket; on the other days, during the session, ordinaries at from Five to Seven Shillings a head: venison, game, and fruit being contributed by Earl Fitzwilliam, the Earl of Carlisle, Paul Beilby Thompson, Esq. and Richard John Thompson, Esq. The Archbishop of York gave a public dinner to the Members of the Association on Friday. The Evening refreshments were furnished by a subscription among the Members of the Yorkshire Philosophical Society.
[page] 21
ings, as it was proposed to admit a more popular audience to these."
Mr. Phillips having concluded his statement,—Mr. William Harcourt again rose and read extracts from letters which had been addressed to him by Mr. Chantrey, Mr. Faraday, and Dr. Buckland, who had been reluctantly prevented from attending the Meeting by pressing engagements. Mr. Chantrey, he said, had given the Yorkshire Philosophical Society another proof of his liberal disposition to promote science, by presenting to its Museum on this occasion, a Cast of the celebrated specimen of Plesiosaurus in the Duke of Buckingham's collection. He then read a letter which he had received from the Duke of Sussex, who had been invited to honour the Meeting with his presence. The letter stated, that nothing would have afforded His Royal Highness greater pleasure than to have complied with the invitation, if he had not been unfortunately pre-engaged. "You will, therefore," His Royal Highness added, "be so kind as to express my regret on the occasion, accompanied with my best wishes for the success of so praiseworthy an object, and an assurance on my part, of my warm cooperation in promoting any measure which may be suggested, and sanctioned by such a respectable Meeting."
Mr. Harcourt then commenced his exposition of the OBJECTS AND PLAN OF THE ASSOCIATION.
"When we came to meditate, Gentlemen, on the means of giving stability and continuance to such Meetings as these, when we considered how little command of time men of science in this country enjoy, and how difficultly they are drawn from their occupations and homes, we could not but entertain a doubt whether the inducement of meeting one another, without a more imperative call, would be powerful enough to bring them annually together from distant parts of the kingdom. But, if there were objects of more essential consequence, which a yearly aggregate Meeting might propose to accomplish, objects now unattempted, and yet of the highest moment to the advancement of science, then we apprehended, that those who have any zeal to advance it would not lightly absent themselves from such a Meeting, and that thus the benefit of personal intercourse would follow in the train of still more important advantages.
Views of this extent, however, were not to be indulged without consultation; and, before we ventured to bring them forward, we inquired the opinions of several of the most distinguished among the lights of British science: from some of those who were consulted, we received warm encouragement and valuable suggestions, whilst to others we were indebted for cautions, of
[page] 22
which we also knew the value, and for a fair representation of the obstacles opposed to our success. These different opinions have been weighed with the attention which they deserved; and I present this plan to the Meeting, as one of which all the bearings have been considered, and of which the deliberate consideration has led us to hope, that a great preponderance of advantage may be derived from its adoption.
I propose then, Gentlemen, in the first place, that we should found a BRITISH ASSOCIATION FOR THE ADVANCEMENT OF SCIENCE, having for its objects, to give a stronger impulse and more systematic direction to scientific inquiry, to obtain a greater degree of national attention to the objects of science, and a removal of those disadvantages which impede its progress, and to promote the intercourse of the cultivators of science with one another, and with foreign philosophers.
On the first and most important of these objects, some difference of opinion may exist; a difference of opinion, I mean, as to the want in which we stand of a new Association, to give a stronger impulse and more systematic direction to scientific inquiry.
I do not rest my opinion, Gentlemen, of this want upon any complaint of the decline of science in England. It would be a strange anomaly if the science of the nation were declining, whilst the general intelligence and prosperity increase. There is good reason, indeed, to regret that it does not make more rapid progress in so favourable a soil, and that its cultivation is not proportionate to the advantages which this country affords, and the immunity from vulgar cares which a mature state of social refinement implies. But, in no other than this relative sense, can I admit science to have declined in England. What three names, if we except the name of NEWTON, can be shown in any one age of our scientific history which rank higher than those of men whose friendship we have enjoyed, by whose genius we have been warmed, and whose loss it has been our misfortune prematurely to deplore, the names of DAVY, WOLLASTON, and YOUNG! And there are men still remaining among us, individuals whom I must not mention, present in this Meeting, and absent from this Meeting, whose names are no less consecrated to immortality than theirs.
But it is not by counting the great luminaries who may chance to shine in this year, or that,—in a decade of years, or a generation of men,—that we are to inform ourselves of the state of national science. Let us look rather to the numbers engaged, effectually, though less conspicuously, in adding by degrees to Our knowledge of nature; let us look to the increase of scientific
[page] 23
transactions and journals; let us look, Gentlemen, at the list produced this day of Philosophical Societies which have grown up in all parts of the kingdom. The multiplication of these new and numerous institutions indicates a wide extension of scientific pursuits. The funds so liberally contributed to their support bear evidence of an enlarged disposition in the public to promote such pursuits.
It is on this very ground I rest the necessity and the practicability of establishing in science a new impulsive and directive force, that there are new and more abundant materials to be directed and impelled. The mining-field of discovery seems to me to show, on the one part, the ore breaking out on every side; veins of the precious metal scarcely opened or imperfectly wrought; and on the other a multitude of hands ready to work it; but no one engaging them to labour, or showing them in what manner they may employ their industry to the best advantage. And therefore it is that I propose to you to found an Association including all the scientific strength of Great Britain, which shall employ a short period of every year in pointing out the lines of direction in which the researches of science should move, in indicating the particulars which most immediately demand investigation, in stating problems to be solved and data to be fixed, in assigning to every class of mind a definite task, and suggesting to its members, that there is here a shore of which the soundings should be more accurately taken, and there a line of coast along which a voyage of discovery should be made.
I am not aware, Gentlemen, that in executing such a plan we should intrude upon the province of any other Institution. There is no Society at present existing among us, which undertakes to lend any guidance to the individual efforts of its members, and there is none perhaps which can undertake it. Consider the difference, Gentlemen, between the limited circle of any of our scientific councils, or even the Annual Meetings of our Societies, and a Meeting at which all the science of these kingdoms should be convened, which should be attended, as this first Meeting you see already promises, by deputations from every other Society, and in which foreign talent and character should be tempted to mingle with our own. With what a momentum would such an Association urge on its purpose! what activity would it be capable of exciting! how powerfully would it attract and stimulate those minds, which either thirst for reputation or rejoice in the light and sunshine of truth!
The eldest of our scientific Institutions contemplated, in its origin, the objects which we now propose to pursue. The foundation, Gentlemen, of the Royal Society was an attempt to
[page] 24
reduce to practice the splendid fiction of the New Atlantis*. The same comprehensive mind which first developed the true method of interpreting nature, sketched also the first draught of a national Association for undertaking, by a system of distributed and combined exertion, the labour of that work.
This philosophical romance was not composed by its great author to amuse the fancy, but to dispose the minds of the legislature towards the foundation of a public establishment for the advancement of science. His plan for its maintenance
* The actual and immediate effect produced by Bacon on the general spirit of philosophy has been underrated: His writings were quickly circulated through Europe, and their value was appreciated abroad even sooner than at home: he himself translated the New Atlantis into Latin, "in gratiam exterorum apud quos expeti inaudiverat," and his most important works were rendered into that language and into French before his death. His letter to Baranzon, who lectured on Natural Philosophy at Annecy in Savoy, and who, it appears, had consulted him on the substitution of his inductive method for the syllogisms of Aristotle, deserves attention not only as containing the most perspicuous summary of his views, but as showing how far the authority and influence of his writings had reached in 1621. It has been said by Playfair that Descartes, who became afterwards the head of so numerous a school, "does not seem to have been acquainted with Bacon's works;" and another eminent historian of philosophy, Dugald Stewart, has admitted that, "if he ever read them he has nowhere alluded to them in his writings." But the fact is, that in the correspondence of Descartes with Mersenne, published in 1642, there are, in several of his letters, passages in which he has referred to the works of "Verulam" with a respect which he yielded to no other writer, and has shown that be had both studied them and adopted the methods which they contain; so that there is no longer any difficulty in accounting for the remarkable coincidence with Bacon's views and language which Mr. Stewart has noticed in the principles laid down by Descartes for studying the phænomena of the mind. The passages to which I refer are these: "Scribis præterea velle te scire modum aliquem faciendi experiments utilia; ad quod nihil est quod dicam post Verulamium qui hac de re scripsit, nisi quod omissis minutioribus circumstantiis oporteret in qualibet materia potissimum facere generates observationes rerum omnium maxime vulgarium et certissimarum et quæ sine sumptu cognosci possint, ut, ex. gr. cochleas omnes in eandem partem esse contortas, atque utrum idem obtineat trans æquinoctialem; omnium animalium corpus esse divisum in tres partes, caput, pectus, et ventrem, et alia id genus, hujusmodi enim observationes ad veritatis investigationem certo deducunt." (Ep. LXV.) "Gratias tibi ago pro qualitatibus quas ex Aristotele desumpsisti; majorem illorum catalogum, partim ex Verulamio desumptum, partim a me collectum jam conscripseram, illasque imprimis conabor explicare." (Ep. C. V.) " Scripsisti ad me aliquando esse tibi notos viros quibus volupe erat scientiis propagandis dare operam, (these were probably the persons whose meetings at Mersenne's house laid the foundation of the French Academy,) adeo ut nullum non experimentorum genus propriis sumptibus se facturos profiterentur. Illorum siquis vellet conscribere historiam phænomenorum cœlestium secundum methodum Verulamii, atque omissis rationibus et hypothesibus accurate describeret cœlum prout nunc apparet, quem situm singulæ stellæ fixæ respectu circumjacentium obtineant, quæ sit aut magnitudinis, aut colons, autluminis, aut scintillationis, &c. differentia; item numquid ea consentiunt cum iis quæ de illis veteres Astronomi scripserunt, quave in re different, (neque enim dubito quin stellæ situm inter se suum aliquantulum mutent quamvis fixæ habeantur) hisque subjiceret observationes Cometarum, tabellam conficiens de uniuscunque motu, quemadmodum Tycho de tribus aut quatuor a se observatis fecit, denique variationes Eclipticæ et apogoeorum planetarum, opus esset utilius quam forte primo intuitu videatur, essetque mihi magnum operæ compendium; sed non spero id facturum quenquam." (Ep. LXVII.) If any one will compare these suggestions with the letter to Baranzon before referred to, he will find them almost a literal transcript of Bacon's request to the Savoyard philosopher to undertake this identical task. These extracts show the philosophical character of Descartes in a light somewhat different from that in which it is commonly regarded; like other great geometers before and since, he carried the use of abstractions and hypotheses too far and too soon into physical reasoning; but though he did not, with the wisdom of Newton, abide by the fundamental principle, laid down by Bacon, "non fingendum, nec excogitandum, sed inveniendum, quid natura faciat aut ferat," he was no stranger to the inductive method of collecting axioms from observation and experiment. In a letter addressed to Descartes, and prefixed to his celebrated treatise on the passions, a strong appeal is made to the public liberality to enable him to pursue those multiplied experiments for which he had occasion in order to carry on his investigations into nature. It is stated in this letter, that Gilbert had expended more than 50,000 crowns on the magnet alone, and that to execute all the experiments which Bacon had designed, would require more than the revenue of two or three kings. The writer (probably Mersenne) refers to "l'Instauratio magna et le Novus Atlantis du Chancelier Bacon, qui me semble estre de tous ceux qui ont escrit avant vous celuy qui a eu les meilleures pensees touchant la methode qu'on doit tenir pour conduire la Physique en sa perfection."
In England meanwhile an experimental school was forming, more faithful to the principles of the inductive philosophy. Foremost among the founders of the Royal Society, "Mr. Boyle, the ornament of his age and country, succeeded to the genius and inquiries of the great Chancellor Verulam1;" and he has left us no doubt as to the master by whom he had been taught; for in recording his experiments he has retained not only the method, but the peculiar idiom and technical phrases of Bacon. Thus this great interpreter of nature stood among philosophers like the pilot among the crew; he constructed the chart of knowedge, he marked upon it the place of the ship, he took the bearings of the land, he pointed out the variation of the compass, he noted the force and direction of the winds, and taught the adventurer to steer a certain course over the wide and trackless sea.
[page] 25
is detailed in 'A speech, touching the recovering of drowned mineral works, prepared for the parliament by the Viscount of St. Albans, then Lord High Chancellor of England.' For that end he would have proposed, by legislative enactment, 'to bring those deserted mineral riches into use by the assiduous labours of felons and the industry of converted penitents, whose wretched carcasses the impartial laws have dedicated, or shall dedicate, as untimely feasts to the worms of the earth.' 'By this unchargeable way, my Lords, have I proposed to erect the
1 Boerhaave.
[page] 26
academical fabric of this island's Solomon's house, modelled in my New Atlantis, and my ends are only to make the world my heir, and the learned fathers of my Solomon's house the successive and sworn trustees, in the dispensation of this great service, for God's glory, my prince's magnificence, this parliament's honour, our country's general good, and the propagation of my own memory.' From this speech it appears that the basis of the great Institution, which Bacon meditated, was a public provision for the maintenance and promotion of science. It was one of the defects noted by him in his masterly survey of the state of learning, that science had never possessed a whole man; and he exerted all the influence of his high station and commanding talents, to promote the supply of that defect. In a letter to the king respecting the foundation of the hospital at Dulwich by Allen the actor, he remarked, that though he was glad to see him play the last act of life so well, yet he thought Sir H. Savile's endowments of geometrical and astronomical Professorships of much greater necessity and more deserving of royal encouragement; and his own last bequest was one which, had it been executed, would have endowed two similar offices with salaries of two hundred pounds a year. In his opinion it was 'necessary to the progression of sciences, that those who are to generate and propagate them should be placed in such a condition as may content the ablest man to appropriate his whole labour, and continue his whole age, in that function and attendance;' and he added, 'there will hardly be any main proficiency in the disclosing of nature, except there be some allowances for expenses about experiments, whether they be experiments appertaining to Vulcan or Daedalus, furnace or engine, or any other kind; and therefore, as secretaries and spials of princes and states bring in bills for intelligence, so you must allow the spials and intelligencers of nature to bring in their bills, or else you shall be ill advertised.'
"These desiderata no means have yet been found of supplying in an adequate degree; and science, even to the present day, can scarcely be said to possess more than fractions of men. The Royal Society did not attempt to execute this part of Bacon's plan; but in other respects it copied as closely as possible, the model of the six days College. It was not then an association of individuals throwing their contributions casually into a common stock, but a body politic of philosophers acting in a corporate capacity and with systematic views, allotting to its members their respective tasks, and conjunctively debating and consulting for the advancement of knowledge. It had, in the figurative language of Bacon, its merchants of light, who
[page] 27
were dispatched in various directions at home and abroad, to gather information and bring back specimens of the productions of nature; it had its depredators who were deputed to examine histories of countries, and to question the travellers who had visited them, in order that queries might be framed which were then addressed to the Society's correspondents in foreign lands, among whom Consuls and Ambassadors were proud to be numbered. It employed some of its members as auxiliaries to the arts; to some it proposed the solution of the most important problems in mathematics, whilst it referred to others the charge of experimental researches, the mode of conducting which was discussed before-hand, and the results re-examined by a public Meeting. I may mention as examples of the effect of this system, that we are indebted to it, practically, for Evelyns History of Forest Trees, by which the planting of the country was so materially promoted, and, theoretically, for the determination of the law of the collision of bodies, simultaneously obtained from Huygens, Wallis, and Wren.
This was indeed to execute a noble plan in the spirit in which it was designed. The noise of works and inventions resounded on every side; new facts and original discoveries of the laws of the universe were daily brought to light; the conveniences and safeguards of life, the measurements of time, the construction of ships, the tilling and planting of the earth began to be rapidly improved. But the vigour of these exertions soon declined, and within thirty years we find Leibnitz suggesting to one of the original founders* of the Royal Society that it wanted new warmth to be infused into its constitution, and recommending that it should be remodelled after the example of the French Academy.
Leibnitz indeed had no right to consider a Society effete, which within a few years had elicited a work† from Newton, that eclipsed the fame even of the great German philosopher. Nor to this hour has it ever lost its title to public respect. It still embodies in its list every name which stands high in British science; it still communicates to the world the most important of our discoveries; it still crowns with the most coveted honours the ambition of successful talent; and when the public service requires the aid of philosophy, it still renders to the nation the ablest assistance, and the soundest counsel. Nevertheless it must be admitted, Gentlemen, that the Royal Society no longer
* Dr. Wallis.
† The Principia, as well as the Optics, of Newton were published at the solicitation of the Royal Society.
[page] 28
performs the part of promoting natural knowledge by any such exertions as those which we now propose to revive. As a body, it scarcely labours itself, and does not attempt to guide the labours of others.
Hence it happens, that when any science becomes popular, and those who interest themselves in its advancement perceive the necessity of working for it by united exertions, that science is detached from the central body; first one fragment falls off, and then another; colony after colony dissevers itself from the declining empire, and by degrees the commonwealth of science is dissolved. The new Societies distinguish themselves by their diligence and activity; the parts of knowledge which thus receive more distinct attention, and are propelled by more undivided labour, make rapid advances; and each separate undertaking justifies itself by the most promising appearances and undeniable fruits.
This is a new stage, Gentlemen, in the progress of science; a new state of things, which, whilst it is attended certainly with great advantages, has some consequences of doubtful aspect to the highest aims of philosophy. As the facts and speculations in any department of knowledge are multiplied, the study of it has a tendency to engross and confine the views of those by whom it is cultivated; and if the system of separate Societies shall encourage this insulation, science will be in the end retarded by them more than it is at first advanced. The chief Interpreters of nature have always been those who grasped the widest field of inquiry, who have listened with the most universal curiosity to all information, and felt an interest in every question which the one great system of nature presents. Nothing, I think, could be a more disastrous event for the sciences, than that one of them should be in any manner dissociated from another; and nothing can conduce more to prevent that dissociation, than the bringing into mutual contact men who have exercised great and equal powers of mind upon different pursuits; nothing more fitted to shame men out of that unphilosophical contempt which they are too apt to feel for each other's objects; nothing more likely to open to them new veins of thought, which may be of the utmost importance to the very inquiries on which they are more peculiarly intent.
I remember, at the Meeting of a foreign Society, to have heard a memoir read, in which a specific and original difference was inferred between two animals (commonly considered of one species), not from any difference in the higher and more essential parts of their organization, but from a dissimilarity of colour in
[page] 29
the skin or fur, and from minute anatomical distinctions; and I heard the error of the Zoologist corrected by a Botanist, one of the most eminent in Europe, who illuminated the whole subject of generic, specific and individual difference, by the light of a powerful mind which had been directed to the study of the question, considered in a different aspect, and with a more extensive survey. In like manner it is easy to conceive, on the one hand, how much advantage might be derived to geological debates from the presence of a sober and rigorous mathematician; and how, on the other hand, the abstract analyst and geometer might have his calculations restricted or promoted by listening to the detail of facts, which those could give him who cultivate the sciences more directly dependent on observation and experiment.
But there is a defect in these separate Societies, in respect to their own immediate objects, which I am sure no member of them would wish to dissemble, and which arises from the narrow basis on which they are of necessity built. It is not only that the constant converse of men, who, to borrow the expression of Goldsmith, have often travelled over each other's minds, is not half so effectual in striking out great and unexpected lights, as the occasional intercourse of those who have studied nature at a distance from each other, under various circumstances and in different views; but it is also, Gentlemen, that none of our existing Societies is able to concentrate the scattered forces even of its own science: they do not know, much less can they connect or employ that extensive and growing body of humble labourers who are ready, whenever they shall be called upon, to render their assistance. I have the pleasure of seeing here the President of the Geological Society of London; and I beg leave to ask him, whether in a science, the most complex of all sciences in its object, because it aims at deciphering the history of nature not only as it is but as it has been, in a science of which very few even among the lowest generalizations are as yet so settled as to be able to bear the weight of any theoretical superstructure whatever,—I ask him whether in the science of Geology there is not a multitude of facts to be ascertained in every district, on which he would be glad to see a much greater number of observers employed? And if it be so, let me remind him that we have heard today of nine Philosophical Societies in this county alone, which could doubtless find members ready to prosecute any local inquiry that this Meeting might, at his suggestion, request them to undertake. It is the same with all parts of Natural History, with Meteorology, and indeed with every science which is founded upon observation, or even upon
[page] 30
experiment; for the lower order of experiments, in subjects of the utmost ultimate abstractness,—such as the relations, for instance, of heat and of light,—are not only abundantly wanted, but by a moderate degree of industry and talent are by no means difficult to be supplied.
What numberless suggestions, what a crowd of valuable but abortive hints are continually floating in the thoughts of philosophers, for the pursuit of which time is wanting to themselves! Now I say, Gentlemen, that we have among us, scattered through the country, men willing to adopt these unexecuted hints, as they arise out of the profound and varied meditations of more experienced minds, men not incapable of surveying with accuracy a limited district, though they may not pretend to draw the general outline of the map, or fill up the whole of its details. Many such there are who only wait for instructions, and who require no other stimulus than that of being invited, to render the most essential service to researches and calculations of the highest order; and it is upon this ground especially that we venture to pronounce an Institution wanting, which shall not hesitate to make such invitations and to offer such instructions; it is upon this ground that if we now propose to revive in the nineteenth century a plan devised two centuries ago,— we see a difference, Gentlemen, in the probability of success. Scientific knowledge has of late years been more largely infused into the education of every class of society, and the time seems to be arrived for taking advantage of the intellectual improvement of the nation. Let Philosophy at length come forth and show herself in public; let her hold her court in different parts of her dominions; and you will see her surrounded by loyal retainers, who will derive new light and zeal from her presence and contribute to extend her power on every side.
Much, indeed, is not to be gained in the more recondite subjects of investigation from the first essays of inexpert inquirers; but let the number of those inquirers only be increased; collect around you, Gentlemen, a school fired with a zeal for truth, confess to them how little you know compared with what remains to be known, apprize them that there is not a subject to which they can apply themselves where new materials are not wanted to advance the fabric, or secure the foundations; let them see that the more multiplied have been your discoveries, the more additional openings to discovery have appeared,—and if you will then draw the precise line of what is, and what is not made out in every science, if you will indicate to them those promising points and inlets of inquiry which bid fair to lead to valuable results,—if you will thus put before them right sub-
[page] 31
jects, and at the same time suggest the right methods of treating those subjects; whatever more may be wanting to accurate and successful investigation, natural sagacity and a longer experience will not fail by degrees to supply.
But even the experienced in science will benefit by consultation with each other; for there are different degrees of experience, and no solitary industry or talent can ever hope to equal the power of combined wisdom and concerted labour. Above all, consider, Gentlemen, the excitement to exertion which will be felt by those who are solicited to undertake an inquiry at one of these Meetings, and pledged to produce the investigation at another. The greatest minds require to be urged by outward impulses, and there is no impulse more powerful than that which is exercised by publicly-esteemed bodies of men. Even Newton's papers might have remained unfinished, but for the incentive of such a solicitation. In a letter which I have lately received from Mr. Conybeare, and in which he expresses a deep regret at finding himself unexpectedly prevented from attending this Meeting, the benefit in these respects which may be looked for from a general scientific combination is described with the energy of his ardent and comprehensive genius. 'Your proposal,' he says, 'for ingrafting on the annual reunion of scientific men, a system for effecting such a concentration of the talent of the country as might tend more effectually to consolidate and combine its scattered powers, to direct its investigations to the points which an extensive survey thus generalized would indicate as the most important,—benefited by all the aids which the union of powerful minds, the enlarged comparison of different views, and a general system of intellectual cooperation could not fail to afford,—fills me with visions too extensive almost to allow me to write with sufficient calmness of approbation. The combined advantages, including at once the most powerful stimulus and the most efficient guidance of scientific research, which might emanate from such a point of central union, seem to me to be beyond calculation. If views like those you have sketched could be realized, they would almost give a local habitation and a name to the philosophical academy of Bacon's Atlantis, when "divers Meetings and consults" of the united body of DEPREDATORS, COMPILERS, PIONEERS, &c., suggested new experiments of a higher light and more penetrating nature to the LAMPS, and these at length yielded materials to the INTERPRETERS of nature.'
To that great model of a national Institution for the advancement of science, I have already adverted today, as I have formerly directed to it the attention of the Yorkshire Philoso-
[page] 32
phical Society: it is here referred to by Mr. Conybeare; and by a remarkable coincidence of ideas, we have the same reference from Mr. Harvey, who in a letter from Plymouth, which he has addressed to the Secretary of the Meeting, observes, that Bacon alludes to "circuits or visits of divers principal cities of the kingdom" as forming a distinguished feature of the New Atlantis. 'What Bacon,' he adds, 'foresaw in distant perspective, it has been reserved to our day to realize; and as his prophetic spirit pointed out the splendid consequences that would result generally from institutions of this kind, so may we hope that the new visions which are opening before us may be productive of still greater effects than have yet been beheld; and that the bringing together the cultivators of science from the North and the South, the East and the West, may fulfil all the anticipations of one of the greatest minds that ever threw glory on our intellectual nature.'
I have now laid before this Meeting the reasons for which I think it would be expedient to form a national Association, having for its first object to give a stronger impulse and more systematic direction to scientific inquiry. On the remaining objects which I have before mentioned, it is not necessary for me to enlarge much. It is not necessary to recommend the promotion of a more general intercourse among the cultivators of science, to those who have come in many instances from a great distance expressly to enjoy the gratification of meeting men of kindred minds and congenial pursuits. I shall content myself with remarking, that nothing can be better calculated to prevent those interferences, and reconcile those jealousies which sometimes disturb the peace of philosophy, than the mutual intelligence and amicable communion of such a Meeting as this.
On the grounds which subsist for seeking to obtain a greater degree of national attention to the objects of science, I have little to add to what the Chairman has said. In confirmation of his remarks on the obstacles which some of our fiscal laws oppose to the progress of knowledge, I may adduce the recent experience of this Museum. There is nothing more indispensable to the utility of such an Institution than a complete display of the specimens which it contains; and for that purpose, where the specimens are numerous, extensive glazing is required. Now there is a most serious impediment to this in the high price of glass, and of that price we find that two thirds consist in the duty paid to Government. So that more than one department of science is injured by this tax; the weight of the impost restrains the public exhibition of the objects of natural history; whilst the regulations of the Excise oppose an obstacle to the improvement
[page] 33
of astronomical instruments, still more to be regretted. Among the subjects to which a Scientific Association may be justly expected to call the public attention, I would particularly instance a revision of the law of Patents. The protection which is given to every other species of property is not given in the same extent to the property of scientific invention. The protection which it does receive must be bought of the state at a high price; an expense, varying from two to four or five hundred pounds, is first to be sustained. Then, after encountering the risk of this outlay, the Patentee is compelled to specify publicly and with legal precision, the particulars of his invention; thus it is exposed to be pirated, with the redress only of ruinous proceedings at law; and the consequence is, that no Patent is considered of any value till it has actually maintained a litigation; and though Patents are still taken out, their chief use is understood to be, not so much to secure a right as to advertise a commodity. Such is the present policy of our laws respecting the remuneration of practical science, a policy which seems to have no other end than to restrain the multiplicity of inventions.
With regard to the direct national encouragement which is due to scientific objects and scientific men, I am unwilling to moot any disputed or disputable question. There is a service of science to be rendered to a state with which it cannot dispense; and all, I think, must allow that it is neither liberal nor politic to keep those, who employ the rarest intellectual endowments in the direct service of the country, upon a kind of parish allowance. It would be difficult also to withhold our assent from the opinion that a liberal public provision would have a powerful effect in promoting those studies of abstract science which most require artificial encouragement; and that 'to detach a number of ingenious men from every thing but scientific pursuits; to deliver them alike from the embarrassments of poverty and the temptations of wealth; to give them a place and station in society the most respectable and independent, is to remove every impediment and to add every stimulus to exertion*.' But I will not, on this occasion, enter upon a subject on which any difference of sentiment can be supposed to exist, nor pretend to decide whether Playfair judged rightly of the degree in which a provision of this kind has actually improved the state of science in a neighbouring country, when he added, that 'to such an Institution operating upon a people of great genius and indefatigable activity of mind, we are to ascribe that superiority in the mathematical sciences,
* Second Dissertation prefixed to the Supplement to the Encyclop. Brit.
C
[page] 34
for which, during the last seventy years, they have been so conspicuous.'
One great benefit, at least, in addition to her maritime expeditions, England, as a nation, has conferred on the science of the world. She has had reason to be proud of her astronomical observations; though perhaps it is not equally gratifying to reflect that these observations have been turned to account, of late years, less by her own geometers than by the national school of mathematicians in France. But there are many other sciences, Gentlemen, on which the resources of states are no less dependent; and in them also there are physical data, which require to be ascertained by masters in science, with the most rigorous precision, and not without the most persevering labour. And I may be permitted to think with Mr. Herschel, that 'it may very reasonably be asked, why the direct assistance afforded by governments to the execution of continued series of observations, adapted to this especial end, should continue to be, as it has hitherto almost exclusively been, confined to Astronomy.'
The Chairman of the Meeting adverting to this subject, has said that 'there are enterprises in science which none but a nation can undertake;'let me add also, that there are establishments for science which none but a nation cap support. I remember, Gentlemen, to have heard the greatest philosopher of this age for variety and extent of attainments, M. de Humboldt, speak of Great Britain, as he was showing me the splen-did collections of natural history in the Louvre. What country in the world, said he, has such opportunities as England for collecting in her capital specimens of all the productions of the earth! I reflected, Gentlemen, on those unrivalled advantages,— but felt, I confess, no elation of national pride when I recollected the state of the British Museum. Since that time, however, one material step has been taken towards improvement; and when an adequate building shall have been prepared, let us hope that we may at length see a public school of natural history in London, so furnished, and so appointed, as not to be unworthy of the British nation. I am persuaded that even our statesmen would have no cause for regret, if, whilst the stores of this national repository were replenished by scientific missions judiciously employed, a more accurate knowledge were at the same time obtained of our distant possessions, and of their natural riches, than has been sometimes discovered in our diplomatic transactions.
All the remarks, Gentlemen, which I have this day made, have been made with an anxious desire to say neither more nor less than the truth. I have spoken both of scientific societies
[page] 35
and of the national policy with all freedom, because I take free speech upon points in which the interests of science are deeply concerned, to be one of the principal purposes for which we are now assembled; but I hope I have spoken also without any disposition to exaggerate the deficiencies which I have thought it right to notice, or to elevate a new institution by detracting from the merits of elder establishments. It only remains for me to lay before you the particulars of the plan by which we propose to accomplish the objects which I have stated; the subordinate details would be most advantageously revised by a Committee, but the material principles on which it is framed are points to which I would request the attention of this Meeting.
The material principles of the plan are included in the composition of the Association, in the constitution of its government, and in the selection of the work on which it is to be employed.
Having objects in view more extensive, and at the same time more specific than those of the German Association, we do not recommend the adoption of the same rules. It is not our desire in the general composition of the Society to separate writers from readers, the professor of natural knowledge from the student. A public testimonial of reputable character and zeal for science is the only passport into our camp which we would require. We propose, therefore, that all Members of Philosophical Societies in the British empire shall be entitled to become MEMBERS OF THE ASSOCIATION, on enrolling their names, and engaging to pay such subscription as may be agreed upon, the amount of which subscription, we think, ought to be low; and we propose that the members shall meet for one week in every year at different places in rotation; in order, by these migratory visits, to extend the sphere of the Association, to meet the convenience of distant districts in turn, and to animate the spirit of philosophy in all the places through which the Meetings may move, without rendering them burthensome to any.
But the governing or executive power of the Association, we think, should be vested in a more select, though still numerous body, and placed in the hands of those who appear to have been actually employed in working for science. We propose, therefore, that the GENERAL COMMITTEE shall consist of all Members present at a Meeting who have contributed a paper to any Philosophical Society, which paper has been printed by its order or with its concurrence; taking this as the safest definition of the class of persons intended, but leaving
C 2
[page] 36
power to the Committee to add to its own number, and to admit into the Association other Members at its discretion; and we propose that it shall sit during the time of the Meeting, or longer if necessary, to regulate the general affairs of the Association, to manage the business of the session, and to settle the principal scientific arrangements for the ensuing Meeting.
We recommend, however, that these arrangements should be first digested, and the particular advancement of every science specially looked to by SUB-COMMITTEES, which the general Committee shall appoint, placing severally on each those Members who are most conversant with the several branches of science. We propose that the Sub-Committees should select the points in each science which most call for inquiry, and endeavour, under the authority of the General Committee, to engage competent persons to investigate them; that where the subject admits of the cooperation of scientific bodies, the Sub-Committees should recommend application to be made for that assistance; and that they should attend especially to the important object of obtaining Reports in which confidence may be placed, on the recent progress, the actual state, and the deficiencies of every department of science.
On the last of these points I beg leave to quote the opinion of an able and zealous philosopher, the Professor of Mineralogy at Cambridge, who has been prevented by his public duties at the University from attending the Meeting, but who nevertheless takes the deepest interest in its objects. 'A collection of Reports,' says Professor Whewell, 'concerning the present state of science, drawn up by competent persons, is on all accounts much wanted; in order that scientific students may know where to begin their labours, and in order that those who pursue one branch of science may know how to communicate with the inquirer in another. For want of this knowledge we perpetually find speculations published which show the greatest ignorance of what has been done and written on the subjects to which they refer, and which must give a very unfavourable impression of our acquirements to well informed foreigners.'
I must add, however, to Mr. Whewell's remarks, that this want of knowledge is not by any means confined to our own country. I do not remember anywhere a more remarkable instance of it than that which occurred in France, to one of the most distinguished improvers of optical science*. As late as the year 1815 M. Fresnel re-observed Dr. Young's impor-
* M. Fresnel.
[page] 37
tant law of the interference of the rays of light; he re-constructed the same mathematical formulas for the application of that law to various phænomena, and he announced these researches as new, in a memoir read before the French Academy. One of the members of that eminent body*, better acquainted with the progress of optics than the writer of the memoir, happily preserved an author, to whose original and profound researches the science has been so largely indebted, from printing as his own the celebrated discoveries long before published by another philosopher; but had this information been earlier acquired, it would have saved all the time and labour which were lost in a retrograde inquiry. Even four years after this, when general attention had been drawn to the subject, and the prize offered by the Academy for the best Memoir on the diffraction of light was adjudged to M. Fresnel, the following animadversions were made by the Reporter† on the unsuccessful competitor, whom he nevertheless represents as an experienced physical inquirer ('physicien exercé'). 'L'auteur paraît n'avoir connu ni les travaux dont on est redevable au Dr. Thomas Young, ni le mémoire que M. Fresnel avait inséré en 1816 dans les Annates de Chimie et de Physique: aussi la partie de son travail qui se rapporte aux influences que les rayons de la lumière exercent ou semblent exercer les uns sur les autres en se mêlant, loin de rien ajouter à ce qui était déjà connu, renferme plusieurs erreurs évidentes.'
Having thus entrusted to the Sub-Committees, Gentlemen, the most active share in advancing their respective sciences, and considered them as the instruments by which, through the medium of the General Committee, the impulse of the Association must be principally directed, we recommend that they should not be dissolved with the Meeting at which they have been appointed, but continue in action till the Society re-assembles in the following year. We do not presume that the persons who may happen to compose them, far removed as they may be from each other, may often have it in their power to meet in the interval; but we conceive that they will feel themselves engaged individually to keep the objects, which they have agreed to forward, in their view, and that the correspondence which they may be induced to maintain between themselves, and with the Officers of the Association, may be highly conducive to that combined exertion, the introduction of which into science would save much labour and ensure a better progress.
The appointments to the HIGHER OFFICES of the Society,
* M. Arago.
† Rapport lu à l'Academie 15 Mars, 1819, par M. Arago.
[page] 38
we propose should be not only annual, like the rest of the machinery by which it is kept in motion, but annually changed; in order at once to extend the interest which they may be supposed to kindle, and to limit the burthen which they will impose; leaving it for future consideration, whether the appointment of more permanent SECRETARIES may not be necessary to secure a steady and uniform course: and we recommend that, when the General Committee is not sitting, the whole business of the Association shall be committed to the Office-bearers, assisted in scientific matters by the Members of the Sub-Committees, and in promoting the interests and objects of the Association in particular places, by the cooperation of LOCAL COMMITTEES.
I have now arrived at the last point to which it remains for me to advert—namely, the selection of the matter which is to engage the attention of the Meetings. It is evident that if the plan which I have thus far explained should be carried into effect, the deliberations of the Committee to be formed at the present Meeting will provide the chief materials for the consideration of the next. Those investigations and those surveys of science which shall have been suggested and procured by the Committees and Officers of the Association, will be entitled to the priority, though other communications may be accepted as far as the duration of the session will allow. Professor Whewell conceives 'that if this Meeting were to request from one or two among the most eminent men in the various branches of science, statements to be presented next year of the recent advances made in each department, and the subjects of research which they consider at present the most important and promising, such a request would be respectfully attended to.' Gentlemen, I do not doubt that it would; neither do I doubt that a request from this Meeting would be successful in procuring new researches to be made; and should the funds of the Association hereafter admit of its going further, and offering PRIZES for particular investigations,—then would another prolific source be opened from which the scientific materials of our Meetings would be derived."
This, indeed, would only be another and a very powerful method of carrying On the system which we recommend of advancing science in determinate lines of direction; a method, which, though scarcely practised in this country, has been found eminently successful abroad. Dr. Brewster, I believe, will confirm me in this statement; for he will recollect that we owe to a prize-memoir, the first announcement of that great optical discovery, that light may be polarized by reflection from the surface of transparent bodies, a discovery which has since
[page] 39
been productive of so many new and admirable observations, and which has been in no hands more fertile than his own; and he will remember also, that the first accurate investigation of all the phænomena of diffraction, and the first complete explanation of them by the doctrine of undulations, was contained in a memoir produced by a similar competition*. The award of medals, indeed, is an honourable encouragement not altogether withheld from successful researches in British science. But the principle on which they are given is of a more vague and general nature. The objects of these rewards have never been so distinct as to give a direct stimulus to specific inquiries. I may add, without imputing any mercenary feelings to men of science, that where the inquiry involves expense, a sum of money instead of a medal would, perhaps, be found a more useful and operative offer. It is well known that the important improvements which have been made in Chronometers have arisen, both in France and England, directly out of the public rewards munificently offered by the British Parliament; and I see no reason why adequate and well devised premiums should not be efficacious in the sciences as well as in the arts. No man, however high may be his literary or scientific pretensions, disdains to receive a pecuniary remuneration for the labour which he employs in the composition of his works; and there can be nothing derogatory to the character of a man of science in accepting a similar compensation for the successful exercise of his talents in researches especially which require an expenditure of money as well as time.
"Such, Gentlemen, are the provisions of the plan which we propose for your consideration; and you will perceive that the methods which it embraces are new in practice, though not in principle. How otherwise indeed, than by new methods, can we hope to exchange the present desultory and tardy progress of philosophy, for a more regular, energetic, and rapid advancement? There is a light in the distant horizon to which we have long eagerly looked, and complained that the current did not set us more quickly towards it; and the question now before you, Gentlemen, is no less than this: Whether you are satisfied still to float passively on the waters, or whether you will raise the sail, and ply the oar, and take the helm into your hands. The methods now proposed are new, and therefore cannot place us in collision with any other Society. It has never yet been seen in this country, that twenty Chemists for instance, or twenty Mineralogists, have met together, for the purpose of settling the
* This Memoir, written by M. Fresnel, gained the physical prize proposed by the French Academy for "a general examination of the phænomena of the diffraction of light," in 1819.
[page] 40
nomonolature of their respective sciences, or attemptig to fix with one consent the foundations on which they rest. It has never yet been seen, that the Chemical, Mineralogical, and Optical inquirers have assembled for the purpose of mutually explaining and learning what light the sciences of Chemistry, Mineralogy, and Optics are capable of reflecting reciprocally upon each other. You will perceive also, Gentlemen, that the Transactions, which we contemplate, are not to be collected by trespassing upon ground which was already occupied. In this respect there is on our part not only no design, but no possibility of interference. The course of an Association which meets once a year, and but for a few days, is necessarily different from that of more abiding Institutions; we have no time, if we wished it, to encroach upon the office, or to drain away the scientific resources of any other Society. It will be enough for us, if we can compress into the compass of a week's deliberations our own restricted objects,—specific investigations into fundamental points of science, reviews of its recent advances, and recommendations of subjects and methods for future research. Our plan contains within it a new power which may perhaps accelerate the wheels that are already in action; but its machinery is exclusively its own. The enlightened Institutions with which it hopes to be associated will regard it, therefore, not as a rival, but a coadjutor; and I trust it may prove such a coadjutor to them as the steam-engine has been to all other kinds of mechanism, in every mine, and in every manufactory; a coadjutor, by the aid of whose powerful movements all their operations have been facilitated, and their productions multiplied a hundredfold.
An enterprise like this has no danger to fear, but from a deficiency of zeal and union in carrying it into effect. It must undoubtedly fail, if it meets only with imperfect cooperation and cold support. But if it shall recommend itself to the full approbation of men of science, if it appears to you, Gentlemen, desirable to undertake it, the Association will have competent sponsors in the present assembly, who will stand pledged not only for its early encouragement, but for those future exertions which will be required to ensure its success. The Council of the Yorkshire Philosophical Society have not the presumption to dictate to this Meeting the course which it may be for the interests of Philosophy to pursue. They collected, in the first instance, the best opinions which they could obtain, before they proceeded to mature their plan; and they now wait for the opinion of the eminent persons who are here assembled, before they can assure themselves that it is as feasible in practice as it appears in theory. My own judgement waits with theirs, Gentlemen, on
[page] 41
that of the High Priests of the temple, in the porch of which I am only an humble worshiper,—'parcus Deorum cultor et in-frequens',—and I shall be the first to withdraw the resolutions which I am now ready to propose, unless I find them, by the deliberate and cordial concurrence of this Meeting, stamped with authority and endued with permanence."
A strig of Resolutions in which were embodied the Objects and Rules of the Association as stated in Mr. Harcourt's speech, were then moved by him seriatim, and seconded by Dr. Brewster, by Mr. Murchison, President of the Geological Society of London, by Dr. Pearson, Vice-President of the Astronomical Society of London, by Mr. Robison, Secretary to the Royal Society of Edinburgh, &c. It was resolved unanimously—"that an Association be formed, to be called The British Association for the Advancement of Science, the objects of which shall be to give a stronger impulse and more systematic direction to scientific inquiry, to promote the intercourse of those who cultivate science in different parts of the British Empire, with one another, and with foreign philosophers, and to obtain a greater degree of national attention to the objects of science and a removal of any disadvantages of a public nature which impede its progress." In the next Resolution, purporting "that the members of Philosophical Societies in any part of the British Empire may become members of the Association on enrolling their names and contributing a small subscription", several alterations were proposed; but it was finally passed, with the remaining Resolutions, subject to the revisal and report of a Committee, constituted, according to the proposed plan, of all members present who had contributed a scientific paper to any Philosophical Society, which paper had been printed with its concurrence.
The thanks of the Meeting were then voted to the Chairman, and to the Rev. Mr. Harcourt for his statement of the plan of the Association; and the further consideration of it was adjourned till the following day.
On Wednesday, at 12 o'clock, Viscount Milton was again called to the chair, and the Meeting resumed its deliberations.
The Rev. W. V. Harcourt, as chairman of the Committee, announced that the Resolution respecting the admission of members, in which alterations had been suggested on the previous day, had been revised, and that the Committee recommended that the following persons should be entitled to become members of the Association, upon subscribing an obligation to conform
[page] 42
to its Rules:—1st. allpersons assembled at the present Meeting: 2nd. the Fellows and Members of all Chartered Societies in the British Empire: 3rd. the Office-bearers and Members of the Council or Managing Committee of all Philosophical Societies: 4th. all Members of such a Society recommended by the Council or Committee thereof. They also proposed that the amount of the annual subscription should be One Pound, and that the composition for it should be Five Pounds; that the accounts should be audited annually by Auditors appointed by the Meeting itself, and that the Treasurer of the Yorkshire Philosophical Society should be Treasurer of the Association for the ensuing year.
Resolutions founded on these recommendations, having been moved and seconded by Sir Thomas Brisbane, Mr. Robison, Mr. Dalton, Dr. Daubeny, the Rev. Mr. Scoresby, Dr. Pearson, Mr. Murchison, Mr. Marshall, &c. were passed by the Meeting; and it was resolved that any further revision which the Rules might require should be left to the Committee.
On the motion of Mr. Murchison, seconded by Sir Thomas Brisbane, it was resolved, "that the Rev. Mr. Harcourt be requested to publish for the Association the exposition of its objects and plan which he delivered yesterday." Mr. Har-court, in assenting to the desire of the Meeting, asked permission to revise what he had said, previous to its publication.
The business of forming the Association being completed, the Chairman proceeded to announce the papers to be read that morning on subjects of Science, a report of the contents of which, as well as of the other communications made during the Session, will be found in the subsequent account of the Scientific Transactions.
On Thursday morning it was stated to the Meeting that the Committee had chosen Viscount Milton, the Rev.W.V. Harcourt, and the Secretaries of the Yorkshire Philosophical Society, to be the actual President, Vice-President, and Secretaries of the Association; that the Rev. Dr. Buckland had been chosen President elect; Dr. Brewster and the Rev. Professor Whewell Vice-Presidents elect; Dr. Daubeny and the Rev. Professor Powell, Secretaries elect; and that the next Meeting was appointed to be held at OXFORD.
On Saturday evening, the scientific communications and discussions having been closed by some remarks of Dr. Brewster, in which, adverting to a method of rendering visible the legends
[page] 43
of ancient coins, he stated that he had never been more struck than by observing on an old coin, which he had placed on hot iron, an inscription make its appearance which he could read in a dark room, bearing the words 'Benedictum sit nomen Dei" —Viscount: Morpeth rose, and addressed the Meeting as follows:—
"Ladies and Gentlemen, an office has been assigned to me, which, although most entirely without any qualification or pretension to fulfil, I nevertheless accept, and will discharge, to the best of my ability, with the utmost alacrity. To the character of a man of science I have, unfortunately for myself, no claim whatsoever; but I have the good fortune to be intimately connected with the county, and consequently with the city of York; and I feel that they have both received great benefit and additional credit from the Meeting which is now brought to a conclusion. I say this, both with reference to the positive instruction we have received upon so many most interesting and important subjects, and also to the circumstance of this town and this edifice, already so much indebted to the zeal, perseverance, and ability of our Vice-President, having been now selected as the birth-place of an Association, which, I trust, is destined to confer fresh lustre on British science, to give a new motive and a new guarantee to the friendly inter-course and continued concord of nations; to make further inroads into the untravelled realm of discovery, and glean fresh harvests from the unexhausted field of Nature; to promote the comforts and augment the resources of civilized man; and to exalt above and over all the wonder-working hand of Heaven. For it will always come out from the pursuit of knowledge as surely as from the rusty medal of which we have this moment heard, 'Benedictum sit nomen Dei.' Observe well, if you wish to appreciate rightly the true value and nobility of science, that while it proposes to itself distinct courses and definite spheres of its own, its general tendencies conduce to peace, and minister to piety. With these views and these hopes, it is natural and it is becoming that there should be mixed feelings of gratitude to those whose efforts have contributed so largely to our future progress. An assembly like that which I have the honour to address, will appreciate far more justly than I can pretend to do, the several papers and productions which have been submitted to our notice. I have no scruple in leaving to your more competent and accurate discrimination, the indications of enlightened and powerful thought which they have exhibited; but I feel sure that, if you pardon me for this intrusion of myself, the proposition I now make will
[page] 44
command, upon this occasion, both the grave assent of Science and the soft sanction of Beauty. I move that the thanks of this Meeting be given to Dr. Brewster, and the other authors who have favoured us with their communications."
Mr. Murchison then rose, and "on the part of Dr. Brewster and his other scientific friends, begged leave to return thanks for the high honour done to the contributors of scientific memoirs, and for the valuable aid which had been received from the residents of York and its neighbourhood, in the promotion of the objects of the Meeting." He explained the motives which induced the original promoters of it to select the city of York for their first assembly. "To this city," he said, "as the cradle of the Association, we shall ever look back with gratitude; and, whether we meet hereafter on the banks of the Isis, the Cam, or the Forth, to this spot, to this beautiful building, we shall still fondly revert, and hail with delight the period at which in our periodical revolution we shall return to the point of our first attraction." Mr. Murchison, after expressing his sense of the kind reception and hospitality which the strangers there collected had experienced from the Archbishop, and from all classes of the inhabitants of the city and neighbourhood, concluded with a motion of thanks as follows:— "That the cultivators of science, here assembled, return their most grateful thanks to His Grace the Archbishop of York, and the other Members of the Yorkshire Philosophical Society, for the very liberal manner in which, by the use of their Halls and Museum, and by their obliging and unwearied efforts to provide every accommodation and comfort to those who have visited York on the present occasion, they have so essentially contributed to the success and prosperity of this Association."
This motion was seconded by Dr. Brewster, and supported by Mr. Dalton. Mr. Harcourt, who was in the chair, then said, that "it was quite unnecessary, from the feelings which he knew to pervade the breasts of all, both strangers and residents, to put to the vote of the Meeting either of the proposals so eloquently brought forward. In the long period of its existence the ancient city of York had never greater reason to be proud, than of the genius and talent it contained within its walls at that moment, and of the honour it had acquired in being the birth-place of an Association destined, he firmly believed, greatly to enlarge the boundaries of science, and in so doing to advance the many interests of human nature which depend upon the improvement of knowledge." He then declared the Meeting to be adjourned to Oxford.
[page] 45
PROCEEDINGS
OF
THE GENERAL COMMITTEE.
THE General Committee was employed from the 27th of September to the 2nd of October in revising the Rules of the Association, in appointing the Office-bearers, in embodying the Local and Sub Committees, and in receiving the recommendations of the latter, and making arrangements for carrying their suggestions into effect.
The care of completing the objects which the Committee had in view, and of printing the results of its deliberations, together with the proceedings and transactions of the General Meeting, was entrusted to the Officers of the Association at York, who have drawn up from its minutes a summary of the Objects and Rules of the Association*; to which are subjoined the appointments of Officers and Committees. The particulars of the scientific business brought before the General Committee are included in the account of the Recommendations of the Sub-committees; and the success which has attended the applications, made in the name of the Association, to eminent individuals, requesting them to undertake the services in science which had been so recommended, has been stated in the Preface to the Report.
OFFICERS.
President.— Charles William, Viscount Milton, F.R.S. &c. President of the Yorkshire Philosophical Society.
president elect.—Rev. William Buckland, D.D., F.R.S. &c. Professor of Geology and Mineralogy, Oxford.
Vice-President.—Rev. William Vernon Harcourt, F.R.S. &c. Vice-President of the Yorkshire Philosophical Society.
Vice-Presidents elect.—David Brewster, LL.D., F.R.S.
* This summary, with the additional regulations since made, is printed in the present volume at the end of the Second Report.
[page] 46
L. & E. Corresp. Member of the Institute of France, &c. Rev. William Whewell, F.R.S. &c. Professor of Mineralogy, Cambridge.
Treasurer.— Jonathan Gray, Esq. York.
Secretaries.—York.—William Gray, jun. John Phillips, F.G.S. &c. Secretaries of the Yorkshire Philosophical Society.
London.—Rev. James Yates, F.L.S., G.S. &c.
Dublin.—Rev. Thomas Luby.
Edinburgh.—John Robison, Secretary of the Royal Society of Edinburgh, &c.
Oxford.—Charles Daubeny, M.D., F.R.S. Professor of Chemistry, Oxford. Rev. Baden Powell, F.R.S., Savilian Professor of Geometry, Oxford.
LOCAL COMMITTEES.
London.—G. B. Greenough, F.R.S., Vice-President of the Geological Society. R. I. Murchison, F.R.S., President of the Geological Society. Rev. James Yates, F.L.S. &c.
Edinburgh.—James D. Forbes, F.R.S. E. &c. James F. W. Johnston, A.M. John Robison, Sec. R.S.E. &c.
Dublin.—W. R. Hamilton, F.R.S. &c. Astronomer Royal of Ireland. Rev. B. Lloyd, D.D., Provost of Trinity College, Dublin.
India.—George Swinton, Esq., Chief Secretary to the Government in India, has been requested to form a Committee at Calcutta, with the aid of Major Benson, J. Calder, Esq., Dr. Christie, J. Herbert, Esq., J. A. Prinsep, Esq. and Sir Edward Ryan.
SUB-COMMITTEES.
Mathematical and Physical Science.
David Brewster, LL.D., F.R.S. L. & E. &c. Sir Thomas Brisbane, K.C.B., F.R.S. L. & E., Corresp. Member of the Institute of France. James D. Forbes, F.R.S.E., &c. W. R. Hamilton, F.R.S. &c. Rev. William Pearson, LL.D., F.R.S. Vice-President of the Astronomical Society. Rev. Baden Powell, F.R.S., &c. Rev. William Scoresby, F.R.S. L. & E., Corresp. Member of the Institute of France. Rev. W. Whewell, F.R.S. &c. Rev. R. Willis, F.R.S. &c.
Chemistry.
Rev. James Cumming, F.R.S. Professor of Chemistry, Cambridge. John Dalton, F.R.S. President of the Literary and Philosophical Society at Manchester, Corresp. Member of the Institute of France. Charles Daubeny, M.D., F.R.S. &c.
[page] 47
Rev. W. V. Harcourt, F.R.S. &c. J. F. W. Johnston, A.M. Edward Turner, M.D., F.R.S. L. & E. Professor of Chemistry in the University of London. William West, Secretary of the Leeds Philosophical Society.
Mineralogy.
Thomas Allan, F.R.S. L. & E. Robert Allan, F.G.S. &c. David Brewster, LL.D.,F.R.S. &c. J. F. W. Johnston, A.M. Rev. W. Whewell, F.R.S. &c.
Geology and Geography.
Rev. William Buckland, D.D., F.R.S. &c. Rev. W. D. Conybeare, F.R.S. &c. Vice-President of the Geological Society, Corresp. Member of the Institute of France. Sir Philip Grey Egerton, Bart. F.R.S. &c. James D. Forbes, F.R.S. E. &c. G. B. Greenough, F.R.S. &c. William Hutton, F.G.S. &c. R. I. Murchison, F.R.S. &c. John Phillips, F.G.S. &c. Rev. Adam Sedgwick, F.R.S. &c. Woodwardian Professor, Cambridge. William Smith, Author of the Geological Map of England. Henry Witham, F.G.S. &c. Rev. James Yates, F.L.S. &c.
Zoology and Botany.
Charles Daubeny, M.D., F.R.S. &c. J. K. Greville, M.D., F.R.S. E. &c. Rev. J. S. Henslow, F.L.S. Professor of Botany, Cambridge. John Lindley, F.R.S., L.S. &c. Professor of Botany in the University of London. J. C. Prichard, M.D., F.R.S.
Mechanical Arts.
J. H. Abraham, F.L.S. &c. John Robison, Sec. R.S. E. &c. Benjamin Rotch, F.S.A. &c.
[page] 48
RECOMMENDATIONS
OF
THE SUB-COMMITTEES.
COMMITTEE OF MATHEMATICAL AND PHYSICAL SCIENCE.
Mathematics.
THE Committee recommend that the Vice-President of the Association residing at Cambridge be requested to use his utmost efforts to procure, from some competent individual, a Report to the next Meeting on the progress of Mathematical Science.
Astronomy.
That Professor Airy be requested to favour the Association with a Report on the state and progress of Physical Astronomy, together with such remarks on the improvements of Practical Astronomy, as he may deem it useful to add.
Theory of Tides.
That J. W. Lubbock, Esq. be requested to furnish a statement of the means which we possess, or which we want, for forming accurate tables for calculating the time and height of High-Water at a given place.
Meteorology.
That James D. Forbes, Esq. be requested to draw up a Report for the next Meeting, on the present state of Meteorological Science.
The Committee, considering that the science of Meteorology is in more want, than perhaps any other, of that systematic direction which it is one great object of the Association to give, has thought it advisable to propose the following points for investigation.
[page] 49
I. That the Association should employ all the means in its power to procure a Register of the Thermometer during every hour of the day and night, to be kept at some military or naval station in the South of England.
Note*. Until the phænomena and distribution of diurnal temperature are more thoroughly understood than at present, we can hardly hope that any very sure footing has been obtained in the study of meteorology. The hourly register kept for several years at the military station of Leith Fort, in lat. 56°, has shown that we want nothing but the combination of a sufficient number of trust-worthy observations, in order to obtain results of primary importance to the science, and which may one day enable us to arrive at the true form of the daily and annual curves of mean temperature with a precision almost mathematical. In order, however, to extend the benefit of such investigations, it is absolutely necessary that they should be pursued in different latitudes. The application to rendering available registers otherwise almost without value, from not being made at the proper hours, will be best illustrated by a reference to the account of the Leith observations. (Transactions of the Royal Society of Edinburgh, Vol. X.)
II. That the establishment of such an hourly meteorological register be pointed out as a highly interesting object, in reference especially to the important point of intertropical climate, to THE COMMITTEE OF THE ASSOCIATION IN INDIA.
III. That the Committee in India be requested to endeavour to institute such observations as may throw light on the phœnomena of the horary oscillations of the barometer, near the equator. Should the concurrence of the Committee on these points be obtained, it would probably be desirable that the Association should take measures for sending out delicate and accurate instruments.
IV. That Mr. Phillips and Mr. Wm. Gray, jun. of York, be requested to undertake a series of experiments on the comparative quantities of rain falling on the top of the great tower of York Minster, and on the ground near its base. The Committee have been induced to propose this specific question in consequence of the local fitness of the situation, and the facilities offered for its solution by the authorities; but it is to be wished that similar experiments should be made elsewhere,
* The notes appended to the Recommendations have been drawn up by some of the Members of the Committees since the Meeting.
D
[page] 50
that by an extended comparison of observations, light may be thrown upon the anomalies which have been observed at Paris and in other places.
V. That the Association should express its desire to receive a satisfactory exposition of the theory of the moistened bulb hygrometer, and that observers be also invited to institute series of comparative experiments on the indications of the moistened thermometer and the temperature of the dew point.
Note. These indications may be ascertained by Mr. Dalton's process, or by Mr. Daniell's Hygrometer, or by both. Notwithstanding the ingenious and laborious researches of Hutton, De Saussure, Leslie, Anderson, and Gay-Lussac upon this subject, scientific deductions drawn from more extended experiments are greatly wanted. The simplicity and certainty of the experiment by which the cold produced by the evaporation of water is measured, renders an accurate theory of the result peculiarly desirable. The experimenter would do well to consult Mr. Dalton's views on the theory of Hygrometry, contained in his Meteorological Essays, and in the Manchester Transactions, and to examine the investigations of Professor Leslie, (Relations of Heat and Moisture, and Supplement to the Encyclopædia Britannica, Article METEOROLOGY;) of Dr. Anderson (Edinburgh Encyclopœdia, Article HYGROMETER,) and of M. Gay-Lussac, (Biot, Traité de Physique, Tom. II.) A good series of observations at high temperatures will be found recorded in Nos. II. and III. of a Calcutta Journal, entitled, Gleanings in Science.
VI. That experiments on the Decrease of Temperature at increasing heights in the Atmosphere be recommended as an important subject for the contributions of observers.
Note. Series of observations for considerable periods of time on the mean temperature of the air at fixed hours, and at stations of which the difference of height has been accurately measured, are the most valuable. The best hours for observation are those which give most accurately the mean temperature of the period of observation. The hourly observations at Leith Fort have determined the hours which give the annual mean temperature in this country to be about 9¼ A.M. and 8½ P.M. Experimental balloons have lately been employed to assist the solution of this problem, which is one of the most interesting in Meteorology; but the investigation of it is nearly brought to a stand for want of sufficiently
[page] 51
numerous observations. The observer may be referred for information to Ramond, Mémoires sur la Formule Barometrique de la Méchanique Celeste; to the Researches of Humboldt; to Professor Leslie, Supplement to the Encyclopœdia Britannica, Article CLIMATE; to Pouillet, Elemens de Physique; to Mr. Atkinson's Paper on Refractions in the Memoirs of the Astronomical Society; and to Mr. Ivory's Memoir on the same subject in the Philosophical Transactions, and his Papers in the Annals of Philosophy.
VII. That the observation of the Temperature of Springs at different heights and depths should be pointed out as an object of great interest, in prosecuting which insulated inquirers may render essential aid to science.
Note. When springs are copious, a few observations in the course of the year suffice to give with great accuracy their mean temperature. The height of the springs above the mean level of the sea, and the depth of Artesian wells, should be carefully observed; and where the corresponding mean temperature of the air can be obtained, it should be stated. In two points of view these observations are important, independently of the inferences which they may furnish as to the decrease of heat in the atmosphere. The great interest attached to the phænomenon of the progressive increase of temperature of the globe, as we descend through the Strata, renders of value observations on the temperature of springs at considerable heights, of springs in mines, and of those brought to the surface from some depths by the process of boring. This question has been treated with great success by M. CORDIER, in several Memoirs, some of which have been translated into English. Again, the researches of Humboldt, Buch, Wahlenberg, and most recently Kupffer in a Memoir on Isogeothermal Lines, read before the Academy of St. Petersburg, in 1829, have shown that the temperature of the earth differs in many parts of the globe from that of the air, being generally in defect below lat. 56°, and in excess beyond it. The progressive increase of temperature with that of the depth in Artesian wells, and the deviation of the mean temperature of the Earth from that of the Air in different latitudes, have opened new fields for discussion; and by the zealous cooperation of observers cannot fail to present results, of which at present we can form but an imperfect idea.
D 2
[page] 52
Magnetism.
It appears to the Committee highly desirable that a series of observations upon the Intensity of Terrestrial Magnetism in various parts of England be made by some competent individual, similar to those which have recently been carried on in Scotland by Mr. Dunlop.
Should the Committee succeed in finding some individual ready to undertake the task, they propose that an application should be made to the Royal Society of Edinburgh, for permission to make use of the Standard Needle belonging to them, and constructed under the direction of Professor Hansteen of Christiania.
It appears to the Committee of considerable importance, that a certain number of observations should be made throughout Britain with the Dipping Needle, in order to reduce the Horizontal to the true Magnetic-Intensity.
Note. The time of three hundred vibrations should be observed, and the methods of observation and reduction should be the same as have been employed and described by Humboldt, Hansteen, and others.
Electro-Magnetism.
The Committee recommend, as an important subject for further prosecution, the examination of the Electro-Magnetic condition of Metalliferous Veins. The Committee would refer for the details of what has been already done upon this subject, to the Paper of Mr. Fox in the Philosophical Transactions for 1830; and would propose that the experiments should be extended to veins which traverse, as in some of our mines, horizontal and dissimilar strata.
Optics.
That Dr. Brewster be requested to prepare for the next Meeting a Report on the progress of Optical Science.
Acoustics.
That the Rev. Robert Willis be requested to prepare for the next Meeting a Report on the state of our knowledge concerning the phænomena of Sound, and the additions which have been recently made to it.
Heat.
That Professor Powell be requested to prepare for the next Meeting a similar Report respecting heat.
Electricity.
That Professor Cumming be requested to prepare for the
[page] 53
next Meeting a similar Report on Thermo-Electricity, and the allied subjects in which recent discoveries have been made.
CHEMICAL COMMITTEE.
It appears to the Committee of supreme importance, that Chemists should be enabled, by the most accurate experiments, to agree in the relative weights of the several elements, Hydrogen, Oxygen, and Azote, or, which amounts to the same thing, that the specific gravity of the three gases should be ascertained in such a way as would insure the reasonable assent of all competent and unprejudiced judges.
They think it highly desirable that the doubts which remain respecting the proportions of Azote, Oxygen, &c. in the atmosphere should be removed; that the proportions of Azote and Oxygen in nitrous gas and nitrous oxide should be strictly determined; and that the specific gravities of the compound gases in general should be more accurately investigated.
They recommend that the members of this Committee, and British Chemists in general, be invited to make experiments on these subjects, and communicate their results to the next Meeting at Oxford.
That Mr. Johnston be requested to present to the next Meeting a view of the recent progress of Chemical science, especially in foreign countries.
That Dr. Daubeny be requested to undertake an investigation into the sources from which organic bodies derive their fixed principles.
That Mr. Johnston be requested to undertake the inquiries which have been suggested to the Committee, into the comparative analysis of iron in the different stages of its manufacture.
That Mr. West be requested to pursue the experiments contemplated by him, into the combinations of gaseous bodies when passed through heated tubes.
That the Rev. W. Vernon Harcourt be requested to prosecute the inquiries contemplated by him, into the chemical phænomena from which the materiality of what are sometimes called ethereal substances has been inferred.
MlNERALOGICAL COMMITTEE.
The Committee recommend that the Rev. Professor Whewell be requested to present to the next Meeting a Report on the state and progress of Mineralogy.
[page] 54
GEOLOGICAL AND GEOGRAPHICAL COMMITTEE.
The Committee recommend that Geologists be requested to examine strictly into the truth of that part of the theory of M. Elie de Beaumont, in its application to England, Scotland, and Ireland, which asserts that the lines of disturbance of the strata assignable to the same age are parallel, and that a Report to the next Meeting on this subject should be procured.
That Mr. Phillips be requested to draw up, with such co-operation as he may procure, a systematic catalogue of all the organized fossils of Great Britain and Ireland, hitherto described, with such new species as he may have an opportunity of accurately examining, with notices of their localities and geological relations.
The Committee propose that Mr. Robert Stevenson, Civil Engineer, be requested to prepare a Report upon the waste and extension of the land on the East coast of Britain, and the question of the permanence of the relative level of the sea and land; and that individuals who can furnish observations, be requested to correspond with him on the subject*.
The importance which, especially of late years, has been attached to facts of this nature, in illustration of the sciences of hydrography and geology, and the mass of uncombined materials Which have recently been accumulating, have induced the Committee to make the present recommendation; and in doing so, it feels pleasure in being able to have in its view an individual whose practical acquaintance with the coast in general, and more particularly the minute survey made by him some years since, gives reason to expect from his Report much important and accurate information.
BOTANICAL COMMITTEE.
The Committee recommend that Professor Lindley be requested to prepare for the next Meeting an account of the principal questions recently settled, or at present agitated, in the philosophy of Botany, whether in this country or abroad.
That Botanists in all parts of Great Britain and Ireland be invited to compose and communicate to the Meetings of the Association, Catalogues of County or other local Floras, with indications of those species which have been recently introduced, of those which are rare or very local, and of those which
* Communications may be addressed to Robert Stevenson, Esq. Engineer to the Northern Lighthouse Board, Edinburgh.
[page] 55
thrive, or which have become or are becoming extinct; with such remarks as may be useful towards determining the connection which there may be between the habitats of particular plants, and the nature of the soil and the strata upon which they grow; with statements of the mean winter and summer temperature of the air and water at the highest as well as the lowest elevation at which species occur, the hygrometrical condition of the air, and any other information of an historical, (œonomical, and philosophical nature.
Note. If upon this plan a complete botanical survey of the British islands could be obtained, the results would be important when the Flora in the aggregate came to be compared with its relations of soil, climate, elevation, &c.
[page] 56
SCIENTIFIC TRANSACTIONS
OF
THE MEETING.
MONDAY EVENING.
MR. PHILLIPS, one of the Secretaries of the Yorkshire Philosophical Society, delivered an extemporaneous account of the most remarkable phænomena in the Geology of Yorkshire, illustrated by drawings and specimens selected from the Museum, and contributed by the visitors to the Meeting.
He observed, that though the principal design in opening the Museum that evening was to promote mutual acquaintance and friendly intercourse among those who were soon to engage in more important scientific labours, yet it was thought conducive to these objects that some observations should be offered by him from the Lecture Table, on the geological relations of the County in which they were assembled. In attempting, therefore, a rapid sketch of some of the more prominent and peculiar features in the Geology of Yorkshire, he was influenced by a natural desire to call the attention of the eminent individuals now assembled in York to the phænomena most worthy of observation in passing through the County. He should thus have the opportunity of illustrating the value of some remarkable specimens which within a few hours had arrived for the inspection of the Meeting, and offer the most appropriate welcome which the City and County of York, and the Institution they had founded for the advancement of science, could give to those who now came amongst them to lay the basis of a wide Association for the same important purpose.
The points embraced in the continuation of Mr. Phillips's address were the following:—
1. The peculiar character of the Carboniferous and Oolitic systems in Yorkshire,—both of these great systems of Calcareous Rocks being here diversified by large interpolations of sandy and argillaceous strata, with thin seams of Coal, and re-
[page] 57
mains of Plants. In both systems these interpolations thicken to the northwards. Thus the nearly undivided mass of limestone under Ingleborough becomes separated into many distinct calcareous beds, with sandstones, shales, and bad coal, in Teesdale, Tynedale, and Swaledale, which are still further modified by the introduction of coarse pebbly sandstones, and workable seams of coal, in the western and northern parts of Northumberland:—and the Oolites of Lincolnshire, diminished in thickness and debased in purity, are almost lost in several hundred feet of sandstone, shale, and coal, which form the north-eastern Moorlands of Yorkshire. Mr. Phillips referred these interpolations of sandstone, &c. to the originally littoral, or perhaps æstuary, situation of those parts of the calcareous deposit; while the thicker and more homogeneous limestone masses were probably produced under the deeper and more tranquil waters of the ancient oceans. The bearing of these deductions upon the important subject of the relative form and extent of the land and sea in this part of the globe at those periods respectively, was briefly illustrated.
2. The remarkable history of the deposit near Market Weighton (first observed by W. H. Dikes, Esq., Curator of the Hull Society, and afterwards more completely investigated by some of the members of the Yorkshire Philosophical Society), in which the bones of several kinds of quadrupeds, including species considered as extinct, were found mingled with many shells belonging to thirteen existing species of land, marsh, and fresh-water mollusca, and covered with gravel from the neighbouring hills, together with some larger stones from very distant localities.
3. The general character of the alluvial deposits, inclosing timber and many remains of quadrupeds, in the eastern part of Yorkshire, and the peculiar condition of some bones of deer obtained by Mr. W. Casson, from the Peat near Thorne. These bones appear to have been deprived of a large portion of their hardening earth, and are nearly in the state of leather,—quite flexible, and much altered from their original shape.
4. The traces of the action of the atmosphere in the rain channels which furrow the sides of the monumental stones of Boroughbridge, and form miniature valleys on the broad surfaces of the limestone scars on the mountains of Western Yorkshire and Westmoreland.
5. The occurrence of three specimens of unknown scaly fishes, with ferns and other fossil plants, in the ironstone bands in the lower part of the coal formation of Leeds and Bradford;
[page] 58
two of them indicating an individual of considerable size, the third smaller, and perhaps of a distinct species*.
TUESDAY MORNING.
This morning having been almost exclusively occupied in the business of forming the Association, the only communication read was the following extract of a letter from Geo. Harvey, Esq. F.R.S. L. & E.
"It was my intention, had I been able to enjoy the privilege of attending at York, to have drawn the attention of the Meeting to the very remarkable circumstance of the Geometrical Analysis of the ancients having been cultivated with eminent success in the northern counties of England, and particularly in Lancashire. The proofs of this may be gathered from a variety of periodical works, devoted almost exclusively to this lofty and abstract pursuit. I have now before me several exquisitely beautiful specimens of the geometry of the Greeks, produced by men in what, for distinction sake, we call the inferior conditions of life. The phænomenon (for such it truly is) has long appeared to me a remarkable one, and deserving of an attentive consideration. Playfair, in one of his admirable papers in the Edinburgh Review, expressed a fear that the increasing taste for analytical science would at length drive the ancient geometry from its favoured retreat in the British Isles; but, at the time he made this desponding remark, the Professor seemed not to be aware that there then existed a devoted band of men in the North, resolutely bound to the pure and ancient forms of geometry, who in the midst of the tumults of steam-engines, cultivated it with unyielding ardour, preserving the sacred fire under circumstances which would seem from their nature most calculated to extinguish it. In many modern Publications, and occasionally in the Senate-House Problems proposed to the Candidates for Honours at Cambridge, questions are to be met with derived from this humble but honourable source.
"The true cause of this remarkable phænomenon I have not been able clearly to trace. A taste for pure geometry, something like that for Entomology among the weavers of Spitalfields, may have been transmitted from father to son; but who was the distinguished individual first to create it, in the peculiar race of men here adverted to, seems not to be known.
* Since the Meeting, Mr. Phillips has had the opportunity of observing another specimen of a different species of fossil fish, in the possession of C. Rawson, Esq. from a still lower part of the coal strata at Halifax.
[page] 59
Surrounded with machinery, with the rich elements of mechanics in their most attractive forms, we should have imagined that a taste for mechanical combinations would have exclusively prevailed; and that inquiries locked up in the deep, and to them unapproachable, recesses of Plato, Pappus, Apollonius, and Euclid, would have met with but few cultivators. On the contrary, Porisms and Loci, Sections of Ratio and of Space, Inclinations and Tangencies,—subjects confined among the ancients to the very greatest minds, were here familiar to men whose condition in life was, to say the least, most unpropitious for the successful prosecution of such elevated and profound pursuits.
The contrast also between the Northern and Southern parts of England, in this particular, was most remarkable. In the latter the torch of geometry emitted but a feeble ray; while in the former it existed in its purest and most splendid form. The two great restorers of the ancient geometry, Mathew Stewart and Robert Simson, it may be observed, lived in Scotland. Did their proximity encourage the growth of this spirit? or were their writings cultivated by some teacher of a village school, who communicated by a method, which genius of a transcendental order knows so well how to employ, a taste for these sublime inquiries, so that at length they gradually worked their way to the anvil and the loom?"
TUESDAY EVENING.
MR. ABRAHAM delivered a Lecture on Magnetism, and particularly described several useful applications of this science, which he had employed for the advantage of the arts. He exhibited the model of a machine used for needle pointing, the labouring at which has been found so prejudicial to health, owing to the particles of steel inhaled during the process, that although the men were employed at it only six hours in the day, few ever attained the age of forty years, most dying at thirty or thirty-five, and several not surviving twenty-five. These deadly effects had been in a great measure obviated by Mr. Abraham's contrivance of placing several magnets around a mouth-piece, to attract the particles of steel as they came off in the process of grinding, or floated in the dusty atmosphere of the small apartments. This invention, for which the Society of Arts awarded their large gold medal, has not been so universally employed in the manufactories as its importance deserved, owing partly to the disinclination of the workmen to adopt methods which, by rendering their avocation less injurious to health, should lower the price of their labour.
[page] 60
Mr. Abraham exhibited another Magnetical Instrument, intended to guard the eyes of the grinders from the dispersion of fragments of steel, communicated several poles to the same magnetic bar, and detailed the method which he had found most effectual for communicating, combining, and increasing the magnetic influence.
He then exhibited his simple process for demagnetizing the steel balance-wheels of watches. Having dipped a balance-wheel, previously rendered magnetical, into iron filings, and thus discovered the situation of its poles,—he presented to one of these, at the distance of an inch, the similar pole of a small magnet. The filings immediately fell from the wheel, and it was found to be perfectly demagnetized. (Mr. Abraham's inventions having been presented to the Society of Arts, are described in their Transactions, Vol. XL. p. 135; Vol. XLIII. p. 48; Vol. XLIV. p. 19.)
WEDNESDAY MORNING.
DR. BREWSTER communicated a paper, which was read by Mr. Robison, presenting a general view of the progress of the science of mineralogy during the last thirty years, and of the principles of classification now adopted for minerals; and suggesting the propriety of adding to the four systems of crystallization now employed by Mohs and other mineralogists (the Rhomboidal, Pyramidal, Prismatic, and Tessular systems,) a fifth, viz. the Composite system, as combining a series of crystalline structures not included under the other heads, and mostly discovered by the agency of polarized light. This new system of crystallization, the Author proposes to divide into two classes, the first of which embraces those minerals in which the physical properties of the individual crystals are not altered by the combination; and the second, those minerals in which the physical properties of the individual crystals are altered by the combination. These classes were again divided into different orders, and the Composite minerals were enumerated, which the author proposed to place under each division.
The following Essay by DR. HENRY, was then read by Mr. Phillips.
An Estimate of the Philosophical Character of Dr. Priestley, by William Henry, M.D., F.R.S. &c. &c.
The principal source of the materials of the following pages, is the work, in which the discoveries of Dr. Priestley were originally announced to the public. It consists of six volumes
[page] 61
in octavo, which were published by him, at intervals between the years 1774 and 1786; the first three under the title of "Experiments and Observations on different kinds of Air;" and the last three under that of "Experiments and Observations relating to various Branches of Natural Philosophy, with a continuation of the Observations on Air." These volumes were afterwards methodized by himself, and compressed into three octavos, which were printed in 1790. As a record of facts, and as a book of reference, the systematized work is to be preferred. But as affording materials for the history of that department of science, which Dr. Priestley cultivated with such extraordinary success; and, still more, for estimating the value of his discoveries, and adjusting his station as an experimental philosopher, the simple narrative, which he originally gave in the order of time, supplies the amplest and the firmest ground-work.
In every thing that respects the history of this branch of experimental philosophy, the writings and researches of Dr. Priestley, to which I have alluded, are peculiarly instructive. They are distinguished by great merits, and by great defects; the latter of which are wholly undisguised by their author. He unveils, with perfect frankness, the whole process of reasoning, which led to his discoveries; he pretends to no more sagacity than belonged to him, and sometimes disclaims even that to which he was fairly entitled; he freely acknowledges his mistakes, and candidly confesses when his success was the result of accident, rather than of judicious anticipation; and by writing historically and analytically, he exhibits the progressive improvement of his views, from their first dawnings, to their final and distinct development. Now, with whatever delight we may contemplate a systematic arrangement, the materials of which have been judiciously selected, and from which every thing has been excluded, that is not essential to the harmony of the general design, yet there can be no question that as elucidating the operations of the human mind, and enabling us to trace and appreciate its powers of invention and discovery, the analytic method of writing has decided advantages.
To estimate, justly, the extent of Dr. Priestley's claim to philosophical reputation, it is necessary to take into account the state of our knowledge of gaseous chemistry, at the time when he began his inquiries. Without underrating what had been already done by Van Helmont, Ray, Hooke, Mayow, Boyle, Hales, Macbride, Black, Cavendish, and some others, Priestley may be safely affirmed to have entered upon a field, which, though not altogether untilled, had yet been very im-
[page] 62
perfectly prepared to yield the rich harvest, which he afterwards gathered from it. The very implements, with which he was to work, were for the most part to be invented; and of the merits of those, which he did invent, it is a sufficient proof that they continue in use to this day, with no very important modifications. All his contrivances for collecting, transferring, and preserving different kinds of air, and for submitting those airs to the action of solid and liquid substances, were exceedingly simple, beautiful, and effectual. They were chiefly, too, the work of his own hands, or were constructed under his directions by unskilled persons; for the class of ingenious artists, from whom the chemical philosopher now derives such valuable aid, had not then been called into existence by the demands of the science. With a very limited knowledge of the general principles of chemistry, and almost without practice in its most common manipulations;—restricted by a narrow income, and at first with little pecuniary assistance from others;—compelled, too, to devote a large portion of his time to other pressing occupations, he nevertheless surmounted all obstacles; and in the career of discovery, outstripped many, who had long been exclusively devoted to science, and were richly provided with all appliances and means for its advancement.
It is well known that the accident of living near a public brewery at Leeds, first directed the attention of Dr. Priestley to pneumatic chemistry, by casually presenting to his observation the appearances attending the extinction of lighted chips of wood, in the gas which floats over fermenting liquors. He remarked, that the smoke formed distinct clouds floating on the surface of the atmosphere of the vessel, and that this mixture of air and smoke, when thrown over the sides of the vat, fell to the ground; from whence he deduced the greater weight of this sort of air than of atmospheric air. He next found that water imbibes the new air, and again abandons it when boiled or frozen. These more obvious properties of fixed air having been ascertained, he extended his inquiries to its other qualities and relations; and was afterwards led by analogy to the discovery of various other gases, and to the investigation of their characteristic properties.
It would be inconsistent with the scope of this Essay to give a full catalogue of Dr. Priestley's discoveries, or to enumerate more of them, than are necessary to a just estimate of his philosophical habits and character. He was the unquestionable author of our first knowledge of oxygen gas, of nitrous oxide, of muriatic, sulphurous, and fluor acid gases, of ammoniacal gas, and of its condensation into a solid form by the acid gases?
[page] 63
Hydrogen gas was known before his time; but he greatly extended our acquaintance with its properties. Nitrous gas, barely discovered by Dr. Hales, was first investigated by Priestley, and applied by him to eudiometry. To the chemical history of the acids derived from nitre, he contributed a vast accession of original and most valuable facts. He seems to have been quite aware that those acids are essentially gaseous substances, and that they might be exhibited as such, provided a fluid could be found that is incapable of absorbing or acting upon them*. He obtained, and distinctly described†, the curious crystalline compound of sulphuric acid with the vapour of nitrous acid, or, more correctly, of sulphuric and hypo-nitrous acids, which, being of rare occurrence, was forgotten, and has since been rediscovered, like many other neglected anticipations of the same author. He greatly enlarged our knowledge of the important class of metals, and traced out many of their most interesting relations to oxygen and to acids. He unfolded, and illustrated by simple and beautiful experiments, distinct views of combustion; of the respiration of animals, both of the inferior and higher classes; of the changes produced in organized bodies by putrefaction, and of the causes that accelerate or retard that process; of the importance of azote as the characteristic ingredient of animal substances, obtainable by the action of dilute nitric acid on muscle and tendon; of the functions and œconomy of living vegetables; and of the relations and subserviency which exist between the animal and vegetable kingdoms. After trying, without effect, a variety of methods, by which he expected to purify air vitiated by the breathing of animals, he discovered that its purity was restored by the growth of living and healthy vegetables, freely exposed to the solar light.
It is impossible to account for these, and a variety of other discoveries, of less importance singly, but forming altogether a tribute to science, greatly exceeding, in richness and extent, that of any contemporary, without pronouncing that their author must have been furnished by nature with intellectual powers, far surpassing the common average of human endowments. If we examine, with which of its various faculties the mind of Dr. Priestley was most eminently gifted, it will, I believe, be found that it was most remarkable for clearness and quickness of apprehension, and for rapidity and extent of association. On these qualities were founded that apparently intuitive perception of analogies, and that happy facility of
* Series I. Vol. ii. p. 175.
† Series II. Vol. i. p. 26.
[page] 64
tracing and pursuing them through all their consequences, which led to several of his most brilliant discoveries. Of these analogies many were just and legitimate, and have stood the test of examination by the clearer light, since reflected upon them from the improved condition of science. But, in other cases, his analogies were fanciful and unfounded, and led him far astray from the path, which might have conducted him directly to truth. It is curious, however, as he himself observes, that in missing one thing, of which he was in search, he often found another of greater value. In such cases, his vigilance seldom failed to put him in full possession of the treasure upon which he had stumbled. Finding by experience, how much chance had to do with the success of his investigations, he resolved to multiply experiments, with the view of increasing the numerical probabilities of discovery. We find him confessing, on one occasion, that he "was led on, by a random expectation of some change or other taking place." In other instances, he was influenced by theoretical views of so flimsy a texture, that they were dispersed by the first appeal to experiment. "These mistakes," he observes, "it was in my power to have concealed; but I was determined to show how little mystery there is in the business of experimental philosophy; and with how little sagacity, discoveries, which some persons are pleased to consider great and wonderful, have been made." Candid acknowledgements of this kind were, however, turned against him by persons envious of his growing fame; and it was asserted that all his discoveries, when not the fruits of plagiarism, were "lucky guesses," or owing to mere chance*. Such detractors, however, could not have been aware of the great amount of credit that is due to the philosopher, who at once perceives the value of a casual observation, or of an unexpected result; who discriminates what facts are trivial, and what are important; and selects the latter, to guide him through difficult and perplexed mazes of investigation. In the words of D'Alembert, "Ces hazards ne sont que pour ceux qui jouent bien."
The talents and qualifications which are here represented as having characterized the mind of Dr. Priestley, though not of the rarest kind, or of the highest dignity, were yet such as admirably adapted him for improving chemical science at the time when he lived. What was then wanted, was a wider field of observation;—an enlarged sphere of chemical phænomena;— an acquaintance with a far greater number of individual bodies
* These charges, especially that of plagiarism, which had been unjustly advanced by some friends of Dr. Higgins, were triumphantly repelled by Dr. Priestley, in a pamphlet entitled," Philosophical Empiricism," published in 1775.
[page] 65
than were then known; from the properties of which, and from those of their combinations, tentative approximations to general principles might at first be deduced; to be confirmed or corrected, enlarged or circumscribed, by future experience. It would have retarded the progress of science, and put off, to a far distant day, that affluence of new facts, which Priestley so rapidly accumulated, if he had stopped to investigate, with painful and rigid precision, all the minute circumstances of temperature, of specific gravity, of absolute and relative weights, and of crystalline structure, on which the more exact science of our own times is firmly based, and from which its evidences must henceforward be derived. Nor could such refined investigations have then been carried on with any success, on account of the imperfection of philosophical instruments. It would have been fruitless, also, at that time, to have indulged in speculations respecting the ultimate constitution of bodies;—speculations that have no solid ground-work, except in a class of facts developed within the last thirty-five years, all tending to establish the laws of combination in definite and in multiple proportions, and to support the still more extensive generalization, which has been reared by the genius of Dalton.
It was, indeed, by the activity of his intellectual faculties, rather than by their reach or vigour, that Dr. Priestley was enabled to render such important services to natural science. We should look, in vain, in any thing that he has achieved, for demonstrations of that powerful and sustained attention, which enables the mind to institute close and accurate comparisons;— to trace resemblances that are far from obvious;—and to discriminate differences that are recondite and obscure. The analogies, which caught his observation, lay near the surface, and were eagerly and hastily pursued; often, indeed, beyond the boundaries, within which they ought to have been circumscribed. Quick as his mind was in the perception of resemblances, it appears (probably for that reason,) to have been little adapted for those profound and cautious abstractions, which supply the only solid foundations of general laws. In sober, patient, and successful induction, Priestley must yield the palm to many others, who, though far less fertile than himself in new and happy combinations of thought, surpassed him in the use of a searching and rigorous logic; in the art of advancing, by secure steps, from phænomena to general conclusions;—and again in the employment of general axioms as the instruments of further discoveries.
Among the defects of his philosophical habits, may be remarked, that he frequently pursued an object of inquiry too
E
[page] 66
exclusively, neglecting others, which were necessarily connected with it, and which, if investigated, would have thrown great light on the main research. As an instance, may be mentioned his omitting to examine the relation of gases to water. This relation, of which he had indistinct glimpses, was a source of perpetual embarrassment to him, and led him to imagine changes in the intimate constitution of gases, which were in fact due to nothing more than an interchange of place between the gas in the water and that above the water, or between the former and the external atmosphere. Thus he erroneously supposed that hydrogen gas was transmuted into azotic gas, by remaining long confined by the water of a pneumatic cistern. The same eager direction of his mind to a single object, caused him, also, to overlook several new substances, which he must necessarily have obtained, and which, by a more watchful care, he might have secured and identified. At a very early period of his inquiries (viz. before November, 1771), he was in possession of oxygen gas from saltpetre, and had remarked its striking effect on the flame of a candle; but he pursued the subject no further until August 1774, when he again procured the same kind of gas from the red oxide of mercury, and, in a less pure state, from red lead. Placed thus a second time within his grasp, he did not omit to make prize of this, his greatest, discovery. He must also have obtained chlorine by the solution of manganese in spirit of salt; but it escaped his notice, because, being received over mercury, the gas was instantly absorbed*. If he had employed a bladder, as Scheele afterwards did, to collect the product of the same materials, he could not have failed to anticipate the Swedish philosopher, in a discovery not less important than that of oxygen gas. Carbonic oxide early and repeatedly presented itself to his observation, without his being aware of its true distinctions from other kinds of inflammable air; and it was reserved for Mr. Cruickshank of Woolwich to unfold its real nature and characters. It is remarkable, also, that in various parts of his works, Dr. Priestley has stated facts, that might have given him a hint of the law, since unfolded by the sagacity of M. Gay-Lussac, "that gaseous substances combine in definite volumes." He shows that
1 measure of fixed air unites with 1 6/7 measure of alkaline air,
1 measure of sulphurous acid with 2 measures of do.
1 measure of fluor acid with 2 measures of do.
1 measure of oxygen gas with 2 measures nitrous, very nearly;
* Series II. p. 253.
[page] 67
and that by the decomposition of 1 volume of ammonia, 3 volumes of hydrogen are evolved.
Let not, however, failures such as these, to reap all that was within his compass, derogate more than their due share from the merits of Dr. Priestley; for they may be traced to that very ardour of temperament, which, though to a certain degree a disqualification for close and correct observation, was the vital and sustaining principle of his zealous devotion to the pursuit of scientific truth. Let it be remembered, that philosophers of the loftiest pretensions are chargeable with similar oversights;—-that even Kepler and Newton overlooked discoveries, upon the very confines of which they trod, but which they left to confer glory on the names of less illustrious followers.
Of the general correctness of Dr. Priestley's experiments, it is but justice to him to speak with decided approbation. In some instances, it must be acknowledged, that his results have been rectified by subsequent inquirers, chiefly as respects quantities and proportions. But of the immense number of new facts originating with him, it is surprising how very few are at variance with recent and correct observations. Even in these few examples, his errors may be traced to causes connected with the actual condition of science at the time; sometimes to the use of impure substances, or to the imperfection of his instruments of research; but never to carelessness of inquiry or negligence of truth. Nor was he more remarkable for the zeal with which he sought satisfactory evidence, than for the fidelity with which he reported it. In no one instance is he chargeable with mis-stating, or even with straining or colouring, a fact to suit an hypothesis. And though this praise may, doubtless, be conceded to the great majority of experimental philosophers, yet Dr. Priestley was singularly exempt from that disposition to view phænomena through a coloured medium, which sometimes steals imperceptibly over minds of the greatest general probity. This security he owed to his freedom from all undue attachment to hypotheses, and to the facility with which he was accustomed to frame and abandon them;—a facility resulting not from habit only, but from principle. "Hypotheses," he pronounces, in one place, "to be a cheap commodity;" in another to be "of no value except as the parents of facts;" and so far as he was himself concerned, he exhorts his readers "to consider new facts only as discoveries, and to draw conclusions for themselves." The only exception to this general praise is to be found in the pertinacity with which he adhered, to the last, to the Stahlian hypothesis of phlogiston;
E 2
[page] 68
and in the anxiety which he evinced to reconcile to it new phænomena, which were considered by almost all other philosophers as proofs of its utter unsoundness. But this anxiety, it must be remembered, was chiefly apparent at a period of life when most men feel a reluctance to change the principle of arrangement, by which they have been long accustomed to class the multifarious particulars of their knowledge.
In all those feelings and habits that connect the purest morals with the highest philosophy (and that there is such a connection no one can doubt), Dr. Priestley is entitled to unqualified esteem and admiration. Attached to science by the most generous motives, he pursued it with an entire disregard to his own peculiar interests. He neither sought, nor accepted when offered, any pecuniary aid in his philosophical pursuits, that did not leave him in possession of the most complete independence of thought and of action. Free from all little jealousies of contemporaries or rivals, he earnestly invited other labourers into the field which he was cultivating; gave publicity in his own volumes to their experiments; and, with true candour, was as ready to record the evidence which contradicted, as that which confirmed, his own views and results. Every hint, which he had derived from the writings or conversation of others, was unreservedly acknowledged. As the best way of accelerating the progress of science, he recommended and practised the early publication of all discoveries; though quite aware that, in his own case, more durable fame would often have resulted from a delayed and more finished performance. "Those persons," he remarks, "are very properly disappointed, who, for the sake of a little more reputation, delay publishing their discoveries till they are anticipated by others."
In perfect consistency with that liberality of temper which has been ascribed to Dr. Priestley, it may be remarked also, that he took the most enlarged views of the scope and objects of Natural Science. In various passages of his works he has enforced, with warm and impressive eloquence, the considerations that flow from the contemplation of those arrangements in the natural world, which are not only perfect in themselves, but are essential parts of one grand and harmonious design. He strenuously recommends experimental philosophy as an agreeable relief from employments, that excite the feelings or overstrain the attention; and he proposes it to the young, the high-born, and the affluent, as a source of pleasure unalloyed with the anxieties and agitations of public life. He regarded the benefits of its investigations, not merely as issuing in the acquirement of new facts, however striking and valuable; nor
[page] 69
yet in the deduction of general principles, however sound and important; but as having a necessary tendency to increase the intellectual power and energy of man, and to exalt human nature to the highest dignity, of which it is susceptible. The springs of such inquiries he represents as inexhaustible; and the prospects that may be gained by successive advances in knowledge, as in themselves "truly sublime and glorious."
Into our estimate of the intellectual character of an individual, the extent and the comprehensiveness of his studies must always enter as an essential element. Of Dr. Priestley it may be justly affirmed, that few men have taken a wider range over the vast and diversified field of human knowledge. In devoting, through the greater part of his life, a large portion of his attention to theological pursuits, he fulfilled what he strongly felt to be his primary duty as a minister of religion. This is not the fit occasion to pronounce an opinion of the fruits of those inquiries, related as they are to topics, which still continue to be agitated as matters of earnest controversy. In Ethics, in Metaphysics, in the philosophy of Language, and in that of General History, he expatiated largely. He has given particular histories of the Sciences of Electricity and of Optics, characterized by strict impartiality, and by great perspicuity of language and arrangement. Of the Mathematics, he appears to have had only a general or elementary knowledge; nor, perhaps, did the original qualities, or acquired habits, of his mind fit him to excel in the exact sciences. On the whole, though Dr. Priestley may have been surpassed by many in vigour of understanding and capacity for profound research, yet it would be difficult to produce an instance of a writer more eminent for the variety and versatility of his talents, or more meritorious for their zealous, unwearied, and productive employment.
APPENDIX.
Since the foregoing pages were written, I have added a few remarks on a passage contained in a recent work of Victor Cousin, in which that writer has committed a material error as to the origin of Dr. Priestley's philosophical discoveries. "La chimie," he observes, "est une création du dixhuitième siècle, une creation de la France; c'est I'Europe entière qui a appelé chimie Francaise le mouvement qui a imprimé à cette belle science une impulsion si forte et une direction si sage; c'est à I'exemple et sur les traces de Lavoisier, de Guyton, de Fourcroy, de Berthollet, de Vauquelin, que se sont formes et que marchent encore les grands chimistes étrangers, ici Priestley et
[page] 70
Davy; là Klaproth et Berzelius." (Cours de l'Histoire de la Philosophie, tom. i. p. 25.)
It is to be lamented that so enlightened a writer as Victor Cousin, yielding, in this instance, to the seduction of national vanity, should have advanced pretensions in behalf of his countrymen, which have no foundation in truth or justice. Nothing can be more absurd or unprofitable than to claim honours in science, either for individuals or for nations, the title to which may be at once set aside by an appeal to public and authentic records.
It was in England, not in France, that the first decided advances were made in our knowledge of elastic fluids. To say nothing of anterior writers, Dr. Black had traced the causticity acquired by alkalies, and by certain earths, to their being freed from combination with fixed air; and Mr. Cavendish, in 1766, had enlarged our knowledge of that gas and of inflammable air. In England, the value of these discoveries was fully appreciated; in France, little or no attention was paid to them, till the philosophers of that country were roused by the striking phænomena exhibited by the experiments of Priestley. Lavoisier, it is true, had been led, by an examination of evidence derived from previous writers, to discard the hypothesis of phlogiston. The discovery of oxygen gas by Dr. Priestley not only completed the demonstration of its fallacy, but served as the corner-stone of a more sound and consistent theory. By a series of researches executed at great expense, and with consummate skill, the French philosopher verified in some cases, and corrected in others, the results of his predecessors, and added new and important observations of his own. Upon these, united, he founded that beautiful system of general laws, chiefly relating to the absorption of oxygen by combustible bodies, and to the constitution of acids, to which, alone, the epithet of the Antiphlogistic or French theory of chemistry is properly applied. Of the genius manifested in the construction of that system, and the taste apparent in its exposition, it is scarcely possible to speak with too much praise. But it is inverting the order of time to assert, that it had any share in giving origin to the researches of Priestley, which were not only anterior to the French theory, but were carried on under the influence of precisely opposite views. This, too, may be asserted of the discoveries of Scheele, who, at the same period with Dr. Priestley, was following, in a distant part of Europe, a scarcely less illustrious career.
It is the natural progress of most generalizations in science, that at first too hasty and comprehensive, they require to be
[page] 71
narrowed as new facts arise. This has happened to the theory of Lavoisier, in consequence of its having been discovered that combustion is not necessarily accompanied with an absorption of oxygen, and that acids exist independently of oxygen, regarded by him as the general acidifying principle. But after all the deductions that can justly be made on that account from the merits of Lavoisier, he must still hold one of the highest places among those illustrious men, who have advanced chemistry to its present rank among the physical sciences. It is deeply to be lamented that his fame, otherwise unsullied, should have been stained by his want of candour and justice to Dr. Priestley, in appropriating to himself the discovery of oxygen gas. This charge, often preferred and never answered, would not have been revived in this place, but for the claim so recently and indiscreetly advanced by M. Victor Cousin. To the credit of Dr. Priestley it may be observed, that in asserting his own right, he exercised more forbearance than could reasonably have been expected under such circumstances. In an unpublished letter to a friend, he thus alludes to the subject of M. Lavoisier's plagiarism. "He" (M. Lavoisier) "is an Intendant of the Finances, and has much public business, but finds leisure for various philosophical pursuits, for which he is exceedingly well qualified. He ought to have acknowledged that my giving him an account of the air I had got from mercurius calcinatus, and buying a quantity of M. Cadet while I was at Paris, led him to try what air it yielded, which he did presently after I left. I have, however, barely hinted at this in my second volume*." The communication alluded to was made by Dr. Priestley to M. Lavoisier in October, 1774; and the Memoir, in which the latter assumes to himself the discovery that mercurius calcinatus (red oxide of mercury) affords oxygen gas when distilled per se, was not read to the Academy of Sciences before April, 1775†. In evincing so little irritability about his own claim, and leaving its vindication with calm and just confidence to posterity, the English philosopher has lost nothing of the honour of that discovery which is now awarded to him, by men of science of every country, as solely and undividedly his own.
WEDNESDAY EVENING.
MR. R. POTTER, Jun. read a description of his new construction of Sir Isaac Newton's reflecting microscope, and exhibited
* Letter to the late Mr. Henry, dated Calne, Dec. 31, 1775.
† See an Abstract of this Memoir in the Journal de Rozier, Mai, 1775.
[page] 72
the instrument, with a variety of finely executed elliptical mirrors, &c. In the reflecting microscope of Sir Isaac Newton, the object is placed directly in the focus of the speculum, and the image is formed in that of the eye-glass; and thus, having only one additional surface in the essential parts of the instrument, it must be considered as next in simplicity to the singlelens microscope.
In this construction the object must be placed in the axis of the tube, where it is difficult to provide sufficient illumination, and it is this defect which the new construction is intended to obviate. A large hole is cut in the tube between the object and the speculum, to allow the light to fall upon the former when it requires to be viewed as an opake object, and all the lower parts of the tube are lined with black velvet to absorb the irregular light. A large lens is also occasionally employed to concentrate the light. Transparent objects require a small oval mirror to be placed immediately behind them; this mirror receiving a concentrated light from a lens, fixed in a sliding piece on the side of the tube, reflects it through the object to the speculum. The objects, to be placed in the centre of the tube, are attached to thin brass wires in wooden handles, and kept separately in a box.
This construction of the reflecting microscope has a great advantage in point of distinctness, from there being only one necessary reflection between the object and the image, and will be found particularly suitable for the examination of opake objects, on account of the large aperture of the speculum, compared to its focal length.—It is therefore recommended as an excellent working tool to the scientific inquirer, who will disregard the little trouble required in its management.
The Secretary then read a description by Dr. Brewster of an Instrument for distinguishing Precious Stones and Minerals. The object of this instrument is to distinguish mineral bodies by the relative quantity and colour of the light reflected from their surfaces, when placed in contact with fluids of different refractive powers. The surfaces employed for this purpose may be either natural or artificial, so that the method is equally applicable to regular crystals, and to gems cut into artificial forms. If a fluid, of a given refractive and dispersive power, is placed on the surface of a mineral of the very same refractive and dispersive power, there will be no light whatever reflected from their separating surface; but in proportion as the fluid and the solid differ in these respects, in the same proportion will the quantities of light differ which are reflected at
[page] 73
the separating surface, and its colour will undergo corresponding changes.
The principal part of the instrument is a triangular prism of glass, between the lower surface of which and the upper surface of the mineral the oil is placed. This oil will form a parallel film, but, by the mechanism of the instrument, the two surfaces which, bound this film can be inclined to each other, so that an eye looking into the prism will see at once the images of a luminous body, such as the sun, &c., reflected from the separating surface of the oil and the prism, and from that of the oil and the mineral. The first of these images is constant both in its colour and quantity of light, while the oil is the same, but the second will vary with the mineral. The comparison of the colour and quantity of light obtained from different minerals furnishes the nicest tests for discriminating them.
The author illustrated his explanations of the principle of the instrument by means of diagrams, and the instrument itself, as constructed by Dollond, was exhibited to the Meeting.
THURSDAY MORNING.
MR. DALTON read a paper written for the Literary and Philosophical Society of Manchester, containing a series of expertments on the quantity of food, taken by a person in health, compared with the quantity of the different secretions; with chemical remarks and deductions.
Mr. Dalton, whose regular habits of life and uniform good health enabled him to make these Experiments upon himself to great advantage, commenced them about 40 years since at Kendal, and prosecuted them for periods of a week or a fortnight at various seasons of the year, to ascertain the proportion beween the weight of food, and the ordinary evacuations. Particular observations were made on the effects occasioned by drinking an infusion of Carbonate of Potash, and a train of experiments was continued for three weeks to determine the loss of weight by perspiration for the whole day, and for certain hours of the morning, afternoon, and night. The mean daily loss by perspiration was 37½ oz.
From these experiments, the state of organic chemistry 40 years since did not permit Mr. Dalton to make any deductions, but he was now enabled to return to the subject with the powerful aid of exact analysis. He showed that the quantity of Carbon contained in the solid and liquid food taken into the
[page] 74
| stomach daily was | 11½ oz. |
| of which there passes off sensibly | 1 |
| Leaving for the waste by insensible perspiration | 10½ oz. |
Mr. Dalton had ascertained from experiments on his own respiration that the quantity of Carbonic acid gas expelled from his lungs contained of Carbon 10¼ oz.
His daily loss by perspiration of aqueous vapour from the lungs was at the same time found to be 20½ oz. to which adding 10¼ oz. Carbon, we have the total loss by perspiration from the lungs, 30¾ oz., which taken from 37½, leaves 6 ¾ oz. per day for the insensible perspiration of the skin, of which 6½ oz. are water, and ¼ oz. is Carbon.
The element Azote, of which 1½ oz. per day was taken into the stomach, appears to have passed off by evacuation. Of the 6 lbs. of aliment taken in a day, 1 lb. consists of Azote and Carbon, and 5 lbs. of water, and nearly the whole quantity of food taken into the stomach enters the circulation,—the residual part constituting only 1/18 of the whole,—of which about half is thrown off by the kidneys, more or less according to season and climate, another part passes off by insensible perspiration, 5/6 being perspired from the lungs, and 1/6 from the skin.
MR. R. POTTER read a paper containing remarks on a theory of the late M. Fresnel concerning the reflection of light from the surfaces of bodies.
M. Fresnel in a paper read before the Academy of Sciences in Paris, and of which he published an abstract in the Annales de Chimie for 1820, proposed to account for the reflection of light at the surfaces of bodies, on the undulatory theory, by its impinging on the ponderable particles. He appears to have afterwards in some measure modified his views, but not, to the writer's knowledge, ever to have formally renounced his former proposition. Hence the subject may fairly be considered as still open to discussion: and the manner of considering reflection, as caused by the light striking the ponderable parts of bodies, being the one which almost every person would recur to, on first commencing the study of physical optics, it will perhaps be considered not entirely useless, on this account also, to enter into a regular examination of M. Fresnel's hypothesis.
Now if reflection were caused immediately by the ponderable matter in any surfaces, it ought to be some function of the quantity of matter in the bodies furnishing such surfaces; but even a superficial view of the small quantity of light reflected
[page] 75
at a perpendicular incidence in transparent bodies, compared with the large quantity reflected at the same incidence in metals, is sufficient to convince us that reflection has no relation to the densities of bodies. To remove, however, an objection which might be urged, that the extension of the particles of bodies may not bear an invariable ratio to their weight, it will be necessary to examine cases where a metal, by combining with a new element or elements, has acquired the property of transparency, and thus possesses an evident refractive power. By knowing the comparative weights of the metal in the two states, it is easy to calculate the relative numbers of similar particles in equal surfaces, and of course to calculate the relative quantities of light which ought to be reflected, if caused only by the ponderable particles of metal. Experience is so much at variance with the hypothesis under examination, that the other elements in the compound may be considered even as lending no assistance at all.
The results obtained by Photometry show that the metals, with the exception, perhaps, of two or three, reflect two thirds and upwards of the light incident perpendicularly on them. For the reflective power of the transparent bodies we may use the analytical formula of M. Poisson, (which was admitted by M. Fresnel,) to calculate it from the refractive index, though it gives most probably, in all cases, quantities too large and of course proportionally favourable to the controverted hypothesis.
Proceeding in this manner for glass of Antimony, the reflection, according to Fresnel's hypothesis, should have been at least 46 rays of every 100 incident, whilst the quantity given by the analytical formula is only 19·3 rays.
In the white oxide of arsenic or arsenious acid the reflection should have been 31·9 (taking even the old number for the specific gravity of the metal,) in place of less than 8·3, which it really is.
In the red silver ore it should have been at least 37·5 rays if the hypothesis were correct, instead of less than 19·2 as determined by the analytical formula.
If the metals of the alkalies and earths might be assumed of equal reflective powers with the other metals, and it is most likely they are, the chloride of sodium would form one of the strongest cases which could be brought forward; for whilst it really reflects only about two per cent., it ought, according to the controverted hypothesis, to have reflected upwards of 60 rays of every 100 incident, from the metal in the chloride being almost as dense as in its proper state.
[page] 76
MR. WM. HUTTON read a paper upon the Whin Sill of Cumberland and Northumberland.
A bed of Stratiform Basalt is well known to occur extensively in connection with the Mountain Limestone Rocks of the North of England, and is called in Alston Moor and the adjoining mining districts, "the Whin Sill." This bed has naturally a good deal of geological interest attached to it, from the circumstance that rocks of its class are generally found under conditions which indicate that their production is entirely independent both as to antiquity and origin of that of the Strata which they divide,—and upon which, at the points of contact, they have produced mechanical and chemical changes which afford the most conclusive evidence of their violent intrusion since the deposition and consolidation of those Strata.
The Whin Sill is visible in many of the streams running into the South Tyne from the West, and may be seen in the bed of the Tyne itself at Tyne-head. It occurs in the bed of the Wear, in Teesdale, where it is extensively developed, and in the Lune; in short, throughout the whole district wherever the water-courses, or the operations of the miner, pierce deep enough: its basset-edge may also be traced almost uninterruptedly from Helton in Westmoreland to Tindale Fell in Northumberland.—Here the whole carboniferous formation is broken through by the "great Stublick Dyke," which throws down the Whin Sill along with the other beds of the formation to an immense depth; its edge reappears on the north side of this dyke at Wall Town Crags, near Glenwhelt, in Northumberland, rising rapidly to the North, and from this spot it can be traced almost throughout the county of Northumberland to the sea-coast near Newton; it occurs again with other beds of the carboniferous formation in consequence of a general depression of the strata a little south of Bamborough, from whence it sweeps round by Belford to Kyloe on the coast, near to which place it finally disappears.
In the course of this bed northward from Alston Moor, it appears to rise in the series of strata, from 'the putting in' of new beds, as the miners term it; and of course it is found in contact with all the varieties of rock composing the carboniferous formation. It is generally in one bed, sometimes in two, and once at least it occurs in three beds.
The action of heat in hardening the rocks near it and rendering the limestone crystalline, can generally be observed accompanying this bed, but nowhere to such an extent as in High Teesdale.
After an attentive examination of the appearances exhibited
[page] 77
throughout its whole course, the conclusion of the writer is, that this bed of Basalt was produced by an overflowing of lava during the deposition of the Mountain Limestone Group, after those beds, which are found below, and prior to those above it.
After Mr. Hutton's paper had been read,
MR. MURCHISON rose to bear testimony to the general value and accuracy of the memoir. His own observations, however, on the evident violence which in High Teesdale accompanied the arrangement of the basaltic matter, the altered character of the limestone, sandstone, and shale both above and below it, and the occasional ramification of its substance through the contiguous and superior Strata, led him to confirm the opinion of Professor Sedgwick, that the Whin of that district had not been injected into the Carboniferous Limestone till after the deposition of that whole system of rocks. He thought it very desirable that the views of Professor Sedgwick respecting the Whin Dykes of Durham should be further pursued, with reference to this theory, to ascertain whether they were emanations from the great Whin Sill, or were posterior to it. Some of these Dykes break off into various branches, all pointing to the Whin Sill, and thus appearing at least to be related to it in age.
Mr. Phillips had formerly examined the whole range of the Whin Sill, and was happy in being able to agree in opinion with both the author of the paper, and the President of the Geological Society. The definite geological situation, between the same Limestone bands, of the great portion of the Basalt, its wide lateral extension, the general limitation of the effects of its heat upon the contiguous rocks to the lower surface of the mass, its course for miles together without furnishing a single dyke, or even entering at all into the many natural fissures of the Limestone, and its division by metallic veins, obliged him to infer that a large portion of the Whin Sill was formed by periodical submarine eruptions of Lava, at intervals during the deposition of the Carboniferous Strata with which it is associated. On the other hand, the instances described of violent eruption and local intrusion of the Basalt into the Strata above its general range, seemed to show that Teesdale had been the theatre of more than one such eruption. The views of Professor Sedgwick and Mr. Hutton were, therefore, by no means irreconcileable; and it might be very possible hereafter to fix upon the foci or centres from which, as probably at Caldron Snout in Teesdale, the principal basaltic coulees had flowed.
[page] 78
MR. JOHNSTON gave an account of the Metal Vanadium and its ores.
He stated that this metal was first observed, though without being distinctly made out, by Del Rio in Mexico 25 years ago, and afterwards rediscovered by Seftström, and nearly at the same time by himself, about the end of last year. He exhibited and described the mineral from Wanlock-head, in which he found it, detailed the process for extracting it, enumerated its most interesting properties when in the metallic state, and the characters by which in all its states it may be distinguished from Chromium, the only other metal with which the analytical chemist is likely to confound it. He exhibited various compounds and salts of the metal, among which were some beautiful crystals of Vanadic Acid, which were transparent, of a brown colour by transmitted light, but reflecting a purplish tint. They were in the form of flat prisms of a high degree of lustre, and had been ascertained by Dr. Brewster to possess a refractive power approaching that of the diamond, to have at least one axis of double refraction, and to belong to the prismatic system of Mohs.
MR. WITHAM read a paper on the general results of botanical investigation concerning the character of the Ancient Flora, which by its decomposition has furnished the materials of coalseams. He described the discoveries which the art of slicing and polishing the fossil stems of plants had enabled him to make, concerning the internal structure of these large coniferous trees which especially abound in the lower part of the Carboniferous Series of Berwickshire; and stated that Geologists are now agreed that the plants of these ancient periods are of more diversified and complicated types than a distinguished foreign writer supposed.
The following Notice of a fact observed in the torrcfaction of Yellow Copper Pyrites at Amlwch, in Anglesey, by DR. HENRY, was read by the Secretary.
"When on a visit, a few years ago, to the Copper-Mines and Works at Amlwch in Anglesey, a fact was mentioned to me by an intelligent superintendent of the processes carried on there (Mr. Joseph Jones), which struck me to be interesting and curious. The poorer part of the ore (a native mixture of yellow copper pyrites with so much foreign matter as to contain only about 5 per cent. of copper,) is roasted in kilns on the spot, in order to expel a considerable part of the sulphur. The combustion, after the kiln has been once lighted, is supported by
[page] 79
the inflammable matter of the ore itself, and a smouldering heat, never, I was assured, sufficient to occasion fusion, is kept up for several months. On examining the lumps of roasted ore, small nodules are observed of an iron grey colour, with some lustre, resembling in appearance the vitreous copper ore (cuivre sulfuré of Haüy). These nodules have been found, when assayed, to be very much richer in copper than the original ore. Their specific gravity I found to be 4·6 very nearly that of vitreous copper ore. By a few general experiments, I ascertained that they are not entirely soluble in heated nitric acid, and that the solution contains a small proportion of peroxide of iron, with a much larger one of oxide of copper. The yellow copper pyrites, in its natural state, was determined by Mr. Richard Phillips, to consist of
2 atoms of protosulphuret of iron,
1 atom of bisulphuret of copper.
It should appear, therefore, that during torrefaction the bisulphuret of copper, by parting with one atom of sulphur, is converted into protosulphuret, which, by its aggregative attraction, is collected into small nodules. This fact furnishes an additional example, to the few which were before known, of changes of molecular arrangement in bodies heated below their point of fusion; with this further circumstance, that the attraction, which causes the aggregation of the particles, is sufficient to overcome the obstacle of interposed matter of a different kind. The only other instances of similar facts that at present occur to me, are presented by crystallites; and by the products of Mr. Gregory Watt's experiments on Basalt, some of the appearances of which he supposes to have taken place after the fused mass had returned to a solid state.
In the second volume of Breislac's Institutions Geologiques, I was pleased to observe, two or three years after the foregoing fact had occurred to me, that a precisely similar observation had been made by Brocchi on the roasted copper ore of Agardo, which is also the yellow pyrites, and of quality, as to its proportion of copper, not exceeding that of the Parys Mountain. On breaking the lumps of roasted ore, similar nodules were observed; and these, when assayed, were found to contain two thirds of their weight of copper, while the surrounding ore had lost greatly of its original proportion of that metal. In the central parts of some of those nodules, small fibres and plates of metallic copper were visible, an appearance which I have not observed in the roasted ore of Anglesey. The nodules, thus enriched in their proportion of metal, are picked out, and subjected to reducing processes.
M. Breislac adds, that the torrefaction of the ore, at Agardo,
[page] 80
is carried on with the careful avoidance of such a heat as would occasion the fusion of the ore; for this is ascertained to be very injurious to the subsequent operation."
It was remarked by Mr. Johnston, that a similar observation had been made some years ago by Professor Brodberg, of the school of Mines at Fahlun, and was detailed by him in a paper published in the Swedish Transactions, and reprinted in the Edinburgh Journal of Science.
THURSDAY EVENING.
On Thursday Evening MR. SCORESBY gave An exposition of some of the laws and phœnomena of Magnetic Induction, and of the mutual influences of magnets on each other, with an account of a method of application of the magnetic influences for the determination of the thickness of solid substances not otherwise measurable.—In the introductory observations, Mr. Scoresby considered the general nature, as far as it is understood, of the magnetic principle, and described a magnetic bar as a battery of magnetic particles, the arrangement of which being regular and consistent, transmits to the poles, like the galvanic pile, the general aggregate of their individual energies. He defined induced magnetism, "as the development of the latent magnetism in iron or steel by the juxtaposition of any substance in a magnetic condition." His investigations on the law of induced magnetism extended to the different qualities of iron and steel; to the proportion of influence acquired by similar masses at different distances from the proximate magnet; and to the relation of capacity in masses in all other respects similar except as to thickness. The proportion of influence was shown, at different distances, of the magnetism induced upon the nearer and more remote ends of a bar of soft iron, and the quantity transmitted, compared with the portion directly induced, was numerically stated. A bar of very soft iron, placed directly over a magnet of similar dimensions, was found at the distance of 5 inches, to acquire 1/30th of the power of the magnet. At 4 inches above, the inductive influence of the magnet was 1/19th of its own power; at 3 inches, 1/13th; at 2 inches, 1/7th; at 1 inch, ¼th. At ¼ of an inch above, the power induced was equal to ½ that of the magnet, and at the distance of 1/16th, it amounted to 2/3rds. Several new and curious illustrations of the phænomena of induced magnetism were then exhibited to the Meeting.
FRIDAY MORNING.
MR. SCORESBY concluded his account of his Magnetical experiments. He described the action of magnets of different
[page] 81
dimensions on the needle of the compass, and the investigation of the law of the deviation at different distances, and with magnets of various sizes. With a pair of three-feet bar magnets, he was able to produce a very perceptible action on the compass, through a variety of intervening solid substances, at a distance of more than 61 feet; and to measure with tolerable precision various masses of solid rock of from 3 feet to more than 40 feet in thickness by the magnetic deviations. The phænomena now communicated to the Association, with their different important applications, were the results of original investigations and discoveries, accomplished, for the most part, within the last ten months.
A paper by DR. BREWSTER on the structure of the crystalline lens in fishes, birds, reptiles, and quadrupeds, was read by the Secretary, and illustrated with drawings and models by the author. After giving an account of the previous observations of Leeuwenhoek and others, the author explained the method in which he conducted his inquiries. The lenses of almost all animals are composed of distinct fibres, and when any of the laminæ are removed, the surface appears fibrous or grooved. In large lenses, the direction of these lines may be easily traced by the microscope alone, but in many cases this is quite impracticable. In order to get rid of this difficulty, the author observed the image of a candle or bright luminous object, when reflected from a fresh surface of the lens, and he found this colourless image invariably accompanied by coloured images on each side, as in mother-of-pearl, and in Mr. Barton's Iris ornaments. As the direction of the fibres is necessarily perpendicular to the line joining these coloured images, and as the distance of the coloured images varies inversely with the diameters of the fibres, Dr. Brewster was able to trace these fibres to their points of convergency or terminations, even when the fibres themselves were no longer visible. When the crystalline lens is dried, a furrowed impression of its surface may be taken upon wax, and the impression will, like that from mother-of-pearl, exhibit the same coloured images.
By the process now described, Dr. Brewster has examined many hundreds of the lenses of animals brought from all parts of the world, and has found that there are five different modes in which the fibres are arranged, the same mode being invariably found in the same animal. These different arrangements of the fibres were illustrated by elegant drawings from the pencil of Dr. Greville, and by wooden models, which ex-
F
[page] 82
hibited all the inflexions of the fibres and the dimensions of their diameter as they approached to their termination.
In the greater number of lenses the structure is perfectly symmetrical in relation to the anterior and posterior surfaces, or to the poles of the axis of vision: but Dr. Brewster discovered in some lenses a remarkable deviation from this symmetry; the anterior surface having the fibres arranged according to one law, and the posterior surface according to another. The object of this singular structure he conceived to be to obtain a more perfect correction of the spherical aberration of the eye to which it belongs.
MR. MURCHISON, President of the Geological Society, communicated, verbally, observations on certain accumulations of clay, gravel, marl, and sand around Preston in Lancashire, which contain marine shells of existing species.
The marine shells of existing species in this district were first noticed by Mr. Gilbertson of Preston; and Mr. Murchison was desirous of calling the attention of the Meeting to the merits of that able naturalist.
He had this year visited the localities, and found the deposit in question to consist, near the surface, of clay with boulders of distant rocks, covering great thicknesses of marl, gravel, and sand, the sand usually being the lowest. These accumulations are not only spread over the broad delta extending from the coast at Blackpool to Preston in the interior, but they rise at the latter place into considerable eminences extending in plateaux on the banks of the Ribble and the Darwent, for several miles inland.
In certain places the marls, sands, and gravels contain shells of existing species (Mr. Gilbertson enumerates about 20 species), not differing from those of the adjoining sea, above which they were found at various heights from 80 to 300 feet. The accumulations seldom offer proofs of regular bedding or tranquil deposit, but rather resemble the detritus formed upon an agitated shore; although from their diversity of structure they present distinct evidence of having been heaped up during a long protracted period. Seeing the height above the sea at which these shells are found, and that they are usually buried under a cover of clay, containing large boulders of Cumbrian rocks, Mr. Murchison infers that one of the last elevations of the central ridge of the North of England is thereby proved to have taken place after the creation of existing species of animals.
[page] 83
The deposit was described as resting on inclined and contorted strata of millstone grit, and shale, and an overlying red sandstone, (banks of the Ribble and Darwent,) and upon the edges of the productive coal measures near Chorley.
This communication was followed by a discussion, in which Mr. Greenough, Mr. Murchison, and Mr. Phillips took part, on the application of these observations to resolve the question of the change of level on the coast of Lancashire, and on that of Yorkshire, where gravel deposits containing marine shells of existing species have been described by Mr. Phillips as diluvium.
DR. DAUBENY gave a Lecture on the connexion of Hot Springs with Volcanos.
Hot springs, he observed, are met with for the most part in one of these situations. 1st. In the vicinity of volcanos. Of this kind of position, the active volcanos of Iceland, Italy, and Sicily, and the extinct ones of France, Hungary, and the Rhenish provinces, afford numerous examples. In these cases, it cannot be doubted that the heat of the springs is derived from the volcanos contiguous to them.
2nd. At the foot of chains of mountains which have been uplifted. Now as the elevation of such chains may with some probability be referred to a volcanic cause, it seems most natural to attribute the occurrence of their hot springs to the same; and this is confirmed by observing that they are found for the most part either near the line at which the elevation seems to have commenced, or else near the axis of the chain, in places where the valleys penetrate to the greatest depth. Of both these positions, the Pyrenees afford abundant examples.
3rd. Hot springs occur in some cases at a distance from any great chain of mountains, but then there is in these cases often strong evidence of some fracture or dislocation of the strata, such as may reasonably be attributed to a volcanic cause. Instances of this are supplied by the hot springs of Clifton in this country, Carlsbad in Bohemia, and Pfeffers in Switzerland.
It appears, then, that the great majority of hot springs are attributable to volcanic action, and this is confirmed by considering the gaseous products which they evolve, for these are the same as those given off by volcanos.
The first of these is sulphuretted hydrogen, which is common also to volcanos, especially when in a state of languid action.
Another kind of gas given off by many hot springs, is
F 2
[page] 84
carbonic acid, which abounds also in cold springs; but when this is the case, the latter often exists in valleys of elevation, to use Dr. Buckland's nomenclature, which in the structure of the beds surrounding them, bear evidence of sudden uplifting. Such are the springs of Pyrmont in Westphalia, and of Tunbridge in this country.
The third gas given off by hot springs, is nitrogen. It had been previously found at Bath and Buxton; but Dr. Daubeny has likewise detected it in several other tepid springs in Derbyshire, and in that of Taafe's Well near Cardiff in Glamorganshire. He met with it also in a state of purity in the hot springs of St. Gervais, Cormayeur, St. Didier, and others, on the skirts of the Alps, and accompanying carbonic acid in those of Mont Dor, St. Nectair, and Chaudes Aigues in France; and from these observations of his own, combined with those of others, he concluded that nitrogen is disengaged from the generality of hot springs.
The presence of nitrogen is also an argument for adopting that chemical theory of volcanic action, which supposes it to arise from a species of combustion or oxidation, in preference to the mechanical hypothesis which regards it merely as a consequence of the law of distribution of temperature within the earth, and excludes the idea of chemical agency altogether.
On the latter part of this paper, it was remarked, that the gases mentioned by Dr. Daubeny are evolved from decompositions known to be going on at the surface, and at various depths from the surface of the earth, independently of hypothetical causes.
With respect to the occurrence of remarkable dislocations in connexion with mineral springs, Mr. Smith observed, that in the neighbourhood of the Bath waters, the dislocations must have been occasioned in very ancient geological æras; since the strata of the lias series, through which the hot springs rise, are unaffected by the disturbances of the coal and limestone series beneath.
FRIDAY EVENING.
MR. POTTER communicated the following observations on Electrical Phœnomena, exhibited in the Torricellian vacuum.
Though early experimenters had directed their attention to the phænomena of Electricity shown in passing through space as void of matter as they were able to procure, yet the question whether electricity can pervade a perfect vacuum, or can not do so, is still far from being decided. The experiments with
[page] 85
the air-pump would lead us to conclude that electricity would pervade an actual vacuum without sensible resistance, and without exhibiting light. But Mr. Walsh, Mr. Morgan, and other experimenters, had asserted that electricity could not pervade a perfect Torricellian vacuum. Sir Humphry Davy has maintained that the Torricellian vacuum is permeable to electricity, with an exhibition of more or less light according to the temperature. But as the appearances he describes are similar to those given by Mr. Morgan, when a very minute portion of air remained in his tube, it must be considered a question still open to further investigation.
From the writer's experiments, made with the object of learning something which might throw a light on the nature of Aurora Borealis, it has appeared, in conformity with the previous experiments of Sir Humphry Davy, Mr. Morgan, and others, that the passage of electricity through space containing only a very minute portion of air, was attended with a very considerable display of light, and this when the mercury in the tube stood scarcely perceptibly lower than that in a good barometer.
DR. WARWICK exhibited the method of making a powerful temporary Magnet, by coiling round a horse-shoe of soft iron a quantity of copper wire, connecting the poles of a galvanic battery, as originally performed by Professor Moll of Utrecht.
DR. DAUBENY exhibited a new instrument composed of finely reticulated wire, intended to illustrate the effects of capillary attraction.
A description of the New Volcanic Island, by Mr. OSBORN, communicated by Captain Hotham, R.N. was read to the Meeting.
SATURDAY MORNING.
MR. DALTON, President of the Manchester Society, read his Physiological Investigations arising from a consideration of the mechanical effects of atmospheric pressure on the animal frame.
In this essay Mr. Dalton endeavours to answer the question, how the animal body is enabled to resist the pressure of the external atmosphere, which varies in amount from 15 to 20 tons on a middle-sized man, without being sensible of the whole or any part of this enormous and fluctuating pressure.
The average specific gravity of the human body being taken
[page] 86
according to Robertson's experiments at 0·9, Mr. Dalton observes that the mean specific gravity of all the solids and fluids which are in it is about 1·05. The air contained in the lungs and other receptacles of the body is estimated at 150 cubic inches; the average bulk of the body 4500 cubic inches, of which consequently 4350 cubic inches are solid and liquid parts. The mean specific gravity of these parts, taken separately when dead, being 1·05, their total weight should be equal to 4567 cubic inches of water; but it was found by actual weighing, when alive, equal to 4044 cubic inches,—a difference of weight equal to 523 cubic inches of water, or more than 1/9th of the whole weight of the body. The general conclusion deduced by Mr. Dalton from these data, combined with other considerations, is, that the whole substance of the body is pervious to air, and that a considerable portion of air constantly exists in the body during life, subject to increase and diminution according to the pressure of the atmosphere, in the same manner as it exists in water: and further, that when life is extinct, this air in some degree escapes and renders the parts specifically heavier than when the vital functions were in a state of activity. (This Paper has since been printed in the Manchester Memoirs, Vol. V.)
MR. ALLAN communicated a Notice of a magnificent specimen of aqua-marine in the possession of Don Pedro.
The largest mass of precious beryl known to mineralogists is an aqua-marine belonging to Don Pedro; it is nearly as large as the head of a calf, its extreme length being 9 1/8 inches, its breadth 6 5/8 inches; it weighs 225 ounces Troy, or eighteen pounds nine ounces. On one side there are slight indications of the plane of a crystal; but it is otherwise entirely water-worn. Its surface is consequently dull; but beneath it the mass is perfectly clear and transparent, and, large as it is, without a flaw. It is of a beautiful pale bottle-green colour.
MR. ROBISON, Secretary of the Royal Society, Edinburgh, described and illustrated by diagrams the principles and mode of construction of his Linseed Oil Barometer, and detailed the mechanical processes by means of which he had been enabled entirely to free the oil from atmospheric air and other gaseous admixtures.
MR. FORBES read to the Meeting his Essay on the Horary Oscillations of the Barometer near Edinburgh.
In the. former part of this communication, the Author, after a short view of the progress of his researches on this subject,
[page] 87
states the circumstances under which his observations commenced. The place of observation is 4 miles S. W. of Edinburgh, lat. 55° 55′ 20″ N. long. 12′ 57·5″ west of Greenwich, at an elevation of 410·5 feet above the mean level of the sea. Five observations were made daily of the barometer and attached thermometer, from 8 to 8½ A.M., at 10 A.M., about 4 P.M., and at 8 and 10 P.M., in order to detect the morning and evening maximum and afternoon minimum. The number of observations was 4410, which, being reduced to a standard hour (10 P.M.), by methods described at length in the paper, yielded the following results.
The maximum of oscillation occurred in spring and summer at 8 or 8½ A.M. and 10 P.M., in autumn and winter at 10 A.M. and 8 P.M.
Taking these hours, and selecting the actual maxima, the amounts of Oscillation are found to be
| Morning. | Evening. | |
| In Spring | ·0213 | ·0202 |
| Summer | ·0181 | ·0151 |
| Autumn | ·0136 | ·0079 |
| Winter | ·0031 | ·0031 |
After comparing the results of his observations with an extensive collection of those of other observers in various latitudes, Mr. Forbes proceeded to investigate formulae which should express with the least error the general amount of oscillations in different latitudes, at the level of the sea. For this the paper itself, which will appear in the Transactions of the Royal Society of Edinburgh, must be consulted, as also for various indications of the influence which elevation above the sea, the season of the year, and the absolute mean temperature of the place, have in modifying the amount and period of occurrence of the phænomenon.
The following Extract of a letter from SIR JAMES SOUTH to Dr. Brewster, dated Observatory, Kensington, Sept. 29, 1831, was read by the Secretary.
"Should the York Meeting not have terminated its labours, and should you think it worth the trouble, I wish you would call the attention of any astronomers that may be there, to the anomaly which sometimes attends the transits of the satellites of Jupiter, over the planet's face. Generally speaking, the satellite may be seen to glide on the face as a bright planetary disc, and remains so till it has proceeded one sixth or one eighth of the planet's diameter. It then becomes invisible till it approaches the opposite limb within one sixth or
[page] 88
one eighth of the diameter of the planet, when it may be again detected as a bright disc, and remains so till it passes entirely off the face of the planet.
This anomaly I have witnessed three or, I believe, four times, and the last on Saturday fortnight, when the satellite itself instead of being invisible, except near the limbs, was perfectly visible as a black disc, and with its attendant shadow, was distinctly seen with the five-feet equatorial. The point to be settled is,—why should this fact be presented sometimes and not always?
The approaching disappearance of Saturn's Ring, and even its present situation, will be very advantageous for obtaining a knowledge of the various phænomena of the Satellites, and of the actual figure of the planet. Of the former we know next to nothing; of the latter but little that is satisfactory. Sir William Herschel's observations of the planet's figure are entirely at variance with mine.
If Lord Oxmantown, or any person possessing a large Reflector, would turn it every fine night on the Georgium Sidus, it would be well; for although Sir William Herschel expressed to me orally his doubts as to the accuracy of his observations, which assigned to that planet two rings perpendicular to each other, still I know not if this suspicion of his has even been promulgated. One Satellite has certainly been seen with my Achromatic, and one also by Mr. Herschel, myself, and Struve with the twenty-feet Reflector. Laplace doubted the existence of more than two. If the others are not greatly more faint than the one I have seen, Lord Oxmantown will certainly detect them instantly with the immense quantity of light afforded by his Reflector."
SATURDAY EVENING.
DR. DAUBENY read an account of Experiments on the combustion of Coal Gas performed at York, by the Rev. W. Taylor, from which it appeared that by regulating the quantity and mode of admission of the atmospheric air to the flame of a common Argand gas burner, the quantity of light might be much increased without increased expenditure of gas, while the colour of the light so produced varied according to circumstances. He referred the effects to the principle laid down by Sir H. Davy as to the luminosity of flame depending on the amount of solid matter maintained in a state of ignition at any given time.
THE REV. WM. VERNON HARCOURT showed a Lamp constructed upon a new principle, and explained the principle and
[page] 89
construction of it. He gave it the name of an oil gas lamp; not because it was lighted by gas formed at a temperature below that of flame, for this was common to all lamps, but because, as in the gas lights of the streets, the gas issued from a reservoir, and owed the perfection of its combustion not to an ascending current of hot air, but to the force with which it was propelled from the reservoir and carried the air along with it. It differed, however, from the common gas lights in these circumstances,— that the reservoir formed part of the burner; that the gas was formed as it was consumed; and that it was propelled, not by a vis a tergo and in a state of condensation, but by the expansive force of its own heat. In consequence of this circumstance the current of the gaseous jet was more rapid in proportion to the quantity of matter contained in it than in the common gas lights, whilst it was also at a much higher temperature, so that it could issue with a greater velocity without being liable to blow itself out. The practical difficulty of the construction consisted in the obtaining a steady supply of oil, especially with the cheap oils. This difficulty had been in great measure surmounted, but the instrument was still imperfect, and had been charged by some accident that evening with a vegetable oil, from which a clear light could not be obtained.
An Essay by DR. BREWSTER on a new Analysis of Solar Light was read by Mr. Phillips.
According to Sir Isaac Newton's Analysis of Solar Light by the prism, white light consists of seven different colours, each of which has a peculiar range of refrangibility occupying distinct spaces in the prismatic spectrum, "to the same degree of refrangibility ever belonging the same colour, and to the same colour ever belonging the same degree of refrangibility."
While examining the specific action of different coloured bodies in absorbing particular portions of the prismatic spectrum, Dr. Brewster was led to observe that rays of two different colours in the same spectrum had actually the very same degree of refrangibility, the one colour being superimposed upon the other. By extending this inquiry, and availing himself of the aid of various methods of insulating rays which the prism could not separate, he was conducted to the new Analysis of Solar Light, which it was the object of this paper to explain and establish. The following propositions contain a general view of the results.
1. White Light, whether it be that of the sun or of artificial flames, consists of three simple colours only, Red, Yellow, and Blue, by the union of which all other colours are composed.
[page] 90
2. The solar spectrum and that of artificial flames, whether formed by prisms of transparent solids and fluids, or by grooves in metallic and transparent bodies, or by the diffraction of light passing through a narrow aperture, consist of three spectra of equal length, beginning and terminating at the same points (viz. a Red, a Yellow, and a Blue Spectrum), and having their maximum of illumination at different points of their length, and their minimum at their two extremities.
3. All the seven colours in the solar spectrum, as they were observed by Newton and Fraunhofer, are compound colours, each of them consisting of Red, Yellow, and Blue Light in different proportions.
4. A certain portion of White Light incapable of being decomposed by the prism, in consequence of all its component rays having the same refrangibility, exists at every point of the spectrum, and may at some points be exhibited in an insulated state.
(Since this paper was read, an abridgement of it has been published in the Edinburgh Journal of Science, No. X. New Series, pp. 197—207; and the original Memoir, illustrated with coloured drawings, will appear in the next Part of the Transactions of the Royal Society of Edinburgh, Vol. XII. Part I.)
MR. WM. GRAY, jun. read the Translation of a memoir by Professor Gazari of Florence, on a method of detecting the traces of writing which has been fraudulently erased.
The Author of this paper having been frequently appointed by the Tribunals to give professional evidence in trials of this nature, instituted experiments on the subject, which, by showing him the possibility of removing entirely not only the ink, but also the materials employed in its removal, proved that cases might arise, when the fraud could not be detected in any other manner than by examining the condition of the paper or other material written on. For this purpose optical means were tried in vain, and immersion in water did not show such a difference in the absorptive power of the written and unwritten parts as happens in the employment of certain sympathetic inks; but on exposure of the suspected paper near to a moderate fire, the paper, which in consequence of the corrosive effects of the ink, was in those parts altered in its nature, was unequally acted on by the process of carbonization, and thus the number and length of the lines, and often the whole of the erased portion was distinctly revealed.
[page] 91
EXHIBITIONS, &c.
Mr. Gould, Member of the Zoological Society of London, exhibited select specimens of Birds figured and described in his work on the Ornithology of the Himalaya Mountains, and copies of this work were laid upon the tables for inspection.
Mr. R. Havell exhibited Drawings of Birds for Mr. Audubon's great work on American Ornithology.
Mr. Hey, Curator to the Philosophical Society of Leeds, showed some remarkable specimens of Fishes from the Yorkshire coal district, which belong to the Museum of that Institution.
Mr. Williamson, Keeper of the Museum to the Philosophical Society of Scarborough, brought for examination a series of the reliquiæ of fossil Crustacea recently discovered in the strata of that coast.
Mr. Wm. Gilbertson, of Preston, displayed an instructive suite of Crinoidal remains with other remarkable fossils from the vicinity of Clithero, and marine shells belonging to existing species from the gravel deposit on the banks of the Ribble.
Copies of recent publications lay upon the tables from Dr. Boswell Reid, from Mr. John V. Thompson of Cork, from Mr. Ashley of Edinburgh, Mr. Harrison of Barton, and others.
Mr. Smith, Author of the Map of the Strata of England, showed a Geological Map of the district round Hackness.
Mr. Murchison, President of the Geological Society, showed coloured Maps representing the Transition Rocks, the old Red Sandstone, and Carboniferous Limestone, on the border of Wales; the basin of new Red Sandstone (as Mr. Murchison has determined it to be,) in the Vale of Clwydd; and other Maps, Sections, and Notices relating to parts of South Wales, Lancashire, Durham, and Yorkshire.
[page 92]
[page 93]
SECOND REPORT
OF
THE BRITISH ASSOCIATION
FOR
THE ADVANCEMENT OF SCIENCE.
1832.
[page 94]
[page 95]
SECOND REPORT.
PROCEEDINGS
OF
THE GENERAL MEETING.
1832.
ON Monday, the 18th of June 1832, the British Association commenced its sittings at Oxford, in the rooms of the Clarendon Buildings, and proceeded to the election of candidates, recommended by the General Committee. In the Evening a numerous assembly of Members met together in the same apartments.
On Tuesday, at one o'clock P.M., the Chair was taken in the Theatre, by the President, Viscount Milton, who opened the business of the Meeting by a speech to the following effect:
"Gentlemen,
Feeling as I did, when called upon to preside over the first Meeting of this Association, the insufficiency of the individual chosen for that office, and knowing that the choice was to be attributed not to any merits or any desire of my own, but to the circumstance of my official connexion with the Society, by whose invitation that Meeting had been collected,—how much more must I feel sensible of the difficulty of the situation in which your kindness has placed me, when I find myself called upon to address you in this Theatre, within the walls in which are transacted the most important concerns of this great and august University! That difficulty, however, is much lightened by the consideration, that on the present occasion my almost only duty is to resign the office with which I have been invested, which will now devolve into hands more competent to wield it, and on shoulders whose strength is more capable of bearing its burthen. The difficulty also is lightened to me, Gentlemen, by the consideration that I am addressing an audience upon whom it
[page] 96
is unnecessary even to endeavour to press the importance of Associations, which have for their object to extend the bounds of human knowledge, and to give man a larger empire over nature.
I should, however, ill discharge the duty which is placed upon me as your organ at the present moment, and I should ill satisfy the wishes of my own mind, if I surrendered this office into the hands of my reverend and learned friend, who sits near me, without expressing the gratitude which I feel, in common I believe with every other Member of the Association, to the constituted authorities of this University, who have so kindly welcomed us here. Confident I am that they will never have reason to repent of the favour which they have shown us, in permitting the Meeting to assemble within these walls, but will reflect with well-founded satisfaction on the encouragement now afforded to an institution, the object and tendency of which is to promote the highest and most important interests of man;—I say, Gentlemen, his highest and most important interests: for were I to be asked what is the chief use of any new facts which we may be enabled to add to the stock of our knowledge, or what is the greatest value of any new inference which may be deduced from those facts of which we are already in possession, I should answer, that the principal use of such knowledge and such reasoning is to lead man to lift up his mind and his heart to his Maker; and in comparing his own inability (of which the greater is his knowledge, the deeper must his conviction be), in comparing the inability of the creature with the stupendous works of creation, to imbibe a deeper feeling of religious awe, and acquire a stronger sense of the reverence and duty which he owes to the power, the wisdom, and the beneficence of the Creator. It is on this ground especially that every reflecting mind will rejoice in the advancement of Science; and it is, I doubt not, to similar views of the value of every improvement in the knowledge of nature, that we are to ascribe the reception with which our Association is honoured in this ancient seat of learning and religion."
The President elect, the Rev. Dr. Buckland, then took the Chair, and addressed the Meeting in the following manner:
"My Lords, and Gentlemen,
I cannot enter on the duties of my office without acknowledging, with the deepest gratitude, the honour which the Association has conferred upon me, and without thanking my noble predecessor for the undeserved compliment which he has been pleased to pay me. The objects of this Meeting have been so fully, philosophically, and eloquently set forth, in the Report
[page] 97
of the Proceedings at York, by my reverend friend the late Vice-President, who has had so large a share in founding this Association, that I think it needless to occupy your time, either in explaining them, or in proving their importance. If any argument were necessary to justify the attempt now made to stimulate and combine the energies of science; if a doubt has existed on any man's mind as to the probability of its success,—I would only ask him to look round upon the present audience, and observe with how many and what manner of persons this Theatre is filled. Such an attendance leaves no room to fear that the Meeting should fail of its intended objects. Your presence, Gentlemen, adds an indisputable sanction to the proceedings of last year, and fulfils the warmest hopes which the promoters of the Association had indulged.
I will detain you no longer from the scientific business of the Meeting than may be necessary to apprise you of the regulations which the Committee has adopted for carrying it on in such a. method as may enable it to accomplish all its objects. The greatest facilities have been afforded to our proceedings by the authorities of the University, who have granted us every possible accommodation, and shown in all instances the most liberal disposition to promote our views. The Chancellor, Lord Grenville,—a name which I can never utter without grateful veneration,—when I had first the honour of communicating to him the proposal of the Association to hold the present Meeting in this place, was pleased instantly to reply, that it was his ardent desire to be enrolled among its Members. From that moment he has by all the means in his power seconded our wishes; and within these few days,—from his retirement, from that calm and peaceful retirement, in which, after the labours of an arduous political life, after exercising the highest functions of the statesman, he now enjoys the dignified repose of the scholar and philosopher,—he has expressed to me his heartfelt regret that he is debarred by the infirmities of advanced life from being present in Oxford at a moment so interesting as this. The Vice-Chancellor, Gentlemen, and the other Governors of the University, have placed at your disposal the Theatre in which we are now assembled, and the numerous rooms which have been lately fitted up for academical and scientific purposes in the Clarendon Building. These will be found very conveniently adapted to answer the exigencies of a Meeting, before which so great a variety of matter is to be brought: the business of your several Committees and Sectional Meetings will be separately distributed in them; the Committee of Mathematics and General Physics will meet in one room; that of Chemistry and Mineralogy in another; that of
G
[page] 98
Geology and Geography in a third; and that of Natural History and Physiology in a fourth. The Committees of these sciences will transact their own especial business, and hold their consultations, from ten to eleven in the Morning; and in the interval from eleven to one, Sectional Meetings of the whole body will be held, at which there will be read, before such Members of the Association as choose to assemble in any of the rooms, by the Secretary of each Committee, the papers which he has received on the subjects included under its denomination. At one P.M. the Meeting will daily adjourn to this Theatre; and here the Reports on the state and progress of different sciences will be read, which have been drawn up at the request of the Association. In the Evening, at nine o'clock, the Sectional readings will be resumed in the Clarendon Buildings, except on Thursday and Saturday, when Lectures will be delivered in the Music room on the late discoveries in Magnetism, and on Chemical and Geological subjects. Thus, Gentlemen, we hope to conduct the multifarious business of the Meeting, so as to accomplish three objects: first, to lay before the whole assembly the general views of the condition of science, to which it is desirable to invite the attention of all; secondly, to enable every one to listen to, and to join in, those scientific details in which he may be more particularly interested; and thirdly, to give instruction of a more popular nature, to a more miscellaneous audience. On Thursday morning, the University of Oxford will avail itself of the present opportunity to express the deep respect which it entertains for the improvers of science, by conferring on four Members of the Association, of preeminent celebrity in different branches of Philosophy, the highest distinction which it has the power to bestow; and when the ceremonial is concluded, in the afternoon of the same day, I would beg leave to offer to any of the Members who will do me the honour of accompanying me on an equestrian excursion, such familiar illustrations of Geology as the country round Oxford is able to afford."
Dr. Buckland then proceeded, after stating the arrangements which had been made for the personal accommodation and hospitable reception of the Meeting*, to call upon Professor Airy for his Report on the recent progress of practical and
* On Tuesday a public dinner was given to the Association by its Oxford Members, in the Hall of New College, granted for that purpose by the Warden and Fellows. On Wednesday Morning a public breakfast was given to it by the Vice-Chancellor, the Rev. Dr. Jones, in the Hall of Exeter College. Ordinaries, at five shillings a-head, were provided daily, to which venison was contributed by the Archbishop of York and the Duke of Buckingham. Refreshments were furnished to the Evening Meetings in the Clarendon Buildings, at the expense of the Oxford Members of the Association.
[page] 99
physical Astronomy, undertaken at the request of the former Meeting of the Association at York.
Professor Airy gave an account of the contents of his Report, and read those parts of it which he considered as possessing the most general interest.
Mr. Lubbock's Report on the present state of our knowledge respecting the Tides being next in order, the substance of it was delivered to the Meeting, in the absence of the author, by the Rev. William Whewell, and illustrated by the exhibition of a Map of the World on which Mr. Whewell had drawn the co-tidal lines passing through the points where it is high water at the same time.
On Wednesday, at one o'clock, the Meeting having re-assembled in the Theatre, the Chairman of the four Sub-committees read the minutes of the transactions of the Sectional Meetings.
At the conclusion of the minutes of the Geological Section, the President requested the Meeting to allow the Wollaston Medal, which had been awarded by the Geological Society to Mr. William Smith, to be delivered to him in the presence of the Members of the Association. The President of the Geological Society, Mr. Murchison, having in consequence presented the medal to him, in the name of that Institution, as a testimony of respect to the acknowledged "Father of English Geology," Mr. Smith expressed his gratitude for the high honour which had been conferred upon him in the Assembly of the British Association, and in the public Theatre of so distinguished a University,—an honour, he said, which was the more grateful to his feelings, from the circumstance of Oxfordshire being his native county. He little thought in his youth that so proud a moment as the present would ever arrive; and he trusted that his example and success would stimulate others to follow in the same course. In devoting himself to his geological pursuits, and opening a new page of knowledge, he had had the satisfaction of procuring the good will of many kind and indulgent friends; he hoped that he had served his country, and in so doing he had endeavoured to serve his God.
Professor Cumming being then called upon by the President, read a Report on Thermo-electricity.
Mr. Forbes gave an account of his Report on the present state of Meteorology, and read extracts from it.
Mr. Willis delivered a verbal Report on the present state of the Philosophy of Sound, illustrated by diagrams and musical experiments.
In the Evening, at nine o'clock, a Meeting was held in the
G 2
[page] 100
Music Room, and two lectures were delivered, the first by Dr. Ritchie on Magnetic Electricity, with reference especially to the recent discoveries of Mr. Faraday; the second by Dr. Turner on the General Principles of Chemistry.
On Thursday morning the University held a Convocation in the Theatre, for the purpose of conferring the honorary degree of Doctor in Civil Law on the under-mentioned Members of the Association:—
Sir David Brewster, K.H. LL.D. F.R.S. L. & E. Instit. Reg. Sc. Paris. Corresp.
Robert Brown, F.R.S. V.P.L.S. Instit. Reg. Sc. Paris. Corresp.
John Dalton, F.R.S. Instit. Reg. Sc. Paris. Corresp.
Michael Faraday, F.R.S., Instit. Reg. Sc. Paris. Corresp.
The Regius Professor of Civil Law, Dr. Phillimore, in presenting them to the Convocation, adverted to the distinguished services which they had respectively rendered to different departments of science, and the celebrity which they had acquired by their successful labours, not only in Great Britain, but throughout Europe, and expressed the high satisfaction felt by the University of Oxford in enrolling such illustrious names in the catalogue of her Members.
After the degrees had been conferred, a party of the Members of the Association accompanied Professor Henslow on a botanical excursion; and a numerous assemblage attended the President, to hear his Lecture on the Geology of the neighbourhood of Oxford. In the course of the Lecture Dr. Buckland took occasion to enforce the importance of Geological Science, as connected with agricultural improvement, and suggested that there might be great utility in an appointment, by the Geological Committee, of a Sub-committee to devote its attention to this object. He pointed out many defects in the ordinary system of drainage, and explained in what manner large tracts of land might in many cases be permanently drained at a small expense, by methods depending entirely on a knowledge of the structure of the strata. He adverted to the possibility of reclaiming the peat bogs in Ireland, distinguishing those which are capable of being reclaimed, from those where the outlay of capital must exceed any profitable return; and in speaking of Artesian wells*, suggested the advantage which
* The name of Artesian wells has been recently applied to those wells in which water is obtained by boring down and introducing tubes, through strata destitute of water, into a subjacent stratum, which is charged with it, in such a manner that it ascends through the tubes almost, or entirely, to the surface, forming in the latter case perpetual fountains, such as are made, and designated by the name of Blow wells, on the eastern coast of Lincolnshire. The practice is most available in low situations, where the upper stratum is a thick bed of clay, and has been of late years introduced in the neighbourhood of London. It is much used in Artois, the ancient Artesium, whence is derived the appellation of Artesian wells.
[page] 101
might be derived from a more general application of them in the neighbourhood of London.
On Friday, the President having taken the chair in the Theatre at the usual hour, the minutes of the Sectional Meetings were read by the Chairmen of the Committees. Mr. P. Duncan gave notice, that there were laid upon the table some Original Manuscripts from the Ashmolean Museum, recording the early Proceedings of the Philosophical Society which met at Oxford during the Civil Wars, and subsequently gave birth to the Royal Society.
An Abstract of a Report on the progress of Optical Science, by Sir David Brewster, was then read by one of the Secretaries.
Mr. Johnston read his Report on the progress which Chemical Science has recently made, especially in foreign countries.
Professor Powell read his Report on the state of our knowledge respecting the phænomena of Radiant Heat.
The Rev. William Conybeare gave a general account of the contents of his Report on the recent progress of Geology.
The Rev. Dr. Bliss and Mr. John Taylor were appointed to audit the accounts.
On Saturday, the Association having assembled in the Theatre for the last time, Mr. John Taylor made a Report on the state of the accounts, which was approved. The minutes of the proceedings in the Sectional Meetings to their close were read by their respective Chairmen.
Mr. Brunel gave a history of the attempt to carry a Tunnel under the Thames, illustrated by Drawings.
The Rev. William Whewell gave a sketch of the views contained in his Report on the recent progress and present state of Mineralogy.
An essay by Dr. Prichard, on the application of Philological inquiry to the Physical History of Man, was read by the Rev. William Conybeare.
The President then announced, that the place which had been fixed upon for the next Meeting was Cambridge; that the President who had been chosen was Professor Sedgwick; that
[page] 102
the Vice-Presidents elect were Dr. Dalton and Professor Airy; that Professor Henslow and Mr. Whewell had undertaken the duties of Secretaries for Cambridge, and the late Vice-President, Mr. Harcourt, those of General Secretary; and that Mr. John Phillips had been appointed to the office of Assistant Secretary. He added, that a Council had been nominated to direct the affairs of the Association during the interval which would elapse before the next Meeting of the General Committee.
The Rev. Professor Sedgwick said, that it would be at all times and in all situations one of his greatest pleasures to contribute his assistance to the British Association, and that he was willing to give any pledge for the zealous performance of the gratifying but arduous duty which had been imposed upon him, as far as his ability extended. He might have been overwhelmed, indeed, by the prospect of such a task, did he not feel confident in the cooperation of many distinguished Members of the University of Cambridge, possessing much greater powers than his own, and did he not believe that before the next Anniversary the organization of the Society would be so complete that his duties would be light when compared with those of the present President, whom he would take this opportunity of publicly thanking for the delightful manner in which he had presided over the Meeting, bringing the various elements of which it was composed into order and harmony, and diffusing sunshine through all its proceedings. He knew not how to express strongly enough the satisfaction which he had derived from this Meeting, or the delight which he had felt at being associated in such a place with such men as Dalton and Brewster, and Faraday and Brown, in honouring whom the University of Oxford had done honour to itself. Studies like those which had lately occupied the Society, consecrated by the principles which have pervaded it, could not but tend to elevate and purify the mind, to engender mutual friendship, mutual forbearance, mutual kindness and confidence; they prevented the growth of any bad feelings, and caused those which were good to germinate with the greatest luxuriance compatible with our nature. He looked forward with full assurance to the happy results of this union between men of similar sentiments and similar pursuits, who possess one common object,—the improvement of mankind by the promotion of truth; and he thanked the Association most cordially for the honour which it had conferred upon him in electing him to the high office of its President. At Cambridge they would endeavour to follow, though they could scarcely hope to rival, the example of hos-
[page] 103
pitality which had been set them at Oxford, and would receive the Meeting not merely with the forms of courtesy, but with the right-hand of fellowship. In one respect he believed that they would be more advantageously circumstanced as to the means of offering accommodation; for at the time when the Association would meet again, at the latter end probably of the month of June, they should be enabled, he trusted, to receive a large proportion of its Members within the College walls.
The Rev. Wm. Whewell said, that his services had been fully and freely given to the Association, and would be so given as long as they could be useful. He hoped that all who were assembled there would accept the offer of a cordial welcome to Cambridge; he hoped that all the Members of the Association in every part of the empire would equally accept it. "We are desirous," he added, "of seeing as many as possible from as many places as possible; we ask for the company of all who are cultivators of science or interested in its objects. I trust all such persons have felt here that they are united by one common tie; and with so large and united a body of zealous and active men, I think I may reasonably prophesy that the next Meeting will be a worthy successor to the brilliant and successful one which now soon must close."
The Marquis of Northampton moved the Thanks of the Meeting to the Vice-Chancellor, the Heads of Houses, and the other resident Members of the University, for the great hospitality and attention with which they had received the Association. The Resolution was seconded by Sir David Brewster, supported by the President of the Geological Society of London, Mr. Murchison, and passed unanimously.
The President said, that he could not allow the Meeting to quit the public Theatre, without adding on his own behalf, and on behalf of his fellow-academics who are Members of the Association, the expression of their grateful thanks to the Vice-Chancellor and the Heads of Houses, for the kind assistance which they had rendered them in promoting the objects and providing for the accommodation of the Meeting.
The Vice-Chancellor replied in the following manner. "On my own part, Sir, and on the part of the University, I beg leave to assure you, that we are most sensible of the advantages afforded to us by this visit of the British Association for the Advancement of Science, and that we have been most happy to have had it in our power to offer it any accommodation; and I will add, that whilst the pursuit of truth and the advancement of knowledge are its objects, and whilst it pursues those ends by the judicious rules which at present regulate its proceed-
[page] 104
ings, it cannot fail to command the good wishes, the respect and admiration of all, and most especially of those, whose institutions have connected them with the duties, and taught them to appreciate the value, of public instruction."
In the evening a Lecture was delivered in the Music Room, by the President, on the Fossil Remains of the Megatherium, recently imported into England from South America. The lecture was illustrated with Drawings by Mr. Clift.
Dr. Buckland began by stating, that the history of this animal is very remarkable. The Megatherium is most nearly allied to the Sloths, a family which presents an apparent monstrosity of external form, accompanied by many strange peculiarities of internal structure, which, before the discoveries of the immortal Cuvier, were but little understood. "Gentlemen," said he, "I cannot utter the name of Cuvier, and associate with it the term 'immortal', without being at once arrested and overwhelmed by melancholy and painful recollections of mortality. We have at this moment to deplore, in common with the whole philosophical world, the loss of the greatest naturalist and one of the greatest philosophers that have arisen in distant ages, to enlighten and improve mankind. The names of Aristotle, and Pliny, and Cuvier, will go down together through every age, in which natural history and physical sciences, in which philosophy and learning, and talent, and everything which, next to religion and morality, gives dignity and exaltation to the character of man, shall be respected upon earth. Gentlemen, I need not state to you how voluminous are the works of that exalted and most illustrious naturalist, whose recent and irreparable loss we now deplore. For nearly thirty years he has been the leader of that branch of natural philosophy which comprehends the structure and relations of all the kingdoms of animated nature. It was the genius of Cuvier that first established the perfect method after which every succeeding naturalist will model his researches; and which laid the foundation of that analytical process of investigation, of that most philosophical and accurate and uniform system of reducing every organ in every species to a fixed and certain type, which will enable his followers to extend their inquiries over the almost boundless regions of the organized world. He has shown that the frame and mechanism of every animal present an uniformity of design and a simplicity of purpose, which prove to demonstration that every individual, not only of existing species, but of those numerous and still more curious races which have lived and perished in distant ages, and of which our knowledge is due to discoveries in geology,
[page] 105
were framed and fashioned by the same Almighty hand, and were designed and contrived by the same Almighty mind. Gentlemen, to this great and good man not only are the sciences of natural history profoundly indebted, but the higher science of morals also owes a debt of deep and everlasting obligation, for that he has proved to demonstration the high and solemn truth to which I have alluded, viz.—the unity and universal goodness of the great Creator. Of this great man, so lately torn away from us by the mysterious and incomprehensible counsels of the Almighty, we now lament the loss; in the vigour of his mind, and almost in the fulness of his strength, at the age of sixty-three, he has been suddenly summoned to the grave, and the tears of Europe have not yet ceased to stream over his funeral. The gratitude of the great nation to whose philosophic fame his genius has added so bright a wreath, has already displayed itself by a liberal provision for his family, and has fixed his widow, during the remainder of her mortal life, in that honoured and well-known mansion, in the Jardin des Plantes, which during a quarter of a century has ever been open, in noble and friendly hospitality, to every son of Science assembled at Paris from every nation under heaven. The French nation has placed her there, a brief and perishable monument of their gratitude: they have resolved also that a splendid and more lasting monument shall be raised to her immortal husband, and have invited the whole philosophical world to partake in the honour of contributing to its erection. He has raised to himself a monument 'ære perennius,' a monument which will endure even when the pyramids are crumbled into dust;—but this does not absolve ourselves from the pleasing and pious duty of contributing our humble share to that monument of marble which a grateful nation and a grateful world are about to consecrate to his memory. —Gentlemen, I fear my feelings of respect, and love, and gratitude, have transported me beyond the limits which the task I have undertaken should impose on me; still I cannot but rejoice in the opportunity which this august assembly affords of inviting you to partake in this great and glorious work, and thus publicly to record your gratitude to that immortal man, whose friendship I have ever counted among the most distinguished honours of my life, and whose genius will, even by those who have not enjoyed this high and enviable privilege, be ever followed as their guide in the paths of science, so long as science shall be cultivated, or virtue venerated upon earth."— Returning to the subject of the Lecture, Dr. Buckland stated, that this monstrous animal, the Megatherium, has been brought to England by Woodbine Parish, Esq., His Majesty's
[page] 106
Consul at Buenos Ayres. It was discovered by a peasant who, passing along the river Salado in a dry season, threw his lasso at something he saw ·half-covered with water, and dragged on shore the enormous pelvis of this animal; the rest of the bones, consisting of the greater part of the skeleton, with many of the claws and teeth, were obtained by turning aside the current by means of a dam. Dr. Buckland proceeded to illustrate the peculiarities of its structure and mode of life, by reference to the peculiar organization and contrivances in its skeleton. This animal, and its kindred monster the Sloth, have been considered by Buffon and other naturalists to afford the greatest deviations from the ordinary structure of quadrupeds—deviations which they have viewed as indicating imperfection in their organization, without any compensating advantage. The object of the Professor's Lecture was to show, that these anomalous conditions and deviations are so far from being attended with inconvenience to the class of animals in which they occur, that they afford striking illustrations of those rich and inexhaustible contrivances of nature by which the structure of every created being is precisely fitted to the state in which it was intended to live, and to the office which it was destined to perform. The peculiarities of the Sloth which render its movements so awkward and inconvenient upon the earth, are adapted with much advantage to its destined office of living upon trees and feeding upon their leaves; the peculiarities of the Megatherium are not less wisely adapted to its office of feeding upon roots.
This animal was about 8 feet high and 12 feet long; its teeth, though ill adapted for the mastication of grass or flesh, are wonderfully contrived for the crushing of roots, with the further advantage of keeping themselves constantly sharp set by the very act of performing their work. The fore feet, nearly a yard in length, and exceeding a foot in breadth, were armed with three gigantic claws, each more than a foot long, and forming most powerful instruments for scraping roots out of the ground. The head and neck and anterior part of the trunk were comparatively light and small: its posterior proportions much exceeded the bulk of those of the largest elephant. The object of this apparently incongruous admixture of proportions, was to enable the creature to stand at ease on three legs, having the weight of its body chiefly supported by the hinder extremities, and one of its fore paws at liberty to be exercised without fatigue in the constant operation of digging roots out of the ground. A further peculiarity consists in the fact of its sides and back having been armed with a coat of mail like the
[page] 107
armadillos, which also obtain their food by the act of continual digging in the ground; this coat of mail exceeds an inch in thickness. The Professor suggested his opinion, that one use of the bony armour is to prevent the annoyance which this class of animals would feel, without some such protection, from the constant presence of sand and dirt with which the act of digging and scratching for their daily food would otherwise fill their skins; its further use may be to afford protection against the myriads of insects that swarm in the regions frequented by these animals, and also against beasts of prey.
The Professor concluded by stating, that this was but one of the many examples afforded by comparative anatomy of the inexhaustible richness of contrivances whereby Nature has adapted every animal to a comfortable and happy existence in that state wherein it was destined to move; and added, that the researches of Geology tended not only to afford similar examples of contrivance, indicating the wisdom, and goodness, and care of the Creator over all his works, but afforded also to natural theology a powerful auxiliary, showing from the unity of design and unity of structure, and from the symmetry and harmony that pervade all organic beings in the fossil world, as well as in the present, that all have derived their existence from one and the same Almighty and Everlasting Creator.
Professor Babbage then rose, and said, that before the Meeting separated he wished to express a feeling which he believed was general among the Members of the Association, that in the selection of the places at which the Annual Meetings were to be held, attention should be paid to the object of bringing theoretical science in contact with that practical knowledge on which the wealth of the country depends. "I was myself," said Mr. Babbage, "particularly anxious for this, owing as I do a debt of gratitude for the valuable information which I have received in many of the manufacturing districts, where I have learned to appreciate still more highly than before, the value of those speculative pursuits which we follow in our academical labours. I was one of those who thought at first that we ought to adjourn for our next Meeting to some large manufacturing town; but I am now satisfied that the arrangement which has been made will be best adapted to the present state of the Association. When, however, it shall be completely consolidated, I trust we may be enabled to cultivate with the commercial interests of the country, that close acquaintance which I am confident will be highly advantageous to our more abstract pursuits."
[page] 108
Mr. Murchison called the attention of the Meeting to the peculiar obligation which it lay under to one of its Members. "At our first Meeting, Gentlemen, in York, when the Institution was in its infancy and every difficulty hung around us, a Professor of this University came forward and undertook, on his own responsibility, that Oxford would open its gates to receive us. Delighted as we have been with the reception which we have experienced, and sensible how much the Association has been consolidated by this Meeting, we owe an acknowledgement of gratitude to Dr. Daubeny as the primary cause of our having assembled here."
Dr. Daubeny said, that from the situation which he occupied in the University, it was naturally to be expected that he should regard with peculiar interest the Meeting, in that place, of an Association which he considered calculated to form an important epoch in the history of British science. "The attachment," said the Professor, "which I entertain for the cause of science implies in my case no extraordinary merit, placed as I am in a situation of comparative independence, by my connexion with one of the great ecclesiastical establishments of the country. It is to those only who have pursued such studies without partaking of the advantages derived from academical institutions, or that patronage of Government which in other countries supplies their place, to whom the praise is due of a high degree of disinterestedness in preferring the attractions of philosophy to those of emolument. With respect to my office of Secretary, any credit which may be attached to the discharge of it belongs equally to my colleague Professor Powell, and the other Secretaries of the Association, and amongst them to one who I regret to find has been prevented by illness from attending this Meeting, I allude to Mr. Phillips, Secretary for York, an individual whom I regard with peculiar friendship, and to whom the Society was more deeply indebted at a much earlier stage of its progress than to me."
The Marquis of Northampton said, that the Meeting had that evening received from its President an excellent exemplification of the utility of scientific knowledge. "We have seen, Gentlemen, that an animal which has been represented even by Buffon as imperfect in its constitution, and almost incapable of enjoyment, appears, on a nearer and more accurate view of its habits and anatomy, to be no less happily framed, and adapted to its peculiar manner of living, than the other parts of the creation. Thus even in those cases which present difficulties in the way of superficial knowledge, a higher degree of acquaintance with nature is sure to find a satisfactory solution. I rejoice, Gen-
[page] 109
tlemen, in the success with which this great and important effort for the advancement of science has been crowned. Long may our Association flourish, and produce fruits for the benefit of others as well as of ourselves! I rejoice in its success, not only in an intellectual but in a moral sense; for I believe it may be the means of binding together all the portions of this great empire, and even of uniting other parts of the world in the same bond. It is a refreshing thing for a person like myself, to come from the metropolis, from the turmoils of political life, and meet with eloquence and ability dedicated entirely to the promotion of good-fellowship and truth, to see discussion deprived of its sting, and those who are elsewhere opposed, brought here into cordial intercourse with each other, and made to feel that on many points they are able to agree. The pursuits of science have a tendency to associate together the whole human family; and I cannot but remark with pleasure, that we have had at least one eminent individual from the United States of America among us at this time. I hope, Gentlemen, that our next Meeting at Cambridge may have more.We must remember, and I trust our Transatlantic brethren will remember, that they and we are sprung from the same race; that we speak the same language; that we equally rejoice in the possession of free though of different institutions; that their ancestors as well as ours were fellow-countrymen of Bacon and Newton, Milton and Shakespeare, and many other great men who have preceded us in science and literature. I hope that these feelings of mutual sympathy will ever exist between us and them; and I hope that the interests of science will form a bond of intercourse and union between us and all the other nations of the world, that wars and tumults at last may cease, and that our only emulation may be, who shall become the wisest, and who become the best."
The President then said, "Gentlemen, the hour is come for the adjournment of this most happy Meeting. I congratulate the University of Oxford on the compliment that has been paid it by the presence of so many distinguished and illustrious strangers, who have honoured us with their company on this ever-memorable occasion. I congratulate the Association on the perfect harmony which has pervaded its Meetings, and on the vast and inestimable utility which is likely to result from its operations; I congratulate the British nation that it possesses such a Society, comprehending a host of individuals not only qualified, but prompt and ready, to come forward and promote the general interests of science. Gentlemen, I congratulate each individual here present, on the enjoyment of what I con-
[page] 110
sider one of the highest gratifications of which our nature is capable,—the enjoyment of that personal knowledge and familiar intercourse, which this Meeting has afforded, with those whose kindred minds and congenial pursuits have been long familiar to us through the medium of their works; the enjoyment of being thus brought into friendly contact and brotherly association, with those whom we have long esteemed and loved and venerated from a distance; the enjoyment of being thus enabled, though but for a short, yet a most delightful week, to hold sweet counsel and communion together in these our palaces of peace. Gentlemen, it is now my painful duty to announce, that the moment of separation is arrived; it is my more grateful task to remind you, that we are to re-assemble at Cambridge in the latter part of the month of June next year."
[page] 111
PROCEEDINGS
OF
THE GENERAL COMMITTEE.
ON Monday the 18th of June the General Committee made the necessary arrangements for transacting the business of the Meeting; appointed a Treasurer; recommended Candidates for election; and granted to Foreigners and to certain other individuals gratuitous tickets of admission to the Meetings.
On the Saturday following, the Treasurer reported the state of the accounts. The General Committee appointed the Trustees in whom the property of the Association was to be vested; selected the place of meeting for the ensuing year; chose the new Officers of the Association; and nominated a Council to transact the business in the intervals between the Meetings. The Recommendations of the Committees of Sciences were presented to the General Committee, and adopted. A Report of the Proceedings of the Meeting was ordered to be published.
TRUSTEES OF THE ASSOCIATION.
Charles Babbage, K.H. F.R.S. Lucasian Professor of Mathematics, Cambridge.
R. I. Murchison, F.R.S. President of the Geological Society.
John Taylor, F.R.S. Treas. G.S. &c.
OFFICERS.
President.—Rev. William Buckland, D.D. Canon of Christ-church, F.R.S. G.S. &c. Professor of Geology and Mineralogy, Oxford.
President elect.—Rev. Adam Sedgwick, F.R.S. G.S. &c. Woodwardian Professor of Geology, Cambridge.
Vice-Presidents.—Sir David Brewster, K.H. LL.D. F.R.S. L. & E. &c. Instit. Reg. Sc. Paris. Corresp. Rev. William Whewell, F.R.S. G.S. &c. Secretary to the Cambridge Philosophical Society.
Vice-Presidents elect.—G. B. Airy, F.R.S. Professor of As-
[page] 112
tronomy, Cambridge. John Dalton, F.R.S. Pres. of the Lit. and Phil. Soc. of Manchester, Instit. Reg. Sc. Paris. Corresp.
Treasurer.— John Taylor, F.R.S. Treas. G.S. &c.
General Secretary.—Rev. William Vernon Harcourt, F.R.S. G.S. &c.
Assistant Secretary.—John Phillips, F.G.S. &c. Secretary to the Yorkshire Philosophical Society.
Secretaries for Oxford.—Charles Daubeny, M.D. F.R.S. Professor of Chemistry. Rev. Baden Powell, F.R.S. Savilian Professor of Geometry.
Secretaries for Cambridge.—Rev. J. S. Henslow, F.R.S. Professor of Botany. Rev. William Whewell, F.R.S.
Secretaries for Edinburgh.—John Robison, Sec. R.S.E. &c.
Secretary for Dublin.—Rev. Thomas Luby.
COUNCIL.
Sir David Brewster, K.H. LL.D. F.R.S. L. & E. Instit. Reg. Sc. Paris. Corresp. Robert Brown, D.C.L. F.R.S. V.P.L.S. Instit. Reg. Sc. Paris. Corresp. M. I. Brunel, F.R.S. Instit. Reg. Sc. Paris. Corresp. William Clift, F.R.S. &c. J. R. Corrie, M.D. F.G.S. James D. Forbes, F.R.S.E. &c. Davies Gilbert, D.C.L. V.P.R.S. &c. J. H. Green, F.R.S. Sir John Herschel, K.H. F.R.S. &c. W. R. Hamilton, F.R.S. Astronomer Royal of Ireland. W. J. Hooker, M.D. Professor of Botany, Glasgow. Rev. B. Lloyd, D.D. Provost of Trinity College Dublin. Rev. George Peacock, F.R.S. J. C. Prichard, M.D. F.R.S. Rev. William Scoresby, F.R.S. L. & E. Instit. Reg. Sc. Paris. Corresp. J. S. Traill, M.D. F.R.S. N. A. Vigors, D.C.L. F.R.S. &c. Ex officio members,—The Trustees and Officers of the Association.
Secretaries.— Edward Turner, M.D. F.R.S. L. & E. Rev. James Yates, F.L.S. G.S.
COMMITTEES.
I.Pure Mathematics.—Mechanics, Hydrostatics, Hydraulics.—Plane and Physical Astronomy.—Meteorology, Magnetism, Philosophy of Heat, Light, and Sound.
Chairman.—Davies Gilbert, D.C.L. V.P.R.S. F.G.S. &c.
Secretary.—Rev. H. Coddington, F.R.S. G.S. &c.
G. B. Airy, F.R.S. &c. Professor of Astronomy, Cambridge. Charles Babbage, F.R.S. Lucasian Professor of Mathematics, Cambridge. Sir David Brewster, K.H. LL.D. F.R.S. L. & E. &c. Lieut. Gen. Sir Thomas M. Brisbane, K.C.B. F.R.S. L. & E.
[page] 113
Instit. Reg. Sc. Paris. Corresp. David Forbes, F.R.S. L. & E. Mr. E. W. Gill. William Gray, jun. Sec. Y.P.S. Rev. R. Greswell, F.R.S. W. R. Hamilton, F.R.S. &c. Astronomer Royal for Ireland. George Harvey, F.R.S.E. F.G.S. Eaton Hodgkinson, Memb. Manch. Soc. Rev. Thomas Jarrett, Professor of Arabic, Cambridge. Robert Murphy, F.C.P.S. Rev. George Peacock, F.R.S. F.G.S. Rev. Baden Powell, F.R.S. Savilian Professor of Geometry, Oxford. Richard Potter, jun. S. P. Rigaud, F.R.S. Savilian Professor of Astronomy, Oxford. Rev. Robert Willis, F.G.S. Rev. Robert Walker, F.R.S. Charles Wheatstone. Rev. William Whewell, F.R.S. F.G.S.
II.Chemistry, Mineralogy, Electricity, Magnetism.
Chairman.—John Dalton, D.C.L. F.R.S. Instit. Reg. Sc. Paris. Corresp.
Secretary.—-James F. W. Johnston, M.A.
Henry James Brooke, F.R.S. F.G.S. John George Children, F.R.S. Rev. James Cumming, F.R.S. F.G.S. Professor of Chemistry, Cambridge. J. F. Daniell, F.R.S. Professor of Chemistry, King's College. Michael Faraday, D.C.L. F.R.S. Charles Daubeny, M.D. F.R.S. F.G.S. &c. Professor of Chemistry, Oxford. William Gregory, M.D. F.R.S.E. William Snow Harris, F.R.S. Rev. W. V. Harcourt, F.R.S. F.G.S. &c. W. H. Miller, F.G.S. Professor of Mineralogy, Cambridge. William Prout, M.D. F.R.S. Rev. William Ritchie, LL.D. F.R.S. Professor of Natural Philosophy, University of London, and the Royal Institution. Rev. William Scoresby, F.R.S. L. & E. Instit. Reg. Sc. Paris. Corresp. Mr.W. Sturgeon. Edward Turner, M.D. F.R.S. Sec. G.S. Professor of Chemistry, University of London.
III.Geology and Geography.
Chairman.—R. I. Murchison, F.R.S. &c. President of the Geological Society.
Secretary.—John Taylor, F.R.S. Treasurer of the Geological Society.
Sir T. D. Acland, Bart. F.H.S. Rev. William Buckland, D.D. V.P.R.S. Professor of Geology and Mineralogy, Oxford. Rev. W. D. Conybeare, F.R.S. V.P.G.S. Instit. Reg. Sc. Paris. Corresp. Viscount Cole, F.R.S. G.S. Joseph Carne, F.R.S. G.S. M.R.I.A. Sir Philip de Malpas Grey Egerton, Bart. F.R.S. G.S. W. H. Fitton, M.D. F.R.S. G.S. Robert W. Fox. G. B. Greenough, F.R.S. L.S. G.S. William Hutton, F.G.S. Sir Charles Lemon, Bart. F.R.S. G.S. G. Mantell, F.R.S. G.S. The Marquis of Northampton, F.G.S.
H
[page] 114
Rev. A. Sedgwick, F.R.S. G.S. Professor of Geology, Cambridge. William Smith. Major-general Stratton, C.B. F.R.S. Rev. James Yates, F.L.S. G.S.
IV.Zoology, Botany, Physiology, Anatomy.
Chairman.—Rev. P. B. Duncan, F.G.S. Keeper of the Ashmolean Museum, Oxford.
Secretary.—Rev. J. S. Henslow, F.L.S. F.G.S. Professor of Botany, Cambridge.
R. Brown, D.C.L. V.P.L.S. &c. William John Burchell, F.L.S. S. D. Broughton, F.R.S. G.S. Rev. William Clark, F.G.S. Professor of Anatomy, Cambridge. William Clift, F.R.S. G.S. Rev. W. L. P. Garnons, F.L.S. C. Henry, M.D. Rev. L. Jenyns, F.L.S. John Kidd, M.D. F.R.S. Reg. Professor of Medicine and Anatomy, Oxford. J. C. Prichard, M.D. F.R.S. Joseph Sabine, F.R.S. Richard Taylor, Sec. L.S. F.G.S. N. A. Vigors, D.C.L. F.L.S. G.S. Sec. Z.S. G. Williams, M.D. Professor of Botany, Oxford.
[page] 115
RECOMMENDATIONS
OF
THE COMMITTEES.
COMMITTEE FOR MATHEMATICS, &c.
THE Committee recommend that REPORTS should be applied for by the Association on the following subjects:—
I. On the recent theoretical and practical history of the Pendulum.
II. On the present state of the analytical theory of Hydrostatics and Hydrodynamics; stating how far the leading problems recently discussed have been solved theoretically,—on what suppositions,—and what remains wanting to complete the solution.
III. On the present state of our knowledge of Hydraulics as a branch of Engineering; stating whether it appears from the writings of Dutch, Italian, and other authors, that any general principles are established on this subject; what they are; and what are the points contested among authors.
IV. On the present state of our knowledge of the Strength of Materials; whether from the experiments of various authors any general principles have been obtained; what these are; how modified in their application to different substances; and what are the differences of opinion which prevail among authors on this subject.
V. On the state of our knowledge respecting the Magnetism of the Earth.
The Committee recommend as a subject for examination, the law of the Resistance of Water to bodies in motion.
The Committee recommend,—That a request be made to the Portsmouth Philosophical Association, through its deputies, to institute a series of hourly meteorological observations at that place.
That the Secretaries of the Yorkshire Philosophical Society
H 2
[page] 116
be requested to continue their observations on the Quantity of Rain which falls at different heights.
That the invitation to investigate fully the theory of the wet bulb Hygrometer be renewed.
That persons who may have opportunities of travelling on mountains or of ascending in balloons, be invited to observe the state of the Thermometer and the dew point Hygrometer below, in, and above the clouds; and to determine how the different kinds of clouds differ in these respects.
That the fourth Sub-section of this Committee be requested to procure Reports and Researches to be made on the following subjects.
I. On the connexion of Vaporization with Pressure.—On the improvement of the Thermo-barometer as to accuracy and portability.—On the correction for Humidity in the barometrical measurement of heights.
II. On the improvement of Thermometers for rendering them sensible to minute impressions of radiant heat.—On the alleged Polarization of Heat.—On the effect of transparent Screens upon Heat.
III. On the phænomena considered as opposed to the undulatory theory of Light.—On the improvement of the Achromatic Telescope.
COMMITTEE FOR CHEMISTRY, &c.
The Committee recommend,—That Dr. Dalton and Dr. Prout be requested to institute experiments on the specific gravities of Oxygen, Hydrogen and Carbonic Acid, and to communicate their results to the next Meeting.
That Dr. Turner be requested to extend his researches into the atomic Weights of the elementary bodies, and to report to the next Meeting, on the progress recently made in this branch of chemical science.
That Mr. Johnston be requested to report to the next Meeting, on any additional evidence which may be deduced from his own experiments, or those of others, in support of the new theory of the Sulphur Salts.
That Professor Miller be requested to determine the form and optical characters of those Crystallized Bodies which have not been examined by Mr. Brooke and Mr. Levy, and that Chemists be invited to send him specimens of perfect artificial Crystals.
That the Rev. Wm. Whewell, Mr. Brooke, Professor Miller, and Dr. Turner, be requested to cooperate in prosecuting and
[page] 117
promoting the following inquiries, with a view to examine the theory of Isomorphism, and the connexion between the crystalline forms and chemical constitution of Minerals:—
I. To determine whether the angles of varieties of the same species (in the usual acceptation of identity of species,) are identically the same, under various circumstances of colour, appearance, and locality; and if not, what are the differences.
II. To determine the chemical constitution of such varieties, —the specimens, mineralogically and chemically examined, being in all cases the same.
III. To determine what quantity of extraneous substances may be mixed with a crystalline salt, without altering its form.
IV. To determine the angles of the various species or varieties of isomorphous or plesiomorphous groups,—and their respective chemical composition.
That a list be drawn up and printed of substances considered isomorphous* and of those considered isomeric†.
List of simple substances and binary compounds presumed to replace each other.
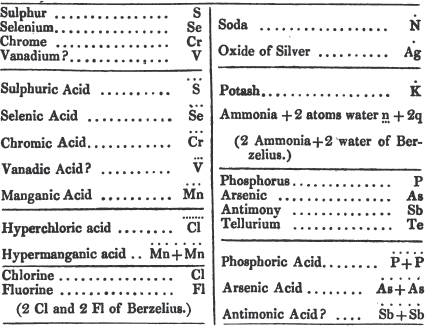
* The lists of isomorphous substances have been drawn up for the Committee by Professor Miller.
† For the list of isomeric substances, see the Report on Chemistry.
[page] 118

Isomorphous Groups.
O. Octahedral. Q. Square prismatic. R. Rhombohedral. P. Prismatic. P′. Oblique prismatic. P″. Doubly oblique prismatic.

[page] 119
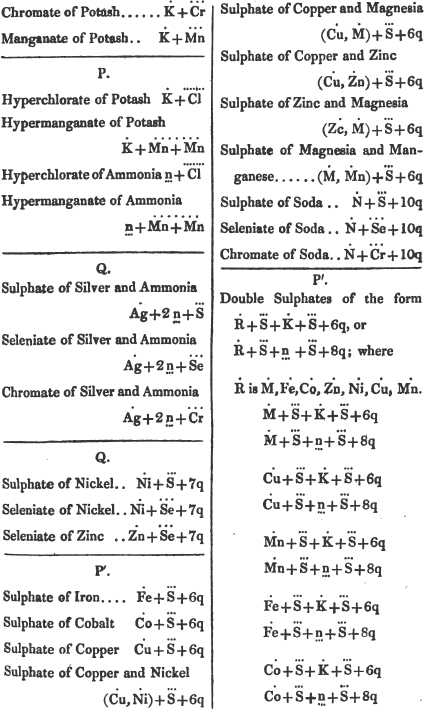
[page] 120

[page] 121
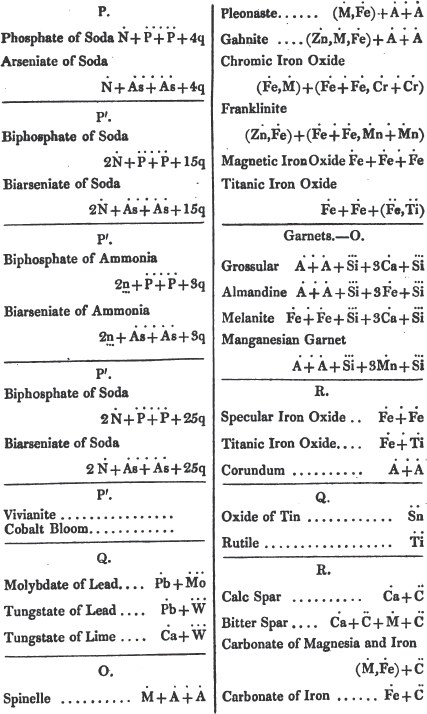
[page] 122

COMMITTEE FOR GEOLOGY, &c.
The Committee recommend,—That Mr. John Taylor be requested to prepare for the next Meeting, a Report on the state of our knowledge of Mineral Veins.
That the attention of Geologists be invited to the Faults or Dykes in the carboniferous rocks in Flintshire, with a view to ascertain whether some remarkable differences in their character may not be observed, as compared with that of veins and dykes in other districts.
That the attention of Geologists be invited to those coal districts in the midland counties of England, where the Carboniferous Limestone and Old Red Sandstone being deficient, the coal measures rest immediately on the Grauwacke and Transition rocks;—with a view to discover whether any circumstances connected with the physical structure of that part of the island can be stated, explanatory of the local absence of the two great formations above mentioned.
That Mr. John Taylor be requested to collect detailed sections of the Carboniferous series of Flintshire, with a view to a comparison with the same series in other parts of England;— with a view also of ascertaining the circumstances under which the Mountain Limestone is developed, after its suppression in certain coal-fields in the central parts of England.
That sections and plans should also be collected of the Coal-
[page] 123
fields of Worcestershire, Shropshire, Staffordshire, Cheshire, Lancashire, and the South Western part of Yorkshire.
That inquiries should be made as to the existence of the Wealden formation in the midland counties of England, and particular attention paid to the character of the Organic Remains.
That a detailed examination of any River in Great Britain should be instituted;—with a view to compare the outline of its bed, with that of the valley in which it runs.
That the quantity of Mud and Silt contained in the water of the principal rivers in Great Britain should be investigated; distinguishing, as far as may be possible, the comparative quantity of sediment from the water at different depths, in different parts of the current, and at different distances from the mouth of the river; distinguishing also any differences in the quality of the sediment, and estimating it at different periods of the year;—with a view of explaining the hollowing of valleys, and the formation of strata at the mouths of rivers.
That the experiments of the late Mr. Gregory Watt, on the fusion and slow cooling of large masses of Stony Substances, should be repeated and extended by those who, from proximity to large furnaces, have an opportunity of trying such experiments on a large scale; and that trial should be made of the effect of long-continued high temperature, on rocks containing petrifactions, in defacing or modifying the traces of organic structure.
That the Members of the Association be requested to institute experiments, for the purpose of ascertaining whether the continued action of steam or of water at a high temperature, is capable of dissolving or altering minerals of difficult solution.
That a request be made to the Board of Ordnance, that the scale of colours adopted in the geological colouring of the Ordnance Maps may be published for general information.
COMMITTEE FOR ZOOLOGY, &c.
The Committee recommend, That REPORTS should be applied for by the Association on the following subjects:
On the recent progress and present state of Zoology.
On the recent progress and present state of Animal Physiology.
[page 124]
TREASURER'S ACCOUNT.
| Dr. | Cr. | ||||||
| 1831. | £. | s. | d. | 1831. | £. | s. | d. |
| Compositions received from 49 Members | 245 | 0 | 0 | Payments at York by Jonathan Gray, Esq. provisional Treasurer, as audited at the Annual Meeting at Oxford, viz. | |||
| Annual Subscriptions from 47 Members | 47 | 0 | 0 | Printing, Stationery, | 95 | 12 | 5 |
| Carriage of Reports, | 3 | 16 | 8 | ||||
| Balance paid to John Taylor, Esq. | 192 | 10 | 11 | ||||
| £292 | 0 | 0 | £292 | 0 | 0 | ||
| 1832. | £. | s. | d. | 1832. | £. | s. | d. |
| Balance from preceding Account | 192 | 10 | 11 | Paid on Account of Expenses at Oxford;— (particulars tobe stated and audited next year) | 112 | 4 | 0 |
| Compositions received from 99 Members | 495 | 0 | 0 | Paid to London Secretary | 1 | 8 | 9 |
| Annual Subscriptions from 435 Members | 435 | 0 | 0 | Postages, &c. to Bankers | 0 | 7 | 1 |
| Purchase of 1000l. s percent consols, vested in the names of the Trustess | 836 | 5 | 0 | ||||
| Composition not yet received | 5 | 0 | 0 | ||||
| Balance in hand, Oct. 1832 | 167 | 6 | 1 | ||||
| £1122 | 10 | 11 | £1122 | 10 | 11 | ||
JOHN TAYLOR, Treasurer.
[page] 125
TRANSACTIONS.
Report on the Progress of Astronomy during the present Century. By G. B. AIRY, M.A., F.R. Ast. Soc., F.G.S., Fellow of the American Academy of Arts and Sciences; late Fellow of Trinity College, Cambridge; and Plumian Professor of Astronomy and Experimental Philosophy in the University of Cambridge.
THE "Committee for Mathematical and Physical Science" of the British Association having done me the honour to desire of me a Report on the progress of Astronomy, I could not but cheerfully comply with their request as far as I was able, though conscious that my Report must in many respects be imperfect. And I must beg the indulgence of the Society for any omissions or erroneous views; and must request them to attribute such, in part, to the circumstance that my own connexion with Astronomy is of short standing, and that since that connexion began I have been much occupied with the minute duties attached to the care of an Observatory, as well as with other official business of a very different kind.
I propose to take a brief survey of the progress of Astronomy since the beginning of the present century. That time must always be regarded as one of the most important epochs in the history of Astronomy. The English theodolite and the French repeating circle had been several years invented, and the advantages of circles were generally recognised; the principal part of the Mécanique Céleste was published, and the theory of perturbations, and especially of inequalities of long period, was beginning to be well understood. But, besides that I have the advantage of commencing from an almost definite point in the history-of the science, I feel that the progress of Astronomy since that time has been such that a correct statement of it must be highly interesting. I will not say that any discoveries of observation within the present century can be compared in general importance to the discovery of aberration and nutation;— that any theoretical discovery of the present century is equal to that of the great inequality of Jupiter and Saturn, or the
[page] 126
acceleration of the Moon's motion,—or that any single effort has been made like that which sent expeditions to Peru and to Lapland: But I will undertake to say, that in no similar period has greater progress been made in the increased number and excellence of observations; in the accuracy of the methods of treating them; in the examination and extension of theory; in the improvement of our powers, both instrumental and mathematical; and, finally, in the diffusion of accurate knowledge, in the increase of the number of persons who are interested in the science, and in the facility of communication among Astronomers.
I shall arrange my Report under the following principal heads:
I. A short general history of institutions and periodical publications.
II. An account of some of the instruments principally in use.
III. A statement of the improvements in the catalogues of fundamental stars, including the discussions of the various corrections.
IV. An account of the more extended star-catalogues, with the tables for facilitating the corrections.
V. Notices upon the measures of double stars, the observations of nebulæ, &c.
VI. An account of the principal observations, tables, &c. of the Sun and Moon, the old planets and their satellites.
VII. History of the new planets and periodical comets: and of comets generally.
VIII. Account of measures whose object is to determine the figure of the earth.
IX. General history of physical theories.
X. Comparison of the progress of Astronomy in England with that in other countries.
XI. Suggestion of points to which it seems desirable that the attention of Astronomers should be directed.
I. At the beginning of the century, the Observatory of Greenwich was the only one (I believe,) in which observations were made on any regular system. The thirty-six stars selected by Dr. Maskelyne, the Sun, and the Moon, were observed on the meridian with great regularity; the planets very rarely, and only at particular parts of their orbits; small stars, or stars not included in the thirty-six, were very seldom observed. A mass of observations was thus accumulating which, though confined in its object, surpassed in regularity and accuracy, and perhaps in general value, any other observations made at that time. The observations also were published in the form in which
[page] 127
they were made, and this circumstance alone gave them great value. So little had this been formerly understood, that Bradley's original observations, and the first part of Maskelyne's, were considered private property; great delays had consequently occurred in the printing of them, and only the first part of Bradley's Greenwich observations was at this time published*. The concluding part, however, was published in a few years: and this work reflects honour on the liberality of the University of Oxford and the care of the Savilian Professors who superintended it. An uninterrupted series of observations thus existed, made on the same plan and at the same place, and published with the fullest details. But this statement cannot be extended to any other astronomical institution. Observations had been made at Oxford, and transcribed, but not published: observations had been made at Armagh, and a standard catalogue had been deduced. I know of no other public observatories in activity in this country; and few observations seem to have been made by private persons. On the Continent, the several observatories of Paris and that of Berlin were the most important. At the national Observatory of Paris observations apparently were not made on any regular plan, and were only published in the Connaissance des Temps; the immense collection of observations of small stars made principally at the Observatory of the Ecole Militaire, was however completely published in the Histoire Céleste. Many irregular observations made at Viviers, Montauban, Mirepoix, &c., were also published, seldom with great detail, in the Connaissance des Temps. In like manner the observations made at Berlin and the various German observatories were imperfectly published in the Berliner Jahrbuch and (I believe,) in the Vienna Ephemeris†: those made in Italy were abstracted in the Effemeridi di Milano‡, &c. Thus, besides the Greenwich Observations, there existed no regular repository of observations, or discussions of observations, except these Ephemerides and (occasionally) the Transactions of the Societies of London, Paris, Berlin, Petersburg, Turin, Modena, &c. The necessary
* Within a few months, the observations made by Bradley before his residence at Greenwich have been published, under the superintendance of Professor Rigaud, at the expense of the University of Oxford. They include the observations by which the most important of his discoveries were made.
† I have not been able to procure a copy of this work.
‡ As I shall have occasion frequently to cite these Ephemerides, it is proper to mention that the Connaissance des Temps and the Berliner Jahrbuch have generally been published three years in advance, and the Effemeridi di Milano one year in advance.
[page] 128
want of detail in these publications has in many instances deprived the observations of much of their value.
With the year 1800 commenced the publication of Zach's Monatliche Correspondenz: it continued without interruption to the end of 1813 (a year in which the order of almost every continental scientific publication is interrupted). Lindenau's Zeitschrift für Astronomie commenced in 1816, and finished with 1818; Zach's Correspondance Astronomique commenced in 1818, and terminated in 1826; and Schumacher's Astronomische Nachrichten, which commenced in 1821, exists still as an astronomical periodical. These works were published at intervals of one or two months (the last of them, as often as matter to fill a sheet could be obtained): and nothing perhaps has contributed more to the progress of the science; especially in those parts (as the observations of comets,) which were useless without immediate circulation. "What," asks Lindenau, "would have been the fate of the small planets, if the Monatliche Correspondenz had not then existed?" But besides the rapid communication of information, these journals also allowed of the publication of observations with greater detail, and of fuller exposition of theoretical or physical views. And in fact nearly all the astronomy of the present century is to be found in these works or in the Ephemerides of Berlin, Paris, or Milan. It is owing, I suppose, partly to political events, and partly to our small acquaintance (in general) with the German language, that the three most valuable of these periodicals and the Berlin Ephemeris have till lately been little known in England.
In 1814 the regular annual publication of the Königsberg Observations (by Bessel) was begun; as well as that of the Dorpat observations (by Struve); in 1820 that of the Vienna observations (by Littrow); and in 1826 that of the observations at Speier (by Schwerd). These are all the Observations regularly published on the Continent with which I am acquainted. One volume. comprising several years' observations, has been published at Paris, but it does not seem likely to be followed by any more; one also has been published at Turin. Several very valuable volumes of observations have also been published at Palermo (by Piazzi and Cacciatore), and, I believe, two at Abo (by Argelander). In all these the original observations are given as fully as in the Greenwich Observations, and some steps of the reductions are in general much more completely explained.
Nor has our own country in the mean time been idle. Soon after the present Astronomer Royal (Mr. Pond) succeeded to Dr. Maskelyne, the regular annual publication of. observations
[page] 129
was changed for quarterly publication. The establishment of assistants at the Greenwich Observatory has been gradually increased, till it now exceeds in numerical strength every other observatory (so far as I know,) in the world. The mass of observations which it produces, of a very laborious kind, but of the very highest value for their accuracy, exceeds those which any other institution has put forth. The plan of these observations is rather confined, but by no means so much as under Dr. Maskelyne: their results have been occasionally published, but without any intermediate step. The Observatory of Dublin, under the direction of Dr. Brinkley, assumed the highest importance. The observations have not been regularly published, but the results (accompanied sometimes with the original observations,) have appeared in various memoirs by Dr. Brinkley. These are confined to observations of the principal stars: but other observations I believe have also been made. In 1823 and 1824 an Observatory was erected at Cambridge: it has been placed successively under the super-intendance of Professor Woodhouse and of the author of this Report. Though at present it is only in part furnished with instruments, the regular publication of observations has commenced, and four volumes (the result of as many years' labour, commencing with 1828,) have appeared. The only difference between the plan of these and that of the others which I have described, is that the reductions are given at greater length; the observation of planets is made one of the principal objects of this Observatory. In 1826 the original observations of Tobias Mayer were published at the expense of the British Government, under the superintendance of M. Mosotti. Within three years the regular publication of observations made at Armagh by Dr. Robinson has been begun: they are nearly on the same plan as the Greenwich Observations.
In 1821 the British Government determined to found an Observatory at the Cape of Good Hope: and Mr. Fallows was immediately sent out with some small instruments. The erection of the Observatory was not completed till 1828: and the two most important instruments arrived in 1829. The observations made by Mr. Fallows have not yet been published: but I have seen them in manuscript, and I can assert them to be most valuable. His successor, Mr. Henderson, has it in contemplation not only to continue the independent observations peculiar to a southern latitude, but also to observe regularly in concert with European astronomers. Let us hope that the publication of Mr. Fallows's observations will not be delayed, and that provision will be made for the regular appearance of
I
[page] 130
his successors', in the same manner as those of European observers.
One addition to our astronomical establishments, the gift of an individual, is yet to be mentioned. In 1822 Sir Thomas Macdougall Brisbane, soon after his appointment as governor of New South Wales, founded an Observatory at Paramatta, and furnished it with excellent instruments. By his personal attention, and by the activity of the assistants whom he procured, a series of valuable observations has been produced. On his leaving the station, he presented the instruments, &c. to the British Government, on condition that the Observatory should be maintained in an efficient state. The condition was accepted, and an observer (Mr. Dunlop) is now maintained by the British Government at this distant station. Among all the instances that we have mentioned, there is not one which reflects higher lustre upon the motives which caused its establishment.
Observatories have also been founded by the East India Company at Madras, Bombay, and St. Helena. The observations made at the first of these places have been published by the Company.
I regret that I cannot attempt to give any accurate history of the increased number and the improvements of Continental Observatories during this period. Several new ones have been erected; several have been much improved both in the character of their buildings (the situation being in some instances changed from an upper story to the ground floor,) and in the nature of the instruments, especially by the general introduction of circular instruments.
At none of these (excepting Königsberg and Vienna, and perhaps one or two more,) is the system of observation so regular as at Greenwich.
The following list includes all the public Observatories with which I am acquainted:—Greenwich, Oxford, Cambridge, Edinburgh, Dublin, Armagh, Cape of Good Hope, Paramatta, Madras, Bombay, St. Helena, Paris, Marseille, Geneva, Turin, Milan, Padua, Bologna, Modena, Naples, Palermo, Abe, Altona, Bremen, Christiana, Dorpat, Copenhagen, Königsberg, Berlin, Gotha, Mannheim, Speyer, Munich, Göttingen, Vienna, Cracow, Warsaw, Wilna, Ofen, Kremsmünster. The Observatories of Brussels and Cadiz, and perhaps some in the above list, are not yet in full activity. I am not aware that there is any public Observatory in America, though there are some able observers.
In 1820 the Astronomical Society of London (now the Royal Astronomical Society,) was founded: and this event, whether
[page] 131
considered as an indication of public feeling on the subject of Astronomy, or as a means for promoting the science, must be considered as most fortunate. Astronomy in England has undoubtedly received a strong impulse from the institution of this Society; and the four volumes of Memoirs which it has published contain some of the most valuable contributions to Astronomy that any country has yet produced.
I must here observe, that nothing appears to me to prove more strongly the extension of accurate science than the increased demand for original observations. Astronomers are now sensible that though observations may be reduced, and the results exhibited in the form most valuable at the time of publication, future researches will generally give the means of improving them; and that the opportunity of doing this will be lost, except the observations are published in the shape in which they are made.
I have spoken above of the Astronomical Ephemerides, without allusion to that which forms their distinctive character. The Nautical Almanac had been followed by most of the others in the system of giving with accuracy only the places of the sun and the moon and principal stars, and such quantities as were necessary for nautical observations; the places of the planets being exhibited very roughly. In two works published by Schumacher (beginning with 1822,) the places of the planets were given more accurately. In the Berliner Jahrbuch for 1830 not only was the accuracy of the solar and lunar places increased, but the places of the planets were given with the same accuracy. Mean time was also adopted in every part, to the exclusion of appaŕent time. The comparison of observations with tables becomes thus an easy work. The English Government, by the advice of the Astronomical Society, have determined on making this improvement in the Nautical Almanac: and the volume for 1834 will appear with these alterations. Among the many additions made by Encke to practical astronomy, the example which he has thus set is not the least.
II. At the epoch from which this Report commences, the mural quadrants were still the only instruments (assisted by the use of the zenith-sector,) for observing zenith-distances, at the Greenwich Observatory, and at most of the Continental Observatories. At Palermo, however, a reversible circle or "altitude and azimuth instrument," of six feet diameter, was in the hands of Piazzi: and a similar instrument was in preparation for the Dublin Observatory, and was mounted early in the century.
I 2
[page] 132
A smaller instrument of the same construction, well known as the Westbury Circle, was in the hands of Mr. Pond: and by the use of this, it may be asserted, the errors of the large Greenwich quadrants were first completely established. In 1809 Mr. Troughton published in the Phil. Trans. an account of his method of dividing circles; and this may be considered as the greatest improvement ever made in the art of instrument-making. The general principle is to make a number of temporary points very near the places of many of the graduations, to compare by microscopes the distances between every pair, and when the errors are found numerically, to set off by a simple apparatus the permanent points at the proper distances from the temporary points*. In 1812 the first mural circle (by Troughton) was erected at Greenwich; and this is an important epoch in the history of Astronomy. I conceive that no instrument but the reversible circle can compete with Troughton's mural circle; and between these I cannot presume to decide. It must be observed that, as the mural circle was first intended to be used, the objects of these instruments were somewhat different. The reversible circle could be turned round its vertical axis in a few minutes, and the deviation of the axis from perfect verticality could be ascertained by the plumb-line; and the body under observation being observed in both positions of the circle, its zenith-distance was directly found (a small correction being necessary to obtain its meridian-zenith-distance, as both observations could not be made on the meridian). But the mural circle, like the mural quadrants, had no reference to the zenith: it could give only the polar distance of heavenly bodies, the position of the instrument corresponding to an observation of the celestial pole being found by observing circum-polar stars above and below the pole. To remedy this want (sometimes felt as an inconvenience,) Mr.Pond introduced (about 1821,) the system of observing sometimes the image of the heavenly body seen by reflection from the surface of mercury. In 1825 another instrument of exactly the same kind was erected; and now the system may be said to have reached its perfection. Whenever the weather permits, the same object is observed directly with one circle, and by reflexion with the other. The determination of zenith-distances from the combination of these observations, though laborious, must I think be unrivalled in point of accuracy.—Several circles on Troughton's plan have been sent to continental observatories.
* Several methods, slightly different, have been founded on this of Troughton's. I do not know what method the continental artists employ.
[page] 133
It had long been thought that an instrument might be constructed which would enable an observer to take at once the transit and the zenith distance of a celestial body. Several such had been made in England; one is particularly described in Wollaston's Fasciculus; and one is well known as the instrument used by Mr. Groombridge. It consists of two parallel circles, firmly braced together, and fixed to an axis similar to the axis of a transit instrument: the telescope also passes through this axis and between the two circles, and it rests near both its extremities on the braces connecting the two circles. The graduations on the circles are read (as in the large instruments,) by microscopes with moveable wires. This I should conceive to be an excellent instrument. In Germany, however, a very different instrument has been constructed with the same object, and (principally through its use by Bessel,) has obtained considerable celebrity. Reichenbach's circle* was first constructed (I believe) about 1820, and a considerable number of instruments of this construction have since been made for observatories in all parts of the Continent. In a journey in the North of Italy in the year 1829, I saw and examined several exactly similar to Bessel's. To the extremity of the axis of a transit instrument, a graduated circle is fastened; this circle turns accurately around another circle carrying on its circumference four verniers (the line of separation between the two circles being almost imperceptible): and this vernier-circle has a fixed as well as a reversible spirit-level, to show how much it deviates from a fixed position. One pivot of the graduated circle passes within that of the vernier-circle, and the latter rests upon the Y: at the opposite end the pivot of the graduated circle rests immediately on the Y. To prevent friction, each extremity of the transitaxis is supported by a lever-counterpoise, and the vernier-circle is supported by an independent lever-counterpoise: and, to prevent flexure of the telescope, each end is supported by a lever-counterpoise whose fulcrum is at the transit-axis. An instrument of this kind would I conceive be below mediocrity unless the workmanship were most exquisite (the German workmanship is very fine); and when made in the best possible way, I cannot but think that its mechanical structure is extremely weak. The first thing to be provided for in an instrument for measuring zenith distances is, that the circle and the telescope shall move together. In Troughton's mural circle this is insured by firmly fixing the telescope by its extremities to the limb of the circle, without any close connexion at the centre.
* I have seen several circles by Reichenbach constructed on different plans that which is used by Bessel is generally known by this name.
[page] 134
In Reichenbach's circle, the connexion is by three weak joints, where a failure of any one will spoil the instrument: and one of these is particularly liable to be strained, on account of the friction which must in time take place between the two circles. I ought not to omit that the Germans consider it a great advantage that the circle is cast in one piece.—It will easily be seen, that the use of this circle requires reversion: this cannot be done readily (as in the Dublin and Palermo circles), and therefore it is only done occasionally.
I have particularly described this instrument, because it is little known in this country, and because it will give a very good idea of the peculiarities of the German school of instrument-making. Its distinguishing features are these:
Telescopes are always supported at the middle, not at the ends.
Every part is, if possible, supported by counterpoises.
To these principles, every thing is sacrificed. For instance, in an equatorial the polar axis is to be supported in the middle by a counterpoise: this not only makes the instrument weak, (as the axis must be single,) but also introduces some inconvenience into the use of it. The telescope is on one side of the axis: on the other side is a counterpoise. Each end of the telescope has a counterpoise. A telescope thus mounted must, I should think, be very liable to tremor. If a person who is no mechanic, and who has not used one of these instruments, may presume to give an opinion, I should say, that the Germans have made no improvement in instruments except in the excellence of the workmanship.
The French repeating circle has lost much of the credit which it once enjoyed. Reichenbach's repeating circles have been much used, but in most instances by rejecting the principle of repetition; which converts them, in fact, into altitude-and-azimuth instruments. Of Reichenbach's universal-instrument, and several others invented here and on the Continent, I shall say nothing, because they do not seem likely to produce any influence on Astronomy.
Among those, however, which have been made as auxiliaries to the principal instruments, I must not omit to mention Capt. Kater's vertical collimator. The object of this instrument (whose construction is too well known to require description here,) is to supply a mark which shall be visible like a star, revolving in a very small circle round the zenith. By observing this in its north and south distances from the zenith, the reading of a circle corresponding to an observation of the zenith may be found, and thus the zenith distance of any heavenly body may be immediately obtained. Strong testimony has
[page] 135
been produced as to the accuracy of the results which may be expected from using this instrument.
In the equatorials of Dorpat and Paris, a clock-work motion has been given, I believe with great success, so as to keep the telescope steadily pointing on the same star. I have not had an opportunity of seeing either of these in a state of action.
Within a few years considerable improvements have been made in achromatic telescopes. A telescope of nine inches aperture was made by Lerebours: many small telescopes of great excellence, and one of more than nine inches aperture (for Dorpat), were made by Fraunhofer: two refractors by Cau-choix, of eleven or twelve inches aperture, have been imported into this country. All these are made on the common principle of the achromatic telescope. Mr. Barlow has turned his thoughts to the construction of telescopes in which the place of the flint-glass is supplied by a fluid lens (of sulphuret of carbon): and having succeeded with telescopes of six and eight inches aperture, proposes to attempt larger dimensions. I believe that none of these surpass in power or clearness the twenty-feet telescopes which Sir William Herschel and Sir John Herschel were in the habit of using; but the science has undoubtedly gained much by the diffusion of these powerful instruments.
In clocks I do not know of any improvement. Hardy's clock is found very useful with transit-instruments, for the loudness and sharpness of its beat; but for steadiness of rate it is probably inferior to the dead-beat which was in general use at the beginning of the century. The execution of chronometers (without any novelty of principle,) has been very greatly improved.
In the use of many of these instruments an improvement (as I consider it,) has very generally been introduced. It is now the rule at many observatories not to attempt mechanically to remove all the errors of the instrument, but to measure them (which can be done more accurately), and to apply numerical corrections to the observations. This innovation is due principally to the Germans.
III. At the beginning of the century the only good catalogue of stars was that of Dr. Maskelyne for 1790. In the last volume of his observations appeared his catalogue of the right ascensions and declinations of thirty-six stars for 1605. These were by far the most accurate places that had ever been produced. The amount of precession (combined for each star, with the proper motion of that star,) was determined by com-
[page] 136
paring these places with those obtained from Bradley's observations, as reduced by Dr. Maskelyne.
The determination of the mean declination of a star is independent of other observations, and depends only on the refraction and nutation at the time of observation (as in a series of observations the effect of aberration is nearly eliminated). For the mean 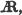 the observations are not independent; the position of the different stars with respect to each other is the subject of one determination, and that of all with respect to the sun, when he has a particular zenith distance, is the subject of another: the values of Æ are affected, therefore, by errors of refraction, as well as by nutation, and a complication of errors of observation. Maskelyne had used Bradley's table of refractions, and had used 9".55 as the coefficient of nutation in declination. The right ascensions were found by comparing all the other stars with α Aquilæ, and comparing α Aquilæ with the sun.
the observations are not independent; the position of the different stars with respect to each other is the subject of one determination, and that of all with respect to the sun, when he has a particular zenith distance, is the subject of another: the values of Æ are affected, therefore, by errors of refraction, as well as by nutation, and a complication of errors of observation. Maskelyne had used Bradley's table of refractions, and had used 9".55 as the coefficient of nutation in declination. The right ascensions were found by comparing all the other stars with α Aquilæ, and comparing α Aquilæ with the sun.
At the beginning of the century Cagnoli determined independently the place of Capella, and founded on this determination a catalogue of stars of which I shall speak again.
In 1806 Mr. Pond gave a catalogue of N.P.D. founded on his observations with an altitude-and-azimuth instrument, but using the same corrections as Maskelyne.
In 1807 Piazzi published, in the sixth volume of the Palermo Observations, his catalogue of 120 principal stars observed with great care, and a greater number of stars on which less attention was bestowed. All the places were referred to Procyon and α Aquilæ; and these stars were compared immediately with the sun. The table of refractions used by Piazzi was one deduced by himself from observations of circumpolar stars in different parts of their diurnal circles, and differed little from Bradley's. This catalogue therefore was strictly independent. In 1814 it was extended so as to include 7646 stars, and published separately; and this large catalogue is at this time referred to by all observers as a standard catalogue.
The subject of refraction had in the last century attracted considerable attention, and had been treated theoretically by Oriani, Kramp, and Laplace. The object of these writers was, from an assumed law of constitution of the atmosphere, founded as far as possible on experiment, to determine, à priori, the magnitude and law of refraction. The experiments made by the French chemists and opticians had determined the relation between the pressure, and density of the air (subject to a very small doubt as to the effect of heat) and the quantity of refraction, without reference to any astronomical observations. Some
[page] 137
doubt remained as to the law of temperature in ascending in the atmosphere: this they attempted to remove by means of observations of horizontal refraction. On these grounds a table was calculated by Delambre (assisted partly by Piazzi's and his own observations), and published in 1806 by the French Board of Longitude; and this table has been always highly esteemed.
In 1806 Carlini published (in the Milan Ephemeris) his astronomical investigations of refraction. His object, besides giving a table of refractions generally, was to show that the refraction on the north side of the zenith at small altitudes was sensibly different from that on the south side. For refractions on the north side he used observations of circumpolar stars; as, the law of refraction as far as the pole being well known, the true zenith distances below the pole are immediately found. For refractions on the south side he referred to observations made at Palermo, where the same stars were seen with 7° greater elevation. In this manner a difference of a few seconds was found below 85° zenith distance. In the position of Milan this difference is quite conceivable: I know not whether the idea has been taken up by any other astronomer.
In 1810 Mr. Groombridge published in the Phil. Trans. a table of refractions founded cn observations of circumpolar stars.
In 1813 and 1815 Mr. Pond published catalogues of the N.P.D. of the principal stars, determined by observations with the Greenwich circle. In reducing these, Bradley's refractions were still used.
In 1813 Bessel published (in the Berliner Jahrbuch for 1816,) the table of refractions obtained from Bradley's observations. In 1818 he published the Fundamenta Astronomiæ pro Anno 1756, deduced from Bradley's observations. This work has always been considered one of the most valuable contributions to our Astronomy. It exhibits the result of all Bradley's observations of stars, reduced on a uniform system, and is always referred to by succeeding astronomers as the representative of Bradley's observations. Various disquisitions on refraction and the mathematical theory of the other corrections are contained in it. From Kramp's and Laplace's theories and Bradley's observations, a new table of refractions was formed. No alteration was made in the coefficient of nutation.
In 1816 Lindenau announced in the Zeitschrift für Astronomie, that by the discussion of 810 Greenwich observations of the right ascension of Polaris, he had found the coefficient of nutation to be rather less than 9"·0. No details of this investigation were given, and none (I believe) have been pub-
[page] 138
lished elsewhere. This, I think, is unfortunate; for I cannot consider the Greenwich transits of Polaris (observed in general on only one wire,) to be very accurate. This coefficient, however, has been adopted by Bessel and all the German astronomers in every subsequent investigation that I know. (Dr. Brinkley's sidereal investigations, and Cacciatore's investigations from the obliquity of the ecliptic in different years, agree in giving something very near 9".3 for the coefficient.) In the Dorpat Observations for 1822, and the Milan Ephemeris for 1819, &c. are many observed right ascensions of Polaris, undertaken with the same object; and I have observed about 400 with much care.
In the Berlin Memoirs for 1818 and 1819, Bessel published a very valuable paper on the right ascension of Maskelyne's 36 stars, from observations with a transit by Dollond and a circle by Cary. This memoir may be cited as a model for all succeeding investigations of the same kind. In the volume for 1825 he published another paper on the same subject, the results being founded on five years' observations with Reichenbach's circle.
In the Konigsberg Observations for 1821 and 1822 appeared a number of observations made by Bessel for ascertaining the amount of refraction near the horizon. They consisted of observations of stars which when on the meridian passed so near to the zenith that there could be little uncertainty about their absolute places. From these observations, and from observations of fifty-nine circumpolar stars on the meridian above and below the pole, he formed a new table of refractions, differing a little from that given in the Funddmenta. This table he applied (amongst other things,) to the solution of a curious diffeculty. Every astronomer (Mr. Groombridge excepted), who had observed the sun's zenith-distance at the solstices, had deduced from the summer solstice a greater obliquity than from the winter solstice. It was impossible that this could arise from any planetary perturbation; and several hypotheses were invented to explain it. Piazzi (Memorie della Società Italiana, 1804,) conceived that solar refraction might depend on something besides the barometer and thermometer, as for instance on the electricity of the air, and that the peculiar state of the air during the prevalence of the sirocco might affect it. Legendre ascribed it to something like nutation of the earth. Olbers thought that the sun's centre of figure might possibly not coincide with its centre of attraction. The general belief, however, was that it depended upon some fault in the tables of refraction, or the method of using them. Now Bessel showed
[page] 139
that upon using his Table the obliquities, from his own observations, came out equal from the two solstices. He had remarked (Berliner Jahrbuch, 1825,) that Bradley's observations gave equal obliquities, and in a paper in the Zeitschrift für Astronomie, vol. i., he endeavoured to show that the observations of all the different observers make the obliquities equal. The difficulty depends (probably) on one of the nicest points about refraction, namely, the thermometrical correction. It is perhaps not easy to ascertain the exact temperature of the air at the time of the sun's passage; and perhaps the difference between the temperature within and without the transit-room (that stumbling-block to astronomers and theorists,) may then be considerable. Perhaps also the colours on the sun's limb, produced by atmospheric dispersion, may produce some doubt. On the whole, I conceive that this question cannot yet be regarded as settled. The subject is well discussed in Cacciatore's Observations.
In the Transactions of the Royal Irish Academy, vol. xii. for 1815, Dr. Brinkley published investigations on refraction, principally astronomical. In vol. xiii. he extended them to observations near the horizon: tables were formed from these materials.
In the Phil. Trans. 1823, Mr. Ivory published a theoretical investigation of refraction: it proceeded principally on the supposition that, on ascending uniformly, the temperature of the air decreases uniformly: the result of this inquiry was given in tables. In the Phil. Trans. 1824, Dr. Young proposed a simple formula for the relation between the density and pressure of the air, which corresponded nearly to Mr. Ivory's.
The most remarkable, by far, of all the investigations of refraction that I have seen, is one by Mr. Atkinson in the Memoirs of the Astronomical Society, vol. ii. To discover the law of the decrease of temperature, this gentleman collected a number of observations of the thermometer, made at various elevations by different persons; and fixed at last upon this law: That uniform decrements of temperature correspond to increments of height which are in arithmetical progression. For the effect of temperature on the density of air, and for the whole refraction of air, the best experiments were referred to. The calculation was effected by a method of quadratures, the air being (for low refractions) supposed to be divided into sixty-four strata. The result is given in tables. It is to be regretted that the untimely death of the author prevented the completion of a second paper on nearly the same subject.
Before quitting the subject of refraction I may point out two
[page] 140
papers in Zach's Correspondance as worthy the attention of the astronomer. In vol. i. the effects of radiation from the thermometer bulb (which had been pointed out by Fourier,Annales de Chimie, vol. vi.)are insisted upon; and the plan proposed by Fourier,namely to use two thermometers of which one has a blackened bulb and the other a clean one, and to apply to the indications of the latter a proportional part of the difference between the two, is recommended to observers. In vol. ii.,Flaugerges proposes to inclose the thermometer in a case consisting of a bright inside surface, and a bright outside surface, with a thin lamina of some nonconducting substance between; the form of the case to be such as will permit the free passage of air. He also observes, that probably the correction for temperature will depend on the hygrometer. It seems to me likely that these precautions might prevent many of the discordances that have been noticed.
Among the various essays which, though less known, are well deserving of attention,may be mentioned, A theory of refraction,by Schmidt; elaborate investigations by Plana, Littrow, Struve, and Schwerd,in their Observations; terrestrial refractions observed by Tralles (Berlin Memoirs, 1804); refractions near the horizon observed by Mechain (Conn.des Temps, 1807), by Oriani (Effemeridi di Milano, 1816), and by Foster (Phil. Trans. 1826); Lee's remarks on atmospheric dispersion,—a valuable paper (Phil. Trans. 1815); and Brinkley's remarks on the observations of the same stars at Dublin and at Paramatta (Memoirs of the Astronomical Society, vol. ii. and Ast. Nachrichten, No. 78.). Rumker's observations also (in his Preliminary Catalogue) are intended, partly, for comparison with European observations,in order to obtain the amount of refraction.
While this subject was pursued at home and abroad, two discussions were going on, confined almost entirely to England. In the Memorie della Società Italiana for 1805 and 1809, and the Berliner Jahrbuch for 1814, Piazzi, Chiminello and Calandreili conceived that they had found a sensible parallax in several stars. Bessel, however, could find no trace of it in Bradley's observations. In the Transactions of the Royal Irish Academy, 1815, a paper appeared by Dr. Brinkley on the same subject; but the parallaxes whose existence he considered to be established, differed considerably from those of Piazzi. In the Philosophical Transactions for 1817, Mr. Fond stated, that with the Greenwich circle no such parallax was discoverable. He proposed however that telescopes should be immoveably fixed to stone piers, for the purpose of observ-
[page] 141
ing stars of nearly the same declination and different right ascensions. This was done at Greenwich, and still the observations gave no indication of parallax. Dr. Brinkley still maintained its existence; and for several years each successive volume of the Phil. Trans, contained a paper on one or the other side of the question. In the course of this discussion, the defects of the different instruments and of the different corrections were closely examined, and in this respect Astronomy has certainly been advanced by the controversy. In the Phil. Trans. 1821, Dr. Brinkley investigated from observations, by the method of equations of condition, the quantities of aberration, nutation, and parallax, and found sensible values of parallax for several stars, especially for α Lyræ. Mr. Pond still doubted of this result; and in vol. i. of the Mem. Ast. Soc. Dr. Brinkley endeavoured to show that the Greenwich observations themselves afforded evidence of parallax. In the Irish Transactions for 1825, Dr. Brinkley attempted to settle the question by an instrumental investigation of the most delicate kind. The quantity of solar nutation (which had never before been extracted from observations,) being smaller than that which he attributed to parallax, it seemed that if the observations were competent to exhibit the former, they might assuredly be relied on for the latter. By equations of condition, therefore, he investigated the quantities of aberration, solar nutation, and parallax, for different stars, and obtained consistent results for the solar nutation, and sensible quantities for parallax. This result would have appeared to me decisive, but for a difficulty which another investigation has made to appear. In the Ast. Soc. Mem. vol. iv. is a most valuable investigation of the quantity of aberration as deduced from a vast number of Greenwich observations, by Mr. Richardson, Assistant at the Greenwich Observatory. The mean result (20"·50) appears to me the most accurate that has yet been obtained. But from different stars different values are obtained; and it is remarkable that the difference between the values for γ Draconis and η Ursæ Majoris,—stars less affected than almost any other by uncertainty of refraction,—is of opposite kinds in the two determinations of Brinkley and Richardson, and that the discrepance far exceeds the quantity which Dr. Brinkley had proposed to investigate. The existence of sensible parallax appears to me, therefore, to be yet undecided.—A few observations and remarks on parallax may be found in Bessel's Fundamenta, and Struve's Observations.
Mr. Pond in the mean time had remarked (Phil. Trans. 1823), that many fixed stars appeared to have an accelerated
[page] 142
motion towards the south. Dr. Brinkley found no traces of this, and in several volumes of the Phil. Trans. the reasons for and against this opinion are fully stated. This question, like the other, seems to be still in doubt.
These discussions, while they have improved the accuracy of the places of the fixed stars, have naturally given rise to different fundamental catalogues. The thermometrical correction of refraction affects the determination of the equinox, because the temperature of the two equinoxes is not the same. As Bradley's refractions are still employed at Greenwich, the Greenwich right ascensions will differ from Brinkley's or Bessel's. The declinations are immediately affected by the refraction. Mr. Pond, several years since found that the right ascensions of all the stars of Dr. Maskelyne's catalogue ought to be increased 0"·31 of time; but he has since thought that 0"·20 is sufficient: no investigations were published with these statements. Several catalogues have been published by him in the Greenwich Observations and in the Nautical Almanac: the last (included in the large catalogue published with the Greenwich Observations for 1829,) is undoubtedly excellent. Dr. Brinkley's catalogue is in the Irish Transactions for 1828. Bessel's catalogues are in various volumes of his Observations, and in the Berliner Jahrbuch. In the Astronomische Nachrichten, vol. v. and vi., are elaborate comparisons of the deelinations of different observers, by Mr. Pond; and in the Berliner Jahrbuch, 1825, by Bessel. In Plana's Observations (Turin Memoirs, 1828), and in Schwerd's Observations, there appear to be good catalogues of declinations.
By comparing late declinations with those of Bradley, &c. the quantity of lunisolar precession is found from different stars, and the difference of each from the mean is held to be the proper motion of that star. In like manner, by comparing late right ascensions with those of Bradley, &c, the quantity of general precession is found. Different values of precession and proper motion are of course obtained by different catalogues. Piazzi's and Maskelyne's depend sensibly on the nutation employed. In the Memorie dell' Istituto nazionale Italiano, 1804, Piazzi gave the proper motion of 300 stars; in the Berlin Memoirs 1801, and the Phil. Trans. 1805 and 1806, Prevost, Maurice, and Sir W. Herschel explained some on the supposition that the sun was moving towards λ Herculis: their Calculations appear very plausible, but the result has not been generally received. Pond's proper motions have been given with his. catalogues; Bessel's precession in the Berlin Mernoirs, 1819 and 1825, and the Astronomische Nachrichten, No. 92,
[page] 143
Brinkley's in the Irish Transactions, 1828. The latter is founded solely on the supposition that (as appears from observations,) the three stars, α Orionis, α. Cygni, and Rigel, have the same relative position as in Bradley's time, and therefore probably have no proper motion. The result as to precession is exhibited as the mean of results from thirteen different stars, but this, taken literally, may convey an erroneous notion to the reader; the result is obtained from one star only, (which, assuming the direction of motion of the pole, is theoretically sufficient,) and any one of the three stars whose relative positions have not altered, or any other star whose place is corrected so as to refer it to the same position with regard to them, would give just the same result. An ample discussion of proper motions may be found in Cacciatore's observations: a valuable paper on the same subject has also been lately communicated by Mr. Baily to the Royal Astronomical Society. The places of some stars in the southern hemisphere have been determined independently by Mr. Rumker.
IV. The places of a number of principal stars being well established, those of other stars are easily established by comparison with them. Catalogues of small stars may therefore be made by astronomers whose instruments are not competent to fix independently the places of fundamental stars. The principal original catalogues in use at the beginning of the century were, one of 387 stars deduced from Bradley's observations, and published in the Nautical Almanac 1773; one by Mayer, of 992 stars; and three by Lacaille*, (of which one included 1942 southern stars; one, 515 zodiacal stars; and one, 307 not confined to any part of the heavens). Besides these there were many compilations (as Wollaston's general catalogue); and many of less authority, and principally catalogues of smaller stars, were published in the Continental Ephemerides, especially in the Connaissance des Temps. In 1800 Wollaston's Fasciculus Astronomicus appeared, including the circumpolar regions to 25° N.P.D. In 1801 the Histoire Celeste was published, comprising observations of 50,000 stars, made principally by Lefrancais Delalande at the Ecole Militaire. Tables for the convenient reduction of these observations, on a principle suggested by Bessel in the Astronomische Nachrichten, No. 2, have since been published by Schumacher (in 1825). These stars were for the most part observed but once. About the same time appeared Bode's Charts of the Heavens, including a con-
* In the Memoirs of the Astronomical Society, Mr. Baily has discussed and republished Mayer's and Lacailie's catalogues.
[page] 144
siderable number of telescopic stars, and his catalogue of 17,000 stars from all authors; it was followed in 1805 by his smaller catalogue. In 1803, Cagnoli's catalogue of 500 stars appeared in the Memorie della Società Italiana (published separately in 1807, with tables for computing the aberration and nutation); and in the same year Piazzi's catalogue of 6748 stars, founded on Maskelyne's catalogue of 1790, was published in the Palermo Observations. The last-mentioned catalogue, revised from the author's fundamental places, and (in his opinion) improved, and extended to 7646 stars, was published as a separate work in 1814. This may well be considered as the greatest work undertaken by any modern astronomer; as not only was every star so frequently observed as to determine its place well, both in right ascension and declination, but every observation was reduced, and the results and their comparison with those of former astronomers exhibited in a clear form. It is still the standard accurate catalogue, as the places deduced from the Histoire Celeste are still the standard approximate catalogue for small stars. In 1806 Zach's Tabulœ Spectales was published. The object of this work, besides giving a catalogue of 1830 zodiacal stars principally from Zach's observations, was to supply facilities for applying the astronomical corrections of aberration and nutation to 494 of the principal stars. And in 1812 the same astronomer published the Tables nouvelles d'Aberration§c. for 1404 stars. In the Greenwich Observations for 1816, Mr.Pond published a catalogue of 400 stars. In 1818 (as I have mentioned,) Bessel published the Fundamenta Astronomiæ, exhibiting the results of all Bradley's observations of stars in a catalogue of 3222 stars; these were compared with Piazzi's, but no means of applying aberration and nutation were given. In 1822, Harding's Atlas Cœlestis was published, comprising a series of charts including every star on the observation of which any dependence could be placed, as far as the 30th degree of south declination.
The principal defect in Piazzi's selection, is the want of stars near the pole; to supply this, Struve at Dorpat observed (in right ascension only)many circumpolar and other stars; he also fixed the places of many minute stars in the neighbourhood of large ones: these observations are contained in the Dorpat Observations. Schwerd also (at Speyer) has observed many circumpolar stars both in right ascension and declination, and has published a chart of this part of the heavens.
Besides these, the following less complete catalogues have been published. Right Ascensions, by Littrow, in the Vienna Observations, and by the writer of this paper in the Cambridge
[page] 145
Observations. Declinations by Schwerd, in the Astronomische Nachrichten. Southern stars by Rumker, in several parts of the same work; and by Fallows and Rumker, in the Phil. Trans. for 1824 and 1829. Declinations by Bianchi, in the Milan Ephemeris 1830. And very lately a catalogue of 632 southern stars by Rumker, with constants for the reductions, has arrived in this country.
In the Greenwich Observations 1829, Mr. Pond published a catalegue of 720 stars lately observed at Greenwich; it is understood that he is now occupied with the extension of it. Mr. Groombridge observed a great number of stars, principally circumpolar, which have been reduced at the expense of the British Government, but are not yet published. Dr. Robinson is, I believe, now employed in re-observing the whole of Bradley's stars.
Bessel has for several years been much employed in observing (with Reichenbach's circle) all stars as far as the 9th magnitude in zones. In general, the transits of these stars are observed at only one wire; their places therefore are only approximate. The unreduced observations, with the elements of reduction, are published in the Königsberg Observations. These zones have been extended from 15° south declination to 45° north declination.
In 1825 the Berlin Academy invited astronomers to join in forming charts of the region of the heavens 15° on each side of the equator (Astr. Nachr. No. 88). It was proposed that each observer should take one hour of right ascension; that having formed a chart including all the stars of the Histoire Céleste and Bessel's Zones, he should put in, by estimation only, all the stars that could be seen with one of Fraunhofer's telescopes of 34 lines aperture. Some parts of this are in progress: three hours (one by Mr. Hussey, one by the Padre Inghirami and Capocci, and one by Göbel,) have been completed and are engraved.
Tables for the reduction of stars were published by Dr. Pearson in 1824; by Mr. Baily in 1827; by Mr. Groombridge and Sir John Herschel, in the Mem. Astr. Soc. vol. 1, and by several others. But in the Astr. Nachr. No. 4. Bessel proposed a method which for most purposes seems likely to supersede every other. The reduction of the right ascension as well as of the declination of a star may be expressed by the sum of four products, one term of each depending only on the year and day, the other depending only on the place of the star. The numbers depending on the day have been published in the German periodical works; and in Schumacher's Hülfstafeln
K
[page] 146
for 1822, the numbers depending on the star's place were given for 500 stars. (Numbers are also given in the Berliner Jahrbuch, depending on the day, and adapted to a different system of reduction.) One of the first important acts of the London Astronomical Society, was to publish (under the super-intendance of Mr. Baily,) a catalogue of 2881 stars, founded on the observations of Bradley and Piazzi, accompanied by the numbers depending on the place of the star; to be used in a method identical (in all essential parts,) with Bessel's. Since that time the numbers depending on the day have been published in the Supplement to the Nautical Almanac: and with these the Astronomical Society's Catalogue, though somewhat less accurate than those founded on late observations (as Mr. Pond's), is far more convenient for use than any other.
Within a short time, a volume of Tables has been published by Bessel under the title Tabulœ Regiomonfanœ, which must have a powerful influence on the state of Astronomy. Besides all tables wanted for ordinary reductions, this volume contains all the numbers depending on the year and day, which are necessary for reducing observations from 1750 to 1850. The advantage of being able to reduce on a uniform system, and by easy methods, all the accurate observations that have been made, can be easily conceived by those who have had occasion to discuss distant observations.
V. At the beginning of the century, our only accurate knowledge of double stars and nebulae was founded on Sir W. Herschel's observations, made nearly twenty years before. A few measures are to be found in Wollaston's Fasciculus. In the Phil. Trans. 1802, Sir W. Herschel published a catalogue of 500 new nebulae of various classes, with remarks on the constitution of the heavens; and in the Phil. Trans. 1803, a paper "On the changes in the relative situation of double stars in 25 years." This may be considered as the epoch of the creation of the science in the form in which it now exists. In the same work for 1804, he continued the subject. In 1811, he published a paper on nebulæ, and on the constitution of the heavens; in 1814 one on the same subject, in which he noticed the breaking up of the Milky Way in different places, apparently from some principle of attraction; and in 1817, one on the local arrangement of stars, and on the Milky Way. These memoirs contained those remarkable ideas on the distribution of the stars in our own cluster between two parallel planes, and on the connexion between stars and nebulae, (the former appearing sometimes to be accompanied by the latter, sometimes to have absorbed a
[page] 147
part, and sometimes perhaps to have been formed from them,) which have since been generally received. The additions made to the subject since that time consist of little more than accumulations of observations (with the exception of one set of deductions, of which I shall speak presently). Sir W. Herschel's last paper was a catalogue of 145 new double stars, without accurate measures, communicated to the Astronomical Society in 1822, and printed in the first volume of their Transactions. In the Dorpat Observations, some measures of the positions and distances of double stars were given, and a catalogue of the places of 795 double stars, from all authorities, but without measures. Some were also observed by Bessel, and a catalogue of 257 is given in the Königsberg Observations part 10, and in the Ast. Nachr. No. 88, with estimations of distance, but no angles of position. In 1827, Struve published his Catalogus Novus, containing the places of 3112 double stars; not measured, but classified by estimation of their distances. This work (the fruit of two years' labour, with Fraunhofer's large telescope,) contains all the double stars of a certain description to 15° S. declination. And though the want of measures renders it inapplicable for the speculations which had been and are now grounded upon measures only, yet this must always rank as a very valuable catalogue. While this was going on, Sir John Herschel and Sir James South published (in the Phil. Trans. 1824,) accurate measures of 380 double and triple stars. Sir James South published (Phil. Trans. 1826,) measures of 458, and a reexamination of 36 of the former list; and Sir John Herschel added remarks on the changes apparently going on. There can be no doubt of the very great value of these determinations. Amici however, in Zach's Correspondance, vol. 8, has called in question the accuracy of some of the measures. In the Mem. Ast. Soc. vol. 2. is a comparison, by Struve, of his own measures with those of Herschel and South. In 1826, Sir John Herschel presented to the Astronomical Society a catalogue of 321 new double stars, the distances and positions being given by estimation, with remarks on the great nebulæ of Orion and Andromeda; in 1827, one of 295 stars; in 1828, one of 384. About the same time Mr. Dunlop published measures of 253 southern double stars (Ast. Soc. Mem.), and remarks on the southern nebulæ(Phil. Trans.). In 1830, Sir John Herschel communicated good measures of 1236 stars, made with a 20-feet reflector; and lately, in vol. 5. of the Ast. Soc. Mem. he has given accurate measures of 364 with an achromatic telescope. This last paper is the most interesting that has been mentioned, exhibiting all the most striking results as to the motion of double
k2
[page] 148
stars that have yet been obtained. In the Monatliche Correspondenz, vol. 26, Bessel had speculated on the relative motion of the stars of 61 Cygni; and in the Phil. Trans. 1824, maps of the apparent relative motions of 61 Cygni and ξ Bootis were given. In many cases it is doubtful whether the apparent motion may not be produced by the motion of our system (supposing the stars unconnected and at very different distances), and whether a part of it may not depend on annual parallax. But in this paper it is shown that ζ Cancri and ξ Ursæ Majorishave nearly completed an entire revolution since they were first observed; that η Coronæ has probably made more than a revolution; and that η Castor, γ Virginis, σ Coronæ, 70 Ophiuchi, 61 Cygni, and others, are undoubtedly connected as binary systems, and have changed their position remarkably.—Other private observers, I believe, in this country, are employed on the measures of these objects.
The belief in the connexion of double stars by some law of attraction naturally excited a desire of reducing their orbits to calculation. Every attempt that has been made has assumed the law to be that of gravitation. In the Conn. des Temps 1830, Savary gave a method requiring four complete observations of distance and position, which he applied to determine the: relative orbit of the two stars of ξ Ursæ Majoris. (In the history of methods it is remarkable that one of the distances actually used by him for £ Ursæ Majoris was concluded from the others by the ratio of the angular motions.) In the Berliner Jahrbuch 1832, Encke gave a method, also requiring four complete observations, which he applied to 70 Ophiuchi. But Sir John Herschel has lately communicated to the Astronomical Society a method which, for elegance and practical utility, must I think be placed above every other that has appeared. For reasons of which only an observer can judge, he rejects entirely the measures of distances; and from the observed angles of position only (of which any number can be used, and the more the better,) he obtains, by a singularly skilful mixture of graphical construction and numerical calculation, all the elements of the orbit. This method has been applied to γ Virginis, σ Coronae, Castor, 70 Ophiuchi, and ξ Ursæ Majoris; and an ephemeris of the first (whose position will change very rapidly in the next few years,) is now published in the Supplement to the Nautical Almanac. This is really a new step in science.
A very extensive series of observations of nebulae, it is understood, is nearly completed by Sir John Herschel; but nothing has yet been published.
Among the changes in nebulæ that have been suspected, one
[page] 149
of the most remarkable is that pointed out by Cacciatore (Ast. Nachr. No. 113). In a place where Lacaille, Piazzi, and Cacciatore himself, had formerly seen a star, Cacciatore in 1826 saw a nebula; and this nebula has since been observed by Capocci and Dunlop. The only doubt is whether the telescopes with which it was seen before were good enough to discriminate between a star and a nebula; and on this point I cannot pretend to decide.
In 1826 a new star (extremely small) appeared in the great nebula of Orion, and was observed by Struve and Herschel (Ast.Nachr. No. 138,Ast. Soc. Trans. vol. 2). Whether it is a periodical star or a new star does not appear certain.
Nothing remarkable, so far as I know, has been added to our knowledge of variable* stars. Many remarks are to be found scattered in the German periodicals, and some in the English Transactions, but none which appeared to be worth extracting.
VI. The planetary tables in highest repute about the year 1800 were those published in Lalande's Astronomy. A review of these will show that astronomers hardly yet expected Tables to represent the places of the heavenly bodies with accuracy, but rather confined their use to approximate prediction; in fact, the theories of perturbation were used no further than was necessary for this purpose. The Solar Tables (calculated by Delambre,) were founded on Laplace's theory and the Greenwich observations; the Lunar Tables (Mason's of 1780, with very small alterations,) were founded on Mayer's theory; but the coefficients of the inequalities were obtained from observation. The Tables of Jupiter, Saturn, and Uranus, (by Delambre,) were founded on Laplace's theory, as the magnitude of their equations made it impossible to dispense with them; but those of Mercury, Venus, and Mars, (by Lalande,) had no effects of perturbation. The Tables of Jupiter's satellites (by Delambre,) were founded on Laplace's theory and one thousand observations. Besides these Tables, however, there were others by Zach, Oriani, Triesnecker, &c, which were also much esteemed.
In several volumes of the Berliner Jahrbuch at the beginning of the century, formulae are investigated for the perturbations of Mercury, Venus, the Earth, and Mars. In the Berlincr Jahrbuch for 1806, Wurm investigated the correction of the mass of Venus from the perturbations of the earth; he found
* I have lately found that the star 42 Virginis, which was observed by Flam-steed and described as of the 6th magnitude, but which was lost in the last century, still exists in the same place, but is not brighter than the 11th magnitude.
[page] 150
that the mass ought to be increased. But as no other errors. were taken into account, no great value can be attributed to this result.
In 1804, Zach published complete Tables of the Sun, founded on the observations made at Gotha. In 1806, the French Board of Longitude published Delambre's Solar Tables, which (till within a short time,) have been generally adopted. They were founded on observations by Bradley, Maskelyne, and Delambre, and on Laplace's theory; the masses of Venus and Mars, as well as the other elements, being determined by the discussion of the observations. In 1809, Zach published his Tables abrégées et portatives, differing little from the larger Tables except in the arrangement, which, giving more trouble to the computer, required less space. In the Milan Ephemeris 1810 and 1811, Carlini published his Solar Tables. By a new arrangement (making the difference of successive values of the arguments the same as the alteration due to one day), he has diminished very much the labour of calculating a solar ephemeris; though for the calculation of an independent place, his system gives no particular facilities. The elements of these Tables are the same as those of Delambre's. In the Conn. des Temps for 1816, Burckhardt gave the results of a comparison of Delambre's Tables with a great number of Maskelyne's observations (far greater than the number on which they were founded). It appeared that the epoch, the perigee, and the eccentricity, required sensible alterations, and that the mass of Venus ought to be reduced about 1/9th, and that of the Moon to be sensibly diminished. In Lindenau's Zeitschrift for 1817, Littrow arrived at nearly the same results, except that he diminished Mars considerably. In the Phil. Trans. 1826, Sir James South gave 86 observations of the Sun, compared with the Tables; which I discussed in the Phil. Trans. 1827. In 1827 the writer of this paper compared Delambre's Tables with 1200 Greenwich observations made with the new transit, and deduced from them the corrections in the elements. These agreed closely, in general, with Burckhardt's, excepting that a diminution of Mars appeared necessary. Some discordancies however led him to suspect the existence of an inequality that had escaped the sagacity of Laplace and Burckhardt, and a new term was at length found and calculated. This was announced in the Phil. Trans. 1828. Corrections founded on these alterations of the elements have for some years been published in the Nautical Almanac. In the Astronomische Nachrichten,Nos.133 and 134, (March 1828,) Bessel gave the result of a discussion of Bradley's and his own observations. Adopting Burckhardt's masses of Venus and Mars, and a mass of the moon nearly corresponding to Lin-
[page] 151
denau's value of nutation, (and therefore smaller than any other received mass,) he put in a tabular form the corrections to be applied to Carlini's Tables. (Some numerical results of these had been published about half a year before.) These have been adopted in the Berlin and other ephemerides; in that of Milan they are adopted, excepting the mass of the Moon, for which mine is substituted. Nothing was added by Bessel to the theory. In Nos. 172, 179, and 217 of the Ast. Nachr. the corrected Tables are compared with observations; in the last place Bessel conceives that something is still wanting to the theory. I have also compared more than 200 Cambridge observations with the Berlin Ephemeris, and I think that this suspicion is well founded. It is understood that Bessel is employed on more complete solar Tables.
The change in the obliquity of the ecliptic, and the length of the solar year, are obtained from discussions of solstices and of solar Tables. The former of these are scattered about very much; but a most able discussion of all the valuable conclusions, with reference to both these objects, is contained in Cacciatore's observations. The annual diminution of obliquity is now almost fixed at 0"·45. The mass of Venus given by this number agreesnearly enough with that obtained from the inequalities of the Sun's longitude.
Little has been done in observing the solar spots, &c. Some observations are contained in the Conn. des Temps 1805, and the Berhiner Jahrbuch 1828; one of the best papers is perhaps that by Mosotti in the Milan Ephemeris 1821. In 1827, the Frankfort Society published some figures &c. of spots observed by Sömmering.
During this century, several astronomers, (in the German periodicals,) from comparisons of the duration of the sun's transit with the difference of zenith distance of the upper and lower limbs, had been led to the conclusion that the Sun's figure is that of a prolate spheroid. As two observers seldom give the same duration to the Sun's passage, this notion seemed in itself to deserve little attention. In the Milan Ephemeris, however, for 1821, is a series of observations with a divided object-glass by M. Mosotti, which seem to establish the sphericity of the Sun.
In 1806, the French Board of Longitude published Burg's Lunar Tables. In these the arguments of the inequalities were taken from Laplace's theory, and the coefficients from the Greenwich observations. In one instance only were so few as 668 equations of condition used to determine the value of a coefficient. They were compared with observations, and received the prize of the French Institute. In these, for the first time, Laplace's (or rather D'Alembert's) equation of long period
[page] 152
was introduced, the coefficient of which was entirely empirical. In 1809, Zach put them in a portable form. In 1812, Burckhardt's Tables appeared, differing a little in the numbers, and a little in arrangement, from Burg's, and containing also a greater number of equations; still the coefficients were obtained from observation. It was felt that the lunar theory was imperfect so long as appeal to observation for more than the six fundamental elements was necessary, and the Institute offered a prize for the lunar theories and Tables which should borrow nothing more. Two able theories were produced, and on one of these Damoiseau's Tables (published 1824,) are founded. They include a greater number of equations than the former, and are simpler in arrangement. In the Conn. des Temps 1828, they are compared with observations. Laplace's long equation is here entirely rejected. In fact, Burckhardt (Conn.des Temps 1824,) shows that other equations will do as well; Carlini (Effemeridi di Milano 1825,) on trying four equations depending on different arguments, shows that some are preferable to Laplace's, but that the best of all is a term depending on the square of the time. The theory of the Moon therefore appears still defective. In the Milan Ephemeris 1827, is a most valuable paper by Carlini on the Moon's variation; in which, after comparing theory with his own observations, he arrives at the conclusion that they are not perfectly reconcileable. Among the materials that have been produced for correcting the lunar Tables, I may mention 215 occupations calcultations by Triesnecker (Göttingen Transactions 1800); 100 by Carlini, and some by Oriani (Milan Ephemeris 1812 and 1814); several for the Moon's diameter, by Wisniewski (Petersburgh Transactions torn. 8); the comparisons by the French Board (Conn. des Temps, and Introduction to the French Tables); comparison of Greenwich observations, by order of the English Board of Longitude; a few by Mr. Henderson (Ast. Nachr. No. 176); some by Carlini (Milan Ephemeris 1830); and nearly 200 right ascensions, and several occultations compared by me (Cambridge Observations). Of unreduced observations, none can be compared with the uninterrupted series made at Greenwich. For distant times, Mr. Baily, on the eclipses of Thales and Agathocles (Phil. Trans. 1811), and Oltmanns (Berliner Jahrbuch 1823 and 1824), and Wurm on 20 ancient eclipses (Zeitschrift vol. 3,) are worth consulting. In No. 102 of the Astr. Nachr. are observations of declination at Paramatta, for the Moon's parallax.
In the Conn. des Temps 1822, is a discussion by Nicollet of 124 observations of one of the lunar spots, for the phænomena of libration. On comparison with theory he is led to the re-
[page] 153
markable conclusion, that the Moon is not homogeneous, and has not the form which it would have, had it been originally fluid.
In 1813, Lindenau published Tables of Mercury. They were founded principally on a discussion of 17 transits over the sun's disk. Lindenau concluded from these that a considerable increase of the mass of Venus was necessary to reconcile theory with observation. The Tables for perturbations are arranged on Carlini's system.
In 1810, Lindenau published Tables of Venus. They are founded entirely on Bradley's observations, and on continental observations of the present century, with the three observed transits. Lindenau would doubtless have preferred a continuous series of observations made at Greenwich, but the observation of Venus has been almost entirely neglected there. The secular variations of the orbit deduced from these observations do not agree with those given by Laplace's (or Lagrange's) theory; and Lindenau thinks that the mass of Mercury ought to be much increased. In Zach's Correspondance, vol. 13, Plana asserts that this difficulty is at present insuperable. Olbers (Monat. Corr. vol. 22,) prefers the theoretical variations. I may mention that it appears from my comparison of observations with the Berlin Ephemeris, that these Tables admit of sensible improvement. These Tables of Venus, (and Bouvard's of Jupiter,) were compared with late Greenwich observations, by order of the English Board of Longitude. In 1811, Reboul published Tables founded on the elements given by Lindenau, Mon. Corr. voL 10. Elaborate discussions of the transits of 1761 and 1769 have since been published by Encke, in separate works.
In the Milan Ephemeris 1801, and the Mon. Corr. vol. 2, are discussions of the elements and perturbations of Mars by Oriani and Wurm. In 1811, Lindenau published Tables of Mars. The Greenwich observations were used as far as possible; but as the observation of Mars was finally abandoned there, he had recourse to continental observations. The variations of the elements agree nearly with Laplace's. In the Ast. Nachr. No. 191, are physical observations of Mars at the opposition of 1830, by Beer and Mädler; they have fixed his time of rotation at 24h 39m. Many physical observations of Mercury, Venus, and Mars, by Schröter, at the beginning of the century, are to be found in his works and in the Berliner Jahrbuch. In the Phil. Trans. 1831, are remarks on Mars by Sir James South; two obsecrations on stars seen very near the planet, lead him to doubt the existence of any extensive atmosphere. In No. 29 Ast. Nachr. are observations by Rumker at Paramatta, for the parallax of Mars. Tables of Jupiter and Saturn, founded
[page] 154
on Laplace's theory, were published by Bouvard in 1808, but were soon suppressed, as it was found that in Burckhardt's addition to Laplace's theory, several terms had been applied with the wrong sign (in consequence of mistaking the perihelion for the aphelion). A new set of Tables was therefore published in 1821, with the improved theory, and founded on all the good observations of modern astronomy. In discussing these, values are obtained for the masses of Jupiter, Saturn, and Uranus.
In the Ast. Nachr, No. 97 and 139, are micrometrical measures of Jupiter and Saturn by Struve. He determines the flattening of Jupiter to be 1/3·7, and the inclination of Saturn's ring to the ecliptic to be 28° 5′. Bessel (Berlin Ephemeris 1814 and 1829,) had made it about 28° 22′. These values are considerably less than that formerly received (about 31° 20′). In No. 189 Ast. Nachr. are measures of Saturn by Bessel, with a divided object-glass.
In the Phil. Trans. 1805, 1806, and 1808, Sir W. Herschel gave observations of Saturn's figure. It appeared that about latitude 45° the planet projected above the elliptic form. I think it worth mention that I have myself witnessed an instance in which a person, who had never heard of this observation, on seeing the planet very distinctly, made spontaneously the same remark. I have many times seen the planet with extreme distinctness, and have on one occasion thought that it certainly had this shape; and on another, have been equally convinced that it is rather flattened at latitude 45°. The shape assigned by Sir W. Herschel (See Monat. Corr. vol. 15, and Cambridge Transactions, vol.2) cannot be reconciled with theory.
In 1821, Bouvard published Tables of Uranus (in the same volume with those of Jupiter and Saturn). With respect to this planet a singular difficulty occurs. Seventeen observations of Uranus have been found in the observations of Bradley, Mayer, &c. (for discussions of which see the Zeitschrift, the Conn. des Temps, &c.); and since its discovery as a planet in 1781, observations have not been wanting in any year. Now it appears impossible to unite all these observations in one elliptic orbit, and Bouvard, to avoid attributing errors of importance to the modern observations, has rejected the ancient ones entirely. But even thus the planet's path cannot be represented truly; for these Tables, made only eleven years ago, are now in error nearly half a minute of space.
Delambre's new Tables of Jupiter's satellites (for eclipses), published in 1817, were founded on all the observations that he could collect from 1662 to 1802, and on Laplace's theory; and will probably want little alteration for some years. It is to be
[page] 155
regrettedthat no measuresof the elongations of these satellites have been made, as they would throw much light on the mass of Jupiter, upon which (as I shall mention hereafter,) there is at present considerable doubt.
The motions of Saturn's largest satellite have lately attracted some attention. In the Berliner Jahrbuch 1814, is a discussion of Bessel's; from the motion of its apse he concluded the mass of Saturn's ring to be 1/213 that of the planet. In the Zeitschrift 1817 be predicted a series of conjunctions which it was desirable to observe. In the Ast. Nachr. Nos. 193, 194, 195, and 214, he gave new investigations (from observation of conjunctions and of the passage of its shadow on Saturn); he condueled the mass of the ring to be 1/118 that of Saturn, and found for saturn's mass a value agreeing nearly with Bouvard's. In No. 208 of the same work, is a prediction of eclipses by Mädler.
On the satellites of Uranus nothing is known except what was published by Sir W. Herschel, Phil. Trans. 1815; though it is understood that his conclusions as to the positions and dimensions of their orbits have been verified by Sir John Herschel.
A new method of giving the places of planets has been introduced, principally by Gauss (Monat. Corr. 1812, and Theoria Motús), namely, of giving the place, referred to the sun, by rectangular coordinates, two of which are parallel to the earth's equator. The sun's place, referred to the earth, being given in the same way, the coordinates of the planet referred to the earth are found by simple addition, and from these the right ascension and declination are found with great ease. This method is generally used for comets: in the Astron. Trans. vol. 3, Littrow proposed to use it for planets: and Weisse in 1829 published Tables for all the planets. These Tables admit of the introduction of secular change of the elements, but not of periodical perturbations: and on this account I think that they will now be little received.
A vast number of observations of planets is to be found in the Transactions, the Ephemerides, and the astronomical periodicals. Their object however is generally rather confined. The inferior planets are little observed: the superior, little except at opposition. At the regular observatories they have been much neglected. In the Berliner Jahrbuch 1816, it is remarked that in two years there were only six observations of planets at Greenwich. The foreign observations are sometimes given without any comparison: sometimes however (especially in the Milan Ephemeris,) they are compared with the
[page] 156
Tables, and even the equations of condition for correcting the elements are formed (as in Milan Eph. 1822). In reflecting on these circumstances, it appeared to me desirable that one set of good instruments should be devoted to the observation of planets: and when the Cambridge Observatory was put under my care, I determined on making the planets my principal object. I hope in a few years to collect a mass of observations directed to this point that will possess great value. I have already obtained and compared with Tables about 1100 right ascensions of planets, besides numerous observations of the sun and moon.
VII. At the beginning of the century the only bodies recognised as belonging exclusively to the solar system were Mercury, Venus, the Earth, Mars, Jupiter, Saturn, Uranus, the satellites of these planets, and Halley's comet. As to Lexell's comet of 1770, whose orbit appeared to have been changed by the action of Jupiter from a parabola to an ellipse of short period, it was generally believed that by again passing near to Jupiter it had been so much deflected that probably it had completely left the system.
On Jan. 1, 1801, Piazzi discovered a moveable body. It was generally observed in Europe during 41 days, in which time it described an arc of 3 degrees; when it was lost from its proximity to the sun. The calculation of its orbit was taken up entirely by the German astronomers. They soon found that the supposition of a parabolic orbit (which was the only one that had usually been made,) could not be applied with the least success: and Gauss invented a new method (which with some alteration was afterwards published in his Theoria Motús). He at length announced that this body was a planet, moving in an orbit rather more eccentric and more inclined to the ecliptic than those of the old planets, and intermediate in distance from the Sun to Mars and Jupiter. Its discoverer gave it the name of Ceres Ferdinandea. The joy of the German astronomers at this discovery was undoubtedly increased by the circumstance, that the mean distance of the new planet gave continuity to a curious law empirically established (as a rough representation of the distances of the successive planets,) by Bode, in which one was wanting between Mars and Jupiter. Their essays are generally headed, "On the long-expected planet between Mars and Jupiter," or with some similar title. So accurate were Gauss's elements, that in the beginning of December of the same year it was found again, and has since been regularly observed at most observatories (at least the
[page] 157
continental ones). At every fresh opposition the German astronomers have corrected the elements of the orbit: the perturbations have been regularly applied: and the place is now predicted with very great accuracy. The principal information respecting this is contained in the German periodicals: but much will be found in the Milan Ephemeris, and some in the Connaissance des Temps.
In March 1802, Olbers, in the examination of stars near Ceres, discovered another planet (Pallas), smaller than the former and moving in an orbit much inclined to the ecliptic. The general history of the discovery and improvement of its elements is the same as that of Ceres: but one curious consideration was suggested by the comparison of the two orbits. Their major axes were so nearly equal, (the order of magnitude being sometimes changed by the perturbations of Jupiter,) and their orbits approached so near at the intersection of their two planes, that Olbers started the hypothesis of their having been originally parts of a larger planet. If this were true, it seemed probable that there might be other parts; and if these were describing orbits round the sun, the intersection of their planes must fall nearly at the same point. By examining the parts of the heavens corresponding to the two intersections, such planets must infallibly be found.
On this principle, the German astronomers proceeded in a systematic look-out for new planets. Olbers in particular examined, once in every month, a certain portion of the heavens. In September 1804, Harding discovered Juno: and in March 1807, after monthly examinations during three years, Olbers discovered Vesta. No others have been found, though the same system of examination was long kept up. In Lindenau's Zeitschrift, vol. 1, is a notification by Olbers, that he had examined the same parts of the heavens with such regularity that he was certain no new planet had passed between 1808 and 1816. Nothing can give a more forcible idea of the perseverance which led to these discoveries*.
The elements of all these orbits have been successively improved (entirely by the Germans); the perturbations are calculated; and the places for some time before and after opposition are now given in the Berlin Ephemeris. I have lately observed, and compared with the Berlin Ephemeris, the right ascensions
* In the Berlin Ephemeris 1817, is a list of eight lost stars, none of which is either of the new planets; and in the Monat. Corr. vol. 15, Harding states that he misses 24 stars of the Histoire Celeste, and that he has six times observed stars which he has not been able to find again. One such instance (apparently quite free from doubt,) has occurred to myself.
[page] 158
of Juno and Vesta, and I find that they are rather more accurate than those of Venus.
Of the successive steps in the theories of these planets, the following are the principal.
In the Milan Ephemeris 1803, Oriani gave formulae for the perturbations of Ceres, on two suppositions of the value of the major axis; also for the perturbations of Pallas, as far as the third power of the inclination, and the second dimension of the eccentricity and its combination with the inclination. In the Berlin Ephemeris 1805, Schubert gave expressions for the perturbations of Ceres by Jupiter; and in the volume for 1809, Pfaff gave similar expressions for the effect of Saturn. In the Monatliche Correspondenz, vol.7, Gauss gave Tables for the perturbations of Ceres, In tom. 1. of the Göttingen Transactions, the same writer discussed the elements of the orbit of Pallas, taking no account of perturbations. In the Memorie della Società Italiana for 1810, Santini gave Vesta's secular variations and formulae for her periodic inequalities to the first order of small quantities, on two hypotheses of the value of the major axis. In the Milan Ephemeris 1815, Carlini gave Tables for the equation of the centre and the reduction of Ceres; in 1816, expressions for the equation of the centre of Vesta. Lindenau remarked, in vol. 1. of the Zeitschrift, that Carlini's Tables for the equation of the centre would be of little use, because the enormous perturbations produced by Jupiter would alter the eccentricity so much that the term depending on a given variation of the eccentricity, would soon be found inaccurate. In the Milan Ephemeris 1819, Carlini gave the equation of the centre for Pallas and Juno, with two values of the eccentricity, together with the alteration for each depending on alteration of eccentricity. In the Monatliche Correspondenz, vol. 28, Burckhardt had given formulae for the perturbations of Vesta, on two suppositions as to the magnitude of its semi-major axis: in the Mem. della Soc. Italiana, Santini gave elements deduced from observations, and complete Tables, including those for the perturbations to the first order of small quantities. In the Connaissance des Temps for 1818 and 1820, Daussy gave very complete Tables for the perturbations of Vesta, including 40 equations. These are still considered standard, except that the Germans prefer calculating the perturbations produced by Jupiter, by the method of quadratures. In the Berlin Ephemeris 1826, Nicolai gave a short paper containing results of great importance deduced from the discussion of observations on Juno. In all the calculations hitherto made, the mass adopted for Jupiter was either that assumed by Laplace (founded on
[page] 159
Pound's observations of the elongations of Jupiter's satellites), or that given by Bouvard (from the perturbations of Saturn), differing little from the other. Now Nicolai stated, that the observations of Juno at 15 oppositions required an increase of about 1/80th in the mass of Jupiter; but that even then the observations could not be well represented; and that he conceived the absolute attraction of Jupiter on Juno, must be different from that upon the Sun. The last conclusion, attacking one of the most important principles in the theory of gravitation, required further examination. In the Berlin Memoirs 1826, Encke discussed all the observed oppositions (fourteen) of Vesta, separating the perturbations produced by Jupiter into two parts, one being Jupiter's attraction on the Sun, and the other, Jupiter's attraction on Vesta, and considering the assumed mass of Jupiter in these two attractions, as liable to two separate errors. The result was, that the absolute attraction of Jupiter on Vesta did not differ from that on the Sun, by more than 1/10000 of the whole, and that Nicolai's mass ought to be increased about 1/300 of the whole. Encke remarks however, that Nicolai's mass will represent the observations very nearly as well; and Gauss has found the same for Pallas. Nicolai's mass is generally adopted by the German astronomers.—In the last-mentioned paper, and in the Berlin Ephemeris 1827, the reader will find an account of the method of quadratures used by the Germans (to which I intend to refer hereafter). In the Astronomische Nachriciten, No. 165, Heiligenstein has given the outlines of the calculation of the perturbations of Ceres for the opposition of 1830.
The methods of determining from observations the orbits of comets may be divided into those which assume parabolic motion, and those which do not: of the former, at the beginning of the century, Olbers's was best known on the Continent, and Lagrange's and Boscovich's in this country: of the latter; Laplace's was the only received one. In the Berlin Mem. 1801, is a method by Trembley. In 1806, Legendre published his methods, (the last Supplement appeared in 1820,) which began without the parabolic assumption, but finally adopted it. It is curious that the only two examples which he has taken for the parabolic orbit, are comets now known to move in very short ellipses, and in which the preference of an elliptic to a parabolic orbit was shown, at the time, by Gauss and Bessel. This is a striking instance of the danger of making our calculations on too restricted suppositions. In 1809 appeared Gauss's Theoria Motûs, still considered in Germany the classical work on this subject. Of the variety and contrivance in the methods given there, it is impossible to give any idea; parabolic motion
[page] 160
is not assumed, (the methods being best adapted to the small planets,) and the tentative part of the operations differs from that commonly used in this respect, that two unknown quantities must be tried. In the Göttingen Transactions, tom.2, is a parabolic method by Gauss. In the Phil. Trans. 1814, Mr. Ivory gave a parabolic method, amounting to the same as Olbers's. In the Berlin Ephemeris 1820, Olbers has given a method of correcting the approximate elements, and introducing the supposition of ellipticity; this, however, had been done by Laplace in the Mecanique Céleste. In the Milan Ephemeris 1817, and the Berlin Ephemeris 1824, Mosotti and Littrow have given methods. Pontécoulant has given a parabolic method in his Théorie Analytique. In the 5th book of the Mécanique Céleste, Laplace pointed out an alteration in his own method, and showed that the preliminary calculations (whose difficulty and inaccuracy had been considered the most formidable objection,) might in fact be made very easy and accurate. Lagrange left some remarks on the orbits of comets, which are published in the last edition of the Mécanique Analytique. In the Mem. Astr. Soc. vol. 4, Mr. Lubbock has shown that supposing the orbit to be parabolic, or supposing the major axis to be known, the equation may be reduced to a quadratic; and in a Supplement he has increased the accuracy of the method, so as to make it applicable to observations at a greater interval. In this method, after an approximate determination of the orbit, on the supposition that it is parabolic, the major axis may be easily found, and may be applied to determine more exactly the orbit; in the present state of the science of comets, this is an important point. Finally, in the Berlin Ephemeris 1833, Olbers has made some additions to his old method.
All these methods (except Laplace's,) require three complete observations, and can use no more; and in every part of the calculations they require accurate numbers for those observations, and calculation with 7-figure logarithms. Laplace's can use any number of observations, and after the preliminary calculations requires no extreme accuracy in any part. The general methods (including Pontécoulant's and Mr. Lubbock's,) tail when the apparent geocentric path passes nearly through the Sun's place.
The calculation of the true anomaly for a given time, by the common elliptic formulæ is troublesome and liable to error when the ellipse is very long. In the Monatliche Correspondentz vol. 12, Bessel gave Tables for finding the true anomaly in a long ellipse; Posselt, in vol. 5 of the Zeitschrift, has also given Tables.
In the Mon. Corr. vol. 14, Gauss found apparently a short
[page] 161
period (1731 days) for the last comet of 1805, which it has since been ascertained is periodical, though with a rather longer time. In 1810, Bessel's "Untersuchungen" &c. on the comet of 1807 was published; and this is an important epoch in the science of comets. The accuracy and long continuation of observations on this comet, seemed to show clearly that an elliptic orbit, though of great length, must be adopted. The author then took into account the perturbations, by methods invented for that occasion and now generally adopted. He then estimated the greatest possible deviation which the determination admitted of; by giving to every observation the greatest error that he thought it could bear, and giving to each such a sign that all their effects were positive or all negative. His conclusion was, that after the comet had left the sensible disturbances of our system, its periodic time could not be less than 1404 years nor greater than 2157 years, and that 1543 years was most probable. In the Berlin Ephemeris 1815, Bessel has found a period of 3383 years for the great comet of 1811. Argelander has published a treatise on this comet; he finally fixed on 2888 years. The most remarkable however of these long comets is Olbers's of 1815. All the calculations of different observers agreed in giving a period of between 72 and 77 years. In a masterly paper printed in the Berlin Memoirs for 1813, Bessel, after correcting the Sun's place, discussing all the observations, calculating the perturbations during and after the time of observation, &c. has fixed on 1887, Feb. 9, as the time of its next return to perihelion. Since that time many periods have been found for comets, of which some have been afterwards rejected. The second comet of 1819, as calculated by Encke, has a period of about 5½ years; and the fourth comet of 1819, a period of 3¾ years. These numbers may be correct, (though these bodies have not been seen again,) as many comets are so small that they can be seen only when near the earth. In the Zeitschrift, vol. 2, Encke gives a period between 66 and 76 years to the comet of 1812, and it seems impossible that it can exceed 100 years; in the Nctchrichten, No. 22, the same writer gave 194 years to the second comet of 1822, which he soon extended to 1550 years; in No. 37, Rumker fixed on 1817 years for the third comet of 1822; in No. 90, Hansen gave 556 years to the fourth comet of 1825: in Zach's Correspondance, vol. 7, Mosotti thought that the first comet of 1822 moved in an ellipse with a period of three or four years, but it was finally judged to be a parabola: in vol. 14, a period of 265 years was given to a comet of 1825. The orbit of the comet of 1824 (Ast. Nach. No. 69,) appears
L
[page] 162
to be hyperbolical. None of these determinations, I suppose, deserve much credit, except where the comet has been long observed and very ably discussed.
In 1818 and 1819, in examining the coincidence of the observed places of a comet discovered by Pons, with the places given by parabolic elements, Encke* found that the supposition of an ellipse of very short period was absolutely necessary; and his first calculations gave 1310 days. It was soon remarked that its elements were similar to those of the first comet of 1805, in calculating which Bessel had remarked (Mon. Corr. vol. 14,) that a parabolic orbit would not represent the observations. The interest excited by the discovery that we had a real periodic comet of short period will best be gathered from the successive parts of Zach's Correspondence, vol.2. Olbers pointed out its identity with that of 1795, on which he had long before remarked (Berlin Ephemeris 1814,) that different calculators had found very different elements. Encke, in the Berlin Ephemeris 1822, showed that the sums of the squares of errors of observation in the comet of 1795, were reduced to less than half by taking an ellipse of 1200 days instead of a parabola. In the same volume, the perturbations for these periods were given. Olbers soon after pointed out that the same comet had been observed before; and this discovery is very curious. In the Conn. des Temps 1819, are given two observations of a comet in 1786; from these alone no orbit could be determined. But Olbers found from the approximate elements, that these were certainly observations of the new periodic comet. Thus a series of observations extending through 33 years, or 10 revolutions of the comet, was established. After very short examination, Encke found (Berlin Ephemeris 1823,) that the periodic time given by the late observations was shorter than that from the earlier, or that the comet was gradually approaching the sun; which would seem to prove the existence of a resisting medium. He however predicted its place approximately for 1822, when, on account of its southern declination, it could not be seen in Europe; happily the Observatory at Paramatta
* In a French elementary work, it is stated that M. Arago first remarked the similarity of the elements of the comet of 1819, with that of 1805. But the discovery was certainly made by Encke in the manner stated in the text. That M. Arago may have conceived there was some similarity, (not much, as may be seen on examining a table of comets,) is quite possible; but nothing followed from this conjecture. Every calculation respecting this comet (except one by Damoiseau, which was a duplicate of one of Encke's,) has been made by the German astronomers.
[page] 163
was established, and it was observed by Rumker. The approach to the sun was confirmed by this observation (Berlin Ephemeris 1826). Damoiseau however, rejecting the earlier observations, found in the later ones no proof of resistance (Conn. des Temps 1827); and Encke himself (Ast. Nachr. No.123,) acknowledged that the supposition of resistance would not reconcile all the observations. It was predicted and generally observed in 1825; and so anxious were astronomers to discover it, that two new comets were found in looking for it; but this return was not favourable for deciding on the question of resistance. Finally, it was predicted and generally observed in 1828 and 1829; and now at last the point was cleared up. The axis of this comet's orbit lies nearly in the plane of Jupiter's orbit, and its aphelion is very near to Jupiter's orbit. Consequently, when Jupiter is in that part of his orbit while the comet is at aphelion, the perturbations of the comet are excessive; and if an erroneous mass is used for Jupiter, its calculated place will be very erroneous. This was nearly the situation of Jupiter between the appearances of 1819 and 1822 (when the perturbation produced by Jupiter in one revolution of the comet retarded the perihelion passage nine days); and the mass assumed for Jupiter by Encke and Damoiseau, in their calculations, was that of Laplace. Upon proceeding in the equations of condition with a term for the determination of Jupiter's mass, a value was found very nearly agreeing with that which Nicolai had found from the perturbations of Juno, and Encke from those of Vesta; and now with the supposition of a resisting medium everything was reconciled. The magnitude of the resistance is such as to diminish the periodic time about 1/10000 of the whole at each revolution; a quantity so large that there can be no mistake about its existence. The history of this discovery is undoubtedly the most curious that modern astronomy has presented. An abstract is given in the Ast. Nachr. No. 210 and 211, and the first part of a longer paper in the Berlin Memoirs has lately arrived. The place of this comet is predicted for the present year; it must be difficult to observe it in Europe, (I know not whether it has yet been seen,) but it has probably been observed at the Cape of Good Hope.
In 1826, M. Biela (a military officer at Prag,) discovered a comet which it appears he had partly expected. Calculation showed that its path was elliptic, and it was soon found that its elements agreed with those of the comet that passed its perihelion about the first day of 1806, (for which Gauss had found a short period.) The elements of a comet of 1772 agreed so nearly, that in 1806 Gauss had thought it probable they might
L2
[page] 164
be the same. It was now found that the later observations might be reconciled by supposing a periodic time of 2460 days, but the earlier observation required 2469 days. There seems no doubt of the identity of the three comets; but as the earlier perturbations have not been computed, it is doubtful whether this difference depends on perturbation or resistance of a medium. In an elaborate paper in the Mémoires de l' Institut, torn. 8, Damoiseau has calculated the perturbations of the mean anomaly and axis major from 1806 to 1826, and those of all the elements from 1826 to 1832; and an ephemeris for the present year, grounded on these, is printed in the Supplement to the 'Nautical Almanac. This comet will pass in the present year, within 20,000 miles of the earth's orbit. The motions of the three new periodical comets (including Olbers's of 74 years,) are in the same direction as those of the planets. The motion of Halley's comet, however, is retrograde.
Much labour has been employed in calculating the elements of Halley's comet for 1835. In the Ast. Nachr. No. 180, Rosenberger has deduced from observations the elements at the last appearance; and in No. 196, the elements at the appearance of 1682. In the results he has given the effects of an error in the assumed value of the major axis. In the Turin Memoirs 1817, is a most elaborate paper by Damoiseau on the perturbations of its elements between 1682 and 1759, and also between 1759 and 1835. I am not aware that the whole of these (which are undoubtedly the best materials,) have been combined to give a prediction for 1835. In the Conn. des Temps 1833, Pontécoulant determines the elements for 1835 by a similar calculation of perturbations applied to the elements which Burckhardt had obtained (Conn. des Temps 1819,) for -1682 and 1759.
A great number of old comets have been calculated, principally by Burckhardt and Olbers, but I know of no interesting result. In the Mémoires de l' Institut 1806, is an elaborate paper by Burckhardt on Lexell's comet of 1770. There seems no doubt that, from the perturbations of Jupiter, its parabolic orbit was changed into an elliptic orbit of about 5½ years, and that this was much altered by the earth's perturbation: but the further' history of the comet is unknown. Burckhardt is inclined to think that it may possibly still be a periodic comet; or possibly a satellite of Jupiter, as it would not at the distance of Jupiter be visible to us.
On the physical constitution of comets we have learnt nothing, except that they appear to be wholly gaseous. In the beginning of the century there were many discussions in Germany re-
[page] 165
specting a comet which some observers conceived they had seen upon the sun's disk. In 1826 M. Gambart found that a comet would cross the sun's disk: he watched the sun most carefully at the time predicted, but nothing was visible. The dilatation of Encke's comet as it receded from the sun, has given rise to some speculations on the nature of the ether pervading space.
I may mention in this place that the method of minimum squares and estimation of probable errors, though applicable to almost all physical calculations, have been most extensively used in calculations for comets, and were in fact first proposed in treatises on comets (Legendre's Nouvelles Methodes, and Gauss's Theoria Motûs). I will not undertake to say that I think the method of minimum squares is unexceptionable in all its applications, or that I attach much more than a relative value to the estimation of probable errors. But I think there is no doubt that these methods have contributed much to the accuracy of modern astronomy, and that in many doubtful cases they have been admirable assistants to the astronomer's judgement.
VIII. The materials upon which a knowledge of the earth's figure was grounded, at the beginning of the century, were the following. The arc measured in Peru by Bouguer, Lacondamine, &c.; that measured in Lapland by Clairaut, Maupertuis, &c.; that in America by Mason and Dixon, &c.; that from Rome to Rimini by Boscovich; and that from Barcelona to Dunkirk, measured by Delambre and Mechain. Besides these there were some others, as one in Piedmont by Beccaria, one in Austria by Liesganig, and one in India by Reuben Burrows, to which little credit was given; and there was Lacaille's measure at the Cape of Good Hope, which could not be reconciled with the others. One arc of parallel had also been measured in France: and one of much greater value in England. The pendulum experiments (serving, with the help of Clairaut's theorem, to determine the proportion of the earth's axes,) were principally scattered observations by De la Croyére, Campbell, Mairan, Bouguer, Godin, Maupertuis, Lacaille, Legentil, Phipps, Malaspina, and Borda. The last of these (confined to Paris,) were the only ones from which great accuracy could be expected; of the others, the only set in which a series of considerable geographical extent were observed by the same persons and with the same instrument, was Malaspina's. The observations of the attraction of Schehallien, and Cavendish's experiments with leaden balls, had given a pretty good knowledge of the earth's mean density.
[page] 166
In the years 1801, 1802, 1803, the arc measured in Lapland (which, according to the calculations of Clairaut and Maupertuis, seemed to present a strange anomaly,) was remeasured and extended by Ofverbom, Svanberg, and others, so as to embrace an amplitude exceeding 1½ degree. For the geodesic part, as well as for the astronomical determinations, the new repeating-circle was used. The conclusions at which they arrived differed from those of Maupertuis, and are more in accordance with those given by other measures. But they did not succeed in pointing out the cause of their difference; and, as far as their measures admitted of comparison, they confirmed greatly the accuracy of the former measure. The former measure has lately been much discussed, especially by M. Rosenberger in various numbers of the Ast. Nachr.; and the general opinion I think is now, that the first measure was the best, and that its anomaly depended only on the ruggedness of the country. In the Phil. Trans. 1803, is an account of the English measure of an arc from the south-eastern part of the Isle of Wight to Clifton in Yorkshire. The bases were measured with Ramsden's steel chain, and the horizontal angles with a large theodolite: the astronomical observations were made with Ramsden's zenith-sector. There is no doubt that, for its length, this was the most accurate arc that had been measured. Yet a point near the middle of this arc presented an anomaly in regard to the direction of gravity. The measure was afterwards extended to Burleigh Moor: and it thus comprehends an arc of nearly four degrees. Two arcs (of which the details are to be found in the Asiatic Researches,) were measured by Colonel Lambton in India. The first of these, near Madras, was of 1½ degree: the other, beginning near Cape Comorin, nearly 10 degrees. The latter has lately been extended by Captain Everest to nearly 16 degrees. The methods adopted in these measures differ in no respect from those of the English measure: and this arc is undoubtedly the best that has ever been surveyed. The French arc from Dunkirk to Barcelona has been extended by Biot and Arago to the little island Formentera in the Mediterranean (near Iviza), and its whole length is now nearly 12½ degrees. Of the excellence of the geodetic part of this there is no doubt; but there seems some reason to doubt the goodness of the astronomical determinations, though no labour was spared by the observers. The account of this forms a conclusion to the Base du Systeme Metrique. The Piedmontese arc of Beccaria has been re-measured with much care by Plana and Carlini: and the account is published in the Operations Géodésiques et Astronomiques en Piémont et Savoie. It is clearly proved that the astronomical part of Beccaria's
[page] 167
measure was erroneous: but the result of MM. Plana and Carlini's measure is still anomalous; perhaps not more so than the form of the country would lead us to expect. I may mention here that Zach, in the Monatliche Correspondentz and in the Correspottdance Astronomique, has shown clearly that Liesganig's measure is worth nothing. An arc has been measured by Gauss from GÖttingen to Altona, of 2 degrees; the astronomical observations being made with Ramsden's zenith-sector: some accounts of it will be found in the Ast. Nachr., and in a small work entitled Bestimmung des Breitenunter-scheides,§c. An arc of 3½ degrees has been measured by Struve, the northern extremity being on an island in the Gulf of Finland. In many parts of this operation, new instruments and new methods have been used: in particular, for the determination of the latitudes, great reliance was placed on the method of observing stars with a transit instrument whose motion is confined to the prime vertical: accounts of this measure are in the Astronomische Nachrichten, The distance on the arc of parallel between Dover and Falmouth having being ascertained in the course of the English survey, and the difference of longitude between them being determined, by Dr. Tiarks, by the transportation of chronometers, the length of an arc of parallel for one degree in a definite latitude is found, and this determination assists much in determining the Earth's figure. But a far longer arc of parallel has been measured, on the Continent, from Marennes (near Bordeaux) to Padua. The geodesic part of this measure had been nearly completed by the French Government, while the country was in their possession; all that was wanting was to connect the surveys on opposite sides of the Alps. This was effected (though not without difficulty,) by Austrian and Sardinian officers. It was then necessary to determine the difference of longitude of the extremities. This was done by dividing the arc into six portions, in each of which a point could be found visible at both its extremities, and observing at each extremity the absolute time at which small quantities of gunpowder were fired at the middle point. The French part was undertaken by MM. Nicollet and Brousseaud: the rest by MM. Plana and Carlini. The result thus obtained is perhaps liable to considerable doubt, as the errors of all the different observations are accumulated. It is unfortunate that the difference of longitude of the extremities has not been determined without any intermediate determination.
The above, as far as I am aware, are all the measures that have actually been made within the present century. But there
[page] 168
are others to which we may look as not far distant. The survey of Ireland that has lately been and is now going forward, is, I suppose, in accuracy and in excellence of arrangement, (I am not speaking of the minutiae of the map, but of the principal triangles, by which the great distances north and south or east and west are to be measured,) superior to every preceding survey. Little is now wanting for the measure of an arc of meridian but the observation of zenith-distances of stars at its extremities. The country is also favourable for the measuring an arc of parallel of considerable extent: and a new method of producing intense light, introduced into practice by one of the gentlemen employed on the survey, will probably give the means of determining the differences of longitude on a long arc without the errors produced by intermediate stations. It is also understood that our Government have long contemplated the repetition or extension of Lacaille's measure at the Cape of Good Hope: and several circumstances lead me to hope that this undertaking, which would perhaps contribute more than any other to our knowledge of the earth's figure, will ere long be seriously taken up. The extension of Struve's arc is in contemplation.
I may state here (though not immediately connected with the subject,) that a vast number of latitudes and longitudes have been determined, accounts of which are to be found in the Transactions and periodicals. Of the longitudes, one of the most important is that of Paris, determined by instantaneous signals as above described (see Phil. Trans. 1826 and 1827). The method of determining longitudes by transits of the moon has been pretty generally introduced (for which in this country we are indebted principally to the zeal of Mr. Baily); and the longitude of Paris has been determined by this means also (Conn. des Temps 1825). Surveys also, of different degrees of merit, have been going on in almost every part of the Continent.
Of pendulum experiments, the most valuable series is that made by Captain Sabine in almost every practicable latitude. Invariable pendulums which had been observed in London (to ascertain the number of vibrations made per day,) were observed in the same manner at all the stations, and again in the same manner on returning to London. In this manner, without ascertaining the absolute force of gravity at any one place, the proportion at different places is found probably with greater accuracy than by any other method. This is the method commonly adopted by the English experimenters. Experiments were previously made at several places in Britain by Captain Kater; and others have been made in different parts of the
[page] 169
world by Captain Hall, Sir Thomas Brisbane, Mr. Goldingham, &c. A vast number of most careful observations by Captain Foster, in his last voyage, has been received in England, and is now (I believe) preparing for the press. Advantage has also been taken of our repeated expeditions to the North Seas to observe pendulums at high latitudes. The method commonly used by the French philosophers was, to observe the absolute length of the seconds pendulum at each station: thus they experimented at several stations in France and Italy, in the Mediterranean, and in Britain. An extensive series, however, made in Freycinet's voyage, and a few in Duperrey's, were made with invariable pendulums. In the course of experiments for ascertaining the absolute length of the seconds pendulum by a new method, Bessel found that the correction applied in all former experiments for the buoyancy of the air was defective. This has been fully confirmed by Captain Sabine's experiments in a vacuum; and Mr. Baily has been actively employed in determining, with superior accuracy, the correction that ought to be adopted. This error, however, produces very little effect on the determinations of the proportion of the force of gravity at different places.
A series of pendulum experiments was made by Carlini, at the Hospice of Mont Cenis, to ascertain the diminution of gravity at the height of a thousand toises. The account of these is given in the Milan Ephemeris for 1824. The result obtained for the mean density of the earth agrees pretty well with that generally received; but the changes which experiment has shown to be necessary in the elements of reduction, throw a little doubt upon its value. The mountain Schehallien (on which Maskelyne's observations of attraction were made,) has been surveyed, and some alteration made in the numerical results: the calculations of Cavendish's experiments have also been corrected. See various volumes of the Phil. Trans.
In the theory, no improvement has been made, I believe, since the time of Clairaut. No satisfactory rule has been given for taking into account the elevation of the station: perhaps the considerations suggested by Dr. Young in the Phil. Trans. 1819, may be regarded as the most useful.
It is generally thought that the measures of arcs give an ellipticity of nearly 1/300 to the earth; some persons considering it a little greater, and others a little smaller. The pendulum experiments, with Clairaut's theorem, give an ellipticity rather greater, though not without remarkable anomalies.
IX. About the year 1800 the following may be considered
[page] 170
AS nearly the state of physical astronomy. The method of investigating the perturbations of the radius vector and longitude and latitude of a planet, and of expressing them by means of a single function, was well understood. The treatise in which this (and nearly everything relating to planetary perturbation,) is given with the greatest extension, is the Mécanique Céleste, a work which contains, without any acknowledgement, a vast quantity of the labours of preceding and contemporary writers. The method commonly referred to by the Germans is Klügel's, given in vols. 10 and 12 of the Göttingen Transactions: it differs little from that of the Mécanique Céleste. The general conception of the variation of elements had long been formed, and expressions had been given for each variation; but as they depended on differential coefficients of the perturbing function with regard to the coordinates of the disturbed planet, and not with regard to the elements themselves, they could not easily be applied to the planets. Still it was possible to use them, and Laplace has used them in one instance. The theory of the secular variations of the elements, the limits of variation of the eccentricity and inclination, the unlimited variation of the perihelion and node, and the permanency of the axis major, were (to a certain degree of approximation,) well understood. The perturbations depending on the second order of the disturbing force were well understood by Laplace. The long inequality of Jupiter and Saturn (a discovery which has been stated, though not quite correctly, to have "banished empiricism from astronomy,") had been calculated, and even the terms of the second order had been included (by proper application of the expressions for the variation of the elements): the acceleration of the moon's mean motion had also been explained, and the inequality depending on the sun's parallax had been pointed out, as well as that depending on the earth's ellipticity. And (which appears to me the greatest step of all,) the remarkable relation between the motions of Jupiter's three first satellites, which exists in consequence of their mutual perturbations, and depends on the second order of the disturbing force, had been explained. These theories had been numerically applied to all the planets, the terms depending on the second and third powers of the eccentricities being (unnecessarily) developed by a method different from that used for the first powers. The lunar theory was almost perfect. The general methods of computing the perturbations of comets had been well explained by Lagrange. With regard to the figure of the planets, Laplace's remarkable theory had appeared. The theorems for precession, change of obliquity of ecliptic (depending on the change of both the
[page] 171
equator and the ecliptic), &c., were almost complete. It will be seen that comparatively little has been added since the beginning of the century.
In 1808, Laplace presented to the French Bureau des Longitudes a Supplement to the third volume of the Mécanique Celeste. Lagrange immediately after produced equivalent results obtained in a different way (Mémoires de l' ζ Institut 1808). These essays may be considered as completing the theory of planetary perturbations. Their object was to express the variation of all the elements by differential coefficients of the perturbing function (supposed to be expanded in terms of the elements and the time), taken with respect to the elements only, and multiplied only by functions of the elements. The perturbations of the elements can therefore be found from the usual expansion of the perturbing function; and then the true position in longitude and latitude can be found by using the elements corresponding to that time as if they were invariable. It has been objected, that whereas we want only three quantities (perturbations of radius vector, longitude, and latitude,) we in fact investigate six (those of the six elements). I believe, however, that in any case the investigation is not more difficult, and in many cases the saving of time is very great. For instance, in an inequality of long period, which is always accompanied by other terms; if the method of variation of elements is used, the development of one term only of the perturbing function is sufficient: if the original methods were used, the development of several terms would be necessary, and the treatment of each of these would be more troublesome. But it is principally with regard to terms of the second and higher orders of the disturbing force that its advantage is felt: it is necessary to substitute in the expressions values of the elements as near as possible to the true ones, and the method therefore becomes a very simple successive approximation, no reference to the longitude &c. being necessary till the whole is completed.
To reduce the calculation of perturbations to a mere me-chanftca]operation, nothing was wanting but the expansion of the perturbing function. This was given in part by Burckhardt in the Mémoires, 1808: the terms depending on the inclination were not included; but those depending on the eccentricities and their combinations were given to the sixth order.
On the variation of constants generally (in mechanics as well as astronomy), and the secular variation of the elements, the most able papers are by Lagrange, Mémoires, 1808 and 1809; Poisson, Journal dc l' Ecole Polytechnique, vol. 8, and Mé-
[page] 172
moires, 1816. The point to which greatest attention is paid is the variation of the axis major. Laplace had previously shown that it contained no permanent terms to the third powers of eccentricity, &c. and the first order of the disturbing force: Lagrange had extended this to all terms of the first order of the disturbing force: Poisson now extended it to the second order of the disturbing force, as far as fourth powers of eccentricity, &c.; and Lagrange showed that the same theorem is true generally to the second order of forces, whether we consider the perturbing body to be itself liable to perturbation, or not.
In the Göttingen Transactions 1816-1818, Gauss investigated the secular variations of the elements, supposing the disturbing body extended over the line of its orbit, the proportion of the thicknesses at different points being the same as that of the time actually occupied in describing a given length. The ingenuity of the transformations, &c., deserves notice, but the theory of perturbations has gained nothing.
In the Memoirs of the Astronomical Society, vol.2, M. Plana made some remarks on the correctness, in point of form, of Laplace's investigation relative to the constant alteration in the axis major, and on the accuracy of his results as to the effect of the attraction of the stars. In the Conn. des Temps 1829, Laplace made some alterations in his investigation of the latter.
In Nos. 166, 167, 168, and 179 of the Astronomische Nackrichten, Hansen has presented the theory (with reference to practical applications,) in a form that well deserves attention. Instead of determining the true longitude by means of the usual elements, all which (including the mean longitude corresponding to any given instant,) are variable, he assumes that the true longitude shall be determined by the usual expression for longitude applied to invariable elements, the mean longitude only (at any fixed epoch) being considered variable. He assumes also that the true radius vector shall be determined by applying the usual formula for the elliptic radius vector to the same invariable elements and variable epoch of mean longitude, and adding to this expression certain variable terms. This method was probably suggested to its author by the observation that, in the great inequalities of long period, the variation of epoch is much more important than the other variations. At all events, it is a form particularly well adapted to the construction of astronomical Tables, and the more so as Hansen found, in application, that the convergence of the terms in this method, especially for the higher orders of the disturbing force, was more rapid than in any other. Laplace's or Lagrange's expres-
[page] 173
sions for the variation of the elements are of course assumed as a foundation for the first investigations.
In the Berlin Memoirs, 1824, Bessel has given a method of investigating separately the effects of perturbation produced by a planet's action on the sun and its action on another planet. This was done in consequence of the agitation of the question, to which I have before alluded, whether the absolute force of the planet on these bodies was the same; a question first started (I believe,) by John Tobias Mayer, Gött. Trans, 1804-1808. The physical investigation consists merely in taking the two terms of the perturbing function separately: this paper however is remarkable for the mathematical part of the process, which by a mixture of general integration and definite integration, assisted by special Tables, seems well adapted to the accurate calculation of planetary inequalities. The subjects of investigation are the perturbations of radius vector, longitude, and latitude (that of the longitude being expressed independently of the radius vector,) to the first order of the disturbing force.
In the Phil. Trans. 1830, 1831, and 1832, Mr. Lubbock has given four papers on the general problem of perturbations. The object of the first of these is to give expressions for the variation of the elements which shall be true to all orders of the disturbing force, (which however holds with regard to Laplace's and Lagrange's expressions,) together with equations in which the eccentric anomaly is the independent variable. In the second it was shown that the perturbations of the reciprocal of the radius vector might be found more readily than those of the radius vector itself. The rest of these papers (relating to perturbations in general,) is occupied with expansions, and with theorems equivalent to those of Laplace, but in a different form. In the Phil. Trans. 1832, Mr. Ivory has also given an investigation of the perturbation of elements, and Mr. Lubbock has shown the identity of the results obtained by perturbation of the elements and by perturbation of the co-ordinates: it is not the object of these papers to extend the theory of perturbations. In the München Denkschriften and the Turin Memoirs, Pfaff and Cisa de Grésy have given various expressions, which however are only equivalent to those of preceding writers.
In the Milan Ephemeris for 1818, and the Memoirs for 1823, Carlini and Laplace have shown that some of the series by which a planet's place is expressed in terms of the mean longitude, cease to be convergent when the eccentricity exceeds 0·62. In the Berlin Memoirs 1816-1817, Bessel has expressed
[page] 174
the co-efficients of these series by means of definite integrals: and in the Conn. des Temps 1825, Poisson has done nearly the same thing. This principle, I believe, has lately been extended by Cauchy. In the Conn. des Temps 1828, Laplace has given means of estimating the value of distant terms in the expansion of the perturbing functions.
I am not acquainted with any other additions to the theories of elliptic motion or perturbation in general.
With regard to the solar theory, Nicolai, in the Berliner Jahrbuch 1820, investigated the secular variations of the Earth's orbit, as a verification of those given by Lagrange and Laplace. In the Phil. Trans. 1828, the author of this Report announced the discovery of a small inequality of long period in the Earth's motion produced by the action of Venus, and a corresponding inequality in the motion of Venus produced by the Earth: the details of the calculation are given in the Phil. Trans. 1832. And in the Milan Ephemeris, 1830 and 1831, (the latest volumes that I have been able to procure,) Carlini has given an investigation, not yet completed, of an inequality in the Earth's motion, depending on the Sun's distance from the Moon's perigee. It has commonly been thought sufficient to consider the motion of the centre of gravity of the Earth and Moon the same as if their masses were united there: but it is quite conceivable that a small error in this may grow up into a sensible inequality; and this, I believe, is the subject of the investigation.
The lunar theory has been much discussed. In the Conn. des Temps 1813, is a paper by Laplace on the inequality of long period, which, from observation, seems to exist in the Moon's motion. In the Mécanique Céleste he had been disposed to attribute it to a term independent of the Earth's form, which had been pointed out by Dalembert; in this paper he inclines to that depending on the difference of the northern and southern hemispheres. This inequality, with an empirical coefficient, was adopted in Burckhardt's Tables. In the Conn. des Temps 1823, Laplace re-investigated the equations depending on the Earth's ellipticity, and on comparing their values with those found by Burg and Burckhardt from observations, fixed on 1/306 as its value. The Institut having offered a prize for a complete lunar theory, in which the values of the co-efficients should be calculated from theory only, two that were sent in were deemed worthy of the prize, one by Damoiseau, the other by Carlini and Plana. The latter, I believe, is not printed; the former is in the Savans Etrangers. The general method pursued by Damoiseau is the same as Laplace's in the
[page] 175
Méc. Cél.; the rule for retaining terms being directed principally by the magnitude of their numerical values, and not by the order of small quantities. The immense calculations of this theory are given with great clearness and attention to order. In the Conn. des Temps 1823, Laplace gave some remarks on the two memoirs, giving generally the preference to Damoiseau's, partly because he had followed Laplace's method. Carlini and Plana replied in an elaborate paper in Zach's Correspondance, vol. 4. Without attempting to analyse it, I shall only remark that I think no one can have an idea of the delicacies and difficulties in a theory of the Moon in the present day, without examining this reply. In another paper in the same volume, they considered one of the most troublesome equations, depending on twice the distance of the perigee from the node. Damoiseau, as well as Carlini and Plana, found that the equation, depending on the difference on the hemispheres, would probably be insensible: and Laplace (Conn. des Temps 1823,) assented to this. In the Conn. des Temps 1824, Laplace has given the investigations of several lunar inequalities of long period. In the same volume, Burckhardt, after discussing several occupations, maintains the necessity of some equa tion not yet given by theory. In the Milan Ephemeris 1825, Carlini suggests an equation depending on six times the distance of the perigee from the node diminished by the Sun's mean anomaly: the period of this would be 1760 years. In Mr. Lubbock's papers, before alluded to, the lunar theory is considered. The author has commenced the investigation in a manner different from that of Laplace, Damoiseau, Carlini, and Plana, by making the time the independent variable in the equations; and has given Tables for facilitating the research of the terms arising from the combination of other terms. He has also given developments of the perturbing function adapted to this case.
In the theory of Mercury, a discussion of an insignificant numerical quantity has taken place between Laplace and Plana, Mem. Ast. Soc. vol. 2, and Conn. des Temps 1829. In the theory of Venus, I believe, the only addition is the term investigated by the writer of this paper, (before alluded to,) and depending on the difference between eight times the mean longitude of Venus, and thirteen times the mean longitude of the Earth. This inequality is small; but as the corresponding inequality of the earth has the opposite sign, and as Venus at inferior conjunctions is very near the earth, the effect of the inequality at those times will be very sensible. In the Conn. des Temps 1820, Burckhardt gave the equations for the prin-
[page] 176
cipal perturbations of Jupiter to the sixth order of eccentricities, &c. In the Mem. Ast. Soc., vol. 2, Plana gave remarks on Laplace's investigations of the perturbations of Jupiter and Saturn depending on the second order of the disturbing force. As the greater part of Laplace's investigation was suppressed, it was only possible to compare the results, and to examine the correctness of some equations given by Laplace. Plana's results differed much from Laplace's; and a simple equation between the perturbations of Jupiter and those of Saturn, given by Laplace, appeared to be incorrect. Laplace in answer (Conn. des Temps 1829, published 1826,) allowed that his equation was not perfectly correct, but maintained that Plana's error was much greater. In the Turin Memoirs 1827, Plana in reply said that Laplace's answer did not enter sufficiently into details. In the Conn. des Temps 1831, Poisson pointed out some terms omitted by Plana. In the Turin Memoirs 1830, Plana again made some calculations, and still obtained results differing from Laplace's. Finally, in a memoir of which an extract is printed in the Conn. des Temps 1833, M. Pontécoulant stated that he had found errors in the calculations both of Laplace and of Plana, and that on correcting these, both determinations agreed. In the Turin Memoirs 1831, Plana acknowledges that this is true. And thus the discussion of these terms appears to be finished, and physical astronomy has gained much from this inquiry, prosecuted at first by M. Plana under the repulsive circumstances of comparing a final result with Laplace's without an intermediate step. An elaborate investigation of the theory of Jupiter and Saturn by Hansen, on the principles which I have described as peculiar to him, has lately been received in this country.
On the theory of satellites, little has been done. In the Conn. des Temps 1821, Laplace has investigated the effect of the long inequality of Jupiter and Saturn on the other bodies of the system, and has shown that they are sensible only in the motions of Jupiter's satellites. In the Ast. Soc. Mem., vol. 2, Plana objected to Laplace's theory, in reference to the seventh satellite of Saturn. Laplace, in the Conn. des Temps 1829, maintained its correctness, which Plana (Turin Mem. 1827,) has again denied. In the Conn. des Temps 1831, Poisson has shown that both methods produce the same results: and here, I believe, the question rests. In the Ast. Nachr., No. 193, are expressions, by Bessel, for those variations of the elements of Saturn's sixth satellite which do not depend on its position in its orbit: the permanent variations and variations of long period, in fact, analogous to the secular equations of the planets.
[page] 177
Of the methods used by the German astronomers for the calculation of the perturbations of the small planets, I can give no complete account. I regret this the more, because the magnitude of their perturbations is far greater than those of any other planets. For though it may not appear, as far as their general theory has yet been carried, that they have equations as large as the great inequality of Saturn, which however is 450 years in passing from one extreme value to the opposite, yet the magnitude of their perturbations in a given time, one year for instance, and the consequent irregularity of their motion, is very much greater than that of Saturn. This only I can state, that the Germans do not generally compute the perturbations of longitude, latitude, and radius vector, but the perturbations of the elements of the orbit; and these, I believe, entirely by mechanical quadratures; in other words, by summation instead of integration, in a method analogous to that which they use for comets. Perhaps in some calculations for Vesta, as in part of those by Encke, Berlin Memoirs 1826, they may use Tables and apply the perturbations directly to the radius vector, &c.: but even in this instance, the most important part of the perturbations, namely, those produced by Jupiter, are computed by quadrature, the elements being corrected for perturbation: and Encke conceives this to be more accurate than the use of Tables. The intervals used here are of forty-two days each, and the fresh corrected elements are used after every sixth or seventh interval.
The groundwork of Lagrange's method for the perturbation of comets (Méc. Cél. tom. 4. liv. 9.) consists in estimating the disturbing forces resolved in the direction of three rectangular co-ordinates, finding the effect of these on the elements of the comet's orbit, and performing the integration by quadratures. The method given by Bessel in the Untersuchungen über die scheinbare und wahre Bahn des im Jahre 1807 erschienenen grossen Kometen, referred to by the Germans as the standard work, consists in resolving the disturbing forces in the direction of the comet's radius vector, a perpendicular to the radius vector in the plane of the orbit, and a perpendicular to the orbit. The inclination and node are referred to the ecliptic. From these quantities, expressions are found for the variations of the elements, which are integrated by quadratures. This is the method used by Bessel in the calculations relative to Olbers's comet, Berlin Mem. 1812-1813. For the comet of 1807, Bessel has calculated the quantities for every thirty days; and for Olbers's comet, he has taken intervals of twenty-five days during its visibility, of one year from 1815 to
M
[page] 178
1883, and of two years from 1833 to the time of its reappearance, 1887. In the Ast. Nachr. Nos. 210 and 211, Encke has described the method by which he calculated the variation of elements of the periodical comet bearing his name, undoubtedly the most troublesome of all. Using the same general methods, the perturbations produced by Mercury were computed for every four days; those of the Earth and Venus for every twelve days; and those of Mars, Jupiter, and Saturn, for every thirty-six days. These calculations were carried on till the comet reached a certain distance from the planets, and then its place was referred to the centre of gravity of the sun and planets. For some details Encke refers to Argelander's treatise on the comet of 1811, a work which I have not been able to procure. For Encke's comet the effect of a resisting medium, whose density is inversely as the square of the distance from the sun, was calculated by the same method. This had been done analytically for several laws of density by Plana, in Zach's Correspondence vol. 13; it is also noticed by Mosotti,Mem. Ast. Soc. vol. 2. In the Conn. des Temps 1832, Damoiseau has described his own method. Me refers the co-ordinates to the original plane of the comet's orbit, (taking its original axis-major for the axis of one ordinate,) and resolves the disturbing forces in these directions, and finds the variation of elements in terms of these forces, which he integrates by quadratures. As the ordinates of the comet are conveniently calculated by means of the eccentric anomaly, he calculates the variations for given intervals of that angle. This is the method that he has adopted in the Turin Memoirs 1817-1818, for Halley's comet, varying the eccentric anomaly by 1° each time for the perturbations of Jupiter, by 2° for those of Saturn, and by 6° for those of Uranus. He has also used it in the calculations for the comets of short period,Conn. des Temps 1827 and 1830, and Memoires 1826. In the Conn. des Temps 1883, is an extract from Pontéoulant's Memoir on the same comet; he refers generally to Lagrange's method, and states that having with the first elements computed by quadratures the perturbations of the elements through 30° (of eccentric anomaly, I suppose), he has then used the elements so corrected in the calculations for the next 30°, when he has again changed the elements from the result of these calculations; and so on for each successive 30°.
The following additions have been made to the theories connected with the figure of the earth, &c. In the Phil. Trans. 1809, is a paper by Mr. Ivory, of which the most important part is the very beautiful theorem for finding the attraction of a
[page] 179
spheroid generally, on a point without it, from the attraction of a spheroid on a point within it. In the Memorie della Società Italiana, 1810, and the Göttingen Transactions 1811-1813, Plana and Gauss have given theorems founded on the same kind of integration, and adding little to our knowledge of the subject. In the Journal l' deζEcole Polytechnique, tom. 8, is a paper by Lagrange on a difficulty in Laplace's general theory; and in the Phil. Trans. 1812 and 1822, Mr. Ivory has pursued this objection, and given a method of his own, of very great analytical elegance. In the Cambridge Transactions, vol. 2, the author of this Report supported Laplace's correctness with respect to the point objected (as Laplace had done himself in the fifth volume of the Méc. Cél.), and pointed out what he considered to be another defect in Laplace's reasoning. In a discussion on the figure of the earth, Phil. Trans. 1826, I gave a theorem analogous to Clairaut's, admitting of extension to all powers of the ellipticity. In the Conn. des Temps 1829, Poisson gave a very able memoir on the attraction of spheroids. In the Phil. Trans. 1824, and in a later volume, Mr. Ivory introduced a new equation in the consideration of the equilibrium of fluids of which the particles mutually attract each other; the necessity for this has not been generally allowed, and was explicitly denied by Poisson in a paper (treating also on other points,) in the Conn. des Temps 1831. In the French Memoirs 1817 and 1818, Laplace has applied his general method to the case of an irregular nucleus covered by a fluid; the most general case that can be conceived, and the case that comes nearest to the state of the earth, but which analysis has not yet completely mastered. In the Conn. des Temps 1821, he gave as a consequence of this theory, that the gravity on a continent reduced to the level of the sea by the factor depending only on the distance from the earth's centre, follows the same law as at the surface of the sea. In the Journal de l' Ecole Poly technique, tom. 8, Poisson investigated the motion of the earth's axis of rotation within the earth itself (considering the motion of the axis in space as completely treated in the Méc. Cél. liv. 5). He found that neither the place of the axis nor the velocity of rotation is permanently altered. In the Mémoires, 1824, he has treated of the earth's motion about its centre generally (by variation of constants), and has compared his numerical results for the obliquity, &c., with observation. In the Conn. des Temps 1827, Laplace has alluded to the combined effect of change in the plane of the ecliptic and precessional motion of the earth's axis; and has shown that in consequence of the latter, the limits of the diminution of obliquity are very much contracted.
M 2
[page] 180
In the volume for 1821, Poisson has treated the precession of the equinoxes by Lagrange's method of the variation of constants. In the same volume is a paper by Laplace on the effect which the sea produces on the earth's motion round its centre. In the volume for 1823, he has shown that, supposing the earth's dimensions to have altered by cooling, the effect on the length of the day would not be sensible. In Zach's Correspondance, vol. 14, Plana has deduced from Lindenau's nutation a value for the moon's mass, which however does not agree with that generally obtained from it.
In the Conn. des Temps 1821 and 1822, Poisson has treated of the libration of the moon. His special object is to determine the inequalities in the inclination and node of the moon's equator, depending on her secular inequalities.
I have been obliged almost to confine myself to a bare enumeration of the titles and subjects of these works, partly by the fear of occupying too much space, and partly because it is impossible to give an opinion on the methods and accuracy of many, without having worked through every line of the investigations; a degree of acquaintance with them which, I suppose, no person living can pretend to possess.
I may mention that treatises, of a more elementary kind than the originals, and embracing different parts of the subject of this section, have been published in England, France, Italy, and Germany.
X. In the preceding sections I have endeavoured to give materials for estimating the steps which Astronomy has made in this century, and for understanding its present state, at least in all the important parts. But I cannot forget that the Association which I have the honour to address, while it is a Philosophical Association, is also a British Association, and that while it is anxious to promote science abstractedly, it is also jealous of our national scientific character. I feel therefore that my Report would be incomplete if I did not, in some degree, give means for answering the questions, What has England contributed to the progress of Astronomy? and, How have the knowledge and practice of Astronomy advanced generally in England?
I fear that the answer to the first of these questions will not be very satisfactory. While I allow that in some important parts of Astronomy we have done much, I cannot conceal that in other parts, especially those which cast a lustre on the conclusion of the last century, and those which are peculiarly distinctive of the present century, we have done nothing.
[page] 181
A subject so complicated as Astronomy, may be divided in several different ways, and thus different comparisons may be made as to the progress of its various parts. I shall here view the subject in two different manners, and I will assert:—
First, That in those parts which depend principally on the assistance of governments or powerful bodies, requiring only method and judgement, with very little science, in the persons employed, we have done much; while in those which depend exclusively on individuals, we have done little.
Secondly, That our principal progress has been made in the instrumental and mechanical parts, and in the lowest parts of Astronomy; while to the higher branches of the science we have not added anything.
I must of course refer generally to what has gone before for materials to justify these assertions; but I may here point out a few of the leading facts which have induced me to bring forward these opinions.
With regard to the first, I can assert that we have contributed more than all the rest of the world to furnish materials for ascertaining the figure of the earth. This praise is to be divided, I suppose, between our Government and the East India Company. Be that as it may, I conceive that nothing which has been done by other nations can be put in competition with the arcs of meridian and parallel in England, the great arc of meridian in India, and the pendulum expeditions of Kater, Foster, Sabine, &c. To some of the latter, objections have been made which are in my opinion groundless; but if they were ever so well founded, they would detract nothing from the merit of originating these expeditions. But these expeditions, though they require care and prudence in the persons who conduct them, demand very little science. The vast improvement of chronometers is entirely due to the encouragement offered by our Government. I may also assert that the observatories depending on our Government are maintained with an extent of establishment which few governments would be willing to allow. And in speaking of this, I cannot forbear alluding to one Institution, which I hope some future reporter on Astronomy will be able to describe as having been beneficial to the science. The Observatory at Cambridge was built, not from any fund bequeathed of old for the purpose, nor with the assistance of any other body, but partly by grant of the University as a corporate body, when its funds were ill able to support such an expense, and partly by the private subscription of its members. It was built and is to be furnished on a plan which will enable it to stand in competition with any other at home or
[page] 182
abroad. Whatever may be its success, none is more creditable to the body which founded it.—Now if we examine what has been done by individual attempts, we shall find it small. We have discussed theories of refraction and aberration, perhaps quite as much as our share in the science requires; but we have done nothing in examining the past state of the heavens, or making it subservient to a knowledge of their future state: the reduction of Bradley's observations was left to a foreigner; the formation of Tables of the Sun and Moon, from British observations, even when the theory was put in a distinct shape, was left to foreigners; and, as if we had determined to leave the present state of the heavens also in obscurity, our own observations have too generally been cast on the world unreduced, with a hope, I suppose, that others would have the zeal to reduce them. The observations that require only moderate instruments, with patience and zeal on the part of the observer, as the discovery and observation of comets, and the observation of the small planets, (which on the Continent have generally been made with unmounted telescopes,) have been little attended to. Of the latter, some observations by Mr. Groombridge, some at Greenwich, and a few by myself, constitute, I believe, the whole amount.
I will not deny that there are some exceptions to my general assertion; and in one of these my hearers will anticipate me. I think that I can fix on only two discoveries, the results of combined theory and observation, which are original in the present century, and one of these belongs to an Englishman. New planets and periodical comets had been discovered in the last century; abstract theory of every kind and observations of almost every kind had been produced: but the existence of a resisting medium was established in this century by Encke, and the practical prediction of the phases of double stars is due to Sir John Herschel. Nor can I omit to mention Sir Thomas Brisbane and Mr. Baily, and (for several investigations connected with the physics of Astronomy,) Mr. Ivory, and lately Mr. Lubbock. But after every credit has been given to their labours, it will, I believe, be allowed that the part in which England has contributed most to Astronomy, and which is likely to be mentioned with greatest gratitude by future historians of the science, is that in which she has contributed as a nation.
In proof of the justice of my second assertion, the following remarks may be sufficient. Our instruments I conceive (though a German would not allow it,) to be superior to those of any other nation. The observations at our observatories are conducted, I imagine, with greater regularity and greater steadiness
[page] 183
of plan than those of foreign observatories. This, indeed, is the character which gave (in some respects) preeminent value to the Greenwich observations of last century, and which makes those of the present century highly valuable. In the reduction of these observations we begin to fall off. Though Dr. Brinkley has investigated from observations a new Table of refractions, and applied it to his own observations, yet Bradley's Table, known twenty years since to be sensibly erroneous, is still the standing Table of refractions at Greenwich. The discussion of the reduced observations has been, I think, confined absolutely to the proper motion of stars. On one or two occasions a number of observations of the moon have (by order of the Board of Longitude,) been compared with the then existing Tables, but not with a view of improving the Tables. I have had occasion to mention the correction of the elements of the earth's orbit made by myself (from Greenwich observations), and the discovery, in consequence, of a new equation in the perturbations of the Earth and Venus. As far as I have been able to ascertain, this was the first improvement in the solar Tables made by an Englishman since the time of Halley, and the first addition to the solar theory since the time of Newton. From English observations of planets it has been impossible to extract a result, because scarcely any have been made. To show the extent of this deficiency, I will mention a mortifying circumstance that has occurred to myself. In order to verify completely the equation above alluded to, I was desirous of collecting observations of Venus near her inferior conjunction. In examining the Greenwich observations I found that no opportunity of making this observation was omitted by Bradley or his immediate successor Bliss; soon after the accession of Maskelyne it was wholly neglected; and from that time till several years after his death scarcely an observation is to be found: several conjunctions have been passed over by the present Astronomer Royal; five however have been completely observed. Under these circumstances, (though the deficiency for the latter part of the time only might be supplied from scattered foreign observations,) considering how desirable it is, in a research of some delicacy, to use observations made at the same place, I believe that I shall be compelled to abandon it entirely. The superior planets have been more frequently observed, and those but very little. And generally as to the comparison of theory with observation, and its immediate consequences, the reducing of complicated phænomena to simple laws, or the showing that new supplementary laws are necessary, forming altogether the most glorious employment for the intellect of man, I may state,
[page] 184
in one word, to the best of my knowledge nothing has been done in England. In the lunar and planetary theories we have done nothing, not even in the way of numerical application. In the theory of the new planets and the periodical comets, we not only have done nothing, but we have scarcely known what others have done. With regard to the latter points, the distinguishing discoveries of the present century, our humiliation is great. Some of the new planets are very faint, and all are subject to excessive perturbation. If Astronomy had been confined to England, we never should have rediscovered them, even if we had once made out their orbits. If Astronomy had been confined to England, the paths of the comets would never have been traced, and the consequences deduced from the appearances of Encke's comet, the brightest discovery of the age, would have been lost. While Germans, Italians, and Frenchmen, have emulously pushed on the theory and the observation of these bodies, Englishmen alone, of all the nations professing to support a high scientific character, have stood still.—I am glad to turn from this dispiriting subject.
There are other points to which I can scarcely allude without introducing a degree of personality which cannot be admitted in a public Report. They can be understood perhaps only by those who know the state of observation here, and who have seen the interior of foreign observatories. Of the latter, I can only profess personally to be slightly acquainted with those of France and those of the North of Italy. The characteristic difference between the spirit of the proceedings in England and on the Continent may be stated thus.—In England, an observer* conceives that he has done every thing when he has made an observation. He thinks that the merely noting the passage of a star over one wire and its bisection by another, is all that can be expected from him; and that the use of a Table of logarithms, or anything beyond the very first stage of reduction, ought to be left to others. In the foreign observatories, on the contrary, an observation is considered as a lump of ore, requiring for its production, when the proper machinery is provided, nothing more than the commonest labour, and without value till it has been smelted. In them, the exhibition of results and the comparison of results with theory, are considered as deserving much more of an astronomer's attention, and demanding greater exer-
* I am far from asserting that this is the character of every English observer, and I am equally unwilling to point out any individual to whom it is applicable. My object is merely to explain what I conceive to be the kind of difference which exists between English observers generally and foreign observers generally.
[page] 185
cise of his intellect, than the mere observation of a body on the wire of a telescope. As an instance of the extent to which the reductions are carried there, I may mention that in one Italian observatory where the planets were considered the principal object, not only were the observations freed from instrumental errors and astronomical corrections, but the tabular places were computed by direct use of the Tables, (the ephemeris attached to Schumacher's lunar distances not having reached that country,) and the equations of condition were regularly prepared for the correction of the elements. I suppose such a thing has never been done in England. This system must however contribute powerfully to produce that strong connexion between physical theory and practical observation, which is general on the Continent, but which does not exist in England.
I believe that in the actual state of our institutions, reasons might be found which would seem to render it improbable that there ever can be so strong a connexion; and I can only hope that my view may be incorrect. There is one point with regard to the foreign astronomers to which I cannot help alluding, without however intending to draw any distinct inference. It is, that they have first obtained distinction while in the lower departments of the observatories. Encke's reputation was first acquired, not when he became Astronomer at Berlin, but when he was assistant at Seeberg: and Bessel became known in every part of Europe, not as Astronomer at Königsberg, but as assistant at Lilienthal. Walbeck and Argelander, in similar situations, have arrived at considerable eminence.
I now proceed, and with great pleasure, to consider the second question. And this leads me to explain my opinion on a point respecting which I am anxious that I may not be misunderstood. I am not one of those who have joined in the cry of "the decline of science in England," nor do I believe that in this science there is any foundation for that cry. On the contrary, I assert without hesitation, that it is now and has been for some years rapidly advancing in this country. That there has been a decline, thirty or forty years ago, or rather that we have not kept up with the advances made by foreigners at that time, I am willing to admit. Perhaps this arose from political separation; perhaps in some degree from our pertinaciously retaining a system of mathematics which was insufficient for the deep investigations of Physical Astronomy, (for it was in this principally that we were behind our neighbours). And I have not disguised my opinion that in all the important branches of science we are still behind them. But in all with which I am acquainted a rapid progress has lately been made. In Physical Astronomy more
[page] 186
has been done in England within the last five years than in the preceding century; and this not only with regard to the additions actually made by Englishmen to the stock of results drawn from that science, but also with respect to the number of persons who understand its principles, and who at some future time may be expected to contribute to its progress. In the University with which I am best acquainted, the study of this subject has made great advances. Of the amount and excellence of our geodetic measures and pendulum experiments, and of our discussions of refraction and aberration, I have already spoken. In accuracy of examination and correction of instrumental errors, perhaps something has been gained. In the extension of our star catalogues, much more has been done within a few years than in the whole previous time which followed Bradley's death. In the observation of planets, and the regular comparison of observations with Tables, (the first essential step to the improvement of the latter,) it is hoped that a great advance has been made. The observation of occultations and eclipses has extended; the exhibition of the results also, both for terrestrial and celestial determinations, has increased; and the regular publication of them in the Memoirs of the Astronomical Society, saves from oblivion the past and insures more completely the observation of the future. In the observation of double stars very much has been done. In all this I see grounds for exultation at "the advance of science in England." And when I remark the growing intermixture of physical with observing science, I indulge in the hope that the character as well as the extent of our Astronomy is improving, and that the time is approaching when a person will not in England be considered a great astronomer because he can observe a transit or measure a zenith-distance correctly.
XI. In conclusion, I shall suggest a few points to which it seems desirable that some attention should be directed. In this part however, more than in any other, the judgement of an individual must be considered fallible.
1. After all that has been done in respect to refraction, I suppose that there is no subject of such continual application, in the theory of which so many difficulties occur, and in whose application there are so many discrepancies. It seems not improbable that some of the latter may depend upon anomalies in the indications of the thermometer, owing perhaps to the effects of radiation, the nature of which till lately has been little understood; and scarcely recognised among astronomers. In some of Mr. Fallows's pendulum experiments it appeared to account for many
[page] 187
discordancies. 1 think that astronomers would be glad to receive simple directions for placing a thermometer so as to indicate correctly the temperature of the air at the place of observation. I omit all discussion of the difference of the external and internal thermometer, as I think the only way of overcoming the difficulty is, to make the external and the internal temperature as nearly as possible equal.
2. In the theory of refraction, the following questions present themselves as only to be solved by experiment. What is the law of the decrease of temperature, or rather of density, in ascending? How does this vary at different times? Can any means be contrived for indicating practically at different times the modulus of variation? (The last question is suggested by the remarks in Mr. Atkinson's valuable paper, Mem. Ast. Soc. vol. 2.) Does the refractive power of air depend simply on its density, without regard to its temperature? Is it well established that the effects of moisture are almost insensible? From Carlini's Tables, as well as from general reasoning, it seems likely that refraction may be different in different azimuths, according to the form of the ground: can any rough rule be given for estimating its effect? Finally, when the atmospheric dispersion is considerable, what part of the spectrum is it best that astronomers should agree to observe?
3. I have already stated that I think Lindenau's constant of nutation has been adopted by the German astronomers on insufficient grounds. The value which I should certainly prefer is that determined by Dr. Brinkley, and which Mr. Baily, with his usual judgement, adopted for the catalogue of the Astronomical Society. The Greenwich circles have now been erected, and in a perfect state, long enough (or nearly so,) to determine this constant; and the mass of excellent observations which they have produced, applicable to this question, vastly exceeds any other that has been used for the same purpose. It is highly desirable that the coefficient of nutation should be investigated from the Greenwich circle-observations.
4. Bradley's observations of stars were nearly useless till Bessel undertook to reduce them. In like manner Bradley's and Maskelyne's observations of the sun and planets are still nearly useless. At different times observations of the sun have been reduced (by Delambre, by Burckhardt, and lately by Bessel or Schumacher), and probably much labour has been wasted. A reduction of these observations on a uniform plan (adopting, for instance, Bessel's Tabulœ Regiomontanœ,) would be invaluable in the application of the planetary theories. Many observations of the moon have been reduced
[page] 188
and published; but, for the sake of uniformity of system, it would be desirable to re-compute them.
5. I have mentioned that the perturbations of the small planets and of Encke's comet give good reason to believe that the mass of Jupiter adopted by Laplace is too small. Laplace's estimation is founded on Pound's measures of the elongation of Jupiter's satellites; and I am not aware that any measures have been made since that time. It is extremely desirable that they should be measured, at least those of the fourth satellite. It would be sufficient, in observing the transit of Jupiter, to observe also the transit of this satellite, one or two days before and one or two days after the time of its greatest elongation, as the theory of the satellites could be applied without difficulty to this measure.
6. The dimensions of the orbit of Encke's comet, as investigated by Encke, depend upon the assumed law of density of the resisting medium. In fact, by assuming a law, he has established a relation between the diminution of the aphelion distance and the diminution of the perihelion distance, which would not hold with any other law. It will be interesting now or at some future time to investigate separately from observations the diminution of these two elements, as a means of proving Encke's law, or of suggesting a new one.
7. The perturbations of Biela's comet have not been calculated, I believe, for the interval between 1772 and 1806, nor those of the node and inclination from 1806 to 1826. It is desirable that this should be done, both for ascertaining the identity of the comet of 1772 (which is not perfectly established), and for examining whether this comet, like Encke's, gives any indication of a resisting medium.
8. The most laborious part of the expansions in physical Astronomy is completely dispensed with by the use of Burckhardt's formulae in the French Mémoires for 1808. But Burckhardt expressed himself very doubtful as to their accuracy; and they do not comprehend any terms depending on inclination. It is desirable that they should be verified, and extended to the terms depending on the inclination.
9. The theory of the perturbations of Pallas has so often and so vainly been proposed, that it would seem useless to urge it again, and still more so to propose the theory of the perturbations of Encke's comet. Yet I conceive this to be the problem which at present demands the efforts of physical Astronomy. It is plain that there is no hope of solving it by any of the usual methods, as the series, which in other cases are convergent, here either diverge or converge so slowly as to be
[page] 189
useless. But it may be possible to choose functions for these expansions, by the use of which the series may become convergent (such perhaps as sin θ/1 + b cos θ instead of sin θ). At all events this may be fixed on as being at present the difficult problem of Physical Astronomy.
In the preceding suggestions I have endeavoured to fix on definite points for the attention of astronomers. I need not mention that there are other subjects (the theory of Uranus, for instance,) in which the existence of difficulties is known, but in which we have no clue to their explanation.
G. B. AIRY.
Observatory, Cambridge,
May 2, 1832.
Report on the Tides. By J. W. LUBBOCK, V.P.§ Treas. R.S.
THE connexion between the Tides and the motion of the moon was known to the ancients; but we are indebted to Newton for the discovery of the mechanical principles which regulate these phænomena. Newton contented himself with explaining the most obvious results of observation, and left all the details open to future inquiries. The subject was next taken up by Bernoulli, Euler and Maclaurin, about the same time, in their several treatises which participated in the prize awarded by the Academy of Paris in 1740. Laplace afterwards undertook this difficult investigation, and succeeded in forming the differential equations from which the explanation of the phænomena is to be derived. The integration of these equations presents, however, so many difficulties, that he confined his attempts to a very simple case, namely, that in which the depth of the ocean is constant, and the solid nucleus but little different from a sphere. Even in this case, his analysis is far from complete, and contributes but little to unravel a question which he has characterized, as "la plus epineuse de l'Astronomie Physique."
Finally, Laplace had recourse to the following indirect consideration, namely, "that the state of any system in which the primitive conditions have disappeared through the resistances which its motion encounters, is periodical with the forces which act upon it." Hence he concludes, that if the system is dis-
[page] 190
turbed by a periodic force expressed by a series of cosines of variable angles, the height of the tide is represented by a similar series of which the arguments are the same, but the epochs and the coefficients different. The adoption, however, of the preceding principle must be considered rather as an evasion of the difficulties by an indirect method, than an accurate and complete solution of the problem.
Lately, the Academy of Sciences of Petersburg has proposed the problem of the Tides for a prize question. The programme may be seen in the Number of the Annales de Chimie for February of the present year (1832).
Since the publication of the researches of Laplace, the theory of the integration of partial differential equations has been very materially improved by Fourier, and by MM. Poisson and Cauchy. The small undulations of an incompressible fluid, acted upon by gravity, which were not previously understood, were completely made out by the latter mathematicians, about the same time, in 1815. This case however, in which the force acting upon the fluid is constant, and in parallel lines, is the simplest which can be proposed; while the problem of the Tides in which the motions of the fluid are due to the action of a force of which the intensity and direction are continually changing, presents more serious difficulties, which are further increased by the circumstance that the bed of the ocean is far too irregular to be represented even approximately by any algebraic curve surface, and by the effect of the resistance and friction of the water against the shores, which cannot be considered as insensible.
The attention of Laplace does not appear to have been directed to the construction of Tide Tables for predicting the time and height of high water at any port; and indeed up to the present time the Table for this purpose published in the Annuaire du Bureau des Longitudes, is deduced, by a very slight alteration of form, from that given by Bernoulli in his prize essay. Nor does the subject appear, until very lately, to have met with any attention in this country, no attempt having been made previously to ascertain how far the theories of Bernoulli or Laplace can be reconciled with the results of observation on our coasts.
Formerly, the time of high water at London Bridge was obtained by adding a constant quantity, three hours, to the time of the moon's southing. As the mean interval is now very little more than two hours, we may infer that the time of high water in our river has been considerably accelerated; and this circumstance shows the importance of continual observations, and the
[page] 191
necessity of renewing from time to time these determinations. This method of adding a constant quantity was somewhat improved by Mr. Phillips in 1668, who gave, in the Philosophical Transactions for that year, a Table showing the variations in the interval between the time of the moon's southing and the time of high water. Shortly afterwards, Flamsteed having frequent occasion to pass between London and Greenwich by water, and having caused above 80 high waters at Tower Wharf and Greenwich to be observed, found that the greatest and least differences betwixt the moon's true southing and the high waters were not, as Mr. Phillips had placed them, at the full or new and quarter moons, but the greatest nearer to the neaps, and the least to the highest spring tides. Previously the Tide Tables had only shown the time of that high water which next follows the moon's southing; Flamsteed introduced both in his Tide Table.
In the earliest years of the Royal Society, some attempts were made to set on foot observations of the Tides by some of the active members; but these phænomena have long ceased until lately to excite any attention whatever in this country. Since the establishment of the various Docks at London, the times and height of high water have indeed generally been registered there in books kept for the purpose; and to these, which scarcely deserve the name of observations, must we have recourse if we wish to determine the various constants of the expressions which furnish the means of calculating the time and height of high water, or of ascertaining the agreement between theory and the tides in our river. Nevertheless by taking the mean of an immense number of observations, the error is almost eliminated; and in the unfortunate alternative of being obliged to relinquish the question altogether, or of making use of these imperfect data, I have preferred the latter, and, with the assistance of Mr. Dessiou of the Admiralty, have discussed a great number of observations made at the London Docks. I have found that when the effects of changes in parallax and declination are neglected, the agreement between the results of observation and the theory of Bernoulli (which so far coincides with that of Laplace,) is very remarkable.
With respect to the effects due to changes in the moon's parallax and declination, I am not yet able to speak so positively, although it is certain that the height of high water is about a foot more, at the London Docks, when the moon is nearest to the earth than when she is furthest, of course cœteris paribus. When the moon is in the equator, the time of high water is retarded about half an hour from what obtains when she is in her greatest declination; the height is also about six inches less.
[page] 192
The height of high water appears to vary very little at different months of the year. The difference between spring and neap tides at the London Docks is about 3 feet, and the rise about 19 feet. Mr. Dessiou has undertaken the laborious task of discussing 6000 observations more, in addition to those of which the results are given in the Philosophical Transactions, in order to obtain with greater accuracy the corrections due to changes in the moon's parallax and declination.
As the times and heights of high water at London Bridge are in future to be inserted in the Nautical Almanac, and as Tables for the prediction of these phænomena are to be given in the new edition of the Requisite Tables, we may hope shortly to see this question rescued from the neglect in which it has lain so long and so undeservedly. With this view it is of great importance that observations should be originated in various parts of the world, and with greater attention to accuracy than any which are yet carried on, except perhaps those at Brest, which were instituted by the French Government in 1806, at the request of Laplace, and have been continued ever since. In August last year, at the request of the Council of the Royal Society, directions were sent from the Admiralty to the masters attendant at Woolwich, Sheerness, Portsmouth and Plymouth, to cause observations of the Tides to be made, and to forward reports quarterly. This order has been complied with, and the observations are in the possession of the Royal Society. I have not been able to ascertain that any observations are made on the coast of Scotland and Ireland; and in this country, with the exceptions I have noted, and at Liverpool, these interesting phænomena pass away unheeded and unrecorded. I trust that the influence of the British Association will be exerted to remove in some degree this national reproach.
Observations of the Tides should record particularly,
The time and height of high water.
The time and height of low water.
The direction of the wind and the height of the barometer and thermometer should be noted, and the direction and velocity of the current should also be described.
The circumstances of high water are more interesting, and admit generally of more accurate observation, than those of low water.
The height of the water must be given from some fixed mark or line*, which should be described accurately, so that it
* I consider this of particular importance, and I allude to it because it has not been complied with in some observations transmitted to the Royal Society. Observations of the rise are useless.
[page] 193
may be easily recovered. It should also be carefully stated whether the time in which the observations are given is mean or apparent, and how obtained.
The name of the observer, or his initials, should be attached to each observation. The simplest method of observation appears to be by means of a staff, carefully graduated, connected with a float, and working through a collar where the height is read off. The staff must be kept in a vertical position by means of friction rollers; the float should be in a chamber to which the water has access by a small opening, in order that the ripple may be as much diminished as possible. It would be convenient to have a clock close to the tide gauge; and if made to strike minutes, so much the better. The observer should note the height of the water at the end of every minute, for half an hour before the expected time of high water, and until there can be no doubt that the time of high water is past. The minute at which the water stood the highest, or the time of high water, is then easily seen. This process is tedious, and it might be imagined that it would suffice to note the time when the water reaches a certain height shortly before high water, and the time when it reaches the same line in its descent; but the water rises and falls by jerks, and much too irregularly for this plan to be adopted with safety, at least in our river.
Mr. Palmer has described, in the Philosophical Transactions, a self-registering machine which is intended to give the time and height of high water; and I believe it is intended to set one up at the London Docks, but I have not heard that it is yet in operation. The principle consists in a style, or pencil, which is moved horizontally by the tide along the summit of a cylinder, which is turned round slowly and uniformly; the pencil describes a curve upon paper wound round the cylinder, which curve indicates the fluctuations of the water. The motion of the tide being originally vertical, is changed by a common mechanical contrivance of the simplest kind.
When it is intended to make a long series of observations, it is of course very desirable to adopt every precaution to ensure accuracy; but many persons have it in their power to make observations, which may be useful in determining the establishment of a port, or the mean interval between the moon's southing and the time of high water, without any expensive apparatus.
For this purpose the observations during one lunation, or even less, may suffice, where, as in the river Thames, the rise is considerable and the tides little subject to irregularities. In the open ocean, where the rise on the contrary is small, the tide often hangs half an hour at high water, and the phænomena
N
[page] 194
take place very irregularly. At St. Helena the rise in springs, according to Dr. Maskelyne, is 39 inches, and in neaps 20 inches; and I apprehend that less information could be elicited from a year's observations there, than from a month's observations at the London Docks. When a few observations only are made with a view of determining the establishment, they should not be used to determine that quantity absolutely, but they should be compared with observations at some place of which the establishment is accurately known, or where observations are continually carried on. It would be very desirable for those who are able, to combine so as to effect the monography or detailed description of the tides through some short extent of coast, such as that which has been effected by M. Daussy for the coast of France.
M. Daussy has determined with great care, by means of observations executed by the "Reconnaissance hydrographique des côtes de France," undertaken by the body of ingénieurs hydrographes under M. Beautems Beaupré, the establishment of all the principal places on the coast of France between Ouessant and the coast of Spain. M. Daussy finds that the influence of the wind upon the height of high water is insensible. I have found that the direction of the wind (unless in violent gales,) has no effect upon the phænomena of the tides in the river Thames; but this I attributed to its comparatively sheltered situation; and I should have thought that the tides in the Bay of Biscay would be much affected by gales sweeping over the surface of the Atlantic. M. Daussy has shown beyond doubt that such is not the case, and that the irregularities of the tides there must be due to more remote causes. He has also shown that the atmospheric pressure has considerable influence upon the height of the tide: an inch of rise in the mercurial column depresses the tide fourteen inches. This fact is very remarkable. I have ascertained, however, that in the river Thames the influence of the fluctuations of the barometer upon the tide is insensible, or very nearly so. Beyond the coasts of France, our knowledge of the progress of the tide-wave is very imperfect; and it is difficult at present to trace satisfactorily the course of high water throughout the globe, owing to the paucity of even bad observations. It is generally high water at any given instant at a series of points which form the crest of the tide-wave, and which I have called, at the suggestion of Mr. Whewell, cotidal lines. If the ocean were not intersected by continents, the tide-wave would proceed from east to west; and if the luminaries moved in the equator, the cotidal lines would be meridians.
[page] 195
The continents of Africa and South America may be considered as immense dams in the course of the tide-wave which completely change its direction, so that it is high water at the same time on the opposite shores of the Atlantic. The rudiments of the cotidal lines which would obtain in the case of a perfect spheroid probably exist round the south pole, interfered with, as they must be, by the great continent of ice in those regions. Owing to the obstructions I have mentioned, it is high water nearly at the same instant at the Cape of Good Hope, off the Straits of Gibraltar, off the coast of Scotland near the Murray Frith, and in the river Thames. The wave takes six hours in proceeding form the Land's End to the North Foreland, being at the rate of about 70 miles an hour, and in a direction contrary to the course of the luminary. If the ocean completely covered the solid nucleus of the earth, it would only be high water at the same instant at places of which the longitude differed by 180°; and at the equator the tide-wave would travel at the rate of about 500 miles per hour. The motion of the crest of the tide-wave must be carefully distinguished from that of the particles of water themselves, which forms a current the velocity of which seldom exceeds a few miles per hour: these currents are modified by others due to changes of temperature. The analytical investigation of the motions produced by changes of temperature, and of the propagation of heat in fluids, is one of extreme difficulty, and has not been yet attempted. In order to approach this important question with any chance of success, it seems necessary to consider the problem in the first instance in its most simple form, and one in which the results of theory can easily be compared with those of observation.
Works of navigation and sailing directions supply much information with respect to the velocity and direction of the currents; while the time of high water appears to have been carefully ascertained at very few points only on the earth's surface. Yet the phænomena of the tides are of extreme interest. Laplace says, "Les marées ne sont pas moins intéressantes à connôitre, que les inégalités des mouvemens celestes. On a negligé' pendant longtemps de les suivre avec une exactitude convenable, à cause des irrégularités qu'elles présentent; mais ces irrégularités disparaissent en multipliant les observations." There is indeed no branch of Physical Astronomy in which so much remains to be accomplished.
[page] 196
Report upon the Recent Progress and Present State of Meteorology. By JAMES D. FORBES, Esq. F. R. S. L. & E. F. G. S. Member of the Royal Geographical Society,, of the Society of Arts for Scotland, and Honorary Member of the York-shire Philosophical Society.
I FEEL that, in undertaking a Report upon the recent progress and present state of Meteorology, I have engaged in a task of greater difficulty than most persons are probably aware of; greater, too, than attaches to sciences of which the fabric is more deeply founded and massive, but at the same time more connected.
In the science of Astronomy, for example, as in that of Optics, the great general truths which emerge in the progress of discovery, though depending for their establishment upon a multitude of independent facts and observations, possess sufficient unity to connect in the mind the bearing of the whole; and the more perfectly understood connexion of parts invites to further generalization.
Very different is the position of an infant science like Meteorology. The unity of the whole, or of the individual greater divisions of which it is composed, is not always kept in view, even as far as our present very limited general conceptions will admit of: and as few persons have devoted their whole attention to this science alone, or the whole exertions which they did bestow, to one branch of so wide a field,—no wonder that we find strewed over its irregular and far-spread surface, patches of cultivation upon spots chosen without discrimination and treated on no common principle, which defy the improver to inclose, and the surveyor to estimate and connect them. Meteorological instruments have been for the most part treated like toys, and much time and labour have been lost in making and recording observations utterly useless for any scientific purpose. Even of the numerous registers of a rather superior class, which monthly, quarterly, and annually are thrown upon the world, how few can be expected to afford, or are even intended to afford, specific information upon any one leading doctrine or fact of the science! These hardly contain one jot of information ready for incorporation in a Report on the progress of Meteorology: such of them as are fitted for undergoing an analysis must previously have furnished the raw material, as it were, for the construction of some arbitrary general laws to connect phænomena, when duly combined with
[page] 197
similar elements already in store. The amount of detached facts is absolutely appalling; and the consequence is, that not only in registers of individual observations, but in those where the results are presented in a condensed shape and the arithmetical means have actually been taken, but a few points have been applied in practice to the elucidation of any one theoretical difficulty in the science.
The most general mistake probably consists in the idea that Meteorology, as a science, has no other object but an experimental acquaintance with the condition of those variable elements which from day to day constitute the general and vague result of the state of weather at any given spot; not considering that while such heterogeneous elements can be of little avail, when viewed simply as a group of facts, towards forwarding any one end of the science, or giving us any precise knowledge regarding it, yet that the careful study of the individual points, when grouped together with others of the same character, may afford the most valuable aid to scientific generalization. If instead of aiming at a rude approximation to the mean numerical elements furnished by meteorological instruments for a particular spot, some individual branch were selected and pursued under the most favourable circumstances, a result would be obtained at no more expense of labour,—an insulated one it is true, but capable, by combination with others, of making a real addition to the deductions of the science. As this appears to me the place to insist upon a total revision of the principles upon which meteorologists have hitherto very generally proceeded, I shall explain my views a little more particularly.
It is in the first place worthy of remark, that the most interesting views which have been given in this science, and the most important general laws at which it has yet arrived, have for the most part been contributed by philosophers who, in pursuit of other objects, have stepped aside for a moment from their systematic studies, and bestowed upon the science of Meteorology some permanent mark of their casual notice of a subject which they never intended to prosecute, and which they soon deserted for other and more favoured paths of inquiry. Mr. Dalton descends for a moment from his chemistry in the abstract, to illustrate the constitution of the atmosphere and the theory of vapour. Laplace, viewing nature with the eye of a master, introduces into his Mécanique Céleste an investigation of the mechanical structure and laws of equilibrium of the gaseous envelope of our planet: he applies Meteorology to one of its great objects,— the laws of atmospherical refraction; and gives to the scientific world a new formula for the measurement of heights by the
[page] 198
barometer, which greatly exceeds in accuracy those which had previously been proposed. Yet may the speculations of these philosophers, and the discussions to which they give rise, be more important to the science than the labours of a professed meteorologist, who has made, with minute scrupulosity, all the ordinary entries in his Journal, daily for a life-time.
We must not be supposed to give to theory a pre-eminence over observation. Had the meteorologist just supposed, instead of observing all the ordinary intruments, perhaps not upon the best construction, and at hours dictated by convenience or by accident, directed his attention even to the merely mechanical examination of any one phænomenon, not to the mixed result of a chaos of heterogeneous principles;—had he, with an eminent chemist of our own time, determined the specific gravity of air every day, and watched the unsuspected variations which that amount undergoes;—had he directed his observations to the detection of the lunar atmospheric tides;—had he examined by reiterated experiments, under every varied condition, the solution of the beautiful problem of the barometrical measurement of heights;—had he taken advantage of lofty and mountainous situations to study the formation and dissolution of clouds and the influence of humidity and temperature in their phases, or of a low and flat country for determining the amount of solar and terrestrial radiation in sheltered spots and under different aspects of the heavens;—had he in any of these, or in one of a hundred other equally fertile paths of inquiry, added to our knowledge of the connexion of cause and effect in this intricate subject, he would have conferred, at perhaps even less expense of time and labour, an infinitely greater boon upon the science which he wished to advance.
The mere local meteorology of a country may frequently be a very interesting object in relation to its physical geography and agriculture, and as such may be prosecuted by the systematic establishment of Registers on a small scale; but for the great facts of the science the adequate support of a few great Registers in any country would suffice, provided such be sustained on the most liberal scale and on the most accurate principles, by great Societies, or, still better, by Governments. Instruments must be provided on the best possible construction, placed in the best situations, and observed at the best times, and with undeviating regularity, by fit observers. The critical hours vary in different climates, and should be determined with the greatest care by preliminary experiments; and no greater error has been committed in the establishment of such Registers than the indiscriminate observation of all instruments which
[page] 199
have no bond of connexion in their diurnal changes, at the same hours.
Great numerical accuracy is always of extremely difficult attainment; and it is hoped that the good sense of observers will dismiss from Meteorology, as well as from some other branches of physical science in which it has prevailed*, that superfluity of decimal places, which when they exceed to a great extent the compass of the instrument to verify, create rather a distrust in the observer than confidence in his observations. Even within very moderate limits it is clear that, where accuracy so entirely depends upon the extreme precision of instruments and attention to their condition, and upon perfect regularity and consistency of observation, there are few individuals who can furnish the numerical data now required for the advancement of the science. Five or six Registers in Great Britain and Ireland, carried on by learned Societies or by Government, would afford the great normal quantities required for establishing the numerical data of the climate of this kingdom with regard to the great elements of temperature, pressure, and humidity in relation to that of other parts of the globe. And while we would by all means encourage the continuance and the extension of local Registers aiming at no very high degree of precision, in illustration of the particular climate of different parts of the island, these Registers would be of a very simple description, and might be confided to the hands of merely mechanical observers, under the occasional superintendance of persons of greater acquirements.
Let us conceive for a moment the gain to science, which such a saving as would thus be effected in cude and unprofitable Registers would produce. The whole class of those who profess to study Meteorology, either as an occasional pursuit or as a more constant occupation, would be left almost free to pursue individual objects of inquiry which, though not so simple as the vague mechanical task to which at present they generally devote their time, might in many cases be rendered nearly as much so, and might add every year a stock of information, which, instead of being looked upon with the coolness and indifference with which an ordinary Register is generally glanced over, might be hailed by fellow-labourers in the same field as throwing new light upon their several branches of inquiry.
We have already adverted to some subjects which afford ample scope for judicious and well-directed experiment: it would be needless formally to enlarge such a list of desiderata; but in the course of this Report we shall have an opportunity of point-
* For example, in Chemistry.
[page] 200
ing out some of the more important, and of the methods which should be taken for supplying them. It is much to be desired that other bodies as well as the British Association should use their influence to direct individual effort into the channel most likely to contribute to the advancement of science. In a period like the present, when the stream of knowledge seems to diminish in depth as it increases in diffusion, it is above all necessary for influential bodies to retard the progress of that tendency to merely superficial study, which has injured nothing more than the science of Meteorology,—one which, though in many respects apparently simple and abounding in palpable results, really consists, in its very nature, of a most elaborate piece of mechanism, delicate in its parts, and of which the connexion is anything but obvious.
The true basis of the science rests upon several branches of physics, which are only at the present moment rising to their true level of importance in the scale of human knowledge; and there are few of the sciences which are not more or less directly connected with the progress of Meteorology. Astronomy bears not only the great relation of taking cognizance of the causes of change of season, but it gives the data for estimating the influence of the heavenly bodies in raising tides in our atmosphere, and indicates the causes of alteration of climate which some of their longer periodic motions present*. Geology teaches us the probable state of cooling from an intensely high temperature in which our globe now exists, which most likely exerts a material though till lately unsuspected influence upon climatology. Chemistry analyses the composition of that gaseous atmosphere, the modifications of which it is the principal object of Meteorology to investigate. Pneumatics furnishes us with the grand laws which connect the pressure and density of the air with height, which gives a key to many of the variations indicated by the barometer, and by means of that instrument enables us to attain an accurate comparison of different elevations in the gaseous medium. To the science of Electricity we must look not merely for the explanation of those phænomena which more obviously indicate its presence and action, but likewise of many which at present are almost veiled in obscurity, or can be but partially explained by other agencies.
But most of all is the science of Heat the very basis of all accurate knowledge in Meteorology. No one department is exempt from its influence; no one substance in nature seems independent of the action of this subtile element. Impalpable though it be, yet since we possess such accurate means of in-
* See Sir John Herschel on Astronomical Causes affecting Geology, Geol. Trans. N.S. iii.
[page] 201
vestigating many of its laws, it is surprising how very imperfect are the notions entertained by mankind at large, and even by the scientific world, as to the importance of the part which it assumes in the œconomy of nature. To attempt to study Meteorology without it, is like trying to read a cipher without previously mastering the key. The laws, so far as they are known to us, by which it is regulated, though generally simple in their enunciation, rise, when pursued into their consequences, to highly complicated deductions, and soon (as is the case with every science rising above the limits of first generalisations of facts, and empirical laws,) require all the resources of mathematical analysis to eliminate general laws and to re-descend to the prediction of phænomena*. The propagation of heat in solid bodies, which forms the first problem in the theory of heat to be solved, and one of the greatest importance in the consideration of the globe as a heated mass in the course of cooling, has occupied the attention of some of the first philosophers of France. At an early period in this century M. Biot pointed out the expression for the condition of a solid bar with regard to temperature, receiving a constant supply of heat at one end, and parting with it towards the other by conduction and radiation, which gave rise to a partial differential equation, which has since undergone repeated discussion†. Laplace took up the question, and removed some analytical difficulties in which it was involved. He was succeeded by Fourier and Poisson, who gave greater generality to the solution, and extended it to bodies of various figures‡. Fourier, in his great work the Théorie Analytique de la Chaleur, has extended his profound inquiries to a vast number of problems in the propagation of heat, most important to our present subject, and which, in special relation to the temperature of the mass of the earth, will shortly be noticed more particularly.
A variety of points connected with the relation of many substances to heat have of late years been determined, though there is yet much to be done in this important field. The constants which regulate the passage of heat through various bodies, and which have been termed by Fourier "external conducibility," or penetrability, and "internal conducibility," or permeability§, have been determined for several bodies, but a
* "L'étude approfondie de la Nature est la source la plus féconde des découvertes mathématiques."—Fourier, Théorie de la Chaleur, Disc. Prel. xiii. This beautiful discourse gives some fine views on the application of mathematical reasoning to physical questions.
† Traité de Physique, iv. 669. Fourier, Théorie, chap. i. sect. v.
‡ Mémoires de l'Institut: Journal de l'Ecole Polytechnique: Connaissance des Tems, &c.
§ Théorie Analytique, Art. 30, 37.
[page] 202
much smaller number than would be desirable*. Fourier's "thermometer of contact†," intended for examining the constant of permeability, has not come into general use, as it probably will do when the calculations required to be made from its results are adapted to variable thicknesses of the bodies under experiment.
The specific heats of substances have also formed a subject of nice and successful investigation. MM. Dulong and Petit have determined those of a great number of solid bodies, and rendered it highly probable that the specific heats of their ultimate atoms are the same. De la Roche and Berard, and De la Rive and Marcet have signalized themselves in the more arduous investigation of the specific heats of the gases. From its direct application to the condition of our atmosphere, and to the probable cause of cold as we ascend through its strata, this subject and the whole relation of the gases to heat has offered a most important and interesting field for investigation during the present century. Laplace discussed it in the tenth book of the Mécanique Céleste; the experimental part was taken up by Gay-Lussac and Welter, by Clement and Desormes, by De la Roche and Berard, by De la Rive and Marcet, by Mr. Haycraft, and finally by M. Dulong. Though this question as relating to different gases cannot be considered settled, the most probable result is that obtained by De la Rive and Marcet, and by Mr. Haycraft,—that equal volumes of the different gases have the same specific heat. The consequences to which the variable specific heat of the gases under different pressures give rise, and especially the extrication of heat which attends their compression, have been studied and analysed by Ivory‡, Poisson§, Leslie‖, Avogadro¶, and others.
Our information upon the expansion of solids has not of late years much increased. Several fluids however have been reexamined, and the anomalous expansion of water and its point of greatest density have been elaborately investigated by Hallström, Müncke, and Stampfer. M. Erman has added to our knowledge of the anomalous effects of heat upon several other substances, and upon various phænomena of liquefaction**.
* See Experiments of Despretz in the Annales de Chimie et de Physique, tom. xix. His result for platinum is generally considered erroneous; and some experiments which I have recently made will, I believe, demonstrate it to be so.
† Annales de Chimie, tom. xxxvii.
‡ Philosophical Magazine, 3rd series, vol. i.
§ Annales de Chimie, tom. xxiii.
‖ Encyclopœdia Britannica, Sup., art. CLIMATE.
¶ Mémoires de l'Académie Royale de Turin, tom. xxxiii.
** Annales de Chimie, xxxviii. xl.
[page] 203
One of the most universally admired investigations of a physical law by which science has been recently benefited, is that of MM. Dulong and Petit into the law of cooling, published in the Journal de l'Ecole Polytechnique, and in the Annales de Chimie; one to which, from its universally acknowledged beauty and importance, we need do no more than allude. The radiation of heat, which has been so powerfully illustrated, and whose general laws are so well determined by these experiments, forms one of the most important elements of the science of Meteorology. Baron Fourier has recently deduced from theory the law of radiation experimentally proved by Professor Leslie,— that the calorific rays decrease proportionably to the sines of the angles they make with the radiating surface; and he has drawn some interesting conclusions*. The same author, considering our globe as a radiating body placed in indefinite space, and as having reached a condition of temperature sensibly invariable, has deduced the temperature of planetary space to be —50° cent.† Swanberg, arguing from the observed decrement of heat in the atmosphere, has arrived at almost the same result‡.
Considering heat as the power by which liquids are converted into vapour, the science of Hygrometry has received of late years important additions, not merely from several researches upon the theory of vapour, but from the elaborate experiments, undertaken with praiseworthy zeal under the superintendance of the French Academy of Sciences, upon the force of vapour at different temperatures§. Mr. Faraday, on the other hand, has pointed out the existence of a limit to vaporization‖.
I have thought it necessary briefly to point out the prodigious obligations under which Meteorology lies to the science of Heat, because the truly philosophical procedure of arriving at the great truths of the former seems to be too much overlooked. The results just enumerated have every one been attained by constant and assiduous labour, some by a course of most arduous experiment, others by the application of the most refined mathematical analysis. Till we have the laws of heat more completely unravelled than at present,—till the most important yet profoundly difficult problem of its relation to a gaseous atmosphere of varying density shall have been adequately solved,— Meteorology will stand upon an uncertain basis, and will abound
* Mémoires de l'Institut, tom. v.
† Annales de Chimie, xxvii.
‡ Bibliotheque Universelle, xliii. 367; and Edinburgh Journal of Science, N.S. iii. 13.
§ Annales de Chimie, xliii. 74. The Commission was composed of MM. De Prony, Arago, Ampère, Girard, and Dulong.
‖ Philosophical Transactions; and Journal of the Royal Institution.
[page] 204
in empirical laws and inconsequential reasoning. Let therefore those whose time is too much wasted in a vague study of the chaos of conflicting phænomena which is presented to us, fit themselves, by suitable physical and mathematical inquiries, for grappling with the difficulties individually. Never, we may be assured, will Meteorology attain the true dignity of a science till that of Heat is fully mastered,—till the laws which regulate its distribution generally are recognised, and its peculiar relations to the materials of our globe and the component parts of its atmosphere are ascertained,—till, in short, the motto of Fourier's great work is fulfilled, Et ignem regunt numeri.
It may be proper now to mention the particular course which is to be adopted, in the remainder of this Report, in endeavouring to give a general view of what has actually been done in Meteorology for some time past, and what points most require elucidation.
I shall not be particular in inquiring what are the precise limits to be assigned to the science of Meteorology, nor proceed to discuss the subject with the formality of arrangement which would be required in a treatise. I shall chiefly confine myself to those branches which admit of systematical cultivation, and which have assumed some consolidation of parts, without which any attempt at general views would be premature. On this account, I shall very slightly allude to what are commonly called atmospheric phænomena, unless where their circumstances have been sufficiently classified to admit of being treated, in a general view, as groups of facts connected by some law, whether deduced by reasoning, or empirical.
After alluding to such systematic works as have appeared of late years upon the science, I shall briefly notice any general views which have been presented as to the constitution of the atmosphere: I shall then successively consider the three great elements of Temperature, Pressure, and Humidity; and finally, under the head of Atmospherical Phænomena and Precipitations, notice such points as may especially claim attention, upon Electricity, Auroræ Boreales, Winds, Rain, &c.
With an earnest desire to render my exertions as useful as possible, I conceived that in giving an idea of what has recently been done in Meteorology, I should very inadequately fulfil the object by analysing merely the few works which may be published separately, or the longer papers scantily scattered through volumes of Transactions: I propose therefore to myself, after mentioning simply, in each department, the great steps by which it has been brought to its present condition, to refer to the papers of any interest which touch upon the subject in question in the periodical literature of the last five years, that is, from
[page] 205
1827 to 1831 inclusive. This will preclude my attempting to analyse these papers, unless where they are most important and comprehensive: but I conceive that in the present state of the subject it will be more important to bring together the results, in the simplest connected form, of the mass of floating periodical information, and give means of reference to enable any one to inquire for himself into the steps by which individual authors arrived at the conclusions which may be announced. When opportunity offers, I shall add to this, hints for the prosecution of the subject in the best lines of direction.
I am sensible that in sciences further advanced, a different style of Report would be more useful and more agreeable. In Meteorology however, where the literature is almost solely fragmentary, I believe that by the mode which I adopt I shall produce a more satisfactory work, though at the expense of greater labour of compilation.
The only meteorological work which I know of as having appeared in this country during the last few years, is Mr. Daniell's volume of Meteorological Essays. This book has been too long in the hands of the public to require any extended criticism or analysis. Mr. Daniell has very justly considered that, in the present state of the science, detached Essays were better suited to its imperfections than a more systematic plan of composition: this likewise affords us the opportunity of taking up the particular subjects of which he has treated, when they contain any important novelty, in their regular place in our Report. It is perhaps to be regretted that Mr. Daniell has mixed so much purely theoretical matter with the interesting practical conclusions in which his book abounds: it has certainly had the distinguished merit of directing public attention strongly to the science, and of eliciting some further very interesting experimental inquiries upon topics touched upon in these Essays.
In France several systematic works have appeared in Meteorology, but chiefly of a character purely popular, and with no great pretensions to scientific interest. M. Pouillet's work*
* Elemens de Physique et de Méteorologie, 8vo, 2 tom. 1827-30. Baron Humboldt has recently presented to the Academy of Sciences two new systematic works, of which the authors are M. Schubler and M. Kämtz, both of whom are already known by their contributions to Meteorology. Bulletin de la Société Philomathique, Mars, Avr. 1832.—N.B. December 1832. Since writing the above, though I have not seen the work of M. Kämtz, I have received such information as leads me to believe that it will take a distinguished position among systematic treatises. The first volume only is published. The second will contain many original researches of the intelligent author, with whom I have been fortunate enough to become acquainted.
[page] 206
is the only exception which occurs to me. In the last volume he has given a valuable though somewhat brief and incomplete view of the present state of Meteorology, and particularly of the electricity of the atmosphere,—a subject to which he has carefully attended, and upon which he has published some valuable papers. We shall occasionally avail ourselves of the information contained in this work, as well as of a useful compendium of facts contained in the article METEOROLOGY in the Encyclopedia Metropolitana now in the course of publication.
Constitution of the Atmosphere.
The opinion, formerly general, that the atmosphere is a chemically combined compound gaseous fluid, consisting of nitrogen, oxygen, carbonic acid, and aqueous vapour, has gradually given way to the views entertained by Mr. Dalton, that these ingredients exist merely in mechanical union, and each in precisely the same condition as if it formed a simple atmosphere without foreign admixture. The important consequences to which this theory leads, have been developed by Mr. Dalton in an interesting paper in the Philosophical Transactions for 1826, part ii. p. 174, of the conclusions of which we shall now give a sketch nearly in the words of the distinguished author.—He conceives a mixed atmosphere composed of a heavy gas such as carbonic acid, and a light one such as hydrogen; and after showing the consequences, generally, which would result from the intermixture of equally elevated sections of two independent atmospheres of these gases placed side by side, each exerting a pressure of thirty inches of mercury, he shows that the two gases would be mixed in equal volumes at the earth's surface; that the carbonic acid would diminish rapidly in density, in ascending, and terminate at twenty-eight or thirty miles of elevation; whilst the hydrogen, diminishing slowly in density, would attain the superior elevation of eleven or twelve hundred miles.
Mr. Dalton considers these views established by three experimentally determined facts. 1st, That two gases combined in whatever proportions in a close bottle, are equally diffused through one another. 2nd, That if different gases be placed together in a bottle with water, and shaken, no pressure of one gas upon its surface can confine another gas in the water, each acting as a simple and independent atmosphere. 3rd, That the quantity and force of vapour of any kind will be the same whether there be any air present or none, being entirely regulated by temperature.
"From these three facts," adds Mr. Dalton, "but more especially by the two last, it appears to me as completely demonstrated as any physical principle, that whenever two or
[page] 207
more such gases or vapours as we have been describing are put together, either into a limited or an unlimited space, they will finally be arranged, each as if it occupied the whole space and the others were not present; the nature of the fluids, and gravitation, being the only efficacious agents*." Upon this principle, then, Mr. Dalton conceives that the total weight of the gases existing in the atmosphere which they compose, is proportional to the volumes existing at the surface of the earth. Thus taking the pressure of the nitrogen and oxygen together at thirty inches, he conceives that the particular pressure exerted by each is as 79 to 21, being the ratio of their volumes; consequently 23·7 inches of pressure result from the atmosphere of the former, and 6·3 inches from that of the latter. The weight of the aqueous atmosphere is variable, and may be assumed at 0·4 inch, and that of carbonic acid at ·03 inch. Mr. Dalton computes the height of the respective atmospheres to be fifty-four miles for nitrogen, thirty-eight for oxygen, carbonic acid ten miles, and aqueous vapour fifty miles. He justly observes, that the condition of the earth's atmosphere may be much modified by the disturbance to which it is subjected, and suggests the inquiry as an experimental rather than a purely theoretical one. It is to be hoped that the experiments which Mr. Dalton has promised to publish on the subject, may soon be given to the world.
Mr. Daniell's Essay on the Constitution of the Atmosphere† will require little notice here, both because it has been a considerable time before the public, and because, its object being an extension in some detail of Mr. Dalton's original views, it does not readily admit of abridgement. Mr. Daniell has successively considered the habitudes of a gaseous atmosphere, one of aqueous vapour, and a mixed atmosphere such as the globe actually possesses. He has illustrated at great length what he conceives to be the particular course of phænomena, chiefly by means of Tables, which he has carried to a considerable extent. These tables are intended to give a general idea of the influence of temperature, the rotation of the globe, and other circumstances in producing currents, of which Mr. Daniell has endeavoured to establish the velocity and other characters, and has applied them to the explanation of various meteorological phænomena.
M. Theodore de Saussure has published an extended memoir J upon the variations of the quantity of carbonic acid in the atmosphere, upon which he has made an elaborate series of
* Phil. Trans. ut supra, p. 184.
† Meteorological Essays, p. 1—137.
‡ Annales de Chimie, xliv. 1—55.
[page] 208
observations. He has determined that the upper strata of the atmosphere contain more carbonic acid than the lower ones; that the quantity undergoes a sensible diurnal variation, being greater during the day than during the night; and that the quantity generally is greater in dry weather than in damp, when it is absorbed by the moisture of the soil. The proximity of the ground probably accounts in that manner for the fact of its being less in quantity in the lowest strata of the atmosphere, which otherwise would be hostile to Mr. Dalton's views.
Temperature.
The thermometer is certainly the most perfect of our meteorological instruments. The range of natural temperatures being confined on the surface of our globe within comparatively narrow limits, namely, 96° centigrade or 172° Fahrenheit in the shade*; the indications of the mercurial thermometer may be considered as absolutely accurate. Notwithstanding, too, the difficulties of procuring tubes of perfect calibre or making due allowance for its variation, the deviations of thermometers made by different makers may, when particular care is taken in their construction, be confined within very narrow limits. This I have recently had the means of particularly observing in some comparisons of standard thermometers belonging to the Royal Society of Edinburgh, by Professor Christison and myself†. The relation of the mercurial to the air thermometer has been investigated since Gay-Lussac's and Dalton's experiments, by MM. Dulong and Petit, who extended the examination to a great range of temperature. More recently M. Auguste has taken up the subject, and given a formula of comparison‡. M. Parrot has re-investigated the subject of the fixed points of thermometers§. He finds that the purity of the water employed has a sensible influence on its point of congelation; and has observed one tenth of a degree of Reaumur between that of the water of the river Neva and distilled water. He has likewise determined that the maximum heat of water in a state of ebullition occurs at and below seventeen lines under the surface.
Nothing has lately been done in the way of materially improving self-registering thermometers: Rutherford's are still the best. Magnus has proposed one acting by the expulsion of
* Arago, Annuaire du Bureau des Longitudes, 1825, p. 186.
† Captain Sabine (Account of Experiments with the Pendulum, &c. 4to,) found the difference of above a degree in two standard thermometers by the same maker. Such an error however cannot be considered unavoidable.
‡ Poggendorff's Annalen, 1828.
§ This he has published in a Latin pamphlet, 4to: Petropoli, 1828.
[page] 209
mercury from the end of the tube*,—a proposal made, in the middle of the last century, by Lord Charles Cavendish, and since that revived by Mr. Blackadder†. I have elsewhere taken occasion to point out the principal defects to which it is liable‡. In connexion with the thermometer we must mention an elegant method devised by M. Bessel of finding the correction to be applied to any thermometer on account of inequalities in the bore; which consists in breaking off columns of various lengths of mercury in the tube, causing them successively to traverse the length of the scale; and by noting the spaces occupied by each at the different points, an equation for the scale of the particular instrument may be formed§.
A very interesting discovery has recently been made with regard to the early history of the thermometer, by Signor Libri of Florence‖. In 1829 were found at Florence a large number of the original alcoholic thermometers made under the direction of the Academia del Cimento, which enabled Sig. Libri to restore the true scale of these early instruments so as to afford a direct comparison with those of modern times. The scale was divided into 50 degrees. The zero corresponded to —15° of Reaumur, the 50th degree with 44° R; and it stood at 131/2° in melting ice. The latter fact is interesting because it shows that no sensible change had taken place in the freezing point of those instruments during the lapse of nearly two centuries; for it is recorded that in melting ice the liquid marked 131/2°. It is well known that, from whatever cause, many old thermometers indicate a temperature somewhat above 32° Fahr. when plunged in melting ice. In some cases however the change has not been noticed, and this is one of the most remarkable examples. Some years ago the question excited considerable discussion; but as nothing has been added to our knowledge for some time, I shall merely refer in a note to the papers which have treated of it¶. By an accident almost as fortunate as the recovery of the thermometers, some registers nearly complete for sixteen years and kept by Raineri, a pupil of Galileo, have come to light; and by the discussion of them, with a knowledge of the true scale, Signor Libri has been
* Poggendorff's Annalen, xxii. 146.
† Edinburgh Transactions, vol. x.
‡ Edinburgh Journal of Science, ix. 300.
§ Poggendorff's Annalen, 1826. Also Bulletin des Sciences Mathematiques, viii. 42. and Philosophical Magazine, vol. lxiii.
‖ Annates de Chimie, xlv. 354.
¶ Flaugergues, Bibliotheque Universelle, xx. 117, xxi. 252. De la Rive and Marcet, Ibid. Avr. 1823. Bellani, Giornale di Fisica, v. Arago, Annales de Chimie, xxxii. Moll, Edinburgh Philosophical Journal, ix. 196.
O
[page] 210
able to show that no sensible change has taken place upon the climate of Florence between that period and the present, which had been suspected.
The metallic thermometers of M. Breguet have not been so much used as might have been expected; they are however rather adapted to delicate experiments on heat than to Meteorology in general. The same remark applies to the beautiful thermo-multiplier recently invented by Signor Nobili*, and which he has applied to the investigation of some of the most delicate phænomena of radiant heat†.
In the use of the thermometer, the sources of error from terrestrial and solar radiation have not been sufficiently attended to. Some hints on the subject may be found in the Article THERMOMETER in the Edinburgh Encyclopœdia. The mode of exposure of the thermometer of the Observatory at Paris is described by Pouillet in his Elemens de Physique.
These various sources of error go far to diminish our confidence in the nice accuracy of thermometric results, where they have not been the subject of particular attention. It is surprising at what a distance a sensible portion of heat is conveyed from soil and walls, or even from grass, illuminated by the sun; the maxima of temperature are thus generally too great, and from the near contact in which thermometers are generally placed with large difficultly conducting masses, such as walls, the temperature during the night is kept up, and the minima are thus also too high.
This however has been by no means the greatest difficulty in determining the mean temperature of a given spot. Since it is difficult to have the thermometer observed oftener than twice or thrice in a day, it becomes an object of great importance to determine those hours the mean temperatures of which will give that of the whole twenty-four. This however involves an accurate determination of the curve of diurnal temperature; and as this varies with the seasons, its connexion with the curve of annual mean temperature must also be assigned. It is obvious that for these objects a very extended scale of observations is requisite, but that when once attained, the results will be subject to the same general law throughout a considerable extent of country. The mean of the maximum and minimum temperature measured by a register thermometer is one of the best approximations; it is however by no means absolutely accurate. The multiplication of hourly observations has only in one or two instances been resorted to for filling up this blank; but it
* Bibliotheqve Universelle, N.S. ii. 225.
† Annales de Chimie, 1831.
[page] 211
is to be hoped that the facilities which military stations offer for fulfilling this most important object will not be neglected.
The only thermometrical register of great extent which has been undertaken, except that of Toaldo and Chiminello at Padua, and of Neuber at Apenrade, was executed by the military at Leith fort, under the superintendance of the Royal Society of Edinburgh; and an account of the results of complete hourly observations during the years 1824 and 1825 has been published by Dr. Brewster*. The results I consider most important to science; and they afford a proof, which I hope will not be overlooked,—that meteorological observations have only to be conducted upon a right scale in order to afford results to a degree of precision scarcely exceeded by any of the physical sciences. The result I particularly allude to is the following. One principal object being to establish the particular hours, the mean of the temperatures of which for the whole year should equal the mean of the whole twenty-four hours, it is obvious that one of these critical times must occur in the morning, the other in the evening. The observations for two years have given the following extraordinary coincidence:
| Hour of Morning. | Hour of Evening. | |
| Mean Temp. | Mean Temp. | |
| 1824 | 9h 13′ | 8h 26′ |
| 1825 | 9 13 | 8 28 |
| Mean | 9 13 | 8 27 |
Such a series of normal observations ought to be made in every extensive country; for the critical hours vary materially with the latitude, and also with the height above the sea. At Paris, for example, the mean temperature occurs before 9 o'clock in the morning. At Padua the critical hours were 8h 41′ A.M. and 7h 52′ P.M. But notwithstanding this considerable variation, occasioned by a difference of 11° of latitude, the interval elapsed between the morning and evening mean is remarkably constant. At Padua it was 11h 14′; at Leith 11h 12′; at Apenrade, in Denmark, 11h 11′†. It is to be hoped that the exertions already made by the British Association for the establishment of an hourly register near the equator, and also one in the South of England, will be successful, as the results would be of the highest interest for science.
Some of the other most important consequences deducible from the Leith observations are the following.
* Edinburgh Transactions, vol. x.
† Schouw Beiträge zur vergleichenden Klimatologie. 1827.
O 2
[page] 212
The mean hour of minimum temperature for the year is 5h 0′ A.M; that of maximum temperature, 2h 40′ P.M.
The deviation of any pair of hours of the same name* from the mean of the day, is less than 0°·5 Fahr. And of all pairs of hours, 4 A.M. and P.M. are the most accurate.
The reduction of the results of any register made at regular hours, in this climate, to the mean temperature of the day, is readily deduced.
The mean annual temperature of any hour never differs more than 3°·2 from the mean of the day for the whole year.
The mean daily range is a minimum at the winter solstice, and a maximum in April. The mean daily range in this climate is 6°·065. The result of the two years agree within 1/1000 of a degree, though the mean temperature was considerably different.
By dividing the curve of daily temperature into four parts, by the maximum and minimum points and the points of mean temperature, the intermediate portions of the curve may be accurately represented by parabolic arcs.
On the whole, these most interesting results give us an insight into what may be done, by multiplying observations, towards bringing the science of Temperature under calculation.
An interesting series of comparative observations were undertaken at Christiana under the superintendance of Professor Hansteen, during the warmest and coldest month of the year 1827†. The result is very striking. The daily variation of temperature at Christiana is in February 12°·01 F.; in July, 12°·09. At Leith, in the former month it is only 3°·57; in the latter, 9°·68. The annual variation is also immensely greater at Christiana than at Leith:
| Christiana. | Leith. | |
| Mean of February. | 16°·224 | 40°·621 |
| ———July | 61 · 690 | 60 · 361 |
| Difference. | 45 ·466 | 19 ·740 |
These are striking illustrations of the difference of a continental and an insular climate.
The curves of diurnal and annual temperature have been investigated by Professor Hallström of Abo, but before the appearance of the above-mentioned observations; he was led to
[page] 213
the following equation for reducing the mean of the temperatures at 10 A.M. and P.M. to the daily mean at any part of the year:
* "Heures homonymes," Humboldt. This author has observed that the same results are deducible from the Padua observations. Fragments Asiatiques, ii. 420.
† Edinburgh Journal of Science, ix. 309.
v=1/2 (x f + x e)—0·33 + 0·41 sin [(n — 1) 30° + 124° 8′]
where v is the mean temperature; 1/2(xf + xe) the mean of the morning and evening temperatures at 10; and n the number of the month, reckoning from January (=1). This is intended to apply all over Europe*. M. Poggendorff inquires with some justice, whether it would not be better to avoid all calculation by inclosing the thermometer in a difficultly conducting medium, by which the daily variations of temperature might be diminished.
A mechanical mode of taking the mean of an infinite number of temperatures has been proposed by M. Grassman, by observing the change of rate caused by the influence of temperature upon the uncompensated pendulum of a clock†. The idea is a good one, but was proposed long ago by Dr. Brewster‡.
M. Bouvard's valuable paper upon the meteorological observations at the Observatory of Paris, contains much information, deduced from the registers of many years, upon the form of the annual curve of mean temperature at Paris§. He observes that the days of greatest and least temperature in the year are the 14th January and the 15th July, differing only a day from an accurate interval of six months; and each follows the corresponding solstice at an interval of twenty-five days. Baron Humboldt has observed the remarkable symmetry of the curve on either side of its maximum ordinate‖. The same author has pointed out the near coincidence of the days of mean temperature observed, even in the too short continuance of registers which we possess¶; these are
| Buda, | 18th April | and 20th October. |
| Milan, | 13th—— | and 21st —— |
| Paris, | 22nd—— | and 20th—— |
These interesting inquiries lead to the general subject of Climatology, which, since the publication of Humboldt's masterly Essay on Isothermal Lines**, has assumed a more satis-
* Poggendorff's Annalen, 1825.
† Ibid.
‡ Edinburgh Encyclopœdia, Art. ATMOSPHERICAL CLOCK.
§ Mémoires de l'Institut pour 1824. M. Bouvard has also given an equation for the diurnal curve depending upon the sine of the angle corresponding to the time from noon.
‖ Fragments Asiatiques, ii. 422.
¶ Ibid. p. 426.
** Mémoires d'Arcueil, tom. iii. p. 462—603.
[page] 214
factory character than perhaps any other branch of Meteorology. That work has been too long in the hands of every one interested in the particular subject, or in the skillful generalization of groups of facts, to require any notice here; and in touching upon what has been more recently added to our knowledge of the subject, we must confine ourselves within the narrowest limits.
The opinion that the climate of a particular place, or of the globe generally, has materially changed during historic records is improbable; and all the force of that precise and circumstantial evidence which ought to carry weight with it, is against the idea. The eminent naturalist M. Schouw, who has recently published upon several points of interest connected with Meteorology*, has written an Essay, replete with curious matter, upon the supposed change of climate since ancient times, part of which has been translated into English†, which goes to show that we have no authority for assuming such a change. A similar result has been arrived at by M. Arago, who has collected a number of curious facts relative to great colds which have occurred at Paris, showing, in opposition to an opinion which had been started, that we have no reason to believe the climate to have been deteriorating for some centuries past‡. We have already quoted the results obtained by Sig. Libri from the registers of Raineri at Florence.
The old formula of Mayer for expressing the mean temperature of any place in terms of its latitude, which made the temperature of the equator 85°, and of the pole 32°, though a respectable generalization for the time at which it was made, was not calculated to stand the experience of another century. Captain Scoresby, I believe, first pointed out its great inaccuracy; and Dr. Brewster, in a paper presented to the Royal Society of Edinburgh and printed in their Transactions§, showed that the deductions of Humboldt in his Essay on Isothermal Lines would be far better represented by the simple formula
τ = 81°·5 cos L,
τ being the mean temperature of a place in latitude L. This
* Particularly connected with the geography of plants. See an Essay on the Geographical Distribution of the Vine, Edin. Phil. Journal; and an interesting brochure, entitled Specimen Geographies Physical Comparativœ, Hauniæ 1828, which contains some interesting comparative views of the climate of the Alps, Pyrenees, and Scandinavian range, the position of the snow line, &c. We have already quoted his work on Comparative Climatology.
† Edinburgh Journal of Science, viii. 311.
‡ Annuaire du Bureau des Longitudes, 1825.
§ vol. ix. p. 201.
[page] 215
indeed applies very well to observations made in the meridian of Europe. A more extended view of the subject, and a comparison of the results of Captains Scoresby and Parry, showed, however, that there must exist two poles of maximum cold, one in America, the other in Asia, round which the isothermal lines circulate: and he has lately pointed out, in a letter to Baron Humboldt*, the remarkable analogy of these to the isodynamical lines of magnetic intensity, developed by Professor Hansteen. In a private letter dated 27th March 1830, Dr. Brewster communicated to me his new formula under this form,
t = (T + τ). (sin1/2 δ. sin1/2 δ′) + τ,
t being the mean temperature of a place of which the distance from the two cold poles is δ and δ′: T the maximum temperature of the globe, and τ the minimum. In the meridian of maximum heat which passes through Europe δ = δ′, and the formula becomes
t = (T + τ). sin δ + τ,
which nearly coincides with the formula T = 81°·5 cos L given above, and represents observations extremely well. I was much pleased to see a formula which took into consideration the actual distance† from the cold poles, because it had always struck me that the modifications of the isothermal lines depending upon the accidental figure of the continental masses, it would be better to discard at once the arbitrary coordinates of latitude and longitude, the essential connexion of climate with the latter being nothing, and with the former modified by an infinity of perturbing causes. This seems, in the arbitrary formula just quoted, to be well effected by making the mean temperature a function of the distance from two imaginary poles of greatest cold. Perhaps the modifying circumstances produced by the physical geography of continents are too complex ever to enter expressly into a formula which should exhibit the relation of the temperature, as an effect, to its really active causes.
Mr. Atkinson has published, in the Memoirs of the Astronomical Society‡, an examination of the results of Humboldt's researches, with a view to obtain an accurate expression for the law of climate. He has however considered it merely as a function of the latitude, which can never represent universally the phænomena. His equation
t = 97·08 cos3/2 l — 10°·53
* Poggendorff's Annalen, 1831: i. 323.
† By employing two distinct formulæ for the two cold poles, Dr. Brewster had before introduced the angle of simple distance. Sec Edin. Trans. ix.
‡ vol. ii.
[page] 216
(where t = mean temperature, l = latitude), it will be observed gives the temperature of the equator = 86°·55, and of the pole —10°·53. The former is decidedly too great, and has been opposed by Baron Humboldt* himself and Dr. Brewster†. It is probable that the mean temperature of the equator does not exceed 81°·5 or 82°.
The temperature of the arctic regions has received the greatest elucidation by the recent labours of Scoresby, Parry, and Franklin. The two last enterprising travellers have established the existence of a degree of cold quite unsuspected in the northern part of America. From admirably conducted observations, embracing a large portion of the year, the following mean temperatures have been established:
| Lat. | Mean Temp. | Observer. | |
| Melville Island | 743/4° | - 11/2° | Parry. |
| Port Bowen | 731/4 | + 4 | ditto. |
| Igloolik | 691/3 | + 7 | ditto. |
| Winter Island | 661/4 | + 91/2 | ditto. |
| Fort Enterprize. | 641/2 | + 151/2 | Franklin. |
To Captain Beechey also we owe some interesting meteorological results‡.
M. Arago had concluded§ from the results of Scoresby, Parry, and Franklin, that the mean temperature of the pole is —25° cent., = –13° F. This however is upon the idea that the cold is at a maximum at the pole, which is not probable; it cannot however be much short of that intense degree. The objection to such a result on account of the supposed increase of ice, which would constantly take place if the temperature were below the freezing point of sea water, I have lately endeavoured to combat, and to show that observation presents no opposition to theory‖.
A gradual accumulation of facts all over the globe is paving the way for a very accurate knowledge of the mean temperature of its surface; and in a few years more our mass of observations -will probably be doubled. Great Britain has done most by her arctic expeditions; and it is earnestly to be desired that with the means she possesses of extending this branch of science likewise in equatorial regions, in the vast continent of India, this great and interesting object will yet meet with some attention,
* Annales de Chimie et de Physique, Septembre 1826.
† Edinburgh Journal of Science, vi. 117.
‡ Beechey's Voyage to the Pacific, and Behring's Straits, 4to edit. vol. ii.
§ Annuaire, 1825, p. 186.
‖ Edinburgh Journal of Science, N.S. v. 17; and Bibliotheque Universelle, 1831.
[page] 217
although hitherto totally neglected. In France an excellent register, one of the standard ones in Europe, is kept up, by the assiduity of M. Bouvard, at the Observatory; and several valuable series of observations have been produced by French expeditions in tropical regions. In Switzerland, Meteorology flourishes more than in almost any country in Europe; and though its small extent gives little room for contributions to general climatology, other problems of the greatest consequence have been successfully investigated, as we shall immediately have occasion to notice. From Russia much is to be hoped for, in the prosecution of the science of Mean Temperature, which we believe is even now obtaining daily accessions of facts from observations in the remote regions of Siberia*; the zeal which has established magnetic observatories in various parts of that vast country†, will not, it is to be expected, neglect the union of some of the most interesting meteorological observations with that of phænomena to which they are so intimately allied. But we wish particularly to allude to the exertions making in the United States of North America to elucidate the mean temperature of that important part of the globe,—one of the most interesting points, indeed, which can at present be examined with a view to rectify our knowledge of the course of the isothermal lines, which, except at the equator, are hardly at all known in the new continent. A great number of Academies scattered over this widely extended country, make annual reports of observations on the mean temperature, fall of rain, and natural phænomena, to the Legislature of New York, and the military stations have afforded extensive series of valuable results‡.
Baron Humboldt has recently published an interesting Essay on the Causes of the Inflexions of the Isothermal Lines§. Without containing much of novelty, this little work gives some general and philosophical views upon climatology, pointing out the nature of the hourly and daily variations of temperature, the variable absorbent and emissive powers with regard to heat of the materials of which the visible surface of the earth is com-
* See Humboldt's Address to the Petersburg Academy of Sciences, 28th November, 1829.
† Humboldt, Kupffer.
‡ Edinburgh Journal of Science, viii. 303. x. 267, &c.—In alluding to the exertions of different Governments for the advancement of meteorological science, I am happy to be able to add that of Prussia. I have had the good fortune to meet this summer (1832), in the Alps, M. Kämtz, a zealous member of the University of Halle, who had been sent on a scientific mission by the Prussian Government to establish some most curious facts in Meteorology.— J. D. F. Dec. 1832.
§ In his Fragments Asiatiques, tom. ii. p. 398.
[page] 218
posed; and the changes which cultivation and other circumstances may gradually effect in the elements of climate, especially with regard to the isotheral* and isocheimal† lines, even when the mean annual temperature suffers little variation. The author next considers, at some length, the particular influence of the configuration of soil in modifying the climate, contrasts the continental arrangements of the two hemispheres, and the characters of a terrestrial and marine climate at the equator. He points out in a clear manner the influence of forests upon temperature, from the shadow they produce, from the coolness created by the evaporation at their surfaces, and from the extended radiating surface which they present. Such are the principally interesting points to which this Essay alludes. In the same volume, Baron Humboldt gives some views upon the climate of Asia, which he has collected during his late journey.
The subject of the decrease of heat as we ascend above the surface of the earth, has excited, and especially in Britain, much less attention than it deserves. We hardly know a finer problem for complete solution in Meteorology. Little has been done towards it during the last few years, but we cannot pass it over quite without notice. The principle upon which this diminution of temperature takes place in the higher regions of the atmosphere, is now universally allowed to be the increased capacity for caloric of air when it is rarefied. The first question for solution is, therefore, the specific heat of air under different degrees of condensation,—a point of by no means easy investigation, and which has engaged the attention of some of the first philosophers of the day, as we have already noticed. It has been investigated experimentally by Dalton, and Leslie, De la Rive and Marcet; and theoretically by Laplace, Ivory, Poisson, and Avogadro. The object is the more important, as it is intimately connected with the amount of astronomical refractions. We do not consider, however, that the total effect can be expressed simply by the law of specific heats varying with height; and it appears that the experimental data which have been sought by observations on the atmosphere, have not generally been conducted on principles which can lead to conclusive results. I do not allude merely to the use of insulated observations of temperature at great elevations, which in my opinion can lead to no general result‡, because of the innumerable accidental causes always at work, which can only have their influence multiplied by a long series of observations,—but to the fact, that observations of temperatures at great heights,
* Equal summer temperature.
† Equal winter temperature.
‡ Such as the collection of insulated observations in Ramond Sur la Formule Barometrique de la Mécanique Céleste, p. 189.
[page] 219
whether on insulated points or in balloons, have been directly compared with those close to the massive heating surface of the earth, the radiation of heat from which probably exerts far more influence in producing a decrease of temperature in the very lowest strata of the atmosphere, than the general law which is sought for, and which is usually alone considered. The true law of decrease of temperature, such as it would be if the earth was removed, must be sought for probably by successive stages of balloon observation*, commencing at a considerable height above the surface; or else we must find the means of estimating accurately the calorific influence of the earth by radiation and conduction. There cannot be a question that in the lowest strata the diminution of temperature appears much greater than at higher elevations, if the observations be not made on a naturally inclined surface of soil, but at two stations, one nearly vertically above the other†.
In order that the terrestrial influence may be as much equalized as possible, observations on the mean temperature of table-lands at considerable elevations, (but not covered with perpetual snow, which introduces a new element,) are perhaps the most satisfactory. And hence the reason that Humboldt's equatorial observations are by far the best that we possess‡. Humboldt's general result gives 121 toises of ascent for a diminution of 1° R. The admirable comparative observations at Geneva and St. Bernard, give a surprisingly near approach to this, the difference of mean temperature of the stations being 8°·64 R. for 1069 toises: this gives 1231/2 for 1° R. or 352 feet for 1° Fahr. This probably is the most correct mean result which we can adopt. The influence of the seasons is very considerable, as the following results of M. Guerin at Ventoux near Avignon, lately published, prove§.
Summer, 80 toises for 1° R., or 156 metres for 1° cent.
Winter, 100 ———— or 195 ————
Some good observations made in summer upon the Rigi by M. Eschmann of Zurich, give 97 toises of ascent for 1° R.‖ I have succeeded in establishing, in latitude 56°, a system of observation on this interesting point, from which I hope in due time to be able to obtain the most important results.
* A few observations with balloons have been published by Lord Minto (Edinburgh Journal of Science); it will be necessary however to carry them on upon a much more extended scale, before any general conclusions can be attained.
† See some observations, remarkably confirmatory of this view, by Sir Thomas Brisbane, (made in New South Wales,) in the Edinburgh Journal of Science.
‡ Observations Astronomiques, 4to, i. 126.
§ Annales de Chimie, xlii. 128.
‖ Bibliotheque Universclle, 1827.
[page] 220
Several formulae have been proposed for a general expression of the law of gradation. Lagrange, and many after him, considered the decrement of heat to be simply proportional to the height, and the best observations may be tolerably represented by
t = n H.
Where t, the decrement of temperature, is expressed in degrees of Fahrenheit, and H in English feet, the coefficient n will be a fraction nearly equal to 1/270, or between that and 1/300 for small heights where the decrement proceeds most rapidly: the observations of Humboldt and those at the Grand St. Bernard would, we have just seen, make n about 1/350 Euler considered the progression an harmonical one. Professor Leslie, from experiments upon the heat absorbed by air in rarefaction, proposed theoretically the formula 25 (1/θ — θ) for the diminution of temperature on the centigrade scale; where θ represents the density of the air at the upper station. This formula was first proposed without demonstration*; afterwards the nature of the experiments upon which it rested was explained†: from its principle, this formula only takes cognizance of the influence of the change of specific heat in the atmosphere, without any reference to the effect of the mass of the earth. Professor Leslie's formula has given rise to several discussions, to which it will only be necessary to refer in a note‡.
Mr. Atkinson, in discussing the subject of Astronomical Refractions§, has examined with great care all the actual observations to which he had access, and from them he has deduced the following formula:
h = [251·3 + 3/2 (n — 1)]n,
where h is the height of the station in English feet, n the depression of Fahrenheit's thermometer.
An ingenious attempt was made by M. Mathieu‖ to deduce the law of decrease of temperature in the polar regions, by analysing two observations by M. Swanberg upon the amount of refraction, by means of the formula of the Mécanique Céleste. The result at which he arrived was 243 metres of eleva-
* Leslie's Elements of Geometry.
† Encyclopœdia Britannica, Supplement, ArticleCLIMATE. See also Thomson On Heat, p. 122.
‡ By Mr. Ivory, Philosophical Magazine, 1821; by an anonymous writer, Edinburgh Journal of Science, vol. v.; and a paper by myself relative to the last-mentioned one, Id. N.S. vol. v.
§ Memoirs of the Astronomical Society, vol. ii.
‖ Humboldt, Observations Astronomiques, i. 155.
[page] 221
tion for a decrease of 1° cent. This however is at variance with the only direct observation we have on the subject. Captain Parry found in latitude 69° 21′, by means of a kite, that the thermometer indicated no diminution of temperature at a height of 400 feet; it stood at — 24°. Much however cannot be inferred from a single observation.
Some observations have recently been made by M. Kupffer in the range of the Caucasus, by means of the temperature of springs, but too few to admit of any satisfactory conclusions*.
We must now touch upon a point of the greatest importance, and which daily increases in interest,—I mean the proper temperature of the globe itself. We have already pointed at the fine views of Baron Fourier relating to this subject; and from the experimental confirmation which they receive every year, there is little doubt that they will soon be established on an immoveable basis. He considers the globe as a mass in the process of cooling from an intense temperature. He has proved that the heat may be very intense at a short distance from the surface, and yet, from the extremely bad conducting power of the crust, that it may exert no sensible influence on the climate: he actually computes it as not amounting to 1/30th of a centigrade degree. Towards the centre the heat may be of the most extreme intensity, and the phænomena of earthquakes and volcanos may be imputed to its influence. The process of cooling, though at first of course comparatively rapid, may now be considered to have reached an asymptotic condition. It is well known that the influence of the seasons, or the total difference of the effect of solar radiation in summer and winter, affects the temperature of the soil to a comparatively minute depth. Experiments with thermometers, sunk to different depths, have been made at Zurich by M. Ott, near Edinburgh by Mr. Ferguson, and at Strasbourg by M. Herrenschneider†. The influence of the solar rays decreases rapidly; and it is probable, from experiments made at Paris‡, that at about 30 metres, or 100 feet, it is almost extinct. The position where this takes place is called by Fourier the "couche invariable," or invariable stratum; all variations above this plane are imputed to the influence of radiation, all below to the native or primaeval heat of the globe.
A successive influx and efflux of heat is constantly going on
* Voyage au Mont Elbrontz, 4to, Petersburg.
† See a resumé of these experiments by M. Pouillet, Element de Physique, ii. 642.
‡ The excellent observations regularly made in the caves under the Observatory are regularly published in the Annales de Chimie et de Physique.
[page] 222
in the strata above that of invariability. The heat of the solar rays is constantly acting on it during the day, and with an intensity depending upon the absorbent power of the surface, and the latitude. M. Pouillet, from some ingenious experiments, concludes that the solar rays which reach the surface of our globe in the course of a year, have sufficient intensity to melt a complete stratum of ice over its whole extent of 14 metres in thickness*. The amount of solar radiation in various parts of the globe, presents almost an open field for investigation. Mr. Daniell, in his Meteorological Essays, started the apparently paradoxical opinion that the force of solar radiation increases from the equator to the poles; and though his reasonings have been opposed by an eminent French philosopher†, and by meteorologists at home‡, we think he has at least had the merit of pointing out the fact, that the force of radiation is much less in the equatorial and much greater in the polar regions than might have been anticipated. Dr. Richardson has made some very interesting though not quite decisive experiments on this subject in the late Northern expedition§. I am indebted to Mr. (now Sir John) Herschel, for the remark, that observations on solar radiation seem generally to have been made upon an erroneous principle; the true indication of the force of the solar rays, not being the statical effect upon the thermometer, but their momentary intensity measured by the velocity with which they communicate heat to an absorbent body. We may confidently point out the subject of radiation as one which will reward the researches of the Meteorologist.
When the immediate calorific cause no longer acts, the surface of the globe of course begins to radiate the superfluous heat which it had received, and exactly in proportion to the facility with which it received it, the absorbent and emissive powers of surfaces being equal. Hence a nocturnal radiation of heat takes place from the soil, occasioning that cold which, according to the laws known to regulate this process, is materially affected by the purity of the sky; for it is perfectly certain that clouds or any interposed body, or even the finest films, have a sensible influence in intercepting the rays of invisible heat. To the coolness thus produced in bodies exposed at night to a clear sky the phænomena of dew have been accurately attributed by Dr. Wells in his Essay on that subject,—a work undoubtedly
* Elemens de Physique, ii. 704.
‡ Edinburgh Philosophical Journal, xiv.
§ Franklin's Second Journey, 4to edit.
† Annales de Chimie, Aug. 1824.
[page] 223
both philosophical and important, but which it appears to me has received fully its due share of commendation.
Let us now see what experimental proofs we have of native heat below the invariable stratum. It is nearly a century since it was first suspected that the temperature of the earth increased as we descend. The proof however has been reserved for our own day, by the multiplication of observations which might annihilate every plausible objection,—for many such there undoubtedly are. Nor can we assert, that before the late researches of M. Cordier* truth has decisively been made to appear. The general consequences which have resulted from his inquiries, and which are substantiated by an abundant collection of facts observed in Cornwall, Saxony, Brittany, Switzerland, America, and other points, are as follow:—That the temperature of any stratum below that of invariability is absolutely the same all the year round†;—That in all strata so situated the temperature increases as we descend, without any exception;—That though the results which have been obtained are far from giving the same law of increase for different countries, which from the imperfection of the observations it was impossible to expect, yet the general progression may be stated at from 25 to 30 metres of descent for an increase of one degree centigrade, or from about 37 to 44 feet for one degree Fahr.‡ M. Cordier has elaborately and successfully refuted the idea that these effects could be produced by the lamps of the miners, though he has shown the nature and amount of the influence they actually exert. A more refined objection, imputing the heat to the condensation of atmospheric air descending into the mines, has been satisfactorily answered by Mr. Fox§, whose scientific observations on the mines in Cornwall, and especially on their temperature, and the electro-magnetic properties of their metalliferous veins, promise so much towards the advancement of science. M. Magnus, whose register thermometer we have already alluded to, and who applied it to the present object, has
* Annales du Museum d'Histoire Naturelle, 1827. See also Bulletin des Sciences Mathématiques, ix. III; Edin. New Phil. Journal, vol. v. & vi.; and De la Beche's Manual of Geology (Introduction).
† See proofs of this in Saxony, Annales de Chimie, xiii. 211.
‡ M. Kupffer has lately deduced 36°·81 English feet for 1° F. Poggendorff's Annalen, xv. Edinburgh Journal of Science, April 1832. The recent experiments of M. Gherard in Prussia, communicated by Baron Humboldt to the Academy of Sciences, give 180 feet of descent for 1° R . Bulletin de la Société Philomathique. Mars—Avr. 1832.
§ Philosophical Magazine, 1830. Both to Mr. Fox and to Mr. Henwood we are indebted for some excellent original experiments. Edinburgh Journal of Science, vol. x.
[page] 224
recently published some good observations on a boring near Berlin*. The temperature of the earth, measured by that of deep-seated and copious springs, has conducted to a similar result; for their variation of temperature as connected with latitude is confined within smaller limits than the mean temperature of the air, indicating a proper temperature of the earth, which at the mean depth of springs diminishes that of those near the equator, and increases it near the poles; the mean point, or where the ordinary temperature of the earth and that of the air are the same, being about latitude 56°. Von Buch pointed out some years ago this interesting fact†, which was deduced from his own observations and those of others, especially of Wahlenberg in Sweden‡. The question has lately been treated in its greatest generality by M. Kupffer, who has established to a considerable extent the course of what he calls the isogeothermal lines, and has given formulæ for their computation as well for longitude as latitude; for, like Humboldt's isothermal lines, he finds that they do not regularly follow the parallels of latitude, but are subject to anomalous inflections. The form of the expression given by M. Kupffer is
a— b sin2 l=t,
a and b being constants which vary with the meridian of the place, and which he has computed for a range extending from 85° W. to 60° E. of Paris§. Near the equator, the ground at 25 metres depth appears to have a temperature 2° R. below the mean temperature of the air, whilst in Lapland it is as much above it. I do not think that the connexion of this remarkable fact with the proper temperature of the globe has been pointed out with sufficient distinctness, but this is not the place to insist upon it more particularly.
It is obvious that the temperature of the ocean, which covers so large a portion of the surface of our globe, must have a great influence in the modification of its climate. This subject has therefore occupied particular attention. The most active observers have been Humboldt, Scoresby, Parry, Ross, Sabine, Hall, Davy, and Duperrey. The variations of temperature of the sea being comparatively small, the climate is subject to much smaller fluctuations than on continents. As might be expected, the maximum temperature of the air is greater than that of the surface water; the mean temperature however, it appears from
* Poggendorff's Annalen, xxii. 146.
† See the Edinburgh New Philosophical Journal, vi. 166.
‡ Wahlenberg's observations were originally published in Gilbert's Annalen der Physik.
§ Voyage au Mont Elbrontz.
[page] 225
the observations of Duperrey, is somewhat less. The temperature, as it varies with depth, is of course amenable to the laws of the maximum density of water; though with respect to salt water, this phenomenon still requires elucidation*. Within the tropics, the temperature constantly diminishes as we descend. Towards latitude 70° this decrease vanishes, and an opposite series of phænomena take place; the temperature increasing as we descend. As these points, though interesting in a high degree, are not so intimately connected with Meteorology as those we have been discussing, and those which yet remain, we shall merely give references to those works where general views have lately been given on the temperature of the sea:—Arago, Annuaire pour 1825; Humboldt, Rélation Historique, iii. 514—530, and Fragments Asiatiques, ii. 556 ; Pouillet, Elemens de Physique, ii. 684; Recent Observations by M. Lens, who accompanied Kotzebue; Poggendorff's Annalen, 1830; and the Voyages of Beechey and Duperrey.
From the great and universal importance of the subject of Temperature, and the more general views which admit of being taken of it, we have been induced to extend our review of its different branches to a greater length than we can permit ourselves in the remainder of the subjects which are before us. We proceed therefore to give a brief view of the subject of
Atmospheric Pressure.
Notwithstanding the beauty of the Torricellian method, the barometer must be admitted to be far from that state of constancy, simplicity and perfection, which could be wished, for the purposes to which it is now applied. My attention has long been greatly devoted to the improvement of this instrument, and to the careful study of its desiderata; but I believe we must be content to admit, that on every plan which has yet been proposed, and with any modification of such plans, the barometer will remain liable to considerable objections.
Whilst a barometer is immoveably fixed, its capability of precision is much greater than when it is constructed with a view to portability; indeed there is not perhaps a more difficult problem in philosophical mechanism than a satisfactory portable barometer. I have lately had occasion most particularly to consider the construction of the instrument in both forms, having last year been requested by the Royal Society of Edinburgh to give designs for, and order a standard barometer to be placed in
* From Erman's experiments it would appear that sea water has no point of greatest density above its freezing point. Annales de Chimie, xxxviii. 287.
P
[page] 226
their apartments; and more lately I have had constructed for my own use, a portable barometer intended for a projected tour on the Continent, in which I have endeavoured to unite the great requisites of accuracy, portability, and security from accident. These properties have been considered so much opposed, that hardly any of the various inventions, or modifications of inventions, which are constantly brought before the scientific world, can prefer a claim to all three. Perhaps the most portable barometer susceptible of any considerable accuracy which is in use, is that of M. Bunten, a modification of Gay-Lussac's, having a safety cavity in glass half way up the tube for stopping the progress of any air which may pass the syphon at the bottom*. The instrument is well mounted and graduated; but the principal defect, as well as in Gay-Lussac's, arises from the friction of the mercury in the shorter leg of the syphon, where it never fails to oxidize,—and from the contraction of the scale, which is necessarily much shortened†. I am informed however, by my friend Captain King, that he found it on the whole a satisfactory instrument and extremely portable‡.
An important source of error in portable barometers is the difficulty of finding the actual temperature of the mercury; with a syphon barometer of the kind just mentioned, Signor Bellami and M. Legrand have pointed out methods of converting the instrument into a temporary thermometer, and thus showing its own temperature§.
Among other ingenious devices for diminishing the risk of breakage, one has been proposed by Mr. Jones of Charing Cross, by constructing the tube wholly of iron; such an one has, I believe, been actually completed, but I have not heard of its success. Mr. Robinson, of Devonshire Street Portland Place, London, has lately constructed a barometer in which the tube consists of two parts, capable of being screwed together at the moment of observation.
The adjustment of the lower level of the barometric column is one of the most difficult parts of the apparatus. I am convinced that the French method of bringing up the mercury in a transparent cistern till a fine fixed point impinges on its surface, will gradually come more into use in this country, whenever really good barometers become an object of attention, which at present can hardly be said to be the case. A most beau-
* A good account of this barometer, with M. Arago's Report upon it, made to the Academy of Sciences, will be found in Ferussac's Bulletin des Sciences Mathématiques, x. 187.
† In fact it is generally reduced to one half.
‡ Since writing the above I have used a barometer upon Gay-Lussac's construction with great satisfaction among the Alps. Dec. 1832.
§ Bulletin des Sciences Mathématiques, tom. x.
[page] 227
tiful modification of this plan has been invented and adopted by Dr. Prout in his admirable standard barometer, one of the finest philosophical instruments I ever had the pleasure of seeing; by means of which he informs me he can ascertain the lower level of the mercury to much greater precision than he can read off upon the scale, which is divided to single thousandths of an inch. As no account of it has been published, it would perhaps be out of place to give any description of it here.
Dr. Jacob proposed a cistern in which the mercury assumed a constant level by merely being permitted to overflow*. Mr. John Adie of Edinburgh has contrived a mode of adjusting the level of the mercury without a leather bag, which in great hygrometric extremes may become unmanageable†, by substituting a glass plunger with a stuffing box‡.
The difficulties arising from want of portability, have sometimes brought the instrument back to its earliest stages of simplicity. Some observers now strenuously recommend the practice of constructing a temporary barometer at the place of observation, by filling a tube with mercury, thus dispensing with the precaution of boiling. A distinguished Russian philosopher, M. Kupffer, recommends that air be left above the mercury, and its effect computed§. Neither of these plans can we approve, more especially the latter, as the effect of temperature, the difficulty of ascertaining which we have already noticed, becomes tenfold more important. Upon the whole, we cannot flatter ourselves that the barometer as an instrument has made much progress towards perfection for some time past. In stationary instruments simplicity and solidity are important requisites; and there is one interesting fact which though frequently suspected, can hardly he said to have been substantiated till lately by the observations of Mr. Hudson, the indefatigable observer to the Royal Society of London,—that the sensibility of barometers depends much upon the bore of the tube, which he has found to have a sensible effect even when it is by no means small‖.
The manometer of Hooke was revived about twelve years ago by Mr. Adie of Edinburgh, under the name of the Sympieso-meter, and he has conferred upon it the most essential improvements and the means of giving indications of very con-
* Dublin Philosophical Journal, No. IV.
† As Captain Hall found near the cataract of Niagara.
‡ Edinburgh Journal of Science, N.S. i. 338.
§ St. Petersburg Transactions, 1830; and Journal of the Royal Institution, N.S. vol. i.
‖ Published (since the above was written) in the Philosophical Transactions for 1832, Part II.
P 2
[page] 228
siderable accuracy. The action of this instrument I have examined with great labour and in great detail, being convinced that with some ameliorations it may be made yet more valuable to science, and capable of general adoption, especially by geologists; for it must be admitted, that at present, without the strictest care, it may most seriously mislead the observer. Its portability far exceeds that of any other barometric instrument*.
One of the last donations of Dr. Wollaston to science was a Differential Barometer for measuring minute differences of pressure; but, not being intended for purposes of Meteorology, need not here detain us.
Considering the great attention which is required in conducting continued series of barometrical observations, and the care that is absolutely requisite in having accurate and comparable results by means of really good instruments, we cannot wonder that in but very few places is the mean pressure of the atmosphere accurately known. Much less can we pronounce upon the question whether the general mean pressure over the globe is the same†. The mean height at Paris for fourteen years, being one of the best-determined points, is exactly 756 millimetres. When Meteorology shall have taken its due place among the sciences, and observations are assiduously carried on at several points in connexion in fixed observatories, we shall have some data for determining likewise whether the pressure remains the same from age to age,—a point upon which at present we are wholly in the dark. Professor Schön, from observations at Wurtzburg, thinks that the pressure has increased during the last fifty years‡. But such generalization is quite premature. Some curious anomalies however with regard to mean pressure seem pretty well established, and demand accurate observation. Von Buch observed that the mean pressure on the shores of the Baltic was less than in France, and imputed the difference to what he calls a vallée atmospherique. A similar fact, not less extraordinary, is established by the observations of M. Erman in the East of Siberia§. His barometrical observations would place Jakuzk below the level of the sea of Ockozk, yet the river Lena flows down from Jakuzk to the North Sea, which must be almost if not precisely on a level with the sea of Ockozk. It is well to observe that the mean temperatures of this part of Asia differ very abruptly at short distances, which are probably intimately connected with the phænomenon. M. Erman allows for
* My papers have been published in the Edinburgh Journal of Science, x. 334; N.S. iv. 91. 329.
† See Humboldt, Rélation Historique, 4to edit. tom. iii.
‡ Kastner, Archiv, viii. 475.
§ Poggendorff's Annalen, Oct. 1829.
The variations of pressure may be considered as periodical and accidental.
[page] 229
the effect of air in his barometer, which, considering the variable temperatures to which it must have been exposed, rather diminishes our confidence in the observation. Captain King communicated to me a remarkable barometric anomaly observed by him at Port Famine in the Straits of Magellan. The instrument was compared with that at Greenwich on leaving and on returning to this country, and was observed five times a day, with all care, for five consecutive months. The result gave the very small mean height of 29·462 inches at five feet above high-water mark*. This fact seems intimately connected with those just mentioned.
Of the periodical variations, that which first demands our attention is the horary oscillation. This phænomenon, somewhat indistinctly pointed at by observers in the tropics above a century ago, has within the last thirty years acquired great interest. Baron Humboldt by his observations near the equator gave an impulse to inquiry, and the observations have been pursued with assiduity and success throughout a great range of latitude. The general fact that the barometer attains a maximum in the tropics at 9 A.M. and P.M., and a minimum at 3 or 4 A.M. and P.M., it must be hardly necessary to recall. Nor does it fall within our province to recapitulate the labours of Ramond†, or the individual results of the earlier observers. It is sufficient to mention the well known names of Humboldt, Caldas, Horner, Boussingault and Rivero, and Simonoff, as observers in the tropics; and of Marqué-Victor, Billiet, Gambart, and Herrenschneider in Europe. Of the recent contributions which fall more particularly under our notice, M. Bouvard's Memoirs are the first. In an excellent analysis of the meteorological observations made at Paris‡, he has determined with great accuracy the amount at that station, which gives for the morning period 0·76 millimetre, and for the evening 0o37, by the mean of eleven years. In a later paper he has analysed the law of diminution of the oscillation from the equator to the poles, and likewise the influence of height and of seasons§. He has adopted the Table given by Humboldt in his admirable Essay on this subject‖, and enlarged it by new observations, especially the manuscript ones
* The results have been published, but without any remark, in the first Number of the Royal Geographical Society's Journal, p. 172.
† Mémoires de l'Institut, 1812; and his excellent Mémoires sur la Formule barometrique de la Mécanique Céleste.
‡ Mémoires de l'Institut pour 1824.
§ Bibliotheque Universelle, 1820.
‖ Relation Historique, 4to edit. tom. iii.
[page] 230
of Duperrey and Freycinet; and where reduction on account of the hours employed has been necessary, he has introduced a formula similar to that for temperature depending upon the sine of the arc corresponding to the time from noon. Such a formula has also been employed by Carlini* and Hallström. The general result to which M. Bouvard's interesting inquiry led him was, that at the equator the amount of the oscillation is proportional simply to the temperature, on the centigrade scale, of the period during which the oscillation is observed at the given spot, the oscillation and temperature at the level of the sea being unity;—that in any other latitude the same law is to be modified by introducing the additional proportionality to the square of the cosine of the latitude. Or representing by m and t the oscillation and mean temperature at any place and for any period in latitude 0, and by m′ and t′ those quantities at the equator, we have, according to M. Bouvard,
m′ = t′/t m/cos2θ
or, the latitude and temperature being given, to find the oscillation
m = t cos2θ/t′ m′
When the temperature (on the centigrade scale) becomes negative, whether by change of latitude or from height, the oscillation will become negative also and take place in an opposite direction; this inference M. Bouvard confirms by the fact, that the mean oscillation at the convent of the Grand St. Bernard is actually negative.
The observations by Mr. Goldingham at Madras, printed (not published) by the East India Company, confirm the results of former observers. As an attached thermometer seems to have been neglected, the amount of oscillation cannot be depended on, but the critical hours are extremely well fixed.
Dr. Russell at Berhampour (lat. 24° N.), and Mr. Prinsep at Benares (251/2°) have also added to our list of recent observations†. In more northern climates we are also lately indebted for valuable observations to the Royal Society of London,—an abstract of the results of which has recently been published by Mr. Lubbock‡, giving an oscillation equal to 0·57 millimetre§;—
* Memorie della Società Italiana, tom. xx.
† Philosophical Transactions, 1828.
‡ Ibid. 1831, p. 223.
§ Since the publication of Humboldt's Essay on this subject, millimetres have become the more usual reference for the measure of the oscillations.
[page] 231
to M. Carlini*, whose Memoir is rather upon the general subject of the variations of the barometer, than containing minute original observation, his register having been continued only for a short time;—to the Geneva and St. Bernard Observatories, where the registers are kept with a regularity and precision worthy of the greatest commendation, and which annually are affording data of the highest value for science†. I have already noticed the important fact that the mean annual oscillation is actually reversed at the St. Bernard, 8000 feet above the aea. M. Eschman has since noticed that it is almost extinct on the summit of the Rigi‡.
Professor Hansteen has published some observations, continued however for only six months, at Christiania, where he gives 0mm·53 for the morning oscillation, and 0mm·40 for the evening§.
M. Hallström from observations at Abo, (likewise of too short continuance,) has endeavoured to deduce the principal oscillation, which he states at 0mm·44; and he has attempted to assign in a general way the law of diminution from the equator to the poles; it is however formed on imperfect data‖. V being the oscillation in latitude l, he gives
V = 0·3931 – 2·3536 cos l + 4·5687 cos2 l
for millimetres. This would give a positive oscillation, at the pole, of 0mm·39 which is quite improbable.
In 1828 I published some observations made by myself at Rome the previous year ¶. Though continued only for a short time, yet, as I frequently made twelve or fourteen observations in a day, I was enabled to trace out very well the diurnal curve of variation, establish the critical hours of morning and evening maxima and afternoon minimum, and give an approximation to the amount.
Since that, I have investigated with great care, during the years 1827–30, the oscillation in latitude 56°,—the most north-
* Memorie delta Società Italiana, tom. xx. In this paper, which is of considerable length, M. Carlini has aimed at giving a type of the mode of treating such observations generally, rather than affording extremely accurate results by the analysis to which he has submitted his own, which were only continued for a few weeks in summer, and again in winter. These observations, however, having been made during part of the time every two hours, are well worthy of being consulted on their own account.
† The annual means are regularly published in the Bibliotheque Universelle.
‡ Bibliotheque Universelle, 1827.
§ Bulletin des Sciences Mathématiques, ix. 32.
‖ Poggendorff's Annalen, 1826. Bulletin des Sciences Mathématiques, ix. 190.
¶ Edinburgh Journal of Science, January and April 1828.
[page] 232
erly point in Europe at which any observations of long continuance on this subject have been made. The results have been published at length in the Edinbuvgh Transactions*; and I have also entered into an analysis of all the existing information on the subject. The following are the general results at which I have arrived.
1st, That near Edinburgh, in lat 56°, the mean annual oscillation between 10 A.M. and 4 P.M. is ·0106 inch, or 0mm·27.
2nd, That the hours of maxima are further from noon in spring and summer than in autumn and winter; and that the amount of oscillation of both the diurnal periods diminishes regularly through the seasons from spring to winter. These conclusions, derived directly from my own observations, I have shown to be the most probable for all parts of the globe, as far as existing observations guide us.
3rd, That the St. Bernard observations, and those of Captain Parry in the arctic regions, both indicate a true negative oscillation, though the second result has been overlooked by M. Bouvard.
4th, That M. Bouvard's hypothesis and formula mentioned above, are founded upon too hasty generalization. This I have shown upon various grounds, but especially from his own quotation of the St. Bernard observations, where, as the mean temperature is much above 0° cent. in summer and below it in winter, the oscillation should be distinctly positive in the former case, and negative in the latter. This I have shown to be precisely the reverse of the fact.
5th, Availing myself of M. Bouvard's excellent Table, with such additions as I could make to it, I proceeded to investigate, from observations made near the level of the sea alone, the influence of latitude in modifying the oscillation; and from a careful combination of the best results, by reducing the squares of the errors to a minimum, I obtained the following equation, which represents wonderfully well the existing observations:
z = 3·031 cos5/2 θ – ·381
for millimetres, z being the oscillation in latitude θ This gives for the equatorial oscillation 2mm·650, and for the poles—·381. The latitude where the oscillation changes its sign, or is = 0, is 64° 8′.
* Vol. xii. The title of the paper is, "On the Horary Oscillations of the Barometer near Edinburgh, deduced from 4410 Observations; with an Inquiry into the Law of Geographical Distribution of the Phænomenon." An abstract has been printed in the Edinburgh Journal of Science for April 1832.
[page] 233
6th, In the course of this investigation, having selected the observations at Cumana and Toulouse, (both being places where the oscillation is positive,) for obtaining approximate values of the constants in the formula, I found to my surprise and satisfaction, that from these observations alone, we might have inferred, à priori, not merely a negative oscillation in the arctic circle, but one not differing sensibly in amount from the actual observation of Captain Parry*.
7th, I have determined from the formula the mean atmospheric tide from the equator to the pole to be equivalent to the weight of a stratum of air 101/2 metres in thickness; and the mean for the whole surface of the earth to be 16 metres, the air being considered under the usual pressure and temperature.
I hope I shall be excused for dwelling so long upon this paper, as it contains not my own observations merely, but the results of all those which I could collect, made up to the present time, in every part of the globe.
In the southern hemisphere, the excellent observations of Captain King at Port Famine† have given us the amount of the oscillation in a much higher latitude than any previous experiments. They correspond very well with my formula.
With regard to the cause of this remarkable and very general phænomenon, extending from the equator to the poles, we are very much in the dark. We must be content to wait for much more complete information before hazarding conjectures. The connexion of it however with other meteorological changes, by whatever means related, seems certain. The diurnal cycle which it so strictly follows, and its modification by the seasons, show the influence of the sun. M. Bouvard's views are certainly so far correct, that temperature appears to be intimately connected with its variations. M. Dove, who is known by several essays on subjects connected with Hygrometry, has pointed out the connexion of the horary oscillations with the state of humidity of the atmosphere‡; and Mr. Snow Harris of Plymouth has kindly put into my hands the results of a number of original experiments, which show in a very striking manner the diurnal changes in the force of the wind,—a subject quite in its infancy, and to which almost no attention is paid,—which correspond closely with the barometric oscillations; the mean force of the wind being much greater at the period of afternoon minimum than at the morning and evening maxima. This is
* See Art. 15. of the paper.
† The observations are given in the Royal Geographical Society's Journal, No. I., and in my paper, Art. 21.
‡ Poggendorff's Annalen, 1831.
[page] 234
analogous to the usual influence of the wind on the barometer, a point not yet quite satisfactorily elucidated, and presents a connexion not to be lost sight of. Mr. Harris, with his usual diffidence, suggested it to me merely as a coincidence worthy of notice, not as the foundation of any hypothesis, and has permitted me to mention his observation here.
The other principal oscillation strictly periodical which we have to notice, is one of which even the existence is hypothetical; I allude to the lunar atmospheric tides. It is remarkable that the observations most lately made, and now before us, are of the most contradictory character. M. Bouvard, by his deductions from the observations at the Paris Observatory, has been led to the conclusion that the minimum pressure takes place at new and full moon, the maximum at the quadratures*. Mr. Lubbock, by the discussion of observations carried on for three years at Somerset House, concludes that the maximum takes place at the syzygies, the minimum at the quadratures, or precisely the reverse†. M. Flaugergues, on the other hand, to complete the contrariety of opinion, has stated, in a memoir on his own observations, the maximum to take place at the last quadrature, and the minimum half-way between the first quadrature and full moon‡. He states the difference of height at lmm·48. Laplace is disposed to consider the lunar influence as not yet established§. On the whole, we must be content to leave this interesting question quite open to discussion.
Among the variable causes which affect the barometer, we shall first notice the direction of the wind. Upon this point observations are more at one. Both the observations at Paris and at London, just referred to, indicate a maximum of pressure when the wind is N.E., decreasing in both directions of azimuth till it reaches a minimum between S. and S.W. This fact may therefore be considered quite established in this climate. The difference of extreme heights amounts at Paris to no less than seven millimetres; at London (from a smaller number of observations,) it amounts to above 5/10ths of an inch, or nearly eight millimetres. Burckhardt, from the observations of Messier, made it 5mm·146‖. The fact of the rise of the barometer in this country with an east wind, is one of the commonest subjects of remark. It is probably in a great measure owing to the cold which accompanies our east winds
* Mémoires de l'Institut pour 1824.
† Philosophical Transactions, 1831, p. 227,
‡ Bibliotheque Universelle, xl. 265.
§ Mécanique Céleste, tom. v. Supp. p. 30.
‖ Connaissance des Tems, 1805.
[page] 235
in spring, connected as they probably are with the melting of the snows in Norway*. Mr. Meikle, however, has lately remarked, and we think with justice, that the circumstance of their opposition to the direction of rotation of the earth, will cause an atmospheric accumulation by diminishing the centrifugal force of the aerial particles†.
The accidental variations of barometric pressure are greatly influenced by latitude. At the equator they may be said to be almost reduced to nothing; for it rarely happens that any change takes place to interfere with the regular course of the diurnal tides. A hurricane creates almost the only exception. The amount of variability increases towards the poles, in a great measure owing probably to the irregularity of the winds beyond the tropics. The mean amount of variation may be stated at the equator at two lines, in France at ten lines, in Scotland at fifteen lines, throughout the year; but this quantity has its monthly oscillations. Hence, a series of lines of equal variation of pressure, or isobarometrical lines as they have been termed, may be constructed‡. These do not appear to follow the parallels of latitude, but, like the isothermal lines, undergo inflections, and are stated to have a striking similarity to the isoclinal magnetic lines of Hansteen. If so, it is probably by the medium of temperature that these two are connected.
The great extent of country over which the accidental variations of the barometer take place, is one of their most striking features; and in a future and more advanced state of Meteorology we may be able to draw the most interesting and important conclusions from the great atmospheric tidal waves which are thus perpetually traversing oceans and continents. The best example we possess of a systematic examination of these great progressive fluctuations, is, it is to be regretted for the present character of the science, of rather old date. The Meteorological Society of the Palatinate was set on foot in 1780, and by the distribution all over Europe of instruments of the best construction then known, made at one common establishment, founded a set of observatories which annually afforded comparable results of the most intrinsic interest§. It is to be lamented that a system which at the present time has no successor, should have lasted only ten years, having ceased with the life of the Secretary of the Society: not
* See Mr. Marshall "On the Causes of the East Winds in Spring," in the Edinburgh Journal of Science.
† Edinburgh New Philosophical Journal, iv. 108.
‡ Kämtz, Jahrbuch der Physik und Chemie, 1827; and Bulletin des Sci. Math. x. 199.
§ Published at Mannheim under the title of Ephemerides Societatis Meteorologieœ Palatinœ.
[page] 236
only has it afforded many important results, especially upon the course and progress of barometric fluctuations, but has left a model of a scheme of combined exertion which the savans of the nineteenth century would do well to imitate. Some account of this Society and of the results of their labours, with projected charts of the barometric oscillations as a specimen, have been given to the world by Mr. Daniell in an interesting article in the second edition of his Essays*.
The connexion of barometric changes over large districts is very important in the determination of heights by simultaneous series of observations carried on for a considerable time at points even very distant. Examples of such an application at Paris and Clermont are given by Ramond†; but to avoid incidental derangements, the continuation of the observations for some time is desirable.
More lately, a comparison of the barometric changes at some principal points in Europe has been given by Prof. Schouw‡ , who has been followed by M. Kämtz in pointing out the connexion of the winds with such changes, and who has illustrated the influence of the prevalent aerial currents which traverse Europe, though not with apparent regularity, yet at least subject to some general laws§.
Of all the problems in Meteorology, few appear to me so intrinsically beautiful as that suggested by the fertile genius of Pascal,—the application of the barometer to the measurement of heights. It should, I think, be an object of ambition to bring this elegant method to the utmost degree of perfection of which it is susceptible. The laborious and praiseworthy experimental exertions of Roy, Shuckburgh, De Luc, Saussure, and Ramond, united to the theoretical skill of Laplace, have brought the method to a degree of precision which a century ago might well have been considered unattainable: but we are by no means arrived at the point at which improvement becomes hopeless, nor do we think that all has been done which might have been accomplished since Ramond's last determination of the coefficient of height, and his consequent improvements upon the barometrical Tables.
One important element neglected in the investigations of Laplace (at least only approximately estimated,) has lately begun to acquire the importance it deserves. I allude to the correction for moisture. The degree of Saussure's hygrometer was indeed made an element of calculation in some pretty early
* Meteorological Essays, p. 541.
† Mémoires sur la Formule Barometrique.
‡ Bibliotheque Universelle, xxxix. 260.
§ Ibid.
[page] 237
Tables; but the means of measuring the force of vapour with accuracy being only lately attained, the due correction has but within a few years become an object of adequate attention. Dr. Anderson, who has bestowed great attention upon the subject of Hygrometry, wrote a paper on this correction a few years ago*. Mr. Galbraith, in his Mathematical and Astronomical Tables, has followed Dr. Anderson in giving the correction, and has facilitated its application by the use of Tables.
Considering the problem as one of the highest interest accurately to solve, we approve of the introduction of every correction established upon sound theory and accurate experiment conjoined, even though in amount it may be less than the errors of observation or the unavoidable uncertainties arising from the interference of imperfectly understood active causes. By this process uncertainty will gradually be cleared away; and though there will undoubtedly be a limit beyond which no human perseverance can carry the approximation to truth, and a much wider one within which not one observation in a hundred will come, yet still truth will be separated from error, and the actual anomalies unaccounted for will be eliminated with precision. We do not therefore blame the superfluous accuracy (practically considered,) at which the formula of Laplace appears to aim. And for every-day observations it is easy to substitute those simple expressions which in most cases will give almost as good an approximation to the truth†. But in all experimental investigations where the arrival at truth within certain limits is the object, too great care can hardly be taken to avoid the intrusion of causes always acting in one direction, or which in the mean of a number of observations do not compensate themselves. Such, in fact, are some of the minuter corrections of Laplace, as those for latitude and for diminished gravity in a vertical direction.
The configuration of the ground has a considerable effect on the measurement of heights by the barometer, as has also the season of the year and time of the day. This last point has lately been a more especial subject of attention, although along with the former it was investigated by Ramond with his usual assiduity, who pointed out noon as the best hour for the experiment. It is very clear that the horary oscillation will in the first place affect the barometric measurement, but this is in Europe a very minute quantity and easily allowed for. At the equator, being almost the only variation to which the mercurial
* Edinburgh Philosophical Journal, vol. xii. xiii.
† Among the numerous forms which these have received, there is perhaps none more comprehensive and satisfactory than that given by Mr. Baily in his most valuable portable volume of Astronomical Tables, Lond. 1827.
[page] 238
column is subject, when it is allowed for, the height at the level of the sea may be considered known at any instant,—a most material advantage to the observer between the tropics, and which has conferred much of their accuracy upon Humboldt's beautiful barometric levellings and sections*.
The great obstacle to the accuracy of barometrical measurement, and the most influential change produced by the hour of the day, is to be found in the variable temperature of the strata of air intervening between the stations, as it is clear that the mean of the upper and lower temperatures may often deviate greatly from the true mean of the intercepted column. The currents produced from the plains to the mountains during the diurnal revolutions of temperature are extremely considerable; and hence the errors arising from the hour of the day greatly exceed those which the horary oscillation would produce. This has been pointed out and experimentally investigated by M. Horner, a Swiss Meteorologist of great activity, who made the mountain of the Rigi the scene of his operations†. Still more lately M. Gautier, Professor of Astronomy at Geneva, has published some interesting observations on the same spot, by which he found the error from the hour of the day alone, to amount to 14 toises upon a height of 700, the corresponding observations being made at Zurich‡.
In my papers on the application of the sympiesometer to the measurement of heights, already alluded to, I have given the results of many comparisons of this method with the geometrical one in several parts of Scotland, and a number of heights from original trigonometrical operations§.
I cannot dwell either upon the construction of portable barometers, or upon the precautions required in observation; but I strenuously recommend the subject to the scientific meteorologist, as one which will repay his labour, and which is yet open to most important ameliorations. When we consider the accuracy and extensive knowledge we have arrived at in the position of points of interest on the surface of the globe, with regard to the coordinates of latitude and longitude, and how little has been done for the third coordinate of elevation, we shall have a field before us open to cultivation at every corner. The results to physical geography of what has already been done by the use of the barometer, excite our warmest hopes of its extension. To mention only one instance;—the singular discovery
* Voyage aux Régions Equinoctiales; ATLAS, and OBSERVATIONS ASTRONOMIQUES. Baron Humboldt has recently circulated a beautiful "Carte hypsometrique" of the Cordillera.
† Bibliotheque Universelle, 1831, N.S. iv. 337.
‡ Ibid. v. 337.
§ Edinburgh Journal of Science, N.S. iv. 91. 329.
[page] 239
by MM. Parrot and Engelhart, of the depression of the Caspian and Lake Aral below the Mediterranean*, and the not less extraordinary extension of this anomalous fact by MM. Humboldt, Rose, and Hoffmann, to an immense territory about 18,000 square leagues in surface†. These conclusions, most important for physical geography, might never have been attained but for the barometer; and at the suggestion of Baron Humboldt the Academy of St. Petersburg have undertaken to prosecute the inquiry with the same instrument, to institute "barometrical soundings," as they have been aptly termed, over this vast crater-like depression, and establish the lines of equal altitude.
A most important synopsis of what has been done in Europe in this department will be found in the Orographie de l' Europe,—a collection of above 7000 heights, formed by the industry of M. Brugiere‡ to whom the scientific world is most deeply indebted, and whose work has been deservedly approved and rewarded by the Geographical Society of France. A vast proportion of the determinations of heights in this volume are due to the barometer.
Humidity.
Hygrometry, scientifically considered, has only had justice done to it within a very short period. Till Mr. Dalton established the true views of the connexion of temperature and the tension of vapour, meteorologists had vague ideas of the true expression of degrees of moisture. The labours of Saussure, though most meritorious, were destined to be superseded by a more elaborate analysis of the subject; indeed his views of hygrometry were in some respects so very imperfect, that he was not aware of the fact, that the coolness produced by the evaporation of water from porous bodies, was independent of the rate at which the moisture was carried off by currents of air, —a want of knowledge which gave him much trouble.
On the general principles of Hygrometry I have no intention of dwelling; I shall chiefly confine myself to a notice of the latest additions to the subject. It will be necessary however to premise one or two observations on the general state of the question.
If the views of Mr. Dalton, noticed in an early part of this Report, be true, with regard to the condition in which vapour exists in the atmosphere,—views, which are now universally admitted,
* The depression of the Caspian is 334 English feet below the Mediterranean, and it has recently been ascertained by Captains Duhamel and Anjon that Lake Aral is 117 feet above the Caspian.
† Humboldt, Fragmens Asiatiquets, tom. i. pp. 9. 91. 136.
‡ Mémoires de la Société de Géographie, tom. iii. Paris 1830.
[page] 240
in so far as they consider the tension of vapour as totally independent of that of air, or the presence of air at all,—we must banish all confused notions from our minds about "saturation of air with moisture," "solvent power of air," &c., which are to be found even in the very writings in which Mr. Dalton's principle is assumed as established. This should be guarded against with care, because it may insensibly lead in practice to the most innaccurate ideas regarding the influence of the presence of gaseous matter. We think nevertheless that in some cases the rage for purifying our scientific nomenclature has been carried too far, where even the results of reasoning are arraigned because they include the use of terms suggesting perhaps hypothetical views, but the adoption of which conventionally, need not be objected to.
The easiest way of obtaining a distinct, simple, and accurate knowledge of the hygrometric state of the atmosphere at any moment, is to ascertain by some means the temperature at which the vapour then existing can no longer maintain its aeriform state, or, in other words, to find the temperature of the dew-point. Then being furnished with a Table of the elasticities of aqueous vapour at different temperatures, the elasticity is of course equal to that of vapour which can just subsist at the temperature of the dew-point; whence the weight of grains in a cubic inch may be easily computed from the experiments of Gay-Lussac, and the expression of the sensible state of humidity of the atmosphere at its own proper temperature, must be obtained by the ratio of the vapour actually existing in a cubic inch, to what might have existed without deposition, in the same space.
Such, in few words, is the rationale of the dew-point experiment. Let us see now the means we have of arriving at this result. Regarding instruments, the simplest form of the experiment is that which Mr. Dalton employs. The dew deposited on the surface of a glass of cold water has been observed from the earliest times, and has been particularly alluded to by ancient authors; let therefore the cold liquid be transferred from one glass to another till the deposition ceases,—the temperature then measured will give the dew-point. Mr. Daniell's elegant instrument is too well known to require minute description: he has applied the principle of the Cryophorus of Wollaston to obtain the requisite cold for the production of dew upon a ball of dark-coloured glass, the temperature of the ether inclosed, being measured by a delicate thermometer inserted. This instrument has come into very general use, and notwithstanding some delicacy required in the management of
[page] 241
it, and an occasional difficulty of arriving at a precise result, we may expect the most valuable results from its application to hygrometry.
The dew-point hygrometer of Mr. Jones*, though more simple and compact, is not so satisfactory in its results; it consists of a thermometer with a cylindrical bulb turned upwards and half-covered with muslin, which is cooled by pouring ether upon it, and the deposition of the dew is observed on the upper part of the bulb. A different form has lately been brought forward by Mr. John Adie of Edinburgh†, who incloses the bulb of a very delicate thermometer in an exterior ball of glass, the interval being filled with mercury; and he observes the deposition of dew on that portion of the outer ball from which the covering of muslin for receiving ether has been removed. By agitating the instrument at the time of deposition, Mr. Adie has been able to get results more closely agreeing with Dalton's experiment, than by the hygrometers of Daniell and Jones. The principle of the instrument is very obvious; from the small size of the ball of the thermometer the temperature is more accurately found than in Mr. Jones's apparatus; and the small bulk and better conducting power of the medium interposed between it and its outer case, render it perhaps more sensible than the instrument of Mr. Daniell. To do the latter instrument justice however, (and from my experience of dew-point instruments in their simplest forms, I think the remark of importance,) the temperature at which dew appears should not only be noticed, but that at which it disappears. The errors of the two must almost always be in opposite directions, and the mean should be taken.
A dew-point hygrometer, in some respects resembling Mr. Adie's, has been proposed in America by Mr. Hayes‡.
Another instrument,—and though I have not tried it, I confess it appears to me a very elegant one,—has recently been proposed by M. Pouillet§. He places a delicate thermometer vertically with its ball upwards, which passes into a small cup of polished silver. Ether is poured into the cup till it covers the ball, and when by the coolness produced by its evaporation the deposition of moisture is produced on the silver, the temperature is noted.
There is probably no instrument which gives the dew-point with so much accuracy as Mr. Dalton's simple experiment when
* Philosophical Transactions, 1826.
† Edinburgh Journal of Science, N.S. i. 60.
‡ Silliman's Journal, xvii. 351.
§ Element de Physique, ii. 732.
Q
[page] 242
the glasses employed are thin. This is the result at which Dr. Thomson of Glasgow has arrived*.
The next point is the Table of the elasticities of vapour at different temperatures. Mr. Dalton's excellent Table, or that calculated by Dr. Young from the experiments of Dr. Ure, will be quite sufficient for the range of atmospherical temperatures. The new Table derived from the meritorious labours of the French savans, whose experiments have been carried up to a pressure of steam amounting to 24 atmospheres, will probably become the standard reference on this subject, at least in the case of high pressures†. The formula at which they have arrived, and which bears a striking analogy to that of Dr. Young, is the following:
e = (1 + 0·7153 t)5,
where e is the elasticity in atmospheres (reckoned at =0m·76), and t the temperature reckoned from 100° and computed in centigrade degrees.
From any such Table of elasticities, with Gay-Lussac's result for the specific gravity of aqueous vapour, the weight in a cubic inch under any circumstances may easily be computed. It is hardly necessary, however, to repeat that the expression for the degree of humidity is not the actual weight of moisture in a given space, but the proportion which that bears to the weight which might exist without deposition under the circumstances of temperature and pressure.
Great as are the advantages of simplicity of calculation, which the dew-point experiment affords, there is a less direct experiment which offers great facilities in performance and likewise the means of self-registration. I allude to the moistened bulb hygrometer, in which the coolness produced is a function of the dryness of the atmosphere, without bearing any relation to the force of wind or other circumstances which affect the rate of evaporation. Under the simplest form of two thermometers, one of which had its ball moistened, it was employed by Dr. James Hutton; and afterwards Professor Leslie adapted it to the principle of his differential thermometer; a change perhaps not contributing to the simplicity of the instrument, which still requires a detached thermometer to determine the temperature of the air. Accordingly, the instrument in its first and simplest form (in which for years we have been in the habit of using it,) has recently been reproduced by M. Auguste, under the high-
* Thomson On Heat, p. 256.
† Annales de Chimie, Janvier 1830, tom. xliii. p. 74.
[page] 243
sounding name of the Psychrometer*. This instrument has been employed by Baron Humboldt in his recent journey in Asia, where he had occasion to observe a very high degree of dryness, the coolness by evaporation amounting to 11°·7 cent., the temperature of the air being 23°·7†. It were to be wished that, for the improvement of the theory of the instrument, he had at the same time ascertained the dew-point by experiment. M. Auguste is himself the author of the formula by which the tension of vapour is deduced. He has published not only a paper expressly on this subject‡, but an essay (which I have not been able to meet with,) upon the progress of Hygrometry in modern times§.
The perfection of the method of the moistened thermometer forms an important and an interesting problem. Mr. Leslie's solution, which was the first, offers a near approximation to the truth, but at the higher temperatures will require modification, especially as instead of adopting any of the Tables of the force of vapour now in use, he has contented himself with the general result of some original experiments, that the "capacity of air for moisture," to use his own phrase, is doubled by the increase of temperature by every 15° of the centigrade scale. This leads him into inevitable errors at higher temperatures‖. Dr. Anderson's elaborate investigation contained in an able article on Hygrometry in the Edinburgh Encyclopœdia, to which we can do no more than allude, appears also to be faulty in the higher parts of the scale, if we can depend upon some experiments recently made by an anonymous writer in India¶. Undoubtedly the most valuable application of Professor Leslie's hygrometer will be, by rendering it self-resistering on the simple principle proposed by the Rev. Mr. Gordon, which is similar to that of Rutherford's minimum thermometer**.
* Bulletin des Sciences Mathématiques, vii. 379.
† Fragments Asiatiques, ii. 378. At Geneva, in August last, I observed a coolness by evaporation amounting to 20° Fahr., the thermometer in the shade being at no less than 92°. I then found it quite impracticable to obtain a deposition upon Daniell's hygrometer.—Dec. 1832.
‡ Poggendorff's Annalen, 1828. There is a paper by Brouwer upon Auguste's instrument, in the Amsterdam Journal, entitled "Bijdragent ot de Naturkundige Wetenschappen" 1831, p. 272. See also Bull. des Sci. Math. x. 302.
§ In German. Read to the Society of German Naturalists in 1828.
‖ Professor Leslie's researches are contained in a tract upon "Heat and Moisture," Edin. 1813: and in the article METEOROLOGY in the Supplement to the Encyclopœdia Britannica.
¶ See two clever papers in a periodical work entitled "Gleanings in Science,1* Nos. II. and III. Calcutta 1829. The Author points out the great difficulty of using Daniell's hygrometer in warm climates, from the deterioration of ether.
** Edinburgh Encyclopœdia, Art. METEOROLOGY.
Q 2
[page] 244
The researches of Gay-Lussac upon the scale of Saussure's hair hygrometer, are too well known to require notice here; we only mention them to observe that the analogy to the abscissae of a hyperbola, of the tensions of vapour, the ordinates representing the degrees of the hygrometer, has been further extended by Signor Melloni in a long paper recently published on this subject*.
The hygrometer proposed by M. De la Rive indicating the temperature evolved by the Combination of a film of sulphuric acid with the moisture of the atmosphere, has not as far as we know come into general use.
The distribution of vapour in the atmosphere is a most curious and difficult problem, of which the data are only now beginning to be collected. We know the mean tension of vapour at very few points on the surface of the globe, which, from the influence of temperature, varies exceedingly, and will one day be the subject of connected and scientific discussion as satisfactory as the isothermal lines are at present. Dr. Anderson has given some interesting views upon what we may believe to be the distribution of vapour from the equator to the poles†, and the same subject has been taken up by Mr. Daniell in his Essay on the Constitution of the Atmosphere‡. As to its variation with height, we are almost equally in the dark, but we are certain that intense dryness reigns in the higher regions of the atmosphere‖. The law of decrease is probably not a regular progression: it appears probable from many circumstances, and in particular from some experiments of Captain Sabine, that the dryness is pretty constant for a certain height, and then rapidly diminishes. In fact there is certainly a stratum of air at the height of from 1 mile to 4 miles, which is more frequently saturated with vapour than any other, and which constitutes the region of clouds.
The annual and diurnal variations of temperature produce effects in the distribution of humidity, analogous to those which we observe in passing from one latitude to another. Even with our extremely limited views of the nature and extent of these changes, we can trace, with a little care, the influence of the great fundamental law of hygrometry, in producing clouds, mists, and other phænomena, which, in hilly countries espe-
* Annales de Chimie, xliii. 39.
† Article HYGROMETRY in the Edinburgh Encyclopœdia.
‡ Meteorological Essays, p. 73, &c.
‖ The interesting researches of M. Kämtz in the higher Alps, promise to throw the greatest light on this important point. I had the satisfaction of witnessing last summer along with him, at the height of 8,500 feet, a degree of natural dryness unexampled, I believe, in the annals of hygrometry.— Dec. 1832.
[page] 245
cially, produce such grand and varied spectacles, and indicate in the most beautiful manner the constancy of the laws by which the temperature and variable conducting powers of the materials on the surface of our globe, modify the distribution of atmospheric vapours*. The subject is one of wide extent, and may at a future period disclose very interesting results; it does not appear however to present such definite points of investigation as to be reckoned among the first objects of the scientific meteorologist in search of general laws.
The nomenclature of the clouds, adopted by Mr. Howard, is a happy specimen of a conventional system, and is well calculated to stamp with a definite character the future results of observation; each species of cloud very probably is attended with a characteristic hygrometric condition, and most likely exists between fixed limits of altitude. I do not recollect to whom we are indebted for a suggestion which well deserves attention, but which cannot be accomplished without that essential condition which it seems the fate of Meteorology to want,—Cooperation. If by a series of little maps of the state of the sky we could represent the daily condition of the atmosphere over a large continent such as Europe, what curious results might not be unfolded! The determination of the existence of immense clouds covering whole countries for days together, while others were under sunshine—the watching of the progress of these clouds, not so much by the influence of wind, as by a gradual process of hygrometric dissolution and recomposition, day after day, would give us more insight into the operations of the higher atmosphere on the large scale, than a thousand insulated observations.
The diurnal extremes of the hygrometric state are of course limited by those of the temperature of the atmosphere; the minimum temperature causing a deposition of moisture when it exceeds a certain amount, and the limit within which the maximum temperature of the air is kept, (86° Fahr. being the maximum over the ocean at any point of the globe†,) preventing the existence of vapour beyond a certain degree of tension. Dr. Anderson has in an elegant paper shown the connexion which is hence established between the dew-point at any time of the day, and the minimum temperature of the same period‡.
We cannot propose to meteorologists a finer problem for complete solution, than that of the moist bulb hygrometer; which will require a close analysis of all that has hitherto been
* See Sir Humphry Davy "On the Formation of Mists," Phil Trans.
† Arago, Annuaire, 1825 p. 186.
‡ Edinb. Phil. Journal, vol. xi.
[page] 246
done on the subject. M. Gay-Lussac, who has communicated some valuable observations towards its attainment, thought that it would never repay the labour of complete investigation. In the present state of the science, however, we look upon it otherwise, and feel strongly assured that in a few years the more direct method of the dew-point will be banished altogether*.
Atmospheric Phœnomena and Precipitations.
In the introduction to this Report I have stated my intention of by no means undertaking to examine the wide field which this subject opens to us. My remarks will be confined to one or two individual points, upon which some general views have been entertained. Those I have selected are Wind, Rain, and Electrical Phænomena, including the Aurora Borealis.
The direction and force of the Winds we have already seen to be intimately concerned in the modification of climate, and in the distribution of temperature in the atmosphere. The periodical winds of the equator and tropics correspond in regularity to the uniform course of the seasons and the limited range of the barometer characteristic of that part of the globe: nor has anything particular been added to our knowledge of these great currents, of late years, which requires notice here†.
In temperate climates the irregularity of the wind in general seems so great as to baffle inquiry. There are a few leading points, however, which show that there is something to be seized in this question, and that an analysis of it may one day lead to more general results. It is undoubted that the south-west is the predominant wind of Europe, and the east winds in spring may be considered as almost accurately periodical in the climate of Britain‡. M. Schouw has gone a step further, and has shown that the prevalence of westerly winds diminishes as we advance towards the east of Europe§. The west winds at London exceed the east in the ratio of 1·7 to 1. At St. Petersburg this is diminished to 1·3 to 1‖.
M. Erman has determined the mean direction of the wind in Russia and Siberia to be as follows:
* In addition to the references already given, we may mention, for the use of those who may pursue the subject, a paper by Mr. Meikle on this point, in the Edinburgh New Phil. Journal, ii. 22; and some articles in Poggendorff's Annalen for 1829, by M. Dove, whose remarks on the connexion of the horary oscillations of the barometer with humidity we have already noticed.
† There is a paper by Captain Hall on this subject in the second edition of Mr. Daniell's Meteorological Essays.
‡ See Mr. Marshall "On the Causes of East Wind in Spring," Edin. Journal of Science.
§ Beiträge zur vergleichenden Klimatologie.
‖ Bulletin des Sciences Mathématiques, x. 201.
[page] 247
| St. Petersburg | S. 41° | W. |
| Moscow | S. 35 | W. |
| Kasan | S. 52 | W. |
| Tobolsk | S. 47 | W. |
Baron Humboldt thinks that the western winds diminish in frequency from St. Petersburg towards Central Asia, though they increase towards the North of Siberia*.
The direction of winds is greatly affected by the configuration of a country, their general direction being modified, so as to coincide with its natural lines of elevation and depression. It is probably on this account that in Egypt the winds are generally either north or south, the former prevailing nine months in the year†. Where the climate is tolerably regular, as in the South of Europe, the direction of the wind makes all possible difference in its character. The transition from a sirocco to a tramontana at Rome or Naples is as great as the effect of ten degrees of latitude. It is surprising therefore, that, powerful elements as these aerial currents are, they have been so imperfectly studied.
I have now before me the results of a register of the force of the wind by Lind's anemometer for the year 1826, kept with great assiduity by my friend Mr. Snow Harris of Plymouth. I have already alluded to the connexion which he has pointed out between the force of the wind and the horary oscillations of the barometer, which has not before been remarked: indeed the observation of the anemometer is so rare, that there are few meteorologists who have persevered in the use of it. This renders the register of Mr. Harris the more valuable. The mean force of the wind for the whole year at 9 A.M. was 0·855; at 3 P.M. 1·107; and at 9 P.M. 0·605. Mr. Harris informs me that he has found the anemometer of Lind a more satisfactory instrument than it is usually considered. The improvement of anemometers has been almost abandoned for some time; indeed it may be doubted whether, with an element so momentarily variable, insusulated observations can be of very great value. M. Leroy has proposed what he calls an Eolian clock, which by means of machinery is intended to measure the direction and force of the wind‡. I think that if the anemometer is ever to become an available meteorological instrument, it must be on some principle of self-registration such as I proposed about two years ago§. Either by a piece of clock-work or some simple movement put in action
* Fragments Asiatiques, ii. 353, note.
† Belzoni, Researches and Operations in Egypt and Nubia, vol. i.
‡ Bulletin des Sciences Mathématiques, ix. 32.
§ Edinb. Journal of Science, January 1830.
[page] 248
by the wind itself, I proposed that small spherules of wood or other light matter, or even shot, should be let fall through a free space, suppose of three feet, and that the force and direction of the wind should at once be measured, at every interval of the falling of a spherule, by the amount and direction of the deflexion produced, and which should be ascertained by the dividing into compartments a platform arranged to receive them. I have made some experiments on the subject, and have every reason to believe that the method admits of great accuracy, and that it is perhaps the most satisfactory mechanical one that has been proposed. I conceive that Professor Leslie's ingenious plan of measuring the force of the wind by the cooling of a thermometer exposed to it, is the most satisfactory indirect method, and has not met with the attention which it deserves*.
We have seen that the direction of the winds exerts an important influence on the height of the barometer. There is another source of action which it creates, and which is less understood. M. Schubler has shown, in an interesting paper, that the winds have each their characteristic electric power The precipitations during the winds from the northern half of the circle of azimuth, have a ratio of positive to negative electricity which is a maximum; and in the other half it is a minimum, the negative precipitations when the wind is south being more than double the positive ones. The mean intensity of electricity, independent of its sign, is greatest in north winds. We must refer to M. Schubler's paper for his reasoning upon these facts†.
It is a fact to be attended to, that the progress of a wind and the storm which may accompany it is not always in the direction in which it blows. M. Pouillet terms the modes of propagation of wind by "impulsion" and by "aspiration‡." In the latter case, a vacuum or diminution of pressure being at any point effected, the air which flows to fill it up commences, of course, its motion nearest the point of deficient equilibrium, from which the current gradually retires. Franklin long ago compared it to the flow of water through a canal upon opening a sluice. Mr. Mitchel in America has discussed this view of Franklin, which he thinks will not always apply; indeed the case is one of difficulty, and, unless we can ascend to the first active causes, would only lead into unprofitable speculation. In many cases the deficiency assumed on Franklin's hypothesis cannot be proved, and in some is untenable; but we
* Essay on Heat, p. 284.
† Jahrbuch der Chemie und Physik, 1829, Heft iii.; Bibliotheque Universelle, Nov. 1829; Edinb. Journal of Science, N.S. iii. 116.
‡ Elemens de Physique, ii. 715.
[page] 249
are not sure that we shall gain much by assuming the gyratory theory of Mr. Mitchel*.
We would recommend to any meteorologist taking up this subject, to endeavour to establish observations at two stations, one considerably elevated above the other, and to trace the course of the wind at the two points when it is changing its direction: we know that currents in various directions several times superposed often coexist in the atmosphere, and it is probable that changes of wind generally commence at considerable elevations.
Of all the columns of that too often unprofitable work, a Meteorological diary, one of the most profitless has generally been that devoted to the direction of the wind, as in its usual form it does not admit of having any average taken, and therefore remains an undigested mass of insulated observations. In order to draw any useful conclusion from this observation, we would therefore recommend the adoption of Lambert's numerical form, in which the south is denoted by 0°, and the angle is measured round the horizon by the W., N., and E. In this way S.W. is denoted by 45°, W. by 90°, &c.
On the subject of RAIN,—a very important one in a practical point of view,—we have not lately obtained much new information. The theory of Dr. James Hutton remains nearly unaltered, only strengthened and enlightened by the clearer views of the nature of deposition which we now possess. The connexion of rain with the fall of the barometer has met with one elucidation from Mr. Meikle† which is worthy of notice, because the change of pressure, it is shown, may be a cause as well as an effect. He observes that the expansion of air accompanying diminished pressure being productive of cold, will diminish the elasticity of the existing vapour, and cause a deposition.
M. Arago has collected many interesting facts in the phænomena of rain‡. He has traced the progress of decrease in the annual amount from the equator to the poles. It is now known that on the Malabar coast in lat. 111/2° N., not less than 123·5 inches of rain fall in a year; whilst in lat. 60° it is reduced to 17 inches. The law of decrease is not known with accuracy. The author of the article PHYSICAL GEOGRAPHY in the Supplement to the Encyclopœdia Britannica§, has proposed the following formula for the fall of rain in inches:
75 (rad. – sine lat.) + 8,
* Silliman's American Journal, xix.
† Royal Institution Journal.
‡ Annales de Chimie, xlii. 360; Annuaires du Bureau des Longitudes pour 1824 et 1825.
§ vol. vi. p. 163.
[page] 250
which however but imperfectly represents the observations. The causes which regulate the amount of rain in different latitudes, have been well pointed out by Dr. Anderson in the Essay on Hygrometry, before alluded to*.
A less explicable variation takes place in the fall of rain at different heights. And here a distinction, not always enough attended to, must be pointed out. The quantity of rain which falls on high grounds exceeds that at the level of the sea; but the amount at stations abruptly elevated above the surface of the earth diminishes as we ascend. For example, at Kinfauns Castle, Perthshire, by a mean of five years, 25·66 inches of rain fell; whilst on a hill in the neighbourhood, 600 feet higher, no less than 41·49 inches were collected by a mean of the same period†. On the other hand, at Paris, whilst 56·37 centimetres of rain fall in the court of the Observatory, according to Arago, only 50·47 fall on the tower at a vertical height of 28 metres. The former fact may readily be explained by the influence of a hilly country in retaining clouds and vapours; but the latter seems yet to have met with no satisfactory explanation, nor has any theory having even novelty to recommend it been recently proposed. The interesting observations established at York Minster, at the suggestion of the British Association, and under the active superintendance of my friends Mr. Phillips and Mr. Gray, jun., will soon, I am certain, afford us valuable information on this curious subject.
The very interesting comparative registers kept at Geneva and at the Convent of the Grand St. Bernard, have not failed to illustrate the influence of a mountainous country on the fall of rain. From the results published in the Bibliotheque Universelle, it appears that the amount at the latter point is double that at the former‡. Mr. Dalton, in an interesting paper upon these observations, which has just appeared§, points out in a clear manner the influence of hot currents of air ascending by the surface of the ground into the colder strata which rest upon a mountainous country. The consequence is, that although neither the hot nor the cold air was accompanied with more moisture than could separately be maintained in an elastic state, when the mixture takes place, the arithmetical mean of the quantities of vapour cannot be supported in an elastic state at the arithmetical mean of the temperatures, since we have seen that the weights of vapour which can exist in a given space, increase nearly in a geometrical ratio when the temperatures follow an arithmetical one.
* Edinburgh Encyclopœdia, vol. xi.
† Ibid, Art. METEOROLOGY.
‡ Bibliotheque Universelle, Mars 1828.
§ Manchester Transactions, New Scries, v. 233.
[page] 251
At Geneva by a mean of 32 years the annual fall of rain is 30·7 inches; at the Grand St. Bernard by a mean of 12 years it is 60·05 inches.
The variation in the amount of rain with the seasons follows in a great measure the same law, founded on hygrometric principles, which causes the difference in different latitudes. The greatest quantity falls in summer, the least in winter. The influence of the lunar periods has also met with some attention. The popular belief of the influence of the moon upon the weather is probably too strong and too universal to be totally without foundation. At one time I attended a good deal to the subject, and my observations led me to believe that there was some real connexion between the lunar phases and the weather. The old writings of La Cotte and Toaldo contain some curious observations on this subject, which has more lately been resumed by M. Flaugergues, who has observed the weather at Viviers with great assiduity for a quarter of a century. He has marked the number of rainy days corresponding to the lunar phases, and he finds them at a maximum at the first quadrature, and at a minimum at the last*. This agrees pretty nearly with his corresponding observations on the height of the barometer which we have already recorded.
A similar question to that which has been put in every other branch of Meteorology, whether there is any secular variation, —has been asked in the case of rain; and we are quite as unable as in the other instances to afford any satisfactory reply to it. There are several causes which may tend to change the amount of rain on a particular spot without forming part of any general law; among such changes will be found the destruction or the planting of forests, the inclosure and drainage of land, and the increase of habitations. M. Arago has shown† that the fall of rain at Paris has not altered sensibly for 130 years; and in order to show that the conclusion drawn by M. Flaugergues at Viviers, that the amount of rain is on the increase, cannot be a general one, he has quoted the case of Marseilles, where the amount of rain appears to have undergone a striking decrease in 50 years. M. Arago justly observes that it is very difficult to know how many years of observation are necessary to get a mean value of the fall of rain, the amount being extremely variable: thus at Milan, where an increase of rain has been thought to be decidedly proved by observations for 54 years, the extremes of the annual results between 1791 and 1817 were 24·7 and 58·9 inches‡.
* Bibliotheque Universelle, xl. 283.
† Annuaire, 1824.
‡ Annuaire, 1825, p. 155.
[page] 252
It is worthy of remark that notwithstanding the enormous annual fall of rain at the equator, particular instances of a great depth of rain in a short time have occurred (though rarely) in Europe, which probably have seldom been equalled by authentic observations in any part of the globe. At Geneva, on the 25th October 1822, there fell thirty inches of rain in one day*. An example equally extraordinary has recently been quoted by M. Arago, and which is perfectly authentic. At Joyeuse in the department of the Ardêche, on the 9th October 1827, there fell 29 inches 3 lines French measure (above 31 inches English) of rain in 22 hours†.
The subject of ATMOSPHERICAL ELECTRICITY excited in the middle of the last century an unexampled degree of interest in consequence of the fine discoveries of Franklin; and the application of thunder-rods produced a more vehement spirit of discussion among all classes than is usually to be met with on any purely scientific question. This excitement was naturally succeeded by a degree of apathy; and it must be admitted, that whilst every department of the noble science of electricity has been illustrated with triumphant success by Coulomb, Davy, Oersted, Faraday, and many others, its application to Meteorology has been strangely neglected, and in fact, on this important subject almost everything has yet to be done. On the general subject of atmospherical electricity, the principal contributions which we have to notice are those of M. Pouillet, to whom we owe some very interesting experiments in electrical science.
One great question in this subject is the source of the vast amount of electricity which seems, as it were, perpetually created in the atmosphere, and which, notwithstanding the constant recombinations which are going forward, remains sensible, according to the experiments of Lemonier, Saussure, and others, during the most steady and cloudless weather. M. Pouillet has very happily shown two causes in constant operation which create this abundant supply‡. The first of these is vegetation. M. Pouillet has proved by direct experiment that the combination of oxygen with the materials of living plants, is a constant source of electricity; and he has shown that a surface of 100 square metres in full vegetation disengages
* Pouillet, Elemens de Physique, ii. 758.
† Annales de Chimie, xxxvi. There are some interesting collections relative to the fall of rain at different places, in Schouw's Specimen Geographiœ Physicœ Comparativœ 4to, 1828.
‡ Annales de Chimie et de Physique, 1827. See also his Elemens de Phyue, liv. ix. chap. 5.
[page] 253
in the course of a day as much vitreous electricity as would charge a powerful battery. The second source is evaporation. The experiments of M. Pouillet went to prove the unexpected fact, that the conversion of pure water into vapour excites no electric tension. As however this applies only to water and other fluids in a state chemically pure, it makes no difference in the efficacy of this change as productive of atmospheric electricity. It is needless to observe how extensive and powerful must be the result of this action.
It is hence easy to conceive how the electricity, produced by these and other sources, must vary in different climates, seasons and localities, and at different heights in the atmosphere. The general principle of the formation of electrical clouds, and the production of thunder and lightning, is easily apprehended; but the fact of our almost total ignorance of any one step of the process cannot be disguised, and, as M. Pouillet frankly admits, "il faut avouer si la principe de la formation des nuages orageux ne présente pas des difficultés, les applications en présentent, parceque nous n'avons pas assez de données sur la formation des nuages elle-mêmes."
It is generally believed that in fine weather the electricity of the air is positive, and increases in intensity as we ascend. Upon these points however observers are by no means agreed, and the subject opens a wide field for experiment. From the observations of M. Schubler, it would appear that in the climate of Europe the electricity of precipitations is more frequently negative than positive in the ratio of 155: 100, but the mean intensity of the positive electricity is greater than that of the negative in the ratio of 69: 43*.
There is a subject intimately connected with electricity which we are unwilling totally to pass over in this place, although little has lately been added to our knowledge upon it, because we think that it has not excited the attention in this country which it deserves;—we mean the phænomenon of Hail. The difficulty of accounting for the retention of masses of ice in the free atmosphere till they attain in some cases the diameter of several inches, is certainly very great. Perhaps no hypothesis more satisfactory, certainly none more ingenious, has followed that of Volta, who conceived from the highly electric condition of the atmosphere, almost universally attending the production of hail, that the frozen masses were kept in a state of reciprocating motion between two clouds oppositely charged with electricity, until the increase of the mass rendered the force of gravity predominant, or the electric tension of the clouds was exhausted by mutual reaction. It
* Bibliotheque Universelle, xlii. 203.
[page] 254
were easy to multiply objections to this hypothesis, and some of the reasonings of the author relative to the production of cold are almost certainly erroneous; but at the present moment it would be difficult to point out any explanation more plausible*.
From the rarity of the occurrence of hail storms in this country, the subject has met with little attention compared to what it has received in most parts of the Continent†. In our Indian territories, however, the finest opportunities occur for the investigation of facts connected with the subject. Dr. Turnbull Christie has recently published, on this subject‡, a short notice in reply to some theoretical views of Prof. Olmsted, an active American meteorologist§. We hope that among the scientific objects which engage Dr. Christie's attention since his recent return to India, this will not be forgotten.
This question has appeared of so much importance and interest on the Continent, that the Academy of Sciences at Paris has recently proposed the theory of hail as the subject of a prize memoir.
Before concluding this Report, I am anxious to advert to the very interesting subject of the AURORA BOREALIS,—one which appears intimately connected with the science of Electricity, and upon which we cannot but hope soon to acquire new and extended views.
I shall not dwell for a moment upon older observations, but proceed to state that Mr. Dalton has been led, from numerous and very interesting observations which he has collected upon auroral arches, to conclude that their average height above the surface of the earth is about 100 miles‖,—a conclusion not differing much from what he had long before been led to¶. The frequent occurrence of these beautiful phænomena of late years, has rendered them an object of general observation, and many descriptions have been published by different authors in the periodical works of the day. The one of which Mr. Dalton deduced the height in the most satisfactory manner, was that of the 29th March 1826. The most striking examples which have since occurred, were on the 29th Sept. 1828, and the 7th Jan. 1831**. The last is perhaps the most extensively observed on record.
* See on this subject a paper by M. Arago in the Annuaire for 1828.
† See however an interesting account, by Mr. Neill, of a remarkable hail storm which occurred in Orkney some years since. Edinburgh Transactions, ix. 187.
† Edinburgh New Philosophical Journal.
§ Published in Silliman's Journal, 1830.
‖ Phil. Trans. 1828, p. 291.
¶ In his Meteorological Essays.
** On the last may be consulted papers by Mr. Christie, Mr. Harris, and Prof. Moll, in the Royal Institution Journal, N.S. vol. i. On that of 1828, Mr. Gilbert, Phil. Mag. N.S. iv. 453; Capt. Kater, Ibid. 337; Mr. Harvey, Edin. Journal of Science, x. 146.
[page] 255
The opinions of Mr. Dalton on the height of the aurora have not been received without contest. Mr. Farquharson of Alford in Aberdeenshire has published his views upon this subject in a paper containing many curious facts, and from which he draws the conclusion that the aurora is not elevated above one or two miles*. His estimate however appears to me to be founded on a species of observations somewhat vague, and by no means comparable, as scientific deductions, to the trigonometrical measures of Mr. Dalton. Indeed, the fact of the immense distances of points at which these arches have been seen at the same instant, is alone sufficient to throw great doubt upon any theory which assigns to them a low position in the atmosphere. So strong is this objection, that Mr. Farquharson has been obliged to suppose that the different observers were viewing different parallel auroral bands,—a supposition surrounded with difficulties. The only striking actually observed fact appearing to demonstrate that the aurora sometimes approaches the surface of the earth, is that, related by Captain Parry, of a beam of the aurora appearing to shoot down between the observers and a rising ground only 3000 feet off†. This very extraordinary and unique observation, certainly appears to me more attributable to an optical illusion, than as fitted to become the basis of extensive induction. At least it is very conceivable that a beam of the aurora shooting downwards, as is described, with all the brilliancy peculiar to that meteor in arctic climates, might, as it passed behind an eminence, appear from the quickness of its motion to continue its former course, and shoot across the obstacle which actually intercepted it from view. Such at least seems to me a highly natural explanation:—be this as it may, a single observation cannot, in the face of all those to the contrary, limit the bounds of the aurora to the lower strata of the atmosphere.
I readily admit however that some phænomena of electrically illuminated clouds, such as I remember particularly to have observed on the 10th Sept. 1827‡, are of difficult explanation. Should it however be admitted that these were "clouds highly electrified," as I have stated in the memorandum just referred to, I would beg to draw a very broad line between these and true auroral nebulæ or arches. The evidence which convinced me that these were truly clouds, was especially the fact that "they
* Phil. Trans. 1829, p. 105.
† Parry's Third Voyage.
‡ Edinb. Journal of Science, ix. 138.
[page] 256
obscured bright stars," for the auroral arches which I have observed, generally allowed even minute stars to be seen through their mass; but this I admit to be a question of degree. Very recently an interesting article by Prof. Jameson has been published in the new edition of the Encyclopœdia Britannica, in which the views of Mr. Farquharson on this point are strongly supported*.
In the paper just alluded to, Mr. Farquharson justly observes, that the motion of the auroral arches is from north to south, or rather N.W. to S.E.: he adds, that he never heard of an arch observed whilst low in the north, and traced in its course up to the zenith, and thence southward. I beg to refer to some circumstantial details of an arch observed by myself, 21st January, 1826†, traced almost from its origin in the north till it disappeared close to the southern horizon. This arch had also the peculiarity of moving, not in the direction of the magnetic meridian, but from N.E. to S.W., and diametrically against the wind.
Mr. Potter has recently given some interesting views regarding the height of the aurora, and pointed out a method by which (certain postulates being admitted,) its height may be calculated from observations at one station‡. His results coincide generally with those of Mr. Dalton.
The influence of the aurora upon the magnetic needle has for some years afforded a fertile subject for discussion; and it is to be regretted that no continued series of observations has been undertaken in Britain, adequate to the solution of the question, or indeed materially contributing to our knowledge of the state of the earth's magnetism. M. Arago of Paris gave an account, some years ago, of the connexion, which his observations established, between the phænomenon of the aurora and the irregular motions of the variation and dipping needles§. Prof. Hansteen coincided in the truth of this result; and added the observed anomalies of the magnetic intensity under the same circumstances‖. M. Arago then remarked, that this variation of the horizontal intensity might only arise from the irregularity occasioned in the dip, of which the former is a function¶. Meanwhile, M. Arago's general conclusions were warmly opposed by Dr. Brewster, who considered the fact as not sufficiently established by such obser-
* Encyc. Brit., Art. AURORA BOREALIS.
† Edinburgh Journal of Science, ix. 129.
§ Ibid. N. S. v. 23.
§ Annales de Chimie, 1825.
‖ Jahrbuch der Chemie und Physik, xviii. 353.
¶ Annales de Chimie, 1827.
[page] 257
vations as were published*. In support of his views he quotes the observations of Parry and Foster, particularly on the occasion of the luminous beam to which we have just alluded, which appeared to exercise no energy on the needle. Unaccountable, however, as the discrepancy may be between travellers so well qualified to judge, and under such favourable circumstances, Capt. Franklin and Dr. Richardson detected the most decided proofs of magnetic action of the aurora†. It must be obvious therefore, that independent of the difficulties of the observation, and the delicacy of the instruments required, there must be some innate source of difficulty in the subject.
Mr. Farquharson has endeavoured to point out some explanation of these anomalies in a recent Memoir in the Philosophical Transactions‡; where he states that from his observations on the variations of magnetic intensity with auroral phænomena, and also of the dip and variation of the needle, he has found the effect to be a maximum when the streamers reach the plane of the dip, or when they pass through that region of the heavens to which the south pole of the dipping-needle points. Dr. Richardson had remarked that the effect on the needle was greatest when the streamers passed to the south of the zenith. This observation of Mr. Farquharson must therefore be considered one of importance, though it does not quite explain some anomalies in the circumstances of the observations, especially of those not made in high latitudes. As far as the observations of Mr. Farquharson himself go, they confirm the results obtained on the Continent‖.
In almost every part of the continents bordering on the arctic circle have observations to the same effect been recently made. Prof. Kupffer has observed the influence of the aurora in the most striking manner at St. Petersburg, Nicolajew, and Kasan; and the results of contemporary observations at these points are well worth consultation§. Prof. Hansteen has detected the same at Tornea in Lapland ¶ and M. Riess of Berlin has lately observed with care the influence of the aurora upon the mag-
* Edinburgh Journal of Science, viii. 189.
† See Franklin's Second Journey; the Edinb. New Phil. Journal; and the Bulletin des Sciences Mathénatiques, xi. 293. These observations have given rise to some criticisms by Dr. Brewster, which have been replied to by Mr. Christie in the Journal of the Royal Institution.
‡ For 1830, p. 97.
‖ For an abstract of Mr. Farquharson's papers, see the Edinb. New Phil. Journal, vi. 392; and the Encyclopœdia Britannica, (New Edit) art AURORA BOREALIS.
§ Poggendorff's Annalen, 1831.
¶ Ibid. 1827; and Bulletin des Sci. Math. ix. 252.
R
[page] 258
netic intensity*. In America also, experiments have led to the same result†.
On the occasion of the great aurora of the 7th January 1831, M. Arago observed the magnetic needle powerfully affected, whilst Mr. Sturgeon of Woolwich could not notice it at all‡: on the 19th April 1831, Mr. Christie of Woolwich, in company with Mr. Faraday, observed the most unequivocal signs of auroral action§. This observation, made by two philosophers perfectly habituated to such experiments, must be considered probably the most complete evidence yet obtained in this country. On the whole, it seems undeniable that the aurora borealis, frequently at least, exercises the most marked action on the magnetic needle, with regard to variation, dip, and intensity. The circumstances under which it does not take place, require however the most careful scrutiny, and we hope that Mr. Farquharson will pursue unremittingly his observations. Unfavourable as is the sky of Britain for many kinds of experiment, her geographical position is in other respects highly important as concerns scientific undertakings. Among these especially rank Meteorology and Magnetism; and it were deeply to be desired that she should lead the way in the prosecution of these too much neglected sciences. There can be no reason why experiments should not be as well conducted here as in the cabinet of M. Arago; and when Baron Humboldt boasted to the French Academy of the wide distribution of his "maisons magnetiques," or magnetic observatories, from Paris, the centre of civilization, to the wilds of Siberia, and to Pekin itself, whose gates have been so long shut against the approaches of science, —it is a humiliating fact that he could not with truth have mentioned Britain as possessing a solitary establishment of this description, either within her own limits, or probably even in the range of her much more widely extended dependencies.
It may not be superfluous to add in conclusion, should more errors (especially those of omission,) be found in the preceding Report than might seem to be inseparable from the nature of the work,—that it has been drawn up within a very limited space of time, and under the pressure of a variety of preparations for an extended scientific tour on the Continent.
June 1832.
* Communicated to the Academy at Paris by Baron Humboldt, 10th Oct. 1831.
† Silliman's Journal, 1828.
‡ Philosophical Magazine, N.S. ix. 151.
§ Journal of the Royal Institution, Dec. 1831. p. 271.
[page] 259
Report on the present State of our Knowledge of the Science of Radiant Heat. By the Rev. BADEN POWELL, M.A. F.R.S., Savilian Professor of Geometry in the University of Oxford.
IN attempting to give a condensed account of the present state of our knowledge of the science of Radiant Heat, it appears to me that I shall be best consulting the design of such a Report by offering, in as brief a form as possible, a sketch of what has been formerly done in this department; and thence proceeding to a more detailed survey of what is now doing. And we shall proceed with greater clearness if we distinguish the several different departments into which the subject divides itself, agreeably to certain known distinctions in the properties and species of heat acting under peculiar circumstances. All these have been too commonly confounded together under the general and vague name of Radiant Heat, whence not unfrequently the most erroneous views have resulted. By distributing our subject, however, under the few well-marked divisions which the scanty results of observation as yet supply, we shall at once secure perspicuity in our views, and be treating the subject in a way most accordant with the inductive process; which, it must be distinctly avowed, has not yet enabled us to advance to any such comprehensive knowledge of the facts as can warrant us in generalizing them, or in ascribing to a common principle the radiation of heat from a mass of hot water, from a flame, and from the sun.
We shall take each of these principal divisions separately, and under each shall consider what is known in reference to those properties to which experiment has been directed.
DIVISION I.
Radiation of heat from hot bodies below the temperature of luminosity.
a.) Radiation (or communication of heat to sensible distances,) is distinct from its conveyance by conduction through the air; since,
1.) It takes place perpendicularly downwards:
2.) Only in elastic media.
The relative cooling in different media is seen in the following experiments. (Rumford's Essays, ii. 425; Torricelli; Murray's Chem. i. 328.)
R 2
[page] 260
Thermometer cooled from 212° to 32° Fahrenheit:
| In Vacuo | in 10m | 5sec |
| Air | 7 | 3 |
| Water | 1 | 5 |
| Mercury | 0 | 36 |
Dulong and Petit, in their elaborate researches on the cooling of bodies, have investigated the law of cooling in the most perfect vacuum they could form: but they admit that there was always a minute portion of air present. The radiation therefore of heat in an absolute vacuum is by no means conclusively established. (See Annals of Phil. vol. xiii. p. 241.)
3.) Professor Leslie ascertained, That the effect from a mass of given size is nearly proportional to the angle which it subtends at the thermometer; and that the heat suffers little or no diminution in its passage through the air.
The radiation is most copious in the direction perpendicular to a plane surface of the hot mass, and is proportional to the sine of its inclination to the direction of the thermometer. (Inquiry into the Nature and Propagation of Heat, p. 51, &c.)
For the same position the effect is proportional to the excess of temperature of the hot body above that of the air.
4.) Pictet made an attempt to estimate the velocity with which heat radiates, by means of concave reflectors at 69 feet distance. The effect on the focal thermometer was absolutely instantaneous. (Essais de Phys.)
b.) Reflexion of simple heat from nonluminous hot bodies.
1.) The general principles are established by Professor Leslie. (Inquiry, pp. 14, 51.)
2.) He shows that the quantity of heat reflected is proportional to the sine of incidence on a plane surface.
3.) It is affected by the polish of the surface. (Leslie, Inquiry, pp. 81, 20, 98, 106.)
4.) The most exact experiments are those made with conjugate concave reflectors; a ball of iron below luminosity in one focus, a thermometer in the other: a glass of boiling water may be substituted for the iron ball. In either case a great effect is produced in the opposite focus, though little out of it. (Saussure, Voyages, t. iv. p. 120; Sir W. Herschel, Phil. Trans. 1803, p. 305.)
Professor Leslie made extensive use of reflectors, but ob-
[page] 261
served that there was a very considerable degree of aberration in the focus from an exact position; considerably nearer to the reflector than the true focus, the effect continued undiminished. (Inquiry, p. 64.)
5.) Alleged reflexion of cold.
An account of the earliest experiments will be found in the Memoirs of the Florentine Academy, (Waller's Transl. p. 103; also Gærtner, 1781.)
Pictet with conjugate reflectors found the thermometer sink when ice was in the opposite focus. (Essais de Phys. p. 82.)
Count Rumford employed a tube, a frustrum of a cone, open at both ends; placing ice at the small end, the thermometer at the large end sunk very little. The ice being at the small end, the thermometer at the large end fell considerably. Rays reflected by the inside of the tube from the body at the large end, would be concentrated on that at the other.
6.) M. Prevost (Essai sur la Calorique rayonnant, Geneva 1809, and Recherches sur la Chaleur, p. 15,) proposes a theory of radiation, that heat is a discrete fluid every particle of which moves in a straight line, and such motions are constantly taking place in all directions, whether there be more or less heat present. Hence all bodies, whether of a higher or lower temperature, are supposed to be continually radiating heat; and this going on mutually tends to bring them all to an equilibrium of temperature.
On this theory explanations are given of the apparent radiation of cold.
The thermometer in the conjugate focus, when nothing is in the other, remains stationary, because the rays reflected from all the surrounding space so as to cross at the focus of the opposite mirror, and be reflected in a parallel state to the other, and thence on to the thermometer in the focus, are exactly equivalent to those which the thermometer radiates. But when a mass of ice is placed in the opposite focus, it intercepts and absorbs a portion of the rays which would otherwise have fallen on the first mirror, and so have reached the thermometer, which in consequence radiates more than it receives, and therefore sinks.
A similar explanation applies to Count Rumford's experiment. (See Thomson On Heat, &c. p. 163.)
In the Quarterly Journal of Science (June 1830, p. 378,) some observations are given on this subject, and an explanation offered, which, though very ingenious, appears somewhat complicated.
[page] 262
It may not be improper to observe, that if the above be a correct view of Prevost's theory, it can hardly be conceived as otherwise than partially hypothetical. The idea, viz. that bodies even of a lower temperature than those about them actually give out a small degree of heat, is extremely difficult to conceive; and it does not appear absolutely essential to the explanation of the facts.
Without reference to any theory, I venture to propose the following as the simple experimental law:
All bodies of unequal temperature tend to become of equal temperature; if in contact—by conduction; if at sensible distances—by radiation, of the excess of heat: and (in the latter case) whether the radiation reach the cooler body directly or by an intervening reflexion.
This appears sufficient to include the facts of Pictet's and Rumford's experiments.
7.) Alleged polarization of simple heat by reflexion.
Mons. J. E. Berard (Mémoire sur les Propriétés des différentes Espèces de Rayons qu'on peut séparer au moyen du Prisme de la Lumière solaire," Mém. de la Société d'Arcueil, Paris 1817, tome iii. See also Annals of Phil. O.S.ii. 164; Biot, Traité de Phys. iv.) tried experiments for the polarization of heat. His apparatus was the same as Malus's, having the axis of revolution vertical; but no precautions of screening, &c. are mentioned. He used an air thermometer containing a bubble of alcohol in the tube, in the focus of a reflector moving round along with the second glass: a ball of copper about two inches in diameter was in the focus of a reflector, placed in the position for polarization of light. (His experiments on heat with light will be referred to in another place.) He tried the effect with the metal heated below luminosity, and assured himself that there was a difference in the degree of heat reflected in the two rectangular azimuths of the second glass.
I have attempted to repeat these experiments with the same kind of apparatus, carefully screened and arranged with the tube horizontal; but could produce no diminution in the proper position. (Edinb. Journal of Science, N.S. vol. x. p. 207.)
I also tried the experiment with a delicate mercurial thermometer, comparing this case with others (referred to in their proper place), in which light accompanied the heat; but in the former could detect no difference in a long series of repetitions.
The total effect is in all cases extremely small, and the disturbing causes considerable, especially the heating of the
[page] 263
glasses, &c. The whole experiment was very unsatisfactory. Edinb. Journal of Science, N.S. vol. vi. p. 297.)
c.) Effect of the nature of surfaces on the emission of simple heat.
1.) Count Rumford (Nicholson's Journal, ix. 60,) employed two similar vessels of hot water of the same temperature; one naked, the other coated with linen, glue, black or white paint, or smoked with a candle: the results were,
| Naked vessel cooled | 10° in 55m |
| Coated ————— | 10 — 361/2 |
Mr. Murray supposes a relation between radiating and conducting powers. (System of Chem. i. 326, 334. See Phil. Trans. 1804, p. 90, &c.)
2.) The most complete investigation of this and other parts of the subject has been made by Professor Leslie in his Inquiry into the Nature and Propagation of Heat, 1804.
He first used hot water in a globe of tin, in which the inserted thermometer fell a given quantity, with the tin bright, in 156m; with the tin coated with lamp black, in 81m.
The difference was greatest in still air, and diminished with the violence of its motion:
| Time of Cooling. | ||
| Wind. | Bright. | Blackened. |
| Gentle. | 44m | 35m |
| Strong | 23 | 201/4 |
| Violent | 91/2 | 9 |
Hence the effect is different from conduction by air.
3.) The most exact series of experiments was that in which he used conjugate reflectors, a differential thermometer having one bulb in the focus, and a cubical tin canister of hot water (the temperature of which was seen by the projecting stem of a thermometer), and each side of which could be coated with a different substance, and presented successively towards the reflector.
The following results collected together afford the best view of the general nature of the conclusions relative to the influence of the state of the surface on the radiation of heat. (Inquiry, pp. 81, 90, 110.)
| Lampblack | 100* |
| Water (estimated) | 100 |
| Writing-paper | 98* |
[page] 264
| Rosin | 96 |
| Sealing wax | 95 |
| Crown glass | 90 |
| China ink | 88* |
| Ice | 85 |
| Minium | 80* |
| Isinglass | 80 |
| Plumbago | 75 |
| Thick film of oil | 59 |
| Film of jelly | 54† |
| Thinner film of oil | 51† |
| Tarnished lead | 45 |
| Film of jelly, (1/4 of former quantity) | 38 |
| Tin scratched with sand-paper | 22 |
| Mercury | 20 |
| Clean lead | 19 |
| Polished iron | 15 |
| Polished tin, gold, silver, copper | 12 |
| Thin lamina of gold, silver, or copper leaf on glass | 12‡ |
* From comparing the results marked, it appears that the effect follows no relation to colour. Softness probably tends to increase radiation.
† Thickness of film increased beyond a certain limit does not increase the radiation.
‡ The tenuity is not sufficient to produce any diminution of effect, which probably would take place if thinner films could be applied.
4.) The effect of the surface on radiation is beautifully exemplified in the laws which regulate the formation of dew as developed by Dr. Wells. (Essay on Dew, 1814. See also Dufay, Mem. Paris 1736, p. 352; and Harvey on Dew, Quarterly Journ. of Science, No. 33; Edinb. Journ. of Science, i. 161.)
5.) Dr. Ritchie (Edinb. Phil. Journ. xxiii. 15,) explains his theory of the mode in which the radiating power of surfaces is increased by making them rough, or furrowing, &c. He contends that it is not owing to the increase of surface, but to the quantity of heat reflected by the sides of the furrows.
He adopts the hypothesis of material caloric, and that its molecules are mutually repulsive.
The effect of surface is an essential distinction between radiation and conduction by air; the latter being shown by Dulong and Petit to be absolutely independent of the nature of the surface. (Annals of Phil. xiii. 322.)
[page] 265
d.) Effect of surface on the absorption of heat from non-luminous hot bodies.
1.) De Saussure and Pictet, with the apparatus before described, found that the thermometer rose in two minutes,
| Plain | 41/8° Fahr. |
| Blackened | 31/8 |
2.) By the same apparatus as before described, Prof. Leslie found that on coating the bulb of the thermometer with the different substances, the absorptive powerwas very nearly in the same proportion as the radiative; and by making the same modifications in the surface of the reflector, he found that reflective power is inversely as the radiative or absorptive. (Inquiry, pp. 19, 81, 98.) He also gives a very precise set of experiments on the effect of coatings of jelly of increasing thicknesses. (p. 106.)
3.) Dr. Ritchie has devised a very elegant mode of showing that the absorptive power of surfaces is precisely proportional to their radiating power. (Royal Inst. Journ. vol. v. p. 305.)
The instrument consists of a large differential thermometer, whose bulbs are chambers of considerable size, presenting large and equal plane surfaces on the sides which are towards each other: of these one is plain or polished, the other coated. Midway between them is placed a canister having equal plane surfaces facing each of the former respectively, and one polished, the other coated with the same pigment as before; this canister is filled with hot water, and is capable of turning on a vertical axis; thus the coated surface of the canister can be turned to the coated bulb or to the polished: in the former case a great effect is produced on the coated bulb, and a very small effect on the plain: in the second case the better radiating surface is directed to the worse absorptive one, and the worse radiating to the more absorptive, and the liquid in the tube remains perfectly stationary: the exact equality, therefore, of the absorptive and radiating powers is established. The whole is on a large scale, and can be exhibited to a Class.
4.) The most recent and curious researches on this part of the subject (and extending, as we shall see, to other parts also,) are those of MM. Nobili and Melloni. (Annales de Chimie, Oct. 1831; Recherches sur plusieurs Phénomènes calorifiques, &c.)
The authors commence by describing their thermo-multiplier, by the aid of which their researches were carried on. This
[page] 266
consists in a thermo-electric combination, susceptible of excitation from the feeblest conceivable application of heat, and connected with a delicate galvanometer, which gives a measure of the effect produced, and consequently of the heat.
The pile is in a case coated with the smoke of a flame when used for radiant heat, but left naked wven for heat of temperature, on account (as they observe) of the bad conducting quality of this coating.
They applied this instrument to the examination of the different reflecting, absorbing, and radiating powers of surfaces.
They confirmed in general the results of Leslie and others already mentioned. They found that polish augments the reflecting power much less than usually supposed. Non-metallic substances possess scarcely any reflecting power, whatever be the state of their surfaces.
They examined the absorptive power of different substances, taking laminæ of equal thickness and similarly fixed, &c.: these having been heated for a few minutes in the rays of the sun, were placed in pairs on apertures at the opposite sides of the thermo-multiplier, and in this way the order of their absorptive powers was considered to be obtained by the degree of heat they respectively radiated; and the results were, that the effect increased by blackness of colour and with roughness of surfaces. Also the following surfaces were in this order,—silk, wool, cotton, flax, hemp, (all white,) which is the inverse of their conducting powers. In like manner, with metals of nearly the same colour and polish, the order was—copper, silver, gold, steel, iron, tin, lead, exactly in the inverse order of the conducting powers;—the same with several woods and minerals.
On these experiments I must remark, that the heat acquired from the sun's rays is so obviously dependent on colour, that it is astonishing that any experimenter should adopt this as affording any ground for making conclusions respecting the comparative absorbing or radiating powers for heat in general. The later results, when the surfaces were all of the same colour, are extremely important. Supposing they all acquired the same degree of solar heat which was thus converted into heat of temperature, and then radiated from the surfaces as simple heat, the real conclusion established is, that the RADIATING powers of surfaces for simple heat are in the inverse order of their conducting powers.
[page] 267
e.) Effect of screens on heat from nonluminous hot bodies.
1.) Pictet found a difference in the interceptive effect, according as the plain or the silvered side of a glass screen was towards the source of heat.
| Towards Hot Body. | Ratio of Effects on Thermometer. |
| Glass | 5 |
| Amalgam | 35 |
| Amalgam, blackened | 92 |
| Amalgam removed,—glass blackened | 180 |
(Essai, &c., p. 72.)
2.) He tried to refract simple heat, without effect.
Sir W. Herschel tried with a lens, and supposed it effected: this has been refuted by Sir D. Brewster. (Vide infra; Phil. Trans. 1800, Part II. No. 15. Exp. 19, 20.)
3.) Prof. Leslie's experiments on screens are perhaps the most valuable portion of his inquiry.
He found the effect of a screen increase rapidly with its distance from the source (p. 28), and less so with its thickness (p. 38).
Different substances appear to have a different interceptive power; but this upon examination appears always to be dependent on their conducting power, and the absorptive nature of their surface jointly.
The most decisive experiment on this point was that made with two panes of glass, each having one side coated with tin foil: according as the plain or coated sides were placed in the contact, the compound screen had a greater or less apparent interceptive power; that is, a greater or a less power of absorbing and subsequently radiating the heat. Again, either might be used separately, or the two at an interval. (p. 35.)
4.) Prevost concluded that a certain portion of heat is directly transmitted through transparent screens, by employing moveable screens which continually presented a fresh surface, so that it was supposed all communication of heat and conveyance by way of secondary radiation would be prevented.
But it must be considered that it is impossible to prevent entirely any portion of a screen in the most rapid motion from acquiring heat:—no such experiments therefore can be strictly conclusive.
Dr. Ritchie tried experiments with the same view, by means of a film of liquid adhering to threads stretched across a frame
[page] 268
continually renewed. (Phil. Trans. 1827, Part II. p. 141.) But to this a similar objection must apply.
5.) The results of Prof. Leslie do not apply to temperatures above those of boiling water.
This extension of the inquiry formed the subject of the researches of De la Roche. The complete account of these is given in its proper place; at present we have to consider them only as far as relates to bodies below luminosity. He tried the effect of a screen of glass, first transparent, and then with one surface blackened, on the heat radiating from mercury at 180° centig. and at 346° when it was boiling. (Biot, Traité de Phys. iv.640.)
The results were as follows:
Rise of focal thermometer (centig.) in 1m.
| No Screen. | Transparent Screen. | Blackened Screen. | |
| Mercury at 180° | 3°·94 | 0°·22 | 0°·07 |
| — at 346 | 16 ·33 | 1 ·36 | 0 ·17 |
He hence infers a partial transmission of heat at these high temperatures; and the more so, viewing these results in connexion with the rest of the subsequent series (considered in another place).
These are the only ones of his experiments referring really to simple radiant heat; and the inference of an actual transmission in the way of direct radiation, is open to several objections.
6.) The blackened screen causes a greater diminution of heat than the transparent, and it was therefore inferred that a portion of heat radiates freely through the transparent screen, and is stopped by the opake one: but there are several circumstances which show that this is not a necessary conclusion.
The coating was towards the source of heat, and rendered this screen more absorptive of heat where exposed to it, that is, at its central part,—and a better radiator towards the edges without the area of the incident rays; so that it radiated its heat most copiously on the side away from the thermometer. With the plain screen there was no such tendency to radiate more on one side than on the other; and hence the greater effect on the thermometer.
This explanation I suggested in the Annals of Philosophy, xlv. 181.
Some observations bearing upon this subject, occur in Sir David Brewster's elaborate paper on "New Properties of Heat," &c. in the Phil. Trans. 1816, Part I. His 40th propo-
[page] 269
sition is directed to prove that radiant heat is not susceptible of refraction, and is incapable of permeating glass, like the luminous rays. The truth of this is demonstratively shown from the curious properties examined in the previous parts of the paper, and shown to be communicated by heat to glass; and by the progress of which, the passage of the heat through the glass may be as clearly traced as if the heat itself were visible.
He applies this conclusion to the experiment of Sir Wm. Herschel, in which the concentration of simple heat by a lens appears to be proved. The thermometer must have received the heat radiated by the lens itself; and from the circumstance that the edges will cool first, the most copious radiation of heat will be in the direction of the axis.
In connexion with the same point he also examines the conclusions of MM. De la Roche and Prevost, and observes: "The ingenious experiments of M. Prevost of Geneva, and the more recent ones of M. De la Roche, have been considered as establishing the permeability of glass to radiant heat. M. Prevost employed moveable screens of glass, and renewed them continually, in order that the result he obtained might not be ascribed to the heating of the screen: but such is the rapidity with which heat is propagated through a thin plate of glass, that it is extremely difficult, if not impossible, to observe the state of the thermometer before it has been affected by the secondary radiation from the screen.
"The method employed by M. De la Roche of observing the difference of effect when a blackened glass screen and a transparent one were made successively to intercept the radiant heat, is liable to an obvious error. The radiant heat would find a quicker passage through the transparent screen, and therefore, the difference of effect was not due to the transmitted heat, but to the heat radiating from the anterior surface. The truth contained in M. De la Roche's fifth proposition, is almost a demonstration of the fallacy of all those that precede it. He found that a thick plate of glass, though as much or more permeable to light than a thin glass of worse quality, allowed a much smaller quantity of radiant heat to pass. If he had employed very thick plates of the purest flint glass, or thick masses of fluid that have the power of transmitting light copiously, he would have found that not a single particle of heat was capable of passing directly through transparent media."
7.) I have further attempted a direct experimental examination of the question in a paper inserted in the Phil. Trans. 1826, Part III. p. 372.
[page] 270
The substance of my observations is as follows:
De la Roche found, that if radiant heat be intercepted by two transparent screens, the additional diminution of effect occasioned by the second, is proportionally much less than that produced by the first; and the same conclusion is extended to any number of screens. This was explained by the supposition that the heat in its passage through the first glass undergoes a certain modification, in some respects analogous to polarization, by which it is enabled to pass, with very little diminution, through the second and subsequent glasses.
In those cases where the source of heat is luminous, such phænomena would receive an obvious explanation on the principle investigated in my other paper. Vide infra.
But if the same effect is still observable below the point of luminosity, we must have recourse to some other principle of explanation. That deduced by De la Roche appears at least plausible; and though it should be considered proved, that, in general, heat is incapable of being radiated directly through glass, it perhaps would not necessarily follow, that it might not, under peculiar circumstances, have a power of doing so communicated to it. Though on the other hand it must be confessed, that in the present case some difficulty would attend such a supposition.
It certainly would not be easy to conceive such a property to be communicated to the heat, by the mere act of being conducted through the first glass. Again; a new property of heat is thus introduced, which, it must be conceded, is not absolutely and exclusively established.
It appeared to me therefore a point of some interest to examine, in the case of non-luminous heat,—in the first place, the accuracy of the fact; and secondly, if verified, whether there might not be circumstances observable in the conditions of the experiment by which it might be accounted for, without the necessity of supposing any peculiar property of heat, or a direct transmission even through the second glass.
My apparatus in following up this inquiry was similar to that described by M. De la Roche, and consisted of two tin reflectors;—in one focus the bulb of a thermometer coated with Indian ink, and in the other an iron ball two inches diameter, which was heated to redness, and then cooled till it ceased to be visibly red in the dark, at which point it was placed on its stand, and a thick screen withdrawn. The indications were observed, first, for the direct effect; secondly, with one glass screen interposed; and thirdly, with two. The temperature of the screens was observed by means of a small thermometer
[page] 271
attached to the face of each away from the ball, towards its central part; the bulb being kept in contact by the spring of a wire with which the thermometer was fastened.
The results are: 1st, That the additional diminution occasioned by the second screen, is proportionally much smaller than that occasioned by the first. Thus De la Roche's conclusion is shown to hold good, not only in the case of luminous, but also of non-luminous hot bodies; which is perhaps of consequence, as I believe doubt has been entertained respecting it; and it may be remarked, that here the greater thickness of the second screen would be against such a result. 2ndly, If the progress of the indications of the direct effect be followed, it appears that the rise in the first 30 seconds is the greatest, and that those in the subsequent periods gradually diminish. 3rdly, With one screen, the effect in the first period is equal to, or even less than those in the subsequent ones; and if we follow the temperature of the first screen, it appears to sustain a rapid increase at first, and afterwards continues gradually to rise till some time after the focal thermometer has become stationary. The progress of the focal thermometer exactly accords with what must be the heating effect of the screen as a source, viz. rising slowly at first as the screen acquires heat sufficient to supply it, and remaining stationary so long as the still increasing temperature of the screen could balance its loss of heat. 4thly, With two screens, there is no rise till the second half-minute, when it is not greater than in the next half, after which the thermometer becomes stationary; and this trifling effect exactly accords with what the temperature of the second screen should produce. It does not begin till the second screen has acquired a higher temperature, and it is stationary while the temperature of the screen continues to increase; and the temperature of the second screen is such as is clearly accounted for from the heating effect of the first. It does not begin to rise till after that of the first has risen; it continues stationary some time after the first has begun to cool, as the first screen did when the iron was cooling. But as in this case the source of heat was cooling during the whole time of the experiment, whilst in the other it was heating during the first part of the time, it follows, that a greater proportional temperature should be communicated to the second screen by the first, than to the first by the iron ball.
Other circumstances will partially cooperate in producing this effect,—as the greater proximity of the second screen to the thermometer; also more heat might be lost in communicating an equable temperature to the first screen from its central and
[page] 272
more heated part; whilst the heat would be thus more equally radiated to all parts of the second without such loss.
Thus it appears that the fact stated by M. De la Roche is fully substantiated; while on the other hand it is satisfactorily accounted for, without supposing any new property of heat, or any direct radiation through glass.
In some unpublished experiments of my own, I found upon observing the temperature acquired by a screen exposed to iron below luminosity, first plain, and then coated with Indian ink towards the source of heat, the thermometer being in contact at the central part on the outside, that it rose rather more on the plain, than on the coated screen.
8.) MM. Nobili and Melloni, in the Memoir before quoted, applied their instrument to estimate the effects of transparent screens. Over the thermo-multiplier were placed successively transparent screens of glass, sulphate of lime, mica, and of water, oil, alcohol, and nitric acid (inclosed between plates of glass?), and also of ice.
The source of heat was a ball of iron, heated to a point below, luminosity, suspended, or rather passed rapidly, at a certain distance above the screen.
The index indicated an instantaneous effect, greater or less in all cases except those of water and ice, in which none was produced, even when the iron was kept a longer time over the instrument, or even heated to redness, and the screen reduced in thickness.
9.) A set of experiments presenting some important results with respéct to the absorbing and radiating properties of surfaces, as well as the action of screens in air and in vacuo, are given by Mr. W. R. Fox, in the Phil. Mag. and Annals, New Series, No. 65, p. 245. A brief statement of the results is as follows:
A cylindrical tin vessel of hot oil with its surface polished, and another similar, painted black, had their times of cooling a certain number of degrees observed under a receiver first highly exhausted, and then full of air; the cylinders being respectively 1st exposed, and 2ndly inclosed in one and sometimes more tin cases with intervals; the outer and inner surfaces being one or both polished or blackened. From all the different combinations of these results, of which he states in detail, I collect the following general inferences:
I. In vacuo; (1) the polished vessel had its cooling always accelerated by the cases; and in this order—
[page] 273
| Case | ||
| Inside. | Outside. | |
| Most accelerated | bright | black. |
| black | black. | |
| bright (3 cases) | black. | |
| bright (3 cases) | bright. | |
| bright (1 case) | bright. | |
| Least accelerated | black | bright. |
(2.) The coated vessel had its cooling in all cases retarded; and in this order—
| Inside. | Outside. | |
| Least accelerated | black | black. |
| bright | black. | |
| black | bright. | |
| Most retarded | bright | bright. |
II. In air: both vessels in all instances had their cooling retarded by the cases.
Mr. Fox also found the boiling of water in a bright vessel before a fire accelerated nearly doubly by a case blackened externally.
He considers the results inexplicable, except on the hypothesis of an attraction between matter and heat.
Mr. Fox has also communicated to me in manuscript, an account of some further experiments of the same kind on iron raised to a red heat, but which nevertheless are of such a nature as properly to come under this division of the subject.
The precise temperature to which the iron was raised in each experiment was estimated by the remarkable cessation of its action on a magnetic needle at a certain stage of incandescence.
The iron was inclosed in tin cases of two different sizes, within which the air could be exhausted, the inside being either plain or coated with lamp-black.
The whole was immersed in water, and the temperature communicated to the water in a given time, noted. After observation the iron was plunged in water, and the residual heat thus communicated to the water, noted.
The general results were,—that in the smaller case the cooling was more rapid than in the larger; and in either the internal coating accelerated the cooling; in no case was any material difference produced by exhausting the air.
10.) Dr. Ritchie (Edin. Phil. Journal, xxii. p. 281,) has shown that when a hot nonluminous body is placed between the two bulbs of a differential thermometer, blown out very large and thin, and both remaining plain, the liquid is stationary: the
S
[page] 274
outside half of ohe being coated with black, the liquid sinks from that side.
Hence he infers that the coating has here stopped the heat, which otherwise radiates freely through the very thin glass.
He varied the experiment by using portions of glass blown thin as screens over an aperture: when blackened in a flame or coated with silver-leaf, they intercepted heat; when transparent, not. That this was not from increase of thickness, was shown by using three thicknesses transparent, then removing the middle one, and blackening the inner surface of the others.
He explains the subject by the theory of material caloric and mutual repulsion of its particles.
The same author in another paper (Ann. of Phil. 2nd Series, xii. 123,) gives a variation of the experiment: the hot body is placed between two large and very thin bulbs; one of the hemispheres of one bulb, formed by a plane passing through the centres of both, is coated with China ink; as are also two of the alternate quarters of the other, formed by a plane cutting the former at right angles.
A greater effect is produced on this second bulb.
This is an argument against the effect being due to greater radiation, from the outer surface of the bulb.
Dr. Ritchie has also maintained the same conclusions in his paper before referred to, (Phil. Trans. 1827, Part II. p. 142,) by varying the distance of the screen, which he found to produce no sensible difference in the effect; though with screens of moderate thickness it diminishes rapidly with the distance, according to Leslie's experiments.
DIVISION II.
Terrestrial luminous hot bodies.
a.) Nature of radiation.
The earliest observers noticed differences between this case and that of heat from nonluminous bodies.
The heat from flame, &c., at least in part, passes through air, &c., without heating it.
Scheele observed this with a fire, and that currents of air did not change the direction of the rays. (Treatise on Air and Fire, &c.)
Cavallo (Phil. Trans. 1780,) found a blackened thermometer affected by the light of a lamp.
Leslie (inquiry, p. 448,) found a fire affect his photometer;
[page] 275
also candles, &c. (p. 447),— a distinction pointed out between this and the solar rays, (p. 83, 54.)
The light from putrescent substances does not appear to be accompanied with any appreciable degree of heat, according to Dr. Hulme. (Thomson's Chem. i. 414, 4th edit.) But the effect, if any, must be so small that we cannot positively assert there is none.
The same remark may apply to many other very faint lights.
b.) Reflexion of heat.
1.) Mariotte collected the heat of a fire in the focus of a reflector. (Mem. Acad. of Sciences, 1682.)
Lambert, with burning charcoal in the focus of conjugate reflectors, found a combustible body kindled in the other focus. (Lambert, Pyrometrie; Saussure, Voyage, iv. 119.)
Scheele (On Air and Fire, p. 67-71,) observes that a glass mirror, though it reflects the light of a fire, does not reflect the heat (it is not stated by what means the heat was estimated); but the mirror becomes heated. A polished metallic mirror reflected both the light and heat, and did not become much heated itself; if blackened, it was soon hot.
Pictet extended the experiments with conjugate reflectors to this case, by placing a candle in one focus. The thermometer rose nearly 10° in 6 minutes (Essais de Phys. p. 63.).
Sir W. Herschel (Phil. Trans. 1800, p. 297,) placed a candle at 29 inches from a concave metallic reflector: the focal thermometer in 5 minutes rose 31/4°; another out of the focus was not affected.
The same took place with a fire, and with red hot steel.
2.) Polarization by reflexion.
Berard (Memoir before cited,) tried the polarization of heat from luminous sources, and found a considerable diminution in the position when the light ceases to be reflected.
There was of course here no distinction drawn between the heat accompanying the fight, and the simple heat: of the latter nothing is proved; the former may be merely an effect of the absorption of light, and if so, the term polarization is applied to the heat without any proof.
I repeated these experiments, and, after all precautions, thought there was a small perceptible effect, (when the simple heat was cut off by a glass screen,) which was diminished in the position of non-reflexion for the light; when the whole heat was admitted, no proportional diminution took place. (Edinb. Journ. of Science, vi. 303.)
S 2
[page] 276
c.) Effect of surfaces on emission of heat.
Nothing ascertained under this head, unless we except some remarks in the Edinb. Journ. of Science, No. ii. p. 302.
d.) Effect of surfaces on absorption of heat.
All experimenters have usually blackened their thermometer. (Cavallo, Phil. Trans. 1780.)
Prof. Robison exposed a thermometer on charred oak under a glass cover to the rays of a fire, when it rose to 212° Fahr. (Black's Lect. i. 547; Thomson, i. 127.)
e.) Effect of screens.
1.) Mariotte interposed a glass screen between the fire and concave mirror, and found the heat no longer sensible at the focus. (Biot, iv. 606; Mem. Paris, i. 344.)
Scheele interposed a glass screen in the experiment before mentioned, and found the heat of a fire so much intercepted as to be no longer sensible to the hand: not even sensible in the focus of a reflector.
Pictet with the conjugate reflectors interposed a glass screen. The focal thermometer, which had risen 10°, fell 7° in 9 min.; on removing the screen it rose again. (Essais de Phys. p. 63.)
2.) Sir W. Herschel tried experiments on this point, (Phil. Trans. 1800.) Two moveable objects illuminated by a lamp were viewed by the eye, one through an open hole, the other through a hole covered successively by different transparent media. One object was moved to greater or less distance, till they appeared equally bright; the interceptive power was estimated directly as the illumination required to produce the equalization, that is, inversely as the square of the distance.
Two equal thermometers inclosed in a box, with apertures over the bulbs (which were plain), one open, the other covered successively by the different transparent media, were exposed to different sources of heat, and the interceptive effects compared together and with those of the same media for light. Thus among the results were the following:—
| common Fire. | Candle. | |||
| Light. | Heat. | Light. | Heat. | |
| Coach glass | 0 | 750 | 86 | 625* |
| Dark red glass | 999 | 573 | 999 | 526 |
* Out of 1000.
[page] 277
3.) Refraction by lenses.
Lambert collected the rays of a fire by a large lens, and found the heat scarcely sensible to the hand.
Sir W. Herschel (Phil. Trans. 1800, pp. 272, 309, 327,) received the rays of a candle on a lens, with a pasteboard screen, having an aperture nearly equal to that of the lens; the thermometer in the focus rose 21/2° Fahr. in 3 min.;—the same with the rays from a fire, and from a mass of red hot iron.
Mr. Brande found the rays of a flame, concentrated by a lens, produced an effect on a blackened thermometer in its focus; the lens did not become heated. (Phil. Trans. 1820, Part I.)
4.) Dr. Ritchie found that if Leslie's photometer be placed opposite a ball of iron heated almost to redness, no effect whatever will be produced; but if the temperature of the ball be raised so as to shine in the dark with a dusky red colour, the fluid in the stem of the black ball will sink a considerable number of degrees. If the temperature of the ball be raised still higher, it.will produce a greater effect upon the instrument than the flame of the finest oil-gas, though the one possesses a much greater illuminating power than the other.
Dr. Turner, and Dr. Christison have found that Leslie's photometer, "is powerfully affected by heat" when placed "before a ball of iron heated so as not to be luminous, or even before a vessel of boiling water." The opposite result of Dr. Ritchie may possibly be owing to some difference in the surface, substance, or thickness of the black bulb employed. (Edinb. Journ. of Science, iv. 321.)
I have found differences, which I am at a loss to account for, between the effects on a differential thermometer with the bulbs of equal height, and one in which they are in a vertical line.
5.) That there exist essential differences between the constitution of the heating power of luminous hot bodies, and that of the same power proceeding from those which are non-luminous, was remarked by former experimenters. But it is a point which does not seem to have excited any close or systematic inquiry until the subject was taken up by M. De la Roche, whose researches are justly entitled to the high celebrity they have acquired. The Report of the French Institute upon them will be found in the Annals of Phil. O.S. ii. 161; and a full account of the experiments in Biot's Traité dc Phys. iv. 640.
The whole series of results is as follows:—
[page] 278
| Rise of hermometer in 1 min, centig. | |||
| Soverce of Heat. | No Screen. | Transparent Sereen. | Blackened Screem. |
| 1. Vessel of mercury, temp. 180° cent. | 3°·94 | 0°·22 | 0°·07 |
| 2. Vessel of mercury, boiling, 346° | 16·33 | 1·36 | 0·17 |
| 3. Iron, 427° | 32·8 | 1·70 | 0·31 |
| 4. Copper, 960° (1.) | 38·97 | 11·83 | 0·40 |
| 5. Ditto (2.) | 71·54 | 21·41 | 0·21 |
| 6. Argand lamp–no chimney | 21·12 | 7·29 | 0·21 |
| 7. Argand lamp–chimney | 23·44 | 12·82 | 0·23 |
The two first experiments of this series have been already considered. The 3rd, or iron at 427° centig., was at a red heat, its temperature of luminosity in the dark being about 400°. This, therefore, and the subsequent part of the series are affected by the consideration that light was emitted, which materially alters the case, as we shall presently observe.
De la Roche infers from these experiments, that a portion of simple radiant heat is transmitted directly in the way of radiation through glass; and that this increases as the temperature is raised.
A thick glass, though very transparent, stops heat more than a thin glass less so: the difference is less as the temperature is raised.
A portion of the heat having been intercepted by one screen, a proportionally much less diminution is caused by the introduction of a second; hence he infers that the rays emitted of a hot body are of several kinds, possessing different degrees of power to pass through glass.
He views the results, when the source of heat is raised to the temperature of luminosity, as forming one connected series with those below that point, and thus conceives a gradual advance in the radiant matter or agent, from the state of simple heat towards that of light or "luminous heat."
6.) The theory adopted by De la Roche, as well as by Biot (Tratité de Phys. iv. 640,) and Leslie, is that of one simple agent, which, as the temperature of the source is raised, is gradually brought more into the state of light, which on absorption is reconverted into heat. At low temperatures it is wholly or nearly all stopped by transparent screens. At in-
[page] 279
creasing intensities more of it is enabled to pass in the way of direct radiation.
In order to establish this theory, it would be necessary to show that whatever may be the particular law of relation to the surfaces of bodies by which the action of the "igneous fluid" is determined at any stage of its evolution, the portion transmitted by a screen should act upon any two given surfaces in precisely the same ratio as the part intercepted, or as the whole. Such a ratio will obviously differ at different stages of incandescence or inflammation; but at the same stage it ought to be found exactly the same—only diminished in the actual magnitude of its terms when the glass screen is interposed,—as when there is none.
But no such experimental proof had been offered by any of the experimenters before named. It was obviously called for to Support or refute their theory, and was capable of being easily supplied by experiment. That the conclusion is not a necessary one, will be evident by merely observing that the phænomena may just as well be explained by supposing two distinct heating influences, one associated in some very close way with the rays of light, carried as it were by them through a glass screen without heating it; the other being merely simple radiant heat stopped by the screen, exactly as in the case of a nonluminous hot body.
To ascertain by experiment which of these suppositions was the true one, was the object of an inquiry which I communicated to the Royal Society, and which is published in the Phil. Trans. 1825, Part I. p. 187. I also gave an abstract of the results, accompanied by other illustrative remarks, and some theoretical views in a paper in the Quarterly Journal of Science, No. XIX. p. 45. Some remarks also on the experiments are made in the Edinb. Journ. of Science, N.S. No. VI. p. 304.)
These experiments combine the examination of the effect of screens with those of surfaces. It is assumed, on the authority of previous experiments, that simple heat affects a thermometer in proportion to the absorptive nature of its surface: for example, a surface washed with a paste of chalk is rather more absorptive than one coated with Indian ink; and this kind of heat is stopped by transparent screens of ordinary thickness. It would seem from some experiments already mentioned, that from luminous hot bodies the effect is greater in reference to the darkness of colour of the surface, and is transmitted through glass. But when a body is heated to luminosity, how does this change in its properties take place? Are its relations gradually altered in themselves? or are there two sorts of heating
[page] 280
effect emanating from it at the same time? These are the questions which my experiments were directed to answer, and the mode of trying the point is extremely simple; it is only to ascertain whether of the total heating effect from a luminous hot body, the portion intercepted by a transparent screen is of the same nature as, or different from, the part transmitted, in its relation to the surfaces on which it acts.
The experiments were conducted simply by having two thermometers, one coated with smooth black, the other with absorptive white, observing the ratio of the effects when they were exposed together to the direct influence of a luminous hot body, and comparing it with the ratio similarly observed when a glass screen was interposed.
The screen acquiring and therefore radiating heat from the first moment of the experiment, will affect the thermometers in a ratio (as before observed,) differing little from equality; and these equal quantities added to the terms of the ratio of the direct effects of the luminous body will of course diminish the inequality of that ratio. This cause of error may not have operated to any great degree, but its tendency is obviously to a diminution of the ratio.
Notwithstanding this, the observed result in all cases, with a lamp, or with iron raised to a bright red heat, was, that the ratio of the effect on the black to that on the white thermometer was increased by the interposition of the screen.
A summary of the results of two sets of experiments (conducted with some slight variation), and in the second of which the temperature acquired by the screen was carefully noted, is as follows:
| Rise of Thermomoeter (centing.) in 1 min. | ||||||
| White. | Black. | white. | Black. | |||
| Iron bright hot | (1.) | 1°·25 | 2°·75 | 7°·0 | 8°·75 | |
| (2.) | 0·6 | 1·25 | 2·95 | 3·75 | ||
| Argand lamp | (1.) | 0·6 | 2·0 | 1·8 | 3·4 | |
| (2.) | 1·3 | 2·35 | 2·35 | 3·2 | ||
These numbers are the means of several repetitions.
The necessary conclusion from this difference in the ratio of the direct and screened effects, is, that the portion of heat which has the property of permeating the screen has also the property of affecting the two surfaces in a ratio different from that in which the part intercepted acts upon them.
As in researches of this kind great numerical precision is unattainable, I was especially, at every step of the inquiry,
[page] 281
anxious to devise as many variations of the experiment as possible;—these all tended to confirm the results just given.
Thus I used a large differential thermometer having its bulbs differently coated, and exposed each of them in turn to the luminous source of heat, the other being completely screened, and invariably found the ratio of the effects on the black and white bulbs considerably greater when affected only by the transmissible part of the heat, than when exposed to the whole. As before, the part added on the removal of the screen was of a nature tending to add to the terms of the former ratio, quantities in a ratio much nearer equality; viz. that which the effects o£ simple radiant heat would give when acting respectively on the two bulbs.
Other variations of the fundamental experiment, were as follows:
A differential thermometer having one bulb black, was exposed to the radiation from luminous hot bodies, first with and then without the interposition of a glass screen; the same position being preserved.
If the screen had no influence, it is evident that in whatever proportion the radiant matter affects the two bulbs, if it be of one simple kind, the only difference on removing the screen will be that its intensity will be increased, but will act on the two bulbs in the same proportion as before. Consequently an increase of effect, or motion of the liquid in the tube in the same direction as before, must take place.
In various experiments of this kind, after using several precautions against the influence of the screen, I never found an increase, and generally a decrease; that is, the action on the other bulb was now increased, or the portion of heat before intercepted and now admitted has a different relation to sur* faces from that transmitted. (Quarterly Journal of Science, xix. p. 45.)
Similar experiments were tried with the two bulbs in a direct line from the hot body, each placed nearest alternately, with and without a screen. The difference of ratios in the two cases was very striking. (Annals of Phil. June 1825, p. 401; see also Edinb. Journ. of Science, No. IV. 323.)
Upon the whole, the unavoidable conclusion is, that if the total direct effect were the result of one simple agent, the intervention of the glass would, by intercepting some portion of it, produce no other alteration than a diminution of intensity; the ratio of the two effects would remain unchanged: but the reverse being the case, it follows that there are two distinct agents or species of heat acting together.
[page] 282
Upon combining these results with those of previous experimenters, we are led to the following general statement of the case:—
When a body is heated, at lower temperatures, it gives off radiant heat stopped entirely by the most transparent glass, and affecting bodies in proportion to the absorptive texture of their surfaces.
At all higher temperatures it continues to give off such radiant heat distinguished by exactly the same properties.
At a certain temperature it begins to give out light: precisely at this point it begins also to exercise another heating power distinct from the former; this is capable of direct transmission through glass, and affects bodies in proportion to their darkness of colour.
This second species appears to agree with what the French philosophers have called "calorique lumineux," or the "igneous fluid" of Prof. Leslie; but they seem to have considered it as constituting the entire effect.
The distinction thus established easily applies to the explanation of De la Roche's results before stated. On inspection it appears that the numbers in the column belonging to the blackened screen are almost exactly in the same ratio to the first or direct effect throughout the whole series.
Upon the principle here laid down, the effects with the blackened screen would be those arising from the absorption and subsequent radiation of both species of heat; these in each instance being absorbed in the proportions in which they existed in the original radiation, produce a secondary effect proportional to the primary.
The effect with the transparent screen does not follow any proportion to the primary; and this is explicable as due to the glass intercepting the one kind of heat, which follows no proportion to the other, this last being wholly transmitted. Also by comparison of the latter experiments with the two first of the series, it is probable that, throughout, a certain degree of heat was in this case also absorbed and radiated again by the screen.
The existence of this distinction, and the proportion between the two species of heat in the radiation from different sources, as various kinds of flame, metal at successive stages of incandescence, &c., afford many topics of inquiry, on some of which I attempted some rough determinations, confessedly very imperfect. (Annals of Phil. N.S. liii. 359; liv. 401.) The distinction applies to some results of Mr. Brande on the flames of different gases, (Phil. Trans. 1820, Part I, p. 22,) and
[page] 283
of Count Rumford, on increased intensity of combustion, and on the coalescing of several flames. (Essays, i. 304.)
7.) Melloni states, (Ann. de Chim. Dec. 1831, p. 385,) that by using his thermomultiplier he has found the permeability of transparent bodies to heat to be also dependent on their refractive power. He has compared twenty such media, and finds the order of permeability constantly the same, whatever be the temperature of the source. Chloruret of sulphur has the greatest power, oil next, and water least; he exposed them to the rays of a candle, an Argand lamp, or the sun. He finds the differences of permeability less, the higher the temperature. The full account is promised in another memoir.
All this obviously applies only to luminous hot bodies.
MM. Melloni and Nobili, in their former paper, (Annales de Chimie, Oct. 1831, p. 211,) also speak of the heat from phosphorus having been by these means found sensible, though it is often supposed to give light without heat.
8.) For information on various points connected with the subject, and on the theories of the evolution of light and heat, the following references may be useful.
Wedgewood, Phil. Trans. 1792, p. 28, thinks that light from attrition is produced by a heat of from 400° to 600° Fahr.
Dizé on Heat as the Cause of Shining, Journ. de Phys. xlix. 177. Gilbert, Ann. iv. 410.
Fordyce on Light from Inflammation, Phil. Trans. 1776, p. 504. Morgan, Phil. Trans. 1785, p. 190. M. Hermstaedt, Nicholson's 4 to Journal, v. 187.
Mr. Davies on Flame, Annals of Phil. Dec. 1825.
Mr. Deuchar on Flame, Edinb. Phil. Journ. iv. 374.
M. Seguin on Heat and Motion, &c., Edinb. Journ. of Science, xx. 280.
DIVISION III.
Heat of the sun's rays.
Speaking according to our ordinary sensations, we are accustomed to say that the sun communicates both light and heat. Light is transmitted in a way which we term radiation. The heat from nonluminous hot bodies is transmitted to a distance in a way closely analogous; and to which the same name has been applied.
In the first instance, we might suppose that the sun sends out two separate emanations, one of light, and another distinct from it, and similar to that of radiant heat from a mass of hot water; and this, perhaps, was the first view taken of the sub-
[page] 284
ject, though a confused idea of some very close and intimate connexion subsisting between the solar light and heat appears to have prevailed.
This subject, as might naturally be expected, attracted the early notice of experimenters. A very slight examination sufficed to show that the rays of solar heat (whatever their nature might be,) differed essentially in many properties from those of terrestrial heat, whether radiated from luminous or nonluminous bodies. Whether there existed a separate set of heating rays distinct from those of light, and at the same time differing in many respects from rays of terrestrial heat; or whether these differences depended on some unknown property of the rays of light, was a question which for a long time remained without any direct investigation, and on which even now we have, perhaps, no very precise ideas.
I. Solar rays in their natural state.
a.) Nature of radiation.
1.) The solar heat is transmitted through the air without heating it.
It invariably accompanies the light.
Scheele conceived that the sun's rays of light produced heat not when in motion but when stopped by the interposition of solid bodies. (On Air and Fire, &c.)
Mr. Melville seems to have adopted nearly the same theory, and to have conceived reflexion at an opake surface to be the cause of an excitation of heat from the sun's rays. (Evans on the Calorific Rays, &c. Phil. Mag. June 1815.)
In general, for light of the same composition the heat appears nearly proportional to the illuminating intensity.
2.) Measures of radiation.
Theory of the sensibility of thermometers especially for experiments of this kind. (Sir W. Herschel, Phil. Trans. 1800, Note, p. 447.)
Leslie contends for the exact proportionality of intensity of light and heating power. (Inquiry, pp. 160 and 408.)
Theory and construction of his "Photometer" ch. xix. p. 403.
Ritchie's "Photometer" of the same kind. Phil. Trans. 1825, Part I. p. 141. See his Remarks on Leslie's Photometer, Edinb. Journ. of Science, No. IV. 321, and V. 104.
Mr. Daniell in his work on Meteorology has collected a great number of observations on the heating power of the sun's rays in different latitudes from the polar to the equatorial regions.
[page] 285
Most of these observations were made by comparing two thermometers, one of which was kept in the shade, whilst the other, having its bulb blackened, was exposed to the direct rays of the sun; but, as Dr. Ritchie observes, no correction seems to have been made for the variable causes which abstract caloric from the blackened ball of the exposed thermometer. (Edinb. Journ. of Science, v. 107.)
In the same paper is described the method proposed by Sir J. F. W. Herschel; his object was to ascertain, by direct experiment, the relative heating power of the sun's rays; this he did by exposing in a glass vessel, or large thermometer, at different times and places, a deep blue liquid, for a given time, to the direct rays of the sun,—noting the increase of temperature, which was purposely rendered very small by properly adjusting the capacity of the instrument, then shading the sun's direct rays, and leaving it exposed for an equal time to the free influence of all the other heating and cooling causes, radiation, conduction, wind, &c., and again noting the effect of these. The same difference of these, according to their signs, was the effect of the mere solar radiation. Dividing this by the time of exposure, he had the momentary effect or differential co-efficient, which is the true measure of the intensity of radiation.
Professor Cumming has been engaged in researches, the object of which was to obtain a measure of the total heating effect of the sun's rays. He has communicated for this Report an account of his investigations, of which the following is the substance.
His instrument consists of a bent tube in the form ⋒ one side terminating in a black bulb containing ether, or sulphuret of carbon; the other a graduated tube closed at the bottom; into this, on exposure to the sun, some of the liquid is distilled over from the bulb; and the quantity measured on the scale is proportional to the amount of radiation, when all interfering causes are allowed for; and these are estimated by comparative observations.
The experiments have been varied by exposing the bulb and screening the other part, or by exposing the whole instrument equally to the sun; and by making contemporaneous observations with the instrument wholly uncovered, or covered totally or partially by a glass to protect it from currents of air.
The Professor has endeavoured to make a standard scale by registering the sun's radiation on clear days every half hour, or hour, in the usual manner, and comparing them with the contemporary distillation; or by placing the two sides of the instrument in two vessels of water at unequal temperatures, and
[page] 286
noting the distillations in given times by ascertained differences of temperature.
The instrument is filled with ether in the same manner as Wollaston's Cryophorus (from which the suggestion was taken); but there is an inconvenience, arising from the circumstance of the difference of pressure under which the instrument is hermetically sealed, which renders two instruments not strictly comparable; this he proposes to remedy by sealing a standard instrument when exhausted to a known pressure by the air pump.
The ether or sulphuret of carbon employed must be perfectly pure, or there is a re-absorption. The circumstance of being exposed to the air, or covered, makes great differences in the indications; especially in windy weather. To avoid an inconveniently long scale, there should be two instruments constructed, one for winter and the other for summer. The Professor has kept for nearly a year a register of sunshine.
b.) Reflexion of solar heat.
1.) It takes place exactly by the same laws as that of the light.
The heat is collected in the focus of concave reflectors along with the light.
2.) The sun's rays reflected from the moon, are probably much too feeble to allow of any heat being made sensible.
Dr. Howard however states, that with a peculiar differential thermometer he has obtained an effect. (Silliman's American Journal, vol. ii. 329.
MM. Melloni and Nobili (with the apparatus before described) tried to detect heat in the moon's rays, but without success; they mention however that terrestrial radiation interferes greatly with such experiments, and do not describe fully their contrivances for obviating this cause of error. (Ann. de Chimie, Oct. 1831, p. 210.)
3.) Berard (Memoir before cited,) tried the polarization of the solar heat; that is, polarized the sun's light; and in the position of non-reflexion found that the heat had disappeared with it. (See Edinb. Journ. of Science, vi. 297.)
c.) Under this head nothing known.
d.) Effect of surface on the absorption of solar heat.
1.) I am not aware of any experiments directly showing how
[page] 287
far the same relation to the texture of surfaces which has been found in absorption of simple heat, may hold good in regard to the sun's rays. But for surfaces of the same texture it has been incontrovertibly established that the effect in this case increases in proportion to the darkness of colour, or in proportion to the absorption of light; and it would seem most probable that this relation is the only one which really holds good, the texture of the surface being probably quite indifferent except so far as it tends to the better absorption of the light.
2.) Among the earliest experiments on the subject, if not actually the first, were those of Mr. Boyle, on the different degrees of heat communicated by the sun to black, white, and red coloured surfaces.
He caused a large block of black marble to be ground into the form of a spherical concave speculum, and found that the sun's rays reflected from it were far from being too powerful for his eyes, as would have been the case had it been of any other colour; and although its size was considerable, yet he could not set a piece of wood on fire with it; whereas a far less speculum of the same form, made out of a more reflecting substance, would presently have made it inflame.
It was remarked by Scheele, that the thermometer when filled with alcohol of a deep red colour, rose more rapidly when exposed to the sun's rays than another filled with the same kind of spirit uncoloured; but that the fluid rose equally in both when dipped together into the same vessel of warm water. (On Air and Fire, &c.)
Dr. Franklin found that the hand when applied alternately to a black and to a white part of his dress in the sun, would feel a great difference in their warmth.
He observed that black paper was sooner fired by exposure to the focus of a lens than white.
His well known experiment of placing differently coloured pieces of cloth on the snow in the sun, and observing them sink deeper in proportion to the darkness of colour, was first suggested by Dr. Hooke.
3.) Cavallo observed that a thermometer with its bulb blackened, stands higher than one which had its bulb clear when exposed to the light of the sun, or even of the clouds. (Phil. Trans. 1780.)
Pictet made a similar observation, observing that when the two thermometers remained for some time in a dark place they acquired precisely the same height. He also found that when they had both been raised to a certain point, the clean one fell
[page] 288
much faster than the coated one. (Sur le Feu, ch. iv. Thomson, i. 126.) This last statement is so contrary to all other experiments, that we must suppose some mistake.
De Saussure received the sun's rays into a box lined with charred cork, containing a thermometer with a glass front; it rose in a few minutes to 221°, when the temperature of the air was 75°. (Voyages, ii. 932.)
Professor Robison in a similar experiment employed three vessels of flint glass within each other at 1/3rd of an inch distance, set on a base of charred cork, and placed on down in a pasteboard cylinder; the thermometer within, in clear sunshine rose to 230°, and once to 237°. (Black's Lect. i. 547. Thomson, i. 127.)
Sir H. Davy took several small disks of copper of equal weight, size, and figure, on one side painted respectively white, yellow, red, green, blue, and black. A mixture of oil and wax, which became liquid at a temperature of 76° Fahr., was attached to the other surface of each disk; and on exposing the coloured surfaces together to the sun's rays, the length of time elapsed before the mixture on each began to be affected, was in the order in which they are above enumerated. (Beddoes's Medical Contributions, p. 44.)
4.) The experiments of Sir E. Home (Phil. Trans. 1821, Part I.) are particularly deserving of attention, as exhibiting what might at first sight be considered an exception to the above remarks; a greater effect being produced in some instances on a white, than on a black surface. A more attentive examination, however, will show us that these experiments prove thus much: The heat occasioned by the rays of the sun when received directly, or when in some degree intercepted, as by thin white cloth, on the skin, is greater than that communicated by conduction to the same skin, through a black cloth in contact with it, which is itself, in the first instance, heated by absorbing the rays.
He observes also that a white skin is scorched, and that of a negro is not, in 10 minutes, by the direct rays of the sun; that is, as before, the outer coat of the skin allows some of the direct rays to pass through and affect the sentient substance beneath; whereas in the case of the black, the rays are absorbed and converted into heat of temperature, which diffuses itself equally and does not produce the effect of scorching.
5.) The most singular facts connected with the absorption of the sun's rays, are those exhibited by the substances called "phosphori" or "pyrophori". (Thomson's Chem. i. 17.)
[page] 289
The general fact is, that after exposure to the sun, on being removed into the dark they give out light, but it is after a time exhausted; it is given out more copiously and exhausted sooner if heat be applied. Many solar phosphori will always emit light of one colour only, to whatever coloured ray they may have been exposed. In a short notice given by Dr. Young, in his valuable Catalogue of authors, it appears that M. Grosser found that such phosphori as emitted red light only were made to shine most by exposure to blue light. (Rozier, xx. 270.)
Beccari, in a memoir "de Phosphoris" extracted in the Phil. Trans. 1746, p. 81, gives as one of his results, that the light emitted was brightest when the surface of the mass was of a rough texture; those which were smooth and polished, retained little or none, but (supposing the colour the same,) a rougher surface would evidently absorb more light than a smooth one, and therefore might emit more.
Mr. T. Wedgewood compared two pieces of phosphorescent marble, one naked, the other painted black; on applying uniform heat, the coated marble gave out no light, though the other did. (Phil. Trans. 1792.)
But the coating increased the radiating power, and it therefore probably did not retain heat enough to cause the extrication of light.
Mr. Morgan (Phil. Trans. 1785,) after examining many of the phænomena of phosphorescence, generalizes his views by maintaining that all phosphori emit light proceeding in order from violet to red, in proportion as the process is effected by the application of an increasing degree of heat.
This is a very curious subject, as connected with the whole theory of the relations of light and heat. Some valuable information might probably be obtained as to the degree of heat necessary, and whether there is any loss of heat when light is evolved, compared with cases when no light is evolved; as there should be on the hypothesis of conversion of heat into light, or on that of heat becoming latent in the light.
In Mr. Wedgewood's paper above cited, is an account of the principal researches on the subject.
e.) Effect of screens.
1.) That no diminution of the effect of the sun's rays on a blackened thermometer, is occasioned by a transparent screen, was remarked by several experimenters, particularly De la Roche. (Biot, iv. 611.)
2.) I tried the point by two thermometers, (as in the case of terrestrial heat), and found no perceptible difference in the ratio,
T
[page] 290
with, and without the screen, of the black and white thermometers. (Annals of Phil. xli. 321.)
The same result was found with a differential thermometer, with a glass screen over the bulb; which was not blackened; no difference was observable between the indication under these circumstances, and when both were exposed. (Annals of Phil. xlii. 401.)
Hence, I think we are entitled to conclude, that there does not exist in the solar beam, in its natural state, any simple radiant heat (as before defined); but that the whole emanation consists of the other species, distinguished by the two characteristics of affecting substances with heat in proportion to the darkness of their colour, and being wholly transmissible through glass without heating it; and inseparable from the rays of light.
This applies to the rays of the sun which come within the reach of our examination. It must, however, be admitted, as by no means improbable, that the sun may originally give out a separate radiation of simple heat. None of this kind reaches us, but we must consider the very different degree in which any medium, as air, absorbs or intercepts the passage of those two sorts of radiant agents. The heat from a hot body will not be perceptible at a short distance, while its light will traverse an amazing extent of length; and thus at different distances the ratio between the two sorts of heating effect will be very different. Some degree of simple heat, therefore, may actually be initially radiated by the sun, and be lost before it reaches us. We do not know that there is any medium between the different parts of the solar system capable of absorbing heat. The highest regions of our atmosphere into which observation has penetrated, are uniformly the coldest; but they are known to have a greater capacity for heat. Thus, though it is possible that some heat may reach to that distance, and be absorbed without becoming sensible to us, its quantity must be very small; if, therefore, we suppose any simple heat to be initially radiated from the sun, it must be all, or nearly all, absorbed by some parts or appendages of that luminary exterior to the part where it is generated.
3.) The concentration of the sun's heat by a lens is a familiar experiment.
Sir W. Herschel (Phil. Trans. 1800, Exp. 23,) concludes that there is a focus of greatest heat further from the lens, than that of light; sealing-wax was scorched in the same time when in the luminous focus, and at half an inch further from the lens; —this affords no proof of its being separated from the light.
[page] 291
That the heat is found to accompany the rays of light in the most constant and inseparable manner through various refractions, as in the instance of the four lenses in the eye-piece of a telescope after reflection, is also remarked by Sir W. Herschel (Phil. Trans, 1800, Exp. 11).
II. Solar rays subjected to analysis by the prism.
1.) The different heating powers belonging to different parts of the spectrum, were probably first observed by the Abbé Rochon. (Phil. Mag. June 1815; and Biot, Traité de Phys. iv. 600.) He found the maximum in the yellow-orange rays: the prism was of flint glass: his thermometer was filled with spirits, probably therefore tinged red: this may account for his result.
I tried some experiments with the bulb of the thermometer painted red, which appeared to agree with his result. (Annals of Phil. li. 201.)
Prof. Leslie applied his "photometer" to these experiments. (Inquiry, p. 454.)
Dr. Hutton observed the different heating powers, and that they are not proportional to the illuminating. (Diss. on Light and Heat, p. 38.)
Landriani found the maximum in the yellow rays, as also did Senebier. (Volta, Lettere, &c. 136.)
Berard (Mém. d'Arcueil, iii.; Ann. de Chimie, lxxxv. 309,) repeated the experiment with a heliostat. He found the maximum in the red, but some heat beyond. He repeated the experiment in both the spectra formed by Iceland spar.
2.) Sir W. Herschel (Phil. Trans. 1800, Part II.) first observed the maximum of heat beyond the red end of the visible spectrum, and considered the effect as due to essentially invisible rays of a separate kind from those of light.
Yet he found them subject to the same laws of refraction, and their dispersion corrected by another prism: they were concentrated by a lens (Ibid. p. 317), and by reflexion (pp. 298, 302).
Leslie objects to the conclusion of invisible rays, and tries to account for it as owing to an optical cause. (Inquiry, Note, p. 559; see also Nicholson's Journal, 4to, iv. 344 and 416.)
Sir H. Englefield (Nicholson's Journal, iii. 125,) found heat beyond the visible red; it does not appear whether it was there at a maximum: the rays were such as to be concentrated by a lens, and he compared the effects on a black and a white bulb.
T 2
[page] 292
The exterior effect on the white bulb was in a much less ratio to that within the visible spectrum, than on the black.
Sir H. Davy repeated these experiments in the clear atmosphere of Italy, and with thermometers of extremely minute size, to secure an instantaneous effect: he found the maximum beyond the red.
These experiments were also tried by Ritter and by Prof. Wünsch (Magazin der Gesellsch, &c. Berlin 1807). He used prisms of different substances; with alcohol, oil of turpentine and water, the maximum was in the yellow; with green glass in the red; and with yellow glass on the extreme boundary.
3.) But by far the most important and conclusive researches on this subject are those of Dr. Seebeck, who in a memoir read to the Royal Academy of Berlin, after discussing the conclusions and views of previous experimenters, proceeds to an elaborate series of experiments of his own, in which he has discovered the cause of all their discrepancies. The position of the maximum heat in the spectrum depends entirely on the nature of the medium employed,—a circumstance almost wholly unnoticed by former experimenters.
The heating intensity is very small towards the violet extremity; it thence gradually increases in prisms of water, alcohol, or oil of turpentine; the maximum is in the yellow space: in those of solution of sal-ammoniac and corrosive sublimate, or sulphuric acid, it is in the orange; in crown glass and common white glass, in the middle of the red: in those glasses which contain much lead, it is in the limit of the red: and in flint glass, beyond the visible boundary, but nearer to it with Bohemian than with English glass. In all cases it gradually diminishes from the maximum, and is perceptible to some distance beyond the visible boundary. (Schweigger's Neues Journ. x. 129; Annals of Phil. Sept. 1824; Abhandl. der Königk. Acad. Wissenschaften in Berlin, 1818-19, p. 305; Phil. Mag. Nov. and Dec. 1825; Edinb. Journ. of Science, No. II. 358.)
4.) Analysis of the solar rays by the absorption of media.
In respect to light, the remarkable variety in the absorption of different rays exhibited by different media has been well established, and affords a new sort of analysis of light.
In regard to the solar heat, similar researches have been made, though as yet to little extent. The first observations of the kind were those of Sir W. Herschel (Phil Trans. 1800). He found the absorption of several kinds of glass for his invisible rays and for the middle red, to be proportional to the following numbers out of 1000 rays incident:
[page] 293
| Invisible rays. | Red rays | |
| Flint glass | 000 | 143 |
| Coach glass | 143 | 200 |
| Crown glass | 182 | 294 |
| Dark red glass | 000 | 692 |
5.) Sir D. Brewster has lately been engaged in some researches on this subject, an abstract of which he has kindly communicated in manuscript for this Report. Agreeably to the view he has established of the solar prismatic spectrum as consisting of spectra of three primary colours superposed, and having their maxima at different points, he regards the heating power as due, in like manner, to another primary spectrum superposed in the same way; and similarly the chemical rays. He makes the following statements with respect to the heating rays.
1st, There is no proof whatever of the existence of invisible rays of any kind beyond the red or the blue extremity of the spectrum. Sir W. Herschel's experiments prove the existence of heat beyond the visible extremity of the spectrum which he used; but Sir D. Brewster has succeeded in rendering the spectrum visible at every point where any heat was produced.
By particular processes he has traced the light at that end greatly beyond the place where Frauenhofer makes the spectrum terminate.
The same he considers established in regard to the blue end of the spectrum and of the deoxidizing rays. He thinks it extremely probable that the heating and illuminating rays are different rays; but they have never yet been found in a state of complete separation.
2ndly, Until it is proved, therefore, or rendered probable, that the same intensity of light of different colours, as it proceeds directly from the sun, is accompanied with different degrees of heat, we must assume it as true that the heating power is proportional to the illuminating power of the different rays of solar light.
3rdly, It appears from Dr. Seebeck's experiments on the water spectrum, that this relation holds generally in it, as he found the maximum of heat to be in the yellow rays, or coincident with the maximum of light. Hence Sir D. Brewster draws the important conclusion, that water has the same degree of transparency for the solar heating rays that it has for light, which is the same as all colourless transparent media have for light; that is, water absorbs equally all the different rays of solar heat, in the same manner as it does all the different rays of solar light.
[page] 294
4thly, It has been found by experiment, that with prisms of crown glass the maximum heating effect is in the middle of the red space. Unfortunately the relation between the maximum heat in the water spectrum and in the crown glass spectrum has not been ascertained. If we suppose them equal, it appears that the crown glass must have exercised a greater absorptive action than the water upon the more refrangible rays, and a less absorptive action upon the less refrangible rays; in the same manner as is done by red glasses upon light.
A prism of sulphuric acid gives the maximum ordinate of heat in the orange space; or the fluid absorbs more of the red rays than crown glass, and less of the rays on the other side of the orange.
In flint glass, where the maximum heat is at the very extremity of the spectrum, scarcely any of the red rays are absorbed, while great proportions of all the others are.
Dr. Turner (Chem. p. 84, 3rd edit.) says, that it is difficult to account for Seebeck's results without supposing that different media differ in their power of refracting caloric (i.e. the heating rays of the sun).
Sir D. Brewster considers that the true explanation is that Which the above principles afford, viz. that colourless transparent bodies, in acting upon the solar heat, exercise the same sort of absorptive action upon it, that coloured transparent bodies do upon light; the maximum ordinate shifting its position with the nature of the body. Coloured media give sometimes two or more maxima of light, with large spaces and small lines entirely defective of light, in consequence of the absorption being total at those places.
In like manner he is persuaded it will be found that there are defective spaces and lines in the spectrum of solar heat; these he thinks may possibly be detected by using as thermometers the minute natural cavities in topaz, &c., filled with fluid or vapour, and not more than 0·001 inch in magnitude.
5thly, These views are exactly accordant with the results of Sir W. Herschel above stated.
They are equally consistent with the facts, whether the curve of heat terminate abruptly at the extremity of the red space, or continue beyond the visible spectrum.
Sir D. Brewster has by particular methods of condensation succeeded in detecting both heat and light at considerable distances beyond the maximum of heat, with a flint glass prism; that is, rays undergoing very little refraction.
He considers it highly probable that the deoxidizing rays will be found to be subject to the same laws of absorption as
[page] 295
those of heat and light; the media we commonly use may absorb them copiously, whilst others may be found which may transmit them more abundantly.
Similarly with the magnetizing rays. And thus we may account for the contradictory results hitherto obtained on this point, by supposing that some ingredient rendered one prism absorptive of these rays, and another not so.
6thly, Sir D. Brewster extends these views to the analogies between solar and terrestrial heat.
He considers those rays of the solar spectrum just mentioned, which undergo little refraction, to be analogous to those thrown off by bodies slightly heated. The waves of heat are broad and slow in their motion; as the temperature is raised they are thrown off with more velocity, and become smaller and suffer a greater refraction. When the velocity is such as to give them a refraction equal to that of the red rays, then red light is produced; and successively the other colours are added, till at a very high temperature white light is radiated.
He proposes to examine what transparent body transmits most heat, and by converting it into a lens, expects to find a series of foci at different distances, beginning from that of the violet rays to that of those corresponding to rays of very little refrangibility.
7thly, He applies these views as affording an explanation of De la Roche's result before mentioned, viz. that a second screen intercepts a much smaller proportion of the heat, after passing a first, than the first did of the whole effect: this De la Roche ascribed to something analogous to polarization.
On the principle just stated, the explanation is very simple. The first plate intercepts those rays which it has a tendency to absorb, and transmits the rest: the second, being of the same kind, of course will transmit these with scarcely any further diminution.
He observes, that thick masses of colourless fluid or of glass transmit scarcely any radiant heat in a way analogous to that in which thick masses of coloured glass are opake to all rays of light.
He conceives that substances may be found which are opake to light and yet transparent to heat. These should be carefully sought for, as they would be of great practical value. Red glass, for example, which scarcely transmits any light or 1 ray in 2000, transmits all the invisible rays of Herschel, 692 of the 1000 red rays, 606 rays out of 1000 of solar heat, and 630 of "culinary" heat, according to Sir W. Herschel. We may expect therefore to find an opake metallic glass, or thin plate of metal,
[page] 296
which, though quite opake for light, may transmit heat copiously.
Sir D. Brewster considers Sir W. Herschel's experiment on the refraction of "culinary" heat by lenses, to be very unsatisfactory, as before noticed. He recommends a lens composed of zones, so as to have no greater thickness in the middle than towards the edges, a construction which he has described in his "Optics," p. 322 (Cabinet Encyclop.), and made of glass, which unites the highest refractive power with the smallest absorptive power for heat.
It is also important to find, as sources of heat, bodies which do not become luminous till at extremely high temperatures.
6.) The researches of M. Melloni have also been extended to this part of the subject. (Annales de Chimie, Dec. 1831, p. 388.)
From known observations on the spectrum, he remarks that there exists on opposite sides of the maximum, isothermal points; one in a coloured part, the other without the red end of the spectrum.
On causing the different rays to pass though a plate of water, and noting the effect on the thermo-multiplier; the heat of the violet ray was undiminished, but its isothermal totally intercepted.
That of the indigo slightly diminished; its isothermal not totally intercepted.
Proceeding in this way with the other rays, he found in general that the portions of heating power intercepted in the coloured rays, and those which are transmitted in their isothermal rays, increase in proportion as they approach the position of the maximum, where of course upon the whole the interception is greatest; or, in other words, the rays of the calorific spectrum undergo an interception by water in proportion as their refrangibility is less.
He gives a Table of the numerical results. He views his results as precisely according with and explaining those of Seebeck. With a water prism the heating orange and red rays are more intercepted than the yellow; in this therefore the maximum appears.
Conclusion.
We have thus far taken as close a survey as is consistent with the limits of a Report like the present, of the successive and varied researches which have been made with the view of tracing the laws of radiant heat. In the present state of our knowledge, it must upon the whole be avowed, that we have little to contem-
[page] 297
plate but an assemblage of facts, or alleged facts, determined with more or less accuracy; few indeed with any great precision, many resting upon very vague evidence, and in several instances the results of different observers exhibiting a wide discrepancy or even direct contradiction: whilst, with very few exceptions, any general laws can hardly be said to be established with that certainty which can substantiate their claim to be received as legitimate physical theories.
In offering suggestions for the advance and improvement of this branch of science, the first and most essential point to which attention ought to be directed, is the improvement, or rather invention, of the means of obtaining accurate indications of radiant heat, down to its most minute and feeble effects. In reference to this point, good determinations are much wanted of the degree to which the expansion of the bulb influences the accuracy of air thermometers. The improvement of mercurial thermometers so as to produce an instrument of extreme sensibility to the minutest effects of heat, is an object the attainment of which would probably be more important than that of any other means for accomplishing the end in view. But other methods founded on good principles should be diligently sought for and tried; for example, it might be matter of inquiry whether we could render available to this purpose the incipient melting or softening of some substances by a very slight increase of heat, or the evaporation of volatile liquids.
But it is more particularly desirable that the instrument of MM. Nobili and Melloni should be tried, and a precise examination set on foot of its real accuracy and the causes of error to whose influence it may be liable. This is the more necessary from the very remarkable character of many of their results; whilst the alleged sensibility of the instrument, as they describe it, is such as almost to exceed belief.
When we shall have succeeded in obtaining that prime requisite, an unexceptionable measure of minute effects of radiant heat, we may then proceed with some hopes of success to examine the points on which there at present prevails so wide a discrepancy between different experimenters.
The polarization of heat is perhaps the question which of all others requires the most extreme sensibility in our thermometer, or rather thermoscope, in order to its satisfactory determination. It may be tried either directly with the simple heat from nonluminous hot bodies; or with luminous sources, with and without a glass screen, comparing the total compound result with that due to the transmissible part or heating power of light alone, and thence deducing the part due to simple heat. The
[page] 298
main difficulty is that of getting any indication at all, after two reflexions from plane surfaces.
Another point which requires further investigation is the apparent transmission of simple heat through very thin transparent screens, but not through opake. This should be examined in connexion with the acute remark of MM. Nobili and Melloni, that a thin stratum of soot may retain its low conducting power, and thus intercept the effect. This of itself would form a subject for an accurate series of experiments; viz. whether the ratios of the conducting powers of substances remain the same for all thicknesses.
The very nature of the transmissive aad interceptive powers of screens is little understood. Supposing simple heat transmitted without diminution, how far is the mode of such transmission analogous to that of light? what time is required for a body to commence radiating heat after it has begun to acquire it? whether it acquires it from a distant source instantaneously? how the heat distributes itself upon or through a screen? what is precisely the effect of a coating on one side of the screen in relation to the last question? upon what the singular exceptions and anomalies pointed out by Melloni and Nobili depend? whether any other such apparently anomalous cases can be found?—These are a few of the most obvious questions which arise out of the slightest survey of the present state of our knowledge, and on which accurate determinations are wanted before we can be said to possess even the elements of a scientific theory.
May it not be the law, that if a body be placed in the rays from a source of heat, it will be acquiring and giving out heat, till the intensity of radiation at the points before and behind it, resumes its original proportionality?
The time in which this takes place will depend on the extent of the body, its thickness, its conducting power, its capacity for heat, and the state of both its surfaces.
These may be such that the effect may be sensibly instantaneous, and the radiation therefore appear to go on without interruption. In this case also the distance of the screen from the source (within moderate limits,) may make no sensible difference; though if any of the above circumstances retard the effect to a sensible amount, then there will be a difference with the variation of distance. In this way we may as it were regard the medium between the source and the thermometer, as merely a compound, of which the screen is one portion, and the air the other.
Another class of questions respecting which little if anything
[page] 299
is accurately known, may be put with regard to the modifications (if any) which radiant heat may undergo, in passing through small apertures; this will again be connected with the interceptive power of net-work. A very curious and delicate subject of inquiry is the repulsion exerted between heated bodies at sensible distances, of which a short notice is given in the Quarterly Journal of Science, xxxix. 164.
The reflexion of heat has been little examined, except in the single case of its concentration by spherical reflectors; and here (according to Leslie,) it is not brought to the same focus as light: this requires examination, as well as the simpler case of plane surfaces, and the proportion of heat reflected at different incidences. There will probably in all cases be a very large deduction to be made for the heat acquired by the reflector and radiated again.
But another class of such questions yet remains in connexion with that fundamental point which was the object of my first inquiries. The conclusion from my experiments, viz. that luminous hot bodies are sending forth at the same time two distinct species of heat distinguished by different properties, is the unavoidable conclusion from the experiments, depending on the mathematical truth, that if a ratio be altered by the addition or subtraction of quantities from its terms, the quantities added or subtracted must be in a different ratio from the original one. I here repeat this because the nature of the reasoning has not been perceived by some persons. This conclusion undoubtedly introduces a complexity into the view we must take of the phænomena; whereas if we were at liberty to adopt the simpler theory of De la Roche and others, many of the apparent anomalies would be reconciled. Hence the verification of my results becomes a point of considerable importance. If any experimenter with more accurate apparatus shall succeed in showing them to be erroneous, he will achieve an important step towards simplifying the theory. In this instance again the improvement of the thermometer is a primary requisite.
I may here mention that I have recently had a more delicate apparatus made, with which I have repeated my former experiments, still with the same result; it consists of two thermometers mounted together as before described. They were contrived for me by Mr. Cary, so as to have very large degrees for a small part of the scale a little above ordinary temperature. 1° Fahr. occupies about half an inch; but the bulbs are large, which is unfavourable to the rapid communication of the effect. These experiments are of a very tedious
[page] 300
nature to repeat with precision, owing to the necessity of waiting between each repetition for the thermometers to cool and become stationary.
But it should be observed that there is nothing in my results which contradicts the idea that simple heat may have in a very slight degree a power of transmissibility through glass: all I have assumed is, that it is sufficiently distinguishable in this respect from the heating power which accompanies the light, and which undergoes no diminution. Connected with these points, again, is the question, whether if simple heat can radiate through solid transparent media, it cannot also commence radiating IN them. It is commonly asserted that radiation can only take place, or commence, in elastic media. This, then, is an inquiry which will lead into a wide field of research, and may be found connected with the intimate nature of radiation. It will also be a question, whether, and how far, radiant heat passes through elastic media without heating them, and what support this gives to Leslie's theory of pulsations. The whole subject should be viewed in connexion with the admirable remarks of Sir J. Herschel in his Discourse on the Study of Natural Philosophy, p. 205.
The radiation of heat in vacuo is another point on which further inquiry is much wanted. The greater capacity of air for heat, as it is more rarefied, would occasion a more rapid abstraction from the hot body; and thus in an atmosphere of extreme rarity the cooling ought to be extremely rapid, and this must be accurately estimated in measuring the radiation. But it appears from the experiments of Gay-Lussac, (see Edinb. Phil. Journ. vi. 302,) that when air is reduced to the most extreme degree of rarefaction possible, a very considerable compression makes so little difference in its actual density, that the giving out of heat which ought to take place from diminishing its capacity is absolutely insensible.
But even in this case it is very questionable whether so complete an approach to a real vacuum is obtained as to warrant inferences respecting the radiation of heat in an actual vacuum.
In fact, we want a connected series of determinations to show the order and increase of conducting powers, as connected both with the radiation in and through different media, and the interception which they offer to its passage.
In solids it is presumed no radiation can commence; it is disputed whether it can continue even partially: but conduction goes on rapidly.
In liquids it has been disputed whether there can be radiation; and they are worse conductors than solids.
[page] 301
In elastic media radiation can commence and continue; but they are still worse conductors.
In vacuo it might be presumed by analogy that a yet more free radiation might take place; yet some experiments (as we have seen,) show the contrary; and here there is no conduction.
With regard to that portion of the heat which accompanies or belongs to light, the theory which I originally suggested, (merely as an hypothesis representing the facts) viz. that it was simply the latent heat of light, developed of course when the light was absorbed, is connected with the hypothesis of the materiality of light; but it may be worth inquiry whether it does not apply even better to the elastic æther, in whose undulations light is now proved to consist.
Report on Thermo-electricity. By the Rev. JAMES CUMMING, F.R.S., Professor of Chemistry in the University of Cambridge.
IN communicating to the members of this Society an outline of the progress and present state of Thermo-electricity, I congratulate both them and myself on the allied branches of science having fallen into such able hands, that I should not be justified, even if it were my wish, to extend this Report beyond its immediate subject.
On one point more particularly I am happy,—since we are not so fortunate as to receive instruction from the discoverer himself,—that Dr. Ritchie has undertaken to exhibit and explain to us the recent researches of Mr. Faraday. The continuous electrical currents, now made known to us by these experiments, seem so much more nearly connected with those in the thermoelectric circuit, than with those peculiar either to the common or galvanic electricity, that I should otherwise have thought it incumbent on me to make the notice of them a part of this Report. Divested, as the subject will thus be, of all extraneous matter, I shall therefore be enabled to say all that I think to be really necessary, and yet detain you but a short time from more important communications.
On a review of the labours of different experimentalists on Thermo-electricity, it soon became evident to me, that, to give anything like a luminous account of them, it would be necessary to make some attempt at a classification of their objects. This, I must confess, was no very easy matter; for in this, as in some other branches of experimental inquiry, I have found it difficult,
[page] 302
after reading a detail of an elaborate series of experiments; to discover what object was intended by them.
The first and most obvious inquiry seems to be into the circumstances which are necessary for thermo-electric excitation, or which modify its action. In this respect the original experiment of Seebeck left much room for further investigation. When he had found that a brass wire coiled round the ends of a bar of antimony exhibited magnetic action by the application of heat to one of the extremities of the bar, it was still doubtful whether this effect might not depend either on some peculiarity in one or the other of these metals, on their contact, or on the mode of their juncture.
The remarkable effects produced by helices in the hydroelectric circuit made it not improbable that much might depend on the wire being coiled round the bar. This was soon shown not to be the case, and that a circuit, however formed, provided it were composed of perfect conductors, was all that was necessary.
Reasoning, again, from the analogy of the galvanic circuit, it might have been imagined that as three elements were necessary in the one, so two metallic elements with heat acting the part of the third might be required in the other; but it appeared from some of the earliest experiments, that metallic bars heated in contact with wires of the same metal gave considerable deviations with the galvanometer needle, and therefore that one metal alone sufficed for the development of thermo-electricity. The experiments of Dr. Trail in 1824 may be referred to this class; since, though made with slips of copper attached to the bar of antimony, yet, as the circuit was not completed through the copper, they properly exhibit the thermo-electricity of a single metal. One result, which is too important to be overlooked, is that the application of ice or heat to the centre of the bar produced opposite deviations in two needles placed between the centre and the extremities; whence he infers that "the direction of the compass needle may be considered as the resultant of two forces, the magnetism of composition of the earth, and its thermo-magnetism, which tends to place the needle east and west." How far this coincides with subsequent experiments I shall have occasion to point out to you hereafter.
But the most important researches on the thermo-electricity of a single metal were those made by Yelin in the same year; from which we learn, that all metallic bodies acquire magnetic properties when unequally heated, and that the series of their magnetic intensities when thus excited, is bismuth, antimony, zinc, silver, platina, copper, brass, gold, tin, lead:—that a metal acts differently according as the hot or cold part of it is placed
[page] 303
under the needle;—and that the magnetic action of metals unequally heated depends upon the form given them in casting; for which purpose masses of each metal, in the different forms of prisms, cylinders, and spheres both hollow and solid, were heated successively in different points, and examined by applying magnetic needles to their surfaces. From the different directions of the magnetism in cylinders of bismuth as they were cooled slowly or rapidly, he infers that there is some relation between the crystallization of metals and their magnetic properties. I may observe that I had previously shown that no difference either in the nature or quantity of the deviation could be detected in bismuth under similar circumstances, when forming a circuit with copper wires, &c.; the modification induced by slow or rapid crystallization is confined to the direction of the currents in the bar itself; and since fluid mercury is capable of becoming a thermo-electric element, crystallization is evidently not a primary agent in thermo-electric excitation or conduction, however it may modify its progress.
The latest experiments connected with this branch of the subject, are those of Mr. Sturgeon in 1831, on the thermoelectricity of homogeneous bodies, and the connexion between crystalline arrangement and thermo-electricity. The objects of his two papers appear to be to trace the directions of the magnetic currents in masses of metal, varying the form and the point of excitation; and so far they agree with those of Yelin. With these it appears that Mr. Sturgeon was not acquainted, as he says he is not aware that any experiments are yet before the public, illustrative of thermo-magnetic action in one solitary piece of metal. As a general result, it may be stated that, whether the mass of metal were in the form of a rectangular prism, a cylinder, or a cone, upon heating a point in the periphery of one extremity, the current proceeded longitudinally from the heated point on the same side of the axis, and returned on the other side, accompanied with transverse currents passing in opposite directions nearly at right angles to the longitudinal ones. With a large rectangular plate of zinc, when the heat was applied at one of the angles, the electric current was in the direction of the diagonal advancing, and returned along the edges. In this and all similar experiments, it seems that the direction of the electricity to or from the heated point, depends upon some peculiarity in each metal, which remains to be discovered; but that the course of the currents afterwards, with reference to the figure of the mass, depends solely upon the figures; and I think may be accounted for, by considering the whole as a congeries of wires, from which, ac-
[page] 304
cording to their position with respect to each other and the exterior surface, the heat is conducted away with more or less rapidity;—each portion, so far as the heat extends, acting both as a thermo-electric element and as a conductor of heat, but beyond that space acting simply as a conductor. The effects of crystalline structure in modifying thermo-electric action Mr. Sturgeon considers as arising from the laminæ of each crystal being only in juxtaposition, and that therefore the heat passes more readily through the parts of each than from one to another. This hypothesis it is obvious is inapplicable to metals devoid of crystalline structure, as wires of copper or silver, and still more so to metals in a liquid state: but, by conceiving each wire to be divided into an indefinite number of circular laminæ, we may suppose each of these to act as a layer of cold particles upon the laminæ on one side and of hot upon those on the other, and the total effect of the whole to depend on their aggregate action; each bar or wire acting as an assemblage of an indefinite number of small plates, as the common magnet may be conceived to be composed of an indefinite number of atomic magnets. Still, admitting this mutual action of the metallic particles, the original induction of electricity by heat and its subsequent propagation remain to be explained. This Becquerel conceives may be accounted for on the hypothesis that, whenever a particle of a metal is heated, part of the neutral electric fluid which is attached to it is decomposed, the vitreous fluid being retained, and the resinous driven off and passing into the adjoining particles. In proportion as the heat extends by communication from particle to particle, similar effects take place in each of those that are acquiring heat, and the contrary in those that are losing it. Thus the first effect is only to produce an oscillatory movement of the electric fluid between the adjacent particles; but if the source of heat be permanent, the retrograde movements are prevented, and a continued current takes place. I can only observe as to this theory, that the hypothesis appears to assume the very point that was to be established. I am not aware of any experiments to prove such a decomposition of the electricities of an uninsulated particle of metal.
The next class of experiments to be mentioned are those which relate to the transmission and augmentation of thermo-electricity.
Reverting to the original experiment of Seebeck, the brass wire connecting the extremities of the bar of antimony might act simply as a conductor, or might modify at the same time that it transmitted the electricity, according as the susceptibility to this species of electric excitation was confined to antimony, or
[page] 305
was common to all the metals. In the latter case it was to be expected that the metals might be formed into a series similar to the voltaic; and if such a series were formed, that the order might coincide with that obtained by arranging them according to some of their properties previously discovered. The results have been so far successful as to determine the thermo-electric relations of the metals to each other, (subject to anomalies which it is not now necessary to mention,) but not to connect them with any other of their properties; unless the conjecture of Becquerel be verified, that they are in the order of the specific heats,—in which case the remark of Dr. Ritchie on galvanic electricity will be applicable to this branch of it, and the whole theory of electricity may be intimately connected with that of latent heat.
The real gain to science from the knowledge of this thermoelectric series, is in the increased effect from the proper apposition of the metals; and as it appears that bismuth and antimony in conjunction, as originally proposed by Fourier and Œrsted and myself, are the furthest removed from each other of the available substances, it is not probable that any single pair of elements will be found more efficacious. On endeavouring to obtain an increase of power by augmenting the number of elements, it was soon discovered that this was limited by the want of tension in this species of electricity; in consequence of which, nearly as much was lost by transmission through a number of elements as was gained by their united action; and for the same reason the galvanometer, which had been so efficacious in multiplying voltaic action, was found to be of little advantage by the earlier experimenters. It appears, however, that by the ingenious contrivances of Nobili and Melloni, this difficulty has been overcome, and that they have been enabled to construct a thermo-electric thermometer of almost incredible delicacy. If there be any tension in thermo-electric currents, we may hope that it will be detected by this instrument, in which there may possibly, by the transmission of the electricity through its numerous elements, acting as more or less perfect conductors in their substance and at their junctures, be induced a tension, similar to that in the voltaic series by the passage from metals to fluids. As yet the only semblance of it is that obtained by Fourier and Œrsted in 1823; which was manifested by a slight convulsion of the muscles of a frog placed in the circuit. I am not aware that the experiment has been repeated; and, with all due deference to such able observers, I must doubt its accuracy. Wherever there is tension, however feeble, I should expect it to be attended either with the power of producing
U
[page] 306
heat, or of inducing magnetism. In the magneto-electric currents discovered by Mr. Faraday, which resemble the thermo-electric in the difficulty with which they are transmitted through even metallic conductors, tension has been manifested, accompanied with the faculty of inducing magnetism in steel by the helix; and 1 cannot but suspect that it will be found capable of heating small wires introduced into its circuit. On the other hand, no such effects have been obtained from the thermo-electric current; as I have repeatedly failed (and I believe others have not been more successful,) in magnetizing a needle by a thermoelectric current, of greater deviating power than that from a pair of galvanic elements, which succeeded without difficulty. Similarly, in regard to their heating power, by passing a wire through the axis of Briguet's thermometer, I have detected an increase of temperature in it when connected with a minute voltaic series, but none with the thermo-electric. I cannot therefore but think this a subject worthy of further inquiry.
Having noticed the thermo-electric experiments that have been made on a single mass of metal, and on two metals partly in contact, it only remains for me to call your attention to some very interesting researches on the thermo-electric action of two metals symmetrically united throughout. This part of the subject was first considered by Dr. Trail in 1824; who ascertained that if two plates of different metals were applied together throughout, (which in fact resolves itself into the case of two very short bars connected in the usual manner,) these would form a thermo-electric arrangement. This experiment was varied, and very important consequences deduced from it, by Mr. Christie in 1827. Acting upon a suggestion in the Cambridge Transactions, that as the diurnal variation of the compass needle seemed in some measure dependent on the position of the sun, it might possibly have a thermo-electric origin, he has shown in a paper in the Philosophical Transactions for 1827,—to which I cannot too strongly call your attention,— that these natural phæomena, so far as they can be imitated experimentally, accord with the supposition that the earth and its atmosphere may form a thermo-electric combination put into action by the sun. Imitating this arrangement by a circular ring of copper surrounding a plate of bismuth, and applying heat to a point in the ring, he found that the characters and extents of the deviations, were such as would arise from the polarizing the plate in lines nearly at right angles to the axis of heat,—contrary poles being opposite to each other in the two surfaces; and applying this to represent the state of the equatorial regions of the earth, we should have two magnetic
[page] 307
poles on the northern, and two poles similarly posited on the southern side; the poles of different names being opposed to each other on the contrary sides of the equator.
I cannot refuse myself the pleasure of quoting another result from an apparatus still more resembling the connexion of the earth and its atmosphere. When heat was applied to a point in the equator of a copper shell surrounding a sphere of bismuth, it resulted that the deviation of the end of the needle of the same name as the latitude, was always towards the west when the place of heat was above the horizon, and towards the east when it was on the meridian below. It is unnecessary for me to say to this audience, how perfectly this and the preceding results accord with observation.
I should be happy, if it were in my power, to bring before you a series of researches of equal interest with this paper by Mr. Christie; but, as I am not aware of any of recent date that are worthy of your notice, it only remains for me to point out to you what I conceive to be the present state of our knowledge of thermo-electricity.
Thermo-electricity may be developed in a homogeneous metallic mass, or in two distinct masses of the same metal unequally heated at their point of contact. In the former case the direction of the electricity in the same metal is influenced by the figure; in the latter, as when a metallic disk is touched by a heated wire, the action is anomalous, varying with the metal.
A metallic bar affords as many circuits as there are portions into which it can be divided. Therefore dividing it indefinitely, it may be conceived that each particle has its distinct current, the positive and negative electricities being separated by heat:
The currents between the extremities of a metallic bar are not affected by varying the directions of the intermediate currents.
Charcoal, plumbago, and some, possibly many, of the metallic sulphurets, are capable of thermo-electric excitation. This species of electricity is therefore not confined to the simple metals.
The metals compose a thermo-electric series of which bismuth and antimony are the extremes, and are the most efficacious within certain limits.
A battery may be formed of pairs of such elements, the action of each depending in some degree on the surfaces in contact; and the effect is greater when they are arranged in sequence, as in the voltaic series, than when connected as in Hare's calorimeter; but in neither case increases in the same ratio as the number of elements.
The ratio between the temperatures and the corresponding
U 2
[page] 308
deviations of a needle, in the thermo-electric circuit, is not invariable, the deviations increasing slower than the temperature, the law of which deviation is unknown; hence a pyrometer constructed on thermo-electric principles gives inaccurate indications.
The action of alloys is not intermediate between those of their constituents, and in some instances changes not only its quantity but its character at different temperatures. The action of iron with other metals is anomalous, being positive or negative with the same metal, according to the temperature.
The thermo-electric order of conducting powers in metallic wires, is silver, copper, gold, zinc, brass, platina, and iron; but compared with galvanic electricity, it is conducted with difficulty.
Thermo-electric rotation may be produced by the action of an exterior magnet, but differs from electro-magnetic rotation in not being the result of a continuous action. The action of a magnet upon an indefinite thermo-electric conductor, varies inversely as the distance: hence the same laws seem applicable to hydro- and stereo-electric currents.
The thermo-electric current appears to be incapable of passing through fluid non-metallic conductors, of heating wire placed in its circuit, or of either magnetizing steel, or forming a thermo-electric magnet; and therefore seems to have no assignable tension.
Hence it is somewhat beyond the limits of probability to suppose (as some have fancied,) that by these currents the metals themselves will be decomposed, and that the great revolution in chemistry, commenced by the pile of Volta, will be completed by that of Seebeck.
Report on the recent Progress of Optics. By Sir DAVID BREWSTER, LL.D. F.R.S. &c.
THERE are few branches of science which have been so irregular in their progress as that which treats of the nature and properties of light. With the exception of some general notions respecting its rectilineal propagation, the equality of the angles of incidence and reflexion, and the refraction of light towards the perpendicular in passing from a rare to a dense, the ancient philosophers contributed little to the advancement of physical
[page] 309
optics. It was not till the second century of the Christian æra, that Claudius Ptolemy, the celebrated astronomer of Alexandria, laid the foundation of this branch of the science, by an accurate examination of the phænomena of refraction. This distinguished individual, to whom Astronomy owes such deep obligations, measured with singular exactness the angles of refraction in water and glass for various incidences from 0° to 80°, and he determined the same angles when the light passed from the one medium into the other. These inquiries were no doubt undertaken in reference to the refractions of the atmosphere, in which, as an astronomer, he felt the greatest interest; and such was the success with which he applied them to this important object, that he gave a theory of astronomical refractions more complete than that of any other astronomer before the time of Cassini. While Tycho in the fifteenth century believed that the refraction of the atmosphere terminated at an altitude of 45°, and while others placed the zero of refraction at the pole of the ecliptic, Ptolemy had shown, 1200 years earlier, that the refraction increased gradually from the zenith to the horizon, and that all stars were elevated by it above their true place: nay, he uses the very same diagram upon which Cassini has founded his theory, and he employs almost the same reasoning as the French astronomer in order to determine the quantity of refraction.
Although the "Optics" of Ptolemy, in which these discoveries are recorded, is known to have existed in the time of Roger Bacon, yet the work appears to have been lost sight of by his successors, till two copies of it were lately found, one in the Savilian Library at Oxford, and the other in the Imperial Library at Paris.
The "Optics" of Alhazen and of his disciple Vitello, though. written a thousand years after that of Ptolemy, contain but very trifling additions to the science. Vitello, indeed, obtained more accurate measures of the deviation of the refracted ray in glass and water; but though his numbers were sufficiently exact for the purpose, he did not discover the constant relation which exists between the sines of incidence and refraction. The discovery of this important law was reserved for Willebrord Snellius, professor of mathematics at Leyden, a young man of the finest genius, who died at the age of 35, before he was able to give his own account of it to the world. The attempt which was made by Descartes to appropriate to himself this discovery is unparalleled in the history of Science; and the means which have been taken in later times to deprive the accomplished Snellius of his legitimate and single claim, present to us one of the most striking examples of national partiality.
[page] 310
The same discovery was undoubtedly made a few years afterwards by Mr. James Gregory; but though he never saw the MSS. of Snellius, as Descartes is believed to have done, neither he nor his countrymen have endeavoured to disturb the laurels which have so long and so justly decorated the Flemish philosopher.
The next important step in the history of Physical Optics was the discovery of double refraction by Bartholinus, a Danish Professor, who published an account of his experiments in 1669. After a careful examination of the phænomena as they appeared in Iceland spar, he discovered that one of the refractions was performed according to the ordinary law of Snellius, and the other according to an extraordinary rule which had not been recognised by philosophers. About nine years after the publication of this work, the celebrated Christian Huygens directed his attention to the same subject. He had long maintained the doctrine that light consists in the vibrations of an elastic medium, and he had succeeded in applying it to explain the rectilineal propagation of light;—the equality of the angles of incidence and reflexion;—the constant ratio of the sines;— the total reflexion of light, and the relation between the reflective and the refractive forces. While he was engaged in these researches, he became acquainted with Newton's discovery of the different refrangibility of light; and though it had a tendency to unsettle his previous opinions, yet he viewed it with an unfavourable eye, and remained firmly attached to the undulatory hypothesis.
In order to explain the phænomenon of two separate refractions, Huygens imagined an hypothesis before he had made a single experiment on the subject. He conceived that the ordinary refraction was produced by spherical emanations of light similar to those which take place in the elastic medium that pervades all transparent bodies, while the extraordinary refraction was produced by spheroidal emanations propagated indifferently through the elastic medium and the solid particles of the body. This singular idea was immediately submitted to the test of experiment; and Huygens had the satisfaction of finding that it represented in the most accurate manner all the phænomena which had been observed by Bartholinus and himself. This law, confirmed by every subsequent inquiry, and certainly one of the most remarkable in physics, is now the law of double refraction for all crystals with one axis; and it deserves to be especially noticed, that it was discovered by the hypothetical method which Kepler had employed with such brilliant success.
[page] 311
It will afford a curious insight into the character of great minds, and at the same time a striking proof of the fallibility and even the weakness of the loftiest intellects, to study the conduct of Newton and of Huygens in reference to their mutual discoveries. We have already seen that Huygens rejected Newton's doctrine of the different refrangibility of light, and also his analysis of the spectrum; and it is well known that he opposed the theory of universal gravitation. The discoveries of the Dutch philosopher were received by our illustrious countryman with equal if not greater distrust. Newton not only rejected the undulatory theory which Huygens had so ably expounded, but he rejected also the mathematical law of extraordinary refraction, which was established by direct experiment,—a law the truth of which was independent of the hypothesis from which it was deduced. Anxious, no doubt, to avoid controversy, Newton did not formally attack the law of Huygens, nor did he call in question the accuracy of his experiments; but without noticing this law he brings forward a new one of his own, without the sanction of any general principles, and without a single experiment in its support. "The unusual refraction," says Newton, "is performed by the following rule," which he proceeds to describe minutely with the aid of a diagram. Now this rule, investigated by the first genius of the age, and with all the powers of the inductive philosophy, has been universally rejected as erroneous; while the law of Huygens, explored by a less gifted mind, and originating in a bold hypothesis, enjoys the splendid triumph of having not only laid the foundation of one of the noblest branches of knowledge, but of having led, by its application, to the establishment of that very hypothesis from which it sprung. Historical truth, however, requires us to add that Huygens did not see the universality of his own law, and that in his investigation of the double refraction of rock crystal, both his experiments and his hypothesis utterly failed him. He announced that the double refraction of this mineral was regulated by two spherical emanations, one of which was a little slower than the other. This strange mistake had however a very natural origin. Taking it for granted that the extraordinary ray must always be the least refracted one, as in Iceland spar, Huygens appears to have measured only the refraction of the least refracted ray, which he found to be regulated by the ordinary law of Snellius. This result was perfectly correct; but he had been working only with the ordinary ray, having assumed most improperly that the other ray would have the same properties as the corresponding one in Iceland spar. By
[page] 312
this oversight Huygens failed in making that fine discovery which was reserved for M. Biot, that the double refraction of quartz differed from that of calcareous spar only in its being regulated by a prolate in place of an oblate spheroid.
After Huygens had established his law of double refraction, and even after he had drawn up his treatise on the subject, he discovered what he calls "a wonderful phænomenon" namely, the polarization of the two pencils of light formed by Iceland spar; and he confesses that other suppositions besides those which he has made will be required to explain it. He acknowleges, however, that he was unable to form any satisfactory conjecture respecting this new property of light, and therefore left the investigation of it to future inquirers. Sir Isaac Newton followed Huygens in this difficult research; but he only stated the fact in another way, when he said that the different sides of the ray had acquired, in passing through the first crystal, different properties which either favoured or prevented its passage through the second.
The subject of double refraction and polarization remained in the state in which we now leave it for one hundred and twenty years, without having received any accession of the least importance. The current of optical discovery, however, was not stopped; its direction only was changed, and during the next century it continued to flow in a more practical and useful channel.
The discovery of the different refrangibility of light which caused Sir Isaac Newton to despair of the improvement of common object-glasses, led himself and his contemporaries to perfect the reflecting telescope. The mistake which he had committed in supposing that all bodies produced spectra proportional to their mean refraction, was detected a few years after his death by Hall and Dollond, who discovered the different dispersive powers of bodies, and who were both led, by independent inquiries, to the invention and construction of the achromatic telescope. At a later period the discovery of the irrationality of the coloured spaces in the spectrum by Clairaut and Boscovich, furnished Dr. Blair with the general principle of the aplanatic telescope, and enabled him to construct fluid object-glasses, in which a perfect correction of colour was effected. These two instruments were doubtless the most valuable gifts which one science has presented to another; and the kindred subjects of navigation and practical astronomy exhibit in the perfection of their methods the great benefits which they have thus received.
After having slumbered for a hundred years, Physical Optics began to revive about the close of the eighteenth century. The events of the French Revolution had summoned from a state
[page] 313
of hybernation all the genius and talent of France. Men of high endowments and lofty intellect found an elevated place in society; and the establishment of the National Institute, the mightiest organization of intellectual power that history records, gave a new and a vigorous tone to scientific research. Even amid the convulsions and atrocities of that awful period, Science shot forth some of her brightest radiations; and in the moral and religious darkness which prevailed, her evening star was the only surviving emblem of heaven. The impulse thus given to knowledge was propagated over all Europe; and the quarter of a century which succeeded was one of the brightest periods in the history of philosophy.
Although Sir Isaac Newton had determined the law of the colours of thin plates, and had begun to examine the phænomena of inflexion, yet his experiments on both these subjects, but especially the last, were left imperfect. The young and ardent minds that were now ambitious of following Newton in his career of discovery, chose the inflexion of light as the subject of their first achievements. Lord Chancellor Brougham, Dr. Young, and Mr. Jordan gave an account of their respective labours on this subject in elaborate and valuable Memoirs. Lord Brougham and Mr. Jordan were warm admirers of the Newtonian theory of light, but Dr. Young had adopted the undulatory hypothesis of Huygens; he was therefore led to examine the phænomena under a different aspect, and was conducted to an experimental demonstration of the general law of interference, which was first observed by Grimaldi, and had been subsequently applied by Dr. Hooke to the explanation of the colours of thin plates. About the same time Ritter had discovered the deoxidating rays of the spectrum; Sir William Herschel had observed the invisible rays of heat; and Dr. Wollaston had detected fixed lines in the spectrum, and had confirmed by new experiments the Huygenian law of double refraction. During the same period the Marquis Laplace, who was a keen supporter of the Newtonian hypothesis, had referred the deviation of the extraordinary ray in doubly-refracting crystals to those attractive and repulsive forces by which the ordinary refraction and reflexion of light are performed; and by considering the force which acts upon the extraordinary ray as a function of the angle which the refracted ray forms with the axis of the crystal, he obtained formulae perfectly coincident with the Huygenian law.
The attention of philosophers was now anxiously directed to the subject of double refraction, and in 1808 the Institute of France proposed it as the subject of their Physical Prize. This prize was adjudged in 1810 to M. Malus, Colonel of the Imperial
[page] 314
Corps of Engineers, who not only composed a most valuable Memoir on double refraction, but enriched the subject with a discovery which laid the foundation of a new science. Having accidentally turned a doubly-refracting prism to the windows of the palace of the Luxemburg, which were at the time illuminated by the setting sun, he was surprised to observe that one of the double images of the windows vanished alternately during the rotation of the prism; and after various fruitless speculations on the cause of this singular phænomenon, he was conducted to the great discovery, that light reflected at a particular angle from transparent bodies is polarized like one of the rays produced by double refraction. This singular result opened a wide field of inquiry to philosophers: and the successive labours of Malus, Arago, Biot, Fresnel, and Cauchy in France; Seebeck and Mitscherlich in Germany; and Young, Herschel, and Airy in England—present a train of research "than which," as a distinguished philosopher remarks, "nothing prouder has adorned the annals of physical science since the development of the true system of the universe."
It would be impossible in a brief Report like the present, to convey even a general idea of the relative labours of these eminent philosophers,—of the new laws which they have established,—of the splendid phænomena which they have brought to light, or of the valuable applications which they have made of their discoveries. But, without giving offence to those who survive, we may distinguish two names, Malus and Fresnel, already marked out by the melancholy preeminence of a short and brilliant career,—-names which will ever be united in the history of Science by the extraordinary similarity of their lives and labours, of their honours and their misfortunes.
Devoted from their earliest years to the study of the sciences, they entered with ardour on the same field of inquiry,—the double refraction of light. Versed in the same abstract acquirements, they were both called to the situation of Examiner of Natural Philosophy and Geometry in the Polytechnic School. Their sovereign conferred upon them the same honours, the Cross of the Legion of Honour. The early discoveries of each were crowned by the Physical Prize of the National Institute. Their latest labours were rewarded by the Royal Society of London with the Rumford Medals; and, as if Providence had invigorated their exhausted frames to enable them to receive On their death-bed this brilliant trophy of their success, they both sunk beneath its banners, the one in the 37th and the other in the 39th year of his age. Thus triumphant in the same field, crowned with the same laurels, and doomed to the same early
[page] 315
grave, their names will be indissolubly embalmed in the sympathies of their countrymen; and in recounting their brilliant discoveries, the philosophers of every age will mingle their tears with their admiration.
Heu fortunati ambo!
Nulla dies unquam memori vos eximet ævo.
As the discoveries of Fresnel and his contemporaries have been fully described in several works in our own language, it would be an unprofitable task to give any account of them at present. The nature of this Report, however, requires me to notice those more recent discoveries which are less accessible and less generally diffused; and to endeavour to point out those new paths of discovery which the young philosopher may most successfully pursue, and those applications of optical science which are likely to be most useful in extending the power of man, in throwing light upon other branches of knowledge, and in investigating the structure and properties of organized matter.
I regret that the first part of this task should embrace the researches of so few labourers; and I fear that the future holds out but little prospect of any increase either in their number or in their efficiency. Pursuits more popular and more generally appreciable, and employments incompatible with scientific inquiry, have allured from Optical research many of those distinguished individuals who were most able to grapple with its difficulties; and within these few months Science has lost Dr. Seebeck* of Berlin, one of the most able and successful discoverers of the present century.
The only individuals who have been recently and actively engaged in the higher departments of physical optics, are Mr. Airy of Cambridge, and M. Cauchy of the Academy of Sciences.
In examining the two rays produced by the double refraction of quartz, Mr. Airy was led to a discovery which we consider as one of the most important in its results, and one of the most beautiful in its phænomena that has yet been made in this branch of optics. The circular polarization of the two rays along the axis of quartz had been studied by different philosophers, and had been explained by Fresnel with singular ingenuity on the principles of the undulatory theory. No attempt, however, had been made to account for the existence of this property only in the rays which pass near the axis of the crystal, or to define the limit where the circular polarization ended and the
* Dr. Thomas John Seebeck was born at Reval on the 9th of April 1770. He died at Berlin on the 10th of December 1831, in the 62nd year of his age.
[page] 316
plane polarization commenced. Fresnel, and all who have written on the subject, seem to have shrunk from this difficulty; but Mr. Airy saw that the two kinds of polarization must have some connecting link, and by the aid of theory and experiment he succeeded in discovering it. In place of the two rays in quartz consisting of plane polarized light, as was universally believed, Mr. Airy has shown that they both consist of elliptically polarized light, the greater axis of the ellipse for the one ray being in the principal plane of the crystal, and the greater axis of the other perpendicular to that plane. One of the rays he found to be right-handed elliptically polarized, and the other left-handed elliptically polarized. The proportion of the axes of the ordinary ray is more nearly one of equality than the proportion of the axes of the extraordinary ray, each proportion being one of equality when the direction of the ray coincides with the axis, and becoming more inequal with the inclination according to a law not yet discovered. The results calculated from the theory are in perfect accordance with those which Mr. Airy has obtained from very nice and difficult experiments; so that we may regard this beautiful and singular property of the two rays of quartz as perfectly established.
Mr. Airy has still more recently discovered a remarkable modification of Newton's rings, when they are produced by a lens laid upon a polished metallic surface. This modification possesses much interest when considered only as a detached fact; but its importance is greatly enhanced by its direct bearing upon the two rival theories of light. On the Newtonian hypothesis the colours of thin plates are produced solely by the light reflected from the second surface of the plate; whereas, according to the undulatory hypothesis, they depend on the interference of the light reflected at the second surface, with the light reflected at the first surface. Hence, if we can by any means destroy the light reflected at the first surface, the rings ought to vanish, according to the undulatory hypothesis; while they ought still to appear, according to that of Newton. Mr. Airy conceived the happy idea of using polarized light, which was freely reflected from the second surface, while it was incapable of being reflected from the first; and upon trying the experiment, he found that the rings disappeared,—a result which he regards as "perfectly inexplicable on any theory of emission, and as affording satisfactory evidence that the rings are produced by interference only." We have no hesitation in admitting that this experiment is inexplicable on the Newtonian hypothesis of fits, and that the action of the two reflected pencils, either on each other or on the retina, is necessary to the
[page] 317
production of the rings; but, as Dr. Young and others have allowed that the doctrine of interference is reconcileable with the doctrine of emission, the disappearance of the rings is not necessarily inexplicable on any theory of emission.
I regret that I am unable to give any satisfactory account of the very important optical discoveries of M. Cauchy, and I am not aware, indeed, that he has published any detailed account of his researches. In one of the Memoirs which he has lately printed, among those of the Academy of Sciences, he refers to three important results, which he has obtained from the undulatory theory.
1. The deduction of the law of the tangents which connects the polarizing angle with the refractive power of the body.
2. The explanation of the phænomena of dispersion.
3. The existence of a triple refraction.
The inability of the undulatory theory to explain the phænomena of inequal refrangibility, is almost the only exception to its universal application in accounting for the most complicated phænomena of light. Various attempts, though not very successful ones, have been made to remove this difficulty. Dr. Young supposes that the material particles of transparent bodies are susceptible of permanent vibrations, somewhat slower than the undulations which produce them, and that the velocity of the original undulation will be diminished in proportion to their frequency. The Rev. Mr. Challis, adopting Dr. Young's idea, has endeavoured to explain the manner in which the undulations of the æther within bodies are modified by their material atoms. He supposes that a sensible reflexion takes place at every interruption of continuity in the medium; and he infers that the mean effect produced by a retarding cause proportional to the reflective power of the atoms, will be to make the condensation corresponding to a given velocity, greater in a certain proportion than in free space, and to diminish the velocity of propagation in the same proportion. Mr. Airy has more recently endeavoured to remove this difficulty, by supposing that in refracting media there may be something depending on time, which alters their elasticity, in the same manner as in air the elasticity is greater with a quick than with a slow vibration of particles.
An anonymous writer, in a very recent Number of The Annals of Philosophy, has proposed another hypothesis for obtaining a difference of elasticity. He supposes that the æther accumulates itself round the particles of transparent media, and forms spheres of a density increasing towards their centres; and he infers that a succession of vibrations communicated through a
[page] 318
medium thus constituted, will give rise to new vibrations propagated with various velocities corresponding to those of the different rays in the spectrum.
The complete removal of such a difficulty from the undulatory theory by the analysis of M. Cauchy, must be regarded as one of the greatest steps in physical optics; and philosophers will look forward with the most intense anxiety to the development of that part of the same theory which renders necessary the existence of a triple refraction.
Such is a very brief notice of some of the most recent discoveries and views in physical optics. It would now be a pleasing task to point out the desiderata of this branch of science, and to indicate the locality of those rich mines which yet remain to be explored, did we descry any young and active labourers who were likely to gird themselves in earnest for so difficult a work. But when we see those who are best fitted for such inquiries, either abandoning altogether the study of light, or pursuing it in professional harness as a sort of contraband adventure, we almost despair of seeing acquired for our country the glory of any fresh achievements. Could we count on the unfettered labours of two of our most eminent natural philosophers, who have already evinced such high capacity for optical discovery, we might still cope with foreign genius, even though it does repose under the sunshine of Royal favour and of Academic ease.
There is scarcely any branch of the subjects of double refraction and polarization which does not afford the richest fields of discovery. Even the theory of undulations, with all its power and all its beauty, is still burthened with difficulties, and cannot claim our implicit assent. It has not yet brought under its dominion the phænomena of elliptic polarization in all its varieties, from the rectilineal polarization of transparent bodies, to the almost circular polarization of pure silver. It has not explained the singular influence of the force of double refraction over the force which polarizes reflected light; and it has great difficulties to struggle with, in accounting for certain phænomena of absorption, to which I shall presently have occasion to refer.
The determination of the physical data (or the physical constants, as Mr. Babbage calls them,) of these departments of science, constitutes a new and almost untrodden field, which may be successfully cultivated by almost every variety of talent. The refractive indices of the two pencils in all crystallized bodies, measured in reference to fixed points in the spectrum, as has been lately done by Rudberg;—the angles at which light is polarized by reflexion from crystallized and uncrystallized
[page] 319
surfaces;—the inclination of the resultant axes of crystals having double refraction, for different rays of the spectrum;—the dimensions of the ellipse which regulates the polarization of metals and their alloys;—the circularly polarizing forces of fluids and solutions;—and the refractive and dispersive powers of ordinary solid and fluid bodies, measured according to the method of Fraunhofer,—are some of the points to which we would call the attention of young and active observers.
But important as these determinations would be in a scientific point of view, and particularly in the renovation of mineralogy, the application of the principles of double refraction to the examination of structures, is pregnant with a still higher interest. The chemist may perform the most dexterous analyses;—the crystallographer may examine crystals by the nicest determination of their forms and cleavages;—the anatomist and the botanist may direct the dissecting knife, and use the microscope with the most exquisite skill;—but there are still structures in the mineral, the vegetable, and the animal kingdom, which defy all such methods of examination, and which will yield only to the magical analysis of polarized light. A body which is quite transparent to the eye, and which appears upon examination to be as monotonous in its structure as it is in its aspect, will yet exhibit under polarized light the most exquisite organization, and will display the result of new laws of combination, which the imagination even could scarcely have conceived. Like the traveller who has visited an unknown land, polarized light emerges from bodies bearing with it the information it has acquired during its passage, and indicating the structures through which it has passed, when put to the question of optical analysis. As an example of the utility of this agent in exploring mineral, vegetable, and animal structures, I may refer to the extraordinary organization of Apophyllite and Analcime; to the symmetrical and figurate deposition of siliceous crystals in the epidermis of equisetaceous plants; and to the wonderful variations of density in the crystalline lenses, and the integuments of the eyes of animals.
One of the finest fields of optical inquiry, and one almost untrodden, is that of the absorption of definite rays of the spectrum by the specific action of the material atoms of those bodies through which light is transmitted, or from which it is reflected. The discovery of dark lines in the solar spectrum, is certainly one of the finest which has been made in the present century, whether we view it in its theoretical bearings, or in its practical application to the construction of the achromatic telescope, and to the determination of all optical data which depend
[page] 320
on coloured light*. Fraunhofer found that the spectrum formed by solar light is crossed with numerous dark lines of different thicknesses, while the spectrum of artificial white flames contains all the rays which are thus wanting. Fraunhofer counted about 590 of these lines; and in a fine map of the spectrum which he has published, he has inserted the strongest of them, amounting to about 354. Some of these lines he found to be entirely black, while others were darker than the rest of the spectrum. From various experiments to which he submitted them, he concluded that they have their origin in the nature of the light of the sun, and that they cannot be attributed to illusion, aberration, or any other secondary cause. Sir John Herschel, taking a wider view of the subject, remarks, "that it is no impossible supposition that the deficient rays in the light of the sun and stars may be absorbed in passing through their own atmospheres; or, to approach still nearer to the origin of the light, we may conceive a ray stifled in the very act of emanation from a luminous molecule by an intense absorbent power residing in the molecule itself; or, in a word, the same indisposition in the molecule of an absorbent body to permit the propagation of any coloured ray through or near them, may constitute an obstacle in limine to the production of that ray."
For reasons which I may have an opportunity of explaining in another communication, I conceive that the original light of the sun is continuous from one end of the visible spectrum to the other, and that the deficient rays are absorbed by the gases
generated during the combustion by which the light is produced. But whatever be the manner in which these dark lines are occasioned, it is manifest that while they are of the highest value as affording fixed points in the spectrum, they render the sun's light absolutely unfit for experiments on absorption. We cannot, for
* In the spectrum formed by a narrow "beam of day-light," Dr. Wollaston had, previously to the year 1802, discovered seven lines, which he has designated by the letters A, B, f, C, g, D, E, the first line being, according to his observations, the extreme boundary of the red rays of the spectrum, and E the extreme boundary of the violet rays. The correspondence of these lines with those of Fraunhofer, I have, with some difficulty, ascertained to be as follows:
A, B, f, C, g, D, E, Wollaston's lines.
B, D, b, F, G, H, Fraunhofer's lines.
There is no single line in Fraunhofer's drawing of the spectrum, nor is there any in the real spectrum coincident with the line C of Wollaston; and, indeed, he himself describes it as not being "so clearly marked as the rest." I have found, however, that this line C corresponds to a number of lines half way between b and F, which, owing to the absorption of the atmosphere, are particularly visible in the light of the sky near the horizon.
In order to have seen the lines B and H of Fraunhofer, especially the last, Dr. Wollaston's "beam of day-light" must have come from a part of the sky very near the sun's disc.
[page] 321
example, examine the action of absorbent media on any one of the 590 rays which are deficient; and there is no possible way of recognising them, for the purpose of examination, in the spectra of those white artificial flames where they all exist.
This difficulty, however, has been completely removed by the discovery which I have lately made, of a gaseous substance which produces more than a thousand dark lines in the spectrum of ordinary flames, and thus renders artificial light more valuable even than that of the sun for the determination of optical data, while it enables us to study the action of material bodies upon all the defective rays of solar light. I have mentioned this experiment at present, in order to point out its bearings upon the two rival theories of light. On the Newtonian hypothesis of emission, the fact may be thus stated:—When a beam of white light is transmitted through a certain thickness of a particular gas, a thousand different portions of that beam are stopped in their passage, in consequence of a specific action exerted upon them by the material atoms of the gas,—an action which is powerfully assisted by the simple application of heat. Such a specific affinity between definite atoms and definite rays, though we do not understand its nature, is yet perfectly conceivable; and we may render it more easy of reception by hazarding the conjecture, that the particles of light itself are identical with the ultimate molecules of bodies, and that similar atoms in each may again unite when brought within the spheres of their mutual attractions.
In the language of the undulatory theory, the same fact may be thus expressed. A thousand different waves or rays of light of different velocities or refrangibilities, are incapable of propagating undulations through the æther of a transparent gas, while all waves or rays of intermediate velocities and refrangibilities are freely transmitted through the same medium: that is, a wave of red light, the 250 millionths of an inch broad, and another wave of the same light the 252 millionths of an inch broad, are capable of transmitting vibrations freely through the gas, while another red ray the 251 millionths of an inch produces vibrations which are entirely stopped by the medium. There is no fact analogous to this in the phænomena of sound, and I can form no conception of a simple elastic medium so modified by the particles of the body which contains it, as to make such an extraordinary selection of the undulations which it stops or transmits. We may suppose, indeed, that æther is a compound medium, consisting of other media, whose particles are the ultimate atoms of matter, and that the undulations of the same æther in transparent bodies are somehow affected by the affinity of similar atoms in the æther
X
[page] 322
and in the refracting body*; but this only removes the difficulty a step further, and leaves the mind impressed with the conviction that the production of such a system of defective rays by the action of a gaseous medium presents a formidable difficulty to the undulatory theory.
But whatever hypothesis be destined to embrace and explain this class of phænomena, the fact which I have mentioned opens an extensive field of inquiry. By the aid of the gaseous absorbent, we may study with the minutest accuracy the action of the elements of material bodies in all their variety of combinations, upon definite and easily recognised rays of light, and we may discover curious analogies between their affinities and those which produce the fixed lines in the spectra of the stars. The apparatus, however, which is requisite to carry on such inquiries with success cannot be procured by individuals, and cannot even be used in ordinary apartments. Lenses of large diameter accurate heliostates, and teleseopes of large aperture are absolutely necessary for this purpose; but with such auxiliaries it would be easy to construct optical combinations, by which the defective rays in the spectra of all the fixed stars down to the tenth magnitude might be observed, and by which we might study the effects of the very combustion which lights up the suns of other systems.
Report on the Recent Progress and Present State of Mineralogy. By W. WHEWELL, M.A., Fellow and Tutor of Trinity College, and late Professor of Mineralogy in the University of Cambridge.
MINERALOGY may be said, in a certain sense, to have continued to be a popular science ever since the time when Werner and Haüy inspired their pupils with so much enthusiasm and activity. During the course of the subsequent years very many persons have employed themselves in making collections of minerals, public and private; in arranging and naming the specimens; in referring their forms and characters to the types of acknowledged species. In England, as well as elsewhere, our best
* This supposition is countenanced by the remarkable fact which I have placed beyond a doubt,—that there are in different parts of the spectrum two or more sets of rays which have the same refrangibility, or which undulate with exactly the same velocity; and yet one of these sets of rays will freely permeate certain transparent bodies, or excite undulations in ita æther, while the other sets re absorbed, or are incapable of propagating undulations through the body.
[page] 323
chemists have frequently analysed mineral specimens; and we have had here persons at least as skilful as have appeared in any other country, who have disentangled the crystalline forms and examined the optical properties of minerals, and have thus established differences and identifications among certain varieties of crystalline bodies.
But on the other hand, mineralogy cannot be said to have been of late a popular science, in that higher sense in which we use the phrase, when we apply it to the sciences in which striking advances in theory, new and widening views, and the bright promise of future progress, attract the attention of all, learned and unlearned,—draw to them the energies of those who feel within themselves the vocation of discovery,—and communicate a feeling of scientific exultation and hope, even to those who have the most imperfect knowledge of the nature of the acquisitions, and of the grounds on which more is expected. Such sciences, in our own time, optics, geology, and chemistry have been and are; such, at least in our own country, mineralogy of late has not been.
It is not difficult to point out some of the reasons of the comparatively slow and undistinguished advance of mineralogy for the last few years. Nothing could be more brilliant than the prospects which appeared to open themselves to this science a few years back, at the epoch of Werner and Haüy. The German Professor gave a fixity and clearness to the determination of minerals by external characters, far exceeding anything which had been taught before; he introduced a system of classification which appeared to lend itself very happily to the known relations of minerals; and he announced the possibility of distinguishing, by the mineral characters of the mountains of the earth, the place which their strata occupied in an invariable chronological series, their meaning as the record of remote but ascertainable epochs in the physical history of the globe; —an application of mineralogy, which of itself was sufficient to give to the study a most attractive dignity and interest.
The French crystallographer, on the other hand, laid before his hearers a science which detected the most beautiful symmetry, simplicity, and constancy, in the midst of apparent complexity and instability; which undertook to determine the forms and laws of aggregation of the component atoms of bodies; and which boasted that, in the most remarkable manner, its predictions and suggestions, founded on differences which the unassisted eye could not appreciate, had been confirmed by the testimony of chemical analysis, summoned as a witness for that purpose. It appeared therefore in the highest degree probable, that mineralogy would be found to be, on the one
X 2
[page] 324
hand, a necessary vestibule to geology, and on the other, an entrance to passages, by which a new way was to be opened to the most recondite questions of chemistry and physics.
This promise has undoubtedly hitherto not been fulfilled; on the contrary, the mineralogist appears in some measure to have been disappointed of the advantages anticipated from both his allies, the geologist and the chemist. The former is now far from considering the mineralogist as his main supporter: conchology, zoology, botany, hydrography and general physics; are held to be at least as important as mineralogy, to the examination of the strata of the earth; and our geological teachers, in a playful spirit of exaggeration, have sometimes said that a person may be too good a mineralogist to be a good geologist. In his appeals to the chemist, the student of the mineral kingdom has always had his claims to assistance allowed; but chemistry is very far indeed from having done for him what he might have hoped it would do; not to mention, that the mere chemist seldom bestows a close and technical attention on that peculiar train of characters, which is the basis of the mineralogist's knowledge. Instead of our knowing exactly the chemical constitution of every mineral species; of finding chemistry ever ready to confirm the arrangements and classifications otherwise made, or if not, to offer something steady and unexceptionable in their place, we find that now, forty years after Haüy began to compare the results of crystallography and chemistry, we have very few minerals of which the chemical constitution is not liable to some dispute;—scarcely a single species of which the rule and limits are known, or in which two different analyses, taken at random, might not lead to different formulæ;—and no system of classification which has obtained general acceptation, or is maintained, even by its proposer, to be free from gross anomalies.
Berzelius has given to one new mineral species an appellation derived from the Greek word for shame, (αισχυνη,) acknowledging a sort of disgrace to fall upon science from the analysis of this mineral; inasmuch as two of its elements of very different natures (titanic acid and zirconia,) cannot be separated so as to determine their relative quantities. If we were to give this name to all the kinds of minerals of which the chemist cannot tell us the exact constitution, eschynite would be a large family instead of a single species.
This decided check in the progress of the science has, I think, without question, very much damped the interest with which mineralogy, as a branch of natural philosophy, has been looked upon in England. Indeed this feeling appears to have gone so far, that all the general questions of the science excite with us scarcely any notice whatever. The value of a method
[page] 325
of classification seems to be looked upon as a point not worth discussing; any one method is considered as good, or as bad, as any other. This opinion indeed is openly maintained by some of our best mineralogists. Their labours have been employed solely and exclusively in the crystallographical and chemical analysis of particular species; and I am not aware that any attempt has been made among us to establish any proposition including a class of species of minerals, with the exception of Sir David Brewster's optical researches.
Such is the state of the case in England.—But a more forward and hopeful spirit appears to have prevailed for some time in other countries, especially Sweden, Germany, and more recently France. It may therefore be of service to point out what is the progress which has been made in this branch of knowledge, and what are the views respecting it which have been opened during the last few years in Europe at large.
The subject may be conveniently considered under the following heads.
1. The Physical characters of minerals; as hardness, specific gravity, lustre, &c.
2. Crystallographical speculations, and their application to minerals.
3. The Optical properties of minerals.
4. The Chemical constitution of minerals.
5. The Classification of minerals.
6. Miscellaneous researches and observations; as the discovery of new minerals, the identification or discrimination of old ones, the determination of their crystalline forms, &c.
I purposely omit all that refers to the localities of minerals, as more properly pertaining to the domain of geology; and all that regards their economical uses, and the processes of metallurgy, as forming in itself a distinct subject. The study of the properties which we have now some hope of referring to general laws,—the optical and chemical properties of minerals, —is a topic sufficiently ample for the present occasion.
1. Physical characters.
The discrimination of minerals by their most obvious properties of colour, lustre, weight, hardness, was naturally attended to in the first attempt to obtain some distinct and connected knowledge with regard to those substances. The study of such characters has now been prosecuted far enough and long enough to show that a systematic and solid mineralogy cannot be formed by attention to these alone; and that crystalline form and chemical constitution must be the main elements of our mineralogica! science. Still, the more vague external characters by no
[page] 326
means deserve to he neglected; nor will they be so by any who study the actual minerals with persevering and close observation.
From the nature of this portion of mineralogical study, it is scarcely susceptible of much speculative progress. The increase of the personal skill and sagacity of the observer by practice is the best result of its cultivation. Yet some improvements in method may be pointed out as having been recently made. Werner, eminently acute in his observation of sensible qualities, gave fixity to his discriminations by the introduction of an appropriate nomenclature. His pupil Mohs, formerly his successor at Freiberg, and now Professor at Vienna,—one of the most gifted of his disciples in the same way,—has attempted to fix one of the most important characters, hardness, by a numerical scale. In this scale, the hardness of common talc is 1, of gypsum 2, of calc spar 3, of fluor spar 4, of apatite or asparagus stone 5, of felspar 6, of quartz 7, of topaz 8, of corundum 9, of diamond 10;—thus, to say that the hardness of a mineral is 51/2 indicates that it scratches apatite and is scratched by felspar. Prof. Breithaupt of Freiberg, the pupil and successor of Mohs, has proposed to put 12 degrees in this scale instead of 10, introducing mica between gypsum and calc spar, and sodalite between apatite and felspar, as intermediate degrees. It has been observed by others, that the hardness of several minerals is different in different parts, and even in different directions; thus kyantte gives a different value of the hardness, according as we scratch it along or across the direction of the axis.
The specific gravity has also been scrupulously attended to by the same school of mineralogists, and both Mohs and Breithaupt have determined very minutely the value of this element for very extensive series of minerals. Beudant also has paid great attention to this subject; he has ascertained by experiment (1. x. 331.) that large crystals, and especially bacillary masses, have a smaller specific gravity than small crystal; and he hence recommends us to reduce minerals to powder previously to finding their specific gravity, in order to avoid the influence of these differences in the mode of aggregation. Magnus found that garnet and similar minerals when melted and again solidified in a glassy but uncrystalline state have their density diminished; the Greenland garnet, for instance, was in this manner reduced from sp. gr. 3·9 to 3·05.
In the observation of the colour and lustre of minerals, we have hitherto been left to the unassisted eye and judgement. It was the object of an instrument described and exhibited by Sir David Brewster, at the last Meeting of the British Asso-
[page] 327
ciation, to make this observation more precise and delicate. The principle of the instrument is, to observe, not the whole light reflected from the surface of the mineral, but the excess of light which remains undestroyed when we apply to the surface a lamina of liquid differing but slightly from the mineral in its refractive power: the differences of lustre and colour in minerals, may thus become much more sensible than when the whole effect is compared.
The different kinds of lustre,—glassy, fatty, pearly, adamantine, metallic,—undoubtedly depend upon optical differences in the surfaces, which differences have not however as yet been clearly explained. Professor Breithaupt is in the habit of showing, by the superposition of a number of watch-glasses, that the pearly lustre results from the lamellar structure of a transparent mass. The very curious difference between the optical properties of the surfaces of metals, and of transparent bodies, has been traced, on different roads, by Sir David Brewster and by Professor Airy; and both agree in considering the optical properties of the diamond as intermediate between the transparent and the metallic character; though they do not agree in their representation of the peculiar laws which the diamond discloses. When the connexion of these properties with those of other bodies is clearly made out, we shall probably learn more distinctly than we now can, what is the precise distinction of metallic, adamantine, and vitreous lustre.
The more distinct cleavages of minerals are among their most important characters, and the less distinct are also of value. Sir David Brewster has suggested a method of obtaining cleavages too indistinct to be made visible in any common way, by tearing the surface of the mineral with a dry file. In this manner he made obvious a cleavage of calc spar in the direction of the long diagonal of each of the rhombic faces.
We may notice here, also, the ingenious mode of mechanical analysis described by M. Cordier, and successfully employed by him in the examination of rocks of various kinds which had been considered as homogeneous substances, but which are in fact aggregates of small crystalline portions of various simple minerals. The specimen is reduced to minute fragments, rather by pressure than by trituration*, and the particles of different kinds, being separated by differences of specific gravity or appearance, are examined in various ways, and especially by means of the blowpipe. This method was found to be particularly applicable to the discrimination and discussion of rocks of a trappean character.
* Journ. Phys. 1816, pp. 82, 83.
[page] 328
2. Crystaliegraphy.
Though no change has since been made with reference to the crystalline forms of minerals which has excited so much popular notice as Haüy's establishment of the fixity of forms and the laws of their derivation, the subject has undergone a complete change since his time; and a principle of classification of forms has been introduced, so scientific and yet so simple, that it is irresistibly superseding the older Haüyian arrangements, and the more so, as it is strikingly confirmed by the optical properties of crystals. I speak of the division of forms into systems of crystallization; namely, the tesseral; the tetragonal, or square pyramidal, or pyramidal of Mohs; the rhombic, or oblong pyramidal, or prismatic of Mohs; the rhombohedral of Mohs, or hexagonal of Naumann; and the monoklinohedral, diklinohedral, and triklinohedral of the last-named writer. Some notion may perhaps be formed of the nature of these distinctions from the following representation.
If we conceive a square steeple with all the four sides of the walls and roof exactly alike, so that every slope and face which occurs on one side, occurs similarly on the other three; we have before us a form belonging to the square pyramidal system.
If instead of this we imagine a house of which the two ends are like each other, and the two sides also precisely like each other, but different from the former, this will belong to the oblong prismatic or rhombic system.
If again we conceive a triangular pillar, as an ancient tripod, its three sides being similarly cut and ornamented; this will belong to the rhombohedral system*. In fact, its three faces may be terminated by slopes which may meet and form an apex resembling in all respects the apex of a rhombohedron. And if each of its three faces be formed into an edge by planes sloping to the right and left, the form may be thus converted into a six-sided pillar with no loss of its regularity.
If we conceive the form of the house of which we spoke as representing the prismatic system to be made less regular by sloping its end walls in the direction of one end; we have the monoklinohedral system; and if the side walls slope also, we may have thus the diklinohedral and triklinohedral forms.
The tesseral or tessular system includes the forms which are derived from the regular solids of geometry, the cube, the octahedron, the dodecahedron.
This distinction of different kinds of forms, is one founded on the most general relation of their parts, and regulated by the
* The rhombohedral and rhombic systems are quite distinct. A rhombic prism has its base a rhomb;—a rhombohedron has all its sides equal rhombs.
[page] 329
degree and kind of their symmetry. The claim of priority in introducing this classification of forms has been a subject of controversy between Prof. Mohs and Prof. Weiss. However this question may be decided, the merit of this valuable simplification rests between them; and all must allow the propriety with which Prof. Naumann of Freiberg, the author of the best recent system of Crystallography, has dedicated his work "to Mohs and Weiss, the Coryphæi of German crystallographers."
The distinction of systems is now generally adopted. Thus Germar, (1830,) one of the most recent authors, has the tessular, pyramidal, prismatic, and hexagonal systems, each subdivided into homohedral and hemihedral, as all or half the faces occur; —the oblique prisms are considered as hemihedral and tetartohedral right prisms, according to the method of Mohs, whose notation also is retained. In England, the distinction of systems of crystallization has not been explained, so far as I am aware, except in Mr. Haidinger's translation of Mohs.
Crystallography is essentially a mathematical subject. The striking mixture of simplicity and complexity which here, as in other parts of nature,—but yet more here than in any other part of nature,—offers itself to our notice, depends upon the combination of the primary forms belonging to the above systems with the geometrical and numerical laws by which other forms are derived from these. To trace the properties of such derived forms, and of their combinations, necessarily requires some considerable portion of mathematical calculation, which may however be of several kinds. Spherical trigonometry, solid geometry, and analytical geometry of three dimensions, may any of them be made to answer the purposes of the crystallographer. Haüy and Mohs, proceeding in the manner which, of the three, implied the least extended acquaintance with mathematics, employed in most instances particular constructions and calculations founded on solid geometry; and though they thus want the conciseness, beauty, and generality of other methods, they are perhaps, in consequence of this, intelligible to a wider circle of students. Monteiro, Bournon, Cordier, Soret, and others, have followed the method of Haüy; and denominations and notations borrowed from it are still common in our catalogues. Phillips also, so far as he referred to any method, employed that of Haüy; but his extraordinary merits consisted rather in determining the angles and forms of individual specimens and species, than in referring them to any general law.
Prof. Hausmann of Göttingen, a pupil of Mohs, has laboured in the spirit and according to the method of his master; as has another distinguished mineralogist from the same school, Mr. Haidinger.
[page] 330
Mr. Brooke has, in a great measure, employed the formulæ of spherical trigonometry, in which he has been followed by others. This method has the great advantage of enabling us immediately to perform all our calculations by the help of logarithmic Tables.
The most scientific mode of treating the subject, which coists in reasoning by means of the equations to the planes according to the methods of analytical geometry, was employed from the first by Weiss. It has been adopted by Mr. Levy, and by a number of German writers, as G. Rose, Kupffer, Köhler. Naumann in his Principles of Crystallography, published in 1826, employed processes much resembling those of Mohs: but in a much enlarged and improved work on the subject which appeared in 1830, he states, with great candour, that at the former period "he was not acquainted with the great advantages of an analytical-geometrical mode of treating the subject," and that he has now "arrived at the conviction that this is and must be the simplest and most natural of all methods." This is a conviction which will probably be more widely diffused as the subject is more studied. M. Naumann has by this method calculated all the formulæ which are likely to be needed in a very clear and complete manner, and has exhibited the results of the most common combinations in a tabular form. Ratzeberg has published a similar synoptical Table, with figures of the crystalline forms and their combinations according to the method of Weiss; a very convenient mode of presenting the subject.
Geometrical truth has generally several aspects, each of which by constant contemplation appears to the individual reasoner to become the most luminous possible; and this is especially the case with regard to a system of truths so complex and multiplied as those which the solid geometry of crystals offers to our notice. It is not surprising, therefore, that other authors besides those above mentioned, should have taken other views of the best mode of treating the subject, and should have brought forwards these as considerable discoveries. Thus. Mr. Grassmann (Stettin 1829,) published a Treatise "On Physical Crystallonomy," in which he develops the connexion of forms by means of "a mathematical discipline hitherto never pursued." He determines the position of a plane by means of a "radius constructor" or line perpendicular to it, and assuming three fundamental radii of this kind, he deduces the number and mutual relation of the others by the combination of the relations of these fundamental radii. Neumann (a different person from Naumann,) has also endeavoured to simplify the subject by the introduction of normals and "index planes," as I learn from
[page] 331
Mr. Hessel, who has followed him in the use of these terms. Mr. Hessel himself (Professor at Marpurg,) in his work entitled "Crystallometry" (Leipzig 1831), has adopted several other new denominations and modes of considering forms. Thus, by way of example, he states concerning the rhombic dodecahedron, that its twelve faces "are perpendicular to doubly-two-membered normals, (the edges are doubly-one-membered, like-sided, unlike-ended,) which are perpendicular to doubly-one-membered four-and-three-spaced rays," &c.
The principle of this and similar methods of treating this subject consists in the permutations and combinations of various kinds of symmetry in lines, surfaces, and solids. One kind of symmetry, which occurs frequently in crystals, is not easily described by any common expression; and Mr. Hessel, who justly attaches much importance to the consideration of it, has introduced a peculiar term to designate it. The symmetry here spoken of is that which is seen in comparing the two ends of an oblique prism; and they are called by him "gerenstellig" gore-wise-placed, in opposition to "gleichstellig" alike-placed. One or two new phrases in such cases may perhaps be introduced with advantage: but the systems to which I here refer are so far laden with new phraseology and new views of the relations of space, that they will probably not be found by many a convenient mode of mineralogical study. It may be readily allowed, that when a person has mastered the fundamental views of these methods, the application of them to crystallometry may have some advantages of order or of generality; but this is for most readers too long and indirect a road to the results: and if crystallography leads to new views on the subject of elementary geometry, the prosecution of these will interest the pure mathematician, but the mineralogist will find it necessary to confine himself to investigations more peculiarly professional.
The consideration of the faces of crystals as distributed into zones, points out a mode of transition from one system of crystallization to another. Thus, if a rhombic dodecahedron be placed so that an axis is vertical which is terminated by three plane angles at each vertex, we may then, by prolonging or contracting the axis, make the form pass from the regular to the hexagonal system. But if the vertical axis be one which is terminated by four planes at each end, its prolongation or contraction converts the same form into one belonging to the tetragonal system. This mode of deriving one set of forms from another has been followed up by Breithaupt, who has thus derived all forms from the octohedron; the axis being, in the regular octohedron 720, and in the other cases greater or less.
[page] 332
Particular questions of crystallometry (as the mathematical part of crystallography has been termed by some writers,) have been examined by various persons. Mr. Haidinger in the Edinburgh Journal of Science for 1824, gave an excellent series of papers on twin crystals; in which he pointed out the various laws of combination, and analysed the resulting forms, in each of the systems of crystallization, and in the most important species. The general principle which governs these various combinations is, that the two parts of the twin crystal are such that one would come into the position of the other, by making a half-revolution round a certain axis; and this axis is always a real line in the series of crystalline forms belonging to the species which presents these phænomena. This general law in particular cases gives rise to occurrences as curious and as perplexing to the mineralogist as double and monstrous flowers are to the botanist. These are now for the most part understood.
One of the most common and yet most curious of these cases, is that of the interposed films in calc spar. These films, which were early noticed as giving rise to remarkable optical properties, were shown by Sir David Brewster to consist of crystalline plates of a thickness from 1/1000th of an inch upwards, in a position transverse to that of the crystal. He proved this by an analysis of the optical properties, and also synthetically by imitating those properties by means of crystalline plates purposely interposed.
A question has been raised, whether the oblique prism and the forms referable to it should be considered as a peculiar system, or as a right prism with only one half the number of sides extant in one case (hemihedral), and one fourth in the other (tetartohedral). Thus the twin-crystallization of pyroxene and of wolfram appears to indicate that though they appear as oblique prisms, they have rectangular axes. Yet the more general opinion and evidence seem to be in favour of the existence of a monoklinohedral or hemiprismatic system. And thus wolfram may be an oblique prism of an angle of 90° 1′, or 90° 0′ 1″. Naumann expresses nearly the same thing by saying that it is qualitatively monoklinohedral, quantitatively rhombic. The question must be decided by determining which mode of considering such crystals gives simple numbers and relations for the individual forms and twin crystals which really occur.
The part of Haüy's views which most caught the popular attention was the supposed exhibition of the real structure of crystals as built up of molecules of known shape, the crystalline faces being formed by given laws of decrement in the courses of
[page] 333
this sort of masonry. This doctrine was readily accepted, because it pretended to offer to our curiosity the ultimate analysis of the constitution of bodies; but by reason of this very boldness of promise, it was unlikely to bear the test of time and trial. At present this doctrine is probably not maintained, as a physical truth, by any one who has examined the subject; for though its assumptions may appear obvious à priori, they are not confirmed by observation. In order to support the theory of integrant and subtractive molecules, the facts of the case ought to be quite different from what they are. If indeed, in all minerals, the cleavage planes were such as to bound forms which would join so as to fill space, and if the forms of the crystals could always be referred to these planes with great numerical simplicity, the theory would still be a good mode of grouping the facts. But it appeared very early that it could not claim this praise; and when the author of it was driven (as in the case of fluor spar,) to conceive crystals made up of solids hanging together by their edges, we had an example of a theory in which difficulties were solved by suppositions directly contradicting the only reasons which could be assigned why the theory should be accepted.
Any theoretical mode of representing in general the ultimate structure of crystals, as consisting of elementary particles, whether as an aggregate of plane-faced solids, spheres, or spheroids, will probably not be of great value to the science in its present stage. But it must be considered interesting to know how far that numerical simplicity in the relations of the faces of crystals which led to the hypothesis of decrements, is really found in nature. The greater part of the faces of the most usual crystals are expressible by laws of which the ratios are very remarkable for simplicity. But in not a few cases the numbers run considerably beyond what was supposed to be the admissible limit in the earlier stage of the study. Thus in arragonite we not only have the numbers 2, 3, 4, 5, but 7, 9, 10, 19, occurring in the ratios. In carbonate of lead we have 13, 19, 21, 28. In galena Naumann has 12012, and 36036, according to his notation. In certain crystals of gold from the Ural, Rose finds a face which is (a, a/11, a/19) in the notation of Weiss. It would be important if any one could decide whether there is any limit of magnitude or simplicity to such ratios.
The most generally useful result which has followed from the modern methods of treating the subject of crystallometry, has been a great simplification in the mode of deducing the laws of formation of faces, when we find them on the crystal.
[page] 334
Instead of requiring the trigonometrical and algebraical calculations of Haüy. the law can, in a great majority of cases, be inferred from properties which are obvious to the eye, especially from the parallelisms of the edges of the faces. This mode of reasoning, introduced by Mohs, has been very successfully cultivated by succeeding writers, and especially by Naumann. The fertility and convenience of this resource is greater than any one not acquainted with it can easily imagine. With a collection of diagrams representing the binary combinations of forms, such as Naumann and others have given, the crystallometrical analysis of a very complex crystal becomes comparatively easy.
Still, to determine the laws of all the faces which commonly occur in the known species of minerals, is a task which has necessarily required the labour and skill of many persons. The early labourers in this province have a particular claim to our gratitude. Haüy did much, but he left also much to do. Weiss (Berlin Trans.) first successfully discussed some of the more difficult and complex cases, as gypsum, felspar, epidote. Professor Moh's Treatise contains a vast treasure of such determinations, and has only left for more recent crystallometers the task of supplying special deficiencies. And of such contributions we have excellent examples in recent times, among which we may mention the examination of the crystallization of felspar by Hessel, and of the blue carbonate of copper by Zippe of Prague.
In speaking of crystallometry, it is necessary to say something of notation, a subject which is repulsive to many in consequence of the multiplicity and complexity of the symbols which have been promulgated and which is yet absolutely indispensable to the mineralogist who would economize time, labour, and thought. Perhaps it may be found that the discrepancies of different authors are not so great as they at first sight appear. The notation of Haüy, indeed, belongs to so imperfect a knowledge of the subject, contains so much that is arbitrary, and is so incapable of being rendered either simple or symmetrical, that its reign ought by this time to have become only a matter of history, although in fact, among the disputes of its successors, it retains here and there some little show of authority. But the systems of notation of Weiss, Mohs, and Naumann, have better claims to our notice. That of Weiss is simple, according to his view of the subject, which, it will be recollected, consists in using the equations to the planes of the crystal; his symbol for any plane consists merely of the three coefficients of the equation, included in brackets. Of this method, the main defect is, that it is too general, and does
[page] 335
not very obviously exhibit those relations of the forms and of their edges of combination, which are so useful, as we have already said. Moreover three coefficients are more than we need; for the ratios of one of the three to each of the others are all that we have occasion for. Accordingly Mohs uses two indices only, by which he indicates any plane; and so far his method has an advantage. But he has been most peculiarly unfortunate in the mode he has selected of combining the indices with his fundamental letter. It is quite inconceivable how a mathematician, having to annex the two indices 2 and 3 to the letter P, should, without any support whatever from mathematical analogy, choose to connect one index with the letter by the sign + so as to convert the symbol into a binomial P + 2, and then use the other index as the exponent of a power of this binomial, as (P + 2)3. The violation of all mathematical significance, and the anomalous and useless complexity thus incurred, make such a system truly forbidding. Mr. Naumann has been more fortunate; and indeed his notation is indisputably almost as simple as it is possible for a crystallometrical notation to be; for two indices being necessary, and all that is necessary, he puts one before and one after his fundamental letter, and thus obtains a simple and convenient symbol. Moreover, the mode in which the laws of derivation are treated in his system is such as to bring very well into view the most important relations of the forms*: and as he has both published an excellent treatise on crystallography, and a compendious system of mineralogy, in which all the known forms of individual crystals are exhibited in this notation, we may hope that in time this system, or one resembling it, will supersede more complex and imperfect ones. It may be added that the systems of Weiss and of Naumann approach near to each other, and the notation of the one is very easily translated into the other. They predominate over a great part of Germany, and stamp the language of a great number of the best publications on the subject.
3. Optical Properties
Malus examined many mineral substances in the course of his inquiries concerning double refraction; but he does not ap-
* In comparing, however, the notation of Naumann and of Weiss, it ought to be taken into the account, that Naumann's two indices have often a more complicated appearance than Weiss's three indices. Thus we may compare Naumann's symbols 3/2 P3, 5/2P∞, 15/7 O 15/11 with Weiss's equivalent symbols, (3a, 1b, 6c), (5a, 2b, ∞ c), (a/7, a/11, a/15).
[page] 336
pear to have noticed any differences between the double refraction of crystals of different forms. Biot found grounds for separating doubly refracting crystals into two classes, characterized by opposite properties, which he called attractive and repulsive, or positive and negative; quartz is of the former, calc spar of the latter class. But no constant external distinction of such substances could be detected: the only fact connecting the form of crystals with their optical properties, was that observed by Haüy,—that substances having the cube, octahedron, dodecahedron, &c., for their primary form, had no double refraction.
Sir David Brewster must be considered as in a great degree the creator of the science which studies the mutual dependence of optical properties and crystalline forms; and he not only gave the first impulse to this study, but has enriched it with a vast quantity of most curious and interesting observations;—so great indeed, that all which has been done by other labourers in this field, bears as yet no proportion to the amount of his contributions.
Some of Sir David Brewster's first results* appeared, however, to contradict the general fact which we have just mentioned, as the only one then known on this subject, (Edinb. Trans. viii. 1815.) He found that some specimens of muriate of soda, fluor spar, and diamond, which according to the law just stated should have no optical axes, did, when they were obtained in considerable thicknesses, exhibit the colours which had already been found to indicate double refraction. The crystals seemed to consist of complementary parts, the effects of which nearly neutralized each other, but left in certain different parts of the crystal a small excess of action on one side and on the other.
His next observations were on calc spar. He had already shown that the colours which appear in the specimens crossed by films are produced, not by these films as thin plates, but by the properties of polarized light; and he now found that these films have a crystallization of a position opposite to that of the rest of the crystal, as has already been stated in speaking of twin crystals.
Another of Sir David Brewster's memoirs belonging to this period, is remarkably interesting. It had appeared by his
* I do not dwell on the discovery,—one of the first announced by Sir David Brewster on such subjects,—that doubly refracting crystals have two dispersive powers corresponding to their two refracting powers; which discovery has recently been re-stated as a novelty by Rudberg, and would have been again so restated by Mr; Cooper if he had not learnt from Sir David Brewster these previous publications of it.
[page] 337
experiments, that compression and dilatation give to transparent bodies a structure which produces the same effects as the crystallization which is associated with double refraction. He now tried the effects of compression and dilatation on crystals; and found that in this way the optical phænomena were variously affected, the rings deformed, the colours altered, the curves multiplied, &c. And, as the law which regulates the influence of this mechanical tension on the previous crystalline tension of the substance, he found that "positive crystals, compressed so that the axis (or direction) of compression is parallel to the axis of the crystal, have the order of the tints raised." The terms "negative," "dilated," "perpendicular," and "depressed," of course alternate in the enunciation of this law, with "positive," "compressed," "parallel," and "raised."
The striking and valuable generalization, however, which has established for ever a close connexion between crystallonomy and photonomy,—a connexion rich in the discoveries which it has already given us, and richer still in those of which it gives us no doubtful promise,—is found in the Phil. Trans. for 1818, (the memoir having been sent in 1817). In this Sir David Brewster states that the extreme perplexity of the subject, and the difficulty of procuring proper specimens, had prevented him hitherto from doing, what he has there done,—" reducing under a general principle all the complex appearances which result from the combined action of more than one axis of double refraction."
This law, so far as we are concerned with it as mineralogists, (for I am not now to speak of pure optical investigations,) is this:—That all crystals with one optical axis belong to the hexagonal or the pyramidal system (using the terms already explained; which are equivalent to those of Sir David Brewster,) —that all crystals with three optical axes belong to the tessular system;—and that all the crystals with two optical axes crystallize in other forms. It thus appeared that there was an exact correspondence between the degree and kind of the symmetry of the optical properties and of the crystalline forms.
This important principle was not hastily snatched from a few observations, as men, judging of great discoveries after the event, and struck by their simplicity, are always ready to think might have been done. It was carefully collected from an examination of many minerals, including 23 species with one axis, and 81 with two axes; and there were not wanting some apparent exceptions and difficulties in the application of the rule. These have for the most part disappeared under a closer
Y
[page] 338
examination. Perhaps one of the most striking instances of such an occurrence is the history of a mineral which has been termed by Mr. Brooke the sulphato-tricarbonate of lead. It was placed both by Count Bournon and by Mr. Brooke among the rhombohedral or hexagonal forms, and therefore ought to have had but one optical axis. Sir David Brewster however found that it presented the phænomena belonging to two axes. The difficulty was solved when Mr. Haidinger subjected the substance to an exact crystallometrical examination. It then appeared that a figure which had been supposed to be a right hexagonal prism had not the exact dimensions which the symmetry of that figure implies. Its sides, instead of making an angle of 90° with its ends, make an angle of 90° 29′; and instead of making angles of 120° with each other, they make angles of 120° 20′. The crystal had in fact precisely one of those forms from which its two optical axes would, by the rule, result.
Sir David Brewster had indeed already discovered a similar case in sulphate of potass; which had been arranged as a rhombohedron by previous mineralogists: but when its optical examination had indicated two axes, he found that the apparent bipyramidal dodecahedron of the rhombohedral system was composed of three prisms with angles of 114°.
The memoir of 1818 of which we have spoken, contains also Sir David Brewster's very happy detection of the remarkable' optical law on which the form of the curves seen in biaxal crystals depends; but on this and the other contents of this valuable memoir I must, for the reason already referred to, forbear to dwell.
The properties of doubly refracting crystals which affect all colours similarly, have now been reduced to a theory of singular beauty, which explains the most complex and apparently anomalous parts of their details: but the properties of such crystals, which seem to select certain colours for their action, remain still to be traced to their most general laws. Here also, however, Sir David Brewster has done a large proportion of all that has been done. His memoirs on the Absorption of Light by Crystals (Phil. Trans. 1816,) contain many curious facts on this subject. It is well known that certain tourmalines polarize the light which passes through them, to such an extent that they are commonly used as the easiest mode of obtaining polarized light. Agate and other substances were found, in like manner, to polarize light by transmission. But it appears that these are merely instances of a more general fact: many doubly refracting crystals, perhaps all coloured ones, affect the ordinary and extraordinary pencils with different colours. Thus beryl,
[page] 339
by Sir David Brewster's experiments, when exposed to polarized light, transmitted different colours (blue and greenish white), as the axis of the crystal was perpendicular or parallel to the plane of polarization.
Other species of mineral crystals were found to possess similar properties, and biaxal crystals exhibit it also with certain modifications. Sir David Brewster's list of cases is, as usual, considerable. He found also that many minerals absorb certain portions of common light, the transmitted portion being more or less polarized; so augite, epidote, produce upon light an effect partly of the same kind as tourmaline. Smoky quartz produces the effect strongly; but it is to be observed that a prism of quartz and one of tourmaline polarize in planes, the one at right angles to the axis, and the other parallel to it. Babinet has recently (1832,) enunciated as the general rule of such cases, that one or the other occurs as the crystal is of the attractive or repulsive class: but as it appears in fact by Sir David Brewster's previous researches, that the results in the two positions do not differ as dark and bright merely, but occur by a selection of colours, the general rule thus asserted must require, if true, to be differently expressed.
After these discoveries concerning the optical structure of crystalline substances, we might have here supposed that we could form some conception of the extent of the variety of nature in this class of phænomena. In such cases, however, nature is more fertile than our conjectures. It was soon found that many crystals possessed a structure far more complex than the mere number of axes of a single crystal could give them. This discovery also, and the accumulation of cases in which it is exemplified, are due to Sir David Brewster. It appears from his researches that many kinds of crystals must be considered as composed of a most curious mosaic work of crystals, in various positions, arranged in an order highly complex yet perfectly symmetrical. Thus he found in 1817, and announced in 1819, that amethyst consisted of different portions, which act differently on light in an alternate and complementary manner; these portions being generally wedges, with their vertices to wards the axis of the crystal, or a series of V's one within another, exhibiting the outlines of such wedges.
Again, it appeared that apophyllites from Iceland and from Ferroe were composed of a most curiously tessellated structure, capable of being visibly resolved into its elements by the transmission of polarized light.
And in 1818, (Edinb. Trans.) Sir David Brewster published his representation of the optical structure of analcime, which is in
Y 2
[page] 340
some respects even more curious and complex than the preceding cases. The icositetrahedron, which is the usual form,—a figure belonging to the class of crystals, which exhibit none of the properties connected with polarized light,—is in this species distinguishable into 24 solids, of which the boundaries have peculiar optical properties.
This phænomenon of the composition of a crystal, apparently simple, of portions exhibiting different optical relations, appears in fact to be very common. Thus nitre and arragonite often contain such portions; and in the second volume of the Cambridge Transactions, Sir David Brewster has shown, that the Brazilian topaz possesses a tessellated structure, a central lozenge being surrounded with a border of a different kind, sometimes with additional variations.
There would be something utterly perplexing in this complexity in the structure of objects apparently so simple, if we were to conceive such a kind of composition as formed of independent portions adhering together; but we ought, probably, rather to conceive these relations of parts as the result of a peculiar state of the equilibrium of the elastic æther which exists within the body, and on which its optical properties depend.
An additional principle, still further complicating the apparently inexhaustible phænomena of crystals, was discovered and fully discussed by Sir J. Herschel (Phil. Trans. 1820). The deviation of the succession of colours which many crystals exhibit from that scale of tints which Newton established by observations on thin plates, and which since his time has always been the alphabet of the higher optics, attracted Sir J. Herschel's attention, and he found that it could be fully explained by conceiving the direction of the axis of double refraction to be different for different colours. In biaxal crystals, such a deviation is almost universal, as in Rochelle salt, in which it is very prominent. Bicarbonate of potash, indeed, is said to be the only biaxal crystal yet examined, in which the axes for all colours are found to be strictly coincident. (Herschel, Light, 923.)
But this deviation from Newton's scale of colours perplexed observers more in the first instance, when it was seen in the case of uniaxal crystals. Sir J. Herschel (Camb. Trans. vol. i. Part I.) found certain varieties of apophyllite in which the doubly refracting structure was positive for the red, and negative for the violet rays, while for the intermediate indigo rays there was no double refraction at all. This remarkable circumstance was confirmed by the most decisive experiments, and now offers ho difficulty when viewed in connexion with the undulatory theory.
[page] 341
A somewhat similar circumstance has been discovered by Sir David Brewster in some specimens of glauberite. They are biaxal for red rays, the resulting axes being 5° asunder; but the axes for violet rays coincide, and for such light the crystal is uniaxal. This remarkable peculiarity was detected by the use of homogeneous light.
We have still another fact to notice equally striking, equally unexpected, and having also the name of Herschel associated with its discovery. There existed an optical law which had already attracted the attention of philosophers as being entirely anomalous and sui generis, and a crystallographical peculiarity equally curious. Sir J. Herschel, with singular sagacity and felicity, showed that these two circumstances were constantly conjoined. I speak of the circular polarization of light to the right or left, and the plagihedral crystallization of quartz. In both these eases we had, instead of the geometrical symmetry by which the laws of nature are usually marked, a set of appearances suggesting the idea of progress round a circle to the right or left hand; the deviation of the plane of polarization, as shown by the succession of colours on increasing the thickness of the transparent plate, being the optical fact thus governed, and the oblique position of certain faces of the crystal the mineralogical fact. It was proved that right-handed polarization always accompanies right-handed plagihedral faces, and left-handed polarization left-handed faces. This was established by Sir J. Herschel from the examination of thirteen crystals, and has since been fully confirmed by other observers.
It does not properly belong to our subject to dwell upon Prof. Airy's theory of the circular and elliptical polarization of the rays of light in quartz, by which an extremely complex and apparently unsymmetrical collection of phænomena are reduced to the most complete simplicity and regularity. But we may mention the experiments of the same observer on the optical properties of diamond. It appeared from these, that diamond, instead of completely polarizing light reflected at a certain angle, as other transparent substances are found to do, presents, at the angle at which light is most nearly polarized, phænomena resembling rather those of a surface of metal, than of a diaphanous medium.
Among optical inquirers, several persons have employed themselves in researches on the causes of the play of colour which is seen in Labrador felspar, as Hessel and Genf; and Nordenskiold has attempted a mathematical explanation of these colours, of which however I am not able to give any further account. Sir David Brewster has examined these
[page] 342
curious phænomena; and it appears from his inquiries that the colours are produced on the principles of the colours of thin plates, by cavities bounded by parallel plane surfaces.
In the application of polarized light to the examination of the properties of minerals and other crystals, we have acquired a new instrument, of a use far more extensive and instructive with regard to the structure and differences of substances, than anything which had before been dreamt of. Physical optics and crystallography are for the future two coordinate portions of a vast province of science, of which the limits as yet have not been caught sight of. In the optical examination of minerals there remains much to do; and it would not be difficult to point out branches of inquiry which are of evident importance to the present state of our knowledge of crystals. It would, for instance, be very satisfactory to know the difference of optical symmetry which exists between a right and an oblique prism; —whether the additional deviation from geometrical symmetry, which occurs in the latter case, corresponds, in the optical properties, with the fact of our no longer finding the poles of the lemniscates in the same plane, as would seem to be the case from some experiments of Sir J. Herschel,—or whether this case is marked by some yet unguessed peculiarity.
But while we look forward with hope to the augmentation of the stores of observation in this most interesting department of the study of nature, it is desirable that we should be aware of the treasures already in existence. The discoveries already mentioned as published by Sir David Brewster, and many others which might have been added to the enumeration, form, I believe, but a part of the facts bearing on optical crystallography, which that indefatigable observer and acute philosopher has in his possession. He has long led the mineralogical world to hope to receive from his hands a Treatise on Mineralogy, on optical principles, in which it may be presumed he will state all the most remarkable facts and laws with regard to the relations of mineral species to light, which have come under his notice; and certainly no acquisition could be more interesting to the mineralogist, or more likely to give a fresh impulse to the progress of this science*.
The different optical properties of minerals have been theoretically expressed by speaking of the different elasticity of the crystal in different directions. This induced Savart to examine, by his ingenious methods, whether the acoustical elasticity was also governed by similar differences. We ought not to overlook,
* I am sorry to learn from Sir David Brewster, that he does not contemplate the immediate publication of this long-desired work.
[page] 343
in this comparison, the circumstance that the term "elasticity" is here applied to two different classes of phænomena, referable to different principles;—the acoustical phænomena depending on the elasticity of the parts of the solid, the optical on that of the optical æther. Savart found that some of the acoustical differences of elasticity correspond to the optical relations, but also that there are other acoustical differences following a different law of symmetry. In a common crystal of quartz, a transverse plate (perpendicular to the axis,) has the elasticity of all its diametral lines equal; but though all the plates cut parallel to the axis have the same optical properties, their acoustical properties have a relation to the edges of the prism; such, however, that any three plates at angles of 120° have equal acoustical elasticity; he found also, that by the acoustical properties he could determine the cleavage planes of quartz; he made like observations on calc spar, and some of equal interest on metals. These, it appears from his researches, have a structure neither regularly crystallized nor altogether uncrystalline; their properties are different in different directions, and they give, by their vibrations, corresponding differences of note, amounting to a tone; yet, as it is found that parts taken from the whole have not properties identical with those of the whole, the composition is not a repetition of that of small parts, as in regularly crystallized bodies. It is extremely interesting to see the sciences of colours and of sound thus uniting to give us that information indirectly concerning the internal structure of minerals, which we have so long attempted in vain to obtain directly.
4. Chemical Mineralogy.
In entering upon the Chemistry of Mineralogy, we come to that part of the subject in which undoubtedly the greatest labour has been employed and the least progress made. That this is not too unfavourable a judgement will be clear, I conceive, when it is considered how numerous and operose have been the analyses of mineral substances executed by all the best chemists during the last century, and yet how scanty and unconnected our knowledge on this subject still is. Not only are there no general and generally recognised chemical laws, capable of being predicated concerning extensive classes of mineral bodies, but the constitution of any particular mineral species, with the exception of a few, is a matter of doubt and dispute; and we shall hardly be contradicted when we say that there are very many cases in which, if we were to state to the chemist the ingredients of a substance and their quantities, he would be unable to tell us what mineral species the substance was.
[page] 344
We can hardly flatter ourselves, therefore, that we are at present close upon the discovery of the nature of the connexion between chemical constitution and mineral character. In the mean time, the chemists have been far from idle in the only road which, under such circumstances, offers itself; and a number of excellent analyses of particular substances have been perpetually accumulating. Still, such labour is naturally and inevitably pursued with less energy and connexion than would probably show themselves, if the analysers were tempted or rewarded by the prospect of some general law to be extended or verified,—some anomalous cases to be included in a well-established analogy.
Perhaps, however, such prospects are already opening. Mitscherlich's Law of Isomorphism, published about twelve y ers ago, promises far more fairly than any previous portion of chemical knowledge, to relieve chemical mineralogy from its stationary and helpless condition. According to this law, the ingredients of a given species of minerals are not absolutely fixed as to their kind and quantity; but one ingredient may be replaced by an equivalent (not necessarily an equal) portion of some similar ingredient,—generally some elementary body in the same degree of oxidation. Thus in amphibole, or in pyroxene, the lime may be in part replaced by portions of protoxide of iron, or of manganese, while the form of the crystal and the angle of its cleavage planes remain the same;—or in such cases the angles may vary slightly, while the other properties remain so far unchanged as to establish a strong mineralogical connexion in the group thus related: so the different kinds of felspar vary only by the substitution of one alkali for another; and the carbonates of lime, magnesia, protoxide of iron, protoxide of manganese, and their mixtures, agree in many respects of form, &c., while the angle varies through one or two degrees only. Several other such groups might be mentioned; as garnet; olivine; the carbonates of baryta, strontia, lead, and lime (arragonite); the sulphates of the same bases; the sulphates of iron and of cobalt;—again, the sulphates of zinc, nickel, magnesia; various phosphates and arseniates; and several other compounds.
It is obvious from the mere enunciation of such propositions that we have here chemical laws of a more general and scientific character, than any that can be founded on the analysis of a single specimen of each species. And as this principle is of the greatest importance to the mineralogist, it is interesting to observe that the mineralogist was the first person to perceive the necessity of such a principle. Thus Breithaupt, writing in 1818 (Auswahl der Dresd. Gesellsch. vol. ii. p. 142;) considers
[page] 345
tourmaline as containing potassa and soda along with lime and magnesia; these alkaline substances taking each other's places, so that there may be less of the one in proportion as there is more of the other. And he observes that Fuchs calls such elements "vicarious" with respect to each other,—a term since used, and with propriety, to designate the relations of isomorphous ingredients.
I do not know whether all our English chemists fully acknowledge the reality of the isomorphous groups of minerals; but those who do, will probably agree, that one of the most important objects which the chemist or the mineralogist can at present propose to himself, is to extend such grouping to as many minerals as possible. We have at present a mere mob of species; by brigading them under a system of isomorphism, they may become a well-ordered army.
It cannot be denied that there is something formidable in the prospect of the labour which is thus found to be incumbent upon those who would learn the constitution and relations of mineral species. The exact analysis of one or two specimens of each species has been considered, and justly, as a business requiring no small skill and sagacity, and great care and sacrifice of time. Even the most patient and most industrious of chemists, Berzelius and the Germans, complain of the employment on this ground. But it appears from the isomorphous doctrine, that we cannot hope to understand the chemical constitution of any mineralogical species or group, without subjecting to careful analysis not one or two specimens only, but many, from different localities and forming different varieties. It is only thus that we can obtain the character, common to the whole group, which may be taken as its type or formula.
In expressing the constitution of bodies, many chemists have found it necessary to call in the aid of notation; and the algebraical system introduced by Berzelius is now pretty generally diffused, though modified in parts, by some of his followers, as, for instance, Beudant. Such a notation is convenient, I conceive, in other parts of chemistry; but it is indispensable in mineralogy, where the composition of bodies is often much too complex to be intelligibly expressed by the resources of the language of modern chemistry. The doctrine of isomorphism gives us an additional reason for the employment of such a notation; for the constitution of an isomorphous group can be most conveniently expressed by means of a formula in which one of the letters is subject to be replaced by others indicating the vicarious ingredients.
I say nothing here of the merits or defects of different systems of chemical notation; for though I cannot but think it
[page] 346
unfortunate that algebraical symbols should be used in a manner contradictory of the first principles of algebra, as is the case in the notation of Berzelius, it is probable that the general acceptation of a notation of any kind will be mainly influenced by the amount and value of the information which it is employed to convey; and in this respect the Swedish system possesses an advantage in which it cannot easily or soon be matched by any rival system.
In speaking of the connexion of chemistry and mineralogy, I ought to mention the general law announced by M. Kupffer. This law professes to give the dependence of the crystalline form on the specific gravity and atomic weight of the body, and thus, if well established, would be a principle of a very high and comprehensive character in our science. I do not think, however, that any one who examines M. Kupffer's Memoir critically, will be satisfied with the kind and quantity of facts from which this induction is held to be collected. The selection of substances belonging to one particular system of crystallization, (for instance, the rhombohedral,) for comparison with each other, seems to be quite an arbitrary step, and is in no way explained by the law so asserted. But not to insist on this objection, the mode in which the dimensions of the primary form are compared with the other quantities is such as would enable the author to prove almost any law with equal facility; for he holds it to be an unimportant matter whether he takes what is usually considered the primary form, the primary rhombohedron for instance, or any other rhombohedron which can be derived from it. Thus in comparing calc spar with rhombohedral iron oxide, he takes the number expressing the axis of the primary form of the one substance, but in the other substance he multiplies the axis by four, thus substituting for the primary rhombohedron that which arises from truncating its edges. And it is by using a similar license in other cases that he exhibits an approximate verification of the formula which he states. There appears to be little hope of any valuable result to be obtained by comparison of numerical results, except the properties which the numbers express be clearly the same property in the different cases which are compared. If the cleavage rhombohedron of one case is analogous to anything, it must be analogous to the cleavage rhombohedron in the other.
Still it is impossible for any one to take an interest in this portion of science, without seeing that the connexion of chemical composition with crystalline form is one of the great problems to be solved; and it is very natural that those who feel this should be tempted to hazard a guess concerning the solu-
[page] 347
tion of this problem. Thus in a Memoir which appears in the Transactions of the Wernerian Society of Dresden for 1818, Breithaupt conjectures that boron is the ingredient which gives the electrical and crystalline polarity which he attributes to boracite, tourmaline, anatase, and axinite. Hitherto, however, conjectures and researches on this subject have had little success. In the course of last year, Magnus endeavoured to detect the chemical difference of garnet, a tessular, and idocrase a pyramidal substance; yet after many analyses and conjectures he was obliged to acknowledge that it had escaped him. Berzelius (Brewster's Journal, N.S. iii. 188.) finds no difference between the composition of hexahedral and prismatic iron pyrites. More recently still, Ampech has analysed a number of tessular minerals, spinelle, pleonaste, gahnite, franklinite, and chromic iron oxide: and in this instance he seems to have had some success in giving a common type to their chemical formulæ, as there is a common type in their crystallization. According to him, they consist of ingredients of two classes: the one class containing alumina, protoxide of chrome, peroxide of iron or manganese; the other class containing protoxide of zinc or iron or magnesia; and the rule of composition is, that the ingredient of the former class contains three times as much oxygen as that of the latter. If this law be true, we cannot doubt that many similar laws exist both for tessular and for other forms, and we may hope that after one has been detected others will soon appear.
The discovery of artificial crystals in the slags of furnaces was not unimportant to the chemistry of mineralogy. One of the first and most extraordinary instances was the detection of perfect crystals of titanium in the Welsh iron slag, by Dr. Wollaston and Professor Buckland. It has appeared by examination that these accidental products are more free from any admixture of iron than it is easy to obtain titanium by the ordinary chemical processes. In 1825 Mitscherlich found in the Swedish furnaces bisilicate of iron (pyroxene), mica, and other mineral species. About the same time, Berthier in France obtained in the furnace, by direct synthesis, regulated by the atomic theory, crystals similar to those found in nature. Professor Miller of Cambridge has examined several slags from the furnaces in Wales, and it appears that the crystals in those assume the form of olivine. It is satisfactory thus to find that the same substances affect the same crystalline form in our furnaces and laboratories, and in the great laboratory of nature. Indeed nothing can be more likely to help us in obtaining a knowledge of the chemical laws of crystalline forms, than to have the power of verifying our conclusions synthetically by forming crystals, as well as analytically by destroying them.
[page] 348
In the same point of view, the examination of crystals formed from solutions is of great value to mineralogy; as, for instance, the many excellent measures of artificial salts by Mr. Brooke, Mr. Haidinger, and others. Such crystals may often be obtained in much greater abundance and perfection than natural crystals, and especially than natural crystals of similar chemical composition; and thus they widen very much the field of facts to which our inquiries lead. In former times the mineralogist was professedly restricted to substances which occur in nature; but we may venture to say that a line so arbitrary and accidental cannot be the true boundary of the science. Where-ever crystalline forces act, the crystallographer is called upon to pursue his speculations: these speculations, whether we call them mineralogical or not, are such as give interest and promise to our study. In this point of view mineralogy possesses not only the importance which belongs to its ancient subjects, but also an importance of another kind, which belongs to it as a necessary supplement to chemistry; for it takes into consideration those physical characters of chemical compounds (crystallization, specific gravity, hardness, fracture, lustre,) which belong to them as solid bodies, and which indicate the law and intensity of the corpuscular forces by which each combination is bound together. The study of artificial crystals, therefore, whether obtained in the wet or in the dry way, may be recommended as very useful to the mineralogist.
Haldat (Ann. de Chim. Jan. 1831,) has shown a mode of obtaining artificial crystals of iron oxide by the decomposition of water; and these resemble the natural crystals of "fer oligiste" from Elba. So Becquerel has obtained the oxides of copper, lead, zinc. But by far the most valuable and important of such experiments appear to be those of M. Becquerel on the sulphurets, iodurets, and bromurets of metals, which he has obtained by artificial chemical action in a perfectly crystalline form. The agency which he employs is very weak galvanic tension; and he has succeeded thus in producing sulphuret of silver in small octohedral crystals resembling the native mineral, and sulphuret of copper, also closely resembling the native sulphuret. The sulphurets of zinc and iron require additional precautions, but are also obtained like to the native species; and iodurets, bromurets, and seleniurets of various metals are procured as crystals by similar processes. (Ann. de Chim. Oct. 1829.) These important steps in synthesis will probably throw a new light upon known analytical results.
M. Beudant has made a number of interesting experiments on the subject of another class of causes which modify the forms of crystals, and of which the general laws are, if pos-
[page] 349
sible, more unknown and obscure than those which determine different fundamental forms to different compounds. He has examined the circumstances which determine the various modifications which a given fundamental form undergoes; and has proceeded so far as to be able to produce at will one or other of certain possible modifications. Thus (Mineralogie, i. 190.) common salt crystallizing in pure water affected almost always the cubical form; if it crystallized in a solution of boracic acid, it assumed the form of the cube with truncated angles. Alum in nitric acid had the same form; in muriatic acid it was a figure of twenty sides, the octohedron and dodecahedron combined, the faces of the former being much the larger. An addition of alumine to the liquor, produced, in addition to the former faces, those of the cube; in pure water this salt is the simple octohedron. Sulphate of iron has commonly a simple form; by adding a few drops of sulphuric acid, more complex forms are obtained; and this rule respecting the effect of the addition of acid appears to be extensively true. The sulphate of iron mixed with sulphate of copper has its simple form, an oblique rhombic prism: the mixture of sulphate of nickel produced the same effect, but that of zinc an opposite one, the crystals becoming less simple. It has long been known that common salt mixed with urea, affects the octohedron instead of its usual form, the cube; and that in similar circumstances, sal ammoniac becomes the cube instead of the octohedron. Alum in a concentrated solution of alumine assumes the cubical form; an octahedral crystal of alum placed in such a solution soon assumes a cubical form; by being placed again in a solution adapted to give octohedral crystals it may be made to assume the octohedron. It is impossible not to be tempted to refer phænomena similar to hese, occurring, as they so often do, in natural crystals, to similar circumstances which have prevailed when the crystals have been forming.
Several statements of a curious kind have been made concerning the recent crystallization of substances which we cannot cause to crystallize in our laboratories. Thus (Brewster's Journal, vol. x.) Repetti observed quartz in a pasty state and in the act of crystallizing. The same kind of occurrence is said to have been observed of various other substances, as beryl, opal, heavy spar.
The chemical changes to which minerals are subject have been well described by Haidinger, (Brewster's Journal, vol. x.) who applies the name of parasitic minerals to those which retain the form of a substance while the substance has undergone a change, particle by particle. This change is of various kinds; weaker
[page] 350
affinities give way to stronger; carbonates are changed into sulphates; metallic substances are oxidized; copper is replaced by iron, &c.
I have not spoken of improvement in the methods of analysing minerals, of which many made in recent times might easily be enumerated; since these processes-seem rather to belong to the history of chemistry. Nor have I attempted to give the results of analyses of particular minerals; for these, though valuable materials of mineralogical knowledge, cannot be introduced into a general view like the present one, till they have been connected by some principle of dependence or relation.
5. Classification.
I. Distinction of species.—It will probably give to common hearers and readers a strong impression of the confusion still prevailing in the science of mineralogy, when we state that it is still a matter of dispute what are the limiting conditions and definition of Species in general; and that very wide and numerous differences of opinion prevail as to the identity and diversity of species in particular cases. Indeed it would be almost difficult to mention a species which is free from such doubts. This uncertainty is however not so fatal to real science as might at first sight appear. The formation of definitions, and the establishment of unerring distinctions, are among the last, and not the first, steps of systematic knowledge.
Haüy's definition of a mineral species, "the same ingredients combined in the same form," acquired a kind of celebrity at the time, and it has been adopted by many succeeding mineralogists. The definition which seems to be recognised in the crystallometrical school of more modern times is, "the same primary form with the same fundamental angles of cleavage, combined with an approximate identity of chemical and physical characters." But both these definitions were announced as axioms when they should have been tried as guesses. It was impossible to know, independently of experience, that the sensible differences of minerals corresponded universally to determinate differences in their ingredients. It is now certain that they do not: for, without calling in the doctrine of isomorphism, we know that scarcely two analyses of minerals of the same kind give identical results; so that the Haüyian definition of species is inapplicable without some reformation of its terms, and to make it unexceptionable will be found no easy task. In like manner it was imposible to know, independently of experience, whether minerals which resembled each other so as to have no constant
[page] 351
distinction to the eye of the common observer, would coincide exactly in their angles when tried by the severe test of the goniometer. Whether they do so or not, may be considered as a question still under decision. We know that we have, in some instances, groups in which the angle varies slightly in correspondence with certain variations of the physical properties and ingredients. And the question is, whether this is a variation per saltum,—so that the carbonate of lime has one invariable angle, that of iron and lime another, that of iron a third, and so on;— or whether it is a variation by insensible degrees, the angle passing from one magnitude to another by gradations corresponding to the minutest gradations in the proportions of the ingredients. Of these opposite opinions Mr. Brooke maintains the former, M. Beudant the latter. The most exact and multiplied observation alone can decide the point.
It may be observed that the groups of which we have already spoken as isomorphous groups, are not, in all cases, such according to the etymological sense of the term; for the forms and angles of the members of these groups are near to each other, but not equal. They have hence been termed plesiomorphous groups by Professor Miller, who has examined some of them; and as the majority of the so-called isomorphous groups are certainly of this character, it may perhaps be questioned when there are any groups strictly isomorphous (those belonging to the tessular system being of course excepted in all such assertions). Thus Rose has shown that several varieties of minerals, crystallizing like pyroxene, agree in composition by the vicarious substitution of one mineral for another; and Bonsdorff has shown the same thing for amphibole. But I am not aware that in these instances the exact identity of the angles of the crystals compared was ascertained by means of the goniometer, so that slight differences in the angles of the different kinds of pyroxene or of amphibole which were compared, may still have existed, as we know that such differences do exist in felspar.
M. Breithaupt has carried still further this scepticism concerning the constancy of crystalline angles. He finds by measurement that crystals of calc spar, apparently equally pure, from different localities, vary in their angles to the amount of half a degree. What is a still more unexpected blow to the fixity of these angles, he finds (1829,) that corresponding, or, as a geometer might call them, homologous angles of the same crystal, which ought to be precisely equal by the law of symmetry, are perceptibly different under the goniometer. Thus two pairs of opposite planes of the square pyramid of anatase were found
[page] 352
to differ in their inclination no less than 3°. Similar irregularity was found to exist in the crystallization of idocrase, and of tetragonal copper pyrites; and at last, as he says, it seemed to be time to satisfy himself that there really was such a thing as a regular square pyramid in nature. This he fortunately discovered in a perfect crystal of zircon. In attempting to systematize these anomalies he has been led to introduce a number of new terms and laws, which perhaps may be less necessary when we have a fuller view of the facts of these and similar cases.
All this unexpected uncertainty shows us how assiduously we ought to measure and compare crystals, if we wish to bring mineralogy into the form of a science either certain or systematical. The invention of the reflecting goniometer by Dr. Wollaston, was an invaluable gift to the crystallometer; and every step of our progress makes us more sensible of the importance of this elegant and well-devised instrument. But unless we can acquire some knowledge of the laws of constancy and of change in the angles which we measure, this instrument is valuable only as an ingenious means for an undiscovered purpose, a precise expression in an unknown tongue.
Perhaps we cannot deny that at present we have still to learn the true place of the isomorphous or plesiomorphous groups, and that we are ignorant on what step of the ladder of classification they ought to stand;—whether they correspond to species, or to some higher division;—and whether they contain, within the groups themselves, a further definite subdivision into subordinate members.
The confusion and perplexity in which this branch of the subject is still involved, may be judged of in some measure from the fact, that in the course of last year Gustavus Rose published a very interesting memoir "On the necessity of uniting Augite and Hornblende into one species"; thus throwing a doubt on the distinction of two minerals which had hitherto been supposed to be as clearly separated by their form and physical properties as any two species composed of similar ingredients. His grounds for maintaining their identity are not slight;—they are, the possibility of reducing the one form to the other, the resemblance of their chemical composition, but especially the mode of their occurrence in combination, and the fact that melted hornblende crystallizes as augite.
Another indication of the same kind may be found in Mr. Kobell's memoir on Diallage and Hypersthene. He conceives that these minerals may be traced to an agreement with pyroxene, both as to their crystallometrical and their chemical pro-
[page] 353
perties. Both consist of bisilicates of a mixed base, the ingredients of the mixture being lime, magnesia, protoxide of iron and of manganese; but in the case of pyroxene the lime is equal to the sum of all the other ingredients of the base; in diallage the magnesia takes this predominant place; and in hypersthene, or bronzite, an intermediate composition prevails.
Nor does it appear that the optical properties of minerals, valuable as they are, can help us out of this uncertainty. It may hereafter be found that the distinctions marked by such properties are more clear and constant than any others. But this is at present uncertain, and there exists a possibility, as yet not disproved, that a very minute change of composition may affect the optical properties much more than it affects any others. However this be, it is clear that the optical distinctions cannot take the place of the familiar divisions which the mineralogist is accustomed to use. The kinds of Apophyllite which have been termed Tesselite and Leucocyclite, in consequence of the curious optical phænomena which they exhibited to Sir D. Brewster and to Sir J. Herschel, do not appear to be distinguishable by the eye, or by the common tests of chemistry, from other kinds of the same mineral. Sir J. Herschel found in a single fragment of a crystal of this substance,three portions, each possessing distinct and peculiar properties. Sir David Brewster's tessellites had, in like manner, a difference of optical structure in different points. It is clear that species, discriminated by such differences, cannot easily be employed in classifying mineral specimens; and it is at present difficult to foresee the place which these differences, when they are more fully known, will occupy in our mineral systems.
The angle between the axes of topaz from different localities, is also said to vary very considerably. But one of the kinds of minerals in which this perplexity appears to be greatest, is the micas. Substances which have been referred to this species (or group, of whatever kind it be,) have been found (Beud. ii. 149,) to have, some of them a single attractive, some a single repulsive axis: others (the more common kinds,) have two axes, and the angle between the resulting optical axes has been found to vary from 50° to 76°; and though the chemical analyses of these different kinds of topaz have given results sufficiently variable, it does not appear that any steady connexion between the composition and the optical properties has yet been discovered. Sir D. Brewster (Journal, 1825, ii. 205,) has shown that a particular kind of mica which he examined (lithion mica), contained a combination of both uniaxal and biaxal crystals.
z
[page] 354
Among the properties which have been proposed as bonds of connexion between the members of plesiomorphous groups, may be mentioned Naumann's formulæ expressing certain simple algebraical relations between the dimensions of the different axes; thus the right prismatic primary forms of carbonates of baryta, strontia, &c. have all the relation 2 a = b + c, though the magnitudes of a, b, c somewhat vary.
II. Systems of classification.—While the lowest member of our mineralogical classification, the Species, is in this state of uncertainty and confusion, it cannot be surprising that the superior departments should be not yet satisfactorily adjusted. The fact has been, that in England the imperfection and inutility of the systems commonly put forwards have been so obvious, that a general impression has established itself among our mineralogists, that a system is a useless source of perplexity, and that any system, however arbitrary, is nearly as good as any other. On the Continent, however, the case is widely different; and an extraordinary number of mineralogical systems, published of late years in Germany, Sweden and France, show how earnestly foreign philosophers have struggled with the difficulties of the problem. Much indeed of the past failures and present apathy of our countrymen on this subject, is to be attributed to their having undertaken the task somewhat hastily and lightly. If any one endeavours to construct a complete classification on that which is perhaps the most obvious principle, the leading ingredient, he will soon be led into endless inconsistencies, and will obtain none of the great advantages which a good system will certainly afford. It might easily be shown by examples that this is what has occurred; and any attempts to patch up the manifest defects of such undertakings would probably lead us, after various wanderings and struggles, to the point at which the Swedish, German, and French chemists have already arrived.
There has taken place in Germany a somewhat vehement dispute respecting the merits of two apparently opposite methods of classification;—that which proceeds by external characters, and that which depends on chemical composition.
In Werner's system, chemical differences were recognised as the great leading divisions; but the subordinate distinctions were established by means of that nice discrimination of external characters which Werner so successfully applied and taught. It was, I believe, with Professor Mohs, the successor of Werner at Freiberg, that the design originated of demonstrating the possibility and the propriety of founding our mineralogical classification on external resemblances and differences alone, borrowing nothing from chemistry; so that mineralogy
[page] 355
should in this respect be brought into agreement with the other provinces of natural history. The general analogy of natural history supplied one motive to this attempt, but another arose from the conviction that we cannot compare the chemical and physical characters of minerals, without first studying them independently. The chemist cannot assert that arragonite does or does not contain strontia, except the mineralogist can tell him what is or is not arragonite.
To the execution of this very remarkable undertaking Professor Mohs brought a consummate acquaintance with the mineral kingdom. The plan of his task was regulated in a great measure by the analogy of the science of botany, in which system has been so successful; and the publication in which he first gave to the world the results of his labour was his "Characteristik," (Dresden, 1820,) a work of not many pages, but one which excited a very extraordinary interest in Germany.
The "characteristik" in any branch of natural history is a portion of the science distinguished from the "systematik," in-asmuch as the latter arranges individuals and species into their classes by a consideration of all their properties; while the former selects certain marks by which we may easily recognise in each instance the class in which any given individual has been placed. Thus the varieties and the species of the genus Lamium in Botany are placed together because of their general affinities; but in the "characteristik" of the science, the genus is distinguished from other flowers of similar form by a small tooth in the outline of the corolla on each side; and the species Lamium album (White Dead-nettle,) is further distinguished by having the tube of the calyx shorter than these teeth.
To devise characters of this kind which should mark a series of successive and subordinate distinctions in the mineral kingdom, was a very curious and difficult task, and was executed with no small skill. We cannot here go into any detail on this subject; but as examples of the method we may take the characteristics of the order Pyrites, the genus Copper Pyrites, and the species Octohedral Copper Pyrites*.
Immediately after the publication of the "Characteristik"
* X. Order Pyrites.
Metallic.
H = 3·0 to 6·5.
G = 4·1 to 7·7.
If H ss 4·5 and less, G is under 5·3.
If G = 5·3 and lew, colour is yellow or red.
V. Genus Copper Pyrites.
Tessular or Pyramidal.
Colour brass-yellow, copper-red.
H = 3·0 to 4·0.
G =4·1 to 5·1.
I. Species Octohedral Copper Pyrites.
Tessular.
Cleavage, octohedron very indistinct.
Colour copper-red.
H = 3·0; G=4·9 to 5·1.
Z 2 V. Genus
[page] 356
of Mohs, in 1820, appeared also a "Characteristik" by another excellent mineralogist, likewise a pupil of Werner,—Breithaupt. Whether this coincidence is to be looked upon as an indication of the general tendency of thought in the school of Freiberg, and how far it resulted from any more direct communication, it is not necessary here to determine. Since that time Professor Mohs has removed to Vienna, to superintend the Imperial Cabinet, and Professor Breithaupt has succeeded him at Freiberg; so that the "natural history method" is now taught to no small or insignificant portion of the mineralogical students of Germany; and the translation of Professor Mohs's Treatise by Mr. Haidinger (1825), enriched as it is with much valuable additional mineralogical information, has done all that could be done for the diffusion of the system in this country,
Berzelius may be considered the head of the chemical classifiers, as Mohs is of the natural history classifiers. In 1816 he published his Essay to establish a purely Scientific System of Mineralogy by means of the Application of the Electro-chemical Theory and the Chemical Doctrine of Definite Proportions. In this Essay he proceeds upon the great principle of his school, that the relation of electro-positive and electro-negative is the foundation of all chemical relations. Of the strict truth of this principle chemists must decide; the application of it to Mineralogy was made with great consistency. Minerals were arranged into families according to their electro-positive element, and these families disposed according to the place of this element in the general electro-chemical scale. Thus there was a family for sulphur, another for azote, another for carbon; others for each of the electro-negative metals; other families for each of the electro-positive metals; others for each of the bases of earths and alkalies. Each of these families was subdivided according to the electro-negative elements. Thus, Copper had the subdivisions,—1. Pure Copper; 2. Sulphurets of Copper, of which there are nine or ten (including mixtures of
[page] 357
iron, lead, tin, bismuth); 3. Oxides of Copper; 4. Sulphates of Copper;—and so on.
Nothing could be more complete and symmetrical than such a system, if the object had been to arrange the products of the laboratory, or substances as definite and distinct as those. But it very soon appeared that the system contained in itself germs of inevitable confusion. Very many minerals are so complex and so imperfectly known as to their chemical composition, that it remained doubtful where they were to be placed; and the parts of the system which appeared to have equal claims to them were widely removed from each other. Nor did any approximation of substances apparently of the same kind, or any analogies and rules with regard to the association of chemical formulae, come into view by means of this classification, and thus give it the air of a successful conjecture.
The same may be said of the system of Brongniart, the successor of Haüy, which proceeded on the chemical principles then (1806) generally recognised, and of that of Leonhard; in both of which there may be observed what was considered as a blemish in the first system of Berzelius,—a struggle between the "scientific" or electro-chemical principle, and the ancient customary views which tended to place similar minerals together. Thus tellurium, like sulphur, is a mineralizing substance; and hence there is no more reason, on the electro-positive principle, for making a family for tellurium than for sulphur. Yet both Brongniart and Leonhard have made such a family, and have arranged the telluriurets in it, while the sulphurets stand each under its metal. This inconsistency is one of the marks of the unsatisfactory impressions produced by a rigid chemical system on such principles, and of the difficulty of adapting such a system to the mineral kingdom.
When in the due course of time the examination of the chemical difficulties of mineralogy had led to the doctrine of isomorphism (1821), the untenable nature of Berzelius's first system, and of all similar ones, became more obvious. With a candour and alacrity worthy of his elevated position in the world of science, Berzelius was himself one of the first to acknowledge the necessity of some change. In 1824 he published in the Transactions of the Academy of Sciences at Stockholm, a Memoir "On the Alterations in the Chemical Mineral System which necessarily follow from the property exhibited by Isomorphous Bodies, of replacing each other in given proportions." In this Memoir he gives a classification of mineral substances according to their electro-negative element; explaining that the fact that one isomorphous electro-positive element may take
[page] 358
the place of another with no definite change of external character, renders an arrangement, such as he had before proposed, impossible in practice. Thus, in this new system one of the families is sulphur, and under this are arranged the sulphurets of zinc, of iron, of cobalt, of nickel, of copper, &c He acknowledges, however, that by this means the difficulty arising from isomorphism is only diminished and not removed: for Mitscherlich had found that electro-negative as well as electropositive elements may replace each other isomorphously;—for instance, arsenic and phosphoric acid.
In 1824 Beudant also published his Traité Elementaire, which professes to found its arrangement on two leading max ims: 1. The electro-negative element imparts its character to the combination more frequently than the electro-positive one, and hence is rather to be taken as the principle of classification; 2. The electro-negative elements are to be arranged in a circular series according to their natural relations. The circular series thus adopted, is founded upon certain views of Mr. Ampere, according to which elementary substances are divided into three great classes, termed, 1°,Gazolytes; 2°, Leucolytes; 3°, Croicolytes; according as they form gases, uncoloured solutions, or coloured solutions. In this system also the form of the chemical symbol of composition is taken into the account. Thus under the family of sulphurets we have (a) simple sulphurets, (b) double or multiple sulphurets.
The doctrine of isomorphism, however imperfectly developed at present, undoubtedly promises much fairer to disclose to us the true chemical relations of minerals than the views previously entertained. It was probably in consequence of this promise that an extraordinary number of new systems of mineralogical classifications on chemical principles were published about the period of which we are speaking. Gmelin (1825) was the author of one which appeared about the same time as that of Berzelius, and like that founded its leading distinctions on the electro-negative or formative element of bodies; but besides this, it took into account the numbers which occur in the chemical formula, distinguishing, for instance, simple, double, and triple silicates. The method of Nordenskiöld (1827) has a similar groundplan, but proceeds still more decidedly upon the proportions of the number of atoms of the different elements; and thus, as Berzelius observes, (Jahr Bericht, viii. 188.) presupposes a complete knowledge of the chemical composition of each species.
Bonsdorff's Essay on this subject was printed at Abo in Finland, in 1827; but its publication was prevented, the im-
[page] 359
pression being destroyed in the calamitous conflagration of that city (Sept. 1827). Its plan was in some respects similar to the, preceding ones; but it made the number of ingredients, and not that of the atoms, its basis. Keferstein also published a mineral system in the fourth volume of his Geognostiches Textschland; he has imitated Beudant, who formed his system upon Ampere's circular arrangement of simple bodies, having, constructed for himself another circular arrangement of eight members, on which his method is grounded.
Notwithstanding the confusion which may naturally be expected to arise from the conflict of so many different plans of arrangement, we may see, I think, a tendency in the chemical and mineralogical methods to approach towards each other. It has, been now proved that neither course can by itself lead to a satisfactory classification. Those who wished to arrange by external characters alone, trusted much to the acuteness of their senses, and believed that they could, by a sort of instinct, make out which of the perceptible properties of substances were most important. The chemists, on the other hand, deeming that their knowledge of the constitution of a mineral must be sufficient to determine its nature and place, did not consider that we require observation to teach us in what mode such knowledge is to be applied;—for what but the comparison of external characters can teach us, for instance, that tellurium, sulphur, selenium, arsenic, discharge similar functions in the composition of minerals, and must be similarly employed in our classification?
It will probably be allowed that a system of arrangement proceeding on strictly chemical principles, which should bring together in all cases the substances which most resemble each other in external properties, would satisfy the requisitions of the science, and that nothing short of this would do so. Such a system is not at present within our reach; but it will perhaps be useful to look upon all methods of classification now proposed, as attempts to approximate to such a perfect system, whether they be founded upon external characters or on chemical principles. Our knowledge of neither of these branches of the subject is as yet complete enough to lead us to expect from it a system which shall be exact according to both; but we may be held to have made some progress in the requisite series of trials and conjectures, when we have constructed any chemical classes which consist of substances of similar character and properties.
That some progress of this kind has already been made cannot be denied. The new system of Berzelius, or that of Beudant, or indeed any of the new chemical systems, would
[page] 360
produce a grouping of substances which would at once be recognised as far more natural than that of Haüy or Phillips.
The new system of Berzelius has been adopted in the arrangement of the minerals of the British Museum in their new apartment, under the intelligent and industrious superintendence of Mr. König; and every one will probably be struck by the evidence which the aspect of the collection offers, of the advantage of this over the ancient mode of arrangement.
Besides the natural historical and the purely chemical, we may observe that there are some which may be called mixed systems of classification. These, proceeding as if our knowledge were as yet too incomplete to allow us to apply any one principle with logical severity, borrow their resources from various quarters, and may thus perhaps make the nearest approximation to their object which is now possible.
One of the best of such systems appears to be that of M. Naumann, of whose crystallographical labours we have already spoken. His Mineralogy (Berlin 1828,) contains, in a very comressed form, very full and systematic accounts of the different kinds of minerals, their properties and crystalline forms; and the species are there arranged in classes and orders, which bear chemical titles, and which bring together similar bodies. Thus the Silicides are— Unmetallic Hydrous Silicides (the zeolites),Unmetallic Anhydrous Silicides (felspar, &c),Mixed (of metallic and unmetallic)Anhydrous Silicides (pyroxene, amphibole, &c.),Metallic Hydrous Silicides (dioptase, silicate of zinc, &c.); and so on. In the sulphurides Naumann retains the distinction of pyrites, glance, and blende, though its chemical signification has not yet been discovered; and this seems to be done not without reason, for the difference of octohedral copper pyrites (cuivre pyriteux, =2 (2 cu +s) + fe + s), and rhombic copper glance (cuivre sulfuré, =2 cu + s,) is of the broadest kind: the sulphuret of zinc (zinc blende), and the sulphuret of iron (iron pyrites) have scarcely any resemblance.
I may also notice the work of M. Kobell, who has published a "Characteristic (Nürnberg 1830,) founded on mixed physical and chemical characters, as those of Mohs and Breithaupt were on physical alone. M. Kobell has however, in some parts of his classification, returned to the arrangement by the electro-positive element, which appears to be a retrograde step on our road to a permanent system. It may be mentioned here that the Mineralogy of M. Beudant contains an excellent chemical "characteristic," that is, certain and universal formularies of tests for determining the place of a given mineral in his arrangement. This part of the work has been adopted by Naumann.
[page] 361
III. Nomexclature.—Some of the authors of mineralogical classifications have endeavoured to introduce a systematic nomenclature into the science. Mr. Mohs in particular has given a series of names almost wholly new to the species of the mineral kingdom. There is no doubt that mineralogical nomenclature has long been in such a state of perplexity and disorder, so defective in all valuable qualities, and so overloaded with useless synonyms, that any reformation of it would be a most important service. It appears reasonable to suppose also, that the introduction of a right and consistent classification ought to be attended in Mineralogy, as it was in Botany, with the introduction of a reformed and simplified language. But we may well doubt whether we have yet reached the point at which such a systematic reform is possible. Our genera and orders are probably too unstable to be made the basis of permanent names. Some of the groups of species, indeed, are pretty well characterized, and have in some degree influenced the common names; thus,Iceland spar, or calc spar, fluor spar, heavy spar, iron spar, seem, by these names, to be referred to a natural order Spar. In like manner native gold, native platinum are connected by the form of their names, as they are by the simplicity of their constitution. The oxides, as red copper oxide, red iron oxide, are a chemical order which can generally be recognised by their appearance, though at one extremity the metallic silicides approach near to them. The orders Pyrites, Glance, Blende have been already noticed. Names referring to such groups as these seem more likely to be permanent than any others, though it must be acknowledged that such groups are often vague; nor can we at present draw their boundary lines. In forming the names of species, the crystallization seems to give one of the best, because the most certain and definite, grounds of nomenclature:hexahedral iron pyrites, rhombic iron pyrites, hexagonal iron pyrites are names which admit of no confusion.
The disorder of our mineralogical nomenclature has been much increased by the facility with which new species have been assumed, and new names applied to them. The rebuke of Berzelius (J. B. vii. 180,) has not been uncalled for. "The mineralogists par excellence," says he, "that is, they who do not trouble themselves about the internal nature of minerals, appear to hold new names for an essential thing; for they hasten to give them before they can possibly know whether they have before them a combination already known or not." In another place (J. B. v. 197,) he appears to consider our countrymen as peculiarly given to offend in this respect. "It is the fashion in England," he says in 1824, "to seek new forms by crystal-
[page] 362
lometrical examination, to give a new name to each conjectural new mineral, generally the name of a living person, and then to give the mineral to Mr. Children to try with the blowpipe, if the pieces are sufficient."
The best method (as appears to me,) to control in some degree this inconvenient multiplication of names and species, will be to require that the name of the species should contain, besides the distinctive term, the name of the order to which it appears to belong (as Spar, Oxide, Pyrites), and an adjective designating the system or some peculiarity of the crystallization (as Hexahedraliron pyrites). The termination of the word, where it is a new one, might also be made to imply some distinction. This has been considered a matter of indifference. The termination ite has hitherto been most common: but ose, ine, and various others, appear to be coming into favour. Thus we have in Beudant's last edition (1830,) not only leadhillite, lanarkite, chamoisite, proustite; but also scolexerose, opsimose, argyrose, argyrythose, exanthalose, rhothalose; diacrase, panabase, neoplase; neoctese; rhodoise, stibiconise, crocoise, malaconise; marceline, wilhelmine, carbocerine, mysorine; exitele, and many more of the same kind; and these terminations are employed without any regard either to any etymological principle or to any difference in the minerals. Even where these names are not superfluous, or superfluously long, or superfluously learned, they are superfluously varied; and to make the variety depend on caprice alone, is to throw away a resource of which chemical nomenclature may teach us the value.
M. Beudant himself has pointed out the advantage which would result from retaining in the names of species a substantive marking the order to which the substance belongs (vol. i. 525); thus he would say silicate stilbite, silicate chabasie, silicate scolezite, &c.; carbonate calcaire, carbonate witherite; sulfate couperose, &c. Mr. Mohs had long before, in the nomenclature which he proposed, founded on characters altogether different, proceeded upon this as an indispensable condition of the specific designations.
6. Particular Discoveries and Researches.
Several of the labours of mineralogists which would naturally come under this head have already been referred to in speaking of the general views which they illustrate; and to speak of the examinations of particular minerals would lead us into too long an enumeration. Such inquiries are best conducted when the chemist and mineralogist are joint labourers, as in the ad-
[page] 363
mirabe examination of the ores of manganese by Dr. Turner and Mr. Haidinger. For similar reasons I shall not dwell on the researches which have been carried on with regard to other physical properties of minerals; as, for instance, Köhler's on their electricity, and Sir D. Brewster's on their pyro-electricity. The discovery of a new metal,vanadium, by Sefström, is closely connected with mineralogy, but will probably appear as part of the history of chemistry, and therefore need not here be dwelt upon.
In reviewing the account which has been given of the recent labours of mineralogists, it is impossible not to be struck with the small share which Englishmen have taken in all that relates to System in this science. With regard to optical researches, we have already mentioned that one person in our own country has done incomparably more than all the experimenters of the Continent together; and in the measurement of the angles of crystals, the goniometer, without which no measure would have any value, is the invention of an English philosopher; and Mr. Phillips and Mr. Brooke have contributed to the stock of crystallography, observations more numerous and exact, probably, than any other two names could rival. Yet in the adoption of new generalities we have been slow: the distinction of the crystalline systems is not commonly employed among us; the doctrines of isomorphism are contested by some and applied by few Englishmen*; and no attempt has recently been made, nor any interest excited, with regard to scientific views of the classification of minerals. This prosecution of details, and apathy or contempt with respect to methods, appears to be a part of the intellectual character of this country. Men here appear to feel no interest with regard to rules and systems till they are so complete, so clearly developed as to principle, their apparent difficulties so far explained, that the general rule will bear a strict application in each particular instance. They are disposed to despise the dim glimmerings of dawning principles, in cases where, though a connexion may be probable or certain,the asserted connexion is clearly not exact. Our countrymen thus often lose much of the pleasure and honour which belong to those who labour to unfold an obscure and imperfect truth: but yet, on this very account, their discoveries, when made, have a more positive character and a more original tone than they might otherwise possess. The step to which mineralogy at
* It will be seen by reference to the proceedings of the Chemical Section, that Dr. Turner, Mr. Brooke, and Professor Miller have undertaken to bring before the next meeting of the Association the result of experimental researches on this subject.
[page] 364
present owes the best portion of its scientific character, was made by an Englishman,—the doctrine of Definite Proportions: and if Englishmen seriously propose to themselves the task, we are justified by the history of Science in asserting that none are more likely than they to solve the great problem of mineralogy which now offers itself,—the connexion of chemical composition and crystalline form. Besides this great problem, it has appeared in the course of this Report, that various other questions of narrower extent remain to be decided by experiment. We will recapitulate a few of these.
1°. To determine the optical differences on which depend the distinctions of the different kinds of lustre,metallic, adamantine, vitreous, resinous, pearly.
2°. To determine whether the oblique rhombic prism is a real system, or is a hemihedral form of a right prism.
3°. To determine the limits of magnitude and simplicity in crystallometrical ratios.
4°. To determine whether chemical groups are strictly isomorphous, or only plesiomorphous,
5°. To determine whether the angles of plesiomorphous crystals are separated by definite or by indefinite steps.
6°. To determine what are the differences of chemical composition corresponding to differences of optical structure in resembling minerals, as apophyllite, tesselite, leucocyclite.
I will further add, that the formation of good collections of well-crystallized minerals, (in which should be included suites of artificial Crystals, both from fusion and solution,) and all arrangements which make such collections accessible to the working mineralogist, are circumstances highly important to the progress of mineralogical knowledge.
The determination of many of the above and similar very essential questions, must depend on observations made on crystals which are generally difficult to procure sufficiently perfect and transparent for such researches, there being often only a few known specimens in the world which would answer the purpose, and these having an enormous and fantastical money value affixed to them as rarities. I conceive, therefore, that all persons and Societies possessing splendid and beautiful minerals, if they are desirous that such possessions should be of use to the advancement of science, cannot in any other way have nearly so good a chance of furthering this object, as by placing these treasures at the disposal of the intelligent and skilful optical or chemical experimenter. While the unique crystal stands on its shelf unmeasured by the goniometer, unslit by the optical lapidary, unanalysed by the chemist,—it is merely a piece
[page] 365
of furniture, and has no more right to be considered as anything pertaining to science, than a curious china tea-cup on a chimney-piece:—given up to the mercy of the philosopher, there is no possibility of determining what valuable information it may not convey,—what grand series of truths it may not originate or establish.
Report on the Progress, Actual State, and Ulterior Prospects of Geological Science. By the Rev. W. D. CONYBEARE,F.R.S. V.P.G.S. Corr. Memb. Institute of France, &c. &c. &c.
IT cannot be necessary, before an assembly like the present, to expatiate on the interest of the science to which I have now to call your attention; a science which by investigating the traces indelibly impressed on the surface of our planet by the successive revolutions it has undergone, proposes to elucidate the history of these stupendous physical actions; and thus fully to develop what may be termed the archæology of the globe itself,—a science which associating itself to those branches of our knowledge which relate to organized nature, to zoology, and to botany, affords to each the important supplementary information of numerous species which have long vanished from the actual order of things;—thus unexpectedly extending our views of the various combinations of organic forms; and in many instances supplying links, otherwise wanting, in uniting the different terms of this series in a continuous and unbroken chain.
Nor, if from these higher views of scientific interest we advert to the more practical considerations of utilitarian importance, and applicability to those œconomical arts on which our national wealth and strength depend, can we think meanly of a science which guides us in the full development of our mineral resources; which, (to mention only a single instance,) in indicating the proper line in which researches for coal may offer the prospect of success, extends, facilitates, and œconomizes the supply of this article, the great element not only of domestic comfort, but of mechanical power.
In tracing the progressive development of this science, it might have been interesting, had the bounds necessarily prescribed by an occasion like the present permitted, to have commenced our examination with the records of classical antiquity. We might have noticed the apparent connexion of many of the
[page] 366
cosmogonical tenets of the philosophical schools with our subject, and indicated the allusions in the writings of historians, geographers, and naturalists, to some of the more striking phænomena of geology; those especially which, by the occurrence of marine remains in the midst of our continents, attest the displacement of the ocean from the regions which it must once have covered: even at this early period we should have seen volcanic agency referred to, as affording the most probable explanation of these striking facts, and the elevation by these forces of considerable tracts, such as that near Methone, pointed out as analogous cases.
On the revival of literature and science, we might have observed that Italy, the earliest and most active country in that bright career, by no means neglected this subject. Numerous and interesting are the anticipations of subsequent discoveries in this science, which may thence be gleaned. From the age of Boccaccio the subject had there received frequent attention, and long before a similar spirit had extended to other countries we find Steno in possession of many of the fundamental facts of geology,—a distinct recognition of various successive formations; of the dislocations and fractures of the strata; of the orderly distribution of organic remains, &c.: but to these subjects I can now only briefly allude; and I regret this the less as I have years ago submitted to the public a concise statement of these historical particulars, and the outline I then offered has been far more ably filled up by subsequent writers, especially by Mr. Lyell, to the early chapters of whose important work I would particularly refer those who may desire satisfactory information on this part of our subject.
I will only therefore allow this earlier period of the progress of geological science to detain us a very few moments while I point out the claims of one most distinguished philosopher, the universal Leibnitz, who honoured this branch of physical speculation by devoting to it a portion of his attention, and anticipated, with the prophetic sagacity of a powerful mind, its future progress, and the very methods of investigation which would most effectually contribute to its successful development. I am induced to pause on the consideration of the geological treatise of this most eminent writer (his Protogœa), because I am persuaded that its merits have been seldom sufficiently appreciated, and admit of being most prominently exhibited by being brought into immediate contact with the subsequent discoveries of our science.
In the 4th section of his Protogœa, Leibnitz presents us with a masterly sketch of his general views; and perhaps, even in
[page] 367
the present day, it would be difficult to lay down more clearly the fundamental positions which must be necessarily common to every theory, attributing geological phænomena in great measure to central igneous agency. He attributes the primary and fundamental rocks,—"id enim potissimum de prima tantum massa, ac terræ basi, accipio,"—to the refrigeration of the crust of this volcanic nucleus;—an assumption which well accords with the now almost universally admitted igneous origin of the fundamental granite, and with the structure of the primitive slates; for the insensible gradation of these formations appears to prove that gneiss must have undergone in a greater, and mica slate in a less degree the same action, of which the maximum intensity produced granite*. The dislocations and deranged position of the strata (phænomena for which he cites the writings of Steno,) he attributes to the breaking in of the vast vaults which the vesicular and cavernous structure assumed by masses during their refrigeration from a state of fusion, must necessarily have occasioned in the crust thus cooling down and consolidated. He assigns the weight of the materials, and the eruption of elastic vapours, as the concurrent causes of these disruptions;—"denique vel pondere materiæ vel erumpente spiritu fractâ fornice:" to which we should perhaps add, that the oscillations of the surface of the still fluid nucleus may, independently of any such cavities, have readily shattered into fragments the refrigerated portion of the crust; especially as, at this early period, it must have been necessarily very thin, and resembling chiefly the scoriae floating on a surface of lava just beginning to cool. He justly adds, that these disruptions of the crust, must, from the disturbances communicated to the incumbent waters, have been necessarily attended with diluvial action on the largest scale,—"maximæ secutæ inundationes." When these waters had subsequently, in the intervals of quiescence between these convulsions, deposited the materials first acquired by their force of attrition, these sediments formed, by their consolidation, various stony and earthy strata: —"Nec dubito postea materiam liquidam in superfìcie telluris procurrentem, quiete moxredditâ, ex ramentis subactis ingentem materiæ vim deposuisse, quorum aliæ varias terræ species formarunt, alia in saxa induruere, e quibus strata diversa sibi
* Whatever theory be entertained as to the origin of these rocks, a gradual transition of character from granite to gneiss and mica slate, assuredly exists; and it is foreign to our present purpose to pronounce on the more minute question, concerning their origin,—further than to observe, that if we admit the igneous origin of granite, this gradation of character appears to indicate a gradation of igneous action on gneiss and mica slate also.
[page] 368
superimposita diversas præcipitationum vices atque intervalla testantur" Thus, he observes, we may recognise a double origin of the rocky masses, the one by refrigeration from igneous fusion, (which, as we have seen, he considered principally to be assignable to the primary and fundamental rocks,) the other by concretion from aqueous solution:—"Unde jam duplex origo intelligitur primorum corporum, una, cum ab ignis fusione refrigescerent, altera, cum reconcrescerent ex solutione aquarum." We have here distinctly stated the great basis of every scientific classification of rock formations. By the repetition of similar causes (i. e. disruption of the crust and consequent inundations,) frequent alternations of new strata were produced, until at length, these causes having been reduced to a condition of quiescent equilibrium, a more permanent state of things emerged:—"Redeunte mox simili causa strata subinde alia aliis imponerentur, et facies teneri adhuc orbis sæpius novata est." Have we not here clearly indicated, the data on which what may be termed the chronological investigation of the series of geological phænomena must ever proceed? But I would particularly invite to the following clause the attention of those writers of the present day, who appear to assume it as an essential condition of their theories, that the same physical causes can never, under any former circumstances, have acted with more intense energy than they actually exert:—"Donec quiescentibus causis, atque æquilibratis, consistentior emergeret rerum status."
The beginning of the following section is very remarkable, as exhibiting a clear anticipation of the importance and of the prospects of the new science, of which he foresaw the dawn:— " Hæc de incunabulis nostri orbis semina continent scientiæ novæ quam Geographiam Naturalem appelles." Leibnitz proceeds even distinctly to indicate the line of future research into the geographical distribution and extension of the various formations, which might be expected to place this new science on a firm basis:—"Rectius tamen omnia definient posteri, ubi curiositas mortalium eo processerit, ut per regiones procurrentia soli genera et strata describant." And then, after making judicious remarks on the distinctions of general and local causes, he modestly and prudently adds, that before we are able to determine to which of these the phænomena we observe are to be attributed, we must wait patiently until the whole surface of the planet shall have been more accurately examined:—"sed quid privatts imputandum sit, aut publicis causis, facilius aliquando statuet poateritas, exploratâ melius humani generis sede." How much of the Wernerian doctrine of universal formations would not a proper attention to this caution have
[page] 369
spared us! Nor can we even yet pronounce that our knowledge of the general structure of the earth's surface is sufficiently advanced, or a hasty spirit of speculation sufficiently moderated, to render the same caution inapplicable or superfluous in the present day.
Another distinguished philosopher of the same age, well worthy of being mentioned in connexion with Leibnitz, our countryman Hooke, (who may almost be considered as having anticipated Newton in his application of the great principle of gravitation to the mechanical system of the universe,) affords an additional example that the greatest minds of this period fully appreciated the high importance of geological inquiries. Much of his posthumous works is dedicated to this subject, especially to the investigation of the arguments derived from geological phænomena in favour of the hypothesis of the volcanic elevation of our continents.
For the reason already assigned, I must pass equally rapidly over the many other interesting topics connected with the earlier history of our science, until Werner—closely, however, treading in the steps of his countrymen Lehman and Fuchsel—at length combined the results previously obtained into a more methodical and systematic arrangement, and, by the ardour of his genius and the influence of his popular lectures*, attracted to geology a degree of general attention which it had assuredly never before received. The previous labours, however, of
* We are chiefly indebted to the reports of his pupils, especially to those of Jamieson, for our knowledge of Werner's general views as fully developed in his lectures, and these only; for his own two short publications, the Kurze. Klassification and Essay on Veins, are confined to partial subjects. Prom these reports of his lectures, I feel convinced that it is to him we are indebted for the first general announcement, that the various species of organic remains grouped together in the rock formations bear a constant relation to the age of those formations: the Italians much earlier, and more recently Rouelle in France, had recognised their regular distribution in certain associated groups; but the distribution to which they referred appears to have been, according to their views, rather topographical than stratigraphical, whereas Werner clearly regarded it in the latter light; thus he characterizes the transition limestone as containing corallites, encrinites, &c., which though not absolutely confined to this formation, yet gradually disappeared in the newer rocks, becoming replaced by other species which never appeared in the transition series. The organic remains of the floetz rocks he regarded as increasing in quantity and variety, the newer the formation; he particularly specifies the most characteristic fossil shells of the gryphite limestone, the muschelkalk, chalk, &c. We should also mention that during the progress of Werner's observations, Saussure, in the excellent geological agenda published at the conclusion of his "Voyages," suggested the solution of the same great problem in the following terms, which state its conditions with the most admirable clearness and precision, "Constater s'il y a des coquillages fossiles qui se trouvent dans les montagnes les plus anciennes; et non dans celles d'une formation plus récente, et classer ainsi, s'il est possible, les ages relatifs et les époques de l'apparition des différentes espoces."
2 A
[page] 370
Saussure in the Alps, of Palassou in the Pyrenees, and of Ardouino Ferber and Fortis in Italy, had at that time collected such a rich store of materials as required only the intervention of a compiler and digester to apply them at once to the purposes of a more comprehensive system. In a former publication, before referred to, I have endeavoured to show what England had contributed to this store; but I am happy to find that at the present moment this interesting subject is about to receive a much fuller illustration from the pen of my friend Dr. Fitton*.
The progress of geology from the period at which it thus began to assume the systematic character of a regularly digested science, may be considered as having presented three marked stages, distinguished by three successive schools; each of these schools has selected for the more especial object of its attention a single member of the three great geological divisions in the series of formations, i. e. the primitive, secondary, and tertiary; and the succession of these schools has, by a singular coincidence, followed the same order with that of the formations to which they were devoted: it may also be observed that the leaders of each school have been distinguished geologists of three different nations,—Germany, England, and France. The first, or German school, is that of Werner: this directed its attention principally to the primitive and transition formations†, in which the distinctions of mineralogical character assume the greatest importance; and the imbedded minerals, from their variety, and relations to the rocks containing them, become the chief objects of the geologist's notice. The second, or English school, has distinguished itself by the ardent and successful zeal with which it has developed the whole of the secondary series of formations: in these the zoological features of the organic remains associated in the several strata, afford characters far more interesting in themselves and important in the conclusions to which they lead, than the mineral contents of the primitive series. This school generally recognises the masterly observations of Smith, first made public in 1799, as those which have principally contributed to its establishment; although the regular distribution of organic remains had before
* Now published in the 1st and 2nd vol. of the Lond. and Edin. Phil. Mag.
† In the early works of one of the ablest British disciples of this school, whose meritorious labours undoubtedly contributed very largely to the diffusion of an ardour for geological inquiries in this island, there occurs a curious illustration of -the exclusive attention to the older rocks. In the general view of geology contained in the Introduction to Professor Jamieson's Account of the Hebrides, 1800, after a sufficiently full detail of the various primitive formations, we find the whole secondary group dismissed in these few vague words: " They consist of limestone and argillite, with numerous petrifactions; also basalt, porphyry, pitchstone, greenstone, wacke, and the various coal strata."
[page] 371
been recognised in Italy by Steno, and in France by Rouelle; and although Werner in his lectures, and Saussure as above quoted, appear to have indicated generally, that the laws of this distribution bore a relation to the geological age of the formations containing them, yet a degree of vagueness hung over the whole subject, which precluded any extensive or useful application of this great principle, until the acute observations of Smith first brought it prominently forward in all the precision of exact detail as applied to a vast succession of formations, including the most important portion of the geological series; and as from his situation in life we must consider the discoveries of Smith as the extraordinary results of native and untaught sagacity of intellect, they must on this account be held to challenge a still warmer tribute of approbation, and may be regarded as strictly original in him, even where faint traces of anticipation may be found in Continental writings little likely to have fallen beneath his observation. The third school, or that of Tertiary Geology, owes its foundation to the admirable Memoir on the Basin of Paris, published by Cuvier and Brongniart, 1811. Although this school was certainly subsequent in point of date to that of Smith, yet those who had already directed their attention to such pursuits at this period, must well remember that the Wernerian school of primitive and mineralogical geology having previously obtained an undisputed and exclusive ascendancy in the minds of most of those who possessed any influential station in the scientific world, the observations of the individual alluded to had little chance of recommending themselves at first to public notice, and that in fact the knowledge of them appears to have been for ten years chiefly confined to a small circle in the neighbourhood of Bath,—-until the high scientific distinction of Cuvier, and the striking and interesting nature of the facts developed in his brilliant Memoir, excited a marked sensation and commanded the general attention of men of science; for none such could peruse with indifference those masterly descriptions, which exhibited the environs of one of the great metropolitan cities of Europe as having been successively occupied by oceanic inundations and fresh-water lakes; which restored from the scattered fragments of their disjointed skeletons the forms of those animals, long extinct, whose flocks once grazed on the margins of those lakes; and which presented to our notice the case of beds of rock only a few inches in thickness, extending continuously over hundreds of square miles, and constantly distinguished by the same peculiar species of fossil shells.
The public mind being thus fully awakened to a perception of the vast importance of zoological geology, as superadded to
2 A 2
[page] 372
mineral geology, became thus ripely prepared to appreciate the value of the materials previously collected by the unassisted acuteness and industry of Smith, which had illustrated the whole secondary series of formations in the same spirit as Cuvier and Brongniart had applied to a portion of the tertiary class, and which thus, after an interval of neglect, assumed their just place and rank in the geological system*.
From this period the views of the zoological school were universally adopted by the most active and efficient labourers in the progress of English geology, and were by them from time to time greatly extended.
The establishment of the Geological Society of London in 1808, afforded also, about the same time, a central point of reunion to those engaged in this pursuit,—an establishment eminently calculated to stimulate their endeavours by the promotion of mutual intercourse, and the comparison of the information individually obtained,—a point in every science very important, but most emphatically indispensable in one which can never be effectually advanced without the steady cooperation of numerous independent observers. Besides accomplishing this, the Geological Society was also most useful as affording the facility of publication to the researches thus prosecuted: indeed it has been well observed, that if we consider our philosophical Societies merely in the light of publishing engines, we shall have no mean idea presented to us of the very important advantages which they yield to science.
The first volume of the Transactions of the Geological Society was published in 1811, and it well illustrates the actual state of the science at that date: the greater part of its contents obviously belong to the Wernerian school, which we have characterized by its almost exclusive attention to primitive and mineralogical geology. The paper by Dr. Berger on the Geology of Dorset, Hampshire, and the Isle of Wight, will well exhibit the low state of secondary geology at that period; but another paper of Mr. Parkinson on the Organic Remains of the neighbourhood by London, including a comparative view of Cuvier's then recent discoveries in the basin of Paris, sufficiently evinces the dawn of a more intelligent system; and it deserves remark, that the introduction of this, the first respectable paper on secondary geology
* As the bases of this advanced geological system mainly depend on an exact knowledge of the zoological characters of the remains contained in the strata,— a knowledge extending to the most minute specific differences,—this could scarcely have been attained anteriorly to the considerable additions made by the French systematic writers, especially Lamarck, to the arrangements of the Linnæan school.
[page] 373
published by the Society, expressly refers to the discoveries of Smith, as the great basis on which all sound and really scientific researches on this subject must be established.
We cannot better illustrate the rapid march of geology from the period when this new light burst in upon the system, than by comparing the Memoir on the Isle of Wight and the Dorsetshire Coast, published by Mr. Webster in the second volume of the Geological Transactions, with the meagre notices of the same district by Dr. Berger, already alluded to as having appeared in the former volume. In this paper Webster ably follows the admirable model presented by Cuvier and Brongniart's Memoir of the Basin of Paris; with the geological structure of which he shows that of the Isle of Wight closely to agree, both districts exhibiting the very same alternations of marine and fluviatile beds* reposing on the chalk; while in one respect the phænomena observed in the Isle of Wight are rendered even more interesting than those of the Parisian basin, by the violent convulsions which have here dislocated the strata and thrown a large portion of them from a horizontal into a vertical position. If we compare this Memoir of Mr. Webster with the preceding one of Dr. Berger, they at once show themselves to belong to two very distinct eras of science; and it is difficult to believe that the interval which elapsed between their respective publication was only three or four years.
The publication in 1815 of Smith's general geological map of England†, succeeded by his more detailed separate county maps, illustrated by the work of the same author on "the English Strata identified by Organic Remains," and by the contemporaneous production of Sowerby on Mineral Conchology, filled up the whole great outline of English geology, and left to those who followed little more than the task of condensing and concentrating what was already ascertained, and enlarging and rendering more precise the detail. I should speak, however, in more distinguished terms than these, of the great geological map of England drawn up by Mr. Greenough, and published by the Geo-
* The same anoplotheria, &c., have subsequently been found in the fluviatile formations of the Isle of Wight as in those of the basin of Paris.
† It is quite erroneous, however, to attribute, as has been sometimes done, to Smith the earliest attempt to execute such maps; their construction was originally proposed by Lister, 1684; in 1746 Guettard published many such maps, although their execution was necessarily at this early period vague and imperfect; and before 1796 Dr. Maton had thus delineated the geology of the southwestern counties; and the various Reports of the Board of Agriculture had included similar representations of Yorkshire, Nottinghamshire, Derbyshire, Kent, and Devonshire.
[page] 374
logical Society in 1819. This map, as compared with the earlier publication of Mr. Smith, will be found to present, in all the districts occupied by formations older than the lias, corrections of the most material description; and in the more recent formations, where both maps generally agree, that agreement is in itself important as a confirmation of the accuracy of each, as that of the Geological Society was in no instance a copy from its predecessor, but entirely the result of independent observations collected during frequent, extensive, and laborious journeys. Those who have never seen the immense collection of materials in Mr. Greenough's most valuable manuscript geological note-books, can have little idea of the immense labour which he bestowed upon this object: his library also contains a vast collection of materials, equally important, in illustration of Continental geology; and it is greatly to be regretted that these still remain entirely unpublished. To no one individual does the progress of our science stand more deeply indebted than to the first President, and I may well add principal founder of the Geological Society, which, without his unwearied zeal and unstinted devotion of his talents, time, and pecuniary resources, could never have struggled through the numerous difficulties which embarrassed the first years of its existence*.
While geology on the Continent was advanced by the labours of Von Buch in Germany and Scandinavia, and by the able general systematic works of Daubuisson and the universal Humboldt, we may here pause to observe what had been accomplished by our own London Society in its earlier years before the close of its first series of Transactions in 1821. Already had its contributions completed to a high degree of perfection all the most important details of English geology; and besides this, the eastern half of Ireland had been very exactly described in the Memoirs of Dr. Berger and Mr. Weaver,—the latter especially well deserving the highest consideration, both from the copiousness and precision of its details, and the extent and beauty of its graphic illustrations†. The primitive districts and the West-
* Considering that this Report was originally delivered within the walls of the theatre of Oxford, I cannot refrain on this occasion from repeating the acknowledgement which I formerly made in the Introduction to my "Outlines,"—"that we owe the introduction of these pursuits into our University to lectures delivered between 1805 and 181O by my much valued friend Dr. Kidd, whose more private exertions in encouraging the rising talents of others were as successful in effect as liberal in design."
† The same author has since, in 1831, communicated to the Geological Society a similar Memoir of the south-western counties; so that the north-western portion of Ireland is all which remains undescribed at the present time.
[page] 375
ern Islands of Scotland had also received very important illustrations in several Memoirs communicated by Dr. MacCulloch* In relation to Continental geology, the very able Memoirs of Mr. Strangways, which so greatly extend our knowledge of the physical structure of an important portion of the Russian territory, claim especial notice; and the first series of our Geological Transactions also contained some valuable papers on portions of our Indian empire, on Ceylon, and on Madagascar.
I have been principally induced, in the present summary of the progress of geological science, to draw a line at the close of the first series of our Geological Transactions in 1821, because an author already alluded to has asserted in a recent publication, that "since that year geology has received scarcely any valuable additions, and not a single fundamental one." Drawing a line at this point, therefore, I shall endeavour to give a slight sketch of the contributions which have really marked the progress of the science during this supposed period of inanition, leaving it to your judgement how far they really deserve the above depreciating character.
Now although previously to this period the main features of English geology had been very amply illustrated, yet even in this province, where least remained to be accomplished, our additions have neither been few nor unimportant; and if we turn to Continental Europe, we shall find that what was then comparatively a blank, has been now filled up to such a degree that we are actually in possession of nearly as good materials for a general geological map of Europe at the present day as we were for one of England only at the former date; and to this, observers from our own country have contributed no less than their ablest Continental brethren. Nor let it be imagined that this only supposes an extension of our knowledge in insulated details: it is in truth far otherwise; since extensive comparative geology affords the only materials for obtaining the fundamental facts of our science. It is by this inductive process alone that we can hope to collect and combine the data which exist for what may be termed a general geological chronology. It is thus only that we can ascertain to what extent and under what modifications the same geological causes have acted at the same epochs. It is thus only that we can learn, what have been the violence, extent, and epochs of the disturbing and ele-
* These Memoirs were embodied in the work of that author on the Western Islands, published in 1819. His treatise on the Classification of Rocks, published in 1821, also claims notice as a very useful manual. Those who may have looked into his recent System of Geology, will feel why, in kindness to his reputation, his friends must here wish to close their survey of his publications.
[page] 376
vating forces which have affected the strata,—whether similar groups of organic remains universally, and in the most distant countries, characterize contemporaneous geological deposits,— or whether those zoological species are not rather restricted (like most of the species of the actual period,) to different geographical districts. All these are evidently questions at the very root of any sound geological theory, whenever the time shall be fully ripe for constructing such a theory; and although it were assuredly premature to assert that this time is even yet completely arrived, we may nevertheless boldly assert that no eye at all capable of appreciating these problems, or the appropriate evidence tending towards their solution, can glance over the discoveries of any single year since 1821 without observing a very rapid accumulation of the most valuable materials for their elucidation. During the same period, moreover, our knowledge of the principal volcanic districts, both those which are still in activity and those now extinct, has been advanced to the greatest degree of precision; and the whole of that which is perhaps the most important geological series*,—that of the tertiary formations, with the lower members of which alone the previous researches of Cuvier, &c. had made us acquainted, —has within the few last years received an additional development, no less important than that which, in an earlier stage of geological progress, the secondary system of the Wernerians received from the discoveries of Smith.
To confirm this general statement, it will be necessary to enter more minutely into the detail of the recent progress of geological discovery. To begin, then, even with that part of our subject which we have admitted to have been far the least promising of interesting novelty; with reference to the series of English strata alone, the corrections and additions to our previous information since 1821 have not only supplied such details as were of local interest, but such as were moreover often pregnant with important general consequences: the rectification, for instance, of the previous arrangement of the subcretaceous sands has brought to light in the Weald of Kent† a fresh-water formation, previously unknown as such, between these sands and
* The tertiary period is especially important in systematic geology, inasmuch as since, on every hypothesis, the geological causes must have acted during this period under conditions most nearly approximating to those which belong to the actual order of things: the formations of this age therefore afford the most essential link in connecting our actual experience with our speculations on the former state of our planet.
† The lowest bituminous clays of this formation have also been noticed on the opposite side of the Channel in the Boulonnois; and traces of them are said to have been observed, in our midland counties, in Buckinghamshire.
[page] 377
the subjacent Purbeck limestone; thus showing that the alternation of oceanic and lacustrine deposits was certainly not confined to the tertiary epoch, but had equally occurred in the more ancient periods*. Messrs. Murchison and Sedgwick have observed similar lacustrine deposits in still older rocks in the Isle of Sky. The elaborate Memoir of Prof. Buckland and Mr. De la Beche on the Weymouth district is not only valuable as having imparted minute and accurate precision to our knowledge of the interesting geological phænomena exhibited in the western extremity of the tract affected by the great convulsions which have elevated the chalky ranges of Purbeck and the Isle of Wight, but it informs us that this tract also, like the Weald, furnishes facts pregnant with remarkable consequences, as to the circumstances of the general surface at the period when the strata of Portland limestone were deposited; for we find interposed between these and the superincumbent Purbeck limestone, a bed of black vegetable mould, full of the stems of Cycadeæ and of large Coniferæ, many of their roots being fixed upon and still adhering to the subjacent limestone; so as to evince that they must have originally grown in their present position: the surface of the Portland limestone must therefore at that time have been dry land, bearing a thick growth of tropical vegetables†.
In the inferior oolite of Yorkshire, associated with the coaly
* A similar conjecture had indeed been previously entertained concerning the fluviatile origin of still older rocks, including portions of the coal measures; but the evidence resting only on the occurrence of obscure shells, referred perhaps too hastily to the fluviatile genus Unio, must be regarded as very insufficient, and appears opposed by the undoubtedly marine shells of the associated carboniferous limestone. It is however certainly by no means improbable that the coal strata have in part at least originated in the drift timber of vast æstuaries like that of the Mississippi; and in such localities this intermixture of fluviatile shells might naturally be expected.
† It must surely be unnecessary to insist on the fundamental importance of a fact thus affording direct evidence of the repeated oscillations by which the relative level of the ocean and land has been affected; for the Portland beds, subsequently to their having been thus exposed as a dry continental surface, appear to have been again submerged, first by an æstuary in which the fluviatile deposits prevailed, although with a partial intermixture of marine fossils as is shown in the Purbeck beds; in the adjoining district of the Weald the fluviatile character of the beds is more unmixed; a second oceanic submersion must have produced the vast mass of the cretaceous superstrata. And lastly, the alternating oceanic and fluviatile deposits of the Isle of Wight seem to attest a recurrence of similar oscillations. Prevost indeed, in a Memoir on this question, opposes the idea of reiterated oceanic submersions, and endeavours to explain the phænomena of the basin of Paris by the hypothesis of a basin originally oceanic, but converted by the gradual subsidence of the sea level successively into an æstuary and inland lake of brackish water, subject occasionally to accidental irruptions of the oceanic water on one hand and of the land floods on the other; but it does not appear possible thus to explain the vast fluviatile and oceanic deposits above described: and whereas this writer builds much on his attempt to prove that no bed in the geological series can be pointed out which appears to represent an ancient continental surface on which vegetables once grew, &c.; the facts stated in the text present a complete answer to this negation.
[page] 378
beds of the eastern moorlands, Equisetaceæ were observed by Mr. Murchison under circumstances exactly parallel, so as to warrant similar inferences.
The very valuable details concerning these Yorkshire oolites, published by Mr. Phillips, will, if we compare the series of rocks as there exhibited with the characters of the same series at its opposite extremity on our southern coast, sufficiently illustrate the great changes which took place in different parts of the very same deposits, inasmuch as the calcareous sands of the inferior oolites in Yorkshire present nearly the characters and mineral constitution of the rocks associated with the older coal formation. We thus collect a series of facts calculated to throw considerable light on the modification of circumstances which may have concurred in different ages to produce carboniferous deposits; and we see convincing proof how far we must depart from the doctrine of universal formations, (if that term be supposed to convey the notion of anything like an identity of character,) in order to approach to the truth of nature*.
The comparative view of the contemporaneous rocks of the Scotch oolitic coal-field at Brora, by Mr. Murchison, is equally important; indeed in many respects it may be considered as having suggested the line of examination pursued by Mr. Phillips†. Still more remarkable is the discovery, by the same geologist and Prof. Sedgwick, of an entirely new formation (seemingly occupying the relative position of our own carboniferous group,) in Caithness, of bituminous schist containing fish‡ and shells apparently fluviatile.
The disruption and disturbance of these newer strata by the elevation of the subjacent granite (at a period, however, evidently
* We can scarcely feel authorized from analogy to conclude that distant portions of a contemporaneous geological deposit in Dorsetshire and Yorkshire should possess absolute identity of mineral character, any more than that the mud banks deposited at the present moment on the coasts of the two counties should so correspond. At the same time, however, we must allow that geological causes appear to have acted on a much greater scale, and homogeneous depositions to have prevailed to a very considerable extent, subject however to material local modifications.
† Mr. Murchison first observed at Brora a considerable number of species of fossil shells previously unknown in the oolite; these were figured in Sowerby's Mineral Conchology, and subsequently the same species were observed by Phillips in Yorkshire.
‡ The vertebrae, teeth, and radii of fish have also previously been observed in carboniferous and even in transition limestone.
[page] 379
subsequent to the consolidation of the latter rock,) presents a fact of great importance, inasmuch as it clearly evinces that the adjoining primitive chains of mountains must have been subject at the least to two æras of disturbance; the first when the injection of the granitic mass, yet in a fluid state, rent the incumbent micaceous slates, injecting veins of its substance into their fissures*; and a second at a much later period, subsequent not only to the refrigeration of the granite, but even to the depositioh of the Brora oolites, which partook in the motions occasioned by this latter elevation, and have been in places shattered by this convulsion into fragments which have been reunited into a brecciated conglomerate.
The next geological group which requires our notice beneath the lias and oolites, is that which is universally characterized by the new red or variegated sandstone, the grés bigarre of the French, and bunter sandstein of the Germans, which is associated in its lower portion with the magnesian lime or zechstein, and (on the Continent) in its upper part with the muschelkalk and keuper. Geology stands much in need of a convenient name for this group; and I will venture therefore to propose the term Pœcilite (from the Greek ποιхιος), as expressing its characteristic rock the grès bigarré, and hence denominate the group, pœcilitic. Brongniart has already adopted the Gallicised form Pœcilien.
The elaborate Memoir of Prof. Sedgwick on the Magnesian Limestone of the Northern Counties is doubly valuable as showing at once the variations and also the identities presented by the comparative view of the same formation in distant points; while in our southern counties this formation exists only in the form of a conglomerate, derived from the debris of the older carboniferous lime united by a dolomitic paste, thus illustrating the original mode of its formation; in the northern counties it becomes fully developed in a regular series of calcareous beds, distinguished by peculiar organic remains, exactly corresponding with the zechstein and rauchwacke of the contemporaneous German deposits; while the organic remains contained are very important as forming a link between the types of the older subcarboniferous and successive newer rocks. Prof. Sedgwick has well shown how, if we take into account the intermediate formations of muschelkalk and zechstein, so amply developed in Germany, but not yet discovered in these islands, we may trace a regular graduation in the
* I believe, indeed, that at the Ord of Caithness, where the granite is in contact with the oolite, the mica slate is absent; but there is surely no reason to believe this particular granitic mass different in age from the other granites of the same portion of the Highlands, which are thus related to the mica slates.
[page] 380
types of the imbedded organic remains, thus almost observing a law of continuity between the carboniferous lime and lias. I cannot conceive that Dr. MacCulloch can ever have read these remarks; otherwise common prudence must at once have shown him the necessity of cancelling his negation, that geology had recently received any fundamental additions*.
The comparative view of our northern coal-fields† has equally extended our knowledge of the varying modifications affecting contemporaneous formations. The geology of this important district is now fully illustrated by a series of elaborate Memoirs by Messrs. Wood, Winch, Witham, Buddle, and Hutton, accompanied by detailed and accurate sectional views representing the whole Northumbrian coast, &c., published in the first volume of the Transactions of the Newcastle Philosophical Society, a work which reflects the highest credit on one of our youngest provincial Societies, and without which no geological library can be esteemed complete. It was indeed previously known that the millstone grit and limestone shale of Derbyshire became in Northumberland complicated into an extensive series of alternating limestones, shales, sandstone, and coal-beds; but an important addition to this fact has been now distinctly established, for we find that to the north
* Prof. Sedgwick has also fully illustrated the beds of sandstone lying beneath this conglomerate as seen at Pontefract, &c., which he has fully shown to be equivalent to the rothe todte liegende of Germany (an identity originally suggested by the author of this Report): a dolomitic conglomerate like that of the southern counties is often interposed between this sandstone and the regular magnesian limestone; and indeed in the southern counties, wherever the dolomitic formation is most fully developed, the uppermost beds are finely grained and gradually pass into a compact limestone. The Pontefract sandstone is completely unconformable to the subjacent coal measures, and partially unconformable also to the superincumbent magnesian lime. M. Elie de Beaumont has observed the same unconformity between the equivalent grès de Vosges and the coal measures on the one hand and the zechstein on the other. A comparison of the equally able Memoirs of the French and English geologists will be found very interesting. I cannot, however, entirely agree with Prof. Sedgwick in assigning to the Heavitree quartzose or porphyritic conglomerate a place in the series younger than the rothe todte, and equivalent to the dolomitic conglomerate; a comparison of the German and Heavitree conglomerates has convinced me of their close connexion. Mr. Hutton in the Newcastle Phil. Trans., has greatly extended our knowledge of this rock.
† The labours of Prof. Buckland, in which I had the honour of being associated with him, had before illustrated the Somersetshire and Gloucestershire portions of our south-western coal-fields, with every desirable copiousness of detail. I am at present actively employed in completing our survey of the South-Welsh portion of these districts. It is greatly to be regretted that we have hitherto no good account of the extensive coal-fields of Lancashire: in the vicinity of such philosophical institutions as those of Liverpool and Manchester, surely this desideratum ought not to be permitted to remain.
[page] 381
of Cross Fell the lower series of the carboniferous limestone, locally termed Scar lime, is subdivided in like manner, and alternates with sandstone and carboniferous shale*; the limestone decreasing and the coal beds increasing as the strata approach the transition chains of the Scotch border. The coal measures occupying the great valley of the Scotch Lowlands on the north of these chains, are with great probability referred to the same lower series; but this Scotch district has as yet been very imperfectly described.
The immense faults and dislocations of our great northern coal-field have also received the fullest illustration in the Memoirs above cited; and no geological subject can be considered more pregnant with fundamental information than this for it is only by a careful and detailed examination of the phænomena attendant on these great convulsions that we can ever hope to be enabled to speculate satisfactorily on the causes which have produced them. Now in the Newcastle district we find the strata shattered at every two or three miles interval, with fissures extending to many leagues distance, and producing subsidencies of occasionally not less than 140 fathoms, which, if they affected equally the configuration of the surface, would produce precipitous escarpments near 1000 feet high; yet is the actual level of the surface found absolutely uniform, and affording no trace whatever of the vast subterraneous disturbances;—a most striking proof of the vast mass of materials which must have been removed subsequently to their occurrence. Here we remark one of those great problems for the
* Thus in the two important groups just noticed (viz. the pœcilitic and carboniferous series), recent observations have enabled us to supply those great lacunae which previously occasioned the appearance of an abrupt transition (per saltum) from one order of geological products to another totally different; the connecting links before missing are now restored, and seem to establish a graduated and continuous order in this most important portion of the geological series. Thus in the transition formations we see alternations of slate, coarse gritty grauwacke, and beds of entrochal limestone, often associated with seams of anthracite, and, according to Mr. Weaver's late Memoir, with regular coal in the South of Ireland. In the carboniferous group we have precisely similar alternations, only that the limestone frequently prevails most in the middle regions, and the coal in the uppermost; the organic remains of the limestones approximating very nearly to those of the preceding transition series. In the succeeding pœcilitic group the organic remains still in many instances belong to the same class, and partake of the earliest type, although species of a more recent character begin to be introduced: the subjacent Pontefract sandstone (rothe todte) also exhibits a regular approximation to the coal grits. We have before seen how the graduation is continued from the magnesian limestone through the muschelkalk to the lias and oolite, and we shall hereafter learn that the same intervention of gradual links exists between the cretaceous and tertiary groups at Maestricht and in the eastern Alps.
[page] 382
solution of which we require the action of diluvial currents on the most vast and violent scale*. Trap dykes are so common in this district, and so frequently associated with the lines of fault, (see especially Mr. Wood's description of the great Statlick fault,) that we cannot but refer those dislocations principally to volcanic agency.
To proceed to the lower formations: The transition districts† of our island, for a long period after the introduction of the more modern schools, were, as if in revenge for the exclusive attention devoted to the older rocks by the Wernerian school, abandoned to comparative neglect. These, however, have recently received important elucidation from the researches of Prof. Sedgwick, who has already fully described the transition chains of the Cumbrian lake district, and is at present engaged in prosecuting a similar examination of North Wales; while his friend Mr. Murchison is simultaneously exploring the junction of the transition and secondary districts along the Welsh border. In his description of the adjoining Island of Anglesea, Prof. Henslow had already presented us with an Essay, which may well serve as a model of the manner in which such investigations ought to be conducted‡.
* On this subject I would quote an interesting note from Mr. Greenough's "Examination," p. 156.
"Mr. Hutchinson, who wrote about 1750. of whose geological opinions I have more than once had occasion to speak with much respect. was the first by whom this important fact was noticed. His words are, It is extremely rare to find a lifted edge of strata standing up above the general surface. The faults, however large the rise which they occasion, being rarely discernible by any sudden inequality of the ground, numerous ascliffs, facades, mural ascents, or precipices are, very few of them are owing to faults; in general the matter has been carried off.'"
Mr. Greenough gives other similar references to the works of Catcot, Williams, Desmarest, Playfair, Deluc, Richardson, and Farey.
† Here geology again stands in need of a term less barbarous than grauwacke slate, which would conveniently denominate the characteristic rock of this æra. Might not clasmoschist (from the Greek χλασμα,) be conveniently adopted? It would afford a term well contrasted to mica schist, the characteristic rock of the primitive group. We should thus obtain a series of convenient denominations for the various geological groups which are principally distinguished: the primitive we might call the mica schistose group; the transition the clasmoschistose group; the denomination of the carboniferous group is already sufficiently established; for the new red sandstone with the associated magnesian zechstein and rothe todte in its lower, and muschelkalk and keuper in its superior portion, I have already proposed the term, the pœcilitic group. The oolitic group, for the lias and oolites; the cretaceous group, for the chalk and subjacent greensand; and the supercretaceous group, for the tertiary formations, are appellations already commonly received.
‡ This Memoir, published in the first volume of the Cambridge Phil. Trans., is peculiarly valuable for its accurate description of the phænomena of the numerous trap dykes, and the changes and crystallized minerals which they have produced in the rocks traversed by them.
[page] 383
Mr. De la Beehe has recently been appointed to colour geologically the Government trigonometrical survey of Devon; and a complete geological map of Cornwall is at length promised by Mr. Henwood, under the auspices of the Geological Society of that county: when these works shall be executed, we may trust that the history of the older formations of this island will receive as full and satisfactory an elucidation as our secondary series has long since obtained from the labours and acuteness of our geologists.
If it be said that these questions as to our older rocks are only questions of detail, be it remembered that the boldest and happiest generalizations of science must rest on such details in the first instance. While we remain imperfectly acquainted with the various modifications exhibited by our earliest formations, (variations which must have resulted from corresponding changes in the causes which produced them,)—while we are as yet unable precisely to distinguish the disturbing forces and intrusive ignigenous masses of this period,—how can the bases of any geological theory be securely laid? Mr. Weaver has recently added to his former important paper (before noticed) a continuation which completes the geology of the South of Ireland, and now leaves only the north-western portion of that island a desideratum. The most important general feature of this paper appears to be the having ascertained the fact that the coal beds of the South-east of Ireland present an older carboniferous formation than any previously known, being associated with the transition rocks.
Before we advert to that wider and more important field, the comparative geology of the Continent, it is most gratifying to remark, that as we shall there find our countrymen distinguishing themselves no less than at home, so in return the geology of our own island has been indebted for many valuable contributions to the labours of the ablest Continental observers. The indefatigable and acute Boué (whose name we shall have such repeated occasion to cite as connected with almost every department of our subject,) commenced his career by exploring Scotland, concerning which he has presented the public with a most masterly sketch, ably condensing every important previous observation before spread over diffuse and voluminous works, and adding original materials of at least equal value*.
* A second edition of this work, incorporating the important subsequent observations of Messrs. Murchison and Sedgwick, would be very desirable; but it would require a previous detailed examination of the coal-fields of this part of our island to render it complete. Should this be accomplished, we might hope that Mr. Weaver might be induced to complete and condense into one volume his most important Memoir on Ireland. I trust also shortly, with the assistance of my distinguished friend Prof. Sedgwick, who has so largely contributed to the elucidation of our older rocks, to complete the survey of England in a second volume of the "Outlines." We should then possess a complete geological history of the British Islands, reduced within the manageable compass of four portable volumes.
[page] 384
Those eminent French geologists to whom the important task of preparing a grand geological map of their own country is intrusted by its Government,—Brochant, Elie de Beaumont, and Dufrenoy,—also began their labours by carefully examining all the most important points of English geology, as affording an ascertained basis for comparative observations on the general structure of Europe; the published results of their journey are indeed rather statistical (relating to the position, extraction, and preparation of our mineral ores,) than strictly geological; but the spirit of these comparative examinations will fully appear in the several valuable Memoirs they have published from time to time on the Central and South-eastern districts of France, the countries to which the survey has been first directed*. Still more recently two of the first German geologists, Messrs. Oeynhausen and Decken, have visited our island, and contributed several important Memoirs on the Granitic Veins of Cornwall, on several of the Scotch Islands, &c.
This general intercourse of observers of different nations is not only, from the liberal spirit which has ever on all sides pervaded it, most gratifying in itself, but it is also especially important to the advancement of a science in which all the great general views require the most widely extended comparative observations for their establishment and development.
Before I proceed to submit to your attention an outline (necessarily brief and slight,) of the rapid progress which geology has recently made in developing the structure of foreign countries, it may be convenient here to premise the general geographical order which it is my intention to adopt in adverting to the investigations thus successfully pursued. I shall begin by those constituting or bordering the great European basin, which I shall take in the following order:—France, the Alps, Germany, the Baltic coasts and Scandinavia, ending with Russia. Next I shall proceed to the countries connected with the Mediterranean basin,—the Spanish Peninsula, Italy, Turkey, and the African coasts. The other quarters of the globe will
* I would especially refer to M. Elie de Beaumont's Memoirs "On the Formations in the Vosges intermediate between the Coal and Lias," or what I would call the pœcilitic group, (Annales des Mines, 1827); and " On the Uniformity of the Jurassic Zone environing the Basins of London and Paris," (Annales des Sciences Nat. 1829); also to those of Dufrenoy "On the Upper Beds of the Lias in South-west France," (Annales des Mines, 1827); " On the Central Platform of France," (Ibid, 1828), and " On the Chalk of the South of France," (Bulletin de la Soc. Géol 1830).
[page] 385
follow,—Asia as divided into the northern and central provinces explored by the Russian Government, and those of India by our own nation. I shall conclude with North and South America: but in all these instances I shall reserve what relates to the two great points of tertiary and volcanic geology, as demanding a distinct notice rather in their relations to the general questions of the science than to the geographical distribution of formations.
To begin with France.—The geological map of this country now in progress has been already alluded to, and some of the preparatory essays of those to whom its execution is intrusted cited. The scientific publications of that country also contain many other most important Memoirs, of which I would especially mention those of Boué on the South-west of France, of Roget on the district of Boulogne and on the Ardennes, and of Voltz on the two departments of the Rhine, as presenting the most important contributions to comparative geology. The work of Charpentier on the Pyrenees is excellent as a descriptive essay, but in many points connected with the secondary rocks appears to belong to the older rather than more modern geological school.
English geologists have ably contributed to the elucidation of the comparative structure of the two countries,—especially, as was to be expected, of those districts of France which bordering on the Channel present the direct prolongations of our own formations;—we may particularly refer to the examination of the Boulogne district by Dr. Fitton, and of the Norman coast by Mr. De la Beche. The institution of a Geological Society in France, in 1830, cannot fail to promote the development of our science equally with its English prototype; and the travelling geological class of M. Boubée, whose members are conducted successively over the most interesting districts, may be considered as advantageously introducing a peripatetic school in geology.
In the Netherlands, before the late disturbances, the Government had in like manner proposed to undertake the publication of a geological map. The scientific commission consisted of that venerable and zealous veteran in our science Omalius D'Halloy, so well known for his important Memoirs on these districts and the adjacent portion of France, published at an early period of modern science, when such communications were among its first models*. With him were associated Van Breda and Von Gor-
* Omalius D'Halloy has since published a small outline geological map of France, and more recently a general work on the classification and history of the various formations.
2 B
[page] 386
kum. With respect to this country the most valuable recent Memoirs are those of Oeynhausen and Dechen on the carboniferous and transition districts of the South; with these we may compare Cauchy's Essay on the province of Namur, to which a prize was awarded by the Brussels Academy; and the communications of MM. Dumont and Davreux on the province of Liege, in which it appears that the coal of that district is, like that on the northern edge of our Northumberland district, an inferior series, beneath the carboniferous line. The district of Brabant has been fully described by Morven, and that of Luxembourg by La Riviere.
Our geographical survey of the progress of our science will next conduct us to the Alpine districts. The interesting excursions of Saussure through these magnificent chains in the last century were among the first causes which attracted the attention of European science to subjects of geological investigation; but we still remained without anything like a precise and systematic general view of their structure, until Ebel, in 1808, published his treatise Ueber die Bau der Erde in den Alpen Gebirge, which contains a detailed account of the central primitive axis and of the lateral zones of secondary Alpine limestone and tertiary nagelflue, together with a comparative view of the other principal European mountain chains, and a more minute description of the Jura; the whole being illustrated with geological maps and sections exhibiting a near approximation to correctness. Soon after this date Brochant also greatly extended our knowledge of these mountain groups, by proving that much of the formations immediately bounding the central axis, to which previously a far higher antiquity had been assigned, belonged undoubtedly to the secondary class; still however the correct identification of the members of the lateral calcareous zones with the equivalent formations in other portions of Europe remained without any satisfactory elucidation,—a fact which may be partly explained from the very unusual characters which these formations here assume; a variation to be attributed to circumstances which during the grand convulsions of this chain have greatly modified the more recent depositions accumulated on its flanks*. Prof. Buckland,
* In approaching and entering into the composition of these vast mountains the ordinary characters of the secondary rocks undergo a very material change, exhibiting that degree of compact consolidation which usually marks rocks of much older formation. A similar alteration of character, and assumption as it were of features of higher antiquity, may even be observed in the transition rocks under the same connexion; for we find, according to Murchison, limestone as highly crystalline as any primitive marble, and alternating with micaceous slate containing garnets, yet presenting encrinites and other transition fossils; the Alpine limestones, considered as equivalent to our oolites, are also distinguished by their containing masses of galena, and the middle portion of the series contains the principal deposits of rock salt. Is it too rash a speculation to attribute these modìfìcations to the more continued and intense agency of the volcanic forces which we may suppose to have elevated these colossal ridges? M. de Beaumont even considers the crystalline slates of St. Gothard as altered secondary rocks.
[page] 387
in his journeys across these mountains in 1816 and 1820, was the first to throw a more accurate light on this subject (see his Notice on the structure of the Alps, and tabular arrangement of the rocks that occur in them, with their equivalents in England, published in the Annals of Philosophy, June 1821); about the same period Brongniart published his discovery of green-sand on the Diablerets; but it is to the recent investigations of Mr. Murchison and of Prof. Sedgwick (who became the companion of the former in all his later tours), that we owe the satisfactory completion of this great work; and it is with their names that the accurate comparative estimation of the members of the secondary series as exhibited in this the grandest of our mountain chains, must remain associated. Boué has indeed ably cooperated in the same work; nor can the trifling difference of opinion on the age of the Gosau rocks, (whether belonging to the beds immediately below or immediately above the cretaceous group,) materially interfere with the many and important points of their agreement. Lilienbach on the Alps of Saltzbourg, Studer on the Southern Alps, and with reference to Other portions of the chain, Hagi's observations on the Bernese Oberland, and Lusser's section of St. Gothard, may also be advantageously compared with the writers before named. Risso has fully described the Italian portion of the Maritime Alps, and De la Beche and Buckland have also published on the environs of Nice.
But while we justly refer to these later authorities for the complete development of the formations constituting this great chain, it must not be forgotten, that as early as 1759 Arduino, in his description of the Alpine slopes bordering the Vicentin Veronese and Padua, had fully anticipated much that has been confirmed by more recent observation: his general sketch of the Alpine chains, as subdivided into their primitive, secondary, and tertiary ranges, could scarcely be improved in the present day, and is much more complete than the contemporaneous division of Lehman into primitive and secondary only; he has enumerated generally the characteristic fossils peculiar to the several beds, and seems quite aware of their constant relation
2 B 2
[page] 388
to the strata and their importance: from his descriptions alone I was enabled satisfactorily to identify the scaglia as equivalent to our chalk, and to point out our oolites as represented by much of the Alpine secondary limestone, while as yet the Continental war precluded English geologists from the possibility of personal examination. In 1813, in preparing a general section of Europe for the Oxford Lectures of my friend Prof. Buckland, I coloured the formations on the southern slope of the Alps on this principle. Arduino's views on the volcanic action which has affected this district in the phænomena of the Euganean hills, Monte Bolca, &c., are equally excellent.
However wide be the discrepance of mineralogical character, yet the relations of position and the organic remains contained may be considered as satisfactorily identifying the great mass of the Alpine limestones with our own oolitic series, and the upper members with our cretaceous formations; but it has been well observed by Prof. Sedgwick, that while the aggregate of the organic remains of a formation generally presents an assemblage nearly identical, yet the subordinate distribution of these remains is materially affected by the diversity of the mineral constitution of different portions of contemporaneous formations: this indeed might have naturally been expected, just as we find some shells and zoophytes actually requiring a solid bottom to which they can firmly attach themselves, and others indifferent to this and equally found among drifting sand and mud; on this principle we may account for the occurrence of certain shells principally in beds of limestone, while these are absent from the alternating shales and sands. "Thus," the Professor proceeds, "we perceive that in these Eastern Alps whereever a secondary deposit approaches the mineral type of the English equivalents, the same approximation extends to the organic remains:" from such observations we may hereafter hope satisfactorily to infer the causes which have induced such remarkable alterations throughout these vast mountainous groups.
Germany.— Proceeding to the lower regions of Germany, we find, as the preceding remarks would lead us to expect, a much closer agreement, both mineralogical and zoological, between the oolitic series as there exhibited and our English equivalents*.
* The Lias.—The inferior ferruginous oolite occasionally (e.g. in Westphalia at the Buckeberg,) containing coal like that of Brora and the Eastern Yorkshire moorlands, are shown by Mr. Murchison to be easily identified with the corresponding formations of our own island: he is inclined, though still with some hesitation, to compare the Solenhofen calcareous slates with the similar beds of Stonesfield. It is unnecessary to repeat, that in Western Germany and Eastern France the oolitic group immediately reposes on the arenaceous keuper and the associated limestone called muschelkalk, two considerable formations here separating the lias from the variegated sandstone (pœcilite), but hitherto undetected in England. I am, however, now inclined to believe that the shelly chert reposing on the dolomitic conglomerate on the north border of the Mendip Hills at East Harptree, and the singular bed occurring immediately beneath the lias on the south of that chain near Shepton Mallet, which at the time I was led to refer to the lower lias, may, on further examination, be found to represent these Continental deposits; but however this may prove, their general absence is remarkable, and if we view the question more generally, it will be found to involve phænomena among the most inexplicable in geology. In Northern Europe,viz. the British Islands, the zechstein is extensive, but the muschelkalk generally wanting. About the latitude of the Hartz and Thuringerwald both formations occur; but south of this, where the muschelkalk is most prevalent, eastward from the Vosges and westward from the Schwartzwald, the zechstein in its turn is absent; the absence of the carboniferous group and its lime also characterize the latter localities. As the conglomerates which in some of our counties appear as the equivalents of the zechstein are clearly derived from the carboniferous lime, are we authorized in attributing this source to the formation generally? if so, the absence of the one will naturally account for the absence of the other.
[page] 389
It is again to the labours of Mr. Murchison that we are indebted for the most complete comparative view of these German formations; but the native geologists have well maintained the reputation of a country which in many respects has taken the lead in the researches of this science; and other Continental observers have also been active in this field. The name of Von Buch will here always hold the first rank; his indefatigable labours have enabled him to complete, in forty-two sheets, a general geological map of Germany: this has been published by Schrop, and is one of the most perfect models of its kind. Berghaus, well known by his map of the Hartz, has undertaken to continue his representation of the whole country on a still larger scale, and the maps by Hoffman of the North-west of Germany, and by Oeynhausen and Dechen of the provinces bordering the Rhine, are of the very highest merit. Elaborate Memoirs have also illustrated the geology of all the most important districts. Boué's*Geognostisches Gemaelde von Deutschland, 1829, contains a full summary of the results of all those published up to that time, with many original observations of his own. Keferstein in his Teutschland Geol. dargestellt, of which the publication commenced in 1822 and has since been periodically continued for many years, has also incorporated a vast series of important essays, partly original and partly compiled from the best authorities, and illustrated throughout by maps and sections, which now include almost the whole of
* M. Boué has also published or submitted to various scientific societies many separate Memoirs on particular districts,e.g. the Carpathians, Moravia, Transylvania, Gallicia, Bavaria, and South Germany.
[page] 390
that great country. With regard to particular Memoirs, I would especially refer to Hoffman's Natursicht der geognostischen Verhãltnisse vom N. W. Deutschland, 1830, accompanying his map: this should be carefully collated with Murchison's comparative views, as also should Helh on the lias of Wurtemberg, Bronn on the fossils of the lithographic lime, Munster on the German oolites, and Huggy's section of St. Jura, together with those of Merian and Rengger on its northern slope.
The names of Freisleben, Nœgerroth, Heininger, and Von Reaumer, belong to an earlier period than that of which we are now treating, and have long been familiar to every geologist; but it may convey some idea of the zeal with which the science is actually cultivated in their country, to append in a note a topographical list of the districts which have received additional illustration from important Memoirs published within the last five years*.
In Poland we are informed that M. Pasch has now in the press a great work illustrated by detailed maps of the geological structure of the whole country, having previously published in 1830 an abstract of his observations. M. Schneider has also published a Memoir on a particular district, in which we find copious details of the secondary rocks of the pœcilitic group and lias, and are informed that coal is associated with the latter rock as in Yorkshire.
Turning from Central Germany to the coast of the Baltic, (which may be considered especially interesting to the English
* Places. Described by
Wutemberg Alberti.
Schwartszwald Walchner.
Heidelberg Bronn.
Hundsruck Schmidt.
Odenwalsd, Spessart, and wetteravia Kilpstein.
Hartz Zincken and Hoffman.
Cobourg Hoff.
Thuringerwald Tauschner.
Bohemia, and Erzegebirge Kilpstein.
Riesengebirge Moteglek.
Lusatia Peschek.
Silesia Gloker.
Carpathians Pasch and Lilienbach.
These are in addition to the Memoirs already cited in the text. The general geological reader may be referred for concise abstracts of all these to Férussac'a excellent periodical the Bulletin des Sciences Naturelles. These abstracts have materially assisted me in compiling the present Report. I have to regret that I did not receive Boué's excellent Resumé des Progres de la Géologie en 1830 et 1831, addressed to the French Geological Society, January 1832, until after it was delivered at the Oxford Meeting;—at the late period, however, of preparing the Report for the press it has suggested some important insertions.
[page] 391
geologist because exhibiting the prolongations of our cretaceous formations on the north-east, as France does on the south-west,) we find that Hoffman has observed the chalk in contact with new red sandstone in the Isle of Heligoland. Forchammer and Bigel the Danish geologists, have traced the chalk and tertiary deposits of Jutland and Zealand: the same thing has been done in the adjoining districts of Mecklenbourg by Bruchner and Blucher, and in Pomerania by Oeynhausen.
Hisinger has explored the transition islands of Bornholm, Oeland, and Gothland*, and Eichwald has traced the continuation of this series through Courland, Livonia, and the shores of the Gulf of Finland, as far as St. Petersburg†. The same transition district, chiefly characterized by its limestone, extends to Volhynia and Podolia on the south.
On the north of the Baltic, Sweden, distinguished by its early zeal in the prosecution of the kindred sciences, and which in the geological maps of Baron Hermelin presented one of the first examples of the kind, has still continued her activity: the chalk, including green-sand, extending through Scania, has been described and its fossils represented by Nilsson. Hisinger has also published Memoirs on the transition groups of Sweden, as has Esmark on those of Norway.
Russia, whose vast possessions contain so many sources of mineral wealth, has recently devoted much attention to their development. An Imperial Geological Society was established in 1817; a regular journal of the mines (Gornoi Journal) has been published since 1828, and is full of interesting Memoirs, but rather calculated to illustrate detached districts, than as yet to supply the materials for a connected and complete survey‡: we may cite Erman's journey from Moscow across the Ural to Lena, Olivieri on the coal formation of the Donetz, and Eursman's Steppes south of the Volga§; the structure of the Ural chain has been elucidated by Kupfer, Anosof, and Engelhardt. We shall hereafter have occasion to refer to the measures undertaken for exploring the Asiatic dominions of Russia.
* The Isle of Gothland is not exclusively transition; a small deposit of oolite with its characteristic fossils is found in the isthmus connecting the southern peninsula with the rest of the island.
† The Russian Government has caused the publication of a geological map of Lithuania, Courland, Esthonia, and Livonia, executed by MM. Ulprecht, d'Engelhardt, Ulmann, and Linchnicky.
‡ We have already cited the map and Memoirs on the Baltic provinces of Lithuania.
§ From these Memoirs it appears that chalk forms a platform on the north of the Government of Toula extending to the Waldai Hills, that it appears near the course of the Donetz and towards the Sea of Azof, and again at Ouralsk on the river Ural; the saliferous steppes bordering on the Caspian are probably tertiary; the variegated sandstone, zechstein, rothe todte, and coal formation reposing on grauwacke, constitute the hills on the right bank of the Donetz; variegated sandstone prevails from the banks of the Volga to Perm; east of which zechstein occurs, and transition limestone skirts the western flank of the primitive crest of the Oural chain.
[page] 392
But although geographically included in that division, yet as being geologically connected with the central European basin, the consideration of the Caucasian district appears to belong to this place. We have descriptions of the chain itself by Klaproth and Kupfer: and M. Boué cites several memoirs on the northern borders, on the Circassian Steppe, and on the coasts of the Aral and Caspian seas; from these it appears that the tertiary rocks (of which the youngest beds contain shells actually existing in those seas,) approach to the foot of that chain, and being elevated by it, attain a height of 2500 feet; they alternate with fluviatile beds, and repose on a cretaceous formation which in its turn covers the oolitic series, forming mountains 3000 feet high, and skirting the older crest, of which these circumstances must refer the final elevation to a very late date.
The countries hitherto considered belong to the great central European basin, in which the depositions having been continuous we may naturally expect the greatest uniformity in the structure and arrangement of the formations. The Southern peninsulas of Spain, Italy, and Greece, to which we shall next advert, are included in the Mediterranean basin, in which it appears clear that the tertiary formations are considerably distinct from their northern equivalents; and it may be a question whether corresponding differences will not be found in the secondary series occupying these separate basins. Yet as a partial answer to this inquiry, we may observe that the formations in the northern and southern slopes of the great ridge of separation, the Alps, are closely analogous.
To begin with Spain. In the present state of the literature of that peninsula, we must necessarily look to foreigners for our only information concerning any point connected with it involving scientific views. Prof. Hausman however, who has travelled through it, has submitted to the Royal Society of Göttingen an admirable memoir De Hispaniœ Constitutione geognostica; he has described the various primitive rocks, and distinguished among the secondary formations pœcilite, lias, oolite, green-sand and chalk; Dufrenoy is of opinion that the salt deposit of Cardona is tertiary, and the adjacent puddingstone of Montserrat equivalent to the Swiss nagelflue.
In Italy we have various memoirs from Catullo and others
[page] 393
concerning the basin of the Po, the Genoese and other northern portions; but we still require a good description of the great chain of the Apennines, the labours of Sari being hitherto confined to its Tuscan extremity; a general geological map of the North is now in preparation, but it does not extend to the South of Tuscany; we can only cite the memoir of Dr. Capello on the district of Accumoli in Abruzzo Ulteriore for a single point of the central portion of the Apennines, and that of Giovene on the Apulias for the southern extremity.
With regard to Greece we of course possess only the general indications of hasty travellers; among these however Boblaye deserves particular mention. Boué has conferred a benefit on the science in collecting the substance of their scattered remarks, connected by his own examinations of the specimens from that country, to be found in many Museums, into a well-digested Resumé, (Zeitschrift für Mineral. 1828); he has included Asia Minor in the same essay.
Of the Mediterranean Islands, the Balearic group has been described by Elie de Beaumont. There does not appear to have been any recent publication on the geology of Corsica and Sardinia. With relation to Sicily, the map and memoirs of Daubeny and the observations of Lyell and Christie appear the most important.
Of the Mediterranean districts of the opposite continent of Africa, we have obtained far more information than the circumstances of the case would have authorized our expecting. The journals of our own travellers Lyon and Denham are far from destitute of interesting notices on this subject; but much more important are the contributions of Rozieres to the great French description of Egypt; the travels of Caillaud, who penetrated up the Nile to Meroe and the White River; and those of Hemprich and Ehrenberg from Libya into Arabia are still more extensive; and the latter (Naturgeschichtliche Reisen durch Nord Africa, &c. 1828,) especially valuable from the precision of its statements*.
In Asia, the Russian Government has zealously promoted the
* It hence appears that the platform ofthe Libyan desert between the Katabathmi and Siwah is tertiary, including shelly lime, clay and gypsum; the banks of the Nile from Cairo ascending to Siout or Lycopolis present nummulitic lime, possibly cretaceous; from Siout to Esne (Latopolis) oolites prevail; above Syene everything is primitive. Proceeding from Cairo to Suez, after leaving the nummulite lime, we observe red sandstone: dolomitic limestone occurs on the West shore of the Gulf of Suez, through which porphyritic masses emerge; the Eastern coast of the gulf is principally occupied by tertiary lime, but the red sandstone reappears at the foot of the porphyritic group of Sinai. The fossils of the various formations are fully described by the several authors.
[page] 394
physical examination of the northern portion under their dominion. The celebrated Humboldt has also been enabled by the enlightened patronage of the Russian Government to exercise in Central Asia those powerful and generalised habits of research which had previously so greatly extended our physical knowledge of the American Andes; and although the personal observations of this distinguished traveller have been restricted to the countries lying north of the Altai, between the Ural chain on the west and Chinese Tartary on the east, yet he has been enabled to collect from various sources the most important information concerning all the adjacent regions, and especially with regard to the very interesting interval between the Altai and Himmaleh mountains, which he has ably condensed in his Fragmens de Géologie et Climatologie Asiatiques*. We have already cited the European portion of Erman's journey from Moscow to the Lena; that which relates to Asia is equally important.
Abstracts will be found in Férussac's Bulletin, of an account of the Altai mountains, by Ledebour, and of the environs of Lake Baikal, by Dr. Hess; the volcanised districts of Kamschatka, and the vast accumulations of fossil bones of the Elephant along the Siberian coast, have also received further illustration.
* The most important general results thus collected are:—1. The correction of the notions previously entertained concerning the orography of Central Asia, which instead of consisting, as was supposed, of a vast elevated platform, is shewn to be traversed by distinct chains separated by valleys not more elevated above the sea than the vale of Geneva. 2. The circumstances of the great depression beneath the level of the sea ranging around the Caspian and Lake Aral are much more precisely stated than in any former authority: this tract includes a space exceeding 18,000 square leagues, its lowest depression, the surface of the Caspian, being 320 feet below the level of the Black Sea. Humboldt inclines to attribute this vast subsidence to the protrusion of the elevated masses forming the adjoining mountain chains of the Caucasus, the Hindoo-Kho, and Iran. 3. Humboldt's researches have greatly extended our knowledge of the volcanic tracts of our globe; he has shown the whole country round the Caspian to be a vast district of this nature, a "pays cratere" exactly resembling in its general outlines the telescopic appearance of the moon: he has also pointed out another great seat of volcanic action, still more important in its extent, and interesting in the geological inferences to which it leads; this is the chain of Thion Chon, south of the Altai and ranging about 42° lat. N. and between 70° and 90° long east of London. This vast ignigenous district extends over 2,500 geographical square leagues, and being generally remote from every sea, shows that marine contiguity, although a common, is by no means an indispensable concomitant of volcanic action. It is Justly remarked, that in the cases where such a proximity does exist, we may sufficiently account for it on the principle that in the neighbourhood of marine basins there would probably be less resistance to the disruption of the crust of the globe, here attenuated by excavation, than in more inland localities; without having recourse to the supposed access of infiltrations from the sea to imaginary beds of potassium, &c., thus causing an explosion.
[page] 395
In Southern Asia, many of the British residents in India have been far from inactive; among these we may specify the names of Franklin, Voysey, Herbert, Christie, Low, Hardie and Govan; but Calder's general memoir on the Geology of India (Asiatic Journal, 1828,) conveniently and ably brings together in one view the substance of the insulated observations of others*.
* From these sources we learn that primitive formations in which granitic rocks bear the principal proportion, occupy not only the great Himmalayan northern chain, but also three fourths of the entire peninsula, from the vale of the Ganges below Patna to Cape Cormorin; although these rocks are frequently overlaid by a thin crust of laterite, (a ferruginous clay considered as associated with the trap formation.) The transition formations have not been clearly distinguished; the secondary formations described are:—1. The carboniferous group. Coal has been said to occur extensively in the grits bounding the southern slope of the Himmaleh; but it has been questioned whether this formation is the older coal, or only lignite associated with nagelflue, (as on the slope of the Alps); it has been particularly described however where the river Tista issues from this chain (88° 35' long. E.), and there undoubtedly bears all the characters of the older formation; its strata are highly inclined, whereas the tertiary beds, and even most of the secondary in this part of India, are horizontal: but the only coal district regularly worked is that on the river Dumoda, about 100 miles N. W. of Calcutta; this extends on the banks of that river about 60 miles, and appears from its fossil Lycopodia to be undoubtedly the older coal; it reposes apparently on the surrounding primitive rocks, but it has been conjectured that it may possibly extend across the delta of the Ganges to Silhet (almost 306 miles distant at the eastern extremity of Bengal); it seems doubtful however whether the Silhet coal be not really modern lignite, as tertiary rocks certainly prevail in that quarter. No carboniferous limestone has been observed.
2. Next to the coal we have to notice a great sandstone formation, which is usually considered equivalent to our new red sandstone; this includes many variations of character, comprising, besides sandstone and conglomerates, shales often approximating to older slate; the diamond mines of Panna (in the Malwah country) and of the Golconda district (on the Coromandel coast) are situated in this formation, the matrix being a conglomerate bed with quartzose pebbles; rock salt and gypsum are found where this formation extends on the N.W. into the great basin of the Indus; the stratification is uniformly horizontal: no organic remains occur. Beginning at the Ganges on the east, this formation first shows itself, supporting basalt, on the Rajmahal Hills; it again prevails throughout the interval between the confluences of the river Soane and of the Jumna with the Ganges, and thence stretches W.S.W. through the Bundelcund district to the banks of the Nermuda (which flows into the Gulf of Cambay), as far as 79° long. E.; where it is overlaid by the eastern extremity of the great basaltic district of North Western India near Sagar; the red sandstone shows itself again, emerging from beneath the north western edge of the basaltic district, at Neemuch, near the western sources of the Chumbal (the great southern branch of the Jumna), and at Bang, in the valley of the Nermuda. In both places, as also along the central portion of the platform before described, stretching through Malwah, it is frequently covered with a thin crust of grey argillaceous limestone, supposed to represent our lias, but nearly destitute of organic remains, although a single Gryphite is said to have been found. The general absence of organic remains in the secondary rocks of India is remarkable; but Mr. Voysey mentions an argillaceous bed full of fossil shells (species not stated,) beneath the trap of the Gonilgesh Hills, (between the confluence of the Tapti and Purnah in the Berar district;) the same lias-like beds occur with the red sandstone of the Golconda district. A primitive range extending from near Delhi to the head of the Gulf of Cambay, separates the secondary rocks of Malwah from those of the great basin of the Indus; but on the western borders of this ridge through Ajmeer, the red sandstone again shows itself, containing rock salt and gypsum. The whole of this immense basin appears to have been hitherto geologically neglected, although it would probably best repay such an examination; for here, if anywhere in India, we might most probably expect a fuller series of secondary rocks. Mr. Govan has observed at the very source of the Sutlej, one of the chief tributaries of the Indus, amid the highest primitive peaks of Himmaleh, a small basin of secondary limetone containing Ammonites and Cardia.
3. Tertiary rocks occur at the foot of the first rise of the primitive rocks of the Himmaleh, in the North-west of Bengal, where the Brahmaputra issues from them at the pass of the Garrow Hills; Cerithia, Turritellæ, remains of lobsters, sharks, crocodiles, &c., are here found; and further east nummulite limestone prevails at Silhet. The soil throughout Bengal is often occupied by deposits of clay, containing concretionary lumps of limestone called Kunkaer: this, which affords the principal supply of lime in India, is probably of very recent origin. It remains only to notice the great basaltic district of the northwest. This extends from Nagpur in the very centre of India to the western coast between Goa and Bombay, occupies the whole of that coast to its termination at the Gulf of Cambay, and thence penetrates northwards as far as the 24th parallel of north latitude.
In the Burmese empire we find primitive rocks in the chains above Ava, but tertiary beds, with the characteristic shells, in the valley of the Irrawady near Prome; also remains of the Mastodon, &c., in the diluvial gravel. West of this the whole chain of the Malayan peninsula is primitive, consisting principally of stanniferous granite.
I believe that the above, condensed as it is, will be found the fullest general account of the progress as yet made in Indian geology hitherto presented to the public.
[page] 396
Of the coasts of Australia we have derived much information from the circumnavigation of Capt. King; and all the geological notices he was able to procure have been excellently digested by his friend Dr. Fitton in a general resumé appended to his Voyage. Since this we have obtained some additional details with regard to the neighbourhood of Swan River; but the interior of this vast country still remains a terra incognita, not only to the geologist, but even to his precursor the geographer.
Turning from the Old to the New World, we first naturally look for information to the United States.—In Sillliman's Journal we find many very good detached memoirs; but the general outlines of the geology of that continent, so long ago and so ably sketched by Maclure, are as yet but very imperfectly filled up. In the imperfect state of secondary geology at that time, his errors in this respect, confounding these formations with alluvial, &c., are not to be wondered at. Subsequently the progress of this department has been much retarded by the strange no-
[page] 397
menclature adopted by Mr. Eaton, and his subdividing a single formation, that of transition limestone, into many members, of which some were absurdly identified with the English lias, &c.; but more recently Mr. Featherstonhaugh, a geologist eminently qualified from his intimate acquaintance with European formations, to advance those comparative views which demand the principal attention in our science, has undertaken a philosophical journal in which these will be especially attended to; he has also presented us with a correct table of the correspondence of the American and European formations, from which it appears that in the regions west of the Alleghany, nothing newer than the carboniferous series has yet been found; the saliferous sandstone appearing, as an equivalent to our old red sandstone, to under-lie these*.
Bigsby has ably explored the geology of the great Canadian lakes. This district principally consists of transition limestone; indeed the line of junction of this formation and the primitive rock throughout the whole northern extremity of America is accurately marked by the great chain of lakes, the primitive zone ranging along their eastern, and the transition along their western borders. Franklin's celebrated expeditions have made us acquainted with all the arctic portion of this tract; and Giesecke and Scoresby have explored the nearly contiguous and equally inhospitable shores of Greenland; the American expeditions to the source of the Mississippi have made known the structure of the great central valley, and enabled James to continue Maclure's sections as far as the Rocky Mountains†.
Burkart and Sartorius have published a geological map and Memoirs on Mexico‡.
On the east of the Alleghanies, in New Jersey, formations which appear from their character and fossils equivalent to the galt of our cretaceous group, have been described by Mr. Moreton. I suspect, from ancient notices in Woodward,
* In this there is nothing that ought to surprise geologists; the saliferous character appears common to rocks of every age, and no view can be more narrow and incorrect, than one which should regard it as restricted to the pœcilitic æra. We have long been acquainted with saline springs in our coal measures; in the more recent secondary rocks, we find the salt of the Alps chiefly in the midst of the oolitic series. The very modern tertiary clays of Sicily and the Sub-Apennines are saliferous; and Boué considers the salt deposits of Transylvania as of the same age; the salt of Wielilzka is also in the tertiary formation.
† At St. Louis on the Mississippi is a limestone with impressions, vulgarly referred to human footsteps. From the representations of these in Silliman, I should conjecture them to be casts of a large Perna, in which case this limestone may be analogous to our oolites.
‡ Hence it appears that near Catorze red sandstone occurs covered by limestone containing Ammonites.
[page] 398
that tertiary deposits occur in Maryland; but I am not aware that they have been recently explored.
With regard to the West Indian Islands, Jamaica has been fully described by De la Beche; and De Jonne's Histoire Physique des Antilles Françaises contains much geological information. As to South America, the completion of that part of Humboldt's Travels which relates to Peru is announced; while on the eastern side we have the works of Spix and Martius*, and of Pohl describing the structure of the Brasils: at the southern extremity of the continent Capt. King has observed chalk and green-sand in Patagonia near the Straits of Magellan.
Having thus cursorily reviewed the great progress of what may be termed geographical geology during the last few years, we have to advert to those particular branches which, from their especial development within this period, and peculiar relations, appear to demand a distinct consideration.
And here we may first notice the great advancement of the history of the tertiary series of formations,—an advancement which does not merely consist in multiplying details, but in tracing the precise facts† which mark an epoch perhaps the most important as to geological theory, inasmuch as the geological causes then acting, evidently most nearly approximated to the physical circumstances still operating under our own experience and observation; and the former appear to have passed into the latter, not by an abrupt leap, but by a gradual transition. We shall be more sensible of the importance and extent of the additions thus introduced, if we remember how short a period has elapsed since every geologist used to talk of the tertiary formations of London and Paris as the very youngest of rocks; but these have been now elevated to the rank of a
* These geologists describe a sandstone, considered by them as equivalent to the German keuper, as prevailing in the basin of the Amazons; and they assign the same formation, as the matrix of the diamond mines, a near approximation to the geological site of the Indian mines of this gem; the sandstone of the plains of Paraguay is however said to be tertiary molasse.
† The discussions by Prevost and De Serres, whether the succession of those formations, and the alternation of marine and fluviatile beds indicate such an oscillation in the level of the surface as may have caused reiterated elevations of the ocean, so as repeatedly to submerge again continents once abandoned, or whether, on the contrary, an uniform and constant depression of the oceanic level, so as to convert oceanic basins successively into gulfs and æstuaries, (the character of whose waters varied as the tidal inundations of the ocean or land floods prevailed,) and into lakes generally of fresh water, (but still so situated as to be exposed occasionally to the incursions of still neighbouring seas,) can only be shortly alluded to in a Report like the present, but require to be studied at length by every one interested in the subject; the same remark will apply to the views of De Serres on the distinctions of the tertiary basins which appear to have been originally connected with the ocean, and those dependent on the Mediterranean.
[page] 399
venerable antiquity as compared with the more recent tertiaries of the Loire, and still younger deposits of the Sub-Apennines and Sicily. These distinctions will be found as well marked as any between the secondary formations; and still more important in the inferences to which they lead. The researches of Desnoyers, Prevost, and Marcel de Serres appear to have led the way in these investigations; but Des Hayes, Basterot, Tournal, Reboul, and the Venetian Catullo, have ably cooperated; our own countrymen also, especially Murchison and Lyell, have brought to this subject all their usual zeal and intelligence: the former has materially contributed to the elucidation of the tertiary formations of the Alps and Germany; and the latter has, in conjunction with Deshayes, produced complete lists of the fossil shells of the several successive tertiary formations, designating in each the proportion which the fossil species apparently identical with those still existing, bear to these which appear to be extinct*.
From the tertiary formations we naturally ascend to the
* It has been ascertained that the inferior, middle and superior group of tertiaries contain respectively the increasing proportions of 3, 19, and 52 per cent. identical with actually existing species among their fossil shells. It is interesting also to observe that while the inferior group passes insensibly into the subjacent secondaries by a kind of transition beds at Gosau and Maestricht, the superior group in its upper limits equally approximates to the actual order of things, some of the highest beds at Nice and in Sicily (although here elevated many thousand feet above the present level of the sea,) containing 94/100 of recent species. May not the sands with recent shells observed at a height of 1000 feet by Mr. Trimmer in Snowdonia, and those mentioned by Mr. Gilbertson in Mr. Murchison's Report to the former Meeting of this Society at York, as occurring, though at a less elevation, in the inland parts of Lancashire, belong to the same æra?
The mammiferous remains imbedded in these groups observe a similar progression, extinct palæotheria, &c., alone occurring in the lowest group, while the middle group exhibits these with a mixture of the elephants, &c., of our own diluvial gravel, and the latter class become prevalent in the superior group to which our own crag is referred.
It is further important, as displaying the great variety in the mineral constitution of rock formations of the same æra, and evincing how impossible it is to take that character as any criterion of their geological age, to observe that the single group of tertiaries (that occupying the middle place) contains, near Aix, rocks which, without an examination of their geological position and organic remains, might have been confounded with those of our own coal measures: and again in the nagelflue and molasse of Switzerland, &c., conglomerates and sandstones like those of the pœcilitic group; similar rocks at Cordona in Spain, and probably also in Poland, containing salt and gypsum, which constitute a further resemblance; and lastly, in Lower Styria, coralline limestone likeour own middle oolites alternates with this series.
M. Boué has recently suggested that much of the tertiary districts of Belgium, North Germany, and Poland appear to him to belong, not to the inferior or Parisian group, but to the superior or Sub-Apennine systems.
[page] 400
modern rocks of volcanic origin, since it is from the tertiary period that the monuments left by that mighty geological cause assume a character so distinctly marked, as neither to be overlooked nor mistaken by even the most cursory glance. The most important group of extinct volcanoes with which we are acquainted, that of Central France, attracted the attention of geologists from a very early period; they were first noticed by Guettard 1751, and soon after described by Desmarets and St. Fond, and many others*. The more precise researches of the present day have produced the detailed and exact labours of Le Coq and Bouillet (still in the course of publication); but an English geologist, Scrope, has presented us with perhaps the most interesting account of this important district. By the peculiar mode he has adopted in the graphical illustrations of his beautiful work, (combining geological colouring with picturesque representation,) he places all the most important features of the district distinctly before our eyes, thus enabling us as it were to travel ourselves over the whole country with him, while his letter-press insures us the society of a most intelligent guide†.
The volcanic district of Eiffel and the Rhine, was years ago most ably described by Nœgerrath and Steininger; and one of its most interesting portions, the basin of Neuwied, has very recently been made the subject of an elaborate monograph by our own countryman Hibbert; Schultz (Archiv für Bergbaa) has also published on the same subject. Boué has also recently materially enlarged the information which Beudant had previously communicated on the volcanic districts of Hungary. With regard to active volcanoes and their general theory, we have also to notice many most valuable recent contributions: the general essays of Daubeny and Scrope require to be carefully consulted: but by far the most important contribution which volcanic geology has ever received is Von Buch's physical description of the recent volcanic district of the Canaries; in this he has included the most masterly general survey of the whole volcanic subject, tracing the general range of volcanic lines over the globe, the particular configuration and relations of single groups, the characters which distinguish the volcanic products of different periods, and their general analogies with the early unstratified and probably ignigenous rocks ascending to
* Desmarets in 1768, from his observations in Auvergne, first ventured on the hypothesis that the basalt of that district was a volcanic product.
† Mr. Scrope has also communicated to our Geological Society some important papers on the Ponza Isles.
[page] 401
the primordial granite; he points out also the general relations existing between the granitic axis of the mountain chains, the lines of elevation and dislocation of the strata, &c.; and the lines of the actual volcanic craters in countries where such still continue active; or, where these are absent, the points marked by the kindred phænomena of earthquakes and thermal springs; concluding that from these relations it is impossible to propose as explanatory of volcanic phænomena any probable theory, which does not at the same time embrace the entire structure of the globe in all its generality.
Viewing the question of volcanic agency under these general relations, the problem of the proper temperature of our globe as emanating from some central source demands our first attention. The Essay of Cordier* on this subject is at once the most extensive and exact; and Fourvier has also illustrated this important subject, which has been well termed "the fundamental basis on which a rational system of geology must rest," by the careful application of his general theory of heat. The observations of Fox in our own Cornish mines have been among the best data supplied to this investigation.
The inquiries instituted by Elie de Beaumont concerning the prevailing lines of direction† in which the principal European chains of mountains have been elevated, and the epochs at which the disturbing forces have acted in the disruption of the terraqueous crust, have also afforded the first example of an attempt to generalize some of the most important phænomena above alluded to by Von Buch; but on the details of this theory and its application to the phænomena of our own island, I am called upon to report at length to the geological section of our own general body‡; and I shall only now observe that while I warmly admire the powerful grasp of mind which it displays, I must still consider many of its principles as rather locally than generally applicable; the wide extent embraced by its general enunciation will undoubtedly require much modification; and the unguarded manner in which the entire elevation of chains like the Alps appears to be referred to a single convulsion,
* The same author had previously supplied us with our most accurate information on the constitution and varieties of actual volcanic products.
† De Beaumont's theory on the general direction of our lines of stratification may suggest to us another recent attempt of M. Necker to point out a relation between these lines as indicated by the general outline of our continental masses and the lines of equal magnetic intensity, (Biblioth. Univ. 1830). Should there prove to be any foundation for such a theory, it ought undoubtedly to be brought into connexion with Fox's observations on the electro-magnetic phænomena developed by the metalliferous lodes of Cornwall, &c.
‡ This Report will be found in the Annals of Philosophy for July 1832.
2 C
[page] 402
rather than to the repeated disturbances of a long succession of geological epochs, I must regard as neither consistent with antecedent probability nor with the most careful examination of the phænomena; yet it is impossible to speak too highly of the value of the general mass of geological information concentrated in his treatises on this subject.
The effects of diluvial action in the transport of rounded fragments of rocks to vast distances from their original site, present a subject of investigation certainly not less important than the preceding questions connected with volcanic agency and the theory of the elevation of our strata. On this subject late years have afforded us many important Memoirs,— viz. those of Sedgwick on our own country; of Haussman on North Germany; of Von Buch and De Luc, jun. on the transported blocks of the Alps; of Brongniart on those of Sweden; Sokolof on those of Russia; and of Lariviere and Schall on those of the Netherlands.
Palœontology,
or the study of the remains of the extinct animals brought to light in these researches, has made no less progress than the other branches of geology within the last ten years: this period has witnessed the complete restitution, and I may almost say the resurrection, of the long-extinct and monstrous Saurians of the lias; the oolites of Stonesfield and the Wealden limestone of Tilgate have yielded the Megalosaurus, and the Iguanodon, to the researches of Buckland, and Mantell.
Adverting to the history of the fossil mammalia of the most recent geological period, Buckland has exhibited the remains of the numerous bone caverns (a subject previously buried in confusion and obscurity,) in their true general relations to the congenerous remains of the diluvial gravel; and his acute observation and extensive information on the general habits of the animal kingdom have thrown a light, which antecedently it would have appeared almost chimerical to expect, on the particulars of the history of these long-extinct races, and proved beyond a doubt that they were originally the inhabitants of the districts where their remains are now found: but still on many questions connected with this curious and interesting subject, especially the relative age of the human bones occasionally found in the saine cavern (as at Bize in the South of France), we are bound to compare the opposite views of De Serres, Christal and Tournal with those of Buckland, with whom however Desnoyers appears entirely to agree.
One of the most interesting recent discoveries in this depart-
[page] 403
ment has been the importation from Buenos Ayres of far more complete remains of the Megatherium than had been previously obtained; hence we have learned that this gigantic animal approximated more nearly to the Dasypus (Armadillo) than to any other of the congenerous Edentata, in the possession of an osseous coat of plate armour; a circumstance hitherto regarded as peculiar to the latter animal: it also possessed a long tail*.
We have to deplore within the present year the loss of that illustrious naturalist who was the first to raise comparative anatomy to the rank of an exact science, and who, by his highly philosophical generalization of the constant coordinate relations of the animal structure, became at once the Newton of that science. Many an investigator, following in the paths he first pointed out, has reaped brilliant discoveries by the comparatively easy labour of deciphering the remains before him by the key fully furnished in the Ossemens fossiles. It is especially to be regretted that we have lost our great instructor before the completion of his elaborate work on Ichthyology had brought the fossil remains of this class under his review. On this subject our knowledge is as yet extremely confused. In our own island the Caithness schist, the magnesian limestone, the lias, the chalk, and the London clay, each present numerous species, and it is much to be desired that some competent naturalist should undertake a connected and exact anatomical investigation of all these remains.
In the department relating to the invertebral fossil remains, every one interested in the subject must hail the splendid work of Groldfuss, which already includes the corals and radiaria, as the most copious, beautiful and accurate which has yet appeared.
The accurate knowledge of the vegetable remains preserved
* Prof. Buckland's observations on these remains have afforded a model of the most philosophical inferential reasoning; deducing the probable habits of this extinct being from the data afforded by the organization of its skeleton; and he has thus shown that organization, considered under these relations, to be as perfect and exquisitely adapted to the wants of the animal, as in any other work of the same creative intelligence: firmly planted on its colossal hinder extremities and one of its fore-feet, the construction of the parts enabling it to bear such a position for any length of time without fatigue, it may have almost incessantly employed the remaining fore-foot, by a swing backwards and forwards (for which it is expressly fitted), in scratching from the ground, by the three powerful claws with which it is furnished, those tubercular roots the common produce of the countries where it is found, and which the structure of its grinders indicate to have been its proper food. Its maily covering would have protected it from the annoyance which the sand and dust raised by this operation must have occasioned to any animal merely invested with an hairy coat; and its powerful tail would have defended it from the attack of any animal which might venture to approach it in this quarter.
2 C 2
[page] 404
in a mineral state, is a branch of our science which may almost be said to have been created within the last ten years. Schlotheim and Count Sternberg were among the first to enter on the field, and opened it very ably; but still we must always look to Adolphe Brongniart as the great introducer of complete and general views into the department of Fossil Botany. Every one interested in the subject must be familiar with his exact assignment to the great formations of the successive geological æras, of different classes of vegetables peculiar to each, and his inferences as to the higher temperature of the surface of our planets at these earlier periods, in some of which every analogy points out an insular tropical climate as alone capable of having nourished the vegetable forms detected.
In one instance, however, it appears that we are now probably authorized to make an important addition to his statements; for while he had detected only vascular Cryptogamiæ and a few Monocotyledoneæ, as Palms and arborescent Liliaceæ, in our carboniferous rocks, Mr. Witham appears fully to have established the occurrence of Coniferæ* also, by obtaining such thin and transparent slices of the fossils as allow a microscopical investigation of the structure of the sections thus procured, as perfectly as if they were those of recent vegetables: these Coniferæ appear to have been species of gigantic growth, and pefectly distinct from those now existing; they are found to prevail in the Scotch coal-fields, while that of Newcastle presents a great predominance of vascular Cryptogamiæ. Mr. W. has boldly conjectured that the former may perhaps have been derived from the drift timber of forests once growing on the Grampian chain already an elevated island; while the latter may possibly have formed the vegetation of more swampy lowlands, and grown nearly in the present site.
Messrs. Lindley and Hutton are now publishing a Work, in numbers, on the fossil vegetables of our own island.
Many general treatises on geology, of which the leading object must necessarily be the systematic arrangement or classification, and comparative views of the several formations, have appeared since those of Daubuisson and Humboldt, the merits of which however have not yet been surpassed; but among the best of the more recent publications we may mention Keferstein's Tabellen, Brongniart's Terrains, and D'Halloy's
* Mr. W. has indeed as yet published only transverse sections of the allegedConiferæ, while our best botanists are of opinion that transverse sections alone cannot afford certain characters of this class; yet assuredly the sections he has already given appear decidedly remote from the monocotyledonous and closely approximate to the coniferous structure.
[page] 405
Elemens. De la Beche's Manual is the best work of this kind which has yet appeared in our own country.
It is much to be regretted that while we fìnd a general agreement as to the facts of the science in all these works, that agreement is sometimes obscured from the want of a simple and uniform terminology of classification*, which should be generally adopted by geologists; at present, indeed, such a terminology can be only considered as provisional, inasmuch as we are as yet only competently acquainted with the European series of formations; and it would be too hasty to pronounce that when those of the globe generally shall be equally known, many modifications may not be required in our arrangements. Still I am persuaded that the general adoption of some such confessedly provisional classification would materially advance the present interests of our science.
A recent Essay of Boué's, however,Considerations sur la Nature et sur l' Origine des Terrains de l'Europe, assumes a character of far higher philosophical speculation, than any mere systematic treatise on geological arrangement: from the distribution and phænomena of each formation it endeavours to deduce the condition of the globe which prevailed at the period of its deposition, and the probable causes which concurred in its production. Leibnitz would have seen in it an able attempt to fill up in the detail from the results of geological investigation, the history of those successive changes of the terraqueous crust, of which his powerful mind anticipated the general outline. We may not, indeed, implicitly concur in all the views ad-
* It would be one of the advantages of a scientific reunion, such as our present Society, if at some of our future sectional meetings such discussions were entered into, as might tend to promote this object; should any leading foreigners be present, such a design appears by no means chimerical. I have already suggested some occasional hints on this subject. I will venture here to submit to the attention of geologists the following sketch of a general classification, founded on an eclectic principle of borrowing from the works referred to in the text the arrangements in which they seem most likely generally to concur: as we have in other natural arrangements families, orders, classes, genera and species, so in geology we may have the aqueous and igneous families; the terms primitive, transition, secondary and tertiary, confirmed by long use, may be retained for the four orders. The primitive would contain only the mica schistose group; the transition might contain three groups,—1, the clasmoschistose or grauwacke; 2, the anthraxiferous or lower coal measures; 3, the carboniferous. I include 2 and 3, as does D'Halloy, in the transition order, being convinced, from the affinities of their organic remains, and every other relation, that such is their most proper place.
The secondary order would include, 1, the pœcilitic group; 2, the oolitic; 3, the cretaceous. The tertiary order has also three groups, for which Lyell has proposed the names Eocena, Meiocena, and Pleiocena. I would inquire however whether some such designations as hypotrital, mesotrital, and hypertritai, would not be more simple.
[page] 406
vanced in this Essay; but it may be fairly said of it, that it contains the most complete and condensed general view of the geological phænomena, ascertained from observation, which we have yet seen, and that by exhibiting these phænomena in a constant bearing upon all the great questions of geological theory involved in them, it affords a store of materials for the investigation of their true relations, with which no one interested in the science can dispense.
Mr, Lyell's recent Work, in itself sufficiently important to mark almost a new æra in the progress of our science, may well close this imperfect survey. He has done well to call the attention of geologists to a generalized examination of the various changes still effected in our planet by the physical causes in operation at the present day, under their precise actual conditions; because no real philosopher, I conceive, ever doubted that the physical causes which have produced the geological hænomena were the same in kind, however they may have been modified as to the degree and intensity of their action, by the varying conditions under which they may have operated at different periods.—It was to these varying conditions that the terms, a different order of things, and the like, were, I conceive, always intended to have been applied; though these terms may undoubtedly have been by some writers incautiously used. But as all are probably agreed, that the causes of nature are permanently the same in kind, however their operation at different periods may have been modified by the varying conditions under which they may have acted, it is an obvious consequence that we shall be altogether unqualified to speculate on the former action of these causes, unless we are previously fully acquainted with their actual operation; this must ever be the great key to the analogies of their earlier geological operation; and he who has so ably extended our knowledge on this fundamental point as Mr. Lyell has done, must ever be considered a most important benefactor to our science. The manner, especially, in which he has brought all the stores of a richly informed mind to bear on his subject, and, if I may so speak, by a kind of process of assimilation, converted as it were everything within the grasp of that mind into geology, is admirable as augmenting the domain of our science, and evincing over how wide a field that domain may be extended, when administered by a powerful intellect. His application also of actual causes to the solution of the geological phænomena of the tertiary epoch, is felicitous and satisfactory; for no one can dispute, that the general conditions under which physical causes operated on the surface of our planet, at this most recent geological period, approximated most closely to the conditions
[page] 407
of the present day; but many of us may nevertheless be permitted to doubt, whether the same identity can be predicated of the general physical conditions affecting the surface of our planet at those earlier primordial periods, when as yet the series of rocks, constituting the whole mass of that portion of the terrestrial crust with which we are acquainted, were only beginning to be deposited; since, even after the long interval which must have elapsed from the first primordial epoch of the micaceous slates, to the deposition of the carboniferous rocks, we are led to infer that, at this later and comparatively modern period, the surface of the globe was still chiefly oceanic, interspersed only with scattered groups of islands, having a tropical temperature; while the animal inhabitants of that surface were restricted to shell-fish, Zoophytes, and possibly a few vertebrated fish, and still fewer Saurians; and its Flora exhibited only gigantic ferns, palms, Equisetaceæ, and Coniferæ. I would repeat then, that we may still perhaps be permitted to doubt, that an identity of physical conditions can be predicated of the surface of our planet between these periods and the present,— even without going as far back as a reviewer (and he a very favourable one,) has done, and referring to the original state of fluidity of the planet, as deduced from its spheroidal form. It may perhaps be the most truly philosophical rule to guide the spirit of our investigations, that whereas the actual operations of nature, and those indicated by geological observations, present certain points of analogy, and other points of difference,—so it is equally contrary to a sound spirit of inductive reasoning, to confine our attention exclusively to the one class of facts or to the other. It may be that former geologists have erred in restricting their considerations too much to the class of differences,— and Mr. Lyell has done well to recall their minds to the contemplation of the many analogies; but that these alone deserve our regard is quite another thing.
I now in conclusion proceed to offer the views, which are most impressed on my own mind, as to the present prospects of our science, and the objects which most claim our attention at this time, and promise most fairly to reward that attention.
The first points of the science are undoubtedly those which connect it with its elder and far superior sister, Physical Astronomy. I mean such questions as those relating to the spheroidal figure*, and to the density, of the Earth;—the inquiry
* It has been well observed in a very able article in a late periodical, that "the most conclusive argument against the fact of any disturbance having, in remote antiquity, taken place in the axis of the earth's rotation, is to be found in the amount of the lunar irregularities which depend on the earth's spheroidal figure. However insufficient the mere transfer of the mass of the ocean, from the old to the new equator, might be to ensure the permanence of the new axis, the enormous abrasion of the solid matter of such immensely protuberant continents as would, on that supposition, be left, by the violent and constant fluctuation of an unequilibriated ocean, would (according to an ingenious remark of Professor Playfair,) no doubt, in lapse of some ages, remodel the surface to the spheroidal form; but the lunar theory teaches us that the internal strata, as well as the external outline of our globe, are elliptical, their centres being coincident, and their axes identical with that of the surface,—a state of things incompatible with a subsequent accommodation of the surface to a new and different state of rotation from that which determined the original distribution of the component matter.
[page] 408
entered into by Sir J. Herschel, how far the secular diminution of the eccentricity of its orbit may have tended to the decrease of its temperature; and the like. Now one of these points, and that very important to geological theory, appears to me to require further investigation; I mean the conclusions fairly deducible from the known density of the earth, as to the solid structure and composition of its interior. As its density is known to be considerably greater than that of a solid sphere composed of any such rocks as we are acquainted with (granite, for instance), our prima facie inference would naturally be, that the interior is solid; and that heavier materials than our ordinary rocks (such as metalliferous masses,) enter into its constitution. But to this it may be objected that the rocks alluded to have in themselves a principle of elasticity and compressibility, and therefore may, under the vast pressure existing in the interior of the globe, be condensed to such a degree as is far more than sufficient to account for the excess of the earth's density, as compared with their specific gravity, and thus still to allow for considerable vacuities. To this, however, a counter argument may be fairly adduced; that, as the resistance to further compression increases with every additional pressure, that resistance may soon, in the case of these rocks, become practically infinite. A more accurate examination of the whole circumstances of this problem appears highly desirable*.
The next branch to which I would call your attention may,
* I am well aware of Prof. Leslie's ingenious Essay on this subject, in which he concludes that compressed light occupies the hollow centre of our globe, being the only substance which possesses a sufficient elastic force to resist and balance the enormous pressure of a vertical column of 3,500 miles; so that the interior of our planet is not, as falsely supposed, obscure, but filled with luminous æther, the most pure, concentrated, and resplendent. I will only observe, with reference to the calculations which are supposed to lead to this truly brilliant conclusion, that it is assumed that the modulus of compressibility of air, water, &c., is invariable, however greatly the pressure may be increased, so that we may have air condensed first into the density of water, and then, still following its former law and not that of the fluid whose place it has taken, to marble, and so on ad infinitum. In answer to this I shall only inquire whether it is actually found that the condensed gases, the subject of Faraday's beautiful experiment, after they are metamorphosed into fluids still retain the same modulus of compressibility which originally belonged to them in their gaseous state.
[page] 409
I think, be termed the true dynamics of geology, with far more justice than that appellation has been applied to other branches of our science. I would so denominate the general consideration of the forces which appear to have been the agents in dislocating and elevating our strata; whether in the earlier geological disturbances, or in the actual phænomena of volcanoes and earthquakes. We have already seen how much Von Buch has contributed, of the most important generalizations, to our knowledge of actual volcanic phænomena; and Elie de Beaumont has been nearly the first to call attention to anything like generalized views with regard to geological elevations. But still it is but a very small portion indeed of the totality of these phænomena, which have yet been brought under our cognisance. Indeed, the more general science which includes this, our knowledge of the physical geography of the different regions of our planet, is still in its infancy; but, as it shall advance, is it too much to anticipate the most important conclusions, when we shall be able to speak of the periods of elevation of the Himmalaya, the Andes, and the American Rocky Mountains, with as much evidence as we now do of the Pyrenees and Alps? When we shall have a generalized view of the principal disturbances of the great continents, may we not hope to enter, with a prospect of satisfactory solution, on the great problem of the elevation of those continents, and the determination of their general forms, on the consideration of the forces which have produced these effects, and even on the dynamical investigation of the laws which those forces appear to have followed? There is one source of analogy which has always appeared to me as likely to throw illustration on this subject, and which I yet almost hesitate to allude to, lest I should incur the charge of indulging speculations altogether rash and visionary. However, I would premise the observation, that we must surely in no respect consider our planet as an isolated body in nature; it is one only of the general planetary system, and every fair presumption of analogy favours the supposition, that similar general causes have acted in all the members of that system. Now one of these members, our own satellite, is placed sufficiently near us to enable our telescopic observations to distinguish accurately the general outlines of its mountain chains, and other similar features of its physical geography. We have been able to discern even the eruption of volcanoes; and any one who has viewed its surface through a telescope, must be struck with the exact identity of the forms which he there contemplates with the maps and descriptions of the volcanic districts of our own globe. If we recall Von Buch's account, already referred to, of crateriform
[page] 410
amphitheatres of many leagues in diameter, encircling central conical craters; of lines of these generally disposed in a linear direction; of such linear trains often radiating from a central focus of principal disturbance; we may almost fancy that this description was intended as an exact portrait of what we observe on the lunar surface. Is it, then, altogether unfounded to believe that by more carefully observing these phænomena, where we have a whole hemisphere of a planet at once open to our inspection,—by comparing the best of the early delineations of its telescopic appearance, with its exact actual forms, and watching diligently those forms, so that we may be able to detect any changes in them,—is it too much to hope that we may thus effectually extend our knowledge of the general laws of the volcanic forces, which should appear to be among the general planetary phænomena*?
The great branches of the comparative geology, and comparative palæontology (or study of fossil remains) of distant countries, much as they have recently advanced, have as yet even a still wider interval to pass over than that which they may have already accomplished, before they shall have obtained that degree of completeness which alone can qualify them to serve as sound bases in any geological theory.
First, as to comparative geology. The very introductory question is yet inadequately answered, Is there or is there not anything like such a general uniformity of type in the series of rock formations in distant countries, that we must conceive them to have resulted from general causes, of almost universal prevalence at the same geological æras? Now it is clear that this question, if intelligently proposed, does not require, for its affirmative solution, anything like an exact identity of formations in remote localities. It does not require any one to be able to take to Australia a detailed list of English strata, and to be able at once to lay his hands on the exact equivalents of our lias, oolites, and chalk. Such an idea would be almost to caricature the Wernerian dogma of universal formations. We are indeed unable to trace many of these formations, even through our own island, without observing such considerable modifications in their comparative types, in our northern and southern counties, as may sufficiently remind us that we are to look only for such analogous rather than identical results, as would naturally proceed
* The ancient selenographical maps of Hevelius, Ricciolus, and Cassini, are too defective in precision of outline to be of much use. Russel's lunar globe and Schrœter's detailed plates afford every desirable information; and Mr. Blunt has recently published in a cheaper form a very beautiful engraving on a single sheet, extremely accurate, and amply sufficient for the purpose.
[page] 411
from the contemporaneous action of similar causes in distant localities; in each of which many varying local circumstances must have affected those results; for two conditions obviously enter into this problem:—first, the contemporaneous prevalence and extent of similar geological causes; and secondly, how far these causes, even where active, may have been modified by varying local circumstances. Now, at present, our materials for answering these questions accurately are confined to Europe: of America indeed we have some information; and although this may as yet be considered too vague to be fully satisfactory, yet as far as it goes it is undoubtedly favourable to the presumption of even a greater degree of geological uniformity, than we should have been justified in anticipating a priori.
Humboldt indeed has remarked, that while on entering a new hemisphere we change all other familiar and accustomed objects,—while in the plains around we survey entirely new forms of vegetable and animal being, and in the heavens over our heads we gaze on new constellations,—in the rocks under our feet, alone, we recognise our old acquaintances. And with regard to the primordial rocks, there is undoubtedly much truth in this pointed remark. Granite, mica slate, and their contained minerals, present the most identical resemblance, whether we collect from Dauphiné, Norway, the Alleghanies, Egypt, India or Australia. But concerning the secondary series, our information is far less precise.
With regard to the comparative geology of secondary districts, the fossil zoology of the various districts, or comparative palœontology, requires to be called to our aid as our surest guide. And these investigations are the more interesting, because an important primary question here suggests itself. In the actual state of things, the limited geographical distribution of identical species, both animal and vegetable, is one of the most striking phœnomena that presents itself to our view. In distant continents, the specific differences of the animal races are wide and strongly marked; and sometimes, as in Australia, the difference extends to the character of genera, and even families; and this even in countries of similar conditions, as to latitude, climate, and temperature. Now we may naturally inquire, whether it does not seem most probable, that in the ancient geological æras the species then inhabiting our globe were grouped together under similar restrictions as to geographical habitation; and if so, how far we are entitled to expect to find in other countries the same series of successive organic remains, (each group characterizing a distinct geological æra,) which we meet with in Europe. To illustrate this inquiry:—If in the present
[page] 412
age the recurrence of violent convulsions were again to submerge Europe and Australia, and cover their surface with fresh sedimentary depositions, these new formations, though absolutely contemporaneous, would, in either continent, (should they again be laid dry and exposed to observation,) be found to exhibit in the organic bodies thus imbedded, differences to the full as marked as those, which, in the same continent, characterize formations of the most distant ages. Now with regard to the secondary rocks of distant countries, our information is as yet far too limited to enable us to return, to the above questions, answers on which we can rely with any degree of condfìdence: but the evidence, as far as it goes, does undoubtedly again seem to indicate a greater degree of resemblance than we could have reasonably anticipated: this may particularly be instanced with regard to the older rocks containing organic remains,e. g. the transition limestone: we are extensively acquainted with this rock in Russia, in the islands of the Baltic, in Scandinavia, and in North America; and its fossils everywhere exhibit so near a generic resemblance, that it would often puzzle even a practised eye, if two groups of specimens, one from Dudley and another from Melville Island, were placed before him, to say which specimen came from which locality: the same Cateniporæ, Caryophylliæ, and Encrinites, being present in both. The fossil vegetables of the coal-fields of Europe and America also appear very similar; but we shall probably often, on a more accurate investigation, observe specific differences combined with generic resemblances: still, undoubtedly, the impression on my own mind, from a tolerably careful examination of all the specimens I have hitherto seen, is, that a much nearer approximation may be observed between the fossil animals and vegetables of the old and new continents than between those occupying them at the actual period. And, what is peculiarly important, we find, as I have already observed, even in the highest latitudes of the arctic ocean, types which appear characteristic of a tropical temperature; and the general diffusion of those types in the rocks of the transition and carboniferous periods, in whatever latitude they are found, appears to imply a much greater equality of temperature to have then prevailed than in the present state of things. It seems also inconsistent with the existence of these beings that such wide variations of temperature between the different seasons should then have occurred, as must necessarily accompany, in high latitudes, any temperature derived entirely from the sun,—a consideration which renders inapplicable to this case the hypothesis, that the higher temperature required by geological inferences, may be accounted for by the diminution of the eccentricity of the earth's orbit, since this
[page] 413
would still leave the inequality of the seasons in the higher latitudes as great as at present. For this more equable temperature, then, it seems difficult to account, without having recourse to the hypothesis, which so many other geological arguments render probable, of an internal source of heat proper to the globe itself.
Of more recent secondary strata than the carboniferous series, there appear few traces in the parts of America yet explored, excepting, as I have already observed, the marls of New Jersey, of which the fossils exhibit a close generic agreement with those of our subcretaceous formations, such as the galt, although they are certainly often specifically distinct. In Virginia we have also extensive tracts of shells, approximating to recent species, as in our own tertiary deposits; and in the diluvium we may observe that the Mastodons approximate to those of Ava and Tuscany, although nevertheless mostly specifically distinct, while the Megalonyx is peculiar to America.
We have yet collected from India and from Australia little information on which we can rely, only that it appears that the usual remains in the bone caves of the latter, are generally those of kangaroos, wombats, and other animals still inhabiting that continent, mingled however with other bones belonging to some animal resembling an hippopotamus, now unknown in those parts. To India and to Australia, however, it is that we must look, no less than to America, with full confidence that we shall speedily thence obtain sufficient evidence on all these fundamental questions to afford us a basis of induction sufficiently extensive and firm to enable us, at no distant period, steadily to lay the foundation, and securely to raise the superstructure of an enduring and general geological theory.
England, the mistress of such vast and remote portions of the globe, seems peculiarly called upon to take the lead in this task. And the increased attention to scientific pursuits, now diffusing itself among her military and naval classes,—one of the most favourable characteristics of the age,—promises to supply her every day with observers more and more competent to achieve this honourable duty.
As an appropriate illustration of the recent progress and actual state of geology, I have presented to the Society an engraved section traversing Europe from the northern extremity of Great Britain to Venice, being, as I believe, the first attempt at so extensive a design as yet submitted to the public. In its execution the merit of a careful compiler is of course all that I can pretend to claim: the English portion is all which I can fairly appropriate to myself on the title of original observation; for both the extremities,viz. the
[page] 414
northern section of Scotland, including the Brora coal-field; and the whole Alpine section (by far the most important and instructive part of the whole), I am indebted to my friend Mr. Murchison, the present President of the Geological Society whose recent contributions to our science have so abundantly vindicated his claim to our highest office; his laborious, exact, and scientific surveys of the Alpine chains, of which a specimen is thus presented, are especially a credit to the English school of geologists. Oeynhausen and Dechen have been my authorities for the central portion of the section.
A scale of one degree of a great circle of the globe is given with the section, which however is to be understood only as an approximation and not strictly to be applied throughout, as some portions, especially in the Alpine districts, have been given on a somewhat larger scale to display the phænomena. No regular scale of elevations has been attempted: to have adopted such a scale would have reduced the English chains to imperceptible undulations, or exaggerated the Alps into proportions most inconvenient for the purposes of the section.
Report on the Recent Progress and Present State of Chemical Science.* By JAMES F. W. JOHNSTON, A.M. &c.
Introduction.—THE science of Chemistry is every year so greatly enlarging its dominion over nature, and adding to the already vast accumulation of facts it embraces,—and to this advance so many minds contribute, and in so many different places, that few even of those who devote themselves exclusively to chemical research can keep pace with its progress or make themselves acquainted with the experimental results continually published in various languages by its numerous votaries. The scientific journals of our own country might be expected to diminish this difficulty, by presenting in an English dress a summary, at least, of the results of foreign experimenters; but want of encouragement chiefly has hitherto confined these channels of in-
* The following Report has undergone considerable alterations since the Oxford Meeting, at which it was read. This has been rendered necessary chiefly by the publication, in the interim, of the fourth edition of Dr. Turner's Chemistry. Many facts which are already incorporated with that work have been omitted, and little more than a notice given of several recent investigations which were previously treated of in detail. Some isolated facts which may appear of minor importance have found a place because they are not yet to be met with in our chemical works.
[page] 415
formation within a narrow range. A similar cause also has prevented the appearance among us of any more extended digest, such as the yearly statements of Berzelius, from which might be gleaned a general knowledge of the new views and researches to which the chemical philosophers of other countries are advancing.
The object of the following Report is in some measure to supply this defect in our literature. I regret only that the necessity of condensing it into as small a space as possible, has compelled me reluctantly to pass over many interesting facts, and to confine myself chiefly to those reasonings and researches which in the present state of our knowledge appeared to occupy the more important place.
The work on the History of Chemistry lately published by Dr. Thomson, has rendered any historical details unnecessary, and enabled me to confine my attention exclusively to the more recent chemical investigations. I shall therefore first give a brief view of those differences which now prevail among chemists in regard to certain points connected with the atomic theory, and of those new and interesting doctrines which late experiments have raised to a distinguished rank in the philosophy of the science; and then present an outline of the recent progress of organic and inorganic chemistry.
The investigations of analytical chemistry,—which have of late years been prosecuted with great zeal and success,—have had two objects in view; first, to determine with accuracy the true nature of the substances which compose the various products of the organic and inorganic kingdoms; and secondly, the exact ratio which the weights of the different constituents bear to each other. Among the earlier analysts the former inquiry necessarily assumed the higher place; since the promulgation of the atomic theory the latter has become of the greater importance.
Combining ratios.—In regard to these combining ratios of bodies, or their atomic weights, two differences of opinion prevail among chemists; first, as to the true weight of the atom of each of the simple substances; and second, as to the relation they all bear to a common submultiple.
All chemists admit as a fundamental principle that the only combining proportions of bodies on which we can depend, are those in which the most accurate experimenters have found them to enter into the composition of compound bodies; and that these proportions (weights) may from time to time be corrected by the results of new experiments more skilfully conducted. On this principle, by the comparison of experimental results alone, the earliest atomic weights received in this coun-
[page] 416
try were deduced, and as yet no other principle is recognised in the most eminent foreign schools.
All atomic weights multiples of that of hydrogen—But in Dr. Prout's well-known paper on the relation between the specific gravities and the atomic weights of gaseous bodies, another principle was brought under the notice of chemists. It was there shown that the atomic weights of such of the simple aëriform bodies as had been determined with tolerable accuracy were all capable of being represented by some multiple of a common weight, probably of twice the atomic weight of hydrogen, by a whole number. The simplicity of this relation drew the immediate attention of chemists; for, assuming it to be a law of nature, it was seen that could we accurately determine the true weight of hydrogen, we might at once correct all other atomic weights, and if not obtain for each substance the precise combining ratio, at least bring very close together the extreme limits of error. The analytical researches of Dr. Thomson detailed in his First Principles of Chemistry, were undertaken with the view of testing this law, and they are considered by him as completely establishing it. The multiplied analyses of Berzelius and other foreign chemists, on the other hand, do not coincide with it; and therefore, however probable the truth of the law may be, and however desirable it would be to have it fully investigated, it cannot yet be considered as proved. For experimental purposes the numbers deduced by the most accurate analysts from careful experiments, though no exact multiple of the atomic weight of hydrogen, must still be considered as the standard atomic weights; and in this view those of Berzelius are most entitled to confidence.
Relation between atomic weights and specific gravities.—It might be supposed that the relation between the atomic weights and specific gravities of simple bodies, so ably treated in the paper of Dr. Prout already referred to, should aid us in determining between two numbers differing little from each other, and be in fact a test by which the truth of the law above stated may be tried. If, for example,
W = G/G′
where W is the atomic weight of any simple substance, G its specific gravity in the gaseous state, and G' the specific gravity (or half* the specific gravity) of oxygen gas, then the true specific gravities being known, the weight of the atom should be
* If we suppose the volumes of hydrogen and oxygen each to represent one atom, G' = the entire specific gravity; if one volume oxygen = 2 atoms G'= 1/2 specific gravity of oxygen.
[page] 417
easily determined. But while chemists differ as much in regard to the true specific gravities as in regard to the atomic weights, it is obvious that to correct the latter by the above equation would be to escape one error by combining two others of perhaps equal amount. The true numerical expressions therefore for each series, the specific gravities and the atomic weights, must be sought for by separate and distinct experimental researches; and the relation between them can be employed only as a check upon the results thus obtained. It was in reference to this point that the Chemical Committee of the British Association, at the Meeting in York, deemed it of high importance to draw the attention of chemical philosophers to the necessity of a more accurate experimental inquiry into the true specific gravities of the simple gases.
Can we obtain the true atoms, or only multiples of them?— The above remarks have had reference to the minute differences between the weights received in this, and in other countries, and which differences, in most cases, do not amount to the atomic weight of hydrogen. Though these differences are the greatest in importance, they are not the only ones to which the limited nature of our present knowledge has given rise. It is still a question, whether the atomic weights we obtain for each simple substance, supposing them correct, represent the true weights of the atoms, or only multiples or submultiples of them; —whether, for instance, the weight of hydrogen be ·0625 or ·125, that of mercury 25 or 12·5. It is in many cases easy enough to come to a probable conclusion on this point, from the consideration of the different compounds which the simple substances form with each other; but in some instances the evidence in favour of the greater or the less number is so equally balanced as to render the decision a matter of great difficulty, and one on which the most eminent chemists disagree.
An indirect auxiliary to these determinations has been sought for in the investigation of the specific heats of bodies. Ever since the experiments of Dulong and Petit showed that in the case of simple substances there was reason to suppose that the specific heats multiplied by the atomic weights give a constant quantity, the confirmation of their results by an accurate determination of the specific heats of all bodies, both simple and compound, has become of great importance in atomic chemistry. It is not probable that we shall ever determine these heats with sufficient accuracy to enable us to employ them in discriminating among the series of minute differences to which we have above adverted; but an establishment of the law of Dulong and Petit by tolerable approximations to the true specific heats, would enable
2 D
[page] 418
us to decide at once on the second series of differences, which refer to the multiples and submultiples of the true atomic weights.
Many chemists, and, among others, Berzelius and Dr. Thomson, have adopted the law above enounced; and the latter has employed it as an argument in fixing upon the weights of hydrogen, gold, copper, and some other simple substances*. Still the theory cannot be considered so well established as not to require that further experiments should be made, and especially that it should be rigorously determined whether or not the law hold also in regard to compound bodies.
Among the experiments lately undertaken with the view of trying the results of Dulong and Petit, those of Mr. Potter, an ingenious and zealous member of this Association, cannot have escaped the attention of persons who have interested themselves in this subject. The results of these experiments are detailed in two papers in Dr. Brewster's Journal†, the second containing a series of corrections upon those in the first. The former results of Mr. Potter led him to a confirmation of the law; the latter induce him to consider it "not capable of proof;" but a further consideration of certain objections he has advanced against it may probably incline him to modify this opinion.
In confirmation of the law, results have been obtained by various experimenters. Weber‡, by a very ingenious application of the change of elasticity and temperature which thin bars of metal undergo by a sudden lengthening through mechanical agency, has deduced for iron, copper, silver, and platinum, specific heats which coincide with and extend those of Dulong and Petit§. The change of elasticity was measured by the number of vibrations in equal times before and after the lengthening, and the heats calculated by the known laws of elasticity.
The specific heats of tin and lead, as determined by Dulong, have also been confirmed by a direct experiment of Dr. Erman, jun.‖, who obtained a ratio between them differing from his only in the fourth decimal place. But the strongest confirmation of the law has been derived from the recent elaborate researches¶ of Neumann of Königsberg.
* Inorganic Chemistry, vol. i. p. 9.
† N. S. vols. v. and vi.
‡ Poggendorf's Annalen, vol. xx. p. 177.
§ Those of Dulong and Weber are as under:—
Dulong. Weber.
Iron ·1100 ·1026
Copper ·0949 ·0872
Silver ·0557 ·0525
Platinum ·0314 ·0259
‖ Poggendorf's Annalen,¶ vol. xx. p. 290.
¶ Ibid. vol. xxiii. p. 1.
[page] 419
The experiments of the French philosophers led them to believe in the existence of a very simple relation between the specific heats and the atomic weights of compound bodies; but they had not led them to a definite expression for that relation. Neumann has shown that the relation is the same in compound as in simple bodies, and by experiments on many chemical compounds, both natural and artificial, has generalized the conclusion of Dulong and Petit, and rendered it probable that a given quantity of heat will elevate the same number of degrees a portion of every solid body represented by its atomic weight. This extension of the law, should it be confirmed, will enable us not only to correct the atomic weights of the simple bodies directly, but to test their accuracy through the compounds they form with each other.
We are not yet in a condition to apply this law with perfect confidence to gaseous bodies. The experiments of De la Roche and Berard on the specific heats of the gases, gave results for the simple gases which were very nearly conformable with the law; but in some of the compound gases the same coincidence did not appear: the researches of Haycroft and of De la Rive and Marcet were still less conformable in the compound gases. Dulong pointed out a source of error which he considered as entirely invalidating the method of experimenting employed by De la Rive and Marcet, and as rendering it probable that the older determinations of De la Roche and Berard were better deserving of confidence. More lately he has published a highly ingenious investigation of these specific heats, obtained indirectly from the unlike tones produced by the different gases when caused to pass at the same temperature and under equal pressures through a small wind instrument*. They are as follows:—
| Specific Heats | |||
| Const. Vol. | Const. Press. | ||
| Atmospheric air | 1· | 1· | 1·421 |
| Oxygen | 1· | 1· | 1·415 |
| Hydrogen | 1· | 1· | 1·407 |
| Carbonic acid | 1·249 | 1·175 | 1·339 |
| Carbonic oxide | 1· | 1· | 1·428 |
| Nitrous oxide | 1·227 | 1·16 | 1·343 |
| Olefiant gas | 1·754 | 1·531 | 1·240 |
* Ann. de Chim. tom. xli. p. 313.
2 D 2
[page] 420
The third column exhibits the relation of the specific heats at constant pressures to that with constant volumes.
On the specific heats of the four-compound gases here given, M. Dulong observes, that as far as they go they are in agreement with the law before announced by him, namely, that there exists a very simple relation between the capacity of compound and elementary atoms.
Dr. Prout has suggested an idea, which, if correct, would take away all force from the arguments which, as above stated, have been drawn in favour of some atomic weights, from the relation supposed to exist between them and the specific heats. He contends that instead of combining always in the ratios indicated by the received atomic weights, bodies may combine in many multiples and submultiples of those ratios, constituting a series of combining numbers. Thus water, the atomic weight of which as generally received is 9, may combine in quantities represented by 3, 6, 9, 12*, &c. This involves a principle similar to that of Dumas, who represents atoms as made up of groups of chemical molecules†.
Doctrine of Volumes.—The doctrine of volumes is another point on which chemists are not agreed. The difference which obtains among them has reference to the relation between the volumes and the atoms of the permanent gases and bodies capable of being volatilized. This relation is stated in chemical works in three different ways. The first, that of Ampère and Dumas, presents it in its simplest form. According to them, the atom and the volume are the same in all gases and vapours; or equal volumes of all gases, simple or compound, contain exactly the same number of atoms. This opinion is founded chiefly on the law of Mariotte, that the volume of any gas whatsoever is inversely as the pressure applied to it. For, it is argued, were the number of atoms in each gas not the same, the law of the diminution of volume under pressure ought to vary for each gas. But either this law is not correct beyond certain limits which experiment has not yet reached, or it is independent of the quantity of matter which the gaseous substance contains;—for carbonic oxide unites to half its volume of oxygen to form carbonic acid without increase of bulk, and yet both gases obey the same law of compression. Besides, the adoption of this simple relation between atoms and volumes leads to considerable difficulties, when we examine the constitution of the compound gases.
If, for example, one volume of hydrogen be combined with
* Daubeny's Atomic Chemistry.
† Traité, toni. i. p. 40.
[page] 421
one volume of chlorine, we obtain two volumes of muriatic acid. Now, according to Dumas, the volume being always equal to the atom, we have two atoms of the acid formed out of one atom of each of its elements, or half an atom of hydrogen combined with half an atom of chlorine produces a whole atom of muriatic acid. To obviate this objection, Dumas supposes that heat when it imparts the gaseous form does not subdivide matter into its chemical atoms; so that when they are acted upon by chemical affinity, a further separation of the entire atoms may take place, and thus the apparent combination by fractional parts in the union of volumes be accounted for.
Berzelius, again, considers the atom and the volume equal only in simple gaseous bodies; or that equal volumes of all the simple permanent gases contain an equal number of atoms. This view does not give rise to the anomalies which the theory of Dumas presents, while it retains the simplicity implied in the idea of equal atoms being contained in equal volumes. It has been adopted by most of the continental chemists.
The third mode of comparing atoms with volumes, which is generally followed in this country, differs from that of Berzelius in making oxygen an exception to the general rule. All other simple gases or vapours are supposed to contain equal atoms in equal volumes;—oxygen in an equal volume to contain twice as many atoms. This opinion involves a departure from the supposed simplicity of nature, for which there is, à priori, no sufficient reason. Whether or not it may ultimately prove a true representation of the relation between atoms and volumes, will depend upon the result of researches at present beyond our reach, by which some or all of the gases and vapours we now consider simple may be shown to be compound. In the mean time it is adopted by British chemists chiefly on account of the simpler view it enables us to take of the compounds of oxygen with hydrogen, chlorine, and other elementary substances.
Dumas's researches on the density of Gases and Vapours.— A very interesting inquiry connected with this subject has been opened by some late experiments of Dumas. His opinion above stated, that equal volumes of all simple substances in the state of gas or vapour contain an equal number of atoms, led him to determine the relative atomic weights of several volatile solid substances by a rigorous determination of their respective specific gravities in the state of vapour. For mercury, phosphorus and sulphur, he obtained the following results:
[page] 422
| Sp. grav. | |||
| Mercury, vapour | = 6·976 ∴ | atom. weight | = 6·327* |
| Phosphorus, vapour | = 4·42 ∴ | — | = 4·005 |
| Sulphur, vapour | = 6·667 ∴ | — | = 6·0012 |
These results give for the composition of
| Protoxide of mercury | 4 atoms mercury | + 1 atom oxygen. |
| Phosphoric acid | 1 atom phosphorus | + 5 atoms oxygen. |
| Sulphuric acid | 1 atom sulphur | + 9 atoms oxygen. |
To account for the difference between the atomic weights of mercury and sulphur obtained by this process, which if true would introduce such striking changes into the received theoretical composition of their compounds with oxygen and the other electro-negative elements, Berzelius supposes, with Dumas, that in the less volatile bodies the number of atoms contained in a given volume of their vapour may be less than in an equal volume of bodies so easily converted into vapour as to constitute permanent gases at the usual temperature and pressure of the atmosphere. The subject is one of great interest, and further experiments can alone clear up the obscurities which at present invest it.
The valuable results published by Dumas in 1827 (Ann. de Chim. xxxiii. p. 337), exhibiting the density of iodine, mercury, the chlorides of phosphorus, arsenic, silicon, boron, tin and titanium, and the fluorides of silicon and boron in the gaseous state by direct experiment, and those of phosphorus, arsenic, silicon, boron, tin and titanium by inference, are only exceeded in interest and importance by those above stated for sulphur and phosphorus.
Isomorphism, use of, in determining the composition of oxides, &c. —The doctrine of isomorphism has lately proved of much use in determining the true atomic constitution of many compound bodies. This application of it, though often made by Berzelius, is in a great measure unknown in this country.
The law of isomorphism, as announced by Mitscherlich in its utmost generality, is as follows:—"The same number of atoms combined in the same way produces the same crystalline form, and the same crystalline form is independent of the chemical nature of the atoms, and is determined only by their number and relative position."—This law has undergone a slight modification† since it was promulgated by its distinguished author, but in no way to affect the conclusions to be drawn from it in regard to the atomic constitution of bodies.
Now in applying the law to the composition of the metallic
* Taking the sp. gr. of oxygen at 1·1026.
† See page 425.
[page] 423
oxides, let us take for an example the peroxide of iron and alumina. In the analyses of crystallized minerals, these two bases are found to replace one another in various proportions, without altering the form of the crystal. The peroxide of iron is universally regarded as a compound of two atoms of iron with three of oxygen; but the law of Mitscherlich requires that whatever number of atoms are removed by replacement, the same number must be contained in the body which replaces it; the combining proportions of iron and alumina must therefore contain the same number of atoms. It is a further deduction from the discoveries of Mitscherlich,—first pointed out by the isomorphism of the phosphoric and arsenic acids, and afterwards confirmed by numerous other examples,—that binary compounds which replace each other contain not only the same absolute number of atoms, but also the atoms of the two elements in the same relative proportion. If therefore in peroxide of iron the iron is to the oxygen in the atomic ratio of two to three, the atoms of aluminum and oxygen must in alumina have the same ratio,—or both bases must be sesqui-oxides. Similar reasoning leads to the same conclusion in regard to the peroxides of manganese and oxide of chromium, which form part of the same isomorphous group with alumina and peroxide of iron.
Oxides of Chromium.—The compounds of chromium with oxygen afford a beautiful illustration of the application of the law in the way now adverted to. The oxygen in the oxide of chromium is to that in chromic acid as 1:2. Now this is compatible with, and indeed naturally suggests, the idea, that in the oxide one atom of the metal is combined with one of oxygen, and in the acid with two; but the oxide is isomorphous with the peroxide of iron, alumina, and the peroxide of manganese, while the acid is in like manner isomorphous with the sulphuric and selenic acids: the oxide therefore must be composed like the peroxide of iron, and the acid like sulphuric acid; that is to say, the former must contain two of base to three of oxygen, and the latter one of base to three of oxygen. Thus the single datum given by analysis,—the ratio, namely, of the oxygen to the metal in the two compounds,—proves sufficient, when aided by the principle of isomorphism, to lead us with certainty to the true atomic constitution of both compounds.
Silica.—Silica affords another striking example of an oxide in which a knowledge of its isomorphous relations would remove the obscurity attending the relative proportions of its two elements. It is the only oxide we possess of the metal silicium; and though we can determine, by analysis, that the metal and
[page] 424
the oxygen in this compound are to one another in absolute weight as 277·312:3, yet we have no means of determining whether the weight thus found denotes one or more atoms of silicium. Were we acquainted with any other compound isomorphous* with silica, we should be enabled at once to come to a probable solution of the difficulty. In the mean time, two opposite views are entertained among chemists, drawn from considerations which do not appear to possess anything of a decisive character. By Berzelius it is regarded as a compound of three atoms of oxygen and one of metal  like sulphuric acid; while by Dr. Thomson it is viewed as a compound of the two elements, atom to atom. The former agrees best with the constitution of felspar, which may be considered as anhydrous alum
like sulphuric acid; while by Dr. Thomson it is viewed as a compound of the two elements, atom to atom. The former agrees best with the constitution of felspar, which may be considered as anhydrous alum 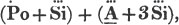 in which the sulphuric acid is replaced by silica; the latter view agrees better with the composition of some other silicated minerals, and with the experiments of Dumas on the density of the chloride and fluoride of silicon in the gaseous state.
in which the sulphuric acid is replaced by silica; the latter view agrees better with the composition of some other silicated minerals, and with the experiments of Dumas on the density of the chloride and fluoride of silicon in the gaseous state.
Application of Isomorphism to mineral compounds. —The doctrine of isomorphism is susceptible of many other important applications in chemical science. It has proved eminently useful in clearing up the constitution of crystallized mineral substances, and of many artificial compounds, in which the presence of apparently foreign bodies seemed to set at defiance the theory of definite proportions. Many varieties of the same mineral occur in nature, agreeing in form and other external characters, and distinguished from one another only by slight shades of difference; in all of which, while the chemist found the same predominating ingredients, he detected in some specimens the presence of small quantities of bodies not generally occurring in the species. As these small quantities bore no atomic ratio to the other constituents, they were supposed at first to be only accidental mechanical mixtures. But when more extended analyses showed that in some instances one of the supposed essential constituents of the regularly crystallized mineral might almost or altogether disappear, while its place was supplied in the true atomic proportion by the same substance, which, when present only in small quantity, had been considered only as an accidental impurity, it became necessary to substitute some other idea for that of mechanical mixtures.
* The analyses of the amphiboles, by Bonsdorf, led him to consider that three of alumina were equivalent to two of silica; but though many other instances have since been found, in which the silica is replaced by alumina, no exact ratio between the replacing substances has yet been made out.
[page] 425
The conclusion to which all analyses pointed was, that the atom of certain classes of acids, of certain classes of hases, and of certain classes of elementary substances, possessed each the same ultimate form, and might therefore be substituted for, or made to replace each other without altering the form of the crystalline compound, into which they entered as constituent parts*. Such suites of bodies constitute the isomorphous groups already referred to.
Isomorphous Groups.—The following list of isomorphous bodies is as perfect as my means of information permitted; and I have endeavoured to arrange the groups in such a way as to show the links by which they are already known to be connected with each other.
List of Isomorphous Bodies, arranged in their several Groups.

*The analytical researches, which, next to the important labours of Mitscherlich, have contributed most to the establishing of this important conclusion, are:
1°. Rose's Analysis of the Pyroxenes: K. V. Acad. Handlingar, 1820.
2°. Bonsdorf's Analysis of the Amphiboles: Berzelius,Årsberättelse, 1821.
3°. Wachmeister's Analysis of the Garnets:K. V. Acad. Handlingar, 1823.
4°. Köhler on Diallage: Poggendorff's Annalen, vol. xiii. p. 111.
5°. Kobell on Talc: Kastner's Archiv, vol. xii. p. 29, on which, however, so much confidence is not to be placed.—And more recently an elegant Memoir of
6°. Abich on Spinell and other Minerals occurring in the form of Octohœdrons.
[page] 426

[page] 427
On this list it is to be remarked,—
1°. That the isomorphism of the several groups of the elementary substances, with the exception of the first four of those constituting group I., is inferred from that of their compounds with an equal number of atoms of oxygen.
2°. That the groups I. IV. VIII. IX. are connected to each other by one or two links, which render it highly probable that these and all other elementary substances will prove isomorphous, conformably to Mitscherlich's law, quoted in page 422. Manganese forms the link between the groups I. IV. and VIII.; the hypermanganic being isomorphous with the hyperchloric acid; the manganic with the chromic, and the protoxide and peroxide of manganese with the protoxide and peroxide of iron.
3°. The connexion between groups VIII. and IX., by the elements calcium and lead, will be explained more fully under the head of Isodimorphous Bodies.
4°. The three bases constituting group XV. form isomorphous compounds with the hydracids.
5°. Gold and silver have been selected (XVII.) from the other electro-positive metals, because G. Rose has lately established their isomorphism by an extensive examination of their secondary forms; they are probably isomorphous with all the other electro-positive metals, but no measurements have yet been made of their secondary forms. Sodium is added to the same group, in consequence of the identity of form of the sulphate and seleniate of silver, and the anhydrous sulphate and seleniate of soda, as shown by Mitscherlich.
Besides these groups of isomorphous elements and binary compounds, other instances of identity of form have been observed, which cannot be comprised within these groups.
Thus one of the forms of sulphur is identical with those of the bisulphate and biseleniate of potash.
Relation of the forms of Potash and Soda.—The form of the nitrate of potash and those of the carbonates of barytes, strontian and lead, are alike:
While nitrate of soda is isomorphous with the carbonates of lime, magnesia, and the other bases in group V.
From these two latter observations on the alkaline nitrates, it appears that soda and potash have the same relation to each other as the bases included in groups VIII. and IX.
Forms of Mineral substances.—In the mineral kingdom, bodies belonging to the same isomorphous group are occasionally met with in identical forms, thus confirming the conclusions drawn from the isomorphism of their similar compounds. Thus we have
[page] 428
| Specular iron | 2 F + 3 O |
| Corundum | 2 Al + 3 O |
| Peroxide of manganese | 2 Mn + 3 O |
all of which belong to the same group, occurring in the same form; and isomorphous with this we have also

in which it will be seen that the titaniate of iron 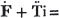
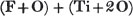 contains exactly the same number of atoms as the peroxide of iron, which in the ilmenite it partially, and in the kibdelophan entirely replaces*.
contains exactly the same number of atoms as the peroxide of iron, which in the ilmenite it partially, and in the kibdelophan entirely replaces*.
Differences between the angles of similar crystals of the same group.—It has been already mentioned (p. 422,) that the general law of Mitscherlich has undergone some modification since it was first announced by that distinguished philosopher. This modification has arisen from the necessity of admitting two new elements into the investigation; namely, temperature, and the relative chemical affinities of the constituent atoms.
The chief support of the doctrine of isomorphism is drawn from the observed identity of the forms and angles of similar salts of the same acid with different bases, or of the same base with different acids. But while, in general, the similar compounds of the isomorphous groups agree perfectly in crystaline form, a few instances have been found in which the angles of the similar crystalline compounds of members of the same group differ from each other by a quantity sometimes exceeding two degrees. Thus the obtuse angle in the right rhombic prisms of the following substances has been found to differ.
| ° | ′ | |
| Sulphate of barytes | 101 | 42 |
| —strontian | 104 | 00 |
| —lead | 103 | 42 |
| ° | ′ | |
| Carbonate of barytes | 118 | 30 |
| —strontian | 117 | 32 |
| —lead | 117 | 18 |
| Arragonite | 116 | 10 |
| ° | ′ | ||||
| Carbonate of lime | 105 | 05 | |||
| —iron | 107 | 00 | These form rhomboids, not rhombic prisms. | ||
| —manganese | 107 | 20 | |||
| —zinc | 107 | 40 | |||
* A valuable list of isomorphous mineral groups, by Professor Miller of Cambridge, will be found in p. 118.
[page] 429
In the arseniate of ammonia, M' or M'' = 85° 54', while in the phosphate it is only 84° 30'.
Now, according to Mitscherlich, the errors of observation in good crystals, and the differences between the angles of different crystals of the same mineral, in good specimens, rarely exceed 10', though they sometimes amount to 20'. In the same crystal differences equally great sometimes occur. In a crystal of binarseniate of potash, he found a difference between the inclinations of two series of similar faces, of 59'. These differences depend on the nature of the surfaces, and the degree of concentration of the mother liquor in which they are formed.
Plesiomorphism. —As the differences between the angles of the carbonates and sulphates above quoted cannot be accounted for by any accidental causes similar to those now detailed, some crystallographers have been led to reject the term isomorphous as applied to such crystallized compounds, and to substitute in its place the term plesiomorphous.
To this term there can be no objection while it is employed only to denote certain groups of crystals belonging to the system, whose forms approximate without becoming identical, but it can never supersede the term isomorphous, or be employed to designate groups of elementary substances, or of their binary compounds. Because lead in the plumbo-calcite* replaces lime without altering the form of the crystal, we infer that these two bases, lead and lime, are isomorphous. But, because we find that the angle of arragonite differs 1° 8' from that of carbonate of lead, were we to infer that the bases themselves are only plesiomorphous, we should have the same substances at once plesio- and iso-morphous, which is a contradiction. While then the cases in which the angles of crystals of similar compounds of the members of an isomorphous group differ from each other, are so few in number as to constitute only exceptions to a general law, the cause of the anomaly must be sought for in something else than a difference in the forms of the ultimate atoms. The fact itself is a very interesting one, —that there exist plesiomorphous groups of similar compounds of isomorphous bodies.
Homoiomorphism.—The differences under discussion have given rise in Germany to another term, homoiomorphous (όμοιος like, μορρη shape), of more extended signification than plesiomorphous. It groups together crystalline forms differing widely in their angles†, provided they belong to the same
* For an account of this interesting mineral, see Brewster's Edinb. Journ. of Science, N. S. No. XI. p. 79.
† See a paper by Kobell, in Schweigger's Jahrbuch, iv. p. 410, where several such groups are to be found.
[page] 430
system of crystallization. Thus specular iron and common calc-spar are members of the same homoiomorphous group, though the obtuse angle of the former is only 93° 50', while that of the latter is 105° 5'. It is possible that the same cause which produces deviations of one or two degrees in certain sulphates and carbonates, may also cause differences of ten or twelve degrees in other minerals; but in the present state of our knowledge such a supposition is void of all probability. The term therefore, though well fitted, apart from theoretical views, to express the connexion of forms as belonging to the same system of crystallization, has not as yet come into general use.
Cause of these differences.—Mitscherlich accounts for the anomalies in question by supposing that the nature of the chemical affinity exerted between the acid and base in certain compounds, may have an influence on the dimensions of the crystals they form. If in all the other salts of the phosphoric and arsenic acids an absolute identity of form was observed, and a slight difference of 1° 24' found only between the angles of the salts of ammonia, are we to reject the general testimony of the salts, and to conclude, from the difference in this one case only, that the ultimate forms of the two acids are different? or are we not rather to suppose that some special cause has interfered to modify the dimensions in this solitary instance? That chemical affinity is the modifying cause cannot be proved, but we know it to be capable of producing similar effects. For, "if chemical affinity," says Mitscherlich*, "have an influence on the dimensions, or axes of the crystals on which the dimensions depend, then this influence must cease entirely when the axes are alike; and such is the case. If oxide of lead or strontian produce a primitive form different from barytes, when they combine with the same substance, and if this dissimilarity be due to the unlike form of the bases themselves, and not to such a modifying power, then, since barytes in combination with nitric acid gives a salt which crystallizes in regular octohædrons, the nitrates of lead and strontian should take a form which deviates from it; but all the three nitrates crystallize in the same form." The probability therefore at present is, that the acids and bases comprehended under each so-called isomorphous group, have like forms, and that chemical affinity in certain cases is capable of modifying the form of their crystalline compounds.
Influence of temperature on crystalline forms.— That these differences in form are not inconsistent with perfect isomorphism in the acids or bases, is further proved by the very interesting
* Köng. Vetens. Acad. Kandlingar, 1821, p. 48.
[page] 431
observations of Mitscherlich*, confirmed by Fresnel and Rudberg,—that the angles of the same crystallized substance vary in dimension with the temperature. Crystals belonging to the regular system expand equally in every direction; other crystals expand more in one direction than in another, showing a tendency to approach to the nearest regular form. Thus the angle in calc-spar varies 81/2′ between 32° and 212°, the obtuse angle diminishing, and the form approximating to the cube. We have only to suppose, therefore, that there is a certain zero point below the mean temperature of the atmosphere, at which the forms of all similar compounds—carbonates and sulphates, for example—of the isomorphous groups are identical; and that the law of expansion by heat, for certain of them, differs from that obeyed by the others,—and we have a cause adequate to account for all the phænomena of plesiomorphism. The rate of deviation in calc-spar, however, is too slow to permit us, without further experiments, to attribute to this cause the entire deviation observed in the carbonates; but if change of temperature can produce such alterations, it becomes more probable that variations in chemical affinity can produce similar and greater differences.
Isomorphism of Potash and Ammonia + two atoms of water.—Before leaving the subject of isomorphism, one other very important observation of Mitscherlich must be adverted to. In the several groups of isomorphous bodies given above, with one exception, every member of each group is supposed to contain the same number of elementary atoms. The application of the doctrine to the determination of atomic weights, of which several illustrations have been given, and to the unravelling of the analytical results obtained from complicated mineral substances, depends entirely upon this supposition. In all cases it is supposed that the atoms of different elements replace each other in equal numbers. But Mitscherlich has found that when the salts of ammonia and potash are isomorphous, the potash salt is anhydrous, while that of ammonia contains two atoms of water. Thus (N +3 H) + 2 (H + O) is isomorphous with Po + O, and could they replace each other, eight atoms must take the place of two. In like manner, the nitrates of potash and soda having respectively the forms of calc-spar and arragonite, if we suppose potash, soda, and lime to consist each of one atom metal and one atom oxygen, we have nitric replacing carbonic acid,—six atoms N + 5 O replacing three C + 2 O.
Dimorphism.—Native sulphur and sulphur deposited from a solution in bisulphuret of carbon, crystallizes in octohædrons with rhombic bases; but when it is melted and allowed to cool
* Annales de Chimie, tom. xxxii. p. 111.
[page] 432
slowly, till part of the mass is consolidated, and the remaining fluid poured off, crystals are obtained in the form of an oblique rhombic prism. These two forms are incompatible, that is, are not derivable from one common form. Pure carbon occurs in nature in two states, constituting diamond and graphite. The former crystallizes in regular octohædrons; the latter in six-sided plates striated parallel to some of their sides. These forms are also incompatible. Sulphur and carbon therefore possess two forms, or they are dimorphous (δις twice, (μορφη shape).
There are also compound bodies which are capable of assuming two forms. Carbonate of lime, in calc-spar and in arragonite, is a well-known example. Carbonate of lead forms also prismatic crystals, but it replaces carbonate of lime in the primitive rhomboid of plumbo-calcite. These two carbonates therefore, and probably also their bases, are dimorphous.
Iron pyrites (bisulphuret of iron) is met with in two incompatible forms, constituting the common or cubical, and the white or prismatic pyrites of mineralogists. The biphosphate of soda likewise crystallizes in two forms, the more common being right rhombic prisms, the more unusual rectangular octohædrons, the form of the binarseniate. Both forms have the same composition. The sulphate of nickel, the sulphate of magnesia, and the sulphate and seleniate of zinc, assume also two forms, but they appear to contain unlike quantities of water*.
In the mineral kingdom also compounds are met with of like composition, but having incompatible forms. Thus the garnet and idocrase are represented by the same chemical formula, but their forms are irreconcileable; and there are many instances of minerals occurring in what are called pseudomorphous or parasitic forms, supposed to be mere casts of the forms of other minerals, some of which may yet prove to be really capable of assuming two forms, or to be dimorphous.
List of Dimorphous Bodies.
I. Simple Substances.
Sulphur.
Carbon.
II. Compounds of two Elements.
Bisulphuret of iron.
III. Compounds of three Elements.
Carbonate of lime.
—lead.
Biphosphate of soda.
IV. Compounds of four Elements.
Garnet, or idocrase.
Cause of Dimorphism.—It would lead us into too great length to enter into a detailed examination of the different
* Mitscherlich, Poggendorf's Annalen, vol. xi. p. 323; Ann. de Chim. 1828.
[page] 433
causes to which, under different circumstances, dimorphism may be traced. In the case of elementary substances, like sulphur, if we suppose the atoms spherical, then different modes of grouping these atoms may produce two or more resultant forms. If the atoms have a definite and constant geometrical form, they may build up unlike figures, by uniting in the direction of their unlike axes. In binary compounds, as in the iron pyrites, sulphur, uniting in each of its two forms with an atom of iron, would produce two forms of the sulphuret, such as we find in nature; and thus the dimorphism of the compound would be due to that of one of its elements. Or if neither atom be dimorphous, the two forms of the compound may arise, as in simple substances, either from a different mode of grouping the atoms, or from their uniting by different faces. By similar suppositions we may form an idea how ternary and more complex compounds might be capable of assuming, not two merely, but several forms. There is indeed no good theoretical or physical reason why even elementary bodies may not be tri- or tetraki-morphous.
Isodimorphism.—There are certain of the dimorphous bodies contained in the list given above, the two forms of which are isomorphous each with each. These are the carbonates of lime and of lead. In one of their forms they give right rhombic prisms, having angles which approach very nearly to each other, as is stated in page 427; in the other they give a rhomboid of 105° 5', the primitive form of calc-spar. I have already mentioned the interesting minerals from the constitution of which we deduce this important fact. Carbonate of lime occurs prismatic in the arragonite, while the carbonate of lead is rhomboidal in the plumbo-calcite. As these compounds are at once dimorphous and isomorphous, I have called them isodimorphous compounds; and if from such compounds we were justified in drawing any conclusion regarding the forms of their bases, we might infer that the two bases, lime and oxide of lead, and, carrying the analysis still a step further, that calcium and lead are also isodimorphous. These bodies are members of the two isomorphous groups VIII. and IX., given in page 245. It is probable that we shall soon be able to embrace the whole of these two groups in one large isodimorphous group.
It is a fact not unworthy of observation, that nearly all the instances of plesiomorphism we yet know, occur in certain compounds of the two bases now mentioned, and of two or three others, which are probably possessed of the same property, as they belong to one or other of the two isomorphous groups referred to.
2 E
[page] 434
Isomierism.—The differences in chemical and mechanical properties among simple and compound bodies, were the first to attract the attention of the early chemists. When methods were discovered in more recent times by which the elements of compound bodies could be separated from each other, it was natural to expect that those which were possessed of unlike properties should also prove unlike in composition. Nor did the results of analysis disappoint this expectation. It was found that substances differing in properties were composed either of unlike elements or of the same elements in unlike proportion; and if results of a contrary character were at any time obtained, they were at once set down as erroneous, and further research generally proved them so. But as the art of analysis improved, and the chances of error were confined within narrower limits, the views of chemists in regard to the composition of bodies became more extended. The vast variety of organic compounds which Nature, by her mysterious processes of elaboration, has formed out of the same four simple elements, taught them that the characteristic properties of different compound bodies depended less on the presence of unlike elements than had hitherto been supposed.—The near approach to equality in the proportions of the elements of many widely different vegetable products, showed them how closely substances might stand to each other in composition, while they were far separated in properties.;— and when at length it was proved by convincing experiments, that the elements may be the same, and their proportions identical, and yet different compounds result, it became necessary to recognise the mode of grouping or arranging these elements, as alone sufficient to produce the most striking sensible differences. This last conclusion was first distinctly pointed at by the compounds of carbon and hydrogen; it has been confirmed and established by many more recent investigations.
Until lately the atomic weights of compound substances containing the same elements in the same relative proportion were always found to differ, and in this difference there appeared still a sufficient reason for their unlike nature. It was conceivable that in bodies differing as to their atomic constitution in this one point only, the elements might be more or less condensed, or otherwise so differently grouped as to give rise to the observed difference in their properties. But the progress of the science has removed this distinction also, and made us acquainted with instances in which like elements grouped together in like number and proportion, constitute unlike compounds having the same atomic weight.
[page] 435
Dr. Dalton, in his reasonings on atomic arrangement, had early shown that the atoms of compound bodies might be supposed to group themselves in one of several different ways; —Berzelius in 1814 had proved, by his experiments on Tin, that there existed two chlorides and two oxides of that metal, having the same atomic constitution but possessing unlike properties;—and Dr. Thomson, in his First Principles, in treating of the then supposed identical composition of the acetic and succinic acids, had made it exceedingly probable that there did actually exist very unlike chemical compounds, in which the same elements in the same relative proportion were so grouped together as to produce the same atomic weight;—but it was not till the appearance of an admirable paper by Berzelius, on the Composition of the Tartaric and Paratartaric (Racemic) Acids, that the doctrine was fully established. In this paper* he showed that these two acids on the one hand, and the phosphoric and paraphosphoric on the other, are identical in composition, and for such bodies he proposed the term Isomeric (ισος equal, μερος part). The able and interesting researches† of MM. Wöhler and Liebig on the acids of cyanogen, added to the list, by showing that the soluble and insoluble cyanuric acids 3/2 (Cy + 2 O + H);—the cyanic and fulminic acids were also isomeric. Many other examples have since been brought forward, and the investigation of organized compounds is daily adding to our knowledge on this important subject. The doctrine itself has likewise met with general reception; and in adverting to the enlarged ideas it has already given birth to, we cannot help regarding the establishment of it as a new bound the science has taken towards that vast extension it is destined to attain.
Polymerism and Metamerism.—Since the introduction of the term Isomeric, it has sometimes been misapplied by chemical writers from not properly understanding the exact meaning it was intended to convey. To prevent such mistakes in future, and to designate compounds approaching in atomic constitution very nearly to those properly called Isomeric, Berzelius has proposed the introduction of two new terms,polymeric and metameric. The distinction between the three terms is as follows.
Isomeric bodies are those which contain the same absolute and relative number of atoms of the same elements, and have consequently the same atomic weight. Of this kind are the two oxides of tin, the two phosphoric acids, &c.
* Kong. Vet. Acad. Handlingar 1830, p. 49.
† Annales de Chimie, xlvi. p. 25.
2 E 2
[page] 436
Polymeric are those which contain the same relative but not the same absolute number of atoms of the same elements, and whose atomic weights are consequently unlike.
To this class belong olefiant gas (2 C + 2 H) and oil of wine (4 C + 4 H), in which the relative proportions of the two elements is the same, but the weight of the atoms as one to two.
Metameric are those which while they contain the same absolute and the same relative number of atoms of the same elements yet constitute substances belonging to an entirely different class of bodies, or a different order of chemical compounds.
It is not easy to express this definition clearly, but it will be understood from the following examples. Sulphate of protoxide of tin  and sulphite of peroxide
and sulphite of peroxide 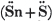 if it exist, contain the same absolute and relative number of atoms of the same elements,—but they are not isomeric. If by heat or by any other process one of these compounds could be made to change entirely into the other, we should not have two isomeric compounds, but two salts belonging to entirely different classes, distinguishable by their appropriate designations. But similar changes may occur among compound bodies; and it is to distinguish such from really isomeric changes, that the term metameric is employed. One beautiful example of this kind is exhibited in the cyanuric and hydrous cyanic acids. The former, without giving off or absorbing anything, is wholly converted by heat into the latter,—that is, it is changed from a compound atom of the first order, or from an oxide of a ternary radical 3/2 (Cy + 2 O + H), into a compound atom of the second order,—into an acid chemically combined with water (Ċy + Ḣ). These two substances Berzelius calls metameric modifications of each other*.
if it exist, contain the same absolute and relative number of atoms of the same elements,—but they are not isomeric. If by heat or by any other process one of these compounds could be made to change entirely into the other, we should not have two isomeric compounds, but two salts belonging to entirely different classes, distinguishable by their appropriate designations. But similar changes may occur among compound bodies; and it is to distinguish such from really isomeric changes, that the term metameric is employed. One beautiful example of this kind is exhibited in the cyanuric and hydrous cyanic acids. The former, without giving off or absorbing anything, is wholly converted by heat into the latter,—that is, it is changed from a compound atom of the first order, or from an oxide of a ternary radical 3/2 (Cy + 2 O + H), into a compound atom of the second order,—into an acid chemically combined with water (Ċy + Ḣ). These two substances Berzelius calls metameric modifications of each other*.
Isomeric bodies,—The following lists comprise nearly all that is at present known on this interesting subject.
I. List of Isomeric Bodies.
1°. The phosphoric and paraphosphoric† acids and their salts.
2°. The tartaric and paratartaric acids.
3°. The two states of the peroxide of tin‡.
* See Berzelius, Årsberättelse, 1832, p. 66, from which the illustration of these terms is taken.
† When two acids differ as these do, Berzelius proposes for the second the prefix πχζχ, as denoting change.
‡ See Berzelius,Traité de Chimie, iii. p. 162.
[page] 437
4°. Silicic acid and the silicates, and the same after heating to redness*
5°. Antimoniates and antimonites, and the same salts after heating to redness†
6°. The two states of the acid and oxides of tungsten.
7°. Telluric acid‡  and oxide of tellurium in their two states.
and oxide of tellurium in their two states.
8°. The soluble and insoluble cyanuric acids 3/2 (Cy + 2 O + H).
9°. The cyanic and fulminic acids (Cy + O).
10°. Oil of wine, and Faraday's light liquid carbo-hydrogen (4 C + 4 H)§.
11°. The pyrotartaric and pyroparatartaric acids?‖
12°. The two states of the phosphate of copper.
13°. The two states of the bisulphuret of mercury.
14°. The two states of phosphuretted hydrogen,—that which inflames, and that which suffers no change when mixed with atmospheric air,—shown by Rose to have the same composition.
To these might be added several other compound bodies, the second state of which is induced by mechanical agency only. Iodide of mercury is a beautiful example in which a slight scratch sets the atoms of the crystal in motion, and changes the colour from a sulphur yellow to a deep red. Chloride of lithium
* The change which takes place in the silicates by heating has been long known;—the principle of Isomerism enables us now to explain it. See Mitscherlich's Lehrbuch der Chemie. i. pp. 393 and 426.
The gadolinite is a fine example of a silicate undergoing this change. When heated it appears to burn, emits light, and becomes yellow, but undergoes no change in weight.
† Antimoniate of copper prepared by the moist way is very easily decomposed by acids; but when heated in vacuo it glows all over without change of weight, and is converted into so intimate a compound that no acid will now decompose it. A similar change takes place in the antimoniates of cobalt and iron, and in some of the antimonites. Phosphate of magnesia when heated exhibits the same phænomena of emission of light and heat during its conversion into paraphosphate.
‡ An account of this new acid and its properties will be given in another part of this Report.
§ For the first of these compounds Berzelius proposes the name of Etherine, as being most probably the base of the ethers.
‖ I have inserted these two acids with an interrogation, as their isomerism is only probable. The two isomeric acids, the tartaric and paratartaric, when subjected to destructive distillation give each acetic acid an insoluble crystalline acid which sublimes towards the end of the process; and a third acid, which from the former is crystallizable, and from the latter is incrystallizable and very volatile. It is these last two acids which are here meant. The crystallizable (pyrotartaric) has lately been examined and analysed by Dr. Gruner. I have commenced an investigation of the latter, by which the isomerism of the two is rendered probable; but my results are not yet in a fit state for publication.
[page] 438
presents another instance. If the crystals of this salt, rectangular four-sided prisms, be taken between the fingers and then laid on bibulous paper, they become opaque at the point which has been in contact with the fingers, and the opacity spreads over the whole crystal, which by a slight touch falls to a white deliquescent powder*. A third example is the mellate of ammonia. This salt, according to Wöhler, crystallizes in two forms, one of which immediately on being taken from the mother liquor becomes opaque and pulverulent either in the air or in vdcuo, and without loss of water†.
All these phænomena are probably dependent on the same principle which gives rise to the more distinct isomeric modifications of the tartaric and paratartaric acids. They are analogous, in the facility with which the change is effected, to the phænomena observed by Mitscherlich in the crystals of the sulphate of nickel and seleniate of zinc. If the prismatic crystals of the latter be laid on paper and exposed to the rays of the sun, in a few moments they become opaque, and are found when broken to be made up of minute octohædrons with square bases,—the other form of which the salt is susceptible. The similar crystals of sulphate of zinc undergo a like change, but more slowly.
The nitrites and vanadiates are sometimes yellow and sometimes colourless, and from one and the same solution may occasionally be obtained colourless and beautifully reddish crystals of the sulphate of protoxide of manganese identical in composition. Both classes of phænomena, and many others of a similar kind, may probably find their proper explanation in isomerism.
II. List of Metameric Bodies.
| 1° | Cyanuric acid 3/2 (Cy + 2 O + H). Hydrous cyanic acid Ċy + Ḣ. | 2 atoms of the former = 3 atoms of the latter. |
| 2°‡. | Naphthaline 5 C + 2 H, density of vapour = 4·528. Paranaphthaline 5 C + 2 H, density of vapour = 6·741. | ∴3 vol. naphthaline = 2 vol. paranaphthaline. |
* This is an observation of Hermann,—Poggendorfs Annalen, xv. 480.
† Berzelius,Traité de Chim. vi. p. 607.
‡ It is difficult to say whether these two are rightly placed in this group, as we have no means of determining how many volumes of paranaphthaline vapour constitute an atom. According to Dumas, four volumes of naphthaline vapour constitute an atom. We are indebted to Dumas for the discovery of paranaphthaline, and I have followed him (Ann. de Chim. 1·183,) in stating the composition of both substances. Opperman lately found naphthaline to be C3 H2, so that there is still considerable doubt as to the metameric nature of these two substances.
[page] 439

III. Polymeric Substances.
| 1. | Paraffin* | 2C + 2H |
| Olefiantgas | 2C + 2H | |
| Trito-carbo-hydrogen (if it exist) | 3C + 3H | |
| Oil of wine | 4C+4H | |
| Faraday's volatile liquid carbo-hydrogen | 4 C + 4H |
| 2. | Oil of lemons. | |
| Oil of turpentine. | 10 C + 8 H.—Dumas. | |
| Camphogene†. |
3. Cyanogen.
Black substance left when the cyanide of mercury is decomposed by heat.‡
4. Arabine and cerasine =6C + 5H + 5 O.—Guerin.
| 5. | Phosphoric and paraphosphoric acids. |
| Metaphosphoric acid. |
From the analyses of Dr. Prout it appears also that cane sugar, diabetes sugar, and lignin, are nearly identical in composition; while, according to the same chemist, gum-arabic, sugar of milk, and manna, differ no more from those three substances than different kinds of cane sugar do from each other. These results have led him to adopt some peculiar hypothetical views§ in regard to the composition of organized bodies.
* This remarkable substance is composed, according to the analysis of Jules Gay-Lussac, of carbon 85·22, hydrogen 14·98,—the same composition as olefiant gas; but we have as yet no means of knowing its atomic weight.
† Dumas thus names (Annales de Chimie, l. p. 225,) the liquid carbo-hydrogen of Opperman, the base of the artificial, and as Dumas thinks also of common camphor. Opperman however gives for the composition of his liquid 12 C + 9 H.
‡ I insert this as a modification of cyanogen on the faith of my early experiments. Some facts however, communicated to me by MM. Liebig and Wöhler, seem to indicate that the subject requires further investigation.
§ For a summary of Dr. Prout's views on this subject, see Dr. Daubeny's Introduction to the Atomic Theory.
[page] 440
He supposes that these compounds owe their differences to the presence of some unknown element in so small a proportion as to escape detection, but which is sufficient to modify the entire properties of the substance. The effect thus produced he calls merorganization: thus starch is merorganized sugar. But while we can so easily conceive that by a different grouping of the elementary atoms, even when present in the same number and proportion, very different compounds may be produced, it would appear superfluous to suppose the presence of any foreign or unknown substance.
Of the speculations to which the law of isomerism has given rise, those of M. Dumas* regarding the probable isomerism of the metals are the most interesting. He supposes that those metals whose atomic weights are equal, or which have a simple relation to each other, may be only isomeric or polymeric modifications of the same elements. Thus in several of the groups which he names we find a remarkable similarity in chemical properties as well as in atomic weight. Thus we have
| Atoms. | |
| Cobalt | 368·99 |
| Nickel | 369·67 |
| Platinum | 1233·26 |
| Iridium | 1233·26 |
| Molybdenum | 598·5 |
| 1/2Tungsten | 596·5 |
In all these cases there is a remarkable similarity in chemical properties, and in the forms and localities in which they are found in nature, as well as in the atomic weight. Can molybdenum and tungsten be different modifications of the same elementary substance? Such inquiries are far from being without their use in experimental sciences; they often suggest trains of research from which most important results are obtained.
Sulphur Salts.—The most important modification which the received views in regard to the nature of saline combinations and the mode of naming them has of late years undergone, has been brought about by the elaborate researches of Berzelius into the constitution of the sulphur salts. The results of these researches were first published in six Memoirs in the Swedish Transactions for 1825 and 1826, and afterwards in Poggendorf's Annals for the latter year. In the outset of his first Memoir, he divides all electro-negative bodies into three classes:
1st, Such as combine directly with and neutralize the electropositive metals, forming salts. These are chlorine, iodine, bromine and fluorine, and are named salt-formers.
2nd, Such as do not neutralize, but form acids or bases
Annales de Chimie, xlvii. p. 324.
[page] 441
when they combine with other bodies. These are oxygen, sulphur, selenium and tellurium, and are called acid-and-base formers.
3rd, Such as possess neither of these properties, but which with bodies of the second class form acids. These are azote, hydrogen, phosphorus, boron, carbon, silicon, arsenic, and the electro-negative metals.
He then proceeds to describe the preparation and properties of a vast number of compounds of sulphur with the simple bodies, in which he demonstrates the striking analogy between that substance and oxygen, and shows—
1°, That sulphur gives a numerous and interesting class of salts in which the oxygen in the oxygen salts is replaced by an equal number of atoms of sulphur, and that this exchange in many oxygen salts may be effected by a current of sulphuretted hydrogen, the hydrogen of which combines with the oxygen both of the acid and the base, and the sulphur takes its place.
2°, That many of these salts, and generally all those of the metals which form with oxygen alkalies or alkaline earths, dissolve in water, crystallize, combine with water of crystallization, unite with one another and with oxygen salts to form double salts, exhibit different degrees of saturation, and in these follow the same multiples as the oxygen salts.
3°, That the sulphur salts are formed in such proportions as generally to have corresponding oxygen salts, but that several classes of sulphur salts have been obtained, the oxygen salts corresponding to which are as yet unknown. Thus, for example, he formed three sulphur acids of arsenic, two of molybdenum, three of antimony, and one of tin, while of arsenic we know only two oxygen acids, and of molybdenum one. Considerable obscurity still attaches to the compounds of oxygen and tin.
4°, That the radicals of all the oxygen acids do not give sulphur acids, or at least that they have not yet been formed. Thus with chlorine, iodine, bromine, fluorine, azote, boron, silicon, titanium and selenium, he could form no sulphur acids or salts. The compound radicals of the organic acids are in the same condition, though a mode of replacing their oxygen by sulphur may yet be discovered.
5°, Negative or acid combinations of sulphur (and consequent classes of sulphur salts,) were formed with hydrogen, carbon, phosphorus, arsenic, molybdenum, tungsten, tellurium, antimony, tin; and less distinct ones with chromium, tantalum, gold, platinum, rhodium, and probably some other metals.
[page] 442
The views advanced in these Memoirs were borne out by such a mass of experimental evidence, that they were at once adopted by nearly all the continental chemists, and Rose in particular soon applied them to a simple and happy explanation of the hitherto obscure composition of many native sulphurets. In this country also they are beginning to make their way, and they have been incorporated by Dr. Thomson in the last edition of his Inorganic Chemistry.
Chlorine salts.—;While Berzelius was pursuing his investigations into the nature of the sulphur salts, Bonsdorf, according to his first paper on the subject in the Annales de Chimie, had been led into similar views in regard to the chlorides and iodides. Led by the analogy which exists between oxygen, chlorine, and iodine, in producing combustion when uniting with simple substances, and in forming soluble compounds with many fixed bodies, he adopted the opinion that chlorine, iodine, bromine and fluorine, like oxygen, were acid-and-baseformers, producing, by their union with the metals and other simple bodies, compounds which possess the properties of acids or bases. In support of this view, he showed that many simple chlorides and iodides might be made to combine into what were formerly called double chlorides, in which he supposed the one chloride to act the part of an acid, the other the part of a base, and he invented a nomenclature in accordance with his peculiar opinions. If the views of Bonsdorf are correct, the distinction which Berzelius makes between his salt-formers and his acid-and-base formers is not founded in nature. Dr. Thomson has adopted these views and incorporated them in his system of inorganic chemistry, and Professor Daniell assumes that we cannot adopt the views of Berzelius in regard to the sulphur salts, without making those of Bonsdorf our own also; but it appears to me that the two opinions stand upon very different grounds, and that however simple the views of Bonsdorf may render the consideration of the chlorine &c. salts, they can never be ranked in the same family with those of oxygen and sulphur.
The main chemical distinction between oxygen and chlorine is this,—that while chlorine neutralizes an electro-positive metal and forms a salt with it, oxygen never neutralizes the metals, but forms with them compounds possessing either acid or alkaline properties. The opinion of Bonsdorf is, that the chlorides are not neutral compounds, but that, like the compounds of oxygen with electro-positive elements, they are all either acids or alkalies, and that when they unite, they do so precisely as an oxygen acid and an oxide do, forming a simple chlorine
[page] 443
salt, in which the chlorine acts the part of the oxygen, as the sulphur does in the sulphur salts.
The evidence brought forward by Bonsdorf in support of this opinion, is as follows:
1°. The bichloride of mercury and other electro-negative metallic chlorides redden litmus, and they lose this property when they unite with a certain quantity of a more electro-positive chloride to form a double salt. Such chlorides, &c. must therefore be held to possess acid properties.
2°. Sugar has a strong affinity for bases: it forms also a crystalline compound with common salt; and therefore it is probable that the chloride of sodium is a base. He has endeavoured to confirm this conclusion by showing that though the chlorides of lime, sodium, &c. exhibit no immediate action on reddened litmus or cudbear paper, yet that a slight change of colour may be observed in the course of half a day or a day upon cudbear paper that has been dipped into solutions of the chlorides of calcium, magnesium, manganese and zinc. That the slowness of this action is no proof of the absence of alkalinity he endeavours to support by the example of the caustic alkalies, which do not act upon reddened litmus paper until they are moistened. It is not the alkali alone then, he infers, which alters the colour of the paper, but the alkali and water together.
Such I believe is the substance of all that has been advanced in support of the views of Bonsdorf; its only other recommendation being that it affords a more simple way of stating the composition of the double chlorine and iodine salts, than that hitherto adopted. The former of the two arguments is the only one that merits much attention; but it is entirely neutralized by two facts advanced by Berzelius*,—1°, that the protochlorides of the older metals, including iron and manganese, give in solution a red colour to litmus paper, and therefore should, on Bonsdorf's views, be called acids. 2°, that the proto-sulphate of iron also reddens litmus, and forms with sulphate of potash a crystallizable double salt which is perfectly neutral. The first of these sulphates therefore should be an acid, and the second a base, and the compound a simple salt, were we to proceed upon such evidence as has been brought forward by Bonsdorf in favour of his chlorine salts†.
* Årsberättelse, 1830, p. 122.
† Other facts of a similar nature may be adduced. Sulphate of alumina, sulphate of zinc and nitrate of lead redden litmus, while boracic acid acts both upon litmus and turmeric paper.
An objection to his class of bases also is derived from the fact that there exist crystallizable double chlorides of magnesia and potash, ammonia and potash, alumina and ammonia, nickel and ammonia, and zinc and ammonia, in which, according to Bonsdorf, one chloride must act as an acid, and the other as a base, and yet these six chlorides are all bases in his salts.
[page] 444
The object of Bonsdorf's papers is not merely to afford a simpler theoretical explanation of the composition of the salts of chlorine, iodine, &c., but to destroy the distinction hitherto made between this class of simple substances and that which comprehends oxygen, sulphur, selenium and tellurium; to place in short the chlorides, iodides, &c., on the same footing with the oxides and acids. To establish the complete analogy between the sulphurand oxygen salts, we have seen, that Berzelius showed the existence of sulphur acids agreeing precisely in composition with the oxygen acids, the latter element being merely replaced by the former;—that suites of acids were formed by sulphur with the same metal, as is the case with oxygen; and that in some cases the analogy was so close that metals, which like tellurium form weak acids with oxygen, gave corresponding weak acids with sulphur. But in the chlorides we have no such analogies. There are no acid chlorides corresponding to the oxygen acids, no suites of acids with the same base corresponding for instance with the oxygen acids of arsenic or antimony. The chlorides which Bonsdorf calls acids have mercury, gold, platinum and palladium for their bases, with none of which does oxygen form an acid.
If we adopt the view of the chlorides advanced by Bonsdorf, then it must be entirely as a matter of theory. If we think it explains their nature more simply than the method of regarding them as double salts, we may adopt his theory and his nomenclature, but we shall still have as many families of simple substances and as many families of salts. Oxygen and sulphur possessing decidedly different properties must still belong to a class of simple bodies different from that to which chlorine and iodine belong; and the oxygen and sulphur salts, being equally distinguished from the double chlorides and iodides both in the nature of the metallic bases they contain, and the relative proportions of the several elements, must also constitute two very different natural families*. It will be matter of consideration for chemists whether the advantages likely to be derived from the adoption of Bonsdorf's theory,—and, as simplifying the arrangement of saline compounds, they are not small,—will com-
* If we were to lay it down as a principle, that compounds of two elements shall be considered as either acids or bases, then the theory of Bonsdorf cannot be rejected; if we take the properties of these compounds as the elements of our conclusion, then analogy alone must be our guide,—and hitherto that has been considered sufficient to place the chlorides among the salts.
[page] 445
pensate for the inconvenience arising from the great change of nomenclature.
Bonsdorf is not unsupported in his views by the opinions of other chemists. In the same volume of the Annales de Chimie (xxxiv.) in which appeared his first paper on the double chlorides, is contained a valuable Memoir of Boullay on the double iodides, and on some compounds of the iodides with the chlorides. These compounds he represented as simple salts, in which the one iodide acted as an acid, the other as a base, agreeably to the views of Bonsdorf in regard to the chlorides. Dr. Thomson also in his system of chemistry now publishing, has adopted the nomenclature of Bonsdorf.
On the other hand his views are opposed by Berzelius and most of the German chemists. Liebig also, who, about the same time with Boullay, investigated many of these double salts, and among others those which the cyanide of potassium forms with the chloride and the iodide of silver, the iodide of potassium with the cyanide of mercury, and the nitrate with the iodide of the same metal, (Jahrbuch der Ch. & Ph. xix. p. 251,) met with difficulties which induced him to reject it. The objections that have been advanced against his theory, Bonsdorf has endeavoured to remove in a valuable paper on some new double bromides, published in the Stockholm Transactions for 1830.
Chemical Notation.—Some discussion has lately taken place in the English journals on the subject of chemical and mineralogical notation. Berzelius, and after him the German chemists, have long been in the habit of using symbols to denote chemical substances and their compounds. In contriving these symbols the initial letter of the Latin name of each substance was selected as the sign of that substance. Thus Fe (ferrum) denotes iron; Sn (stannum), tin; Tu, tungsten; O, oxygen; S, sulphur, &c. Adding these, Fe + O, or Ḟe, is protoxide of iron; S + 3 O, or  , sulphuric acid; Fe + 2 S, or
, sulphuric acid; Fe + 2 S, or  bisulphuret of iron. When two or more binary compounds are united, as
bisulphuret of iron. When two or more binary compounds are united, as  + Ḟe, forming sulphate of protoxide of iron, for the sake of shortness
+ Ḟe, forming sulphate of protoxide of iron, for the sake of shortness 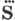 Ḟe are placed together without any sign between them: and in organic compounds where three or more elements are present, as in cyanic acid, instead of placing the elements N + 2C + O, they have been grouped thus, N C2 O, where the number of atoms of carbon is denoted by the index placed over its symbol.
Ḟe are placed together without any sign between them: and in organic compounds where three or more elements are present, as in cyanic acid, instead of placing the elements N + 2C + O, they have been grouped thus, N C2 O, where the number of atoms of carbon is denoted by the index placed over its symbol.
[page] 446
This notation possesses the two great requisites clearness and brevity, and it would be very difficult to devise any other system which should possess them in an equal degree. It has been objected, however, that it has appropriated a mode of notation already employed in algebra with a very different signification; and it would certainly have been very desirable if such an appropriation could have been avoided.
In the simple cases given above as illustrations, it is not very much longer to write N + 2 C + O for cyanic acid, which is algebraically correct, than to express it by N C2 O; but if we go to more complex cases, we shall find pure algebraic notation become so cumbersome as to impair very materially both the requisites of a chemical notation: thus cyanate of ammonia N C2 O + N H3 + Ḣ represented algebraically, is (N +2 C + O) + (N + 3 H) + (O + H), a form of notation which is much longer: but if we take an extreme case like the double ferrocyanides formed by Mosander, we shall see how the clearness as well as the brevity of the notation will be impaired. One of these salts is composed of an atom of ferrocyanide of potassium united to an atom of ferrocyanide of magnesium thus expressed, (Fe N C2 + 2 K N C2) + (Fe N C2 + 2 Mg N C2), which, with all the algebraical signs interposed, becomes
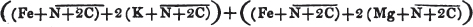
While it is very desirable therefore to express the composition of chemical compounds by a notation as nearly algebraical as possible, it would obviously be to sacrifice both brevity and clearness to insert all the algebraical signs in all chemical formulae.
Arbitrary Symbols.—For the purpose of simplifying notation, several arbitrary signs have been introduced by Berzelius. Thus the dot (·) over a letter denotes an atom of oxygen, the comma (') an atom of sulphur, a horizontal line (¯) selenium, a cross (+) tellurium. A longer line (¯¯) over a letter denotes that it is an organic acid. Thus,
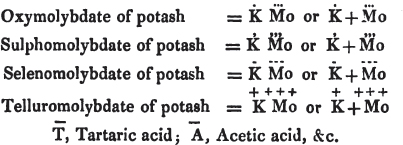
Abbreviations also are employed: thus Cy is cyanogen, = N + 2 C; Bz is benzule=(14 C +10 H + 2 O).
[page] 447
Chemical formulæ have not hitherto obtained much favour in England, but the state of the science makes their adoption now imperative, and Dr. Turner has with much judgement introduced them into the latter half of the fourth edition of his Chemistry.
PART II.—1. Inorganic Chemistry.
Physical relations of the gases.—Döbereiner first observed the curious fact, that when a receiver having a crack in its upper part is filled with hydrogen gas, and placed over water in a pneumatic trough, the gas slowly escapes by the fissure, while the water rises sometimes to the height of three inches.
Magnus* explained the phænomenon by supposing the crack to absorb and condense the gas, as a porous body does; the hydrogen again evaporating from the external surface as it comes in contact with the atmosphere. This explanation he strengthened by showing, that if an open tube be closed at one end by a piece of bladder, be then filled with water, inverted over mercury and left at rest, the water escapes by evaporation from the external surface of the bladder, and the mercury rises in the tube.
Mr. Graham, in a paper on the Diffusion of Gases†, in which he confirmed the previous observations of Dr. Dalton, added another interesting experiment to our knowledge of this subject. Into a receiver filled with carbonic acid he introduced a bladder two thirds full of coal gas,—when he found that the bladder gradually swelled till it became full almost to bursting. The carbonic acid had made its way into the bladder, while, at the same time, a small quantity of the coal gas had escaped into the receiver. Berzelius‡ explained this experiment on the same principle as those already mentioned. The moisture in the bladder becomes saturated with the carbonic acid, which it gives off again from the inner surface, when it is in contact with the other gas.
This experiment was repeated with modifications by Baumgartner, of Vienna; but it was investigated much more fully by Mr. Mitchell§. He employed thin membranes of caoutchouc, and found that when a vessel filled with atmospheric air was closed with such a membrane, and placed in an atmosphere of hydrogen gas, the latter made its way into the vessel till the
* Poggendorf's Annalen, x. p. 153.
† Journal of the Royal Institution, September 1829, p. 74.
‡ Årsberättelse, 1830, p. 54.
§ Journal of the Royal Institution, N. S. ii. pp. 101, 307.
[page] 448
membrane burst outwards. On the contrary, when the vessel was filled with hydrogen gas, and placed in another vessel containing common air, the hydrogen escaped from the inner vessel, till by the increased pressure in the outer the membrane burst inwards.
This suggested to him the construction of an instrument by which the relative velocity and force, with which gases pass through such a membrane may be ascertained. He bent a long tube into the form of a siphon, widened the end of the shorter arm like the mouth of a funnel, and covered it with the membrane of caoutchouc. Mercury was then poured in, so as to be of equal height in both arms; and the shorter, filled with common air and closed with the caoutchouc, was introduced into a receiver containing the gas to be experimented on. Under these circumstances he found ammoniacal gas to pass through the membrane more quickly than any other, and equal quantities of different gases in the following times:
| h | m | |
| Ammoniacal gas | 0 | 1 |
| Sulphuretted hydrogen | 0 | 21/2 |
| Cyanogen | 0 | 31/4 |
| Carbonic acid | 0 | 51/2 |
| Protoxide of azote | 0 | 61/2 |
| Arseniuretted hydrogen | 0 | 271/2 |
| Olefiant gas | 0 | 28 |
| Hydrogen | 0 | 371/2 |
| Oxygen | 1 | 13 |
| Carbonic oxide | 2 | 40 |
| Azote | 3 | 15 |
The gases continued to enter without sensible diminution of velocity, till the mercury in the longer arm rose to the height of sixty inches, when the membrane gave way.
In all these experiments it had been observed that a mutual interchange of the gases took place to a greater or less degree, but it had not been made out that this interchange was regulated by any fixed law. Mr. Graham first pointed out the extent of this mutual diffusion, and returning* to the subject, he has shown,—
1°. That the tendency of two different gases, separated by a porous diaphragm of any kind, is to mutual equable diffusion.
2°. That this mutual diffusion is not necessarily in equal volumes, being inversely proportional to the square root of the density of each gas.
Thus, when a bladder of common air, or, as in Mr. Mitchell's experiments, a tube covered with bladder, is introduced into an atmosphere of hydrogen gas, the tendency of the two gases is to diffuse themselves through each other equably, till the ratio between their volumes within the bladder, and in the re-
* Edinb. Transactions, xii. p. 222.
[page] 449
ceiver without the bladder, is equal. But this diffusion proceeds in such a way, that for every volume of common air which passes out of the bladder, 3·79 volumes of hydrogen pass into it; and hence its remarkable expansion. 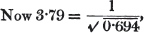 where 0·694 represents the density of hydrogen gas.
where 0·694 represents the density of hydrogen gas.
Again, if hydrogen be introduced into the bladder, and it be left in the open air, the tendency is the same; but as there is no proportion between the volume of hydrogen in the bladder and that of air in the atmosphere, the latter replaces the former entirely; but for every 3·79 volumes of hydrogen at the commencement, only one of air will be found in the bladder at the conclusion of the experiment.
The instrument employed by Mr. Graham in his experiments —his diffusion tube—is a graduated tube, half an inch in diameter and twelve or fourteen in length, with or without a bulb blown into it towards the upper end, which is closed with a plug of plaster of Paris dried at 200° Fahr. A plug of this description he found to absorb 6·5 times its volume of ammoniacal gas, 0·75 of sulphurous acid gas, 0·5 of cyanogen, 0·45 of sulphuretted hydrogen, and 0·25 of carbonic acid; and for these quantities allowance was made in deducing the results of experiment. The other gases tried were not absorbed in any sensible quantity.
The diffusion tube was filled with the gas, whose volume of diffusion was to be ascertained, and inverted over water in the open air. The diffusion took place through the plug, and one volume of air replaced the other gases in the proportions noted in the following Table.
| By Experiment. | By Theory,or 1/D1/2. | Spec. Grav. | |
| Hydrogen | 3·83 | 3·7947 | 0·694 |
| Carburetted hydrogen | 1·344 | 1·3414 | 0·555 |
| Olefiant gas | 1·0191 | 1·0140 | 0·972 |
| Carbonic oxide | 1·0151 | 1·0140 | 0·972 |
| Nitrogen | 1·0145 | 1·0140 | 0·972 |
| Oxygen | 0·9487 | 0·9487 | 1·111 |
| Sulphuretted hydrogen | 0·95 | 0·9204 | 1·1805 |
| Protoxide of azote | 0·82 | 0·8091 | 1·527 |
| Carbonic acid | 0·812 | 0·8091 | 1·527 |
| Sulphurous acid | 0·68 | 0·6708 | 2·222 |
[page] 450
The numbers in the second and third columns of this Table correspond very closely, showing that the theory is probably a true expression of the phænomena.
Granting the existence of a law of nature by which gases are induced to diffuse themselves mutually as liquids do, and as solids in solution do, it might be supposed that the difference of the volumes in which they replace each other through a diaphragm, is due to the greater facility with which one gas passes through a given porous body than another can. But Mr. Graham caused each gas to pass through a stucco plug into the exhausted receiver of an air-pump; and marking the time which elapsed before the attached barometer fell two inches (from 29 to 27), he found that equal volumes of dry air, of air saturated with moisture at 60°, of carbonic acid, of azote, and of oxygen, entered in 10′; of carbonic oxide in 9′ 30″, of olefiant gas in 7′ 50″, of coal gas in 7′, and of hydrogen in 4′. Thus, though they pass through with different velocities, the expression for the rate is very different from that for the volume with which they replace each other. Hydrogen, for instance, passes through only 2·4 times more rapidly than common air, but its diffusion volume is 3·79 times that of air.
It would be interesting to inquire if the diffusion volumes undergo any modification when the diaphragm is formed of different organic products, as in the endosmose experiment of Mitchell, who found that when alcohol or æther is separated from water by a diaphragm of caoutchouc, the alcohol or æther diffuses in greater quantity with the water,—while with a moist bladder the water passes through in greater quantity to the alcohol. These effects are due to the unequal capillary attraction of these substances for the different fluids.
Condensation of the Gases.—Nieman* has repeated Faraday's experiments on the condensation of the gases with nearly the same results. Nitrous oxide, condensed by Faraday with a pressure of upwards of fifty atmospheres, he did not succeed in reducing to a liquid, while in chlorine and sulphuretted hydrogen he found a much higher pressure necessary. Their comparative results on these two gases are as follows:
| Atmosph. | ° | ||
| Chlorine (Faraday) | 4 | at | 15·5 C. |
| — (Nieman) | 61/2 | — | 0 |
| 81/2 | — | 12·5 | |
| Sulphuretted hydrogen (Faraday) | 17 | — | 12 |
| — (Nieman) | 54 | — | 0 |
| 58 | — | 12·5 |
* Brandes, Archiv, xxxvi. p. 175.
[page] 451
He found liquid euchlorine to have a density of 1·4 to 1·5, protoxide of chlorine 1·5, sulphuretted hydrogen 0·6, carbonic acid 0·6 to 0·7. Mr. Kemp has found liquid sulphurous acid a conductor, liquid chlorine and cyanogen non-conductors of electricity.
Law of Mariotte.—A question of great importance has lately been raised regarding the law of Mariotte. From the experiments of Oersted in 1825, it appeared that sulphurous acid gas showed a deviation from the law, diminishing in volume, under increased pressure, more rapidly than common air. Thus, their volumes were equal at the following pressures.
| Air. | Gas. | Air. | Gas. |
| 1 | 1 | 2·8207 | 2·7819 |
| 1·0229 | 1·1215 | 2·9556 | 2·9057 |
| 1·1750 | 1·1729 | 3·0974 | 3·0407 |
| 1·3644 | 1·3634 | 3·3186 | 3·1889 |
This difference was attributed by Oersted to an incipient condensation. Despretz has more lately made* some experiments on sulphurous acid, sulphuretted hydrogen, cyanogen, and ammonia; in which he confirms the observation of Oersted, and renders it probable that the unequal march of the condensible gases, under increasing pressures, is due to some law of their constitution. He gives for ammonia and common air the following numbers:
| Ammonia. | Air. |
| 1·819 | 1·85 |
| 2·582 | 2·663 |
| 3·863 | 4·132 |
denoting the pressures under which the volumes remain equal.
If these gases, then, diminish in volume more rapidly than common air under increasing, they should also expand more rapidly under decreasing pressures; and there is no reason for believing that the pressure of the atmosphere constitutes a fixed point, at which the volumes of all gases accord with theory, and from which the deviations in contracting or expanding commence. We may therefore find in this fact a probable cause why the specific gravities of the condensible gases is generally obtained higher by experiment than by theory it ought to be.
* Annales de Chimie, xxxiv. pp. 335, 443.
2 F 2
[page] 452
It is very desirable that this important point should be thoroughly investigated, as it would appear to throw a shade of uncertainty over the densities of the vapours of solid bodies, determined by Dumas, which have been adverted to in the former part of this Report.
Simple substances— non-metallic— Hydrogen gas.—When two volumes of hydrogen and one volume of oxygen are exploded at the ordinary temperature and pressure, the light emitted during combustion is very feeble. This fact, with others of a similar kind, were brought forward by Davy in confirmation of his opinion that the intensity of every flame depended upon the presence of solid matter in the state of combustion. But if the above mixture of gases be inflamed under a pressure of two atmospheres, the light is of the brightest and most vivid character, resembling lightning; or in the dark, illuminating a room like the sun's rays, and imparting the well-known phosphorescent property to oyster-shells calcined with sulphur.
From this fact Döbereiner* infers, that the intensity of light depends upon the condensation of the heat by which it is caused. But though this may be the true cause of the light emitted in the above experiment, it cannot be the source of the great light which is produced by introducing certain solid substances into flames otherwise scarcely visible.
Basifying power of hydrogen.—The gaseous compounds of hydrogen may be divided into several groups.
1°. Its compounds with chlorine, bromine, iodine, and fluorine, which all contain in one volume half a volume of hydrogen + half a volume of the radical.—They form a series of strong acids, which are decomposed when they combine with the oxidized bases, and form water. Without decomposition they have hitherto been combined only with ammonia and phosphuretted hydrogen (N + 3 H and P + 3 H), and with some metallic chlorides, to which they act the part of acids.
2°. Its compounds with oxygen, sulphur, selenium, and tellurium.—These consist of one volume of hydrogen + half a volume of radical, and form a series of weak acids, acting also as bases to the stronger acids. Thus, water acts as a base in the liquid sulphuric acid, while it is an acid in the hydrates†.
Like the former group, they form water on combining with
* Schweigger-Seidel's Neues Jahrbuch, ii. p. 89.
† Mitscherlich has shown that the bisulphate of potash may be regarded as a double salt =  Sulpho-naphthalic and sulpho-vinic acids may also be so represented, the former being
Sulpho-naphthalic and sulpho-vinic acids may also be so represented, the former being  Na, the latter
Na, the latter  E; where Na denotes naphthaline and E etherine (4C + 4H).
E; where Na denotes naphthaline and E etherine (4C + 4H).
[page] 453
basic oxides. With ammonia also they combine without decomposition: their combinations with phosphuretted hydrogen are not known.
3°. Its gaseous compounds with nitrogen, phosphorus, and arsenic.—These act in all cases as bases. In the case of ammonia this is well known. Serullas and Rose have shown it to hold also with phosphuretted hydrogen, and from analogy we infer the same of arseniuretted hydrogen.
The composition of this group is such, that one volume of the compound contains one volume and a half of hydrogen + half a volume of radical, in the gaseous state.
4°. Its compounds with carbon.—In these there exists still more hydrogen than in the third group, and they act more powerfully as bases.
Hydrogen, therefore, seems to be the principle on which the basic property of very many compounds depends. It has also an analogy with oxygen in forming both acids and bases,—but with this difference, that while oxygen forms acids when present in large, and bases when in small quantity, hydrogen, on the contrary, forms bases when present in large, and acids only when combined in small quantity with electro-negative substances.
Water, maximum density of.—The question as to the temperature at which the density of water is a maximum, does not seem to be yet quite settled. De Luc first fixed it at 40° Fahr.; Sir Charles Blagden and Mr. Gilpin reduced it to 39°; Dr. Hope's elegant method gave 39°·5; Biot, in his Tables (Traité, i. p. 425), gives, by calculation, 38°·156; and the French, in fixing their standard weights and measures, adopted 40°. More lately, the elaborate researches of Hällström* fixed it at 39°·38, in which determination great confidence was placed. Prof. Stampfer†, of Vienna, has renewed the investigation with the adoption of new precautions. His method was to weigh a hollow cylinder of known bulk, made air-tight, at about 66° Fahr., in water of different temperatures; and to insure accuracy, he continued his weighings during a whole year, so as to have the temperatures of the water and surrounding air nearly alike. From a great number of results carefully corrected, he deduces 38°·75 for the maximum density. Muncke‡ also has made experiments on the same subject, and found water to have a maximum at 38°·804. The cause of differences so great must be determined by further investigation,—the thermometers are
* Köng. Vet. Handlingar.
† Poggendorf's Annalen, xxi. p. 75.
‡ Neues Physicalisches Wörterbuch, iv. p. 149.
[page] 454
the most likely source of error; for though Erman* has shown that a very minute admixture of a saline substance would cause an important difference in the temperature of maximum density, we cannot suppose such experimenters to employ water that had not been several times distilled.
Mr. Crichton† of Glasgow, by employing a thermometer tube with a large bulb filled with water, and allowing for the expansion of the glass, has more recently arrived at a determination agreeing very nearly with those of Muncke and Stampfer. The true point of maximum density he fixes at 38°·97 Fahr.; consequently that at which water acquires the same absolute magnitude as at 32°, is 45°·94.
Steam.—Mr. Johnson, of Philadelphia, has published the results of a series of experiments on the influence of temperature and the powers of different metals in the generation of steam. He finds that more steam is generated in a given time by immersing in boiling water a mass of iron at a red heat, just visible in daylight, than by the same mass raised to a white heat. The steam generated bears a direct relation to the weight of the metal, being about one pound of steam for every nine pounds of iron. In comparing cast iron with malleable, he found that cast iron, raised to the same temperature, generates more than wrought iron, being about one pound of steam for every eight pounds and a quarter of iron‡.
The former of these observations in regard to iron at a white heat corresponds with that of Leidenfrost, of the mobility and permanence of a drop of water on a very hot plate of iron; and of Perkins, that an aperture in the side of a cylinder, which, at low temperatures, permits the passage of a jet of steam, ceases to do so when the cylinder is raised to very high temperatures. They call to mind also Pouillet's observation, that water might be kept for a quarter of an hour in a platinum crucible, heated to whiteness, without sensible diminution.
Ice.—Osann§ has determined the specific gravity of ice at 32° Fahr., and found, as a mean of ten weighings, of which the extremes were 0·9352 and 0·9198, the true specific gravity = 0·9268. It floated on oil of turpentine whose density at 32° was 0·9313, so that the specific gravity of the ice by this test cannot exceed 0·93.
Persulphuretted hydrogen.—The bisulphuret of hydrogen, the hydrosulphurous (hydrothionige) acid of the German chemists, acts the part of an acid in the salts formerly called sul-
* Poggendorf's Ann. xii. 466.
‡ Silliman's Journal, xix. p. 292.
† Trans. Roy. Soc. Edinb.
§ Kastner's Archiv, i. p. 95.
[page] 455
phuretted hydrosulphurets, and is understood by Berzelius to consist of
| Four atoms sulphur. | = H + 5S. |
| One atom sulphuretted hydrogen. |
From the analysis of the octohædral soda salt, Dr. Thomson states its composition to be two atoms sulphur + one atom hydrogen, and therefore, in his System, he retains the name Bisulphuret*.
M. Thenard† has published some observations on this singular substance, the object of which is to point out its strong analogy to the deutoxide of hydrogen, in its action upon the skin and other animal textures, in bleaching and destroying them; its power of discharging vegetable colours; its action upon the metals, and the more easily reduced metallic oxides when in the state of fine powder; in the facility with which it is decomposed, and the influence of a few drops of very diluted acid in retarding decomposition for a considerable time.
Finely divided charcoal, several of the metals in the state of powder, as platina, gold, iridium, many oxides, the peroxide of manganese, and all the earthy bases, are acted upon by it with brisk effervescence; with potash and soda it is so violent as to resemble ebullition. The oxides of silver and gold are instantly reduced with incandescence.
M. Thenard prepares it by boiling quicklime in water, with an excess of sulphur, and pouring the filtered solution by degrees into muriatic acid, diluted with twice its weight of water, and shaking the mixture. Prepared in this way, the composition and density must vary, as it is always contaminated by a mixture of sulphur or sulphuretted hydrogen. He obtained it of the specific gravity 1·769, and two different specimens gave him, on analysis, eight atoms of sulphur to one of hydrogen, and six atoms of sulphur + one of hydrogen. The process of Berzelius, who directs a solution of the crystallized potash salt to be employed, would probably give more uniform results.
The sulphurets decompose this compound, disengaging sulphuretted hydrogen gas, and causing a deposit of sulphur;— hence the reason why it is not obtained when an acid is poured into a solution of any of its salts. The excess of sulphuret present decomposes the bisulphuret of hydrogen, as it is formed, causing sulphur to precipitate and gaseous sulphuretted hydrogen to be given off.
Nitrogen.—One of the easiest methods of preparing nitrogen
* Chemistry of Inorganic Bodies, i. p. 278.
† Annales de Chimie, xlviii. p. 79.
[page] 456
is to pass a current of chlorine gas through liquid ammonia. The ammonia is decomposed, muriatic acid formed, and nitrogen liberated, which may be collected in a receiver. Mr. Emmett* has recommended an equally easy and simple process for obtaining this gas. It consists in fusing nitrate of ammonia in a retort with some fragments of metallic zinc. This metal decomposes the nitric acid, and nitrogen and ammonia are given off. When collected over water, the latter gas is absorbed. Mr. Emmett employs a small cylinder of zinc attached to a rod passing through the tubulure of a retort, by raising or depressing which into the fused nitrate he can regulate the emission of the gas.
Phosphuretted hydrogen.— Prof. H.Rose has established the very interesting fact, that the two kinds of phosphuretted hydrogen,—that which inflames spontaneously when brought into contact with atmospheric air, and that which does not possess this property,—hitherto considered as different compounds, and described in our chemical books under the names of proto- and per-phosphuretted hydrogen,—have in reality the same composition and specific gravity. They both consist of one volume phosphorus vapour + three volumes hydrogen condensed into two volumes. They are therefore isomeric combinations of the same elements.
Sulphuric acid absorbs both modifications, and gives both off again by heating; but in the non-inflammable state both gases combine with the chloride of titanium, forming a brown mass, which water, acid solutions, and solutions of the fixed alkalies decompose, always liberating the gas in the non-inflammable state. Liquid ammonia decomposes it also, and gives off the gas always in the self-inflammable state. The same takes place with the compound of phosphuretted hydrogen and some other chlorides†.
This remarkable fact is analogous to the conversion of the phosphoric into the paraphosphoric acid, by boiling its solution in water, as described by Stromeyer.
An observation of Serullas confirms these results of Rose: he found that water decomposes the compound of hydriodic acid and phosphuretted hydrogen, giving off a gas (P H3) which does not take fire; while oxide of silver strewed upon it evolves a gas which does take fire spontaneously.
Magnus has directed the attention of chemists to a solid compound of phosphorus and hydrogen, similar to that of hy-
* Royal Institution Journal, i. p. 384.
† As in that with bichloride of tin = 3 (St+2 Cl)+2 (P+3 H). The formula for the compound with chloride of titanium = 3 Ti Cl2 + 2 (Cl H + P H3).
[page] 457
drogen and arsenic formerly known. This compound was also discovered by Rose, and is obtained in the form of a yellow insoluble powder when phosphuret of potassium is dissolved in water. It is more difficultly fusible than phosphorus, and at the moment of melting gives off hydrogen and vapour of phosphorus. To prepare the phosphuret, Magnus* recommends that the two elements should be heated together under naphtha, by which means the danger of breaking the tube, which exists when they are heated alone, is avoided.
Phosphoric acid.—The remarkable property possessed by phosphuretted hydrogen, of assuming two mutually convertible isomeric modifications, is possessed in a still more striking degree by phosphoric acid. It is several years since Engelhart and Berzelius remarked that phosphoric acid, prepared by burning phosphorus in oxygen gas, or by oxidizing it with nitric acid, and fusing at a high temperature, if dissolved immediately in water, possessed the property of coagulating albumen, which it again lost by remaining in solution a few days. Soon after, the attention of chemists was more particularly directed to this object by the discovery of Mr. Clarke,—that a similar change was produced on the common phosphate of soda, by heating to redness. In its usual state the solution of neutral phosphate of soda precipitates from nitrate of silver a yellow precipitate, which is a sesquiphosphate of silver  after heating to redness it precipitates a white salt, which is a neutral phosphate,
after heating to redness it precipitates a white salt, which is a neutral phosphate, 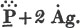 A similar white salt Berzelius found to be thrown down by the heated acid,—an observation afterwards confirmed by Gay-Lussac, thus connecting the appearances observed by Engelhart with those of Clarke's heated phosphate. For the acid in this second state Mr. Clarke proposed the name pyrophosphoric acid. When the discovery of the identity of the tartaric and racemic acids rendered it desirable to have a general prefix to denote change of properties without change of composition, Berzelius proposed πaga for this purpose, as involving no theory, and merely marking out that an alteration had taken place. Thus the racemic is the paratartaric; the fulminic, the paracyanic; &c. And as the first state of the phosphoric acid seems to be that in which it is obtained immediately on burning phosphorus in oxygen gas, from which, in a few days, it changes into the common phosphoric, he proposed to call the new acid the phosphoric, and the old acid the para-
A similar white salt Berzelius found to be thrown down by the heated acid,—an observation afterwards confirmed by Gay-Lussac, thus connecting the appearances observed by Engelhart with those of Clarke's heated phosphate. For the acid in this second state Mr. Clarke proposed the name pyrophosphoric acid. When the discovery of the identity of the tartaric and racemic acids rendered it desirable to have a general prefix to denote change of properties without change of composition, Berzelius proposed πaga for this purpose, as involving no theory, and merely marking out that an alteration had taken place. Thus the racemic is the paratartaric; the fulminic, the paracyanic; &c. And as the first state of the phosphoric acid seems to be that in which it is obtained immediately on burning phosphorus in oxygen gas, from which, in a few days, it changes into the common phosphoric, he proposed to call the new acid the phosphoric, and the old acid the para-
* Poggendorf's Annalen, xvii. p. 527.
[page] 458
phosphoric. The advantage to be gained by the adoption of such a general prefix is obvious, both in simplifying nomenclature and in keeping before the mind the true relations subsisting between the several groups of isomeric compounds: but it is to be regretted that Berzelius should have thought it necessary to transfer to the new modification a term so long applied to the old one. The only reason given for the change will also be set aside, should the observations of Mr. Graham on the metaphosphoric acid prove correct.
Metaphosphoric acid.—The differences between the two acids above mentioned, as they are at present received, are the following:—
1°. The common acid gives a yellow precipitate with nitrate of silver; the fused, a white precipitate.
2°. The common does not affect a solution of albumen; the fused, coagulates it.
3°. The common is changed into the new acid by fusion at a high temperature; the new again into the common by solution in water, more speedily by boiling, and by the aid of a small quantity of another acid.
4°. The common phosphate of soda contains 121/2 atoms of water; after heating to redness and resolution it crystallizes with only ten atoms of water. It has been stated that the precipitates with nitrate of silver by solutions of newly heated phosphoric acid and of the heated phosphate of soda, are both white, and similar in appearance. But by the analysis of Berzelius the precipitate from the acid is a bisalt 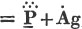 , while that from the solution of the heated soda salt is neutral
, while that from the solution of the heated soda salt is neutral 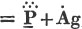 .
.
This difference in composition it was easy to account for from the presence of an excess of acid in the case where the double salt was formed, without supposing that there was any difference between the acids combined with the silver in the two precipitates.
Dumas in his Chemistry (vol. ii. p. 239,) states distinctly, however, that there is a third modification of the phosphoric acid, though he does not specify its distinctive properties. He says that when the common phosphate of soda is boiled for some time it crystallizes in a peculiar form, which it obstinately retains.
Mr. Graham has lately directed his attention to this subject, and has made out, it would appear satisfactorily, that there is in reality a sufficiently distinct third acid, which, with his consent, I shall here distinguish by the term metaphos-
[page] 459
phoric acid. According to his experiments, it would appear that the acid after fusion, or as obtained by burning phosphorus, is not the same as that contained in the fused phosphate of soda. The common acid, the pyro acid obtained by decomposing the pyrophosphate of lead by sulphuretted hydrogen after the method of Gay-Lussac, and the meta or fused acid, are thus distinguished by Mr. Graham:—
The common phosphoric does not affect solutions of silver, albumen, or barytes.
The pyrophosphoric gives with silver a white pulverulent precipitate 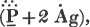 but does not affect solutions of albumen or of chloride of barium.
but does not affect solutions of albumen or of chloride of barium.
The metaphosphoric gives with nitrate of silver a white gelatinous precipitate 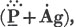 coagulates albumen, and gives a white precipitate with chloride of barium.
coagulates albumen, and gives a white precipitate with chloride of barium.
The acids in applying these tests are supposed to be in the state of weak solutions, in which state they are perfectly stable. Mere concentration is sufficient to change the meta into the common acid.
The metaphosphoric acid is obtained by burning phosphorus in an excess of oxygen gas, or by heating to redness the common acid. It may also be obtained in composition by fusing the biphosphate of soda, dissolving in water and saturating with carbonate of soda. To saturate the common biphosphate, an atom of carbonate of soda is necessary; the fused biphosphate requires for saturation only half an atom, and the salt is incrystallizable. If the whole acid therefore has undergone a like change, its saturating power has been diminished one fourth, and consequently the weight of the atom of the meta is to that of the common acid as 4:3. Such a constitution of the acid, if made out, would be sufficiently remarkable.
But the composition of the fused biphosphate after saturation 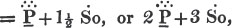 may be represented
may be represented 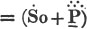
 being a compound of one atom of a biphosphate with two atoms of a neutral phosphate. This view obviates the necessity of supposing that there is any change in the saturating power,—a necessity which is not borne out by the known composition of the white biphosphate of silver thrown down by the fused acid. If the acid have a less saturating power when combined with soda, it ought to have it also when combined with silver. It is possible, however, that during fusion the free acid in the biphosphate has alone suffered this new change. If that
being a compound of one atom of a biphosphate with two atoms of a neutral phosphate. This view obviates the necessity of supposing that there is any change in the saturating power,—a necessity which is not borne out by the known composition of the white biphosphate of silver thrown down by the fused acid. If the acid have a less saturating power when combined with soda, it ought to have it also when combined with silver. It is possible, however, that during fusion the free acid in the biphosphate has alone suffered this new change. If that
[page] 460
change is such as to reduce its saturating power one half, it would at once account for the silver salt containing the elements of two atoms of phosphoric acid, and for the fact that half an atom of soda is sufficient to saturate the free acid in the salt after fusion.
Mr. Graham, who has been kind enough to make me partially acquainted with his results, is still occupied with the investigation, and it is to be hoped that he will soon be able to clear up all the difficulties which the subject presents.
In the present state of the science, the isomeric modifications of the compounds of phosphorus and of other elementary substances, form the most interesting and perhaps also the most important subject of inquiry.
Sulphur in mineral waters.—When chlorine and iodine are present together in a mineral water, they are both precipitated by nitrate of silver, and the precipitate is treated with caustic ammonia, which dissolves the chloride and leaves the iodide, which is then collected and weighed. Brandes*, however, has observed that the insoluble residue sometimes contains sulphuret as well as iodide of silver, and this when the quantity of sulphur present in the water was so small as to escape detection by the usual tests. If the quantity of sulphuret present be large, it may be detected by the dark colour it imparts to the precipitated yellow iodide. But if small in quantity, the precipitate is to be fused with caustic potash, when the alkaline solution obtained by treating the mass with water gives with muriatic acid the odour of sulphuretted hydrogen, and by the further addition of nitric acid, vapours of iodine which stain starch paper of a blue colour.
It is often a matter of some interest to ascertain in what state the sulphur exists in hepatic waters. In the greater part of such springs it has been supposed to be merely in a state of solution, not of combination. An examination of some of the German springs has shown it to be generally present, partly in a free and partly in a combined state, in combination with soda. The mode of determining the relative quantities depends upon the employment of reagents of the two classes of metallic oxides, one of which is precipitated either by sulphuretted hydrogen or by hydrosulphurets, the other only by hydrosulphurets. Thus sulphate of copper will precipitate all the sulphur which the water contains,—sulphate of manganese only that which is in combination.
Sulphuretted hydrogen, action of on nitric acid.—I have observed that when a current of sulphuretted hydrogen is passed
* Schweigger's Neues Jahrbuch, i. p. 250.
[page] 461
through a solution containing free nitric acid, as well as through solutions of some nitrates, ammonia and sulphuric acid are formed. If the solution be hot and acid, this proceeds with considerable rapidity, as may be seen by adding nitrate of barytes to the solution when sulphate forms. The presence of the ammonia is beautifully shown by adding protosulphate of iron, and evaporating when the beautiful pinkish-coloured octohædrons of sulphate of iron and ammonia are deposited*. This observation may be found of use in some cases of analysis where the presence of ammonia may retain more or less of a metallic oxide in solution (as oxide of copper), which it may be desirable to precipitate.
Hyposulphurous acid.—M. H. Rose† has published a new analysis of hyposulphurous acid, in which he confirms the previous deductions of Gay-Lussac. When solutions of the hyposulphites are poured into the solutions of certain metallic salts, as those of silver and mercury, half the sulphur in the hyposulphurous acid forms a metallic sulphuret, and is precipitated: the other half is changed into sulphuric acid, and remains in solution. He found the acid contained in the hyposulphite of barytes to consist of
| Two atoms sulphur | = 4 | 6. |
| Two atoms oxygen | = 2 |
When the one atom of sulphur falls, the other unites with both the atoms of oxygen in the acid and with the atom given off by the metallic oxide precipitated in the state of sulphuret; with these three atoms it forms one atom of sulphuric acid. The hyposulphurous acid agrees, therefore, with the supposed subsulphurous acid of Dr. Thomson‡ in containing equal atoms of the two constituents; but it differs from it in the remarkable circumstance of having an atomic weight twice as great. Dr. Thomson states the composition of hyposulphurous acid to be two atoms sulphur + one atom oxygen.
Sulphurous acid gas.—Knezaurek§ has given a very useful and cheap method of preparing sulphurous acid gas. He introduces powdered charcoal into a retort and pours over it concentrated sulphuric acid, until on shaking it the mass appears moist. On heating, a constant stream of a mixture of two volumes of sulphurous acid and one of carbonic acid gases is given off, which continues till the mass becomes dry. This
* Brewster's Journal, N.S. vi. p. 65.
† Poggendorf's Annalen, tom. xxi. p. 436.
‡ Chemistry of Inorganic Bodies, vol. i. p. 266.
§ Baumgartner's Zeitschrift, ix.
[page] 462
method may be used with great advantage in saturating alkalies or preparing the hyposulphites.
Anhydrous sulphuric acid.—Prof. Mosander of Stockholm has communicated to me the following very simple mode of preparing anhydrous sulphuric acid. If oxide of antimony be treated with excess of sulphuric acid till the oxide is saturated, and the excess of acid then driven off by a low temperature, the sulphate  is obtained dry and crystallized. If this dry salt be put into a retort and heated to dull redness, the greater part of the acid is driven off in an anhydrous state, and is easily condensed in a cool receiver.
is obtained dry and crystallized. If this dry salt be put into a retort and heated to dull redness, the greater part of the acid is driven off in an anhydrous state, and is easily condensed in a cool receiver.
Deutoxide of chlorine.—Some chemists have thought that the oxide of chlorine obtained by decomposing chlorate of potash under sulphuric acid by the method of Von Stadion was different from that of Davy and Gay-Lussac. Soubeiran* finds that the gas of Stadion contains an admixture of oxygen which may be separated by passing the gas through water. The deutoxide is absorbed, while the oxygen escapes. A gentle heat disengages the gas from the water, which by this means may be obtained pure. It consists of one volume chlorine + two volumes oxygen.
On the other hand the same writer endeavours to show that the gas† prepared by Davy's method with muriatic acid and chlorate of potash, and hitherto considered a protoxide of chlorine, is only a mixture of oxygen and deutoxide of chlorine. When this gas is absorbed by water, afterwards expelled from it by heat and caused to pass through mercury to absorb the excess of chlorine, a pure deutoxide is obtained of a similar composition to the above. Euchlorine as it is given off from the chlorate contains the two elements very nearly in the proportion of two volumes chlorine to one volume oxygen, and it does not appear from the experiments of Soubeiran that there may not have been a decomposition of the gas during his process for purifying it. Nor, should he be correct in regard to the identity of the two gases he examined, does it follow that there may not still be two gaseous oxides of chlorine.
Chlorous acid.—The existence of a lower acid of chlorine (chlorous acid) in the bleaching compound prepared by means of this gas, and to the presence and easy decomposition of which the bleaching property is owing, is strongly maintained by some of the most eminent German chemists. In the bleaching compounds there are present chlorine, oxygen and a metal;
* Annales de Chimie, tom. xlviii. p. 148.
† Euchlorine.
[page] 463
—does the chlorine combine with the whole metallic oxide, or does it decompose one portion forming a chloride with the metal and a chlorous acid with the oxygen, which acid unites with another portion of the oxide forming a chlorite?
Berzelius found that a solution of carbonate of potash or soda, saturated with chloride of potassium or sodium, and subjected to a current of chlorine, deposited a portion of chloride, while a bleaching liquid was formed. From this he infers that a new portion of chloride had been formed and an acid of chlorine.
Soubeiran found that the chloride of soda formed by decomposing that of lime with carbonate of soda, evaporated to dryness in vacuo, and the dry mass washed with a saturated solution of common salt, lost its odour of chlorine, common salt alone remaining. The solution and the salt dried in vacuo must therefore undoubtedly, says Soubeiran, have contained a chloride and a chlorite. He found also that chloride of lime decomposed by carbonate or oxalate of ammonia, gave a bleaching liquid, though chlorine, as is well known, does not combine with ammonia.
Liebig found that chlorine decomposed acetate of potash and formed a bleaching liquid, which he considers to argue the existence of such an acid. But his strongest arguments are drawn from the analogy of chlorine with sulphur in certain circumstances, which the reader will find stated in Gerger's Ann. der Pharmacie, 1832, vol. i. p. 317.
Soubeiran thinks it probable that the composition of this acid is 2 Cl + 3 O, analogous to the nitrous acid; but this opinion is altogether conjectural.
Sulphuret of phosphorus and chlorine.—When phosphuretted hydrogen is passed through chloride of sulphur, a yellow sirupy liquid is formed, which is decomposed by water, and consists of five atoms sulphur, two atoms chlorine, and one atom phosphorus*. It may be represented by the formula 2 (Cl + S) + (P + 3 S), in which a sulphur acid is united to a sulphur base. It is doubtful, however, which of the sulphurets is the base in this compound. Chloride of sulphur acts the part of a base in the compounds it forms with the chlorides of tin and titanium; it may therefore in the present compound be a base to a sulphur acid of phosphorus 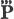 analogous to the oxygen acid
analogous to the oxygen acid 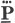 .
.
Bisulphuret of carbon.—Marx† has investigated the tension of
* Rose in Poggendorf's Annalen, xxiv.
† N. Jahrbuch der Chimie, iii. p. 460.
[page] 464
the sulphuret of carbon in the gaseous state from 16° to 139° F. He found its boiling point to be 116° F., and its vapour at 139° sustained a column of mercury of 40·8 Parisian = 43·49 English inches. His experiments seem to show that Dalton's law that the tension of all volatile bodies is equal, an equal number of degrees above and below their boiling points, does not hold in this compound.
Muriate, hydriodate, and hydrobromate of phosphuretted hydrogen.—One of the many interesting facts lately made out in regard to phosphuretted hydrogen is, that it has decidedly the properties of a base, and is capable of forming definite crystallizable compounds with the hydrogen acids.
The first known of these, the hydriodate, was discovered by Dulong, and has been lately studied by Serullas*. The easiest mode of preparing it is to introduce into a retort sixty parts of iodine, fifteen of phosphorus, and eight or nine of water, and apply a gentle heat. Hydriodic acid and phosphuretted hydrogen are generated at the same time, and uniting in the nascent state condense as a white sublimate in the upper part of the retort. From these quantities twelve or fifteen parts of the compound are obtained. A little hydriodic acid passes off in the gaseous state, and may be condensed in water. It crystallizes in cubes, volatilizes at about 290° F., and is not decomposed by a red heat. In vapour it is inflammable; water decomposes it, giving off non-inflammable phosphuretted hydrogen. Chloric, bromic, and iodic acids, and their salts of potash, decompose it with flame at common temperatures; nitric acid does the same, but nitrate of potash, perchloric acid, and perchlorate of potash require the aid of heat. Nitrate of silver in powder strewed upon the salt causes an instant production of iodide and phosphate of silver. With oxide of silver iodide of silver is produced, and a spontaneously inflammable phosphuretted hydrogen. This compound consists, according to Rose, of one atom of each constituent, or it is (I + H) + (P + 3 H).
The hydrobromate was discovered by Serullas†. It may be obtained by bringing together phosphuretted hydrogen and hydrobromic acid in the gaseous state, when they condense on the sides of the vessel into cubical crystals, which may be preserved in close bottles.
The muriate has not yet been obtained in a separate state; but Rose has formed a compound of this muriate with the subchloride of titanium from which its separate existence may be inferred. The formula for this double chloride is 3 (Ti +2 Cl)
* Annales de Chimie, tom. xlviii, p. 93.
† Ibid. p. 90.
[page] 465
 the latter member of which represents the composition of the muriate of phosphuretted hydrogen.
the latter member of which represents the composition of the muriate of phosphuretted hydrogen.
The interest connected with these compounds arises from the analogy in constitution between ammonia and phosphuretted hydrogen and their corresponding salts. The hydriodate and the hydrobromate of phosphuretted hydrogen, the similar salts of ammonia, as well as sal ammoniac, all crystallize in cubes, and are all compounds of the same character, in which a gaseous acid containing one atom of hydrogen is combined with a gaseous base containing three atoms of hydrogen. Whatever opinion, therefore, we form of the constitution of sal ammoniac, the same, it is obvious, must be entertained of the compounds above described.
The similarity in properties and the identity in form of sal ammoniac with the chlorides of the alkalifiable metals, formerly induced some eminent chemists to consider it as a similar compound of chlorine with a metal. This opinion was very much strengthened by the remarkable property of sal ammoniac in forming an amalgam with mercury when placed in the circuit of a galvanic battery. But if instead of comparing this salt of ammonia with those of potassium and sodium, we compare it with that of phosphuretted hydrogen, the atomic constitution of which is precisely analogous, we shall no longer see the necessity of resorting to the supposition of a compound volatile metal (N + 4 H.) Ammonia as a volatile compound base no longer stands alone, and as we cannot suppose the existence of any metal P + 4 H, so all probability of any such as N + 4H is done away.
The very interesting inquiry into the relations of these compounds of phosphuretted hydrogen to mercury in the galvanic circuit was not made by Serullas. It remains for other experimenters who yet survive to follow up the many discoveries made by this indefatigable chemist during the few brilliant years he had devoted himself to his favourite science.
Oxides of chlorine and iodine.—Much has of late years been added to our knowledge of the compounds of chlorine and iodine with oxygen and with each other. Great obscurity, however, as will appear from what has been already stated regarding euchlorine and chlorous acid, still rests on the lower degrees of oxidation of these two substances. From the experiments of Sementini,—who, by passing a mixture of iodine vapour and oxygen through a red-hot tube, obtained an oily, amber-like liquid, which decomposes combustible substances and inflames phosphorus and potassium,—it would appear that there exists one or more compounds of iodine with less oxygen than in iodic acid; but nothing definite is yet known on the subject.
2 G
[page] 466
Perchloric acid.—The perchloric acid of Von Stadion has been studied by Serullas and Mitscherlich, the latter of whom has shown it to be isomorphous with the permanganic. Serullas has shown that the perchlorate of potash may be prepared in any quantity by fusing the chlorate and driving off oxygen as long as muriatic acid causes a yellow stain when dropped on the salt. From an ounce of chlorate heated in a retort till four bottles of oxygen gas were given off, Liebig obtained three drams and a half of perchlorate; what remains in the retort is a mixture of chloride and perchlorate. This is dissolved in hot water and allowed to cool, when the perchlorate, which is difficultly soluble, is deposited. From this salt the acid is obtained by means of fluo-silicic acid. Serullas suggests that the insolubility of the salt of potash may render this acid useful in separating that alkali from soda.
Iodic acid.—Iodic acid has also received considerable attention. Mr. Connell* has established the fact, that iodine may be acidified directly by digestion with nitric acid over a spirit lamp in a large flask, and washing down the iodine as it condenses on the sides of the flask. The process has been repeated by Duflos†, who from half an ounce of iodine and two ounces and a half of concentrated acid, after an hour and a half boiling, obtained five drams and a half of iodic acid. This acid from its weight must obviously have contained water.
Mr. Connell does not find, after repeated trials, that bromine undergoes any oxidation by a similar treatment with nitric acid.
The process recommended by Liebig is to precipitate the iodate of soda by chloride of barium; to every nine parts of the precipitate, well washed and dried, to add two of sulphuric acid diluted with ten or twelve of water, to boil half an hour, filter, evaporate to a sirupy consistence, and expose it to the air for several days. Regular transparent crystals are obtained to the last drop.
Test for chlorine in bromides.— M. Caillot ‡ proposes the chromate of potash as a test for the presence of chlorine in the bromides. This salt decomposes the bichloride, but has no action on solutions of the bibromide of mercury. The bromide to be tested, therefore, is decomposed by a salt of mercury, and brought into the state of bibromide of mercury, as, for example, by subliming it with sulphate of mercury mixed with a little peroxide of manganese,—is dissolved in water and tested with a
* Jameson's Journal, 1831, p. 72.
† Neues Jahrbuch der Chemie, ii. p. 496.
‡ Journal de Pharmacie, March 1830.
[page] 467
few drops of chromate of potash. If any bichloride be present, a number of small red points of chromate of mercuryare immediately deposited.
Löwig* has proposed another mode. The dry mixture of chloride and bromide is heated in a stream of chlorine gas and the vapours made to pass through caustic potash, by which chloride of potassium and chlorate and bromate of potash are formed. A solution of nitrate of silver precipitates the chlorine and the bromic acid. A solution of caustic barytes digested on the moist precipitates takes up the bromic acid only. The excess of barytes is separated by carbonic acid, and the bromate of barytes obtained by evaporation; or the barytic solution may be neutralized by nitric acid, and the bromic acid precipitated again by nitrate of silver.
Oxacids of cyanogen.—The nature and remarkable properties of the compounds of cyanogen and oxygen have been beautifully cleared up in an able Memoir Ann. de Chimie, xlvi. p. 25,) by MM. Wöhler and Liebig. They have shown that the cyanic acid of Serullas contains hydrogen according to the formula l1/2 (Cy + 2 O + H), and have given it the name of cyanuric acid. This acid distills over without loss, and condenses in the cooled receiver into a limpid colourless liquid, which is the cyanic acid of Wöhler, combined with one atom of water. It is represented by the formula (Cy + O) + (H + O), in which the elements are precisely in the same ratio as in the cyanuric acid,—from which it appears that by heat the atoms constituting cyanuric acid are arranged so as to produce one atom and a half of a hydrated cyanic acid containing one atom water. But this new arrangement of the atoms is very unstable; for on acquiring the temperature of the atmosphere it begins to grow turbid, evolves heat, enters into ebullition, and in a few minutes is converted into a dry, compact, brilliant, white solid, of the same composition as the cyanuric and hydrated cyanic acids, and which, from its insolubility in water and nitric and muriatic acids, has been called insoluble cyanuric acid.
I had drawn up a short outline of the properties and several modes of obtaining these acids; but as I find Dr. Turner, in the fourth edition of his Chemistry), just published, has inserted all the most important facts, I cannot do better than refer the reader to that excellent work.
Metals, precipitation of, from solutions in a malleable state. —It is a well-known fact, in chemical science, that certain metals in the metallic state introduced into the saline solutions of certain others, deprive the latter of their acid and oxygen, and
* Geiger's Magazin, xxxiii. p. 10.
2 G 2
[page] 468
precipitate them in the metallic state. Thus, iron precipitates copper, zinc precipitates silver, &c. But in all such cases the reduced metal is generally obtained either in the state of a fine powder, or cohering together in a porous mass. In some instances, however, it has been met with in a solid malleable state, as if it had been submitted to fusion, sometimes even in regular crystals. Thus in the copper pits of Anglesea, where iron is thrown in to reduce the copper from its solutions, malleable and crystallized copper has often been obtained. To ascertain the circumstances under which it was deposited in this form, and above all to be able to produce it at pleasure, became a very interesting inquiry, not to the chemist only, but also to the geologist.
Wach* has investigated these circumstances, and found that wherever the process proceeds with sufficient slowness the metal is deposited in this form. The process he recommends is founded on an observation of Fischer, that when a metal is placed in a tube containing water and separated at the lower end by a diaphragm of bladder from the solution of the metal it is intended to precipitate, the action takes place through the bladder, the precipitated metal is deposited on its outer surface, while the acid liquor passes through and dissolves the other metal. By this method Wach has succeeded in obtaining copper, antimony, bismuth, silver, and platina in a massive state, as if they had been fused. The metal may also be sewed up in two or three folds of bladder and immersed in the solution to be precipitated, when the same effects follow.
Becquerel obtained copper in crystals by means of very weak galvanic currents.
Metallic copper has also been found in large masses deposited in vessels made entirely of wood, into which the solution of sulphate of copper is collected in the vitriol manufactories previous to boiling for crystallization. This has been found by Clement and Bischof to be due to the presence of a portion of a salt of the protoxide in the solution, one part of which gives up its oxygen and is reduced to the metallic state. This phænomenon therefore is quite distinct from those above mentioned.
Electro-negative metals— Vanadium.—The most important addition to our knowledge of the electro-negative metals made during the last ten years has been the discovery of vanadium by Sefström, and the elaborate examination of the properties of the new metal since published by Berzelius.
This metal was discovered by Sefström towards the end of 1830, in the iron from the forges of Eckersholm in Sweden,
* Neues Jahrbuch, i. p. 40.
[page] 469
and by myself in the beginning of 1831 in an ore of lead from Wanlock Head, and before Sefström had published anything upon the subject. It approaches nearest to chromium in its properties, and lies in a natural arrangement between that metal and molybdenum. It is characterized by giving, in the form of oxide, blue salts with acids, with oxygen forming a peculiar acid, which fuses at a red heat without decomposition, and on cooling is reddish brown and crystalline, and which gives with bases, colourless neutral and orange-coloured acid salts. Before the blowpipe it behaves itself like chromium, with this characteristic difference—that the green colour with borax can in the oxidizing flame be changed into a pale yellow.
Vanadium has three degrees of oxidation, represented by  ,
,  ,
, .
.
Protoxide,—The protoxide,  , is dark brown or black, soluble in nitric acid and in aqua regia, but does not form salts. It is obtained by reducing vanadic acid in hydrogen gas.
, is dark brown or black, soluble in nitric acid and in aqua regia, but does not form salts. It is obtained by reducing vanadic acid in hydrogen gas.
Binoxide.—The binoxide,  , is black and infusible, heated in the air attracts oxygen, and is changed into vanadic acid. It forms salts with the acids, which when anhydrous are dark brown, and when they contain water of a deep blue, like the salts of copper. They crystallize with difficulty; the sulphate forms a sirupy liquid in which crystals are gradually formed. Its salts have a sweetish astringent taste. It combines also with alkalies, giving brown soluble compounds; with the other bases its compounds are insoluble, but are convertible, by heating in the air, into vanadiates.
, is black and infusible, heated in the air attracts oxygen, and is changed into vanadic acid. It forms salts with the acids, which when anhydrous are dark brown, and when they contain water of a deep blue, like the salts of copper. They crystallize with difficulty; the sulphate forms a sirupy liquid in which crystals are gradually formed. Its salts have a sweetish astringent taste. It combines also with alkalies, giving brown soluble compounds; with the other bases its compounds are insoluble, but are convertible, by heating in the air, into vanadiates.
Vanadic acid.— Vanadic acid,  , in the state of powder is yellow; at a red heat it fuses readily, and on cooling crystallizes in beautiful prismatic crystals, transparent at the edges, of a reddish brown colour and a high degree of lustre. These crystals belong to the hemiprismatic system, have at least one axis of double refraction, and are remarkable for possessing a higher refractive power than any other known body. In some very huge crystals which I obtained by very slow cooling, Sir David Brewster found the polarizing angle = 69° 37', giving a refractive power = 2·691. That of chromate of lead is 2·5, and the highest determination for diamond that is to be depended on = 2·487.
, in the state of powder is yellow; at a red heat it fuses readily, and on cooling crystallizes in beautiful prismatic crystals, transparent at the edges, of a reddish brown colour and a high degree of lustre. These crystals belong to the hemiprismatic system, have at least one axis of double refraction, and are remarkable for possessing a higher refractive power than any other known body. In some very huge crystals which I obtained by very slow cooling, Sir David Brewster found the polarizing angle = 69° 37', giving a refractive power = 2·691. That of chromate of lead is 2·5, and the highest determination for diamond that is to be depended on = 2·487.
This acid is sparingly soluble in water and alcohol, to both of which liquids it imparts a yellow colour; water does not take up 1/1000dth part. It forms with bases neutral salts, which in general are colourless, and acid salts of a bright orange colour.
[page] 470
With acids it gives, like molybdic acid, saline combinations. A remarkable double salt of this character was obtained by Berzelius in his process for purifying the acid; it consisted of 1 atom phosphoric acid, 1 atom vanadic acid, 1 atom silica, and 3 atoms water. One circumstance for which this acid is remarkable is, that its neutral salts seem capable of existing in a coloured as well as a colourless state. If an acid salt be saturated with caustic potash, in the cold it retains a yellow colour which disappears after some time. If the saturation be effected with caustic ammonia, the yellow colour is permanent till the solution is heated near to the boiling point, when it disappears. If a solution of chloride of barium be poured into one of a neutral vanadiate, the salt of barytes falls of a beautiful yellow colour, which slowly disappears of itself, but the precipitate becomes at once white if heated near to 212°. The same is true in regard to the artificial vanadiate of lead,—except that the colour of this latter salt never becomes pure white. I have observed also that if a drop of nitric acid be mixed with a neutral solution of vanadiate of ammonia, a yellow colour is immediately developed. This might be attributed to the formation of a small quantity of bivanadiate, were it not that by standing the colour disappears, while the acidity remains. It would appear therefore that this acid is susceptible of two isomeric molecular arrangements, one of which being the cause of the colour exists in the bisalts, the other in the neutral salts.
Sulphurets.—Like molybdic and tungstic acids, the vanadic also combines with its oxide. But it unites in different proportions, and the compounds are all soluble in water. The subvanadiate of the oxide gives in water a purple solution; the neutral  +2
+2  a beautiful dark green; the acid 4
a beautiful dark green; the acid 4  +
+  also a green; and a greater excess gives compounds of various shades of yellow.
also a green; and a greater excess gives compounds of various shades of yellow.
The atomic weight of vanadium, according to the experiments of Berzelius, = 855·84, and of vanadic acid 1155·84.
Vanadium gives also two sulphurets, 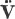 and
and 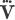 , corresponding to the oxide and acid.
, corresponding to the oxide and acid.
Vanadiate of lead.—It has not yet been made out in what state this metal exists in the ore of iron, from which it has been extracted in Sweden. In the mineral from Wanlock Head it is in combination with lead, constituting the principal electronegative ingredient, although there are present at the same time chlorine, arsenic acid, and phosphoric acid. This mineral was found at one time in a lead mine at Wanlock Head, which has not been wrought for several years. The process for extract-
[page] 471
ing the vanadic acid is very simple. The mineral is dissolved in muriatic acid,—the lead and arsenic separated by sulphuretted hydrogen,—the filtered liquid reduced to smaller volume by evaporation, supersaturated at a boiling temperature with carbonate of ammonia, and filtered while hot. On cooling, the vanadiate of ammonia crystallizes, forming a white crust which adheres strongly to the sides of the glass. It may be purified by a second crystallization, and the vanadic acid is obtained from the purified salt by driving off the ammonia with the aid of heat.
Tellurium.—Berzelius has for some time been engaged in an elaborate investigation of the properties and combinations of tellurium, and has already obtained many very interesting results. Several of these, which he has been kind enough to communicate to me, I shall here insert. The following processes—the only ones yet given—by which it can be obtained perfectly pure from the tellurets of bismuth and silver, he has already published in his Årsberättelse for 1832, p. 104. The telluret of bismuth, from Schemnitz in Hungary, is reduced to powder, washed, mixed with twice its weight of carbonate of potash, made into a paste with oil, and placed in a well-covered crucible, to which heat is at first carefully applied, but which is gradually raised to full redness, and kept so as long as flame appears between the crucible and the lid. When cold, the mass is porous and of a dark brown colour. It is powdered and well washed (with cold water previously boiled to free it from air,) in a filter, by which an opaque purple red solution is carried through, and there remains a dark brown mass consisting of charcoal and bismuth, with very little tellurium. The solution is telluret of potassium. A current of air is passed through it, by which the potassium is oxidized and the tellurium precipitated. In the alkaline liquid remains a little sulphuret and seleniuret of tellurium, which may be thrown down by an acid.
The precipitated metal is washed, dried and melted. It is then placed in a small elongated capsule of porcelain, introduced into a porcelain tube, and exposed to the heat of a furnace while hydrogen gas is passed over it. The tellurium sublimes into the cool part of the tube, and there remains in the capsule a residuum, consisting chiefly of telluret of gold, but containing also tellurets of copper, iron and manganese, which had all been present in the alkaline solution.
From the telluret of silver it is easiest prepared by heating it in a current of chlorine, by which the chloride of tellurium is driven off and condenses in a solid form leaving chloride of silver. The solution of the chloride in muriatic acid is precipi-
[page] 472
tated by hyposulphite of soda, and the precipitate, as before, distilled in hydrogen gas. In this way it may contain selenium, the most of which is carried over by the hydrogen gas in the form of a red vapour; but it cannot be obtained by this process entirely free from selenium, unless it have been previously heated to fusion in the state of oxide, by which the selenious acid is driven off.
Metallic tellurium.—Pure tellurium heated to full redness in a close vessel volatilizes in the form of a yellow gas, which condenses again in drops, and on cooling crystallizes in forms which appear to belong to the regular system. The smell of the vapour is peculiar, slightly resembling that of selenium. Its specific gravity, taking the mean of several trials, is 6·2455; the highest was 6·2578, and is probably nearest the truth.
Its atomic weight, taking the mean of four results, is 802·902. The mean of two nearly coinciding results gives 801·7675.
Berzelius did not succeed in forming an oxide containing one atom of oxygen Ṫ, but he obtained two degrees of oxidation, the one formerly known Ṫ, and a higher one  , which is the telluric acid.
, which is the telluric acid.
Tellurous acid.—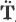 can scarcely ever be made to act the part of a base, and therefore it must be considered as an acid, —the tellurous acid. Like oxide of tin, it takes two isomeric forms, which possess unlike properties. Prepared by digesting tellurium with nitric acid, and precipitating the solution by water or allowing it to crystallize, it is paratellurous acid (acidum paratellurosum). In this state it crystallizes apparently in octohædrons, contains no water, is almost insoluble in nitric acid, and when precipitated by water, and dried on the water bath, is milk white and crystalline. It reddens litmus slowly, and is very sparingly soluble in water. On heating it becomes yellow, and at incipient redness melts into a clear liquid, is volatilized in small quantity, and cools into a milk-white crystalline mass of acid in the same metameric state. By slow cooling it may be obtained in a transparent mass.
can scarcely ever be made to act the part of a base, and therefore it must be considered as an acid, —the tellurous acid. Like oxide of tin, it takes two isomeric forms, which possess unlike properties. Prepared by digesting tellurium with nitric acid, and precipitating the solution by water or allowing it to crystallize, it is paratellurous acid (acidum paratellurosum). In this state it crystallizes apparently in octohædrons, contains no water, is almost insoluble in nitric acid, and when precipitated by water, and dried on the water bath, is milk white and crystalline. It reddens litmus slowly, and is very sparingly soluble in water. On heating it becomes yellow, and at incipient redness melts into a clear liquid, is volatilized in small quantity, and cools into a milk-white crystalline mass of acid in the same metameric state. By slow cooling it may be obtained in a transparent mass.
By dissolving this mass in muriatic acid, or more easily by fusing it with the proper quantity of carbonate of soda to form a neutral salt, and precipitating its solution with nitric acid, the tellurous acid (acidum tellurosum) is obtained in the state of a voluminous flocky precipitate. This must be dried in the air, for if it be even slightly heated it loses its water and becomes metameric.
In this state it is soluble in acids, in ammonia and in carbo-
[page] 473
nated alkalies. It contains 12·47 per cent. of water, and the dry acid is composed of,
| Tellurium | 80·06 | = 1 atom. |
| Oxygen. | 19·94 | = 2 atoms. |
It forms bi- and quadri-tellurets, the latter most easily.
Telluric acid.—The tellurous acid in solution with excess of caustic alkali is submitted to a current of chlorine, till the precipitate, which at first falls, is entirely redissolved. Chloride of barium is added to precipitate any sulphuric or selenic acid which may be present, after which the solution is saturated with ammonia, and the tellurate of barytes precipitated by chloride of barium, collected and washed with cold water. This salt is decomposed by sulphuric acid, and the concentrated solution deposits the telluric acid in beautiful prismatic crystals.
Tellurous acid may also be oxidized by fusion with nitrate of potash, when on treating with water paratellurate of potash remains undissolved. This may be dissolved in nitric acid saturated with ammonia and precipitated by a barytes or lead salt as before.
The acid crystallizes in six-sided prisms terminated by four planes, has a metallic taste resembling that of nitrate of silver, dissolves slowly in cold but rapidly and in large quantity in boiling water. It is very sparingly soluble in alcohol. The crystals contain three atoms of water, being (T + 3 O) + 3 Ḣ. At 212° it loses no water; on a hot sand-bath it loses two atoms, retaining its crystalline form; and is insoluble in cold water. At a temperature below redness it loses the third atom of water and gives a beautiful yellow acid which still retains the crystalline form. At a higher temperature it loses an atom of oxygen, becoming paratellurous acid.
The yellow acid obtained by heating below redness is the paratelluric acid. It is insoluble in boiling water and boiling caustic ley if not very concentrated, in cold concentrated muriatic and in boiling nitric acid. From the concentrated caustic potash solution it is obtained in the usual state. It gives bi- and quadri-salts, those of the common and metameric acid differing in properties.
The hydrated crystals, as they are at first formed, contain 23·428 per cent. = 3 atoms of water; after drying on a hot sand-bath 9·255 per cent. = 1 atom of water; and the anhydrous acid consists of
| Tellurium | 72·799 | = 1 atom, |
| Oxygen. | 27·201 | = 3 atoms, |
[page] 474
which gives for the atom tellurium 8·028, and of telluric acid 11·028.
Persulphuret of tellurium.—Besides the sulphuret of tellurium Ṫ formerly known, Berzelius has formed a persulphuret  , corresponding to the new acid
, corresponding to the new acid  . When a solution of telluric acid in water is saturated with sulphuretted hydrogen and set aside, a metallic-looking substance gradually deposits itself on the sides of the glass, while the liquid rests clear: this is the sulphuret in question.
. When a solution of telluric acid in water is saturated with sulphuretted hydrogen and set aside, a metallic-looking substance gradually deposits itself on the sides of the glass, while the liquid rests clear: this is the sulphuret in question.
Chlorides of tellurium.—Rose has also lately examined the chlorides of tellurium. When the metal is gently heated in chlorine gas, a white crystalline compound is formed, consisting of
| Chlorine | 52·13 | = 2 atoms | = 2 Cl+T. |
| Tellurium | 47·87 | = 1 atom |
It has much resemblance to the solid chloride of selenium. When the metal is strongly heated in the same gas, violet vapours are formed, which condense into a black deliquescent mass. When freed as much as possible from bichloride, this substance was found to consist of
| Chlorine | 37·77* | = 1 atom | Cl + T. |
| Tellurium | 62·23 | = 1 atom |
It is interesting to observe by what analogies the chlorides of sulphur, selenium, and tellurium are connected, and by what differences they are distinguished. Of sulphur we have one chloride, (Cl + 2S), which is analogous in every respect to the dichloride of selenium, (Cl +2 Se); but here the analogy stops, and we have no direct link to connect it with the chlorides of tellurium. But the solid chloride of selenium, (2 Cl + Se), is connected by an analogy equally close and striking with the bichloride of tellurium, 2 Cl + T. These three bodies therefore form a natural group connected together, like the compounds of phosphorus, arsenic, and antimony, by many similar properties, and yet differing in others so widely as to afford the analytical chemists ample means for detecting the presence of each.
Artificial Ultramarine.—A discovery likely to prove of considerable use in the arts is the manufacture of ultramarine by a process founded on the analysis of the native substance by Clement Desormes, and of a similar substance found on the lining of a furnace by Vauquelin. According to Gmelin† this
* Poggendorf's Annalen, xxi. p. 442.
† Ann. de Chimie, xxxvii. p. 409.
[page] 475
compound is a double silicate of alumina and soda, coloured by combining with sulphuret of sodium. He precipitates alum by caustic ammonia, washes and dries the subsulphate, and estimates the amount of water it contains. In like manner he precipitates silica from its solution in an alkali, washes and dries without heating to redness. The silica is dissolved in a concentrated solution of caustic soda to saturation, and for every seventy-two parts thus dissolved, seventy of the alumina are added, each calculated in the anhydrous state, and the whole evaporated to the state of a moist powder. Two parts of sulphur and one of dry carbonate of soda are now fused together, and the moist powder gradually thrown in, and kept at a red heat for an hour in a covered crucible. The ultramarine is then separated by washing.
In what state the sulphur and the alkali exist in this compound has not been ascertained.
Guimet in France first succeeded in producing this pigment, by a process probably more economical, but which has not been made public; and in that country it is already manufactured in considerable quantities.
Electro-positive Metals, reduction of.—Since the brilliant discovery of the metallic bases of the alkalies and earths by Davy, processes have been devised, by means of which those of the alkalies can be obtained in large quantity. Most of the earths, however, continued refractory, and chemists were unacquainted, till lately, with any process by which they could be decomposed. In 1828 this difficulty was overcome by Wöhler, who, by preparing anhydrous chlorides and heating them with potassium, succeeded in reducing alumina, glucina, and yttria. By the same process in the following year Berzelius reduced thorina, and Bussy magnesia.
The metals obtained from these oxides are much more permanent than the bases of the alkalies; they may be boiled in water without oxidation, burn when heated in the open air, and are dissolved by acids with evolution of hydrogen gas.
Potash.—Fuchs* has proposed to prepare potash for commercial purposes from felspar and mica. The minerals are reduced to powder, calcined in a furnace with quick lime, and afterwards exposed to air and moisture for some time; the alkali is then washed out: by this process felspar should give about one fifth of its weight of potash.
Barytes and Strontian, separation of.—Liebig† proposes iodate of soda as an excellent means of separating these two
* Roy. Inst. Journ. i. p. 184.
† Journ. de Pharm. April 1832, p. 214.
[page] 476
earths. Neutral solutions of strontian are not precipitated by this salt, while those of barytes give immediately white flocks, and the precipitation is so complete that no trace of barytes remains in solution.
Lime.—Mr. Andrews* has also given a very simple method of detecting the presence of barytes and strontian in lime. The whole is dissolved in nitric acid, evaporated to dryness, and the acid expelled by heating to redness in a platinum crucible. The caustic residue is boiled with water, when the whole of the barytes and strontian and only a little of the lime are dissolved. Sulphuric acid added to the solution shows if any of these two earths are present, while a boiling saturated solution of sulphate of strontian troubles it if it contain barytes, but causes no precipitate if the earth be strontian.
Thorium.—In a notice of the most important discoveries recently made, that of the metal thorium by Berzelius must not be passed over. In 1816, in analysing the gadolinite of Korarvet near Fahlun, and some other rare Swedish minerals, he obtained an earthy base which differed from all other known substances, and which he therefore concluded to be the oxide of a new metal, to which he gave the name of thorium. More lately, however, he found that he had been led into an error, and that the supposed oxide was a subphosphate of yttria, in which the phosphoric acid could be detected with very great difficulty. But in 1828, Pastor Esmarck, of Brevig in Norway, transmitted to Berzelius a black heavy mineral which he supposed to contain columbium. On analysis, however, it proved to consist chiefly of a silicate of a new earth which constituted about fifty-eight per cent. of the whole mineral. To the base of this earth Berzelius gave the name thorium, partly on account of a resemblance between its properties and those of the subphosphate of yttria to which it had formerly been applied, and partly because that term had already found its way into chemical works. It is allied in properties to yttria, glucina, and zirconia.
The mineral thorite, in which it is found, is very rare. Only one mass of it has hitherto been found in a primitive rock near Brevig in Norway. I visited the locality last summer, but succeeded only in obtaining a small fragment, for which I was indebted to the kindness of its discoverer Pastor Esmarck.
Manganese.—The nature and composition of the several oxides of manganese have been beautifully cleared up by the united labours of Turner, Haidinger, and Phillips; and the
* Phil. Mag. and Annals, vii. p. 404.
[page] 477
acid compounds of this metal have lately been studied by Mitscherlich with equal success.
When peroxide of manganese is fused in the open air with an equal weight of caustic potash, and the mass afterwards treated with water, a green solution is obtained, which must be decanted, not filtered, through paper. This solution, evaporated in the receiver of an air-pump, gives green crystals of manganate of potash. These crystals are isomorphous with the sulphate and chromate of potash, and the acid they contain is composed of 1 atom manganese + 3 atoms oxygen = M + 3 O.
These crystals may be redissolved in caustic potash and crystallized without decomposition; but if the solution be exposed to the air till the alkali attracts carbonic acid, a brown powder, which is a compound or mixture of the hydrates of the pro- and per-oxides, is precipitated, and the solution becomes red. Evaporated on a sand-bath red crystals are obtained, which are permanganate, and are isomorphous with the perchlorate of potash. The acid they contain consists of two atoms manganese and seven atoms oxygen = 2 M + 7 O. This salt in solution rapidly decomposes when heated, and slowly when set aside in a diluted state, and becomes finally green, passing through the series of changes from that colour to red, to which it owes its name of chameleon mineral*.
The known compounds of oxygen and manganese are as follow:—
| Protoxide | = M + O |
| Sesquioxide | = 2 M + 3 O |
| Peroxide | = M + 2 O |
| Red oxide | = 3 M + 4 O |
| Varvicite | = 4 M + 7 O |
| Manganic acid | = M + 3 O |
| Permanganic acid | = 2 M + 7 O |
Zinc.—De la Rive† has made an interesting observation regarding the action of sulphuric acid on metallic zinc. He found that distilled zinc is much more slowly dissolved in dilute acid than the zinc of commerce. This he attributes to the electrical agency of the foreign metals, especially iron, which it contains; and this explanation is confirmed by the fact that when a thick platinum rod was fastened to a piece of zinc it evolved twice as much hydrogen in a given time as when placed in the acid alone. An acid containing from fifty to seventy per cent. of water dissolves the zinc most rapidly.
Oxide of zinc.—By heating metallic zinc in an atmosphere
* Ann. de Chimie, xlix. p. 113.
† Ibid. xliii. p. 425.
[page] 478
of aqueous vapour, cautiously regulating the heat so as not to fuse the metal, M. Haldat* has obtained crystals of oxide of zinc, of a honey colour, almost transparent, and of a rhomboidal form.—By a similar process he has obtained minute groups of brilliant rhombohedral crystals of specular iron, equal in lustre and in play of colours to the freshest specimens from Elba or Framont.
Schindler† has also shown that a hydrated oxide of zinc may be obtained in crystals by uniting a rod of zinc and iron, and placing them in caustic ammonia in a close vessel. Gas is developed, and in a few days the inside of the vessel is covered with small transparent crystals, which are permanent in the air, and consist of oxide of zinc 81·62, water 18·36 = Żn + Ḣ.
Iron, carburets of.—MM. Gay-Lussac and Wilson‡ have published the results of a series of analyses of carbonaceous irons, which show the carbon in the bar irons examined to vary from fourteen to twenty-nine, and in the steels from sixty-two to ninety-three ten-thousand parts. In gray cast-iron it varied from sixteen to twenty-eight, and in white from twenty-three to twenty-seven thousand parts. It would be desirable to know how the carbon was determined, as they make it so much less than former experimenters.
Lead, sulphurets of.—Bredberg has added to our knowledge of the compounds of lead and sulphur by forming two new compounds of these elements: the lowest, 4 Pb + S, is formed by fusing together 25 parts of galena with 21·6 of lead: it is granular and sectile. The other, S + 2 Pb, is obtained by fusing the same mixture in a crucible with borax. It is crystalline, lamellar, and slightly malleable.
Super-sulphuretted.—I have lately analysed the super-sulphuretted lead of Dufton, and found that it is merely a mixture of sulphur and sulphate of lead, in proportions which probably vary.
Bismuth, expansion of, on becoming solid.—Marx§ has established a very important fact in regard to melted bismuth. He finds that at the moment of solidifying it expands 1/53 of its volume. He considers also that, like water, it has in the fluid state a point of maximum density.
Bismuth, oxide of.—Mr. Phillips‖ has shown that a deep blueish black oxide of bismuth is sometimes obtained when the subchloride is decomposed by alkalies. This might be taken
* Ann. de Chimie, xlvi. p. 72.
‡ Journ. of Science, 1830, p. 204.
‖ Phil. Mag. and Ann. of Phil. 1830, p. 410.
† Geiger's Magazin, Aug. 1830, p. 174.
§ Neues Jahrbuch, i. p. 454; ii. p. 114.
[page] 479
for an isomeric oxide, were it not that Simon has described what appears to be a lower oxide, of a black colour*, obtained from the solution of the nitrate, and that we are already acquainted with other appearances which seem to indicate the existence of such a lower oxide. The subject is highly deserving of investigation.
Antimony and lead do not expand at the moment of congelation as was formerly supposed; so that water, bismuth, and cast-iron, are the only bodies which possess this property; and in regard to cast-iron it is still doubtful.
Zinc contracts greatly; potassium also contracts, and arsenic at least three times as much as bismuth expands, if we may judge from the fact that a mixture of one fourth of its weight of arsenic prevents bismuth from expanding on becoming solid.
Copper, phosphuret of.—Phosphuret of copper, prepared by passing a current of phosphuretted hydrogen through a solution of sulphate of copper, is a black powder, which when heated in close vessels assumes the colour and lustre of metallic copper, and gives no phosphorus flame before the blowpipe. It has in this state been mistaken by some chemists for pure copper. Prepared by passing phosphuretted hydrogen over heated chloride of copper, it is nearly black, does not lose its dark gray metallic lustre by heating, and gives a phosphorus flame before the blowpipe. Like so many other compounds of phosphorus, therefore, these two phosphurets are isomeric. According to Rose, they have both the composition 3 Cu + 2 P.
Salts.—The department of salts is so very extensive, and it has within these few years received such large additions, that it is impossible, in a Report like the present, to give even an outline of the great progress that has been made in filling up the chasms in this branch of the science.
To this great advance Dr. Thomson has contributed much in his First Principles; Berzelius, in his elaborate papers on the fluates, the cyanides, the seleniates, the sulpho-salts, those of thorina, vanadium, and tellurium, platinum, and the metals which accompany it; Stromeyer, in investigating the salts of cadmium; Arfwedson, those of lithia; Mitscherlich, in his various memoirs on isomorphous bodies; Gmelin, in forming the red prussiates; Sir John Herschel, in his description of the hyposulphites; Bonsdorf, in his papers on the double chlorides, bromides, &c.; Boullay, on the double iodides; Persoz and Rose, in examining the compounds of ammonia, with many
* Berzelius's Arsberättelse, 1832, p. 112.
[page] 480
chlorine and oxygen salts; Wöhler, the obscure compounds of cyanogen and its acids; Berthier, the compounds which oxides, acids, and neutral salts form with each other by fusion; Serullas, the saline compounds of iodine, chlorine, bromine, and their acids; Liebig, and more lately Edmund Davy, the fulminates; Zeisé the xanthates, and the remarkable hydrocarburetted chlorides of platinum; Dulk, the tartrates; Grüner, the pyrotartrates; Mosander, the complicated salts containing three metallic cyanides;—besides many others whose valuable labours find a place in our systems of chemistry.
The most important theoretical point connected with the history of the salts which at present occupies the attention of chemists,—that regarding the sulphur and chlorine salts,—has already been treated of in the former section. I shall here, therefore, introduce only a brief notice of a few of the most recent and interesting additions to our knowledge.
Iodate of soda.—Liebig* prepares the iodate of soda by passing chlorine over iodine mixed with a little water till the iodine is all dissolved; carbonate of soda is then added till saturation, when a considerable quantity of iodine is separated; this solution is poured off, a little more water added, and the operation repeated till saturation with soda ceases to throw down any iodine; the solutions are then evaporated to one tenth, and while hot an equal volume of alcohol added, and the whole set aside; on cooling the iodate is deposited in starry groups of octohædral prisms, which are to be washed with alcohol to free them from adhering common salt.
The mode of preparing iodic acid from this salt has been already given.
Carbonate of soda and lime.—Bauer has observed that when the solution of carbonate of soda, prepared by the usual manufacturing process from Glauber's salt, was cooled to 32°, a white crystalline powder was deposited, which effloresced in warm air, and consisted of carbonate of soda 36·2, carbonate of lime 34·1, water 29·8, 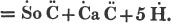 This is the same composition as the Gay-Lussite of Boussingault.
This is the same composition as the Gay-Lussite of Boussingault.
The mother leys do not deposit the whole of the lime when thus cooled to 32°; for when tested with oxalate of ammonia they give a white precipitate. It may therefore exist in the carbonate of soda of commerce.
Carbonate of lime;— Arragonite.—Mitscherlich† has described a very interesting crystal of arragonite, which, had it
* Journ. de Pharm. 1832, p. 212.
† Poggendorf's Ann. xxi. p. 159.
[page] 481
preserved its original form, but by the action of heat had been converted externally into the rhomboidal carbonate of lime. It was found among the detritus of Mount Vesuvius, and it would appear that it had fallen among the lava, the long-continued heat of which had induced a new arrangement of the particles, but had ceased before the whole crystal had undergone the change. An analogous change of arrangement is exhibited in those crystals of augite and hornblende mentioned by Gustaf Rose in his admirable paper* on the identity of these two minerals, in which a kernel of the one form is contained within a crystal possessing externally the characteristic form of the other.
Hydrated carbonate of lime.—Becquerel† and Gay-Lussac have examined the artificial crystals of carbonate of lime, which are formed when a solution of quick-lime in sugar, mucilage or starch, is exposed to the air, or is acted on in close vessels by a weak galvanic energy. Gay-Lussac employs one part quick-lime, three of sugar, and six of water, and in forty-eight hours obtained a considerable quantity of crystals; and in two months no lime remained in solution. The crystals are white, tasteless, insoluble in water, effloresce in air of 30° C., have a specific gravity of 1·788 at 10° C., and crystallize in rhomboidal prisms. They contain 47·08 per cent. = 5 atoms water. Boiling alcohol takes up two atoms of the water, without changing the form of the crystals, which, however, effloresce now more rapidly than when they contain five atoms.
Hyposulphite of barytes.—The hyposulphite of barytes has been analysed by Rose‡, and its composition found as in the formula (2S + 2O) + Ḃ + Ḣ. When this salt is decomposed by heat in close vessels, a sulphate and a sulphuret are formed; sulphur is sublimed, sulphuretted hydrogen is given off, and a portion of the water passes over undecomposed, presenting the remarkable circumstance of no less than five different products from the decomposition of an inorganic body, composed of four elements.
Carbonate of lead.—I have lately analysed and described§ under the name of Plumbo-calcite a mineral crystallizing in the primitive form of the carbonate of lime, but which consists of 92·2 carbonate of lime, and 7·8 carbonate of lead. This proves that carbonate of lead is also capable of assuming two forms, which are respectively isomorphous with those of carbonate of lime.
* Poggendorf's Ann. xxii. p. 321.
‡ Poggendorf's Ann. xxi. p. 440.
† Ann. de Chimie, xlvii. p. 9; xlviii. p. 301.
§ Brewster's Journ., N. S., vi. p. 79.
2 H
[page] 482
Sulphate of copper.—The detection of copper in bread in several parts of France and the Netherlands has led to the discovery that in many places the bakers put sulphate of copper into their dough, for the purpose of improving the colour and lightness of their bread. The many analyses which have been made for the purpose of detecting it, however, have led to some interesting results. Meissner has shown that the detection of copper in grain is not always a proof of adulteration, for that copper exists in small quantity in many kinds of grain. This interesting result has been confirmed by other chemists, and M. Sarzeau in particular states* that he has found traces of it in two hundred vegetables, and that it exists in gelatine and in butchers-meat, in the proportion of one grain to every fifteen pounds, and in the cheese he has examined, in that of four grains and a half to fifteen pounds.
Berthier† has lately analysed a native sulphate of copper, found in large quantities in South America. It occurs in the form of a green powder, and is composed of 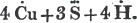
Red salts of manganese.—Mr. Pearsall‡ has published a very ingenious paper on the red colour occasionally developed in the salts and solutions of manganese. This he endeavours to show to be due at all times to the presence of permanganic acid. Allowing that in some instances the colour is due to the presence of this acid, it is sufficient to advert to the coloured salts of manganese to show that the colour is often wholly independent of any higher degree of oxidation than the protoxide.
Pfaff first drew the attention of chemists to the fact that there exist two sulphates of manganese, one of which is colourless, the other of a rose-red tint. Berzelius§, in his experiments to determine the atomic weight of this metal, obtained both the sulphate and the protochloride of this rose-red tint, and therefore saturated the solutions with sulphuretted hydrogen to deoxidize the permanganic acid which he supposed to colour them, but found it impossible by this process to render them colourless. And, lastly, Brandes‖ has shown that the colourless and red sulphate have both the same composition;—we can therefore explain the fact in no other way than by supposing that they are isomeric combinations of the same elements.
Peroxalate of iron, action of light upon.—The peroxalate of iron kept in solution for several hours, at a temperature of
* Journal de Pharmacie, November 1832 p. 653.
† Annales de Chimie, i. p. 360.
‡ Journal of Royal Institution, ii. p. 49.
§ Årsberättelse, 1831, p. 190.
‖ Poggendorf's Annalen, xx. p. 556.
[page] 483
212°, undergoes no change; but if it be exposed to the sun's rays, bubbles of gas gradually show themselves, and soon be come frequent. The liquid assumes a greenish yellow colour, is troubled, and deposits beautiful small shining citron-yellow crystals. The gas is carbonic acid, and the crystals are protoxalate of iron. The action continues till all the persalt is changed into protosalt, and the solution becomes colourless. The changes which take place are thus represented by Döbereiner.
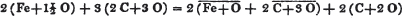
where two atoms of the sesquioxalate of the peroxide of iron are decomposed into two atoms of oxalate of the protoxide, and two atoms of carbonic acid.
Sir John Herschel has lately shown that light exerts a similar action in predisposing the chloride of platinum to be decomposed by caustic lime. If a solution of the latter be poured into one of the former, no change takes place, unless the mixture be submitted to the action of light, when a copious grey precipitate gradually falls.
Submuriate of iron.—In an interesting paper on the composition of several subsalts, Mr. Phillips (Phil. Mag. & Ann. December 1830, p. 406,) has described a remarkable submuriate of iron, containing one atom acid + ten atoms peroxide, which is soluble in water, and is precipitated by a further addition, either of acid or base. It is formed by adding fresh precipitated peroxide to the acid as long as it is dissolved. The solution may be obtained of the density 1·017; further concentration decomposes it. It has only a slight chalybeate taste, and is precipitated by prussiate of potash of a dark brownish green.
Hydrocarburetted chlorides of platinum.—In 1829, (Årsberättelse, p. 159,) Berzelius noticed the existence of a salt of platinum, which appeared to contain the elements of æther. This has led to an elaborate investigation of the subject by Prof. Zeise of Copenhagen, who has shown that the black powder precipitated when slightly acid chloride of platinum is treated with alcohol, contains also the elements of æther, and is probably E + 2 Pt, where E represents ætherine, the base of the æthers = 4H+4C.
When the alcoholic solution of the bichloride is evaporated, a compound is obtained, represented by the formula E + 2 (Pt + Cl). If the solution of this salt be digested on chloride of potassium, a salt is obtained, having the composition
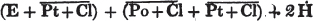
With sal ammoniac and with caustic ammonia, analogous
2 H 2
[page] 484
compounds are obtained, in which these two substances represent the chloride of potassium in the above formula. In these compounds Zeise considers the ætherine to act the part of a base, as, according to the theory of Dumas and Boullay, it does in the æthers.
This able and elaborate paper is well worthy the attentive perusal of the chemist*.
Compound cyanides.—A solution of prussiate of potash throws down precipitates from solutions of barytes, lime, magnesia, &c. These have been investigated by Mosander, who has found them to consist of an atom of each of the cyanides. Thus the calcium salt is expressed by
(Fe + Cy) + (Ca + Cy) + (Po + Cy).
The barium salt crystallizes and contains two atoms of water†.
Mineral Chemistry.—In the first part of this Report I have adverted to the several great steps by which, through the elaborate analytical labours of Bonsdorf, Rose, Wachtmeister and others, several orders of minerals have been established, in which many varieties, distinguished by various trivial names, and erected by mere mineralogists into many different species, have been included under one general formula. I shall insert the results of the two latest of these suites of analyses, by which much light is thrown upon two obscure classes of native compounds.
Phosphates of lead.—Wöhler showed that the native phosphates of lead are composed of one atom neutral chloride of lead with three atoms subsesquiphosphate or arseniate of lead; and Rose, that they might contain lime and fluorine without change of form. Kersten has confirmed these determinations by a series of analyses, of which the following Table shows the results.
| Locality. | Specific Gravity. | Chloride of Lead. | Fluoride of Calcium. | Subsesquiphosphate of Lime. | Subsesqutphosphate of Lead. |
| Freiberg | 6·092 | 10·838 | 1·094 | 11·053 | 77·015 |
| Mies, amorphous | 6·444 | 10·642 | 0·248 | 7·457 | 81·651 |
| —, crystallized | 6·983 | 9·664 | 0·219 | 0·848 | 89·268 |
| Bleistadt | 7·009 | 9·918 | 0·137 | 0·771 | 89·174 |
| England | 10·074 | 0·130 | 0·682 | 89·110 | |
| Poullaouen, crystallized | 7·048 | 10·090 | 89·910 | ||
| —, amorphous | 7·050 | 10·069 | 89·931 |
* Poggendorf's Annalen; xxi. p. 506.
† Berzelius, Årsberättelse, 1832, p. 151.
[page] 485
The last two exhibit the composition of the pure mineral = 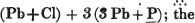 others are represented by
others are represented by
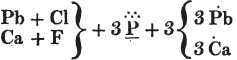
Hedyphan he shows to be the same mineral in which a portion of the phosphoric is replaced by arsenic acid.
An observation of Mr. Harcourt in regard to the phosphates of lead has become highly interesting since the discovery of vanadic acid in minerals having the same form. He found the orange phosphate to contain 1·2 per cent. of chromate of lead. With which of the other electro-negative constituents of these minerals are  isomorphous?
isomorphous?
Octohædral minerals.—Abich, the grandson of the celebrated Klaproth, has investigated the composition of several octohædral minerals, the result of which has been to show that all these minerals have a similar atomic constitution, according perfectly with the views of Mitscherlich in regard to bodies which assume the same crystalline form. Their composition is as follows:
| Locality. | Alumina. | Protoxide of Chromium. | Peroxide of Manganese. | Peroxide of Iron. | Magnesia. | Protoxide of Iron. | Oxide of Zinc. | Silica. |
| Spinell from Åker. | 68·94 | 25·72 | 3·49 | 2·25 | ||||
| — Ceylon | 69·01 | 1·10 | 26·21 | 0·71 | 2·02 | |||
| Pleonast from Ural. | 65·27 | 17·58 | 13·97 | 2·50 | ||||
| — Montzoni | 66·89 | 23·61 | 8·07 | 1·23 | ||||
| — Vesuvius | 67·46 | 25·94 | 5·06 | 2·38 | ||||
| — Iserwises | 59·66 | 0·73 | 9·69 | 18·97 | 1·79 | |||
| Gabnite from Fahlun | 55·14 | 5·25 | 5·85 | 30·02 | S·84 | |||
| — America | 57·09 | 2·22 | 4·55 | 34·80 | 1·22 | |||
| Chromite of Irom, amorphous. | 13·85 | 54·91 | 9·69 | 18·97 | 0·83 | |||
| —, crystallized. | 11·85 | 60·04 | 7·45 | 20·13 | ||||
| Franklinite. | 0·73 | 18·17 | 47·52 | 21·31 | 10·81 | 0·40 |
The spinell from Åker and the pleonast from Iserwiese contained also a trace of protoxide of manganese.
The formulae are as follow:
[page] 486
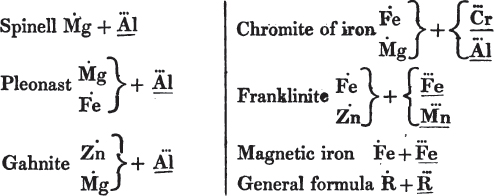
Abich has found that all these compounds can be formed artificially by the moist way*.
Hornblende and augite.—A late Memoir of Gustaf Rose, in which he proves the identity of hornblende and augite, has opened out a new field for crystallographic research, and has confirmed the conclusion to which other researches had led, that crystalline form can no longer be depended on as a characteristic of mineral compounds. To this beautiful Memoir† I can only allude in this place, and recommend it strongly to the attention of the reader.
Artificial mineral compounds.—A similar observation of Mitscherlich, connecting together the slags of the iron smelting furnaces and the pyroxenes (augites), is highly deserving the notice of the mineralogist. These slags are often crystallized, have the form of augite, and are represehted by the same chemical formula. Berthier, to whom mineral chemistry owes so much, has formed silicates of manganese as well as of iron in his furnaces, and obtained them regularly crystallized. Those who have access to his elaborate papers in the Annales de Mines, will recollect many other chemical compounds in atomic proportions, formed by fusing together the several constituents.
Chemical analysis.—A valuable present has lately been made to the analytical chemist by the publication of Prof. H. Rose's work on chemical analysis, a translation of which into English has been executed by Mr. Griffin. Two editions in the original German have already been published, and a third is in progress. These later editions are greatly improved and enlarged.
Systems of Chemistry.—Of the systems of chemistry in progress in foreign countries, the reader is probably already acquainted with the French edition of Berzelius's Lehrbuch, with
* Poggendorf's Annalen, xxiii. p. 305.
† Id. xxii. p. 321.
[page] 487
Dumas's Chimie appliquée aux Arts, and with Leopold Gmelin's elaborate Lehrbuch, the first edition of which has lately been concluded. Besides these, a concise but beautifully clear elementary work, by Mitscherlich, of which two parts have already been published at Berlin, ought to be in the hands of every chemist.
II. Present state of Inorganic Chemistry.
We are at present acquainted with fifty-four elementary substances.
| Elements. | Discovered by | Country. | Year. | Reduced by | Country. | Year. |
| Oxygen. | Dr: Priestley. Scheele. |
England. Sweden. |
1774 |
|||
| Chlorine. | Scheele. | Sweden. | 1774 | |||
| Bromine. | Balard. | France. | 1826 | |||
| Iodine | Courtois. | France. | 1811 | |||
| Fluorine | Scheele. | Sweden. | 1771 | not yet isolated | ||
| Hydrogen | Cavendish. | England. | 1766 | |||
| Nitrogen | Rutherford. | Scotland | 1772 | |||
| Boron | Homberg. | France. | 1702 | Davy. | England. | 1807 |
| Silicon | Acid long known. | Berzelius. | Sweden | 1824 | ||
| Phosphorus. | Brandt. | Hamburg. | 1669 | |||
| Sulphur. | ||||||
| Selenium | Berzelius. | Sweden. | 1818 | |||
| Tellurium | Müller. | Reichenstein. | 1782 | |||
| Arsenic. | Arsenious acid known to Avicenna, 11the century; metal to Para- celsus. | |||||
| Antimony. | Known before the time of Basil Valentine, 15th century. | |||||
| Chromium. | Vauquelin. | France. | 1797 | |||
| Vanadium. | Sefstrom. | Sweden. | 1830 | Berzelius. | ||
| Uranium. | Klaproth. | Berlin. | 1789 | |||
| Molybdenum | Scheele. | Sweden. | 1778 | Hjelm. | Sweden. | 1782 |
| Tungsten. | Scheele. | Sweden. | 1781 | D'Elhuyart. | 1781 | |
| Columbium. | Hatchett. | England. | 1801 | Berzelius. | Sweden. | 1824 |
| Titanium Sodium | Gregor. | 1791 | Vauquelin. | France. | 1796 | |
| Potassium | Davy. | England. | 1807 | |||
| Sodium | ||||||
| Lithium. | Arfvredson. | Sweden. | 1818 | |||
| Barium | Scheele. | 1774 | 1808 | |||
| Strontium | Dr. Hone. Klaproth. |
Scotland. Berlin. |
1792 | |||
| Calcium. | ||||||
| Magnesium | Black. | Scotland. | 1755 | Bussy. | France. | 1819 |
| Aluminum. | Margraff. | Germany. | 1754 | Wöhler. | Germany. | 1828 |
| Glucinum | Vauquelin. | France. | 1797 | |||
| Yttrium | Gadolin. | Sweden. | 1794 | |||
| Cerium | Hisinger& Berzelius. | 1804 | Mosander. | Sweden. | ||
[page] 488
| Zirconium | Klaproth. | Berlin. | 1789 | Berzelius. | 1824 | |
| Thorium | Berzelius. | Stockholm. | 1828 | Berzelius. | ||
| Iron. | ||||||
| Manganese | Scheele. | Sweden. | 1774 | Gahn. | Sweden. | 1774 |
| Nickel | Cronstedt. | 1751 | Bergman. | 1775 | ||
| Cobale | Brandt. | 1733 | ||||
| Zine | Described by | Agricola, 1520. | ||||
| Cadmium | Stromeyer. | Germany. | 1817 | |||
| Lead. | ||||||
| Tin. | ||||||
| Copper | ||||||
| Bismuth | Mentioned by Paracelsus, 16th century. | |||||
| Mercury. | ||||||
| Silver. | ||||||
| Gold. | ||||||
| Platinum. | Known to Wood, assay-master, Jamaica, 1741. | |||||
| Palladium | Wollaston. | England. | 1803 | |||
| Rhodium | Wollaston. | England. | 1804 | |||
| Iridium. | Descotils. Tennant. |
France. England. |
1803 | |||
| Osmium | Tennant. | |||||
In making out this list I have departed from the usual course of attributing the discovery of the metals, &c., to those who reduced them to their simplest state. I have ascribed the discovery to the individual by whom any of the compounds of the body with oxygen or hydrogen were first made known or first shown to differ from every other known body. To reduce an oxide to the metallic state, is much the same in importance as to produce a new compound of one already long known, and cannot be compared to the actual addition of a new element, or of the binary compound of a new element, to our previous knowledge. The only exception to this rule is the splendid discovery of the alkaline metals by Sir Humphry Davy; but this did not derive its main importance from the knowledge it gave us of the metals themselves,—singular and highly valuable as that knowledge has proved,—but from the new law of nature which it revealed, and the new field which it opened to our view, enlarging at once the domains and rectifying the principles of the science.
The following Table presents a synopsis of the actual state of our knowledge in regard to the most important binary compounds of the elementary substances with each other.
[page] 489
From an inspection of this Table a general idea may be formed of the present state of our knowledge in regard to compounds of the first order. Where large gaps occur, the difficulty of the investigation, or the rarity of the substances to be combined, have presented obstacles which it will still take much time and labour to overcome.
The great strength of chemical research has been expended in the formation and analysis of the salts. With a vast number of these compounds we are already acquainted. Two thousand or more have been already either described or indicated; and yet in this more complex branch there are still gaps, wider and more frequent than appear in the list above given. We are in fact only beginning to see how wide the field is which the science of chemistry has yet to explore.
It was my intention to have inserted here a series of general formulae expressive of the composition of the several classes of salts, but the limits assigned to this Report make it necessary to exclude them.
 In the following Table,? denotes that certain phænomena appear to indicate the existence of such a compound, but that nothing certain has yet been made out.
In the following Table,? denotes that certain phænomena appear to indicate the existence of such a compound, but that nothing certain has yet been made out.
"one?" denotes that we are acquainted with one such compound, but that its composition is unknown.
? after a formula, as 2 So+O?, denotes that such a compound has actually been formed or is supposed to exist, but that the composition assigned to it requires confirmation.
[page] 490
[page] 491
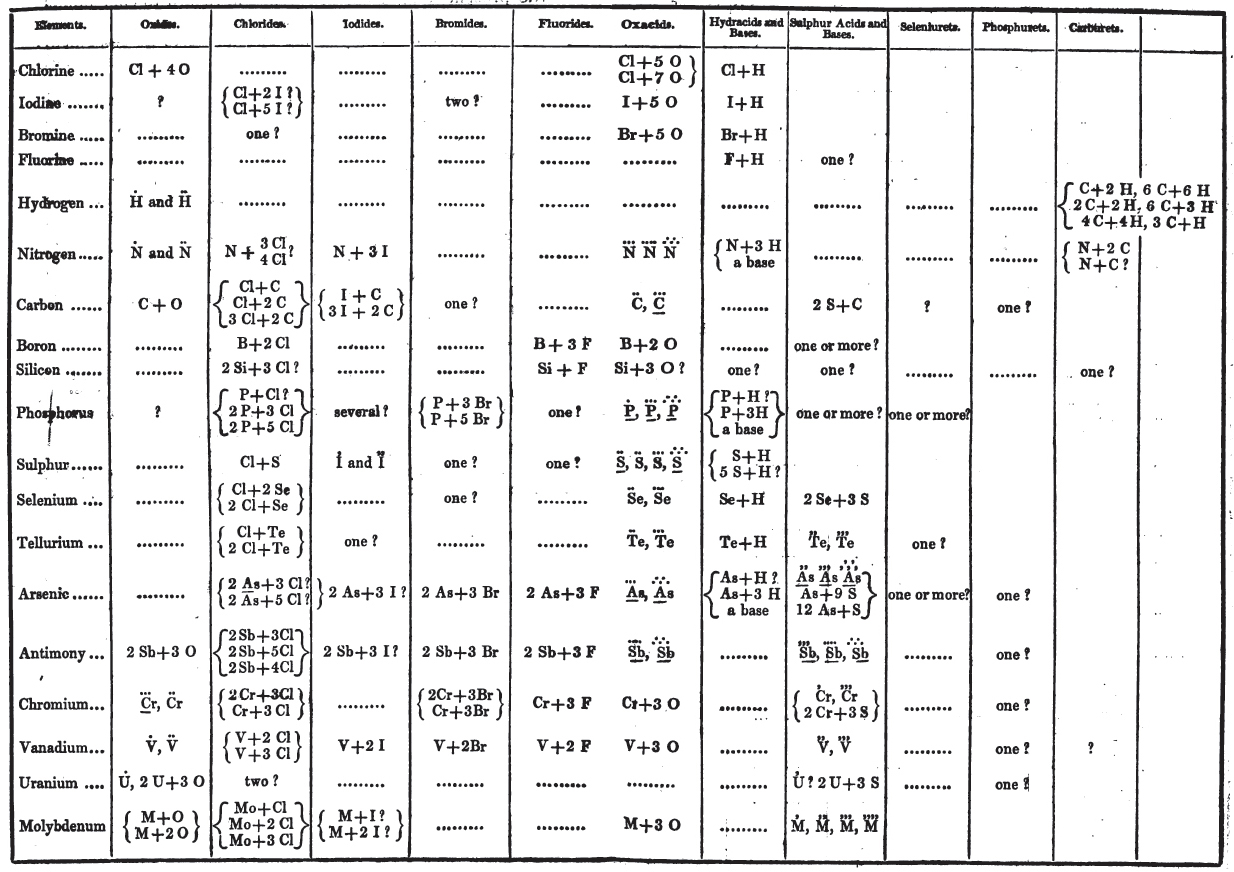
[page] 492
[page] 493

[page] 494
[page] 495
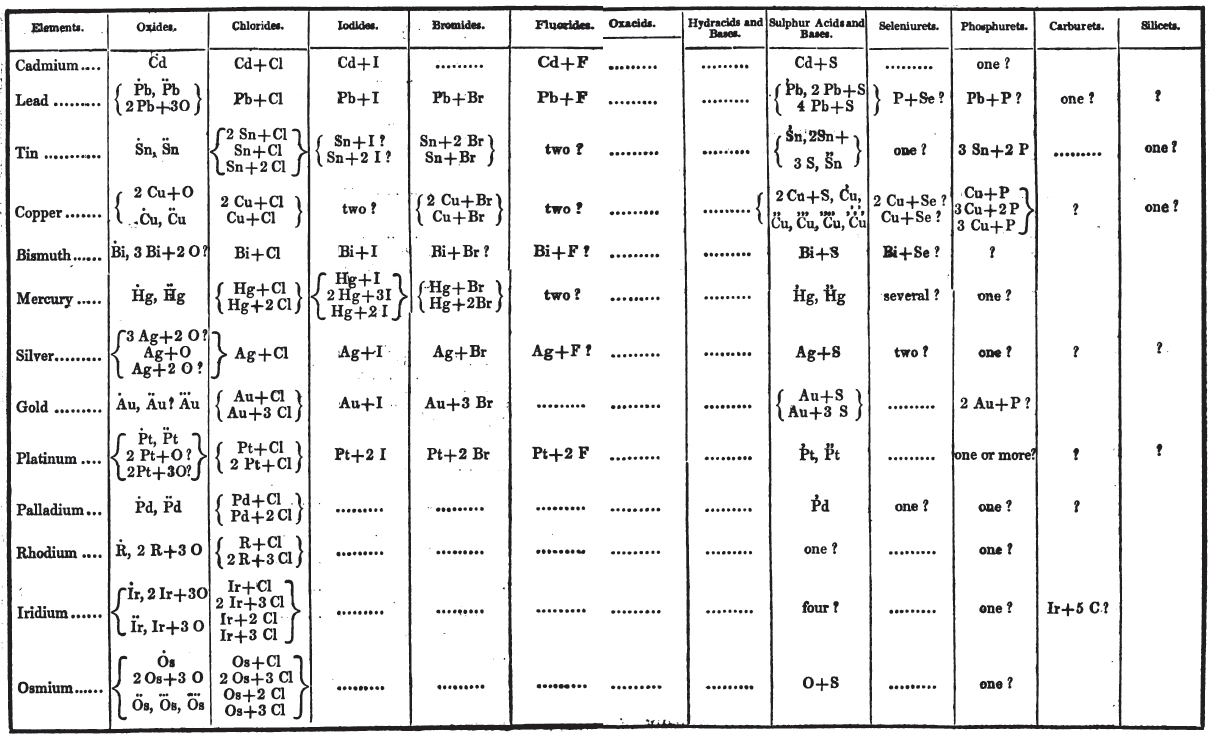
[page] 496
PART III.— Organic Chemistry.
Section I. Vegetable products.—The attention of chemists, long withheld from the department of vegetable chemistry by the obscurity and difficulty of the subject, has of late years been more earnestly directed to this interesting field. The analytical researches of Gay-Lussac and Thenard threw the first distinct light on the nature of vegetable compounds, and gave rise to the first general deductions in regard to their composition. Those of Saussure and of other chemists, whom the apparently simple method of analysis employed by Gay-Lussac induced to undertake similar investigations, speedily added to the number of ultimate analyses. But the dissimilar and often contradictory results obtained by different experimenters in analysing the same substance, showed that few of those yet made known could be regarded as anything more than tolerable approximations. The determinations of Berzelius and Dr. Prout were among the earliest on which confidence could be placed, and have proved almost the only ones which later investigations have not corrected.
In different countries attempts have been made to improve the method of vegetable analysis, so as to secure more constant and more exact results. In England, Dr. Prout's apparatus, though less simple than that employed by others, has in his hands led to results of the greatest precision. In France, Dumas, Pelletier, Henry and Plisson, and others, have paid much attention to this subject. In Germany we owe to Liebig and Wöhler many of the best and most important analyses hitherto published on vegetable chemistry.
Another description of labourers also has done much in this field. The remarkable discovery of Serturner, that the opium of commerce contains a vegetable alkali or salt basis (morphia), to which its soporific virtues are owing, led the way to a train of vegetable research, from which large accessions of knowledge have been obtained. Pelletier and Caventon had the merit of first following up the investigation, and of making us acquainted with several of the most important vegetable principles hitherto discovered. They have been followed by many others, and with such success that during the fifteen years which have elapsed since Serturner's discovery began to attract the attention of chemists, we have been made acquainted more or less fully with upwards of thirty acid and nearly as many- alkaline principles, either existing ready formed in the products of the vegetable kingdom, or produced during the process employed for extracting their active ingredients. Besides these,
[page] 497
upwards of fifty other principles have been described, possessing the properties or virtues of the plants from which they are extracted, but exhibiting neither acid nor alkaline properties.
While our knowledge of ultimate principles and their atomic constitution has thus been extending, several interesting theoretical inquiries have been raised, chiefly in regard to the manner in which the elementary atoms of which they are composed may be supposed to be grouped together. To a few of these I shall here briefly advert, as they will enable the reader better than anything else to form a proper estimate of the true state of our knowledge in this department of the science.
Are vegetable principles binary compounds?—1°. It has long been the opinion of certain chemists, and has lately been ably maintained by Dumas, that vegetable or animal principles containing three or more elements ought, not to be regarded as simple combinations into which the elements enter singly, or as combinations of the first order, but as made up of two binary compounds of the first order, which unite together in virtue of their opposite electrical states, as an acid and an alkali do, Thus sugar may be considered as a compound of carbonic acid and carburetted hydrogen; and the æthers as composed of ætherine which gives the general character to all, and of water or an acid which imparts its peculiar properties to each. This view of organic principles is very simple, and gives a very clear idea of the way in which the elementary atoms are grouped; but while we admit the probability of the hypothesis, so many facts militate against it that it cannot be received as a law. One of the strongest of these is, that we know only of one or two cases in which these compounds can be resolved into their supposed binary elements, and none in which they can be artificially produced by uniting them*. Many of the vegetable acids also appear so obviously to be oxides of a compound radical containing hydrogen and carbon, that we should be neglecting the most striking analogies were we to adopt the opinion of Dumas in regard to them; while on the other hand, the discovery of benzule by Liebig and Wöhler puts beyond question the existence of compound radicals of several elements in which no such binary grouping can be supposed to have place.
Dr. Prout's view.—2°. A view of a different kind has been advanced by Dr. Prout in regard to the arrangement of the
* The interesting observation of Brande, that in the galvanic circuit the vegetable alkalies are not decomposed, appears also to oppose the idea of their being binary compounds.
2 I
[page] 498
elements in a large class of vegetable compounds. In sugar, gum, and starch of different kinds, he has shown that the oxygen and hydrogen exist in the same proportion as in water, and therefore suggests that such substances may in reality be compounds of water and carbon. Such views are valuable as well as interesting when they are drawn as deductions from a large number of analytical results, as those of Dr. Prout are; but they are, on the other hand, productive of much mischief when they are adopted on theoretical grounds, and experiments afterwards instituted to confirm them.
Are the vegetable acids oxides of compound radicals?— 3°. A third theoretical view, not destitute of probability, is that which represents the organic acids as oxides of a compound radical. Thus, if we put 2 C + 0=R, a supposed radical, we have
Ṙ=Mellitic acid.
Ṙ=Carbonic oxide.
 =Oxalic acid.
=Oxalic acid.
 = Carbonic acid.
= Carbonic acid.
But there are difficulties attending this representation of the constitution of these compounds. Carbonic oxide contains more oxygen than mellitic acid, while oxalic the stronger contains less oxygen than carbonic the weaker acid. It is not unlikely that future researches may supply us with a more satisfactory mode of obviating these difficulties than we are yet acquainted with.
Again, if we take C + 1/2H=R, we have the base of the following suite of acids.
Citric acid. = Ṙ
Formic acid = 
Succinic acid = 4R + 3O
Tartaric acid. = 4R + 5O
Pyrogallic acid = 6R + 3O
Tannin =6R + 4O
The crystalline compound discovered by Opperman as the base of artificial camphor =4 C+3 H. If we represent this by R, we have
 |
=Acetic acid. |
| 3R+O | =Camphor. |
| 3R+(Cl+H) | =Artificial camphor. |
 |
=Camphoric acid. |
It is very interesting by means of such formulae to compare the atomic composition of bodies possessing properties so different. That the acetic and camphoric acids are oxides of the
[page] 499
same compound radical, does not appear probable; but the facts to be stated respecting benzule render it highly probable that the three compounds here classed along with it have the same radical as the camphoric acid.
Does the azote in vegetable alkalies constitute ammonia?— 4°. It has been found that the vegetable alkalies, so far as they have been examined with care, contain, as an essential constituent, a considerable quantity of nitrogen; and the question early suggested itself—Does the nitrogen in these salt bases exist in the form of ammonia? If so, it would be easy to understand why they exhibit alkaline properties. The experiments of Pelletier and Dumas appeared to supply a negative answer to the question, as well as the later ones of Liebig, who found that though brucine and strychnine are completely decomposed by nitric acid, yet ammonia is not one of the products. But there are other circumstances which are considerably in favour of the opposite opinion. Thus, in the six vegetable alkalies, morphine, narcotine, strychnine, brucine, quinine, and cinchonine, Liebig found that each atom contained one atom of nitrogen; and that thus the saturating power of each was in proportion to the amount of that element which it contained. The sulphates he found also to contain two atoms of water*, corresponding exactly to the quantity of water found by Mitscherlich to exist in the crystallizable salts of ammonia with the oxacids. These coincidences are extremely curious; and though they prove nothing, yet they show that we are not yet in a state to answer the question, definitively, whether the vegetable alkalies do really contain ammonia or not.
Are vegetable principles educts or products?—5°. I shall only advert to one other point, on which considerable doubt will probably for a long time remain. I have already stated how numerous the proximate principles of the vegetable kingdom—acid, alkaline, and indifferent—with which we are acquainted, have already become, and the rapidity with which their number is increasing. In regard to them, also, an interesting question arises;—Do they all exist ready formed in the vegetables from which they are extracted, or are they not often the product of the lengthened processes by which they are obtained? There is one test by which we can scarcely be deceived in determining this point. When the principle extracted possesses the virtues of the plant from which it is prepared, the probability is very great that it exists ready elaborated in the natural state of the vegetable. When it possesses no such
* The sulphate of strychnine he has since found may be obtained in an anhydrous state.—Pogg. xxi. p. 487.
2 I 2
[page] 500
properties, we can have no evidence, but the simplicity of the process by which it is prepared, that it is not the result of the treatment to which the vegetable has been subjected. And even this is not conclusive evidence; for though the process for extracting oils by distilling the parts of plants with water is very simple, it has been shown that these oils do not always exist ready formed in the substance of the vegetable. Thus, from bitter almonds, alcohol or æther extract no oil, nor is any formed in them by the aid of heat until water is added. The same is the case, also, with the volatile oil of mustard-seed. Now what happens in these cases is likely to take place in many others, so that it will probably be long before we are able to determine in all cases what are and what are not real proximate principles. It will be still longer before we are able to refer each of them to its true place in a purely chemical arrangement, and to make them out to be so many determinate compounds of a series of organic radicals. The first step towards such an arrangement has been made by the discovery of the relation between benzoic acid and oil of bitter almonds; and we may hope that similar discoveries will gradually diminish the confusion and obscurity which at present prevail in animal as well as in vegetable chemistry.
Vegetable acids— Tartaric and Paratartaric acids.—One of the most interesting results lately arrived at regarding the vegetable acids, is that obtained by Berzelius from his analysis of the racemic (paratartaric) and tartaric acids. The former acid had been found by John to be different from the tartaric acid; and Gay-Lussac had determined that their atomic weights were as nearly as possible the same. Berzelius discovered, further, that they have the same elementary composition = 4C+2H+5O; and was led by this discovery into a train of reasoning in regard to isomeric bodies, which has been already adverted to when treating of that interesting subject.
The para- is distinguished from the common tartaric acid, by its inferior solubility in water; by its containing two atoms of water of crystallization, one of which is given off at a moderate heat; by the insolubility of its salt with lime; and by its giving no double salt with soda and potash. This last property may be taken advantage of to separate the two acids. The crude tartar, or other mixture containing them in the state of acid salts, is saturated with soda; the Rochelle salt allowed to crystallize, and separated; the mother liquor precipitated by a lead salt, and the precipitate afterwards decomposed by sulphuric acid.
Modification of tartaric acid.—When tartaric acid is fused
[page] 501
in a retort at a temperature insufficient to decompose it, a yellow transparent gummy-like mass remains, which gives, with lime and alumina, insoluble gummy-like compounds,—with potash and soda, similar but soluble salts. Partially saturated with potash, a precipitate falls, which, saturated with soda, gives Rochelle salt. In this state Braconnot considers the acid to be an isomeric modification of the common tartaric acid. Berzelius* has observed a similar change in the acid by heating, and considers it probable that it may be analogous to the change produced upon phosphoric acid by the same means.
Pyrotartaric acid.—The pyrotartaric acid discovered by Rose, has been lately investigated and analysed by Dr. Grüner†. When tartaric acid is distilled in a retort, there pass over acetic acid, pyrotartaric acid, an empyreumatic oil, and an insoluble acid which crystallizes in the neck of the retort towards the close of the operation. The pyrotartaric acid, freed from these mixtures, is obtained in needles and four-sided prisms, which fuse at 212° F., losing eight per cent. of water, and which volatilize totally in white fumes at 221° F. They are soluble in alcohol, in æther, and in three times their weight of cold water. The acid, as was already known, forms soluble salts with all the alkaline and earthy bases. That of soda crystallizes in cubes. It forms, also, crystallizable double salts with potash and barytes, soda and barytes, and oxide of lead and ammonia. Its composition, according to Grüner, is
By experiment.
| 4 carbon | =24·244 | |
| 3 hydrogen | = 3·030 | 743·8 = atomic weight. |
| 4 oxygen | = 32·326 |
Pyroracemic— Pyroparatartaric acid. — When the paratartaric acid is distilled in a retort at a temperature sufficient to decompose it, there pass over, as in the distillation of the tartaric acid, an empyreumatic oil, acetic acid, an insoluble acid which crystallizes in prisms, and a liquid volatile acid differing in properties from the pyrotartaric acid of Rose. This acid is extremely volatile, distilling over at a temperature much below 212°, and gives crystallizable salts, with potash, soda, barytes, lead, and probably the other bases. Saturated in the cold with any of these bases, it speedily deposits crystals; but if the solutions be concentrated by evaporation, or heated for a short time to the temperature of about 100° F., they refuse to crystallize, and form only, when left to themselves, a
* Årsberättelse, 1832, p. 208.
† Tromsdorf, xxiv. p. 55.
[page] 502
transparent gummy-like mass. This remarkable property is analogous to the change produced by heat on the common tartaric acid; but the effect, in this instance, is produced with extraordinary facility.
This acid was first noticed by Berzelius; and I had the pleasure of determining the above properties in his laboratory during the past summer. To his kindness also I am indebted for a supply of the acid, which I hope will enable me to prosecute the investigation. This promises to be the more interesting, as I have reason to believe that the pyropara- and the pyro-tartaric acids may prove, like the acids from which they are obtained, to be also isomorphous.
Benzoic acid and Benzule.—The most important discovery lately made in vegetable chemistry, is that of Wöhler and Liebig of the radical of the benzoic acid. It is the first example of a radical consisting of three elements, and promises to throw much light on the nature of the vegetable principles in general.
By analysing the benzoate of lead, Berzelius had long ago found the composition of the acid to be = 14C + 6H+ 4O. MM. Wöhler and Liebig analysed the benzoate of silver, and deduced for the composition of the acid 14C + 5H + 3O; being the elements of an atom of water less than that given by Berzelius. The latter chemist, on repeating the analyses of both salts, found that of silver to be anhydrous, while that of lead contains an atom of water. The true composition of benzoic acid therefore is 14 C + 5 H + 3 O.
The close connexion of benzoic acid and oil of bitter almonds, in which crystals of the acid are often deposited, as it was supposed from the oxidizement of the oil, led Wöhler and Liebig to analyse it, when they found it to consist of l4C+6H+2O; that is, it contains one atom of hydrogen more, and one of oxygen less, than benzoic acid. They consider both, therefore, to be compounds of the same radical, composed of 14C + 5H +2O, which they propose to call Benzule, from ύλη, matter or base. If this radical be represented by Bz, then
Ḃz = benzoic acid.
Bz + H = oil of bitter almonds.
If the oil be exposed to a current of chlorine, there are formed two products, Bz + Cl and Cl + H, chloride of benzule and muriatic acid. Treated with water, this chloride gives benzoic and muriatic acids. With hydrated bases, it gives a benzoate of the oxide and a chloride of the metal. Distilled with cyanide of mercury, it gives a cyanide of benzule and a chloride
[page] 503
of mercury. With sulphuret of lead, and iodide and bromide of potassium, it gives, in the same way, a sulphuret and iodide and a bromide of benzule, while the chlorine unites with the metal. The iodide and bromide are crystallizable and volatile, and all three are decomposed by water and bases like the chloride.
The radical itself has not been isolated, but these experiments leave no doubt of its existence. One of the most striking facts in regard to it is the presence of oxygen as a constituent element which remains unaffected in its combinations with chlorine and iodine.
Benzamid.—The chloride of benzule, Bz + Cl, takes up two atoms of ammonia 2 (N + 3H), forming a crystallized volatile substance, which is decomposed by water, giving muriate of ammonia (Cl + H) + (N + 3H) and benzamid = Bz + (N + 2H), the latter of which remains undissolved in the form of a white powder.
This last substance contains the same elements as benzoate of ammonia minus one atom of water, for
Bz + (N+2H) + Ḣ= Ḃz + (N+3H).
Dumas' oxamid consists of 2 C + N + 2 H; or it contains the same compound of azote and hydrogen as the white salt (benzamid) above obtained: and in both cases they are united to a compound radical. We know of similar compounds, not hitherto understood, of potassium and sodium with the same two elements. If, then, we represent N + 2 H by amid, we shall have
2Ċ + (N+2H) = oxamid.
Bz + (N + 2H) = benzamid.
Po + N + 2H = potassamid.
So + N + 2H = sodamid.
Metamorphic oil of bitter almonds.—MM. Wöhler and Liebig have also analysed the camphor which is sometimes deposited in oil of bitter almonds, and have found it to have precisely the same composition—to be, in fact, metamorphic oil of bitter almonds.
Gallic acid.—Dobereiner* obtains pure gallic acid in a few minutes by the following process. A concentrated decoction of gall-nuts, mixed with a little acetic acid to decompose the gallate of lime, is shaken for one minute with a quantity of æther. The gallic acid is taken up by the æther, and by spontaneous evaporation on a watch-glass is obtained in small colourless prisms. If longer digested, the liquid separates into
* Schweigger's N. Jahr. i. p. 380.
[page] 504
three portions. The lightest contains the gallic and acetic acids, if the latter be present in excess; the next, an æthereal solution of tannin; and the heaviest, the water and extractive matter.
Acetic acid.—A most important improvement has recently been introduced into the manufactory of vinegar, which is already extensively practised on the Continent. The introduction of this improvement is chiefly due, I believe, to Mitscherlich. It is founded upon the principle that alcohol, by absorbing oxygen, is changed into acetic acid and water. For,
| Two alcohol + four oxygen | = one acetic acid + three water |
| (6H+4C+2O)+4O | =(3H+4C+3O)+3(H + O). |
This oxidation is promoted by the process of fermentation; and when the fermentation has begun, is much accelerated by the presence of acetic acid. The oxidation is effected entirely at the expense of the oxygen of the air:—to accelerate the process, therefore, by producing as many points of contact as possible between the liquid and the air, the following arrangement is adopted. A large cask is taken, placed upright with a stopcock at the bottom, and a series of holes, half an inch in diameter, bored, one in each stave, a few inches above it. It is then nearly filled with chips or shavings of wood, previously steeped in strong vinegar till they are perfectly saturated. Within the upper end of the cask a shallow cylindrical vessel is placed, nearly in contact with the shavings, the bottom of which is perforated with many small holes, each partially stopped with a slender twig which passes an inch or two beneath the perforated bottom of the cylinder. The alcohol, diluted with eight or nine parts of water, and mixed with the fermenting substance, is now poured into the cylinder, through the bottom of which it trickles, drop by drop, upon the shavings below, becomes oxidized in its passage, and runs out at the stop-cock beneath, already converted almost entirely into vinegar. The air rushes in by the holes beneath, and passes out above by eight glass tubes, cemented for that purpose into the bottom of the cylinder; and so rapidly is it deprived of its oxygen, that when it escapes above, it extinguishes a candle. During the process much heat also is developed; so that from the temperature of 60° (that of the room), the interior of the cask rises as high as 86° F. In the proper regulation of this temperature, much of the difficulty consists.
A second transmission of the acid, thus obtained, through another similar cask, finishes the process. The whole is concluded in a few hours; four-and-twenty is considered amply sufficient to convert a given quantity of alcohol into vinegar.
[page] 505
Chloroxalic acid.—By exposing concentrated acetic acid to the action of chlorine in the rays of the sun, Dumas* has found that broad plates of an acid body are gradually deposited on the sides and bottom of the vessel. By subsequent purification it is obtained in rhombic tables of 100° and 80°, which melt at about 112° F., and boil in vacuo at 390°, volatilizing without decomposition. It is very deliquescent, very soluble in water, and gives very soluble salts. The composition of the crystals is 2C + 2H + 2Cl + 3O; and may be represented by (Cl + H) + (2C + 3O), or an atom of muriatic combined with one of oxalic acid. Dumas therefore calls it chloroxalic acid. It would be interesting to learn the saturating power of the acid, and the characters of its salts.
Oxamid.—We may notice here another singular compound for the discovery of which we are also indebted to Dumas. When oxalate of ammonia is heated in a crucible, at a temperature sufficient to decompose it, among other products is obtained a small quantity of a dirty-white sublimate, insoluble in cold, but dissolving in hot water. This substance is oxamid, and the interest attending it arises from its composition, and from the phænomena it exhibits when acted upon by acids and alkalies. According to Dumas its composition is 2C + N + 2H +2O=2C + (N + 2H), or two carbonic oxide + one amid. It contains, therefore, neither oxalic acid nor ammonia; but when it is heated with a solution of caustic alkali, or with concentrated sulphuric acid, it unites with the elements of water, and forms again equal atoms of oxalic acid and ammonia.
Elardic and Palmic acids.—Fat and drying oils are distinguished by the remarkable property, first noticed by Pontet,— that when mixed with an acid nitrate of mercury, or with hyponitric acid in small quantity, they speedily become solidified. Boudet has lately investigated this subject, and found the solid thus obtained to possess peculiar properties. He has proposed for that obtained from olive oil, oil of almonds, cocoa nuts, &c. the name of Elardine, and for that obtained from palm oil, Palmine. Alkalies change these substances into elardic and palmic acids respectively, and glycerine. Muriatic acid separates the acids from the alkalies in the form of an oil which solidifies on cooling. They possess decided acid properties, and may be distilled without decomposition.
The following List contains the names of nearly all the vegetable acids hitherto described, with the names of their discoverers, and their composition so far as it is known.
* Journ. de Chim. Medicale, vi. p. 659.
[page] 506
| Vegetable Acid. | Discovered by | Composition. | According to | Existing in and obtained from | |||
| Carb. | Oxy. | Hyd. | |||||
| Carbonic | Dr. Black. | C | 2 O | ||||
| Oxalic | Scheele. | 2C | 30 | Many plants. | |||
| Croconic | Gmelin. | 5C | 40 | Gmelin. | |||
| Mellitic | Klaproth. | 4C | 30 | Liebig. | |||
| Chloroxalic. | Dumas. | 2C | 3D | 2 H | 2 Cl | ||
| Formic | Macgraff. | 2C | 30 | H | |||
| Acetic | Long known. | 4C | 30 | 3H | |||
| Tartaric | Scheele? | 4C | 50 | 2H | Prout. | Tartar. | |
| Racemic | John. | Berzelius. | Tartar. | ||||
| Pyrotartaric. | Rose. | 4C | 40 | 3 H | Grüner. | Tartaric acid. | |
| Pyroracemic | Berzelius. | Racemic acid. | |||||
| Citric | Scheele. | 4C | 40 | 2H | Lemons. | ||
| Pyrocitric | Lassaigne. | Citric acid. | |||||
| Malic | Scheele. | 4C | 40 | H | Sorbus aucuparia. | ||
| Pyromalic | Braconnot. | Malic acid. | |||||
| Benzoic | Blaise de Vigenere | 14 C | 30 | 5H | · | Liebig & Wühler. | Gum benzoin. |
| Succinic | Boyle. | 4C | 30 | 2H | Amber. | ||
| Mucic | Scheele. | 6C | 80 | 5H | Gum arabic. | ||
| Pyromucic | Hinton deLabillardiere. | 9C | 60 | 2H | Mucic acid. | ||
| Gallic | Scheele. | Nut-galls. | |||||
| Pyrogallic | Braconnot | 6C | 3O | 3H | Berzelius. | From gallic acid. | |
| Ellagic | Brraconnot. | Nut-galls. | |||||
| Carbuzotic | Liebig. | 10 c | 10 O | 4N | Liebig. | Indigo. | |
| Camphoric | Kosegarten. | 10 C | 50 | 15 H | Liebig. | From camphor. | |
| Rocellic | Huren. | 16 C | 40 | 16H | Rocella tinctoria. | ||
| Pectic | Braconnot. | From pectine. | |||||
| Caffeic | Pfaff. | 29·1 | 6·4 | 6·9 | Pfaff. | In coffee. | |
| Kinic | Vauquelin. | 15 C | 12 0 | 12 H | Liebig. | Cinchona bark. | |
| Pyrokinic | Pelletier and Caventou. | From kinic acid. | |||||
| Meconic | Serturner. | Opium. | |||||
| Lactucie | Pfaff. | Lactuca virosa. | |||||
| Valerianic | Grote. | Valeriana officer nalis. | |||||
| Caincic | François. | 8C | 40 | 7H | Liebig. | Cainca root. | |
| Fungic | Braconnot | Peziza nigra, &c. | |||||
| Boletic | Braconnot | Boletus pseudoigniarius. | |||||
| Igasuric | Pelletier and Caventou. | Strychnos Ignatul &c. | |||||
| Equisetic | Braconnot. | Equisetum fluviatile. | |||||
| Lichenic | Pfaff | Cetraria islandiea. | |||||
| Laccic | John. | In gum lac. | |||||
| Crameric | Peschier. | In Crameria triandria. | |||||
| Aspartic | Henry and Plisson. | 73·56 | 16·58 | 9·86 | H.&P. | From asparagine. | |
| Ulmic | Boullay. | 56·7 | 38·5 | 4·8 | Boullay. | Turf. | |
| Azulmic? | Bonllay. | Prussic acid. | |||||
| Indigotic | Chevreul. | 15 C | 10 O | 2 N | Buff. | Indigo. | |
| Suberic | Brugnatelli. | 37·25 | 46·76 | 15·98 | Biandes. | Cork. | |
[page] 507
| Vegetable Acids. | Discovered by | Composition. | According to | Existing in and obtained] from | |||
| Carb. | Oxy. | Hyd. | |||||
| Fumaric | Winkler. | Fumaria officinalis. | |||||
| Cinic acid | Kahler. | 18? | Semina Cinæ. | ||||
| Alizaric acid | Zenneck. | 20? | 62? | Zenneck | Colouring matter in madder. | ||
| Amylic? | Tunnerman. | Starch. | |||||
| Moroxylic?. | Klaproth. | Gum of the mul berry. | |||||
| Cocco Gnidic | Göbel. | Daphne Gnidium. | |||||
| Aconitic? | Peschier. | Aconitum Napellus | |||||
| Atropic? | Peschier. | Atropa Belladonna | |||||
| Conic? | Peschier. | Conium maculaturn, | |||||
| Daturie? | Peschier. | Datura Stramonium. | |||||
| Ginkoriqne? | Peschier, | Fmitof Gingko- biloba. | |||||
| Polygalic? | Peschier. | Poly gala Senega. | |||||
| Solanic? | Peschier. | Solanum nigrum. | |||||
| Tanacetic? | Peschier. | Tanacetum vulgare | |||||
| Absinthic? | Braconnot. | Absinthium vulgare. | |||||
| Phytolaccic? | Braconnot. | Phytolacca decandra. | |||||
| Kinoric? | Pelletier & Caventou. | China Nova bark. | |||||
| Violic? | Peretti. | Viola odorata. | |||||
| Viridous? | Runge. | In several families of plants. | |||||
| Nitrohemic. | Wöhler. | From indigo. | |||||
| Nitrosacchoric | Braconnot | Sugar of glue. | |||||
| Nitroleucic. | Braconnot. | Leucine. | |||||
| Crotonic | Pelletier and Caventou. | By saponifying croton oil. | |||||
| Cevadic | Pelletier & Caventou. | By saponifying oil of Veratrum Sebadilla. | |||||
| Ceric acid. | Pfaff. | From a soap of wax. | |||||
| Ricinic. | Bussy and Lecanu. | 73·56 | 16·58 | 9·86 | B. & L. | By distilling cas tor oil. | |
| Elaioidic | B. and L. | By ditto. | |||||
| Margaritic | B. and L. | 79·5 | 18·6 | 100 | By saponifying castor oil. | ||
| Elaïdic | Bondet. | From elaidine by muriatic acid. | |||||
| Palmic | Bondet. | From palmine by ditto. | |||||
| Sulphovinic. | Dabit. | (E +  ) + (S+Ḣ) ) + (S+Ḣ) |
Hennell. | ||||
| Sulphonaphthalic | Faraday. | 2 + 20 C+8H + 20 C+8H |
Faraday | Naphthaline. | |||
| Chlorovinous? | Hayes. | Alcohol and chloride of lime. | |||||
| Chlorovinic?. | Hayes. | Ditto ditto. | |||||
| Pinic | Unverdorben | Venice turpentine. | |||||
| Silvic | Unverdorben | Ditto. | |||||
[page] 508
At the head of this list I have placed four acids,—the croconic, mellitic, chloroxalic, and formic,—which are not properly of vegetable origin, but which, from their analogous composition, seem naturally to belong to this class.
The list might have been much swelled out by inserting the numerous resinous substances which Unverdorben has shown to possess electro-negative properties; but it would extend the term 'Acid' too widely to rank all such bodies among the acids properly so called. Other acid substances have also been met with, to which no names have yet been given: among these is one obtained in a crystalline form, by the action of nitric acid on meconine, and which, according to Pelletier, is composed of carbon 49·76, nitrogen 9·50, hydrogen 4·78, oxygen 35·96, or 18C + 10O + 21H + 3N.
Vegetable alkalies.—Duflos has published a series of interesting researches on several of the vegetable alkalies. He has observed that bicarbonate of potash precipitates* narcotin, but not morphine, and upon this property has founded a process for preparing them. He has also increased our knowledge regarding brucine and strychnine, and improved the process for extracting them from the nux vomica. He separates the two alkalies by digestion in absolute alcohol, which takes up most of the brucine, and afterwards boiling in water as long as a brown colour is imparted to the cold solution by a few drops of fuming nitric acid. This test is so sensible, that water is tinged when it contains only 1/10000dth of its weight of brucine†. Quinine he finds to fuse at 248° F., losing 41/2 per cent. of water, while cinchonine requires a temperature of 329° F., loses nothing, and is partly sublimed into crystals resembling benzoic acid.
Mode of testing Peruvian bark.—The following method for testing Peruvian bark, given by Duflos, is highly deserving of attention. A dram of the bark finely powdered is boiled for a few minutes with an ounce of water and half a dram of concentrated acetic acid, the whole thrown on a filter, the residue washed with water, and the whole evaporated to dryness on a water-bath. If the mass be still acid, it is dissolved again and evaporated to drive off all the acetic acid, the dry mass is digested in absolute alcohol, the solution freed from colouring matter by animal charcoal, and precipitated by bichloride of platinum added drop by drop as long as any deposit takes place. The precipitate washed, dried in the air, and weighed, corresponds to half its weight of the vegetable alkali. The
* Schweigger, N. Jahrbuch,i. 105—217.
† Ibid. ii. p. 304.
[page] 509
precipitate is soluble in water, and is a double salt, consisting of one atom of bichloride of platinum with one atom of a muriate of the vegetable alkali.
Test for cinchonin in sulphate of quinin.—Kindt* gives the following method for detecting the presence of cinchonin in sulphate of quinin. A grain of the salt in fine powder is shaken with one dram of æther, and a dram of ammonia is added, and the whole well shaken. If no cinchonin be present, the line of separation of the two fluids is clean; if the smallest quantity be present, it is deposited at this line.
Atropin— Hyoscyamin.—Brandes states, that if leaves of belladonna be distilled with water and caustic lime, a liquid passes over, which besides the smell of ammonia has that also of the fresh plant. By saturation with muriatic acid, evaporation to dryness, treating with alcohol, evaporating this solution, and distilling the dry mass with a little water and caustic lime, an alkaline poisonous liquid is obtained, which in the open air speedily decomposes.
Coniin.—By a similar treatment of the seed, flowers, or fresh stems of hemlock, Geiger has obtained a volatile alkali analogous to that contained in tobacco. The dry plant is almost destitute of it. Coniin is a colourless oily liquid, which gives a temporary oily stain to paper, has a peculiar penetrating smell, brings tears to the eyes, causes giddiness in the head, and has a sharp tobacco-like taste. It is poisonous, and more so than its salts, has a specific gravity = 0·89, boils at 308° F., and distills with only slight decomposition. It burns like a volatile oil, and reddens moist litmus paper. In the air it is decomposed, giving off ammonia and leaving a brown resin-like mass. At 59° F. it takes up 1/4 of its weight of water without losing its oily appearance, and still more as the temperature decreases; so that at 23° F. it takes up its own weight. If the temperature be increased, the water is separated, so that coniin containing water always becomes opaque when heated, and clear when cooled again. For perfect solution one part requires 100 of water; with absolute alcohol, it mixes in all proportions. It has been analysed by Liebig, and found to consist of carbon 66·913, hydrogen 12·, nitrogen 12·805, oxygen 8·282. The atomic weight by calculation = 1369·986; Geiger by experiment found 14·06†.
The following List contains all the vegetable salt bases hitherto described, and their composition as far as is known.
* Brandes' Archiv. xxxvii. p. 254.
† Geiger's Magazin, xxxvi. p. 161.
[page] 510
List of Vegetable Alkalies.

[page] 511
Of those which have been analysed, meconine and picrotoxin contain no azote, and therefore will most likely be found, on a more careful examination, to be destitute of basic properties. Buchner has also found a base in columbo root, Quassia Simaruba, &c., to which he has given no name, and Pelletier has described two alkalies differing from cinchonin and quinin, found in the Carthagena and Cusco varieties of bark, and Miell one in white bark (China ovifolia), which he has called blanquinine: over these, however, considerable obscurity still rests.
Indifferent vegetable substances.—Becquerel* has pointed out some interesting distinctions between cane sugar, sugar of milk, and gum, which may probably be employed with advantage in distinguishing between them, or in detecting their presence in solution. Caustic potash, soda, or lime, is added to the solution, which is then digested on fresh precipitated hydrated oxide of copper in the cold. If gum be present, it is wholly precipitated; if saccharine matter, the copper is dissolved in whole or in part, and the solution is blue, yellow, or red, according to the quantity of sugar present. If the coloured solution be boiled, the copper remains in the state of protoxide; if cane sugar be present—if it be sugar of milk—boiling reduces the copper to the metallic state.
Manna sugar (Mannite).—Manna sugar has been analysed by Henry and Phsson and by Opperman, with the following results:
Henry and Plisson = C = 38·77 H = 8·487,O = 52·743
Opperman. = C = 40·32, H = 7·728,O = 51·843
Opperman's result gives 4C + 9H + 4O.
Guerin has published an elaborate examination of the gums. He divides them into three classes: 1°. Arabine, of which gum arabic is the type, soluble in cold water; 2°. Bassorine, which swells into a jelly, but does not dissolve in water: Bassora gum is the type of this class; 3°. Cerasine, from the gum of the cherry-tree (Cerasus), is also insoluble in cold, but soluble in boiling water, and when treated with nitric acid gives about one fourth less mucic acid than bassorine.
Arabine, by his analysis, consists of carbon = 48·81, hydrogen 6·2, oxygen 49·85, nitrogen 0·14 = 6C + 5H + 5O. Gum Senegal and the soluble parts of gum tragacanth and Bassora gum consist of arabine.
Bassorine = carbon 37·28, hydrogen = 6·85, oxygen = 55·87 = 10C + 11H+ 11O.
Cerasine appears to be metamorphic arabine, for it has pre-
* Ann. de Chimie, lxvii. p. 15.
[page] 512
cisely the same composition, and is changed into it by solution in boiling water. The gums of the cherry-, apricot-, prune-; peach-, and almond-tree are of this kind.
Vegetable and bees-wax.—Opperman has analysed two species of vegetable wax and one of bees-wax, with the following results:
| Car. | Hyd. | Oxy. | |
| Vegetable wax from Japan. | 70·96. | 12·07. | 16·95 |
| — Brazil | 72·25. | 12·70. | 14·07* |
| Bees-wax | 81·29. | 14·07. | 4·63 |
All that we can gather from these analyses is, that the several kinds of solid fat of which each of the above is a mixture, are probably of the same nature in the two kinds of vegetable wax, but different from those contained in bees-wax.
Oil of turpentine— Artificial camphor.—He has also analysed oil of turpentine and artificial camphor, and obtained from them
| Car. | Hyd. | ||
| Oil of turpentine,. | 84·59, | 11·73, | Oxy. 3·67 =60C +51 H+O |
| Artificial camphor,. | 72·80, | 9·47, | Chl. 17·71 =12C+9H+(Cl+H) |
These analyses establish two interesting points,—that oil of turpentine contains oxygen, and that in artificial the carbon and hydrogen are in the same proportion as in natural camphor (4:3), according to the results of Liebig.
Resins and Gum resins— Acid bodies.—The resins and gum resins have been much investigated, and with great success, by Unverdorben. In a series of elaborate memoirs he has shown that many of the known resins are mixtures of several substances of the same class, which may be separated from each other generally by the action of alcohol and æther. Thus Sandarak is a mixture of three gums, Copal of five, Benzoin of three, Guiac of two, and Gum lac and Colophony also of several. He has shown likewise, that all these resinous bodies possess electro-negative properties, forming salts with oxides in definite proportions. These compounds are decomposed by the electric current, the resins being attracted to the positive, and the oxides to the negative wire. The alcoholic solutions of these bodies also redden litmus, so that there can be no doubt of their electro-negative character, though it would not only unnecessarily extend the meaning of the term acid, but also unnecessarily overburden the nomenclature, to apply the term to all these faintly negative substances, and to form classes of salts of their many obscure compounds. The labours of Un-
* Henry has also analysed a vegetable wax obtained by Nicollet from juniper berries, in which he found carbon 65·4, hydrogen 7·3, and oxygen 27·3.
[page] 513
verdorben alone have already brought together such a mass of observations on this subject, that, as has been justly observed by Berzelius, were each of the resins to be examined with equal care, the detail would speedily occupy as much space as the whole of our present systems of chemistry.
Basic resins.—Buchner and Herberger have advanced one step further in the investigation of resinous bodies. Unverdorben had found the fifth resin of copal to be indifferent; these chemists describe some to be possessed of weak basic properties. Thus the resin extracted from jalap and from euphorbium they find to be each a compound of two; one of which is a weak acid, and the other a weak base; and they consider all the drastic gum resins to be similar compounds*. The subject, however, requires further investigation.
Subresins.—The following substances belonging to Bonastre's class of subresins† have been analysed by Henry and Plisson‡.
| Car. | Hyd. | Oxy. | |
| Amyrine | = 81·040 | 10·474 | 8·486 |
| Subresin from arbre à brais | = 79·728 | 10·651 | 9·621 |
| Alonchi | = 82·640 | 11·006 | 6·354 |
| Ceroxylin | = 83·2 | 11·05 | 5·75 |
Opium.—Few substances have undergone more repeated investigations than opium, or been subjected to more varied chemical torture. Of this some idea may be formed from the following list of immediate principles obtained from it, as given by Pelletier:
Morphine, a base discovered by Serturner.
Meconic acid, discovered by Serturner.
Narcotine, a base discovered by Derosne.
Meconine, an indifferent substance? Dublanc and Couerbe.
Narceine, an indifferent substance? Pelletier.
Brown acid and extractive matter; a peculiar resin strongly electro-negative; a fatty oil; caoutchouc; gum; bassorine; lignine, and a volatile principle.
Many new immediate principles, possessing neither alkaline nor acid properties, have recently been extracted from vegetable substances: the greater number of these are noticed in the following list, containing nearly all the indifferent vegetable principles with which we are yet acquainted.
* Buchner's Repertorium, xxxvii. p. 17.
† Bonastre's subresins are insoluble in cold, but soluble in hot alcohol, from which they precipitate in a crystalline form on cooling.
‡ Journ. de Pharm. xvii. p. 450.
2 K
[page] 514
List of Indifferent Vegetable Principles
| Names. | Discovered by | Remarks. |
| Pelletier. | Boullay. | In the Menispermum Cocculus. |
| Brandes. | Brandes. | In the Bryonia alba. |
| Piperin. | Pelletier. | Car. H O 76·10, 10·27, 13·63. Henry and Plisson. In pepper. |
| Imperatorin | Osann. | PA crystalline principle, resembling piperin, in the root of Imptratarim Ostruthium. |
| Cathartin | Lassaigne and Feneuille. | A crystalline principle resembling J senna leaves. |
| Cytisin | Lassaigne & Chevalier. | A crystalline principle resembling J senna leaves. |
| Zanthopicritin | Chevalier and Pelletier. | A crystalline principle in Zantho xylum carribæum. |
| Scillitin | Vogel. | A crystalline principle in Scilla maritima. |
| Senegin | Gehlen. | Very like Scillitin. |
| Caffein | Runge and Pelletier. | C H N O39·8+6·6+20-8+32·8. Pfaff. In L coffee. |
| Rheine | Vaudin. | From the root of rhubarb, by ether. |
| Rhæponticine | Heememan. | ––––, by water. |
| Salicin | Leroux. | 4C +5H + 20 55·49+6-38+38-13, Jules GayLussac. The willow bark. |
| Meconine | Dublanc and Couerbe. | 9C +9H + 40 60-247+4-756+34-997. Couerbe. In opium. |
| Narceine | Pelletier. | 6C+24H+ 80 + N 54·73+6·52 +34·42+4·33. Peltier. In opium. |
| Plombagin | Dulong.d' Astrofort. | Root of the Plumbago europæa. |
| Hesperidin | Lebreton. | In pomegranates. |
| Populin | Braconnot. | In the bark of the poplar-tree. |
| Corticin | Braconnot. | The colouring matter of the same bark; probably only a compound of tannin and extractive matter. |
| Columbin | Wittstock | 7C+7H+ O 66·36+6·17+27·47. Liebig. By alcohol from colwnbo-root;—cty-j stallizes in four-sided prisms with rhombic bases. |
| Cinin | Kahler and Alms. | Crystalline yellow needles, by alcohol, from semina cinæ. |
| Santonin | Alms. | C=51·34+H = 9·76 + 0 = 38·9. Alms. From cinin by nit or mur. acids. |
| Arthanition | Saladin. | In the roots of the Cyclamen europæum and Primula Veris. |
| Asparagine | Plisson. | 6C +12H+3N + 40 I 38·38+6·24+22·46+34·41. Henry and Plisson. This substance was 1 first found by Bacon in mallows, and called Altheine. |
[page] 515
| Names. | Discovered by | Remarks. |
| Amanetin | Le Tellier. | The poisonous principle in Agaricus bulbosus and vernus. |
| Betulin | ? | Obtained from birch-bark, and lately redescri bed as new by Good, in SilliL man's Journal. |
| Sinapisin | Henry and Garot. | C=57·92·fH=7·795+ 0=19·6881 +N=4·94+S=9·657. In mustard seed. |
| Mudarin | Dr. Duncan. | An extractive matter from Caloptrist Madarit. |
| Elatine | Hennell. | A crystalline substance in the juice of the wild cucumber (Elaterium). |
| Liriodendrin | Emmett. | A crystalline substance from the bark of the root of Liriodendron tulipifera |
| Fagin | Herberager. | A volatile narcotic bitter substance X by distilling beech-nuts with water. |
| Esculin | Minor. | Called by him Schillerstoff;—found X in the horse-chestnut. |
| Berberin | Buchner. | C H O N 60·3+4·4+22·l+13·2. Anextractive matter from the root of the barberry. |
| Ceratrin | Herberger | so calls the bitter principle of Ice-land moss. |
| Gentianine | Pollctier and Caventou. | The active crystalline principle of gentian. |
| Granadin | Latour de Trie | So names the crystalline substance J found by Mitowart in the root of pomegranate, and which appears to be only manna sugar. |
| Saponine | Osborne and Bucholz. | A peculiar matter, which froths in water like soap, in the roots of the Saponaria officinalis. |
Colouring Matters.
| Name | Ddiscovered by | Remarks |
| Xanthin | Kuhlam. | The yellow colouring-matter of madder. |
| Polychroite | Bouillon Lagrage and Vogel. | The yellow colouring-matter in saffrom. |
| Orcin | Robiquet. | Red colouring-matter of Variolaria dealbata. |
| Erythrin | Heeren. | Red colouring-matter of Lichen roccella. |
| Pseudo Erythrin. | Heeren. | By boiling erythrin in alcohol;- alkalies change it into erythrin. |
| Morin | Chevereul. | Ayellow crystalline matter in Morus tinctioria. |
2 k 2
[page] 516
| Names. | Discovered by | Remarks. |
| Luteolin | Chevreul. | A yellow crystalline matter in Reseda luteola. |
| Vulpulin | Bebert. | A yellow crystalline matter in -Ever-nia vulpina. |
| Quercitrin | Chevreul. | A yellow crystalline matter in quercitron bark. |
| Santaline | Red colouring-matter in sandalwood. | |
| Hematine | Chevreul. | Red colouring-matter in Campeachy (log) wood. |
| Carotin | Wackenroder. | A ruby-red colouring-matter in carrots. |
Gums, Resinous, Substances, Stearoptes, &c.
| Names. | Discovered by | Remarks. |
| Arabine | Chevreul. | 6 C+5 H+5 O. Guerin. Gum arabic. |
| Bassorine | Guerin. | 10C+11H+11O. Guerin. Gum bassora. |
| Cerasine | Guerin | 6C+5H+50. Guerin. Cherry-tree gum. |
| Amyrine | Bonastre. | C=81·04+H=10·474+O:=8·486. Henry and Plisson. |
| Jalappine | Buchner. | One of the two resins in jalap. |
| Abretine | Caillot. | An indifferent resin in four-sided prisms, in the turpentine of the genus Abies. |
| Camphor | 12C+9H+0. Liebig. | |
| Cerin Myricin | John. | In wax. |
| Cerain | Boudet and Boissenot. | By saponifying cerin. |
| Amygdalin | Robiquet. | A stearopte deposited in oil of bitter almonds. |
| Caryophillin | Bonastre. | C=81·92+H =12·25+0 = 5·73. H. and P. Clove stearopte. |
| Laurine | Bonastre. | Probably also a stearopte. Zawttt nobilis. |
| Aurade | Henry and Plisson. | C =83·76+H=: 15·09+O = 1·15. X H. and P. Oil of orange flowers. |
| Styracin | Bonastre. | C=76-273+H=5-563+0=18'22. Henry. Styrax liquida. |
| Variolarin | Kuhlman. | Kind of fatty matter in Variolaria X dealbata. |
| Elaidine | Boudet. | By the action of hyponitric acid X olive oil. |
| Palmine | Boudet. | By the action of hyponitric acid on X palm oil. |
[page] 517
The following substances may also be classed together.
| Names. | Discovered by | Remarks. | ||
| Lignin. | ||||
| Fungin | Proust. | Probably a mixture of the powder of the pericarp with the insoluble integuments of the flower. | ||
| Fungin | Braconnot. | The fleshy part of the Mushroom tribe. | ||
| Tremellin | Brandes. | Has the same relation to Tremella mesenteria. | ||
| Pectin | Braconnot. | The gelatinizing principle in fruits. | ||
| Pollonin | Braconnot and John. | The vegetable matter of the pollen of the cedar and lycopodium gave to Macaire Prinsep | Ced. | Lycop. |
| C. 40·0 | 52·21 | |||
| 0·48·3 | 39·2 | |||
| H.11·7 | 8-6 | |||
Products of destructive distillation,§c.
| Names. | Discovered by | Remarks. |
| Naphthaline | Dr. Kidd. | 5C+ 4 H 3C +2H 93·75+6-25, Faraday: 94·68+5*31, Opperman. |
| Petroline | Christison. | From petroleum, probably a variety of paraffin. |
| Paraffin | Reichenbach. | C = 85·22+H = 14·98 = C+ H, Jules Gay-Lussac. |
| Eupion | Reichenbach. | This and Paraffin are both products of the destructive distillation of vegetable and animal substances. |
Besides these, there is the vast variety of vegetable oils; those of sugar, starch, tannin, and bitter principle, &c., many of which are characterized by properties so little distinct, and their true differences in composition are so little understood, that it is unnecessary here to set them down as so many recognised vegetable principles.
The stearoptes, distinguished by particular names and placed in the above list, are probably only metamorphic states of the oils in which they are deposited, as is the case with that deposited in the oil of bitter almonds (Amygdalin), as shown by the analyses of Wöhler and Liebig.
Etherine.—Berzelius proposes for the radical or base of the æthers, the term Etherine = E, and represents them as follows:
[page] 518
| Etherine | =4C+4H*=E=oleum vini dulce. |
| Ether | = E+Ḣ |
| Alcohol | = E + 2Ḣ |
| Muriatic ether | = E + (Cl+H) |
| Nitric ether | = E+ 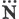 +Ḣ +Ḣ |
| Acetic ether | = E+ 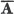 +Ḣ +Ḣ |
| Pyro-acetic spirit | =2E+ 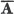 +Ḣ +Ḣ |
| Benzoic ether | = E+Bz+Ḣ |
| Etherine oxide | = E+O=Ė. A hypothetical compound. |
| Döbereiner's Oxygen ether acetal | =2Ė+Ḣ |
| Pyroligneous spirit | = Ė+Ḣ |
| Sulphovinic acid | 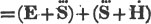 |
| Oil of wine,—the sulphate; of ether of Serullas | 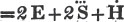 |
| Platinum ether | = E+2Pt This composition is hypoibetical, as the compounds have not been rigorously analysed. |
| Platinum oxide ether. | = E+2Pt |
| Chloride of platinum ether | = E+2(Pt+Cl) |
Chloral.— When chlorine gas is passed over alcohol till it ceases to act upon it,—the liquid being occasionally heated to 120° or 130° F to drive off the muriatic acid and muriatic æther which are formed,—a liquid of a sirupy consistence is obtained, which after some time crystallizes into a solid white mass. From this mass concentrated sulphuric acid takes water, and liberates a colourless oily liquid which boils at 202° F., distilling over unchanged, and has a specific gravity of 1·502. This substance Liebig, to whom we owe the discovery, has named chloral. It is composed of 9 carbon =18·09,6 chlorine = 70·24, and 4 oxygen =11·659, by experiment. The crystals contain two atoms of water =9C+6Cl+4O+2Ḣ.
New chloride of carbon.—When chloral is treated with caustic potash it is decomposed, and an oily body separated, which is a new chloride of carbon, consisting, according to Liebig,
* According to Macaire Prinsep, this is the composition of the variety of mountain tallow found at St. Gall, and called by Stromeyer Sehererite.
[page] 519
of 4C + 5Cl. It may also be obtained from chloric æther, or by distilling very dilute alcohol with chloride of lime.
Bichlorine æther.—Liebig has also analysed the chlorine æther obtained from a mixture of chlorine and olefiant gas, and has found it composed of 8C1+ 16C + 15H, the other atom of hydrogen forming muriatic acid with a portion of the chlorine. He considers it probable that it may consist of three atoms of muriatic æther, and one of the new chloride of carbon above described = (4C + 5Cl) + 3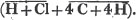 It is usually considered as E + 2Cl.
It is usually considered as E + 2Cl.
Perchlorine (æther.—Soubeiran* has found that if 1 part of alcohol be distilled with 30 of a concentrated solution of chloride of lime, an æthereal liquid passes over which, by his analysis, consists of carbon 14·39, hydrogen 2·35, chloride 83·26, being one atom of each constituent. We have therefore
| Chlorine æther. | =E + C1 | Formed by the action of chlorine on alcohol. |
| Bicholrine æther | =E + 2C1? | The oil of the Dutch chemists. |
| Perchlorine æther | = E + 4Cl. | The æther of Serullas. |
Chlorovinic and Chlorovinous acids.—If the liquid which remains after precipitating the first of these æthers with water from the alcohol in which it is formed be saturated with lime,— or if alcohol be mixed with an equal weight of chloride of lime, set aside for 30 hours, and then treated with water,—a solution is obtained containing, according to Mr. Hayes†, salts of lime with two new acids, which he calls the Chlorovinic and the Chlorovinous. The chlorovinate forms pale yellow rectangular crystals, which at 300° or 400° fuse and give off fumes of nearly pure chlorovinous acid, which condense in water. The residuum is a mixture of charcoal and chloride of lime. It gives no precipitate with nitrate of silver, and with protonitrate of mercury a sparingly soluble salt. The chlorovinite is more soluble in water, and forms six-sided transparent colourless prisms, truncated or terminated by pyramids. It contains water, and effloresces in dry air. The solution gives a curdy white precipitate with nitrate of silver. Oxalic acid precipitates the lime from both salts, and gives free acid.—This interesting subject requires further investigation.
Naphthaline.—Brocke‡ has shown that naphthaline may be prepared in large quantity by distilling coal tar with water, chloride of lime, and sulphuric acid.
Paraffin.—Dr. Reichenbach§, a chemical manufacturer in
* Ann. de Chim, xlviii. p. 137.
‡ Buchner's Repert. xxxviii. p. 268
† Silliman's Journal, xxii. p. 141.
§ Jahr. der Ch. 1830, ii. p. 436.
[page] 520
the Austrian states, has discovered two very interesting substances among the products of the destructive distillation of vegetable and animal substances. The first of these—Paraffin, (parum— affinis) from the little affinity it has to most bodies—he obtained from the heavy tarry matter which is found at the bottom of the receiver in the process for preparing pyroligneous acid from beech-wood. This heavy tar is distilled. The first portion which comes over is rejected, and a stronger heat applied. An oily mass is obtained, filled with shining scales. Mixed gradually with six or eight times its weight of alcohol the paraffin is precipitated, is afterwards washed with and dissolved in alcohol, from which it crystallizes in minute scales and needles. It is white, shining, soft to the touch and flexible, without taste or smell, has a sp. grav. =0·87, melts at 78° F., and at a higher temperature may be distilled without change. It kindles with difficulty, but burns clearly. It is not acted upon by potassium, chlorine, the caustic alkalies, or by acids. Æther dissolves 1·4 time its weight, alcohol only 3·45 per cent. of paraffin. It is soluble in oils, and may be melted with sulphur, phosphorus, fat, wax, resins, &c. It may be obtained from all kinds of wood, and in large quantity. According to the analysis of Jules Gay-Lussac, it consists of carbon 85·22, hydrogen 14·98; or it is C + H.
Eupion.—The other product he calls Eupion (ευ good, πιων fat). It exists in all kinds of tar obtained from the distillation of animal or vegetable substances. The tar is mixed with an equal weight of sulphuric acid, and heated gently, by which the greater part of the empyreumatic oil is destroyed, and the paraffin and eupion rise to the top. They are decanted, again treated with sulphuric acid, nitre added, and three fourths of the oil distilled. The eupion passes over, and a mixture of eupion and paraffin remains in the retort. The oil in the receiver is treated with sulphuric acid and caustic potash as long as they are coloured; after which it is distilled with water and rendered hydrous by exposure to sulphuric acid in vacuo. When pure, potassium has no action upon it; it is colourless, transparent, without taste or smell, remarkably fluid, has a sp. gr. of 0·74 at 71°·5F., remains liquid at —4° F., boils at 336° F., and distils without change. It is a non-conductor of electricity, and so remarkably expansible, that from 66° F. to its boiling point it expands one fifth of its volume. It kindles with difficulty alone, but with a wick it gives a clear flame without smoke or soot. It dissolves without combination—sulphur, phosphorus, selenium, chlorine, bromine, and iodine. It is insoluble in water; alcohol dissolves it in large quantity, and æther five times
[page] 521
its weight. Concentrated acids and alkalies, even at a boiling temperature, do not decompose it.
This remarkable substance is recommended, by its great expansibility and high boiling point, as a fluid likely to be useful in the construction of thermometers. Its power of resisting the action of all chemical reagents may render it, as is remarked by Berzelius, applicable in many cases as a solvent. He draws attention also to the liquid found by Sir David Brewster in the cavities of the topaz, to the highly expansive power of which this new fluid presents an approximation*.
Organic matter in mineral waters.—The slime deposited in the bath of Tatenhausen has lately been examined by Brandes, and found to contain an organic substance in combination with the peroxide of iron. The slime is treated with caustic potash or ammonia, the solution decanted, saturated with acetic acid, and evaporated to dryness. Alcohol separates the acetate and leaves the organic matter. It constitutes a dark brown gummy-like mass, which is tasteless, soluble in water, and contains azote. It exists in the water when it issues from the spring, and is precipitated with the iron on the latter attracting oxygen from the air and becoming peroxidized.
Berzelius has recently commenced an examination of a mineral water found at Porla in Sweden, in which an ochreous precipitate is formed, and has found in it a similar organic principle, which may be separated by the process above described. He has found it to possess decidedly acid properties; and the probability is that it exists in the water in the state of an acid holding iron in solution, with the protoxide of which it forms a soluble, and with the peroxide an insoluble compound. This is supported by the fact that if the insoluble sediment be mixed with water, and subjected to a current of sulphuretted hydrogen, the iron does not fall in the state of sulphuret, but is retained in solution by the acid. To the publication of Berzelius's results we may look for much information on this curious subject.
Organic matters of this kind have been met with in several instances in springs which, like those above mentioned, issue from primitive rocks. What can be the origin of the organic matter which such geological formations afford?
SECTION II.—Animal Principles and Products.
The number of proximate animal principles yet known is very small compared with those discovered in the vegetable kingdom. Animal chemistry has chiefly been studied in connexion with
· Årsberättelse, 1832, p. 320.
[page] 522
physiology; most of the investigations by which our knowledge in this department has been extended, having had reference to the functions, either healthy or diseased, of the human æconomy. In so narrow a field it was not to be expected that results should be obtained equally numerous and varied as those daily elicited in the boundless field of vegetable life.
It would be out of place to introduce any physiological discussions into the present Report; I shall therefore merely notice a few of the more interesting purely chemical observations made upon animal substances during the last two or three years.
Blood.—A very valuable analysis of healthy blood has been published by Lecanu*. He found in it a solid and liquid fat, which appear to resemble the similar substances found in the brain. This observation has been confirmed by Dr.B. Babington and by Denis†. He observed also a difference between the relative proportions of the ingredients of the blood in men and women. Denis made a more extended examination of this point, with similar results. The following Table contains those obtained by Lecanu from the blood of ten men and ten women, compared with those of Denis, who made eighty-three analyses of human blood.
Men's Blood.
| Mean. | |||||
| Max. | Min. | Diff. | Denis. | Lecanu. | |
| Water | 805 | 732 | 73 | 767 | 789·32 |
| Albumen | 63 | 48†5 | 14·5 | 57 | 67·50 |
| Colouring-matter. | 186 | 110·5 | 75·5 | 149 | 132·49 |
| Fibrin | 4 | 2 | 2 | 2·75 | |
| Saline and extractive matter | 10·69 | ||||
Women's Blood.
| Water | 848 | 750 | 98 | 787 | 804·37 |
| Albumen | 68·4 | 50 | 18·4 | 59 | 69·72 |
| Colouring-matter. | 167·1 | 71·4 | 95·7 | 127·7 | 115·96 |
| Fibrin | 3·1 | 2 | 1·1 | 2·6 | |
| Saline and extractive mnatter. | 9·95 | ||||
Globuline—Hematosine.—The colouring-matter of the blood Lecanu proposes to call globuline, considering what is generally known by the name of colouring-matter to be a compound of globuline with albumen. To this compound he gives the name of hematosine. The two substances being precipitated from their solution in water by muriatic acid, and the precipitate boiled in alcohol, the muriate of globuline is dissolved, while the compound of muriatic acid and albumen remains insoluble.
* Ann. de Chim. xlviii. p. 308.
† Journ. de Pharm. xvii. p. 522.
[page] 523
There does not, however, appear any reason to suppose that the colouring-matter and the albumen are chemically combined. The term globuline, therefore, has not come into use, and the colouring-matter freed from albumen is generally distinguished by the term hematosine.
colour of the blood.—A fact of great interest to the physiologist has been stated by Dr. Stevens in his Treatise on the Blood, and confirmed by Dr. Turner (Chemistry, p. 903), and others who have repeated the experiment. If perfectly florid arterial blood be allowed to coagulate, and the clot be washed with repeated portions of pure water, its colour gradually darkens so as at last to appear quite black. Exposure to the air does not restore the colour, but a solution of common salt, carbonate of soda, and other neutral salts, restore it to the original colour of arterial blood. Hence Dr. Stevens concludes, "that the florid colour of arterial blood is not due to oxygen, but to the saline matter of the serum."
Manganese in the blood. —Wurzer* has found peroxide of manganese in the blood, in quantity equal to one third of the weight of the peroxide of iron. The ashes of the colouring-matter are dissolved in muriatic acid neutralized by ammonia and precipitated by succinate of ammonia, by which the iron and the phosphate of lime are both separated. Carbonate of soda precipitates the manganese, which, heated to redness and treated with nitric acid, gives peroxide.
Volatile principle in blood.—M. Barruel states that the blood of animals has in each case a peculiar smell by which it may be recognised. This smell is stronger in the male than in the female, and is probably due to the presence of some volatile principle. Zenneck† has investigated the subject, and confirmed Barruel's statement that such smell is evolved when the blood is mixed with sulphuric or phosphoric acid; he finds the detection of it, however, to be very difficult.
Albumen,—Bourdois and Caventou observed some years ago that cheese, albumen, fibrin, and mucus, are dissolved in the cold by concentrated muriatic acid; and if kept at a temperature between 60° and 70° F. for 24 hours, gradually assumed a beautiful blue colour. This fact has since been controverted, but it has also been confirmed by several chemists. Robiquet observes that heat is not indispensable, but that a large quantity of acid is necessary to produce the effect. Nitric acid gives a yellow, and sulphuric acid a reddish brown. Dr. Hope has obtained from albumen in the latter acid a beautiful red solution.
Bile.—Perhaps the most important additions which animal
* Neues Jahrbuch, i. p. 481.
† Geiger's Mag. xxxiii. pp. 68, 172.
[page] 524
chemistry has received of late years are contained in the valuable work of Tiedemann and Gmelin on the phænomena of digestion*. They examined with much minuteness the various fluids concerned in the process of digestion; and in particular they investigated very closely the constitution of the bile, and found in it many substances not previously detected. According to them, the bile of the ox contains 91·5 per cent. of water, and the remainder consists of a volatile substance having the smell of musk, cholesterine, resin, bile asparagine, a crystalline substance they have since named taurine, picromel, colouringmatter, a substance resembling gluten, caseous matter, ptyaline or salivary matter, albumen, mucus from the gall-bladder, osmazome, an extractive matter insoluble in alcohol, together with bicarbonates, acetates, oleates, margarates, cholates, sulphates and phosphates of potash and soda, common salt, phosphate of lime, and a little carbonate of ammonia.
MM. Tiedemann and Gmelin consider all these substances to exist ready formed in the bile; Berzelius†, however, whose extended researches in animal chemistry are well known, thinks it more likely that some of them are formed by the action of the various reagents employed in extracting them from the bile.
Urea.—A very interesting discovery, lately made by Wöhler, is the artificial formation of urea. If cyanate of silver be treated with a solution of sal ammoniac, or cyanate of lead with caustic ammonia, a substance is obtained in transparent, colourless, rectangular four-sided crystals, which contains the elements of cyanic acid and ammonia, but which contains no ammonia, and possesses all the properties of urea. Wöhler has since succeeded in forming a cyanate of ammonia possessing the characters of a salt. The following formulae show the identity of composition of urea and cyanate of ammonia with one atom water.
| Urea. | Cyanate of ammonia. |
| 2C + 2N + 4H+2O. | =(N+2C) + O +(N+3H) + (H+O) |
Uric acid.— Uric acid, according to Dr. Prout, consists of 6C + 2H + 3PO + 2N. Kodweiss‡ has lately analysed it under the direction of Liebig, and obtained carbon 39·79, hydrogen 2, nitrogen 37·4, oxygen 20·81 = 10 C + 3H + 4O + 4N. The atomic weight =5·298.
Cyanurate of urea.—He has also found that the sublimate obtained by the dry distillation of uric acid, which was shown by Wöhler to consist of cyanuric acid and urea, is not a mixture but a compound of these two substances, which may also be ob-
* Die Verdauung nach Versuchen: Heidelberg, 1826.
† Årsberättelse, 1827, p. 311.
‡ Pogg. xix. p. 1.
[page] 525
tained by boiling cyanuric acid with a saturated solution of urea. It is soluble in alcohol, and decomposed by nitric acid into cyanuric acid and nitrate of urea.
Purpuric acid.—Some degree of light is thrown upon the nature of purpuric acid by the process for preparing it given by Quesneville*. He dissolves uric in nitric acid in the proportions given by Dr. Prout, saturates the acid with ammonia, and precipitates with acetate of lead. The precipitate is of a beautiful rose-red. Decomposed by sulphuretted hydrogen in excess, the colourless acid of Vauquelin is obtained; if not employed in excess, the acid is of a red colour. This would seem to argue that a deoxidation takes place when the colour is destroyed.
Erythric acid.—Kodweiss has found that the nitric and purpuric acids combine when the latter is dissolved in a weak solution of the former, and give by gentle evaporation small rhomboidal crystals, which imperfectly saturated with ammonia have the characters of Brugnatelli's erythric acid. This agrees with the previous observations of Dr. Prout.
On the other hand, if uric be dissolved in excess of nitric acid, colourless crystals of a compound of purpuric and oxalic acids are obtained. They may also be prepared by dissolving purpuric in nitric acid, and adding oxalic acid. Bases decompose this double acid, giving an oxalate and a purpurate. Kodweiss observes also, that in every case of the decomposition of uric by nitric acid, urea is formed; which may be separated by alcohol from the solution previously saturated with oxide of lead, and concentrated to the consistence of a sirup.
Lactic acid.—Mitscherlich (Lehrbuch, i. p. 50,) gives the following process for preparing pure lactic acid. Lactate of lead prepared in the usual way is decomposed by sulphate of zinc, the sulphate of lead separated, and the lactate of zinc crystallized by evaporation: at first it is yellow, but by repeated crystallization is obtained of a pure white. The solution of this lactate is decomposed by caustic barytes, the oxide of zinc separated, and the lactate of barytes which is in solution decomposed by sulphuric acid, and evaporated, gives a clear, colourless, sirupy acid, not volatile, but decomposing and leaving a residue of charcoal when heated to a sufficiently high temperature.
Milk.—Braconnot† has published some observations on milk and cheese.
The following preparation suggested by him might probably be advantageously employed at sea. Fresh curd is boiled in
* Journ. de Chim. Medicale, iv. p. 225.
† Annales de Chim. xliii. p. 337.
[page] 526
water to extract everything soluble, is pressed and cut into small pieces. To every five hundred parts of it in this state twelve of bicarbonate of potash are added, and the whole boiled in a sufficient quantity of water till solution takes place: evaporated on the water-bath it leaves a mass which may be drawn into strings or sheets, and dried like glue. In this state it may be preserved for any length of time, is soluble in water, and with sugar &c. forms an agreeable article of diet.
Bubulin.—Morin* has extracted from cow-dung a substance to which he has given the name of Bubulin, and which he considers to be the ingredient to which the utility of cow-dung as a mordant is owing. It is obtained by taking up the soluble parts with water, evaporating to dryness, treating the extract-like matter with æther and alcohol, after which water dissolves the bubulin. It is precipitated by alum, by acetate of lead, and by sulphates of copper and iron. The soluble matter in dry cow-dung amounts to about 20 per cent., of which the bubulin constitutes upwards of one fourth. Berzelius considers this substance not peculiar to the dung of graminivorous animals.
Odorin, Animin, &c.—Unverdorben, to whose researches upon resinous bodies we have already referred, has discovered no less than four substances possessed of basic properties in animal (Dippals) oil. The first, Odorin, is obtained by distilling the rectified oil previously neutralized by a little muriatic acid, and changing the receiver as soon as what comes over ceases to be entirely soluble in water. It is a colourless, oily, powerfully refracting body, soluble in water, and restoring the colour of reddened litmus-paper. Animin is procured by continuing the distillation till only one twentieth part of the oil remains in the retort. The oily matter in the receiver is a mixture of odorin and animin: water dissolves out the former, and leaves the greater part of the latter. It is also in the form of an oil soluble in, 20 times its weight of water, and acting like odorin on litmus-paper.
Olanin (oleum, animale,) constitutes the greater part of what remains in the retort: if this residue be treated with 20 times its weight of watery the animin it contains is taken up, and a thick oily fluid remains, which is olanin. It acts also upon reddened litmus-paper, and when exposed to the air gradually becomes brown, and is changed into a substance which Unverdorhen calls fuscin.
Ammolin.—Ammolinis obtained only from the unrectified oil: the oil is digested for several hours with dilute sulphuric acid, decanted from the acid solution, washed with water, and the washings added to the solution. The acid has taken up the three
* Journ. de Chim. Med. vi. p. 545.
[page] 527
bases already mentioned, ammolin, and a portion of empyreumatic oil: the last is separated by long boiling, during which it is either volatilized or converted into a brown resinous matter. A fortieth part of nitric acid is now added to the brown solution, and the whole evaporated to one fourth: this is again diluted, nearly saturated with carbonate of soda, and distilled so long as what passes over smells of odorin or animin; the residue is a mixture of sulphates of ammonia, soda, and ammolin. The sulphuric acid is completely saturated by carbonate of soda, and evaporated: the carbonate of ammonia flies off, and a brown oil separates, which by distillation gives ammolin. It is a colourless oily body, heavier than water, more powerfully alkaline than any of the other bases above described, expelling ammonia from its salts at a boiling temperature, dissolves in 40 boiling and 200 cold water, is volatilized in very small quantity by boiling in water, and dissolves in all proportions in alcohol and æther. It is decomposed by chlorine.
These substances form salts with the acids, most of which have an oily appearance. With the chlorides of gold and platina the muriate of odorin forms double salts in beautiful yellow crystals. The muriate of animin gives a similar crystallizable salt with chloride of platina.
Paraffin and Eupion.—It has already been mentioned that Reichenbach had discovered two remarkable substances, Paraffin and Eupion, in the products of the destructive distillation of vegetable substances. He has since found that they may be obtained also from the products of the distillation of animal substances, the paraffin being less, and the eupion more abundant in the latter case.
The train of research in which he was engaged led Reichenbach to investigate the basic substances of Unverdorben; but he did not succeed in obtaining anything which resembled any one of them: he therefore infers that they do not exist. It is probable that circumstances not stated by Unverdorben may have influenced their production; for that he did produce them is certain, as I have seen in the possession of Berzelius specimens of them sent to him by the discoverer.
The following List contains nearly all the animal principles with which we are yet acquainted.
[page] 528
Acid Principles.
| Names. | Discovered by | Remarks. |
| Oleic acid | Chevreul. | 70 C+17 H+5 O. |
| Margaric | Chevreul. | 34 C+65 H+5 0. |
| Butyric | Chevreul. | 8 C+11H+3 0. |
| Delphinic | Chevreul. | 10 C+14H+3O. |
| Stearic | Chevreul. | 70 C+75 H+5 O. |
| Capric | Chevreul. | 18 C+29 H+3 O. |
| Caproic | Chevreul. | 12 C+19 H+3 O. |
| Hircic | Chevreul. | |
| Cetic | Chevreul. | By saponifying spermaceti. |
| Ceric? | Pfaff. | By distilling a wax soap with sulphuric acid. |
| Cholesteric | Pelletier and Caventou. | By treating cholesterine with nitric acid. |
| Ambreic | Pell. and Cav. | By treating ambreine with nitric acid. |
| Castoric? | Brandes. | By treating castorin with nitric acid. |
| Uric | Scheele. | |
| Hippuric | Liebig. | In the urine of graminivorous animals. |
| Purpuric | Dr. Prout. | |
| Lactic | Berzelius. | |
| Formic | Margraff? | |
| Allantoic | Buniva and Vauquelin. | In the urine of the foetus of the cow, = 5 C +8 H+4 N+4 0.—Liebig. |
| Cholic | Tiedemann and Gmelin. | In bile. |
Indifferent and Alkaline Principles.
| Names. | Discovered by | Remarks. |
| stearine | Chevreul. | |
| Stearine | Chevreul. | |
| Butyrine | Chevreul. | |
| Phocenine. | Chevreul. | |
| Hircine | Chevreul. | |
| Cholesterine | Chevreul. | |
| Glycerine. | Chevreul. | 3 (C+H)+2 O.—Gusseron. |
| Cetine | Chevreul | so calls spermaceti. |
| Ambreine | Pelletier and Caventou. | Resembles cholesterine, and is extracted J from ambergris. |
| Castorine. | Bizio | Resembles cholesterine, and is extracted from musk. |
| Ethal?. | Chevreul. | Obtained from spermaceti soap. |
| Taurin | Tiedemann and Gmelin | have so named the resin of the bile. |
| Picromel | Tied. and Gm. | Called at first bile asparagin. |
| Erythrogen | Thenard. | From bile. |
| Erythrogen. | Bizio. | In a diseased biliary fluid. |
| Ptyalin | The name given by Hunefeld to salivary matter. | |
| Urea | Cruikshank. | 2 C+4 H+2 N+2 O.—Dr. Prout. |
[page] 529
Indifferent and Alkaline Principles,—continued.
| Names. | Discovered by | Remark s. |
| Hematosine. | Lecanu | so calls the colouring-matter of the blood. |
| Osmazome | Thenard. | An extractive matter from flesh. |
| Fibrin. | ||
| Albumen. | ||
| Gelatine. | ||
| Caseum | Braconnot. | The curd of milk. |
| Aposepidin. | Braconnot. | The caseous oxide of Proust; the product of the putrefaction of (all?) animal substances. |
| Cantharidin. | Henry and Plisson. | Obtained from Spanish flies, = 68·56 C +8·43 H + 9·86 N + 13·15. O |
| Bubulin | Morin. | |
| Paraffin | Reichenbach. | From the destructive distillation of animal substances. |
| Eupion | Unverdorben. | By distilling Dippel's animal oil. |
| Odorin | Unverdorben. | |
| Animin | Unverdorben. | |
| Olanin | Unverdorben. | |
| Ammolin | Unverdorben. | |
| Melain | Bizio | so calls the pigment of the cuttle-fish; and to a substance obtained from the horny covering of beetles he gives the name of Chitin. |
Many other principles have been met with possessed of peculiar properties, but their characters in general have been so obscure, or their purity so uncertain, that they have not been distinguished by any appropriate names.
In regarding the above list, we cannot help remarking how much this department of the science owes to Chevreul. Almost the only branch of animal chemistry which has been thoroughly investigated is that of the fatty bodies; and for nearly everything that has been done in it we are indebted to that distinguished chemist.
Abstract of a Comparative Review of Philological and Physical Researches as applied to the History of the Human Species. By J. C. PRICHARD, M.D. F.R.S.
THE object of this Essay is to furnish a survey of the progress of knowledge in relation to ethnography, with a critical account of the attempts which have been made to distribute the human species into departments constituting what are termed families of nations, and especially of that classification of races which has been adopted by Baron Cuvier, and is now very
2 L
[page] 530
generally received.—The author commenced with preliminary remarks on the resources of knowledge available in researches of this kind, and stated it to be his principal design to consider and estimate the means of information respecting the history of mankind which are furnished by two different methods of inquiry,viz. by philological and physical investigations; the former including those researches into the structure and affinity of languages which have been undertaken with a view to elucidate the relations of tribes and races to each other; the latter, the attempts which have been made to classify nations by their mutual resemblances in figure, complexion, and other physical peculiarities.
"Philology, in this point of view an important study, dates its origin from an sera glorious in the history of modern discovery and the achievements of science. It begins with the voyage of Magalhaens, who first led the way in the circumnavigation of the globe, and whose fame has been recorded by the gratitude of posterity upon the heavens as well as upon the earth. While Magalhaens was employed in tracing in the sky nebulae, and new seas and oceans on the globe, his companion Pigafetta bethought himself of acquiring the means of rendering intelligible and of comparing with each other the various dialects of the new races of men, whose existence this voyage was destined to make known. He began the practice of collecting vocabularies which might furnish specimens of the idioms spoken in distant islands of the ocean. His example has been followed by succeeding navigators, and has led by degrees to results of great interest. The native tribes found in remote groups of islands in the great Southern Ocean, looked upon themselves as the offspring of the sun and moon, or of the soil; they knew nothing of other branches of the human family; their whole world and sphere of existence was limited by their shores, or by the small circle of their imperfect navigation. Accordingly, by some writers it has been confidently assumed that these tribes of men, like the bread-fruit and coco-nut trees by which they are fed, are the indigenous produce of the coralline or volcanic soil on which they exist. This notion might have been strenuously maintained, if researches into the structure and affinity of languages had not furnished its refutation, and displayed, in the idioms of these insular tribes, sufficient evidence of their mutual relationship and of the derivation of the whole stock of people from a common centre."
The author proceeded to give a brief survey of the history of philological inquiries and of the various collections exemplifying the diversity and affinity of languages which have been made since the year 1555. "In 1555 was published the first general essay on this subjeet,—the Mithridates of the learned Conrad
[page] 531
Gessner, which may be considered, however, as an abortive attempt, the author having aimed at more than it was possible to attain in his age. The Mithridates of Adelung and Vater, which followed 130 years afterwards, is the last general history of languages which has appeared. Particular portions, however, of the field of philology have been cultivated with great success, either by private individuals or by societies of learned men.
"1. Much light has been thrown on the languages of Asia, their affinities and relations, by M. Julius Klaproth, who, in various journeys in Caucasus, Siberia, and the provinces of the Russian empire bordering on China, has enjoyed extensive opportunities of acquiring information: he is likewise acquainted with the Chinese and Mongolian languages, and has made diligent use of the historical information extant in the works of Chinese annalists and literary compilers. The principal results of his studies are contained in his great work, entitled Asia Polyglotta, to which is appended a Sprach-xtlas, containing comparative tables of vocabularies.
2. A great mass of information was collected by Dr. Seetzen, in reference to the languages of the African nations. On the geographical discoveries of this traveller in Palestine, the eastern parts of which he was the first among modern travellers to explore, I have no occasion for remark. The principal theatre of Seetzen's researches was Africa, where he spent a long time in collecting vocabularies and historical and geographical information from intelligent men whom he met with among the woolly-haired races. Such of his papers as reached Europe were either put into the hands of Professor Vater of Königsberg, or were published by Baron Von Zach in the Monatliche Correspondenz. I shall briefly advert to one point, in reference to which he has illustrated the ethnography of Africa. The origin of the Felatahs, in the interior of that continent,—a red or copper-coloured race, who have lately made extensive conquests over the negro nations,—was, for some time after that people became known, a matter of uncertain conjecture. It is now known that the Felatahs are a branch of the same race who have for many centuries inhabited the high-lands of Guinea, where the Gambia and the Rio Grande have their sources, and who have been visited in their mountainous capital of Teembo by more than one European adventurer. They are the Foulahs of English travellers, and the Red Poules of M. Mollien. Seetzen obtained a vocabulary of the Felatah language, which was published in the Königsberg Archivs filr Philosophie; and this led to a discovery of the real origin of the people.
3. In reference to the languages of America, which are
2 L 2
[page] 532
known to be very numerous and complex in their structure, much information was collected by Hervas,—the result of his own personal researches, and those of other Jesuits. Baron Alexander Von Humboldt brought back with him from America a large collection of vocabularies, dictionaries, and devotional offices, and other books, prepared by the Catholic instructors, in different parts of that continent, for the use of the native tribes who came under their spiritual jurisdiction. These were put into the hands of Professor Vater, the continuator of the Mithridates. Since the publication of that work, the Historical Committee of the Philosophical Society of the United States have devoted their attention to the languages and history of the aborigines of the Western Continent. The names of Hecke-welder and Zeisberger, and that of Mr. Duponceau, the learned Secretary of the Committee, stand highly distinguished among those of contributors to this department of human knowledge."
The author then stated the most important results, in reference to the history of languages, which he considered as established by these inquiries.
1. It appears that the number of human idioms, widely differing from each other, is very great—much greater than many persons supposed. Mr. Jefferson, President of the United States, used to argue from the great number of distinct languages found in America, and the comparatively small number existing, as he supposed, in the old Continent, that America was the most anciently peopled. Most persons will be of opinion that this conclusion requires further proof; but the fact is undoubted that a great variety of languages are spoken in America. According to Hervas, who relied on the information given him by Lopez, 1500 languages, which are said to be 'notabilmente diverse,' are spoken in different parts of America. According to Dr. Seetzen, the number of distinct languages in Africa amount to 100 or 150. If these calculations are nearly correct, we may, without much danger of exceeding the truth, consider the probable number of languages spoken in all the world, to be not less than 2000.
2. We may observe in the second place, that a comparison of various languages displays two different relations subsisting between them. These relations may be termed those of affinity and of analogy. I shall give a few examples of each.
1.) The relation of affinity, or, as it has been termed by German writers, the Stammverwandschaft, or family relation of languages, subsists between idioms which have a great proportion of their elements or roots common to all of them, together with a general resemblance in grammatical structure. It is generally
[page] 533
allowed that nations, whose idioms have this sort of affinity, are allied in origin. Groups of idioms thus related are termed families of languages.
One strongly marked family of languages consists of the dialects termed collectively the Semitic. To this belong the Hebrew, the Chaldee, the Aramean or Syriac, and the Geez or Ethiopic.
Another family of languages is the Indo-European, in which are included various idioms both of Europe and Asia, whose near affinity has been thought to prove a kindred origin in nations long ago separated from each other. It has been chiefly during the last twenty years that the near affinity of this class of languages has been discovered. They form a most extensive group, including, 1st, the Sanskrit and all its dialects in India; 2nd, the ancient Zend or Medo-Persian language, and all the idioms now spoken in Persia and Armenia; 3rd, the Greek and Latin languages, and all the dialects sprung from them; 4th, the Sclavonic, the origin of the Russian, Polish, and Bohemian languages; 5th, the Teutonic languages; 6th, the Celtic dialects, which belong, if I am not mistaken, to the same family, though on this subject there is some dispute.
We have next to consider analogy between languages. Many idioms which are entirely distinct from each other, being com-pletely different in their vocabularies, and having few or perhaps no words in common, are yet found to bear to each other a striking resemblance in their grammatical structure. This resemblance is such as to admit of no other term than that of analogy, and such languages cannot be said to belong to the same family; they constitute particular classes of languages. I shall mention some examples of this relation.
1. A strongly marked class of languages are those termed Monosyllabic, the words belonging to which are monosyllables, uttered without any inflection of termination, and with merely a sort of intonation to express the relations of words to each other. Idioms of this description are spoken by the Chinese, Tibetans, Burmans, Cochin-Chinese, Siamese, and nearly all the nations of the further Indian Peninsula. The particular languages I have now mentioned are quite distinct from each other; even their numerals and their most familiar and common elements of speech are different.
Another class of languages are those termed Polysynthetic, consisting in long polysyllabic words, and abounding in modes of inflection, refined and elaborate, admitting almost infinite varieties of termination and changes of structure; such varieties of structure and termination expressing numerous modifications in the original ideas which the words were intended to
[page] 534
convey. To this very remarkable class of languages belong all the idioms of America, from that of the Esquimaux at Behring's Straits, to the dialects of Patagonia and Tierra del Fuego.
I shall now terminate what I have to say on this branch of my subject— viz. on philological researches—by one remark, of which the application will hereafter be very obvious. It is, that although we may not be authorized in a positive conclusion, that all nations whose languages belong to the same class are of one race,—as for example, all the nations of the New World, the resemblance between their respective idioms being only analogy and not amounting to affinity,—yet we may determine upon regarding such nations as more nearly connected than those whose idioms belong to different classes; and we may assert, that any pretence for including in one race or lineage, nations whose idioms belong to classes totally different, must be arbitrary and in opposition to all probability. Such, for example, would be an attempt to include some of the American nations whose idioms are polysynthetic, in the same race or stock with tribes who speak monosyllabic languages.
From the survey I have now taken of the progress of philological information, and from the conception which this survey is calculated to produce, of the nature and extent of such information, we are entitled to conclude that it is a department of knowledge which ought by no means to be neglected by those who wish to elucidate the history and affinity of nations or of different races of men; and that any conclusions which may be drawn by such writers from other sources,—as, for example, from anatomical and physical inquiries pursued separately,–will be liable to error, if reference is not occasionally made to the results deduced from philology. Notwithstanding this almost palpable fact, we shall presently perceive that the most popular systems with respect to the history of mankind, and the classification of nations, are not only built on premises altogether distinct from those which depend on affinity in languages, but are completely at variance with the most obvious conclusions derivable from this source of information."
The author, after these general remarks on the application of philology, proceeded to give an account of the attempts which have been made to distinguish and classify the races of men by their physical characters.
"Many late writers on the history of mankind have attempted to distribute the human species into several races, distinguished from each other by peculiarities in the form, structure and colour, of their bodies. Varieties of form have generally been thought to afford a better groundwork for this division than
[page] 535
those of complexion; and since it has been known that there exist national diversities in the shape of the skull, this circumstance has been generally selected as furnishing the most permanent distinctions, and those which admit of the most extensive comparison and classification. Several writers, both French and German, have differed from each other as to the number of human races which they constitute; but the most generally received system is that which has been adopted by Baron Cuvier, though it did not originate with that celebrated writer. Professor Camper had thrown out the first hint of a triple division of the forms of the skull. He distinguished the facial angle as found by his measurement in European, Kalmuc and African skulls. But a more important view of the diversities of form in the human skull seems also to have originated with Camper; for we are informed by Sœmmerring, that in his unpublished commentaries Camper remarked the difference in breadth which exists between the three classes of skulls above mentioned, and observed that the skulls of the Kalmucs have the greatest breadth, those of Europeans a middle degree, and that the skulls of African Negroes are the narrowest of all.
Nobody ever possessed means of observation and comparison sufficient for establishing any conclusions of importance as to the different forms of the human cranium, until Blumenbach had made his admirable collection of skulls. The results of his long-continued study of this collection have been published by himself at different times.
Blumenbach distinguished, in the first place, three principal varieties of form in the human skull,—the oval form, which is that of Europeans; the narrow and compressed, which is that of Negroes; and the broad-faced skull, with laterally-projecting cheek-bones, belonging to Kalmucs and Mongoles. It happened, as I think, unfortunately, that Blumenbach named these varieties of the skull, not from their characteristic forms, but from some nations, in whom they in a conspicuous manner occur, or from the supposed primitive abode of such nations. Thus the broad-faced form is termed by him Mongolian; the compressed, Æthiopic, meaning African; and the oval form, Caucasian. The inconvenience which has arisen from the terms thus used is the hypothesis to which it has given rise, that these three varieties of form are characteristic of three distinct races of mankind. This is not Blumenbach's opinion, but it appears to be that of Cuvier, who, in his Règne Animal and other works, has adopted Blumenbach's terms and divisions. Relying on the diversity of physical characters, which yet he does not consider sufficiently marked to constitute differences of species,
[page] 536
Cuvier proposes to divide mankind into three distinct races. One of these races had, according to his hypothesis, its original seat on Mount Atlas, and its branches are spread over Africa. These are the narrow-skulled, woolly-haired African nations. But there are woolly-haired tribes of men, equally black with the Negroes of Guinea, and resembling them in form and general appearance, in other equatorial countries besides Africa. Such are the black savages who inhabit the mountains behind Malacca, termed Samang; the woolly-haired Papuas of New Guinea and nearly all the larger islands of the Indian Archipelago; and the natives of Mallicollo and some other isles in the Pacific Ocean. These must belong to the same race as the African Negroes, if races are constituted on the principle of physical analogy; and Cuvier accordingly resorts to the hypothesis, that some Negroes from Africa lost their way—se sont égarés—in the great Southern Ocean, in order to account for the existence of woolly-haired people in the countries above mentioned. A second human race in his system are the Mongolians or Kalmucs, whose origin he thinks may be deduced from the high mountains of Altai. The other great division of mankind, consisting of men with oval and symmetrical skulls, to which European nations belong, are in like manner supposed to have drawn their first breath on Mount Caucasus, and are hence termed the Caucasian race.
On surveying the manner in which nations are distributed and associated together, in these three departments, we meet with some facts which are staggering anomalies to those who judge of the affinity of races by that of languages. We shall take for example the enumeration of tribes reckoned by Baron Cuvier as belonging to the Mongolian race. He says:—
'To the eastward of what has been termed the Tartar branch of the Caucasian race, that is, to the northward of the Caspian, is found the commencement of the Mongolian stock, which prevails from thence as far as the Eastern Ocean. Its branches, still nomadic, the Kalmucs and the Kalkas, wander over vast deserts. Their ancestors three times—under Attila, under Genghis, and under Tamerlane—carried far the terror of their name. The Chinese are the branch, the most anciently civilized, not only of this race, but of all nations that are known. A third branch, the Mantschoos, have lately conquered China, and yet govern it. The Japanese and the Coreans, and most of the hordes reaching to the N.E. of Siberia under the domination of the Russians, belong, in great part, to this stock:—except some of the Chinese literati, the whole Mongolian race is addicted to the worship of Fo.'
Here we find two classes of nations, identified and represent-
[page] 537
ed as branches of one stock, who differ from each other in the most decided and remarkable manner, in every respect in which one nation can differ from another, with the single exception that they bear a degree of resemblance in the shape of their skulls. The Mongoles and Kalmucs are tribes of nomades or wandering shepherds who roam about the lofty saline plains of central Asia, living in wagons, and under movable tents, as their ancestors are said to have lived in the time of Æschylus: they are incapable of changing their habits for those of settled and agricultural people. They are all one nation, strictly so termed, and have one language, which is polysyllabic in its structure, admitting inflections and conjugations of nouns and verbs. On the other hand, the Chinese are ever known as a people of settled, uniform, and changeless habits: their historical records deduce them as a separate nation from the earliest ages of antiquity, and especially establish their perpetual enmity and discordance with the Mongolian nomades, who are the very people to exclude whom from their borders the famous Chinese wall was erected in a remote age. The Chinese and the Indo-Chinese nations appropriate to themselves, as we have before observed, one entire class of languages, constituting one of the most strongly marked examples of a distinct assemblage of human idioms, widely differing from all others. It is to these nations that the monosyllabic languages belong, consisting of monosyllables, incapable of inflection or variation, in which a mere change of intonation and juxtaposition alone serves to indicate the relations of words to each other. Before we can admit of an hypothesis which derives one of these nations from the other, we must resolve to shut our eyes against all the evidence that can be brought to bear upon such a subject, excepting merely that afforded by physical resemblances, which, if we are not mistaken, will admit of a different explanation.
The only other connective link between the Mongolian and Chinese nations, is the circumstance that they are all worshipers of Fo. This can scarcely be thought an argument for their unity of race. The religion of Buddha indeed, called in China Fo, is well known to have taken its rise in India, among the Hindoos who belong to the division of nations termed by Cuvier the Caucasian race. It was established at a remote period in Tibet, and thence propagated to China, where however it is but one of several prevailing superstitions. The Mongoles and Kalmucs received it not until A.D. 1250. It is not, therefore, a peculiar and ancient distinction of the Mongolian race.
Many writers have thought fit to associate the native American tribes with the Mongolian race. Cuvier hesitates on this subject; but the excellent naturalists, Von Spix and Mar-
[page] 538
tius, who some years ago visited South America, were struck by the great resemblance between the Chinese, in the form of their skulls and features, and the American tribes near Brasil. Many tribes in the Western World have flatter features, more approaching to the Mongolian, than the nations of North America; and if we were to adhere to a classification founded entirely on the principle of physical peculiarities, it would be difficult to discover a precise line of discrimination by which all the native tribes of Americans are to be distinguished from the group of nations which constitute Cuvier's 'race Mangolique.' If the triple division of skulls is maintained, those of the American nations must be referred to the Mongolian form. Here, then, we have a wide extension of this family, which thus comes to include a great assemblage of nations beyond the limits of Asia, whose languages, though multiplied, have some common characters; and it is worthy of notice that those common characters are the very reverse of the peculiarities which, as above mentioned, distinguish the Chinese and Indo-Chinese languages from all others. The latter are monosyllabic and hardly inflected; the American languages, as we have observed, abound in long polysyllables, and in their modes of inflection are refined and elaborate, admitting almost infinite variety of termination and change of structure. As a class of languages, they have obtained the distinguishing term polysynthetic.
The Malays, a people whose original seat, or, as I would rather say, earliest known position, is in the island of Sumatra, and from whom were descended, as it appears, all the Polynesian tribes of the great Southern Ocean, associate themselves more nearly with this department of nations than any other; and if referrible to either of the three divisions, must be included in the Mongolian department. The history of these tribes will present us with many physical phænomena very adverse to the fundamental principle on which the tripartite division of races can alone be maintained. This principle is the assumption that all physical characters are permanent and immutable. Now we have reason to believe that some of the tribes of Polynesian islanders have deviated in a most remarkable manner from the physical character most generally prevalent in their stock. Individuals are seen among the natives of the Society Islands of fair and sanguine complexion, and the Marquesans are among the finest races of men existing; their skulls have the oval, or, as it is termed, Caucasian form. We thus find that the division of mankind, termed the Mongolian race, includes several groups or classes of nations distinguished by the most permanent and indelible characters, which are known to separate the great families of the human race from each other. They are associated by no common cir-
[page] 539
cumstance whatever, except a resemblance in physical characters, and these are plainly subject to great varieties.
"We now come to Baron Cuvier's Caucasian race, of which he gives the following account.—' The stock from which we are descended has been termed the Caucasian race, because the traditions and filiations of tribes seem to carry it to that group of mountains situated between the Caspian and the Black Sea.' He goes on to say, that 'the principal branches of the Caucasian race may be distinguished by the analogy of their languages.' Here he enters upon the ground of philological investigation, and it is important to observe how far it affords a firm basis for his conclusions. The branches of the Caucasian race are thus mentioned:—1st. 'The Aramean branch, or that of Syria, directed its progress southward: it produced the Assyrians, the Chaldeans, the Arabs, always unconquered, who after Mohammed expected to have become lords of the world; the Phœnicians, the Jews, and the Abyssins, colonies of the Arabs: it is very probable,' he adds, 'that the Egyptians belonged to the same division.' Before we proceed to the account which is given of other branches of the Caucasian stock, we may take an opportunity to observe that some historical paradoxes have been already brought under our view. Both Jews and Arabs are allowed to have ancient traditions; yet none of these, written or oral, represent either people as descended from Mount Caucasus. Again, it is not a little startling to find the red or copper-coloured Egyptians considered as Caucasians, and as belonging to the Semitic stock of nations. How is this to be reconciled with the statements of Herodotus and Manetho, and all the historians who so strongly contrast the Egyptians with the Jews, and even of Moses, who represents them as speaking different languages as early as the time of the patriarch Joseph? And how, indeed, are we to get over the fact, that the Egyptian language which remains to our time is entirely of a different structure and has a totally different vocabulary from the Hebrew? We shall pass on to the next branch of the Caucasian race.
'The Indian, German, and Pelasgic branch,' says Cuvier, 'is much more extended, and was more anciently divided. We can however recognise a multitude of affinities between the following four lauguages. 1. The Sanskrit, which is now the sacred language of India, the mother of all the idioms of Hindostan. 2. The ancient language of the Pelasgi, the common mother of the Greek, the Latin, and many of the extinct languages, and of all our idioms of the South of Europe. 3. The Gothic, or Tudesque, from which are derived all the languages of the North and North-west, the German, the Dutch, the English, the Danish, the Swedish, and their dia-
[page] 540
lects. 4. Lastly, the language called Sclavonian, from which are derived all the languages of the North-east, the Russian, the Polonese, the Bohemian, the Wendish. It is this grand branch of the Caucasian race which has carried to the highest pitch philosophy, science, and the arts, and which has for more than thirty ages been the depositories of them.'
There is indisputable proof in support of the assertion that the nations now enumerated may be identified by means of their languages. But how are they to be connected with the Arabs, Jews, and Egyptians, already referred to the same race, or with the third branch of the Caucasian race, who yet remain to be mentioned?
'The Scythian and Tartarian branch,' it is added, 'extend towards the north and north-east, ever wandering forth through the immense deserts of these regions, and only returning to overthrow the more happy settlements of their brethren. The Scythians, who so early made an irruption into the higher parts of Asia; the Parthians, who destroyed the Greek and Roman empires; the Turks, who overthrew that of the Arabs, and subdued in Europe the miserable remains of the Grecian nation,—were swarms from this horde. The Finlanders and Hungarians were a colony of them wandering among the nations of the Sclavonian and Teutonic races. Their original country to the northward and eastward of the Caspian Sea preserves yet traces of people of the same stock; but they are intermixed with an infinite number of other small tribes of different origin and languages. The Tartar people have remained more unmixed in all this space. They long menaced Russia, and have at length been subjugated by her from the mouths of the Danube as far as those of the Irtisch.'
We are here, in the first place, struck with the circumstance that the Tartar race are joined with the Finlanders and the Hungarians. Now the nations last mentioned are two branches of a stock spread through the northern parts of Europe and some regions of Asia from very early times, and are strongly distinguished by physical character and by manners from the Tartar or Scythian race. What is still more important, the Finnish nations are always to be identified among themselves, and clearly distinguishable from the Tartars by their dialects. The Fenni and Scritifenni, belonging to the stock of the Finns and Laplanders, are described by the Roman writers Tacitus and Pliny, as inhabitants of the North of Europe. They are mentioned by King Alfred in his curious transcript of the Voyage of Ohter the Northman; and according to the most learned investigators of northern antiquities, the Finns are the people who under the name of Jotuni, or giants, had occupied Scandinavia
[page] 541
and the shores of the Baltic before the arrival of Odin and his Teutonic followers from the East. It is said, indeed, that some of the noble families among the Northmen, or Normans, were descended from these aborigines of Scandinavia. Even Rollo, the conqueror of Normandy and the ancestor of the royal dynasty of England, claimed his descent from a Jotune family who had dwelt from time immemorial near Drontheim in Norway. The history of the Finns has been traced among all the writers of the middle ages. It has long been known that all the Finnish and Hungarian tribes are allied by the resemblance of their dialects; but a few years ago this subject was profoundly investigated by a learned native of Hungary, Gyarmathi, who availed himself of his intimate acquaintance with one of those dialects—his own mother tongue,—and applied himself to the investigation of the cognate languages. The result has been to establish a connection in speech, and therefore in race and origin, between the Laplanders, the Finns, the Hungarians, the Ostiaks in Asia, and many tribes scattered on both sides of the great Ouralian chain which separates the North of Europe from that of Asia. Many of these nations are distinguished for flat faces and red hair, in which characters they are contrasted with the Tartars. Their language unequivocally separates them from that people.
But still less can the Tartar or Turkish nation itself be identified with the other members of the supposed Caucasian race. It has never been pretended that any affinity subsists between the language of the Tartars and the Indo-European nations. The dialects of the Tartar tribes are not much varied: all the clans belonging to this great nation, though spread far and wide, and reaching from Constantinople to the Irtisch and Lena, speak one language.
Everything that we can collect as to the ancient history of the Tartar nation, seems to run counter to such an hypothesis. The only ground, indeed, on which it is pretended to associate the Tartar with the European, or, as they are termed, Caucasian nations, is the fact that the skulls of the Turks have a form which belongs to the European type. But even this is by no means universal. Many of the Tartar nations approach nearly to the Mongoles and Kalmucs in their features and in the shape of their heads; and this is particularly the case with those branches of the Turkish stock who have long been settled in the North of Asia, in climates inhabited of old by people to whom the Mongolian characters were from early periods appropriate. These deviations from the more general traits of the Turkish race, and approximations to those of the Mongoles, are attributed by writers who maintain the permanent transmission of physical cha-
[page] 542
racters to intermixtures of race. But this is altogether gratuitous. If we may judge of the purity of race by purity of language, the Yakuts, who inhabit the shores of the Lena, must be considered as of unmixed Turkish race. Their speech, as M. Julius Klaproth has proved, is nearly that of the Osmanli themselves, and it has been said that a Turk of Stamboul would be understood among the Yakuts on the Lena. Probability is in favour of the opinion of Blumenbach, that a long residence in the climate of North-eastern Asia has changed the features of the race. The language of the Yakuts being unmixed, we may be allowed to infer from this circumstance the purity of their stock, though their features are those of the Mongoles and Kalmucs.
"Before I take leave of the Caucasian race, I shall offer some further remarks on this designation. It is applied, as we are informed, to nations of this class, because their traditions deduce them from Mount Caucasus. But is this really a fact? The mountains of Asia Minor, of Thrace, and of Hellas, are all famous in Grecian story. Mountains were of old, in the simple and primitive age which long preceded the erection of temples, consecrated to the worship of the unseen power whom all nations venerated. The tops of Olympus and Mount Meru in the poetry of Greece and India were the resting-places where father Zeus and Indra descended from the clouds to converse with mortals. Caucasus came in for its share in the general respect paid to high places; according to a story, of which it is difficult to conjecture the meaning, it was the dwelling-place of Prometheus, where that ambiguous personage, by turns a titan, a teacher of mechanical arts, and a maker of man, and then a natural philosopher, is said to have watched the movements of the heavenly bodies. I cannot remember any tradition among the fabulists or historians of Greece which admits of a construction answering to the hypothesis of M. Cuvier, or deducing the human race from Mount Caucasus. Nor can anything more to the purpose be traced in the mythology of the Oriental nations. The authentic narrative of the Hebrews leads us certainly to Mount Ararat in Armenia for the resting-place of the ark; but that is far from Caucasus.
Another objection to the term Caucasian, as applied to an assemblage of nations consisting principally of the Indo-Europeans and Semitic tribes, arises from the fact that the chain of Caucasus has been from immemorial time the seat of nations who are proved by their languages to be entirely distinct from both of these celebrated races. The idioms of the real Caucasian nations have been carefully examined by Julius Klaproth. The result has been a reduction of these numerous dialects to a few
[page] 543
original languages, all of which, except that of the Ossetes, are destitute of any analogy to the Indo-European idioms. The Ossetes indeed speak a dialect resembling some of the languages of that stock; they are an inconsiderable tribe, who appear to have found their way incidentally into the midst of races foreign to their lineage: and it would be absurd to regard them as the ancestral stock of so many great and anciently-civilized nations.
3. The Negroes of Africa and the woolly-haired natives of the Malayan mountains, and of New Guinea, and many islands in the Pacific at no great distance from New Holland, are referred by M. Cuvier to his third race, which he supposes to have originated in Mount Atlas. The languages of these tribes are multifarious, and the migration of one part of them to the Eastern Ocean improbable and difficult to imagine. It is evident that the attempt to identify the African Negroes with the Papuas of the Eastern Ocean rests on the physical peculiarities of these tribes, and that every other species of evidence is against it. But is it certain that no other principle can be found to account for the existence of nations resembling the Africans in New Guinea and the Easternislands? Are not the torrid climes of these countries similar to that of Old Guinea? and do not all the other productions of nature likewise resemble those of Africa? It is not to be wondered at that the human species should assimilate in these parallel latitudes and analogous situations. The black and woolly-haired variety of the human species is that which has ever thriven best in equatorial countries, and there is probably something in the nature of the torrid clime which favours its rise and propagation. If physical agencies produced it once, similar agencies may have produced it wherever their influence has been exerted with a certain degree of intensity and under favourable circumstances."
The following are the general inferences which the author has deduced from the preceding statement.
"It appears, on the whole, that the attempt to constitute particular families of nations, or to divide the human species into several distinct races, upon the principle of a permanent and constant transmission of physical characters, is altogether impracticable. In the first place, such divisions of races do not coincide with the divisions of languages. We shall find one class of men as distinguished by physical character, including several races entirely distinct from each other, when reference is made to their languages. Thus the Turkish or Tartar race are separated by their language from the Indo-European nations, and the distinction is not less when we go back to the earliest ages. How distant indeed must have been the period
[page] 544
when the Celtæ and the German nations, and the Greeks; Latins, and Sclavonians were separated from the Hindoos! Yet all these nations have preserved from that period strong proofs of the identity of their speech!—Nor can we imagine why the Tartars alone should have lost all traces of their former language, if they had once partaken of the same idiom with the nations just mentioned, or had a dialect allied to it! The distinction of races, according to the same principle, will, besides, separate nations who are shown to be connected by their language, when they happen to have acquired a different character, diversities of figure or complexion.—I have already alluded to particular instances which exemplify this remark.
2ndly. A second objection to the distributing of men into different races on the ground of physical diversities, is, that it is contradictory to the very principle which has been always professed by the most enlightened writers on the philosophy of natural history, and which, it may be added, had been admirably maintained and illustrated by Cuvier himself in regard to the nature and distinction of species. The clear and broad line which he lays down as constituting the distinction of species in natural history, is that of permanent and constant difference. 'We are under the necessity of admitting the existence of certain forms which have perpetuated themselves from the beginning of the world, without exceeding the limits first prescribed: all the individuals belonging to one of these forms constitute what is termed a species.' 'Varieties,' he adds, 'are the accidental subdivisions of species.' This is his own account of the laws constituting species. By himself the diversities found between different races of men are clearly laid down as varieties. To regard these afterwards as permanent is to contradict what has previously been established. In fact, we must either concede at once that there are several distinct human species,—an hypothesis which would be immediately opposed by a number of nisuperable objections,—or we must allow that no permanently distinct races as constituted by physical characters exist in the one human species.
If these general observations are allowed to be well founded, they will lead towards the conclusion,— that the various tribes of men are of one origin. The diversities of language carry us, indeed, very far back towards the infancy of our race, and are perhaps much more ancient distinctions than the varieties of form and colour. But these diversities require no such explanation as that of a separate origin, or a distinct creation of the several races who are so characterized."
[page] 545
TRANSACTIONS
OF
THE SECTIONS.
1. MATHEMATICS.
PROFESSOR HAMILTON, Astronomer Royal for Ireland, gave an account of a Memoir by JAMES MACCULLAGH, F.T.C.D.,On the Attractions of Spheroids, which had lately been presented to the Royal Irish Academy, and in which is given a very simple demonstration of a rigorous theorem corresponding to a celebrated and contested approximate theorem of Laplace: together with a geometrical construction of the quantity neglected in that approximate theorem, which is shown to be the function of a certain small solid assigned by the author. He likewise gave an outline of a manuscript Memoir on Numeral Evolution, by DR. ALLMAN, Professor of Botany in the University of Dublin, which related principally to a new method for the arithmetical calculation of logarithms.
PROFESSOR HAMILTON also stated a general Theorem of his own respecting differences and differentials of Functions of Zero, which he had presented to the Royal Irish Academy, and to which he had been led by meditating on a method given by Sir John Herschel for the development of exponential functions. He gave a verbal account of his view of Mathematical Optics. of which the following is an abstract.
On a View of Mathematical Optics. By WILLIAM R. HAMILTON,Royal Astronomer of Ireland, &c.
"The Memoirs on Systems of Rays, which have been presented by me to the Royal Irish Academy, and of which some have been published in the XVth, and XVIth volumes of the Transactions of that Academy, contain a view of mathematical optics, which appears to me to be analogous to the view taken by Descartes of algebraical geometry, and likely to lead those who shall adopt it to analogous changes of method. It has been thought desirable, by the Mathematical Committee of the British Association for the Advancement of Science, that a short statement of this view of optics should be given in the forthcoming publication of that body. Such a statement, therefore, I shall now offer, as briefly as I can; endeavouring only to communicate the view itself, and abstaining from giving any account of the results to which it has conducted me.
2 M
[page] 546
The general problem that I have proposed to myself in optics, is to investigate the mathematical consequences of the law of least action: a general law of vision, in which are included, as it is well known, all the particular conditions of reflexion and refraction, gradual and sudden, ordinary and extraordinary. And the central idea from which my whole method flows, is the idea of one radical or characteristic relation for each optical system of rays, that is, for each combination of straight, or bent, or curved paths, along which light is supposed to be propagated according to the law of least action. This characteristic relation, being different for different systems, and being such that the mathematical properties of the system can all be deduced from it, in the same manner as the method invented by Descartes for the algebraical solution of geometrical problems, flows all from the central idea of one radical relation, for each plane curve, or curved surface, in the form of which relation are included all the properties of the curve or the surface. In the radical relation thus contemplated by Descartes, in his view of algebraical geometry, the related things are elements of position of a variable point which has for locus a curve or a surface; and the number of these related elements is either two or three. In the relation contemplated by me, in my view of algebraical optics, the related things are, in general, in number, eight: of which, six are elements of position of two variable points of space, considered as visually connected; the seventh is an index of colour; and the eighth, which I call the CHARACTERISTIC FUNCTION,—because I find, that in the manner of its dependence on the seven foregoing are involved all the properties of the system,—is the action between the two variable points; the word action being used here, in the same sense as in that known law of vision which has been already mentioned. I have assigned, for the variation of this characteristic function, corresponding to any infinitesimal variations in the positions on which it depends, a fundamental formula; and I consider as reducible to the study of this one characteristic function, by means of this one fundamental formula, all the problems of mathematical optics, respecting all imaginable combinations of mirrors, lenses, crystals, and atmospheres. And though, among these problems of mathematical optics, it is not here intended to include investigations respecting the phœnomena of interference, yet it is easy to perceive, from the nature of the quantity which I have called the characteristic function, and which in the hypothesis of undulations is the time of propagation of light from one variable point to another, that the study of this function must be useful in such investigations also. My own researches, however, have been hitherto chiefly directed to the consequences of the law of least action, and to the properties of optical systems, and systems of rays in general. And having stated, in the foregoing remarks, the view that has guided these researches, I must refer, for the results, to the volumes already mentioned, of the Royal Irish Academy, and to the XVIIth volume, not yet published, in which a third supplement to my Essay on the Theory of Systems of Rays has been ordered by the Academy to be printed."
[page] 547
An Abstract of the Solution of the principal Questions which are treated in Fourier's " Théorie de la Chaleur," by an analysis different from that which that author employs, and founded on the Theory of Equations,— was communicated by ROBERT MURPHY, of Caius College, Cambridge.
The principal object of Mr. Murphy was to point out the source of the want of generality in Fourier's two methods of solution.
2. OPTICS.
On the Colours of Natural Bodies. By Sir DAVID BREWSTER, K.H. LL.D. F.R.S. V.P.R.S.E.
THE object of this paper was to submit to a rigorous examination Sir Isaac Newton's theory of the colours of natural bodies. This theory is contained in the two following propositions.
1. "Every body reflects the rays of its own colour more copiously than the rest, and from their excess or predominance in the reflected light has its colour.
2. "The transparent parts of bodies, according to their several sizes, reflect rays of one colour and transmit those of another, on the same ground that their plates or bubbles do reflect or transmit those rays."
In estimating the truth of this theory, the author does not enter into any examination of the postulates, facts, and reasonings on which it is founded; but he proceeds at once to analyse one leading phænomenon of colour, and he then applies this analysis as an experimentum crucis in determining the origin of all colours similarly produced.
The colour chosen for this purpose was the green colour of the vegetable world, and this selection was made for the following reasons:
1. Because the green colour of plants is the one most prevalent in nature;
2. Because it is the colour of which Sir Isaac Newton has most distinctly described the nature and composition;
3. Because its true composition is almost identically the same in all the variety of plants in which it appears.
After determining the exact composition of this colour, the author concludes that the green colour of plants, whether it is examined in its original verdure, or in its decaying tints, has no relation whatever to the colours of thin plates.
To the same mode of analysis the author submitted nearly 150 coloured media, consisting of fluids extracted from the petals, the leaves, the seeds, and the rind of plants; of the different substances used in dyeing; of coloured glosses of minerals; of coloured artificial salts; and of different coloured gases: and in all these cases he obtained results which prove that their colours are not those of thin plates.
2 M 2
[page] 548
From the experiments detailed in this paper, the author concludes that the second and leading proposition of Newton's theory of colours is incompatible with the phænomena; and he infers the incorrectness of the first proposition by stating the fact, that he has found red, yellow, green, and blue media, which are absolutely incapable of reflecting or transmitting certain definite rays of the same colour with themselves.
The paper was concluded with a brief statement of what the author regards as the true theory of the colours of natural bodies. When light enters any body, and is either reflected or transmitted to the eye, a certain portion of it, of various refrangibilities, is lost within the body, and the colour of the body, which evidently arises from part of the intermitted light, is that which is composed of all the rays which are not lost; or, which is the same thing, the colour of the body is that which, when combined with that of all the rays which are lost, composes the original light. Whether the lost rays are reflected or detained by a specific affinity for the material atoms of the body, has not been rigorously demonstrated. In some cases of opalescence they are either partly or wholly reflected; but the author considered it as almost capable of demonstration, that in all transparent bodies, and in that great variety of substances in which no reflected tints can be discovered, the lost rays are detained within the body by absorption.
On the Undulations excited in the Retina by the Action of luminous Points and Lines. By Sir DAVID BREWSTER, K.H. LL.D. F.R.S. V.P.R.S.E.
In this communication the author considers a variety of cases when light affects other parts of the retina than those on which it directly falls,—either by rendering them more or less sensible to light and particular colours, or by altering the tints which are visible there, or by the excitement of undulations in the retina from the illuminated part. The following are the results of Sir D. Brewster's experiments on the last of these phænomena, as exhibited by the action of luminous points and lines.
1. If we look through a narrow aperture, about the 1/10th of an inch wide, at a bright part of the sky, or at the flame of a candle, we shall observe the luminous ground covered with a great number of broken parallel lines alternately light and dark. These lines are always parallel to the narrow slit, and of course change their place as the slit is moved round before the eye. Through a number of parallel slits, such as between the teeth of a comb, the broken parallel lines are seen more distinctly; and if we give the comb a motion oblique to the direction of its teeth, the broken lines become more distinct, though less straight than before, and new black lines appear, lying in different directions, as if they were detached portions of a number of dark ramifications. All these phænomena are seen more distinctly when we look at homogeneous light. If we use two systems of narrow slits, and cross them
[page] 549
at different angles, we shall perceive two systems of broken lines crossing each other at the same angles; and if when the lines of the two systems are parallel we give one of them a rapid alternating motion perpendicular to the direction of its slits, the parallel broken fringes are seen with peculiar distinctness.
2. Phænomena analogous to those now described may be seen by looking at a number of parallel black lines drawn upon white paper, such as those which represent the sea in an engraved map, or by looking at the luminous intervals in a number of parallel wires seen against the sky. If the eye looks at any of these objects steadily and continuously, the black lines soon lose their straightness and their parallelism, and inclose luminous spaces somewhat like the links of a number of broken chains. When this change takes place, the eye which sees it experiences a good deal of uneasiness,—an effect which is communicated also to the eye which is shut. When this dazzling effect takes place, the luminous spaces between the broken lines become coloured, some with yellow and others with green and blue light.
The phænomena produced in these two experiments are obviously owing to rectilineal undulations propagated across the retina; and the interference and crossing of the undulations, by which the dark lines are broken into detached portions, and by which the colours are produced, arise from the unsteadiness of the head or the hand, which causes a want of parallelism in the successive undulations.
3. The action of small and bright points of light upon the retina produces phænomena of a very interesting kind. If we look at the sun through a small aperture at a great distance from the eye, or if we look at the diminutive image of the sun formed by a convex lens or a concave mirror, or seen in a convex surface, the light which falls upon the retina does not form a sharp and definite image of the luminous point, but it sends out in all directions an infinity of radiations, covering in some cases almost the whole retina. These radiations are extremely bright, and are accompanied in some cases by mottled colours of great variety and beauty. The bright point of light propagates around it circular undulations, which are broken and coloured by interference, and which, being in constant motion from the centre of the retina in all directions, occasion the radiations which have been mentioned.
4. If we look at the radiant image just described through a narrow aperture, a very singular effect is produced. A vortex of circular rays appears on each side of the radiant point, and the rays have a rapid whirling motion. The line joining the centres of the two vortices is always perpendicular to the narrow aperture. This remarkable configuration of the rays is evidently produced by the union of a system of parallel undulations with a system of circular ones, the intersections of the parallel fringes and the diverging radiations forming the circular rays, as in the case of ordinary caustics.
The preceding phænomena, continues the author, whatever be their true cause, clearly prove that light incident upon the retina exerts an
[page] 550
action on parts of it upon which it does not directly fall,and that the same action renders other parts of the retina insensible to the light which actually falls upon these parts.
Upon this principle the author explains the experiments of Mr. G. Smith of Fochabers, in which the same object appeared, under certain conditions of vision, of different colours to the different eyes, the colour observed by the one eye being complementary to that observed by the other. He also refers to the same general principle of undulations propagated across the retina, for an explanation of the remarkable experiment on the eye, first made known by Dr. Purkinje of Breslau.
In this experiment, if a candle be held before one eye, at about a foot distance, and in a direction deviating a little from the line of distinct vision,—that eye sees a general mass of reddish light around the candle, and in this light, as a ground, are seen the ramifying blood-vessels of the retina, the base of the optic nerve, and the foramen centrale. Sir D. Brewster states it to be the most prevalent opinion, that the light which surrounds the candle is reflected back upon the retina, either by the inner concave surface of the crystalline lens or of the cornea; and that the objects are, somehow or other, magnified by these concave surfaces. His own view of the subject is, that the light was propagated from the luminous image of the candle, and that though the retina, in contact with the blood-vessels, is sensible to direct light, it is insensible to propagated light, and therefore the blood-vessels must be delineated in obscure lines. As there is no retina across the foramen centrale, it will of course appear as a black spot; and, owing to the obtuse vision of the optic nerve, it will appear less luminous than the surrounding retina.
After the reading of Sir David Brewster's paper, MR. WHEATSTONE said, that having been the first person to introduce Purkinje's beautiful experiment into this country, and having repeated it a great number of times under a variety of forms, he would take the opportunity of stating a few particulars respecting it, which appeared not to be generally known.—The experiment succeeds best in a dark room, when, one eye being excluded from the light, the flame of a candle is placed by the side of the unshaded eye, but so as not to occupy any of the central part of the field of view. So long as the flame of the candle remains stationary, nothing further occurs than a diminution of the sensibility of the retina to light; but after the flame has been moved upwards and downwards, through a small space, for a length of time, varying with the susceptibility of the individual on whom the experiment is tried, the phænomenon presents itself. The blood-vessels of the retina, with all their ramifications, exactly as represented in the engravings of Sœmmerring, are distinctly seen, apparently projected on a plane before the eye, and greatly magnified. The image continues only while the flame is in motion; directly, or soon after, the flame becomes stationary, it dissolves into fragments and disappears.
Mr. Wheatstone dissented from the ingenious explanation of this ap-
[page] 551
pearance offered by Sir David Brewster, and also from that opinion stated to be the generally received one; and begged to repeat the solution he had published, and which he had not since been induced to relinquish. Mr. W. observed, that there was no difficulty in accounting for the image; it evidently was a shadow resulting from the obstruction of light by the blood-vessels spread over the retina; the real difficulty was to explain why this shadow is not always visible. To account for this, Mr. W. adduced several facts, which tended to prove that an object, either more or less luminous than the ground on which it is placed, when continuously presented to the same point of the retina, becomes invisible; and the rapidity of its disappearance is greater as the difference of luminous intensity between the object and the ground is less; but by continually shifting the place of the image of the object on the retina, or by making it act intermittently on the same point, the object may be rendered permanently visible. To apply this explanation to the phænomenon in question, Mr. W. observed, that whenever the flame of the candle changes its place the shadows of the vessels fall on different parts of the retina; which is evident from the motion of the figure while the eye remains still, which is always in a contrary direction to that of the flame. Hence the shadow, being thus made to change its place on the retina, remains, according to the law above stated, permanently visible; but instantly the flame is at rest, the shadow also becomes stationary, and consequently disappears.
Mr. Wheatstone then exhibited an instrument for showing an original variation of this experiment: it consisted of a circular plate of metal, about two inches in diameter, blackened at its outer side, and perforated at its centre with an aperture about as large as an ordinary gun-hole; to the inner face was fixed a similar plate of ground glass. On placing the aperture between the eye and the flame of a candle, and keeping the plate in motion, so as to displace continually the image of the aperture on the retina, the blood-vessels will be seen distributed as before, but will now appear brighter, and the spaces between the ramifications will be seen filled with innumerable minute vessels, anastomosing with each other in every direction, which were invisible in the former experiment. In the very centre of the field of vision there is a small circular space, in which no traces of these vessels appear. Mr. W. remarked, that the absence of these minute obstructions to light will probably account for the greater distinctness with which small objects are there seen, and also for the difference of colour observed by anatomists in that spot of the retina.
On the Effect of Compression and Dilatation upon the Retina. By Sir DAVID BREWSTER, K.H. LL.D. F.R.S. V.P.R.S.E.
In repeating many times the well known experiment, particularly described by Newton in the sixteenth query at the end of his Optics, of the production of light by gentle pressure upon the eye-ball, or a stroke upon the eye, Sir David Brewster saw reason to correct the
[page] 552
statement of Newton, that " the colours vanish in a second when the eye and the finger remain quiet," having found them to continue as long as the pressure is kept up. With respect also to the character of the light thus produced in the eye, the author's experience has only shown him black and white circles, with a general red tinge, arising from the light passing through the closed eye-lids, whereas Newton speaks of the colours as like those in the feather of a peacock's tail.
The author states,—When a gentle pressure is first applied, so as to compress slightly the fine pulpy substance of the retina, a circular spot of colourless light is produced, though the eye be in total darkness, and have not been exposed to light for many hours. If light be now admitted to the eye, the compressed part of the retina is found to be more sensible to the light than any other part, and consequently appears more luminous. Hence it follows, that a slight compression of the retina increases its sensibility to the light which falls upon it, and creates a sensation of light when the eye is in absolute darkness.
If we now increase the pressure, the circular spot of light gradually becomes darker, and at last black, and is surrounded with a bright ring of light. By augmenting the pressure still more, a luminous spot appears in the middle of the central dark one, and another luminous spot diametrically opposite, and beneath the point of pressure. Considering the eye as an elastic sphere, filled with incompressible fluids, it is obvious that a ring of fluids will rise round the point depressed by the finger, and that its pressure from within outwards will dilate the part of the retina under the finger which was formerly compressed, and will compress all that part of the retina in contact with the elevated ring; An increase of pressure will be resisted by the opposite part of the retina, and will thus produce a compression at both extremities of the axis of pressure, occasioning the diametrically opposite spot of light, and also the luminous spot in the middle of the circular black space. Hence the author concludes, that when the retina is dilated under exposure to light, it becomes absolutely blind or insensible to all luminous impressions.
These properties of the retina often exhibit themselves involuntarily, with different variations, according to the state of sensibility of the retina, in consequence of the movement of the eye-ball by its own muscles during the act of sneezing, and on other occasions.
The phænomena above described are those produced in the parts of the retina which are most affected by any given pressure: but it is obvious that this pressure is propagated over the whole retina; and even when it is too weak to produce a luminous impression, it may yet modify other impressions previously produced on the retina. If, from looking at the sun, the eye sees a pinkish brown spectrum, a pressure upon another part of the retina will change it to a green spectrum, which, when the pressure is removed, will again become brown. If the pressure is such as to diminish the sensibility of the retina, it will either diminish or entirely remove a weak spectral impression.
When the eye is pressed in front, by putting the finger on the eyelid
[page] 553
above the cornea, no luminous spectrum is seen, and the author did not venture to increase this pressure so as to produce an impression on the back of the eye. He however mentions a case where this effect was produced accidentally. A person, in a state of intense grief, had been sitting for some time with his hand pressed against his eye;—the moment his hand was removed, and the eye opened, a black spot, the size of a sixpence, was seen in the axis of vision. The pressure of the blood-vessels upon the retina, in particular states of indisposition, occasion floating masses of light, visible in the dark, at first faint blue, then green, then yellow, and sometimes even red, all these colours being occasionally seen at the edge of the luminous mass.
The preceding observations on the influence of dilatation in making the retina insensible to light, render it extremely probable that the disease in that membrane, called amaurosis, may sometimes arise from a general distention of the eye-ball, arising from a superabundance of the fluids which it incloses. If this be the case, the removal of the pressure might be effected by puncturing the eye-ball, (when this can be done with safety,) and letting out a portion of the aqueous humour. How far such an operation would be effectual when the disease is of long standing, can be determined only by experiment.
On the Modification of the Interference of two Pencils of homogeneous Light, produced by causing them to pass through a prism of glass; and on the Phœnomena which then take place, with reference to the velocity of light in its passage through refracting substances. By RICHARD POTTER, Jun.
The principal part of this paper was occupied with mathematical investigations, relating to certain peculiarities which Mr. Potter detected, whilst repeating, in a different mode, an experiment first proposed by Professor Powell. The experiment consists in placing a prism of glass in the direction of two pencils of light, which produce bright and dark bands by interference, and then examining the light which has passed through the prism with an eye-lens. These pencils are most readily obtained by causing the rays which diverge from the image of the sun given by a lens of short focus, to fall upon two mirrors, very slightly inclined to each other. When the prism is placed in the direction of the interfering rays, the interference is not entirely prevented, but takes place between rays which have passed at a greater distance from the angle of the prism, than those which would have interfered if the prism had not been interposed. When the prism is small, or the overlapping of the pencils inconsiderable, there is a small distance from the prism, beyond which all appearance of interference ceases, on account that, the further from the prism is the position where the pencils are viewed with the lens, the further from the angle of the prism do the rays pass which interfere, until at last, from the causes mentioned, the effect ceases to take place.
By altering the incidence on the prism, the breadth of the bands is
[page] 554
also affected: from the angle of minimum deviation, towards a perpendicular incidence on the first surface, the bands become narrower and narrower, and on the opposite side of the same angle they become in the same manner broader.
To find the central points of interference, according to any particular hypothesis on the velocity with which the light has traversed the prism, requires the previous consideration of three distinct questions, namely, first,—the positions of the secondary images of the original luminous point, or the centres of divergence of the rays after the two refractions: secondly,—the simultaneous positions of the luminiferous surfaces: and thirdly,—the figures of the curves of the principal section of these surfaces, the plane of this section being common to both pencils.
Having determined these questions on the supposition, first, that the velocity of light, in passing through transparent media, is inversely as their refractive indices, and then, that it is directly as their refractive indices, and having afterwards calculated the points where interference should have taken place according to each hypothesis, Mr. Potter inferred that neither one nor the other would account for the phænomena which he had observed. He concluded also that it is necessary to consider light as moving through the prism with even less velocity than the reciprocal of the refractive index of the glass, to account for the actual phænomena.
On an Instrument for Photometry by comparison, and on some Applications of it to optical Phœnomena. By RICHARD POTTER, Jun.
When engaged in examining the phænomena of the colours of thin plates, in the form of what are generally denominated Newton's rings, Mr. Potter was surprised to find the rings appear so distinctly in the transmitted light, and particularly when homogeneous light was used. If, as is now generally allowed, these rings are produced by the interference of the light which has been twice reflected, at an incidence nearly perpendicular on the glass, the difference of the intensities of the dark and bright rings is much greater than can be accounted for on any mechanical theories of the intensity of light in interference. For if, as it is found to be very nearly by experiment, the reflection at a surface of common glass is about 1/30th, two reflections would give an intensity of about 1/900th; and the light causing interference would be only 1/900th part of the whole transmitted light,—a quantity of which the presence or absence is, in common circumstances, far beyond the limits of detection by the eye. Hence he inferred the importance of determining the relative intensities of the light in the dark and bright rings, and invented this photometer for the purpose. It consists of a rectangular piece of pasteboard, about 23¾ inches long and about 3 3/8 inches broad, set edgewise, in the form of a semicircle, on a rectangular piece of wood; in the centre is a pivot, round which turn two arms, carrying pieces of plane crown glass, blackened at their further surfaces. These pieces of glass are so mounted, as when they are moved with the arms round
[page] 555
the pivot, to remain, like the pasteboard, perpendicular to the plane of the rectangular board. When the pasteboard is equally illuminated in every part, as on a misty or uniformly overclouded day, the intensities of the reflections of its surface seen in the glasses, depend only on their inclinations to the directions of the visual rays. A fixed position for the eye being given, and a quadrant round the pivot graduated, these inclinations are easily found, and the intensities of the reflections are then to be calculated by the formula which Mr. Potter discovered from photometrical measurements, and published in the Edinburgh Journal of Science.
The apparatus producing the rings with homogeneous light being conveniently attached to the photometer, he compared them with two narrow stripes on the glasses, and found that when a green light, nearly homogeneous, produced by a solution of arsenite of copper in diluted muriatic acid, was employed, the rings were represented in the photometer, when the intensity of the light in the glass representing the dark ring, was to the intensity in that representing the bright one, as 1 to about 2·48. With a very pure red light, produced by a solution of iodine in hydriodic acid, the difference was much greater, and the intensity of the light in the dark ring to that in the bright ring was found to be nearly as 1· to 3·5. The pasteboard surface was brought as nearly to the same colours as possible, to enable the eye to judge with greater accuracy.
Dr. Young and Sir John Herschel have each given formulae for the difference of the intensities according to the undulatory theory, which, excepting that the latter has used certain approximations, coincide.
From the formula of Sir J. Herschel, Mr. Potter finds the ratio of the intensities for pure homogeneous light should, if that theory were correct, be as 1· to 1·1538, a ratio widely different from 1 to 3·5, as he found it to be nearly in fact.
Finding his photometer applicable to comparing the reflections of crown glass with that of other substances, he examined by it the reflective powers of diamond, mica, rock crystal, selenite, Iceland spar, emerald, and amethyst. Diamond he found to reflect at 10° incidence, about 9·4 of every 100 rays incident. By the formula according to the undulatory theory, namely, (μ′-μ/μ′+μ)2, 18·36 rays of every 100 should have been reflected at a perpendicular incidence, in lieu of about half that number which are in reality reflected.
With the other substances, the reflections by mica, rock crystal, Iceland spar, emerald, and amethyst, were rather higher than that of crown glass; the reflection by a recent surface of selenite was so nearly that of crown glass, that he found it impossible to state whether it was in reality higher or lower.
[page] 556
3. ACOUSTICS.
MR. WHEATSTONE exhibited an experimental proof, which he had devised, of the following result of Bernouilli's theory of wind instruments:— that in the fundamental sound of a tube, open at both ends, the portions of air on opposite sides of the centre of the tube move in contrary directions to each other.
It consisted of a leaden tube, about an inch in diameter and thirteen inches long, bent nearly into a circle, so that its two ends were near, and opposite to, each other. Between these ends was held a vibrating part of a square plate of glass, put into vibration either with a violin-bow or a hammer, so as to produce its lowest sound, corresponding with Chladni's first figure. By this arrangement, the plate, advancing in its vibration towards one end of the tube, and receding at the same instant from the other, the effects neutralize each other, and no resonance or augmentation of the original sound takes place. In the middle of the tube was a joint, which allowed each half to move independently round the axis of the tube; by this means the two ends were capable of being brought to the opposite sides of portions of the plate vibrating at the same moment on contrary sides of the neutral plane; in this case, the impulses were made at the same instant towards both ends of the tube, and the augmentation of sound was very considerable. It is obvious that these effects would be reversed were Bernouilli's theory wrong.
Mr. Wheatstone gave an abstract of his Researches on the acoustical figures of vibrating surfaces. In the first part of this inquiry the author confined himself to the consideration of the figures of square surfaces only, and after stating the general results of Chladni's experiments, proceeded to show that all the figures, however complicated in appearance, were the resultants of simpler modes of vibration, exactly similar to, and superposed upon, each other. The nodal lines of the elementary modes of vibration he showed might be either parallel to a side, to a diagonal, or to any other intermediate direction: in the two first cases, the plate admits but of two coexisting similar modes of vibration, but in all other cases of four. He described various processes for combining the component figures and ascertaining their resultants, and exhibited an extensive general table of perfect resultant figures, calculated according to the rules he had discovered. He then proceeded to show the application of these laws to the figures of other rectangular plates, and also to those of plates formed of substances possessing unequal elasticities in different directions. He concluded by showing the possibility of constructing an optical instrument which should exhibit exact resemblances of all the component and resultant figures of vibrating surfaces, and, at the same time, serve as a calculating machine for these phenomena, by performing all the requisite synthetical operations.
[page] 557
On the. Velocity of Sound. By JOHN HERAPATH.
The author, referring to the methods employed by Newton and others for determining, from theory alone, the velocity of sound, took occasion to compare the fundamental postulate usually employed in this investigation, viz. the limited mutual repulsion of aeriform particles, with his own views of the constitution of gaseous bodies, formerly published at length in the Annals of Philosophy for 1821; according to which, the mechanical properties of aeriform bodies depend upon the internal motions of their hard particles. In the present communication, the author, upon the supposition of a particular system of internal motions, deduced formulae for the calculation of the velocity of sound, the results of which, at different temperatures, he compared with the best experiments on the subject in common air. Passing, then, to the consideration of the intensities of sound in different airs, he deduced the relation between these intensities and the corresponding velocities, and compared the results of his hypothesis with the tones produced by various gases, as given by M. Dulong,Annales de Chimie, t. xli. p. 150.
4. MAGNETISM.—ELECTRICITY.
Experiments on the Intensity of Terrestrial Magnetism, at Liverpool and Manchester, with Hansteen's Needles. By W. S. TRAILL, M.D.&c. Performed at the request of the Association.
THE needles employed by Dr. Traill were furnished to him, at the request of the Association, by the Royal Society of Edinburgh. The following are the results.
Botanic Garden, Liverpool.
Experiments with the cylindrical needle, No. 1, corrected for temperature, &c.
| ″ | ||
| March 27, | 1832;—300 vibrations in | 797·70 |
| April 2, | 797·64 | |
| — 19, | 797·76 |
Experiments with the flat needle.
| March 27, | 1832;—300 vibrations in | 1054·95 |
| April 2, | 1052·50 | |
| — 19, | 1051·69 |
Cornbrook, Manchester.
| Cylindrical needle, No. 1. | ″ |
| April 18, 1832;—300 vibrations in | 798·78 |
| Flat needle. | |
| April 18, 1832;—300 vibrations in | 1051·71 |
[page] 558
On the Method of employing vibrating Magnets in the Investigation of the Magnetic Intensity of the Earth. By WILLIAM SNOW HARRIS, F.R.S. &c.
The many irregularities to which the magnetic pendulum is subject, has rendered the method of investigating terrestrial magnetism by the oscillations of a freely suspended magnet, though perfect in theory, liable to much uncertainty in practice. In this paper, the author has entered upon some of the most important of the causes of these irregularities, and has endeavoured to apply practically some general principles, in experimenting with vibrating magnets, by means of which more perfect results may be arrived at.
The leading points treated of in this paper are as follow:
1. The advantages of observing the oscillations of the magnetic pendulum in a rare medium, with a description of a recently constructed apparatus for that purpose.
2. The means of detecting changes in the force of magnetic bars.
3. The influence of temperature on the state of vibration of a bar, either in altering its magnetic conditions, or changing its angular inertia.
4. The possibility of rendering the magnetic state of bars invariable for certain temperatures.
5. The influence of some mechanical conditions incident to the mode of suspension of a bar on its rate of vibration.
6. The influence of the sun's rays, of artificial light, and of free electricity pervading a vacuum, on the state of oscillation.
The author observes, that although the mere presence of a resisting medium is not prejudicial to the rate of vibration, supposing every attendant circumstance to remain the same; yet, from a variety of changes to which such a medium is liable, it becomes indirectly a great source of inconvenience and error. From an extensive series of experiments with vibrating needles, in air and in a rare medium, he is led to conclude, that we cannot depend upon observations embracing extremely minute differences in the times of vibration, at different places, until our instruments of research have become more refined; and that it is essential, in very delicate inquiries, to take the vibrations within very small arcs,—the least possible, so that they be consistent with accuracy of observation. With a bar of about 5 inches long, 1/2th of an inch thick, and about 3/10ths of an inch wide, vibrating in an exhausted receiver, from 200 to 300 vibrations may be obtained, before the arc of vibration becomes reduced from 5° to 3°. The author thinks that the mean of 100 vibrations, determined in the way recommended by Prof. Hansteen, and taken within an arc of 5°, is more to be depended on than a much greater number, taken in larger arcs.
The following is a brief account of the apparatus employed by Mr. Harris for carrying on experiments, with vibrating magnets, in an exhausted receiver.
There is a firm base, somewhat resembling in form a sector of a circle,
[page] 559
sustained on levelling-screws; the diameter of this sector is placed as nearly as possible in the direction of the magnetic meridian: this base supports a parallelogram of wood, on which are fixed the magnetic apparatus and air-pump; the whole of the latter part is moveable, for a short distance, upon a pin concentric with the centre of the sector, and with the suspended bar and cord: by this is obtained the final adjustment, requisite to place the zero of the cord exactly in the meridian.
The magnetic bar is suspended in a light vertical frame of wood, supported on a circular block, which last carries a graduated circle of stout card-board. The block is fixed, by means of a nut and screw, to a circular plate of fine-grained slate, having an air-tight communication, by a horizontal pipe, with the air-pump barrels; this plate of slate is found to answer admirably as a pump-plate,—thus avoiding a large mass of metal in the vicinity of the bar.
The centre of the wood block is hollowed to about three inches of its diameter, in order to admit of the operation of a sort of forked lever, moveable through the centre of the pump-plate, in an air-light collar; by means of this, the bar can be deflected and set free at any given point on the graduated cord. There is a gauge, of an extremely simple kind, for indicating the degree of exhaustion, and a thermometer, both attached to the wooden frame carrying the suspended bar, and the whole is inclosed in appropriate receivers. The bar is suspended in the usual way, by one or two slight filaments of silk, about 15 inches in length, which is attached to a vertical and sliding rod of brass, so as to admit of being raised or depressed, through a small distance, to any required point.
When the exhaustion is complete, the air-pump barrels may be taken away, if required.
The whole of the above-mentioned apparatus may be taken in pieces, for the convenience of carriage, without difficulty.
The possibility of change in the magnetic state of a bar, has necessarily occupied the attention of those who have at various times been engaged in this department of science,—the identity of the magnetic pendulum not being preserved, if its magnetism should vary either in intensity or distribution: it has hence been proposed, as a means of discovering whether any change has taken place in the magnetism of a bar, to return again to the first place of experiment, and observe whether the same rate of vibration takes place, as had been observed in a previous instance under the same circumstances. In the present state of magnetic inquiries, however, this method is by no means free from fallacy, since we do not know of what change a magnetic bar may be susceptible, in consequence of a change of place on the earth's surface; nor are we quite certain, that the directive force of the earth is, in the same place, an invariable quantity for all periods: it seems, therefore, desirable to obtain some method of detecting changes in the magnetism of a bar, which may be available at any time and in any place; as also a method by which its magnetic state may
[page] 560
be rendered invariable, at least as far as the operation of ordinary causes of disturbance interfere.
The author proposes, as a delicate test of change in the magnetism of a bar, the oscillations of a ring of copper about its poles, the bar being fixed in the direction of a diameter of the ring, with its ends at about the 1/10th of an inch distance from the inner surface of the ring. For this purpose, a light ring of copper, about half an inch in depth, and 1/2th of an inch in thickness, is suspended over a graduated cord by two parallel threads, without torsion; the threads being fixed to a cross bar, which is a diameter of the ring, and at about 1/2th of an inch on each side of the centre. The ring is furnished with index points, and is to be suspended in the frame-work of wood of the apparatus above mentioned; the cross-bar is turned to any required angle, from the direction of the parallelism of the threads, in which the ring remains at rest by the same means as are employed to deflect the needle, and again set free; in which case, it will continue to vibrate until it again comes to rest in the direction of the parallel threads. If the number of vibrations made by the ring alone in this way, between any given limits on the graduated cord, be divided by the number made within the same limits, when allowed to vibrate about the bar, then the quotient minus 1, may be taken as a comparative numerical value of the force of the bar, and will be a constant quantity, whatever may be the limits within which the vibrations are taken.
Of the ordinary causes of change in the magnetism of a bar, perhaps an increase of temperature may be considered as one of the most important. The effect, however, seems to be different for different bars, and also to vary with their magnetic intensity; so that in certain bars, and under certain temperatures, the magnetic state may remain unchanged. The results of very many experiments on the influence of heat on magnets, has led the author to conclude, that a bar formed of the best double steel of commerce, well hammered and tempered, may, after being rendered magnetic, obtain an invariable magnetic intensity, for all temperatures under 200° of Fahrenheit; by boiling it for a short time in water, at 212°; or otherwise, by touching the bar, after being prepared as before, whilst its temperature is raised to 250 or 260 degrees.
A decrease of temperature will not restore any of the power which heat has destroyed. Thus a bar, after being magnetized to saturation, was tested by the rate of vibration, and subsequently exposed to the heat of boiling water, when it was again tested in the same way, so as to ascertain the amount of the force it had lost by this process. On being afterwards immersed for an hour in a freezing mixture, which reduced its temperature to 0 of Fahrenheit, it was again vibrated as before, the previous temperature of the bar being first restored; but no increase of power was apparent; rather, on the contrary, the bar appeared to have lost somewhat more of its power, but in a very slight degree. It would seem, therefore, that corrections for the effects of heat on the magnetism of a bar, are limited to such elevations of tem-
[page] 561
perature only as happen to be above that to which it has been already exposed.
The author observes, that the effects of heat on the magnetic distribution of a bar, are very precarious and uncertain, since the magnetic disturbance tends more readily to a state of neutrality in some bars than in others: it is hence requisite to examine the influence of heat experimentally, on each individual bar, previously to its being employed for investigating terrestrial magnetism.
Besides the influence of change of temperature on the magnetic tension, we have likewise to consider the change induced in the bar itself as a pendulum, by which its angular inertia is increased or decreased. A change of 20 or 30 degrees of temperature will cause very sensible differences in this way; for which a correction is requisite, adapted to each particular magnet.
In all experiments with the horizontal needle, it is evident that we do not measure the whole magnetic intensity of the place of experiment; we obtain only one of its resolved portions, except the place be exactly on the magnetic equator: hence it is desirable to preserve the horizontal position perfect. The following ready method is resorted to by the author, for determining, at any time, whether the bar be accurately suspended in an horizontal direction.—Let the bar be held by a fine filament of suspension silk near the surface of a reflecting fluid, such as mercury, or, what answers equally well, near the surface of water which has a little indigo dissolved in it; an image of the bar will, of course, be seen by reflection—when the lines of the bar and its image are parallel, the bar is accurately balanced in the horizontal position; the parallelism of the lines is readily detected by the eye.
The author here details the method employed by him for suspending the bar, and preserving the horizontal position, without changing the angular inertia of the mass.
The bar is suspended by means of a fine loop of silk, fixed in a very small hole, drilled through a piece of brass immediately in the centre; so that the axis of suspension may at all times pass through the centre of gravity of the mass, and the point of magnetic neutrality Previously to the bar being magnetized, it is carefully balanced horizontally, after which two points are impressed on it, on each side of the centre, at midway between the centre and ends. Two small sliders of platinum, of equal weight, are then carefully fitted on the bar, so as to be easily moved on it; these bear a very small proportion to the Weight of the whole mass, and are at first placed with their centres immediately over the above-mentioned points: when the bar is rendered magnetic, the inclination is corrected for any place, by moving one of the sliders from the centre, and the other toward it, each by an equal quantity. The distance through which it is requisite to move the sliders, in order to correct the inclination, may at all times be extremely small: hence, we may consider the angular inertia of the mass as being nearly the same, since the errors, which would otherwise arise from this cause, are made to neutralize each other, or very nearly so. To obtain
2 N
[page] 562
exactly the same angular inertia, we have only to determine a series of points for the slider, on each side, the sums of the squares of the distances of corresponding pairs of which, from the centre, taking one on each side, give a constant quantity. But for all practical purposes, the correction of the inclination, by the more simple method, appears to be sufficient.
For setting the sliders with accuracy, it is requisite to measure off the distances by means of a finely graduated scale, and a fine pair of compasses.
The author has found, that a small difference in the flexibility and length of the suspension silk, frequently gives rise to small differences in the rate of vibration; and therefore recommends that the silk for suspension be carefully tested by experiment, and be previously prepared, both as to length and size; so as to obtain a series of suspension silks, which may be considered as being identical in effect on the bar.
Mr. Christie's very interesting experiments " On the Influence of the Sun's Rays on the Magnetic Needle," (detailed in the Transactions of the Royal Society for the year 1826,) are of great consequence to researches with the magnetic pendulum. If a magnet be allowed to vibrate in air in a closed receiver, first in the shade, and subsequently when exposed to the rays of the sun, the time in which the arc of vibration becomes reduced to a given point, will, in the sunshine, be considerably less, whilst the time in which a given number of vibrations is performed, is slightly diminished.
The author endeavours to discover, whether this effect arises from any pure magnetic quality in the sun's rays, or from some mechanical influence operating on the bar or medium in which it moves.
In order to avoid the very great inconveniences and inaccuracy to which the experimentalist is necessarily exposed under the influence of a burning sunshine, the author was led to employ only a portion of the sun's rays, thrown by plane mirrors upon the needle whilst under a closed receiver; the experiment, conducted in this way, became very manageable and satisfactory. The following are the general results which he states that he has arrived at.
1. The influence of the sun's rays on a magnet, oscillating in air, is to reduce more rapidly the arc, and at the same time to diminish the time of a given number of vibrations.
2. The influence of the sun's rays on a magnet, oscillating in void, is to increase the time of a given number of vibrations, whilst the arc remains very nearly the same: if the retardation of the rate of vibration be small, the arc may be considered as being wholly unchanged; at least the difference is so little, as not to be worthy of notice.
The rays of bright sunshine, having been thrown at first upon the bar in a direction from south to north, they were subsequently thrown upon the bar in the reverse direction, from north to south, but without any difference in the result: hence, it does not appear that the direction in which the rays fall on the needle, in respect of the magnetic meridian, is of any consequence.
[page] 563
Whatever may be the value of the above results, when taken as evidence against the opinion that the sun's rays possess any magnetic influence, yet the author considers that they are fully competent to elucidate the phænomena which ensue in observing the oscillations of a magnetic bar, in the shade and in the sunshine.
Thus, the more rapid diminution of the arc of vibration in the sun's rays in air, is dependent on the change effected in the elasticity of the air surrounding the bar; since the arc is not similarly affected, in a strictly comparative case, when the air is greatly rarefied.
The increase of the rate of vibration in air, in the sunshine, necessarily ensues in consequence of the great differences in the arcs of vibration, which, in the sunshine, become more rapidly diminished; this effect will be more or less prominent, in proportion to its exceeding or falling short of the effects produced by other causes, tending to an opposite result.
The slight decrease in the rate of vibration, in a rare medium, is referable to the effect of expansion on the bar itself, by which its length, as a pendulum, becomes changed; and which effect, being no longer masked by the differences in the arc of vibration, as in the former case, becomes now apparent.
For the purpose of inquiring more minutely into the accuracy of the foregoing deductions, the following experiments were instituted.
1. A copper bar, of the same dimensions as the magnetic bar, was set vibrating by means of a suspension with two parallel threads, as explained in the early part of the paper; and the times and arcs of vibration noted as before, both in air and in an exhausted receiver; the results were precisely the same, except that the slight retardation in the rarefied medium was not very appreciable. In this case, there was no magnetism proper to the bar; hence these effects are not exclusively dependent on that agency.
2. The oscillations of the magnetic bar, employed in the previous experiments, were observed for 25 minutes, during a free exposure to the intense light evolved by lime, under the action of the hydro-oxygen blowpipe; but no differences were observed in the time of vibration on the arc, previously determined in the shade. We may hence infer, that mere light has but little influence on the oscillations of a magnetic needle.
The author arrives at the same conclusion in respect of free electricity pervading a vacuum, which, flashing brilliantly through an exhausted receiver, 6 feet high and 4 inches in diameter, during the space of 25 minutes, and within a foot of the needle, did not in any way affect its rate or arc of vibration, whilst the needle oscillated in vacuo.
The author is impressed with the conviction, that many of the uncertainties which at present embarrass the observer in taking magnetic intensities, may be fairly traced to some of the above-mentioned causes; and expresses a hope that when the object of taking these intensities is considered—no less a one than the accurate measurement of the earth's
2 N 2
[page] 564
magnetism—the few attempts which he has thus made to improve our methods of research, will be of advantage to this important department of science.
An Account of some extraordinary effects of Lightning on the Packet Ship, New York. By the Rev. W. SCORESBY, F. R. S.
The circumstance to which this paper refers, occurred on the passage of the New York packet from America to Liverpool in the year 1827. Soon after the commencement of the voyage, this vessel encountered a severe thunder-storm, and received a stroke of lightning which shattered the masts in several parts, and started some of the exterior planks of the bends. This was in the morning before daytight. The weather continuing unsettled, and the air in a highly electric state, with water-spouts in various directions around—the captain, fearing another explosion from the highly charged atmosphere, put up a lightning conductor which he had on board. In the afternoon of the same day, the ship was a second time struck, but preserved by the conductor, though the iron of which it was composed was destroyed, and fell in melted globules upon the deck. No lives were lost, though some of the crew received heavy shocks; whilst one person, an invalid passenger, derived essential benefit from the electric discharge. Mr. Scoresby had an opportunity of examining the vessel immediately on her arrival in Liverpool, when, on investigating the condition of the iron on board, he found almost every article capable of permanent magnetism, with sensible polarity. Table knives and forks were capable of lifting needles or small nails, and one knife sustained a travelling-trunk key. Most of the watches on board suffered by the magnetic influence, especially those which were under the pillows of their owners in bed. These were all stopped, and on examination were found so highly magnetic, that portions of the steel-work were capable of suspension by each other, in a chain of three or four pieces. Of one of these pieces (the cap-spring) Mr. Scoresby made a pocket-compass, which was exhibited when his communication to the Association was made, and was observed to be, in all respects, a delicate and perfect instrument.
In the discussion which followed the reading of this paper, Mr. SNOW HARRIS stated the following facts to illustrate the effect of Lightning Conductors.
In January 1814, H.M. ship Melford was struck, in Hamoaze at Plymouth, by lightning, which rent the mast. This ship had not a lightning conductor up at the time, but several other ships close by had lightning conductors;—these were not assailed by the electric explosion. There was likewise a powder magazine not above a quarter of a mile distant, armed with pointed iron rods;—this also escaped.
H.M. ship Norge was severely damaged by lightning, in June 1815, in Port Royal Harbour, Jamaica. Several other ships were near which had lightning conductors; but the Norge, with the exception of a
[page] 565
merchant-ship, was the only one struck and damaged; and it is not a little remarkable, that the merchant-vessel and the Norge were the only ships not having lightning conductors.
In the case of the Heckingham poor-house damaged by lightning, an account of which may be seen in the Philosophical Transactions, the electric matter fell on the building, at a point furthest removed from the conductors with which the building was furnished.
In the 14th volume of the Philosophical Transactions an instance is given in which a long building was struck at one end, a lightning conductor being fixed at the other.
Mr. Harris observed, that whenever we erect an artificial elevation on the earth's surface, we do in fact set up a lightning conductor, upon which the electricity of the atmosphere will certainly fall, when it happens to lie in the course of the discharge; and no human power can prevent it. Hence, if metallic bodies be prominent, those will be first assailed; if not, then the bodies next in conducting power. A curious illustration of this, out of many which might be given, is to be found in the Memoirs of the Count de Forbin, and noticed in the Philosophical Transactions. " In the night," says the author of these Memoirs, "it became extremely dark, and it thundered and lightened fearfully. As we were threatened with the ship being torn to pieces, I ordered the sails to be taken in; we saw upon different parts of the ship, above thirty St. Helmos fires; amongst the rest was one upon the top of the vane of the mainmast, more than a foot and half in height. I ordered one of the sailors to take the vane down; but scarcely had he taken the vane from its place, when the fire fixed itself upon the top of the mainmast, from which it was impossible to remove it.
Lecture on Electro-Magnetism. By W. STURGEON.
Mr. W. Sturgeon illustrated by experiments the progress of discovery in developing the magnetic energies of galvanic currents of electricity, and the various means which have been employed for exalting the powers of ferro-electro-magnets. He stated the reasons that had induced him, in 1824, to substitute soft iron for steel, which had been previously employed in these arrangements,—a substitution which at once increases the energy and varies the character of the phænomena; he also showed the method which he had adopted of exalting the power of the electro-magnet by bending the iron into the horse-shoe form. He alluded to the experiments of Professor Moll, who employed larger pieces of iron, one of which, with the aid of a powerful galvanic battery, supported 160lbs. The American philosophers made the next improvement, by greatly multiplying the number of coils of wire round a very large mass of iron, by means of which they were enabled to command a power which lifted 2000lbs. Mr. Sturgeon exhibited two electro-magnets, which he had himself constructed: one of these, the iron of which weighs 4 oz., furnished with 6 coils of copper wire, will support about 50lbs.; the other, of which the iron weighs 16 lbs., will
[page] 566
support about 400lbs. The latter is surrounded by 20 coils of copper wire, separated from each other by pieces of calico. The coiling of each wire proceeds from one of the poles to the other, without inter-ruption, so that all of them terminate at the poles. No electro-magnets, he said, have been made in this country possessing greater proportional powers; a cylindric galvanic pair of plates, which can be placed in a half-pint pot, is sufficiently powerful to excite the largest of Mr. Sturgeon's magnets to its maximum of polar force.
Mr. Barlow, he stated, has employed a globe of wood, covered with coils of wire, to illustrate terrestrial magnetism on electro-magnetic principles: he exhibited an iron sphere, fitted up for the same purpose, which shows a more powerful polarity; it is an 8-inch shell, surrounded between the tropics with 4 coils of wire, and turning on an horizontal axis at right angles to its polar axis.
His concluding observations related to the distribution of magnetic polarity in copper and other non-ferruginous metallic plates. After the discovery, by M. Arago, of the magnetical effects produced by the rotation of such plates, Mr. Sturgeon had diversified the experiment by giving the discs a vibratory in place of a rotatory motion, and had thus succeeded in developing phænomena which he considered distinct from those first shown by the experiments of Arago: he had observed the deflections of a magnetic needle, produced by the force excited in the discs when rotated between the poles of a horse-shoe magnet, and had investigated the distribution of that force.
5. CHEMISTRY.
Abstract of Observations on Atmospheric Air. By WM. PROUT, M.D.
[When this paper was read, different tables, containing the details of the observations, were exhibited, but which were not intended for publication as yet.]
THE observations in the present communication are chiefly confined to two points,—the absolute weight of atmospheric air; and the law of its expansion by heat.
1. Of the weight of dry atmospheric air at 32°. In determining the absolute weight of a gaseous body, there are three things to be especially considered and settled in the first place; viz. the barometer, the thermometer, and the weights and measures to be employed.
The barometer employed in the whole of the present experiments was made expressly for the purpose, with the greatest care. The internal diameter of the tube is ·575 inch, and it is guarded at bottom by platina, in the manner recommended by Mr. Daniell. The distance between the upper and lower surfaces of the mercury is determined by a brass rod, ending at the bottom in a fine point, and having the usual
[page] 567
scale affixed to the top. This rod is movable by a screw, so that the lower point can always be brought in contact with the surface of the mercury in the cistern, while the scale at the upper end of the rod marks, with the utmost precision, the exact distance between the upper and lower mercurial surfaces, without the necessity of further adjustment or calculation. The distances on the scale are set off from a standard formerly belonging to Mr, Cavendish, and which is presumed to be identical with the old national standard. This, however, has not yet been actually verified by experiment. There may, therefore, exist a slight constant error, affecting all the following results, from this cause, and also from another, pointed out in the memoir, and amounting to about + ·003 inch.
The standard thermometer referred to in these experiments belongs to Mr. Daniell, and is that described by Capt. Sabine, in his work on the Pendulum (page 183). The bore of the tube of this instrument has been repeatedly examined, and found to be uniform between 32° and 212°.
The weights employed are of platina, and adjusted with the greatest care by Mr. Robinson, from a Troy pound, expressly verified for the purpose by Capt. Kater with the national standard. The measures were determined from the weights in grains of water at 62°, of which 252,458 are presumed to be equal to a cubic inch. The balance employed was made by Robinson, and devoted expressly to the purpose. It is mounted with a counterpoise of glass, as nearly as possible of the same size and weight as the balloon in which the air is weighed, by which all errors from buoyancy, &c. are completely obviated.
The air to be weighed was first passed through lime water, into a large bell glass receiver, where it was permitted to remain for six or eight hours, with the view of separating the carbonic acid present. One portion of it was then introduced into a similar smaller apparatus filled with the strongest sulphuric acid, while another portion was conveyed into a similar apparatus filled with distilled water. With these two fluids the different portions of air were permitted to remain in contact for at least twelve hours, with the view in the one instance of separating the whole of the water present, and in the other of saturating it with that fluid. A known quantity of air in each state, as determined by a very simple gasometer, was then introduced into the weighing balloon, and its weight carefully determined, with all the necessary precautions. In weighing air at 32°, an apparatus on the same principle was employed, but so constructed, that the whole gasometer would be surrounded with ice for some hours before the air was weighed.
A table was exhibited containing the results of 87 experiments, conducted as above, between the 16th of December 1831, and the 24th of March 1832. The experiments were usually made about noon, and as nearly as possible under similar circumstances. The following is a summary of the results:
The mean of all the experiments (with one exception, to be presently noticed,) is, that one hundred cubic inches of dry atmospheric air, free
[page] 568
from carbonic acid, at the temperature of 32°, barometer 30 inches, in the latitude of London, weigh 32·7958 gr.; the extreme differences between the highest and the lowest observations being '0507 gr. The mean of the first forty-four experiments, between the 16th of December and the 8th of February inclusive, is 32·7900 grs.; the mean of the last forty-four, between the 10th of February and the 24th of March inclusive, 32·8018 grs.; the difference between the two series being ·0118 gr.
The exception alluded to above occurred on the 9th of February, on which day the weight of the air was 32·8218 grs.; and it is remarkable that after this period, during the whole time that the experiments were continued, the air almost uniformly possessed a weight above the usual standard; so that, as above stated, the mean of the 42 observations after this crisis, exceeds the mean of the 44 preceding it by no less than ·0118 gr. The apparatus employed, and the care taken, were the same throughout, and there can be no doubt that the difference, whatever it depended on, really existed, and did not arise from error of experiment. How the circumstance is to be explained, it is difficult to form a conjecture; but perhaps it may be worth while to observe, that almost precisely at the period above mentioned, the wind veered round to the north and east, where it continued for a considerable time, and that under these circumstances the epidemic cholera first made its appearance in London. It would seem, therefore, as if some heavy foreign body had been diffused through the lower regions of the atmosphere about this period, and which was, some how or other, connected with the disease in question. The action of this body is quite unknown; but it could have scarcely possessed acid or alkaline properties, as in the former instance it would have been separated by the lime water, and in the latter by the sulphuric acid. We may probably consider it as a variety of malaria; and what renders the conjecture the more likely, are its effects upon the animal œconomy, which are somewhat analogous to those known to be produced by certain varieties of this poison. Thus, during the present spring and summer, the saliva, and the exhalations from the skin, in almost every individual on whom the experiment was made, have been found to be unusually acid: the state of the urine also, and other secretions, has been most remarkable; and that in so great a number of individuals, as to prove the existence of some widely-acting cause, such as has not occurred in our time, or at least since the author of the present communication has turned his attention to the subject. Should the above conjectures prove to be well founded, they lead us to hope that the cause of the present formidable epidemic will not be permanent, but will pass gradually away; though, from the deep-seated and malignant influence which it has exerted in organic action, it is probable that several years will elapse before its effects will be entirely obliterated.
The weight of the air is observed to be very unsteady, and usually heavier about the new and the full moon. Whether this arises from aerial tides has not been satisfactorily determined. It may, however,
[page] 569
be proper to observe, that many of the minute differences in the weights of the air at different times are more apparent than real, and depend upon the sluggishness of the mercurial barometer, which prevents it from being an exact measure of the movements of the lighter and more mobile fluids.
Another table was exhibited, intended to show the effects of the direction of the wind upon the weight of the air. As these observations were made near the western extremity of the metropolis, they were considered as well adapted for illustrating the important question, whether the quantity of oxygen naturally existing in the atmosphere be sensibly diminished by the innumerable sources of consumption of this most important principle constantly going on around us. As before observed, the carbonic acid gas was separated in all instances; and as this of course would be formed at the expense of an equal volume of oxygen, the air would be necessarily found lighter, if the proportion of this was sensibly diminished. This diminution in weight, of course, if it happened at all, would happen in air from the east, which had travelled over the whole extent of the town; while air from the opposite point might be considered as coming directly from the country. With this view the experiments were arranged in such a way that all the results, obtained when the wind came from between the S.W. and N.W., and the S.E. and N.E.; and between the S.E. and S.W., and N.E. and N.W. inclusive, were classed together. The following are the general results:
| grs. | |||
| Mean of 47 | Observations | West | 32·7964 |
| 28 | East | 32·7944 | |
| 39 | South | 32·7941 | |
| 21 | North. | 32·7943 |
Hence,—
| gr. | |||
| Difference between | West and East | ·0020 | |
| South and North | ·0002 | ||
| West and South | ·0023 | West being + | |
| West and North | ·0021 |
These differences are too trifling, perhaps, to be much relied on either way; but they appear to show, what might indeed have been anticipated, that air from the West contains a very small fraction per cent. of oxygen more than air from the East, South, or North, which are nearly identical; and in air from these quarters, under ordinary circumstances, in London, the place of the oxygen is doubtless supplied by carbonic acid gas.
2. Of the Weight of Atmospheric Air at other Temperatures; and on the Law of its Expansion by Heat.
A diagram and tables were exhibited, showing the results of nearly a thousand experiments made upon dry and moist air, at different tem-
[page] 570
peratures between 32° and 72°. These experiments were commenced in May 1830, and continued daily till August 1831. The air on each day was generally introduced into the apparatus about 11 A. M., where it was permitted to remain, first in contact with lime-water, and afterwards with sulphuric acid and distilled water till the next morning between 7 and 9 A. M., when its weight in the dry and moist states was carefully determined. The results prove beyond a doubt that the mercurial and air thermometers do not go on pari passu between 32° and 212°, as at present generally supposed, but that there is a gradually increasing difference from 32° upwards, and amounting at 72° to upwards of 6/8ths of a degree, the mercurial thermometer being in advance; (that is to say, 62° on the mercurial scale coincides almost exactly with 611/2° on the air scale). How far this difference continues to increase, is not at present known, but there is reason to believe that it goes on to upwards of 100°, and afterwards gradually diminishes till of course at 212° it disappears altogether.
These results show what very little reliance can be placed on experiments hitherto made with gases, or rather on calculations deduced from them. Thus 100 cubic inches of dry atmospheric air at 60°, barom 30 inches, are found by experiment to weigh 31·0117 grs.; but if we wish to know from this datum what the same bulk of air will weigh at 32°, Gay-Lussac's formula will give us 32·8206 grs. instead of 32·7900 grs., being an error of no less than ·0306 gr. in weight, and nearly one tenth of a cubic inch in volume.
The experiments on moist air are expected to throw considerable light on the law of the tension of vapour; but for want of leisure principally they have not yet been completely investigated. - For similar reasons also, some observations on the specific gravities of hydrogen, oxygen, azote, and carbonic acid gases are deferred till a future opportunity. In conclusion, it may be remarked, that the fluctuating weight of atmospheric air renders it a very improper unit of comparison for gaseous bodies: quite as improper, for example, as sea water would be for fluids.
A letter was read from JAMES APJOHN, M.D., Prof. of Chemistry in the Royal College of Surgeons, Ireland, communicating A Formula by which a proper correction for Vapour may be applied to the specific Gravities obtained by Experiments on Gases saturated with Moisture.
In this letter, the writer expresses his opinion that it was with great propriety the Association had directed the attention of chemists to a reinvestigation of the relative densities of the principal elementary gases. He had, in consequence, projected a course of experiments on this subject, which, however, had been interrupted by more urgent duties. The method of operating by a comparison, in two successive experiments, of the weights of equal volumes of the gas, and of atmospherical air, is recommended by the circumstance of its being inde-
[page] 571
pendent of all knowledge of the volume of the gas or of the weight of a given bulk of atmospheric air; but it requires either artificial desiccation, which is extremely troublesome; or if the gas and air are saturated with moisture, then, for rigorous exactness, it requires the correction for vapour; to furnish which, Dr. Apjohn proposes the following formula.
If the specific gravity of dry atmospherical air at any temperature t, and pressure p, be represented by 1, it will become, when the air is charged with vapour, p—f/p + ·625 f/p, f being the tension belonging to vapour at the temperature t For similar reasons, if x represent the specific gravity of any other gas, at the same pressure and temperature, x.p—f/p + ·625 f/p, will be its altered specific gravity, after saturation with aqueous moisture. These expressions follow immediately from the theory of mixed gases and vapours. Hence if a be the experimental specific gravity of the gas, determined by the method of Thomson, the gas and the air employed being both saturated with moisture, x.p—f/p + ·625 f/p/p—f/p + ·625 f/p = a, an equation from which we deduce x =a + ·625 (a–1) f/p–f
From this formula it is obvious, that if the experimental specific gravity a be less than unity, it is greater than the truth; if greater than unity, it is less than the truth; and that the error is greatest in the case of the gases which recede most from unity on either side. With hydrogen, it amounts to 1/4th, and with chlorine to 1/89th of the true specific gravities of the respective gases, assuming for these the values assigned to them by Dr. Thomson. In the case of nitric oxide, however, and even of azote, carbonic oxide, sulphuretted hydrogen, and some other gases, it is so small as to be safely negligible.
On Atomic Weights. By E. TURNER, M.D.,Professor of Chemistry in the University of London.
The author's attention had been principally directed to the atomic weights of lead, silver, chlorine, and barium; and the results which he had obtained convinced him of the inaccuracy of many of the equivalents adopted in this country. He considers the equivalent of lead to be not higher than 103·6, and not lower than 103·4; that of silver to be 108, or perhaps 108·1; he agrees with Berzelius in taking 35·45 as the equivalent of chlorine; and adopts 68·7 as the equivalent of barium. Some of his experiments induce him to admit 14 to be the equivalent of nitrogen, while others are more favourable to 14·1. The analyses of the author agree in general very closely with those of Berzelius.
The author proposes to lay the details of his researches before the
[page] 572
Royal Society during the ensuing winter, and hopes in the intervening period to reduce some of the equivalents within still narrower limits. In the mean time, without denying the possibility of hereafter tracing some simple relation between the equivalents of bodies, he is convinced that the hypothesis, "of all equivalents being multiples by a whole number of the equivalent of hydrogen," is inconsistent with the best analysis which chemists at present possess.
Examination of the Sulphuretted Sulphate of Lead from Dufton. By JAMES F. W. JOHNSTON, A.M.
This mineral,—mentioned in Phillips's System of Mineralogy under the name of Supersulphuretted Lead,—is of various colours from an almost pure white to a deep lead grey. It varies also in hardness, being sometimes so soft as to be easily scratched by the nail; at others, offering considerable resistance to the knife. It occurs only massive, often composed of distinct layers of different shades of colour, and imbedding occasional crystals of common galena. Mr. Johnston has met with one specimen which in the cavities contained minute crystals of sulphate of lead. The specific gravity of a dark lead grey variety was 5·275.
In the flame of a candle it takes fire and burns with a blue flame and smell of sulphur. Heated in a close tube, it gives off sulphur in large quantity. Oil of turpentine and boiling alcohol dissolve sulphur from the mineral when in the state of fine powder. The sulphur present, therefore, is not in a state of combination with the lead.
By heating to redness in the open air, a lead grey variety lost 10 per cent., a white variety only 7 per cent. of its weight.
Treated with muriatic acid in a gentle heat, it was decomposed and dissolved, with the exception of the sulphur. A lead grey specimen left of sulphur 8·71 per cent.; and when the lead was separated by sulphuretted hydrogen, the filtered solution gave with chloride of barium 69·8 of sulphate of barytes, equivalent to 90·38 of sulphate of lead. The mineral therefore consists of
| Sulphur | 8·71 |
| Sulphate of lead · | 90·38 |
| 99·09 |
and is merely a mixture of sulphur with sulphate of lead.
It occurs at Dufton in the midst of the regular veins: it is difficult, without a knowledge of the localities, to understand the source of the uncombined sulphur.
Lecture on a new Safety Tube adapted to the Oxhydrogen Blowpipe. By JOHN HEMMINGS.
Mr. Hemmings commenced with a few general remarks on the advantage to the chemist of the intense and continuous heat of the ox-hydrogen blowpipe, if it could be employed without danger of explo-
[page] 573
sion. The blowpipe of Newman, with Professor Cumming's safety cylinder, were described. The improved blowpipe by Gurney was exhibited, and the well and safety-chamber also described; with the improvement made by Wilkinson on the latter, by introducing layers of asbestos between some of the discs of wire gauze. Mr. Hemmings stated that the latter, which is decidedly superior to all the others, occasionally, however, permitted the flame to recede through it, and that he had found when the gases contained a portion of water mechanically suspended in them, by passing through a long column of water in the well, the flame would return through the chamber many times in succession, and explode the gases in the well, and sometimes in the reservoir also.
He then introduced his own safety-tube, which had withstood every attempt to produce a recession of the flame through it, although tried under circumstances which invariably produced explosion in the others.
It is a cylinder of brass about six inches long and 1/4 inch diameter, filled with fine brass wire in lengths equal to that of the tube. The diameter of the wire should not exceed 1/180th of an inch. When the tube is packed with the wires as closely as possible, a pointed rod of equal length is forcibly driven through the centre of the bundle of wires, which brings them into still closer approximation, and wedges them firmly together: the rod is about 3/4 inch diameter.
The interstices between these wires, when thus closely packed, are extremely small, and become, in effect, congeries of metallic tubes, of smaller bore than the finest capillary tubes of glass. The cooling and conducting power of these is infinitely greater than could be effected, if a cylinder of equal length were filled with discs of the finest wire gauze, as the diameter of these tubes is infinitely less than that of the apertures in the finest wire gauze, and it possesses the important advantage of unbroken continuity in the tube from one extremity of the chamber to the other. Since the invention of this tube, Mr. Hemmings has dispensed with the well, &c. &c. of the ordinary blowpipe, and has operated constantly with the bladder of the mixed gases under his arm; In this manner he ignited, before the members of the Association, a piece of lime from the jet; he then removed the jet, and ignited the gases at the extremity of the tube; and although this aperture is nearly 3/4 inch diameter, and pressure on the bladder was frequently withdrawn to permit recession of the flame if possible, yet no accident occurred.
An approximate idea of the size of the apertures may be formed from the following statement:—the number of lengths of wire in the last tube made for Mr. Hemmings is 4200; the internal diameter of the tube is 8/8 inch, and the diameter of the rod in the centre is rather more than 1/8 inch thick.
[page] 574
6. METEOROLOGY.
Notice of the Thermometrical Observations now making at Devonport, near Plymouth. By GEORGE HARVEY, F.R.S.E. &c.
In this communication Mr. Harvey describes the plan which, through the liberal encouragement of Major-General Sir John Cameron, K.C.B., Commander-in-Chief of the Western District, and the active zeal of Col. Pym, of the Royal Artillery, he has been enabled to put in execution, for the fulfilment of the recommendation of the former Meeting of the Association at York, relating to the establishment of an hourly register of the thermometer at one of the military or naval stations on the south coast of England. The thermometer is placed with a northern aspect, in the open square of the Royal Artillery, within a few yards of Col. Pym's quarters, and is sheltered from the influence of the sun, and the effects of local heat, by a wooden erection, having its sides, its back and its roof, formed of double planks, the intervals being filled with sawdust. The observations are at present taken, every two hours, by the non-commissioned officers who go round with the reliefguard; but arrangements are in progress by which the register will be completed for every hour. The observers show the utmost attention to accuracy.
Notice of the Establishment of a Register of hourly Observations of the Thermometer, in the Dock-yard at Plymouth. By WILLIAM SNOW HARRIS, F.R.S.
Mr. Harris has been enabled, with the sanction of Commissioner Ross, to set up a thermometer at Plymouth, in the Dock-yard, which continues to be registered every hour during the day and night. It is placed in an open spot, under an appropriate screen, at about 80 feet above the level of the sea, and is so contrived, that by means of a T square, which slides in a groove in the scale, marks, corresponding to the height of the mercury, can be made on an adjoining slate, at the time of entering the observation. The marks and entries can be compared every twelve hours, and thus a complete check is obtained on any occasional error. The registering of this instrument is entrusted to the warders at the gate, all of whom are men of character; and there is every reason to hope that a series of unexceptionable observations will be thus obtained. A copy of the register for May and part of June was presented to the Meeting.
Description of a new self-registering Maximum Thermometer. By JOHN PHILLIPS, F.G.S. &c.
The advantage of this invention is stated to be, the acquisition of an instrument, capable of exactly the same delicacy and exactness as the best mercurial thermometer, possessing the same durability as that instrument, and applicable to measure the extremes of heat in a variety
[page] 575
of positions,—objects to which the ordinary maximum thermometer is, from the principles of its construction, entirely inadequate. In the first part of the paper certain facts are stated, as observed in the process of making mercurial thermometers, from which the construction of the instrument flows as a simple inference. They relate to the extrication of air by boiling, to the position of the residual air-speck, and to the size and form of sections of the tubes.
The small residual air-speck, which is supposed to be, without any exception, left in every mercurial thermometer, is employed, in Mr. Phillips's construction, to separate a small portion of the column, and thus to permit that portion to be acted on exactly in the same manner, as the iron, or other cylinder, above the mercury of the common instrument. The air-speck is for this purpose brought to a certain place in the tube, and the bore of the tube is chosen so slender, as to render it extremely difficult, except by artificial refrigeration, to change the place of the air-speck;—consequently instruments of very great delicacy may thus be made, and appear no more liable to injury or deterioration than the finest common thermometer.
In applying this principle to practice, various methods have been tried by Mr. Phillips; and, finally, he has for some time preferred the following plan. 1. The bulb and tube are filled in the common way, carefully boiled, allowed to retain the proper quantity of mercury, and sealed. 2. The end of the tube is melted, and instantly afterwards the bulb is plunged into the flame of a spirit-lamp. The consequence is, that the end of the tube is blown out into a spherule, in which, when cooled, the elastic fluids are so highly attenuated as to offer no sensible resistance to the movement of the mercury. This operation requires address. 3. The air-speck will now be evident;—if its length, when made to enter the tube, is less than half a degree, the instrument may be considered as fit for immediate use, as soon as, by a process of refrigeration, the right position of the air-speck is obtained. 4. If the length of the air-bubble exceed a degree or two, it may be easily diminished, by, first, causing the separated portion of the column to pass into the empty bulb; and, secondly, returning it into the tube, after exposing the mercury in the bulb to some augmentation of temperature.
Finally, by this process a dextrous workman may easily convert any good common thermometer, of a fine bore, into a self-registering instrument.
JAMES D. FORBES, F.R.S.E. &c., announced that he has a Register of Observations of the Thermometer nearly ready to be communicated, which has been kept, for a period of three years, in Scotland, at an elevation of 1100 feet above the level of the sea.
Mr. Forbes also exhibited and explained the construction of an improved portable Barometer, which had been recently executed for him by Mr. Robinson of Devonshire-street, and in which he had sought to combine the requisites of an exact zero for the scale, an accurate indi-
[page] 576
cation of the temperature of the mercury, steadiness during observation, and convenience and security in travelling.
[The Report of the Experiments which were made at York by the Secretaries of the Yorkshire Philosophical Society, on the quantity of Rain falling at different Heights, having been accidentally mislaid, will appear in the next Volume of the Reports.]
7. GEOGRAPHY.—GEOLOGY.
On the Compilation of a General Table of Altitudes of Places in Great Britain and Ireland. By BENJAMIN BEVAN, F.R.S. &c.
IT is well known, that in the common topographical publications,two only of the co-ordinates, out of the three requisite for defining the position of a spot on the surface of a district, have yet been given; that is, the latitude and longitude; whereas, to define the spot completely, the altitude is required.
To have attempted to supply this defect many years ago would have been fruitless; but in the present day, when, from the national trigonometrical survey, and from the numerous canals which have been executed, and from surveys and sections which have been obtained both for canals and rail-roads, nearly the whole of the country has been intersected by actual levelling, the necessary data may be obtained for determining the relative altitude of almost every important part of the United Kingdom.
These data, now scattered over a broad space, would, if collected together, form the basis of a general table of the altitude of places, which, if once commenced by proper persons, would soon be sufficiently mature to lay before the public, and might hereafter be extended and improved.
Mr. Bevan offers on his part to give a list of altitudes, which he has for some years been collecting; and he conceives that many members of the Association, and their friends, will readily contribute towards so desirable an object. He proposes, therefore, that such a number of gentlemen as may be willing to assist in the plan, be appointed a Committee for that purpose, and that one general place of communication should be fixed upon.
About 13 years since, Mr. Bevan attempted to set on foot a measure of this nature, which met with the approbation of most of our learned Societies; but at that period it was considered desirable to adopt some general standard, or zero, from which all the altitudes might be numbered, and to which they might be referred; and it was thought proper that this standard point of levels should be fixed at some public place in or near London. He therefore made search in all the public offices for a section of the river Thames, from which the height of some spot near London, above the natural zero, or lowest point of drainage, might be determined, but did not succeed in finding one.
[page] 577
This difficulty has now been removed by the section lately made by Capt. Lloyd.
From this zero, and the canals, &c. above referred to, the elevation of every important part of the country may be stated, with a degree of accuracy quite equal to that of the latitudes and longitudes, so long considered essential in a geographical definition.
A letter from Mr. ROBERT STEVENSON was read, stating his reasons for postponing to a future meeting of the Association the Report for which he had been asked— On the waste and extension of the Land of the east coast of Britain, and the permanency of the relative level of the Sea and Land;—and expressing his hope that those, whose residence near the coast might enable them to furnish information, would favour him with contributions towards the completion of this inquiry. Mr. Stevenson added, that he had drawn up a paper on the subject, which had been published in 1816 in the 2nd volume of the Wernerian Transactions, and had treated it again, in 1820, in the 3rd volume of the Edinburgh Philosophical Journal; that since that period he had had frequent opportunities of renewing his observations on the British shores, and of extending them upon the Continent, between the Zuider Zee and the mouth of the river Garonne; but that this additional range of observation did not lead him to suggest any new theoretical principle. He had materials, however, for giving further information, both in respect to the figure of the land and the state of the tides; and hoped at the next meeting to be more fully prepared, in reference to a chart showing the direction, progressive motion, and rise of spring and neap tides upon our shores, by adding to the fruits of his own exertions the benefit which might be derived from the aid of other members of the Association. There was one subject which he begged to be permitted to suggest, as highly worthy of the notice of that meeting, viz. to ascertain with greater accuracy than the casual observation of the mariner can be supposed to obtain, the depth of waters in the British seas. A more perfect knowledge of the soundings of the German Ocean and minor British seas, was noticed, a good many years since, to the Board of Admiralty as an object deserving of national attention, in conjunction with the Trigonometrical Survey. In the prosecution of such an object, the Association could not have recourse to better advice, or more valuable assistance, than that of the present Hydrographer to the Admiralty, Captain Beaufort.
The Rev. WILLIAM D. CONYBEARE gave a verbal explanation of A Geological Section of Europe*, which he had drawn to illustrate his Report upon the general progress of Geology.
The Meeting expressed a unanimous wish that the numerous and valuable materials for a Geological Map of Europe, which Mr. GREENOUGH
* This section has beenengraved for the Association at the author's expense.
2 O
[page] 578
is known to have collected, and to which his friends have always had free access, might be given to the public.
Mr. WITHAM read a memoir on Fossil Vegetation. In this communication the author states the results of the observations which he has made on the botanical character of the vegetable remains preserved in the strata of the earth. By his method of reducing the hardest fossils to slices so thin that the structure may be clearly traced, he has been enabled to examine the internal organization of the stems of numerous fossil plants, both in their transverse, and lately to a still greater advantage, in their longitudinal section; and this examination leads him to conclude that the number of gymnospermous phanerogamic plants in the early deposits of coal, will be found greatly to exceed what those who have written upon the subject have formed any conception of. The author mentions various localities near Edinburgh, Berwick, Newcastle, and Durham, where abundance of plants of this character have been found; the fossil trees discovered at Craig-leith, near Edinburgh, between 40 and 50 feet long, with a diameter of five feet at their lower extremity, and that found at Widespen, near Newcastle, measuring 72 feet in length, appear to be Coniferœ; but he thinks that some of these plants vary materially from true Coniferæ, showing a difference of structure, especially in the longitudinal section, in which they bear a nearer resemblance to the true Dicotyledon. The vascular cryptogamic plants are undoubtedly in much greater proportion in some parts of the series of deposits; but in the Lothian basin, which contains 33 beds of coal, as well as amongst the lower coals of Northumberland, Durham, and Yorkshire, the remains of cryptogamic plants, and especially ferns, are exceedingly rare, and the author attributes the contrast observable between the coal system of Yorkshire and Newcastle on the one hand, rich, as to its upper beds, in ferns and cryptogamic reliquiœ, and that of Scotland on the other, remarkable for the quantity of trunks of phanerogamic plants—to a difference in the ancient physical geography of the countries where the coal was formed. The memoir concluded with a description of the traces of structure observed by the author in various kinds of coal. Bovey coal and Jet have both been evidently wood; and in the former an indistinct resemblance to coniferous structure may be observed in parallel series of square or hexagonal marks. In Cannel coal the longitudinal section presents a confused cellular tissue, like that of a vascular plant; but in the fibrous and slaty coal of the mountain limestone, the author has remarked very decided traces of a structure much resembling the coniferous, and leaving no doubt that the plants of which it was formed must be classed among the phanerogamic.
Mr. JOHN TAYLOR wished to call the attention of geologists to the collection and arrangement of vein-stones, and to an accurate examination of their connexion with the rocks in which they occur. Mr. Taylor pointed out the importance of these investigations, both in respect to
[page] 579
geological theory and economical utility: he stated, that by attention to such circumstances, a rich vein of ore had been re-discovered at Vita Grande Zarateras, in Mexico; he mentioned, also, the occurrence of a vein at Rumos, in the same country, under a covering of basalt.— Mr. GREENOUGH adverted to analogous instances which had been observed in this country; and remarked that in Derbyshire the veins are often interrupted by trap, and that, at the point of contact, the vein sometimes separates into branches.—Professor SEDGWICK said, that in some instances in Derbyshire, the veins are traced through the trap rocks; in some they are cut off by these rocks; but in others the veins have unquestionably been formed since the formation of the trap. Certain granite veins, he thought, had been caused by injection under great pressure, and were simply a prolongation of unstratified into stratified masses; but the cause of granitic, and of metalliferous veins, did not appear to him to have been the same; the latter were often mere cracks, traversing the granite veins, as well as other rocks. In Cornwall the contemporaneous veins might more aptly be termed veins of segregation. So that there are three different sets of veins. 1st, Those of injection;—2nd., Those of segregation; which comprehend several of the metalliferous veins of Cornwall in actual work. 3rd, Those which have been plainly mere fissures or cracks, and which have been subsequently filled—for example, by spar, ore, rolled pebbles, &c. No good general view of veins can be given, without much observation of the phænomena bearing upon this subject, which are exhibited by secondary rocks. In these there could be no doubt of certain veins having been produced by fissures; and any theory, therefore, which should assume all veins to be contemporaneous with the rocks inclosing them, must be erroneous. Cleavages, such as had been described as occurring in the rocks of Cornwall, were not incompatible with stratification: in Cumberland, and in Wales, fissile texture is marked by chlorite and mica, running with parallelism; beds occur also there, containing shells, which mark the real stratification, whilst the same beds of shells have, in many cases, vertical cleavages, traversed by the lines of slaty cleavage; and it might be inferred by analogical reasoning, notwithstanding the absence of organic remains, that Cornwall, also, is a stratified country.—Dr. BOASE stated his opinion, that the slate rocks of Cornwall are in their original position, and have not been uplifted by the granite. In the neighbourhood of the granite the slate differs in composition, and becomes metalliferous; a connexion between the rocks which could scarcely have subsisted, if the granite had been injected. The composition of the mineral and metalliferous veins of Cornwall varies according to the nature of the rocks which they traverse: and the veins and the rocks are always intimately connected together by mineral transitions, similar to those which so frequently occur between different rocks. He further remarked, that the phænomena of veins, commonly referred to motion, are often exhibited, even in hand-specimens of Carclaze granite, and of St. Agnes slate; the veins being arranged in the joints of the laminæ: and these facts ap-
2 O 2
[page] 580
pear to admit of no other explanation, than that the production of the veins was coeval with the formation of the rocks. Lastly, he begged to suggest for the consideration of geologists, whether the arrangement of veins, on the large scale, is not perfectly analogous; since the different series of veins cross each other in directions corresponding with those of the joints or seams of the rocks, the latter having the same mechanical structure as the individual concretions and laminæ of which they are composed.—Professor Sedgwick, in answer to a question from Sir Philip Egerton, said that in certain cases of contorted rocks, the contorted masses are the true beds, the fissures being the lines of cleavage; and that these fissures continue parallel to fissures in the undisturbed beds.—Sir PHILIP EGERTON observed, that in the Isle of Man, he had seen veins in grauwacke running at right angles to the lines of cleavage, and that he had examined a vein of granite, found in sinking a shaft, adjoining which the slate had been broken into fragments, and re-cemented; veins of felspar, and a vein of lead, here run parallel to the granitic vein. This vein is supposed to be a ramification from the main body of granite which only appears in one place of the island. Sir P. Egerton also described the occurrence of a vein of copper-ore in the new red sand-stone of Cheshire.
Mr. CARNE explained his views on the relative age and direction of the veins in Cornwall; he concurred with Professor Sedgwick in his distinction of veins of segregation, and remarked that contemporaneous veins, which are sometimes metalliferous, terminate in length and depth; but that metalliferous veins of fissure do not terminate. Mr. Carne exhibited specimens of tin ore from Cornwall, showing that certain varieties, which had formerly been found only in alluvium, have lately been discovered in situ in true veins. Wood tin, or fibrous oxide; toad's-eye tin, or radiated oxide; tin in spherical concretions; have all been observed in a regular vein near the surface, under circumstances which may authorize an inference that the alluvial fragments have been transported thence by a current setting from N. N.W. to S. S. E.
Mr. MANTELL requested the attention of geologists to the zoological characters of the Wealden formation, as furnishing the best evidence of the circumstances of its deposition, and the best means of identifying it in other parts of England. He particularly dwelt on the proofs afforded by the presence of fresh-water shells in the Tilgate beds, and by the absence, from the whole Wealden series, of zoophyta, echinida, ammonites, belemnites, and other decidedly marine tribes, that this formation was principally accumulated under the influence of fresh water. He illustrated the structure of the Iguanodon by specimens of the teeth, bones, horn, claw, &c.; and stated that no remains of this animal had yet been discovered except between the North and South Downs; a fact which, joined to the other zoological peculiarities of
[page] 581
that district, rendered it probable that the Wealden formation had not yet been recognised in any of the midland counties. He also exhibited a specimen of Hippurites from the chalk of Lewes, a genus of which he had formerly obtained only indeterminate fragments, and which he believed was now, for the first time, recorded as an English fossil.
The Rev. J. WILLIAMS exhibited a very perfect specimen of Ichthyosaurus tenuirostris from the Lias of Somersetshire.
The Rev. W. D. CONYBEARE communicated a Memoir, which he had drawn up in answer to a question proposed to him at the former meeting of the Association at York,On the application to Great Britain and Ireland of that part of the theory of M. Elie De Beaumont, which asserts that the lines of disturbance of the strata, assignable to the same age, are parallel.
Mr. Conybeare, in this communication, remarked on the various sources of difficulty experienced in attempting to determine the geological epochs to which particular elevations of the strata should be referred. Direct evidence is attainable in but few instances; where, as in the Isle of Wight, not only the dislocated strata are seen in juxta-position with those which retain their original place, but also immediately follow them in the regular geological series; and in the absence of such evidence, we are reduced to reason from the lesser analogy afforded by similar disturbances of the same rocks, but in unconnected localities. Even as to the convulsions affecting the very same geographical district, it is too much to assume, without distinct evidence, that they have all been produced by one single shock, rather than by a series which may have occurred at intervals, through a long period of ages, in conformity to the operations of modern volcanic forces.
Having shown the difficulties and obscurities which hitherto overcloud this important branch of geological inquiry, Mr. Conybeare proceeded to state the few data on the subject which he considers to be tolerably well ascertained, so far as the geology of Great Britain is concerned, commencing with the convulsions of the most recent order which have been here observed,—those, namely, which have happened during the period of the tertiary formations.
The following extract of his views respecting the convulsions of the tertiary epoch, will serve to illustrate the general plan of investigation employed by Mr. Conybeare*.
"The tertiary formations, and the chalk on which they rest, have participated in the general elevation of all the secondary strata of the Island, of which the general line of bearing is from N.E. to S.W., but there is no appearance whatever of this elevation having been the result of any violent, sudden or single convulsion; on the contrary, everything
* For further details see the London and Edinburgh Phil. Mag, and Journ. for Aug. 1832, p. 123.
[page] 582
indicates that it was a gradual, gentle, and protracted upheaving (to borrow a German term), continued without interruption during the whole period of the formation of all these strata; or perhaps some persons may be inclined to refer it rather to an equally progressive depression of the basins of the surrounding ocean: as all the phænomena simply indicate a relative change of level, they will admit an equally ready explanation on either hypothesis. We may observe a very general tendency to parallelism between this line (although the result of a cause certainly continuing to act in the same direction in the tertiary epoch,) and the earlier and more violent convulsions which we shall hereafter find to have affected the older carboniferous strata before the deposition of the new red sandstone; for the general line (N.E. to S.W.) above indicated, may be more correctly described as a curve running nearly N. and S. in the northern part of its course, and trending towards an E. and W. direction towards the south; and in like manner we find the carboniferous lines of elevation generally ranging N. and S. in our northern counties, and E. and W. in the southern. But independently of this general elevation, we find in the southern counties three parallel lines of elevation ranging E. and W., and indicative of more abrupt and violent action, which appears to have occurred during the tertiary epoch, and which may very probably be regarded as strictly contemporaneous. The first and most important of these lines of disturbance, is that which, having traversed the Isle of Wight, strikes and ranges through the peninsula of Purbeck, and then produces the anticlinal line and parallel faults of the Weymouth district; thus extending over more than sixty miles. It must have produced an angular movement of the strata of many thousand feet, as it has thrown the chalk, plastic clay, and London clay into a vertical position. The section in Alum Bay distinctly exhibiting the contact of the disturbed and undisturbed strata, shows this derangement to have been effected by a single and most violent convulsion, of which the æra is most distinctly marked and precisely limited, being subsequent to the formation of London clay, and anterior to the alternations of fluviatile and marine deposits which characterize the basins of the Isle of Wight and Paris.
The anticlinal line of the Weald of Kent and Sussex, ranging from the North of Hastings to the North of Petersfield.—This is the cause of the elevation of the north and south chalky downs, and its disturbing effects may be most strongly traced in the narrow chalky ridge of the Hogsback (in the former), where the strata are considerably inclined: it may very probably be referred to the same sera as the foregoing line of disturbance, to which it is very nearly parallel. We may consider this anticlinal line as prolonged through the chalk by Winchester, and a little north of Salisbury, and thus reaching the Vale of Wardour, which is what Professor Buckland terms a Valley of Elevation: here the Portland limestone is thrown up, and the strata often considerably inclined. On the whole, however, the line now described is rather an anticlinal line of very gentle curvature, than one indicating violent disturbance. It is impossible to dismiss this line without ob-
[page] 583
serving how exactly parallel it is to the much older lines of elevation of the transition strata of the Quantock Hills and the Forest of Exmoor, which, when the eye glances over the map, appear to be its prolongation, and yet are really anterior to the age of the new red sandstone*.
A third parallel anticlinal line traverses the Vale of Pewsey, another valley of elevation on the greensand, separating the chalky ranges of Salisbury Plain and Marlborough Downs.—The protrusion of the greensand, in the prolongation of this line at Ham and Kingsclere, (see Buckland's paper†,Geol. Trans. 2nd series, vol. ii.) within the western angle of the London basin, may be referred to the same line of elevation, which will give it an extent of about 30 miles. I am not aware that its effect on the contiguous tertiary strata has been noticed, and can therefore only conjecture that it will probably, on examination, prove exactly contemporaneous with that of the Isle of Wight.
The above elevations, that of the Isle of Wight certainly, and those of the Weald and Vale of Pewsey by the most probable analogy, appear to have taken place subsequently to the formation of the inferior tertiary strata, and before the more recent beds. Elie de Beaumont assigns only the systems of Corsica and Sardinia to this epoch, and characterizes them as having a north and south direction; whereas our examples uniformly range E. and W."
In the discussion which followed the reading of this memoir, Professor Sedgwick expressed his agreement with the general views of Mr. Conybeare, but added, that in the older strata of our Island the phænomena appear to be in strict accordance with that part of M. de Beaumont's theory which asserts that mountain chains of synchronous elevation are parallel.
Mr. SEDGWICK afterwards proceeded to give a verbal account of the geology of Caernarvonshire. He described the mineralogical structure of the country, and showed that the range of the beds is parallel to the direction of the mountain chain. The lowest of these strata are composed of mica and chlorite slate, containing masses of jasper, verd antique, and primary marble. The highest mountains are distinctly stratified, and not unfrequently contain organic remains, but are associated with masses of pseudo-breccia, compact felspar, and porphyry generally arranged in masses exactly parallel to, and passing into, the true strata: the laminæ of slaty cleavage, after passing through the various beds of entire mountains, are seldom, if ever, parallel to the stratification. He then showed, by maps and sections, the existence of three or four parallel anticlinal lines, ranging nearly the whole length of the chain, and only deflected from their bearing when they interfere with other lines, produced by a local elevation; and he stated, lastly,
* See Geol. Trans. vol. ii. 2nd series, for Dr. Fitton's Hastings Section, and those of the Western Weald, where the anticlinal line ranges through Hastings, and comes north of Petersfield.
† An abstract of this paper will be found in Phil. Mag. vol. lxv. p. 214.
[page] 584
the extent to which the theory of M. Elie de Beaumont appeared to be confirmed by the phænomena described.
Dr. DAUBENY communicated his views of the geological inferences to be deduced from the chemical constitution of springs and of sea water, and proposed the following queries, as supplementary to those which he had formerly printed and circulated.
1st, Is the quantity of iodine and bromine in the waters of different seas equable at the same or at different depths?
2nd, Are these substances found in all countries in salt springs, and are they confined to such springs?
3rd, Does the flocculent or mucus-looking substance which exists in many thermal springs, always exhibit traces of an organic structure, when recent; and is the same appearance constantly presented in springs of the same chemical constitution?
4th, What are the gases given out by hot springs, and what connexion do such springs appear to have with volcanoes?
A discussion ensued respecting the existence and cause of a central heat, in the course of which Mr. JOHN TAYLOR described the action of hot water upon glass, and gave an account of an experiment suggested by the late Dr. Wollaston, for measuring the corroding power exercised by steam under pressure upon different kinds of glass.
Dr. BUCKLAND communicated a scale of geological colours, which had been adopted by the Board of Ordnance, and which will in future be employed in the colouring of all the different sheets of the Ordnance Map: he thought that if geologists would agree in the general adoption of this scale, the uniformity of geological signs which would be thus introduced would be advantageous to the science.
Notices of the Geological Structure of the Island of Pantellaria. By THE DUKE OF BUCKINGHAM, F.G.S. &c.
The author's description of this volcanic island is derived from a survey in the year 1828, in which he was accompanied by Signor Donati of Naples. It is distant from the nearest coast of Sicily 56 nautical miles, from Africa 36 miles; is of an elliptical contour; in length, 10 miles from N.W. to S.E.; in breadth, 5 miles; its circumference, 25 miles; its greatest elevation above the sea, 3500 feet. The rugged masses of volcanic rock which compose the uninviting shores of this island, continue themselves into the neighbouring sea, of which the bed, bristled with sharp points and edges, affords only a dangerous anchorage.
In the centre of the island rises the mountain called Il Bosco, with conical sides, truncated summit, and elliptical contour, whose longer axis runs N.E. and S.W. It is from this mountain, according to the Duke of Buckingham's observations, that the earliest lavas appear to have flowed; they contain crystals of felspar, but neither pyroxene nor mica; are superficially scorified; tinted with oxide of iron; and split
[page] 585
into large rectangular prisms. Three fumaroli appear in the ascent toward the summit of Monte del Bosco, at a place called Favaro, from whence constantly issues vapour, at the temperature of 60° R. = 135°F.; but no sublimation of any kind was noticed, nor were the pumice and scoriæ in the bank above the fumaroli at all changed by the vapours, which, when condensed, yielded only pure water. Aqueous vapour is the only product of another fumarole, which opens at a place called Il Bagno Secco, on the S.W. side of the mountain, from beneath a trachytic current, containing large crystals of felspar, thickly studded with microscopic crystals of oxide of iron. The current appears to have flowed down direct from the summit of the mountain. This natural vapour-bath is much used in rheumatic cases.
At the S.E. extremity of the island, a conical eminence, called Codia di Scaviri Supra, rising with a truncated summit to the height of 500 feet above the sea, is especially recommended to the attention of geologists. The currents of lava which have flowed from it, and which have all directed their course towards the interior, are composed of semivitreous matter, filled with small crystals of felspar and laminæ of mica,—mixed with pumice, and containing geodes lined with delicate acicular crystals. Fumaroli have at different periods opened in several points in the interior of this crater; but none are now in action. Lithomarge, hyalite, cacholong, and chalcedony of various colours appear in the volcanic products. The chalcedonic formation is found equally at the summit of the cone and at the seaward base of the mountains. Chalcedony was observed in similar lava currents on the seashore on the S.W. by W. point of the island, near a spring of boiling water, by the vapour of which it appeared in some places to have been decomposed.
In the western part of the island is a large crater, well defined, elliptical in figure, the larger diameter from N. to S. being about 1/3rd of a mile; its depth, 300 feet; the interior nearly filled with fallen prismatic blocks of lava. Some of the trachytic currents which have flowed from this crater contain geodes of brown obsidian and pumice. Minute crystals of felspar are observed in the obsidian. All the scoriæ accompanying this lava are cellular and vitreous, showing layers of obsidian and pumice. The volcanic action which opened this crater appears to have entirely ceased, and no fumaroli are observed about it, though the lava has a very recent appearance.
Not far from the village of Il Bagno, at the foot of the mountain of that name, are hot springs (70° R.), from which much carbonic acid gas is extricated, flowing into a lake 1/3rd of a mile in diameter, milk-warm, soapy to the touch and to the taste. The water contains much muriate of lime, with small quantities of sulphur and alkaline carbonate; it is employed for bleaching linen. The mountain of Il Bagno, rising 300 feet above the sea, is a volcanic cone, with a well-defined crater, half filled with detritus, from which a current of vitreous lava, passing into resinous obsidian, and containing in geodes microscopic crystals of a yellowish white colour, has descended toward the N.E. Not very far
[page] 586
from this, another current of lava shows chloritic clay, with laminæ of felspar. Neither of these currents of lava exceeds 8 or 10 feet in depth.
The mountain called Arca della Zelia is another volcanic cone, with the remains of a crater upon its summit, perfectly circular, about 50 feet in depth; covered with vegetation; everywhere exhibiting trachytic lavas, partly pumiceous and partly vitreous; externally scoriaceous; and inclosing great masses of pearlite and felspar.
Monte Saterno, and several other smaller hills, are all volcanic cones, independent of each other, and appear to have been successively elevated on bases of lava and volcanic substances, ejected from the primary cone of the Monte del Bosco.
The shores of Pantellaria are composed of alternating lavas, mixed with breccia, detritus of scoriae, pumice, and puzzolana, conglomerated with sand. The entire S.W. coast consists of trachytic lava, gradually passing into obsidian, &c. To the N.E. a bay, indented by basaltic caverns and surrounded by lavas of all descriptions, forms the principal part,—if part it can be called,—of the island. In all the spaces that intervene between the different currents of lava throughout the whole island, are beds of pumice and scoriæ, united with sand and pebbles of lava and obsidian. Amongst them, at La Codia di Scaviri Supra, are found great masses of granitello, composed of crystals of felspar and pyroxene, and some small pieces of perfect granite.
In the detritus of all these materials, vegetation thrives luxuriantly. The mountain of Il Bosco is entirely covered with evergreen oak and shrubs: the island produces wine, the ordinary fruits of the climate, and sufficient grain for the use of the inhabitants, who amount to about 4000 souls; but there is not to be found in it a single spring of pure water; and he who has a cistern whereby he can collect a few bottles in the year of water not sulphureous, is accounted a fortunate man. Nevertheless, the island is very healthy, and contagious disorders are unknown.
During the Duke of Buckingham's stay on this coast, the following circumstance occurred. A severe gale of wind was blowing in shore from the N.W., having continued all day, with a deep slate-coloured haze filling the atmosphere, and a thick cloud of the same colour concealing the mountains to their bases: at 10 o'clock P.M. a tremendous squall of heated air sprung up in a moment, blowing from the shore, directly opposite to the quarter from which the gale had proceeded. The blast was very suffocating, and continued with great fury about 10 or 15 minutes, when as suddenly it ceased. At the time of the hot blast, the shock of an earthquake, sufficiently strong to make the inhabitants seek refuge in the church, was felt in the village, directly opposite to where the vessel lay, and about a quarter of a mile distant. On his return to Naples, the Duke learned that on the same evening, about 7 o'clock, a similar sudden and transient gust of hot wind had rushed down the bay of Naples towards Castellamare, consequently proceeding from a different point of the compass from that at Pantel-
[page] 587
laria. At the same time Vesuvius, which had been for some time uneasy, threw up a jet of flame and smoke, and again resumed its former state of partial tranquillity: at the same hour also, a shock of an earthquake was felt at Ischia.
8. ZOOLOGY.—ANATOMY.—PHYSIOLOGY.
Observations on the Natural History of the Salmon. By R. KNOX, M.D.
AFTER some observations on the nature of the Parliamentary inquiry in 1824 and 1825, into the state of the salmon fisheries, the author describes the salmon as properly a sea fish, frequenting shores and estuaries, and ascending the neighbouring rivers whenever they are swollen with floods, in obedience to a natural migratory habit, and at certain seasons of the year, for the special purpose of spawning, whether they are flooded or not, yet giving the preference to those which are flooded.
About the beginning of September or latter end of August, salmon, now fast losing condition, begin to ascend most of the rivers of Great Britain and Ireland for the purpose of spawning. This migration continues, and the fish, full of milt and roe, is entirely worthless for food through the months of October, November, December, and January. The middle of March may be considered as the extreme limit of this process, and they retire from the spawning-ground as soon afterwards as any flooding of the river occurs. The kelt, or salmon which has spawned, is known however, during dry seasons, to remain in the rivers until May, and even later, and then return with the first summer rains to the ocean. In the cold part of the spring they conceal themselves carefully in the smaller streams, and eat little; taken near the source of a river they are truly abominable as food, but as they descend the stream and approach the sea at a later period, they improve gradually in quality; never possessing, however, that flavour and delicacy which belong to a fresh river fish taken at the same time, after floods. In December and January, a few salmon in good condition appear in the rivers, which had either not assumed the spawning state during the preceding year, or had spawned early and returned to the ocean, and having recovered their good condition, renewed their usual migratory life. The presence of these few good fishes in the rivers, by affording a pretext for fishing at improper seasons, has done immense injury to the salmon fisheries.
Concerning the natural history of the young salmon (smelt or fry) from the deposition of the ova to the appearance of the young fishes in the tideway of the river, the author describes the result of a series of observations on the deposition and development of the ova of Salmo Stuchio, in the upper part of one of the tributary streams of the Tweed, near its source. The observations commenced on the 2nd of November,
[page] 588
1831, on which day a pair of this kind of salmon were observed-engaged in depositing their ova, and covering them up in a bed of gravel, 4 feet long and 8 feet broad, covered by water 6 inches in depth. At a depth of from 9 to 12 inches hundreds of ova were turned up with the spade: On the 25th of February they were clear, transparent, and to all appearance unchanged. Thus the ova had remained one hundred and sixteen days without any visible change. The winter had been remarkably mild: abundance of larvæ of insects, which serve as food to salmon and trout, were dug up with the ova: these, rising through the gravel, offer ready food to the trout and salmon of all sizes which may be in the river. Some of the ova were transported to Edinburgh in six hours, preserved in moss and in water, but none of them became developed: some were placed in a small glass full of water, in a house situated on the banks of the river; these not having been transported so far, and having consequently been less disturbed, underwent the natural change, and grew to the length of an inch. The period of development in such circumstances varies with the temperature of the apartment, and may thus be produced ten days or a fortnight before their natural evolution in the river; but exposure to the sun kills them infallibly. Having cast the slough, they will live about ten days in water unchanged, apparently thriving, growing, and darkening in colour, (if exposed to the light,) but they have not been observed to eat anything offered them, and they invariably die, whether the water be changed or not, after attaining the length of an inch and a quarter. The author has ascertained that the grown smelt has not an equal capability of living in unchanged water, but when confined in the same manner, quickly sickens and dies.
On the 25th of March, it was found that nearly all the fish in the bed of gravel had cast the outer shell, and, from the appearances, it was concluded that this operation had taken place two days before; and, therefore, that 142 days had elapsed from the time of the deposition of the ova, till the fish were disclosed. The author is disposed to believe that this period of twenty weeks may be subject to some variation, according to temperature and other causes, but he rejects the idea, that ova deposited in the beginning of April, could become smelts in the beginning of June, since this would require the admission that eight weeks in the spring would produce the same effects as twenty in winter.
Several of the young fry, now disclosed, were put immediately into a tumbler of water, and six were carried to Edinburgh; and of these only one survived; it lived five days in water twice changed, and died on a crumb of bread being thrown in, which troubled the clearness of the water: but all those which were kept in a house near the river lived, and were well on the 1st of April, in water which had not been changed.
When first observed in the gravel bed, after bursting the shell, they were somewhat less than an inch in length, with the yolk of the ovum very large, and in the usual situation; they appear to remain in this state under the gravel about eight days, and then emerge and haunt
[page] 589
the edges of the river in shallow places. On the first of April, when the young fry emerged, the temperature of the gravel bed at 9 A. M. was found to be 41°, that of the water 4S°; but by 12 o'clock the water was at 45°, and the air 55°. The author conjectures the temperature of the gravel bed not to have been below 39° during the winter. Neither trout nor salmon devour the ova or the young fry; flies, beetles, worms, and larvae, being the only food found in the stomachs of either of these fish.
On the 20th of April the rivers were fished with fly, and were found full of salmon smelts, from 7 to 9 inches long, in good condition, with insects in their stomachs. Some of these, put into a vessel of rain water, died in an hour or two, and this whether taken with net or fly; and the author mentions facts, showing they will not bear to be carried to any considerable distance.
On the 5th of May the rivers were again fished, and smelts were still plentiful, notwithstanding some intervening floods, but they had descended the river two miles.
The author having thus related a portion of his investigations on the subject of the natural history of the salmon, proceeds to a survey of the Pan trout, or Samlet, which he distinguishes as a peculiar species, and makes some remarks on the hybernation of the trout and salmon. He then enters on topics of a more general nature, and discusses the subject of legal provisions for the regulation of salmon fisheries. It would be difficult to present an abstract of this part of the communication in such a manner as to do justice to the views of the author, who here gives a general review of the effects of the power and improvidence of man, in destroying races of animals which might have been long available for human food; and proposes a plan of legislative enactments, by which the further diminution of the salmon in the rivers of Great Britain may be prevented.
Mr. ARTHUR STRICKLAND communicated Observations on a species of Procellaria, new to the British Fauna, which was shot at the mouth of the Tees, in Yorkshire, in August, 1828. The bird was at first compared to Procellaria Bulweri, but upon further examination of it with Mr. Vigors and Mr. Gould, the author found it to be a different species. Subsequent researches* have given reason to presume that the bird is identical with Nectris fuliginosa of Solander's MS. described in Kuhl's Beiträge, from two unpublished drawings belonging to Sir Joseph Banks's collection, now deposited in the British Museum.
Mr. S. D. BROUGHTON communicated Notices of the Progress of Physiological Research, appertaining to the following subjects:—
1. The Development of the Ovum.
2. The ultimate Structure of Organized Matter.
* See Proceedings of the Zoological Society for July 12, 1832.
[page] 590
3. Physiological Effects of Oxygen and other Gases.
4. Experiments upon the Irritability of Animals.
In the former part of the paper, the author, after a general view of the utility of the microscope, and especially of the most recently invented kinds, in researches into the minuter organization and less obvious processes of nature, presents a connected view of the changes observable in the impregnated ovum during its gradual development into a new being. Rejecting the opinion that it contains within itself the miniature rudiments in detail of the entire foetal fabric, and that the embryo growth is a simultaneous evolution of all the organs which belong to the future foetus,—he follows Professors Wolf, Baer, &c., in describing the gradual changes in the germinal membrane, and the successive growth of all the organs, in the ovum of the fowl; compares them with what is known of the parallel development of the foetus in viviparous animals; and insists on the probability that in both cases the evolutions are conducted on one common principle.
In treating on the subject of the ultimate structure of organized matter, the author compares the results of the examination of the red particles in blood by successive observers from the time of Leeuwenhoeck to the present day. The superior power and clearness of Mr. Lister's microscope had enabled its inventor, with Dr. Hodgkin, to examine the blood and the tissues of the body to great advantage: the particles in human blood appeared to them as described by Dr. Young, —circular, but flattened, with no capsule or central nucleus, of a pale pink colour, and transparent; the same particles in the frog and skate appeared to be oval. In the muscular tissue the fibres, instead of being seen as in an inferior microscope, like so many strings of globules, appeared to be simple uninterrupted fibrillæ, longitudinally placed by the side of each other, and each separately crossed at right angles by a series of striæ. In no tissue of the animal body are distinct particles to be seen, and in no animal fluid are the particles strictly globular.
Thus the modern improvements in the microscope afford no confirmation of the views of those physiologists who imagined the particles of blood and of other animal and vegetable fluids to possess the globular form and the vitality of a monad, and considered their concatenation in muscular fibre as the first step of organization. The author concludes this head by insisting on the necessity of extreme caution in forming theories from the indications even of the best microscopes.
His account of the physiological phænomena attending the respiration of oxygen and other gases, is chiefly derived from his own researches. In this inquiry, rabbits, kittens, guinea-pigs, mice, sparrows, and frogs, were subjected to the influence of the gases in glass jars containing one and two gallons.
It was found that animals immersed in oxygen gas lived longer in it than in an equal quantity of atmospheric air; that the residual gas still consisted principally of oxygen, and was capable of maintaining
[page] 591
the respiration of a second animal, as long as on the first trial. Very little carbonic acid gas was produced in the jar of oxygen, even by the repeated respiration of several successive animals. The average period during which the warm-blooded animals remained unaffected by pure oxygen, was about one hour, the cold-blooded much longer. The immersion of kittens and rabbits in this gas was usually attended with apparent death in about three hours, but they were sometimes brought to life again by artificial respiration, and if the action of the diaphragm had not quite ceased, even by simple exposure to the open air, with warmth; debility, however, supervened in these cases, and occasionally death; if the diaphragm had ceased to act, there was no recovery, though the heart had not ceased to beat. Rabbits, however, in some instances survived longer in the gas: one remained seven hours in the two-gallon jar, apparently unaffected, and at the expiration of 13 hours was not dead. The first symptoms of failing life were gasping, hurried respiration, panting, and strong action of the heart; then followed a stage of increasing debility and decreasing sensibility, the animal falling on its side, drawing deep and slow inspirations, with short expirations, at longer and longer intervals, until a glaziness of the eyes and absence of all motion indicated total insensibility. Examined in this state, the diaphragm was still, but the heart in action, and the peristaltic motion of the viscera maintained. The heart, after being removed, continued for some time to contract on being irritated. In all the examinations, the bright crimson colour of the interior of the animal arising from the action of oxygen upon the blood, whether the animal had breathed in fresh oxygen, or was the second or third in succession in the same jar of gas, was very remarkable. The author terminates his description of these effects of oxygen gas by stating his opinion, that oxygen, pure or in excess above the proportion in atmospheric air, acts as a poison, and produces on the animal system effects very analogous to those of narcotic or sedative poisons.
He next relates the results of comparative experiments with various other gases;—in nitrous oxide, excitement followed by debility, bright redness of the interior of the body, long-continued action of the heart and intestinal canal after sensibility has ceased, and generally effects very similar to those produced in oxygen, took place in a much shorter space of time. Young rabbits are affected in little more than a minute, sparrows in four or five minutes; cold-blooded animals remain a long time unaffected, but ultimately die; a kitten left in the gas half an hour was past recovery.
With regard to other gases, the author states his experience to be at variance with a prevailing notion that they are all incapable of entering the lungs, from a closing of the epiglottis simultaneously with the first-drawn inspiration. About thirty seconds of time are sufficient to manifest the effects of chlorine when the animal falls down insensible. If immediately opened, the heart is found palpitating, and the peristaltic movements going on. This gas is traced into the lungs both by their
[page] 592
deep yellow tinge and acquired odour, and the brain likewise smells strongly of it.
Sulphuretted hydrogen destroyed sensibility in about half a minute, and in two minutes and a half the heart still palpitated. The lungs and brain exhibited a dark brown tint, and smelt strongly of the gas.
In the other gases, animals do not remain unaffected so long as a minute, and contractility is not preserved, as in experiments with oxygen and nitrous oxide, although the period of its surviving sensibility and the motion of the diaphragm may vary a little. All the gases experimented on probably passed into the lungs, with the exception, perhaps, of the carbonic acid gas, immersion in which is borne without any very sensible effects during nearly three minutes, when the animal struggles, and falls down insensible, the blood appearing very dark-coloured, and the heart still and flaccid. From these results, the author extends his deduction of the poisonous character of oxygen in excess to the other gases which enter the lungs, and remarks on the specific analogy which obtains between the effects of nitrous oxide and fermented liquors.
In the last portion of the paper, an account was given of some late researches of Dr. Marshall Hall, who has inferred from certain galvanic experiments on animals, that the muscular irritability of animals is inversely proportioned to the quantity of respiration.
On a new Membrane in the Eye. By GEORGE H. FIELDING, M.R.C.S.
The object of this paper was to prove that immediately behind the retina and in connexion with it, there is a coloured membrane of a peculiar nature, distinct from the pigmentum nigrum. The eyes taken for the purpose of experiment were those of the ox and the sheep, in each of which the part in question, of a fine blue or green colour, appears at the back of the globe of the eye, immediately in contact with the retina, having behind it the true pigmentum. To prove the difference between this membrane and the pigmentum, the author quotes Dr. Young's account of the latter, in which it is described as composed of mucous and carbonaceous matter, as staining white paper, and easily removable from the choroïdes by washing it with water and a soft pencil; but according to the author, the membrane in question will not stain white paper, nor will it part with its colour on the application of water. Its surface is bright and polished, and varies in colour according to the angle under which it is viewed, and according as it is examined by reflected or transmitted light. A portion of it, which was of a pale blue by reflected light, appeared of a yellowish red by transmitted light; dipped in dilute sulphuric or muriatic acid, or in solution of ammonia, its colours begin to fade; if it is then plunged into cold water, they entirely disappear; if again into the acids or alkali, they reappear as bright as ever. The author infers that the colours depend
[page] 593
not oh any peculiar secreted matter, but on the general laws for thin plates.
Examined with a fine achromatic microscope of Chevalier's construction, blood-vessels, and even the red globules contained in them, were visible in the membrane. By careful dissection in water, it is separable in distinct layers from the choroid. Its colour is frequently different in the same species of animals; it is usually blue in the ox, the pigment in the same animal being of a rich brown; in the cat and fox the membrane is of a golden yellow, the pigment a rich black; in the deer the membrane is pale blue, but the pigment a light brown.
The author proposes to name the subject of his researches Membrana versicolor; he enters into some considerations concerning its probable use in the act of vision, suggested by its low reflecting power and immediate connexion with the retina; and supposes that vibrations are excited in it by the converging pencils of light, and that these are communicated to the contiguous retina, and thus transferred to the brain.
On an hitherto undescribed Modification of the Respiratory Organs of certain Crustacea. By J. O. WESTWOOD, F.L.S. &c.
The object of this memoir was to illustrate the principal characters of an animal obtained from Shetland, of the genus Praniza, included among the Malacostraca edriophthalma of Leach; and especially to furnish a description of some peculiarities in its respiratory apparatus, as compared with the variations of that system already known in the several groups of the typical Malacostraca. The author, after giving an account of the history of the species, and of many parts of the organization of the animal, proceeded to describe more particularly the abdomen and the respiratory appendages beneath it.
The branchial organs of lsopodous Crustacea resemble those above described as found in the genus Praniza, by their plate-like form and the number of their pairs; but they are entirely, or in a great measure, covered by the large basal pair resulting from the slight development of the abdomen; those of the Amphipoda are exposed as in Praniza, but acquire a linear form; while those of Stomapoda, equally exposed, are found to consist of bundles of filaments. The group of Stomapoda belonging to the Podophthalmic division of Crustacea, whilst Praniza, with the Isopoda and Amphipoda, forms part of the division Edriophthalma, it follows evidently that it is between the Isopoda and Amphipoda that the true place of Praniza is to be sought in a natural distribution; and here the author places it in a small osculant group; thus reconciling the conflicting opinions of several authors, by whom it was classed in one or other of these groups, according to their peculiar views of its affinities.
The abdomen of the animal is about half the length of the thorax, and formed of five depressed transverse joints of equal breadth, but narrower than the thorax; the lateral caudal plates are representations of the sixth
2 P
[page] 594
abdominal segment, as in the lobster; and the seventh or terminal segment is represented by an elongate conic plate, forming with the lateral ones a quinquepartite caudal swimming organ, as in the shrimps.
The subabdominal appendages were found to consist of flat, very delicate, suboval, deeply ciliated, membranaceous plates, two pairs to every one of the five abdominal segments, inserted in pairs at each side of the joints beneath. These organs are of an equal size, except those upon the fifth segment, which are very slightly larger than the preceding. They are considerably smaller than the joints to which they are respectively attached, and consequently are all naked and exposed to the action of the water; when unemployed, however, and folded close upon the abdomen, the inner plate in each pair is almost covered by the outer one.
Abstract of an Essay on the Poisonous Properties of the Salts of Lead. By ANTHONY TODD THOMSON,M.D., Professor of Materia Medica, &c. in the University of London.
The object of this Essay is to show the probability, that among the salts of lead the carbonate is the only direct poison; and that the seemingly poisonous properties of the other salts of that metal depend on their conversion into this.
The author commences his inquiry by the detail of a few historical facts, to show that the poisonous effects of the carbonate of lead were known at an early period, and that the opinions respecting the poisonous properties of the other salts of this metal are of modern date. Paracelsus, who introduced the medicinal employment of acetate of lead, administered it in large doses with impunity; and instances are recorded in which persons have swallowed from one drachm to six drachms of the salt, without experiencing any injurious consequences.
He then states some experiments which he made to determine the affinity of the different salts of lead for carbonic acid; from which it appears, that subacetate and citrate of lead in solution have so powerful an affinity for carbonic acid, as to take it from the air, and all other substances containing it; that the affinity of the acetate for this acid is comparatively weak; and that carbonic acid effects no change whatever on the nitrate, muriate, sulphate, phosphate, and tartrate of lead.
The next object of the author was to examine the effect which the three salts, convertible into the carbonate, produce upon animals. Eight experiments upon dogs are detailed, which led to the conclusion that these quadrupeds are bad subjects of experiments with the salts of lead. None of the dogs died. He endeavours to account for the deaths recorded by Orfila in similar experiments on dogs, by suggesting that, as the doses were extravagantly large, the irritation excited in the stomach produced inflammation and death, in the same manner as common salt or any other salt in excessive doses, independent of any poisonous property. He also thinks that some fallacy may arise from
[page] 595
placing a ligature upon the oesophagus, as under such circumstances the animal cannot be considered to be in a natural condition. Eight experiments upon rabbits are next detailed. In the first experiment, six grains of nitrate of lead in solution were injected into the stomach of a strong young rabbit; no injurious consequences resulted, nor did any inconvenience follow the repetition of the experiment with nine grains of the nitrate on the following day. Three days afterwards, when the same rabbit appeared in perfect health, six grains of the carbonate of lead, suspended in mucilage of starch, were injected into the stomach of the animal. On the following morning he was found dead. The only peculiarity perceived, on opening the body, was the turgid state of all the cavities of the heart; the blood was slightly coagulated in the right auricle, but it was fluid in the other cavities.
In the fourth experiment, six grains of muriate of lead, in solution, were injected into the stomach of a strong young rabbit, without being followed by any inconvenience. Three days afterwards, six grains of the carbonate were thrown into the stomach of the same rabbit, who died on the morning of the second day.
Two other rabbits were treated with seven grains of the acetate and of the subacetate of lead. No inconvenience followed the administration of the acetate; that of the subacetate was followed by dejection and languor, with a disinclination to move and to take food: the animal, however, was alive at the termination of a week after taking the salt; it died in twelve hours after taking four grains of the carbonate of lead.
The author is disposed to conclude that the subacetate of lead, which approaches nearest to the carbonate in its effects, owes its poisonous property to its powerful affinity for carbonic acid: he points out the consequences which would result to the practice of medicine, if it should appear that the carbonate is the only salt of lead deleterious to the human subject; and remarks that these experiments appear to form an exception to the doctrine, that the activity of a poisonous salt is in proportion to its solubility.
9. BOTANY.
On the spiral Vessels observed in the mucous Matter which envelops the Seeds of Salvia verbenaca. By WILLIAM BAXTER.
In the "Introduction to the Natural System of Botany," by Professor Lindley, at page 220, an account is given of minute spiral vessels contained in the mucilaginous matter which envelops the seed of Collomia linearis; and Mr. Lindley adds, that he knows of "no parallel to this, except in Casuarina, in which the whole of the inside of the testa consists of minute spiral vessels."
After reading this statement, it occurred to Mr. Baxter that the mucous matter which envelops the seed in most species of Salvia,
2 P 2
[page] 596
might possibly also be partly composed of the same kind of spiral vessels as those first observed by Professor Lindley in the mucous matter which envelops the seed in the Collomia. He accordingly placed a seed of Salvia verbenaca on a small bit of glass on the stage of a compound microscope, and subjected it to moisture by dropping upon it a drop or two of clear water, when in a few seconds he had the satisfaction to observe an infinite number of exceedingly delicate and beautiful spiral vessels dart forth from the outside of the testa,—not in separate fasciculi, as he found to be the case in some seeds of Collomia grandiflora, which he had before examined, but in one unbroken circle, forming a complete and beautiful radius round the seed. If the seed on which the experiment has been tried is allowed to dry upon the glass, the spiral vessels will remain in their extended position, (their bases inclosed in the mucous matter which also dries upon the glass,) and may be preserved as an interesting object for the microscope at any future time.
Dr. DAUBENY exhibited A Specimen of an Index to the Flora of Oxfordshire, showing in separate columns the kind of situation most favourable to the growth of each plant, and the geological character of the spot in which it usually occurs. He stated that this Specimen was only intended to convey an idea of such a tabular Index as might enable the botanists of Oxfordshire to cooperate in collecting the data necessary for perfecting a Flora of that county, and might also engage others in different parts of Great Britain to draw up similar Indexes for their own neighbourhood, and thus to realize the views which the Association had suggested of obtaining a general Flora of the kingdom on one uniform plan.
On the Geographical Distribution of the Plants of Cambridgeshire. By the Rev. J. S. HENSLOW,Professor of Botany,Cambridge.
In this communication the author stated, that Cambridgeshire may be correctly divided into three distinct botanical districts, exclusive of a small maritime station. These districts do not coincide with the geological divisions of the county, although their limits are defined by the mineralogical character of the subsoil;—chalk, sandstone, and clay respectively predominating in each. The plants peculiar to each district appear to be sufficiently numerous and characteristic to warrant the proposed division; but the observations have not hitherto been made to such an extent as to enable Professor Henslow to speak with entire confidence on the subject. To the statements in this communication were added lists of such plants as had been detected since the last edition of the Flora Cantabrigiensis, and of those which had apparently become extinct in the county, in consequence, principally, of the extensive drainage of the fen districts.
[page] 597
10. ARTS.
On the State of Naval Architecture in Great Britain. By GEORGE HARVEY, F.R.S. E. &c.
THE vast national importance of the subject of naval architecture, as well as its necessary though not enough considered dependence on mathematical principles, is strongly insisted on by the author of this communication; and the latter circumstance in particular is illustrated by a reference both to the actual state of the art of ship-building in Great Britain, and to the history of its progress in France and other countries. The remarkable deficiency of mathematical theory in the adjustment of the external forms of vessels, on which their sailing performance so much depends, is contrasted with the extreme perfection to which the internal carpentry of the vessels has been brought by Sir R. Seppings and others; and the probable benefit of the application of refined analysis to the various practical problems which ought to interest the ship-builder,—problems of capacity, of displacement, of stowage, of velocity, of pitching and rolling, of masting, of effect of sails, and of resistance of fluids,—is illustrated. The notorious differences in the performance of ships, as well as the extraordinary variety in their external forms, are stated by the author to be at present unaccounted for in British treatises on ship-building; and he proposes the theory of these diversities of structure, and differences of effect, as a specific object of investigation, which, though embracing only a part of the subjects that demand rigorous mathematical inquiry, is capable of yielding immediate and valuable results in practice. The example of the French philosophers, encouraged by Colbert, who, by the application of the resources of a refined analysis to some of the problems of ship-building, have obtained better models than ourselves, is noticed; and the peculiar advantages enjoyed by the geometricians of Britain for collecting the great "constants of the art," and thus combining the established data of practice with the indications of theory, are pointed out. To collect and combine these data—a labour too great for individual exertion—is considered by the author an object peculiarly deserving of the cordial cooperation of men of science, under the auspices of a public Board.
A communication was received from Mr. JEREMIAH OWEN, containing Remarks on the Neglect of Naval Architecture in Great Britain, and recommending that subject to the early attention of the Association, assisted by the Government; for which purpose he further proposed that a correspondence should be opened with the authorities, and means taken to establish regular experiments on a large scale.
A communication on the same subject was also received from Mr. E. W. GILL.
[page] 598
On the Steel Suspension-Bridge recently built over an Arm of the Danube at Vienna; and on the Mode by which the exceeding tough steel employed was manufactured in Styria, at a small advance upon the price of Iron. By JAMES J. HAWKINS.
Experiments on the comparative strength of iron and steel in resisting tension have been from time to time made and published,—establishing, upon unquestionable authority, that it requires more than double the force to break a bar of steel by tension, than one of iron of equal dimensions; and that three times the force is necessary to stretch a bar of steel beyond the power of its elasticity to recover itself, than of an equal-sized bar of iron.
It is also established on the best chemical evidence, that the power of steel to resist corrosion from the operation of air and moisture, is very far above that of iron.
But it does not appear that advantage has been taken of these very valuable properties of steel in bridge-building, until about three or four years since, when the Austrian engineer, the Chevalier Ignace Edlin von Mitis, built a steel supension-bridge, of 230 feet span, at Vienna.
Having attentively examined this bridge during the progress of building,—having conversed with M. von Mitis on the subject,—and being strongly impressed with the opinion that the employment of steel as a material for bridge suspension is of very great advantage, and forms a most important epoch in bridge-building,—I am very desirous of calling the attention of the scientific world, and particularly of civil engineers, to the serious consideration of the question,—How far ought iron to be hereafter used for suspension-bridges, since it is ascertained that a steel bridge can be built of equal strength, and superior durability, with one third or one fourth of the weight of an iron one, and at a much less expense, provided steel can be manufactured in this country upon the same principles as that made in Styria?
The only doubt of this being practicable on the large scale, arises from the circumstance, that in this country iron is made with mineral coal, but in Styria with charcoal of wood.
It is well known that steel is made by decarbonizing cast iron, which is a compound of iron and carbon, down to the state of iron, (in which state it is wrought into bars); and recarbonizing these bars up to the state of steel, in which state the iron is combined with a less proportion of carbon than was contained in it in the state of cast iron.
Now the Austrian improvement consists in decarbonizing the cast iron down to the point at which the proportion of carbon left in the iron is exactly sufficient to constitute steel, in which condition it is wrought into bars, which are found to be of a tougher quality than steel made in the ordinary way.
It would appear that the carbon remains more uniformly combined with the iron, when the surplus quantity is removed, than when the new dose is given to it.
Thus the expense is saved of the extra decarbonization and the whole
[page] 599
of the recarbonization, and consequently the steel is produced at a much less cost than could be effected by the common process.
But whether or not this simple operation will be equally successful upon the large scale, with mineral coal, as with the charcoal of wood, is a problem which I would most earnestly call upon the iron-masters of the United Kingdom to lose no time in endeavouring to solve;—a problem of immense consequence to a large range of the manufactures of the country, and therefore a high national object. The hope that this will be accomplished is strengthened by the fact, that many small articles are now made of cast iron, and afterwards reduced down to steel.
The price of bar iron in Vienna, when I left it, (about a year since,) was 6l. a ton; that of bar steel, formed from the decarbonized cast iron, 8l. 16s.
By means of the Styrian steel, suspension-bridges may be built for less than half the cost at which they could be formed of iron; and a span of double the extent that would be practicable in iron, may safely be ventured on in steel. I have calculated that upwards of 1000 feet span may with confidence be depended on.
Mr. HAWKINS likewise exhibited a specimen of the sand employed in the fine iron castings at Berlin.
The Rev. WILLIAM TAYLOR exhibited a variety of specimens of Ornamental Turning, such as is usually attempted with the rose engine, but executed by himself with the common milling tool, and slight modifications thereof.
Abstract of Researches on the Strength and best Forms of Iron Beams*. By EATON HODGKINSON.
If a beam be fixed at one end in an horizontal position, and be bent or broken by a weight at the other, it is evident that the upper fibres will be extended, and the lower ones compressed; and therefore there must be some intermediate part, which will neither be extended nor compressed. This boundary has been denominated the neutral line, and its position is always dependent on the following equality:—The sum of the forces exerted by the extended fibres is equal to the sum of the forces from the compressed fibres.(1.) We have, moreover, from the principles of statics,—The product of the weight at the end of the beam by the length of leverage equal to the sum of the moments of the forces on both sides of the neutral line, with respect to that line (2.)
If the forces of tension and compression are known, and the situation of the neutral line be obtained in any one form of beam, we may, by equation (1) above, find its place in a beam of any other form; and the strength of the beam itself by equation (2).
* A full account of these researches has been published in the 5th volume of the Memoirs of the Literary and Philosophical Society of Manchester.
[page] 600
The theory from equations (1) and (2) is general, whatever the forces of tension and compression may be; but its utility and accuracy are diminished when the beams are overstrained; for the neutral line is found to shift when the elasticity becomes defective, or perhaps earlier; and therefore its place is difficult to be found in some bodies, as, for instance, cast iron, at the breaking point. In that metal we cannot discover it by inspection after fracture, as in timber.
Cast iron, however, affords a more simple rule for its ultimate strength, in some forms, as will be seen afterwards. Mr. Hodgkinson commenced the research for this rule, and the other objects of the paper, by seeking in that metal for the relative extensions and compressions of a bent piece, under equal weights, through the whole range up to fracture. For this purpose he got several castings moulded, such that a cross section of each was in the form DCE  and the castings several feet long. During an experiment, one end of the casting was fixed in a wall, and weights to bend it hung at the other. It was so fixed as to be easily removed, in order that the casting might be turned the other side upward, as the intention was alternately to compress and extend the vertical rib C of D C E. When the rib was upwards, the deflection arose from its extension, and when downwards, from its compression.
and the castings several feet long. During an experiment, one end of the casting was fixed in a wall, and weights to bend it hung at the other. It was so fixed as to be easily removed, in order that the casting might be turned the other side upward, as the intention was alternately to compress and extend the vertical rib C of D C E. When the rib was upwards, the deflection arose from its extension, and when downwards, from its compression.
The corresponding results from equal weights are placed in order as below:
| Extensions | 37 | 611/2 | 96 | 86 | 156 | 165 | 172 | 203 |
| Compressions | 34 | 53 | 971/2 | 84 | 135 | 163 | 167 | 186 |
From this comparison it appears that the extensions are greater than the compressions, by equal weights, whether the elasticity be perfect or not; the first four pairs of experiments having been made before the elasticity could have been much injured, and the other four nearly at the breaking point. Still the difference between the extensions and compressions is so small, that their equality seems to be a law of nature; the deviations arising only from imperfect elasticity.
In every instance the casting broke by tension; viz. by tearing asunder the vertical rib, that rib being upwards, thus  The rib never showed any sign of being crushed when it was compressed, though sustaining a weight which would have torn it asunder when stretched. To try, then, the effect of a greater weight, it was increased, in one instance, to double what would have broken the casting the other way up. Still the rib was not crushed; and, after sustaining the weight for many hours, the piece seemed only to have lost a little of its elasticity.
The rib never showed any sign of being crushed when it was compressed, though sustaining a weight which would have torn it asunder when stretched. To try, then, the effect of a greater weight, it was increased, in one instance, to double what would have broken the casting the other way up. Still the rib was not crushed; and, after sustaining the weight for many hours, the piece seemed only to have lost a little of its elasticity.
To set this matter in a still clearer point of view, two castings were taken of the same form as the preceding, and apparently precisely alike; they were placed between two props, upwards of 4 feet asunder, and broken by a weight at the middle; one with the rib upwards, to compress it, and the other with the rib downwards to tear it asunder The former required nearly four times as much to break it as the latter.
[page] 601
These experiments show clearly the serious consequences which might result from turning iron beams, of the usual form, wrong side up, as is frequently done in factories;—though, according to the theory which ascribes equal resisting powers to tension and compression from equal forces in this material, the beams ought to bear the same weight turned either way.
To apply the views developed by these experiments, and by theory, to beams of cast iron, the author sought to obtain—1st, By a series of experiments, the form of a beam which would bear the most without breaking;—2nd, The variation in strength arising from changes in the dimensions of such beam;—3rd, A practical rule for the measure of its strength at the time of fracture.
With respect to the general form of the cross section, in the middle of the beam, that was assumed to be the best which has a strong rib at top and bottom, with a thin solid sheet between them; the strengths of the ribs and sheet being in proportion to the strains upon them. The best proportion of this form at which the author was able to arrive, was one in which the bottom rib was more than two thirds of the whole section; and, from a comparison of all the experiments, it appears that in those beams which approach to the best, the strength is nearly in proportion to the size of the bottom rib in the middle, the length and depth of the beam being invariable.
The great size of the bottom rib, in this form, rendered it desirable to reduce it towards the ends of the beam. This was done by making the bottom rib parabolic, and the beam of equal height throughout: so formed it was uniform in strength, made a level floor, and afforded the greatest saving of metal.
A number of experiments were next made to ascertain the effect of a variation in the depth only of these beams, every other dimension being constant. These were made on beams of considerable size, some of them being 9 feet between the supports, and upwards of 10 inches deep. The result from these experiments was—that the strength was very nearly as the depth.
From the above two properties, then, we derive for the ultimate strength of cast iron beams of the best form, the following
General Rule.—Multiply the area of a section of the bottom rib, in the middle, by the depth of the beam, and by a constant quantity, and divide this product by the length.
If these dimensions be taken in inches, and the breaking weight in tons, the above constant is 26 for beams cast erect, and 25 for those cast on their side: and this agrees with the experiments to a great degree of accuracy.
In many of the preceding experiments the elasticity seemed to be perfect when one half, or even two thirds, of the breaking weight was laid on. The ultimate deflections, too, were rather greater than as the length, but were not as the square of the length, as is commonly assumed in rectangular forms.
[page] 602
11. MISCELLANEOUS.
PROFESSOR BABBAGE explained his views of the advantage which would be derived from a collection in Tables of all those Facts which can be expressed by Numbers in the various Sciences and Arts, and which he has denominated "the Constants of Nature and Art" A very valuable collection of this kind he thought might be formed, without much difficulty, by the cooperation of the Members of the Association.
Mr. ROTCH communicated some observations on the state of the laws respecting Patents, and the influence of those laws on the progress of the mechanical arts.
Professor RIGAUD stated, that having discovered the observations which Bradley made at Kew and Wanstead, he had been entrusted by the University with the publication of them, together with the rest of Bradley's miscellaneous Works. Among his loose papers were some observations of Halley's comet in 1759, which suggested the wish of annexing those that Harriot had made of the same body in 1607. The Baron de Zach had published them in 1793 (in the Supplement to Bode's Jahrbuch), but very unfaithfully, as appeared from a careful examination of the originals, with which the Professor had been entrusted by the Earl of Egremont. He stated that the same inaccuracy pervaded the whole of the Baron's account of these papers, and he instanced it particularly with respect to the discovery of Jupiter's satellites. The Baron's statement was originally printed in the Berlin Ephemeris for 1788, and a translation of it is inserted in Hutton's Dictionary (Art. HARRIOT). He argues in it that Harriot probably knew nothing of what had been done by the great philosopher of Florence, and adds, that he had found an observation of Jupiter's satellites made by Harriot as early as Jan. 16, 1610. In answer to this, Prof. Rigaud showed two separate memoranda, in Harriot's own hand-writing, in which he distinctly calls his observation of Oct. 17, 1610, the "first" which he made "of the new-found planets about Jupiter;" and he showed the name of "Galilæus" written on the back of one of these papers. To complete the evidence, he read a letter, which he had found in the British Museum, from Sir Wm. Lower to Harriot, from which it was evident that in June 1610, Harriot had already written to his friend to apprise him of the discoveries which are detailed in Galileo's Sidereus Nuntius. It appears that both were desirous of examining the great phænomena with their own eyes; but they were obliged to wait till Jupiter had passed its conjunction, which at that time took place in the summer.—Prof. Rigaud showed the original papers to those Members of the Association who were desirous of examining them, and pointed out the most remarkable particulars which they contained.
[page 603]
INDEX.
OBJECTS of the Association, ix.
Rules of the Association, ix.
Officers and Council of the Association:—of the First Meeting, 45; of the Second Meeting, 111.
Committees of the Association:—of the First Meeting, 46; of the Second Meeting, 112.
Abraham (J. H.), lecture on magnetism, 59.
Airy (G. B.), report on the progress of Astronomy during the present century, 125.
Allan (Mr.), notice of a magnificent specimen of aqua-marine in the possession of Don Pedro, 86.
Allman (Dr.), notice of his memoir on numeral evolution, 545.
Apjohn (Dr. J.), formula by which a proper correction for vapour may be applied to the specific gravities obtained by experiments on gases saturated with moisture, 570.
Baxter (W.) on the spiral vessels observed in the mucous matter which envelops the seeds of Salvia verbenaca, 595.
Bevan (B.) on the compilation of a general table of altitudes of places in Great Britain and Ireland, 576.
Boase (Dr.), remarks on mineral veins, 579.
Brewster (Sir D.) on the progress of mineralogy, 60.
—— description of an instrument for distinguishing minerals, 72.
—— on the crystalline lens of fishes, birds, &c. 81.
—— analysis of solar light, 89.
—— the honorary degree of Doctor of Civil Law conferred on, 100.
—— report on the recent progress of optics, 308.
—— on the colours of natural bodies, 547.
Brewster (Sir D.) on the undulations excited in the retina by the action of luminous points and lines, 548.
—— on the effect of compression and dilatation upon the retina, 551.
Broughton (S. D.) on the progress of physiological research, 589.
Brown (R.), the honorary degree of Doctor of Civil Law conferred on, 100.
Buckingham (Duke of) on the geological structure of the Island of Pantellaria, 584.
Buckland (Rev. Dr.), his address, 96.
—— lecture on the geology of the neighbourhood of Oxford, 100.
—— lecture on the fossil remains of the Megatherium, 104.
—— on a scale of geological colours, 584.
Carne (J.) on the mineral veins of Cornwall, 579.
Conybeare (Rev. W. D.), report on the progress, actual state, and ulterior prospects of geological science, 365.
—— on the application to Great Britain and Ireland of M. de Beaumont's theory of the parallelism of contemporaneous lines of elevation, 581.
—— notice of his geological section of Europe, 577.
Cumming (Rev. J.), report on thermo-electricity, 301.
Dalton (J.) on the proportion between the quantity of food and the evacuations, 74.
—— on the effects of atmospheric pressure on the animal frame, 85.
—— the honorary degree of Doctor of Civil Law conferred on, 100.
Daubeny (Dr.) on hot springs, 83.
—— new instrument for illustrating the effects of capillary attraction, 85.
—— on the geological inferences to
[page] 604
be deduced from the chemical constitution of springs and of sea-water, 584.
Daubeny (Dr.), specimen of an Index to the Flora of Oxfordshire exhibited by, 596.
Egerton (Sir P.), remarks on mineral veins, 579.
Exhibitions at the First Meeting, 91.
Fielding (G. H.) on a new membrane in the eye, 592.
Forbes (J. D.) on the horary oscillations of the barometer, 86.
—— report upon the recent progress and present state of meteorology, 196.
—— on an improved portable barometer, 575.
Gazari (Prof.) on a method of detecting the traces of writing which has been fraudulently erased, 90.
Gill (E. W.), notice of his communication on the state of naval architecture in Great Britain, 598.
Greenough (G. B.), remarks on mineral veins,.579.
—— notice of his valuable materials for a geological map of Europe, 577.
Hamilton (W. R.) on a view of mathematical optics, 545.
Harcourt (Rev. W. V.), his address, containing an exposition of the objects and plan of the Association, 17, 21.
—— on an oil-gas lamp, 88.
Harris (W. S.) on the method of employing vibrating magnets in the investigation of the magnetic intensity of the earth, 558.
—— notice of the establishment of a register of hourly observations of the thermometer in the Dock-yard at Plymouth, 574.
Harvey (G.) on the cultivation of mathematical studies, 58.
—— notice of the thermometrical observations now making at Devonport, 574.
—— on the state of naval architecture in Great Britain, 597.
Hawkins (J. J.) on the steel suspensionbridge built over an arm of the Danube at Vienna, 598.
Hemmings (J.) on a new safety tube adapted to the ox-hydrogen blowpipe, 572.
Henry (Dr. W.), estimate of the philosophical character of Dr. Priestley, 60.
—— on the torrefaction of copper pyrites, 78.
Henslow (Rev. J. S.) on the geographical distribution of the plants of Cambridgeshire, 596.
Herapath (J.) on the velocity of sound, 557.
Hodgkinson (E.) on the strength and best form of iron beams, 599.
Hutton (W.) on the Whin Sill of Cumberland and Northumberland, 76.
Johnston (J. F. W.) on vanadium, 78.
—— report on the recent progress and present state of chemical science, 414.
—— examination of the sulphuretted sulphate of lead from Dufton, 572.
Knox (R.) on the natural history of the salmon, 587.
Lubbock (J. W.), report on the tides, 189.
MacCullagh (J.), notice of his paper on the attractions of spheroids, 545.
Mantell (G.) on the zoological characters of the Wealden formation, 580.
Milton (Viscount), his address at the First Meeting, 13; at the Second Meeting, 95.
Morpeth (Viscount), his address, 43.
Murchison (R. I.), remarks on Mr. Hutton's paper on the Whin Sill of Cumberland and Northumberland, 76.
—— on marine shells in the deposits about Preston, 82.
—— delivers the Wollaston medal, awarded by the Geological Society, to Mr. W. Smith, 99.
Murphy (R.) on Fourier's "Théorie de la Chaleur," 547.
Osborn (Mr.), notice of the new volcanic island, 85.
[page] 605
Owen (J.), notice of his remarks on the neglect of naval architecture in Great Britain, 597.
Phillips (J.), his statement of the arrangements made for the First Meeting, 18.
—— account of the most remarkable phænomena in the geology of Yorkshire, 56.
—— remarks on the Whin Sill of Cumberland and Northumberland, 76.
—— description of a new self-registering maximum thermometer, 574.
Potter (R. jun.), description of a new microscope, 71.
—— on M. Fresnel's Theory of Reflection, 74.
—— on electrical phænomena in vacuo, 84.
—— on the modification of the interference of two pencils of homogeneous light, 553.
—— on an instrument for photometry by comparison, and on some applications of it to optical phænomena, 554.
Powell (Rev. B.), report on the present state of our knowledge of the science of radiant heat, 259.
Pritchard (Dr. J. C.) on the application of philological and physical researches to the history of the human species, 529.
Prout (Dr. W.), observations on atmospheric air, 566.
Recommendations of the Sub-committees at First Meeting, 48; at Second Meeting, 115.
Rigaud (Prof.), notice relative to the discovery of the satellites of Jupiter, 602.
Robison (Mr.), notice of his linseed-oil barometer, 86.
Scoresby (Rev. W.) on magnetic phænomena, 80.
—— account of some extraordinary effects of lightning on the packetship New York, 564.
Sedgwick (Prof.), remarks on mineral veins, 579.
—— on the geology of Caernarvonshire, 583.
Smith (W.), the Wollaston medal awarded by the Geological Society delivered to, 99.
South (Sir J.) on the satellites of Jupiter, 87.
Strickland (A.) on a new species of Procellaria, 589.
Sturgeon (W.), lecture on electro-magnetism, 565.
Taylor (J.) on the collection and arrangement of vein-stones, and importance of an accurate examination of their connexion with the rocks in which they occur, 578.
Taylor (Rev. W.) on coal-gas, 88.
Thomson (Dr. A. T.) on the poisonous properties of the salts of lead, 594.
Traill (Dr. W. S.), experiments on the intensity of terrestrial magnetism with Hansteen's needles, 557.
Turner (Dr. E.) on atomic weights, 571.
Warwick (Dr.), method of making a powerful temporary magnet, 85.
Westwood (J. O.) on an hitherto undescribed modification of the respiratory organs of certain Crustacea, 593.
Wheatstone (C.) on Dr. Purkinje's experiment on the eye, 550.
—— an experimental proof of Bernoulli's theory of wind instruments, 556.
—— on the acoustical figures of vibrating surfaces, 556.
Whewell (W.), report on the recent progress and present state of mineralogy, 322.
Williams (Rev. J.), a specimen of Ichthyosaurus tenuirostris exhibited by, 581.
Willis (Mr.), notice of his report on the philosophy of sound, 99.
Witham (H.) on fossil plants, 78.
—— on fossil vegetation, 578.
[page 606]
ERRATA.
Page 34, line 26,for Louvre read Jardin des Plantes.
—— 112, — 14,for Secretaries read Secretary.
—— 112, — insert "George Bellas Greenough, F.R.S. & L.S. P.G.S." as one of the Council.
—— 353, — 6 from bottom,for topaz read mica.
—— 331, — 8 from bottom,for regular read tessular.
[page 607]
LIST OF MEMBERS.
[Those who have compounded have an asterisk prefixed to their names.]
*H. R. H. THE DUKE OF SUSSEX, K.G.
PRESIDENT OF THE ROYAL SOCIETY.
Abbatt, Richard, F.R.A.S. Grove House, Tottenham.
*Abercrombie, John, M.D. F.R.S.E. Edinburgh.
Abney, E. H., Exeter College, Oxford.
Abraham, J. H., F.L.S. Sheffield.
*Acland, Sir Thomas Dyke, Bart., Christ Church, Oxford; Killerton, Devon.
Acland, Arthur H. D., Christ Church, Oxford.
Acland, T. D., All Souls College, Oxford.
Adams, John, Christ Church, Oxford.
Adamson, John, M.R.S.L. F.L.S. F.S.A. L.E. and Copenhagen; Corr. Memb. of Royal Academy of Sciences at Lisbon; Secretary of the Antiquarian and Literary and Philosophical Societies of Newcastle-upon-Tyne.
Airy, G. B., M.A. F.G.S. Plumian Professor of Astronomy and Experimental Philosophy, Cambridge.
*Alexander, Edward Nelson, F.S.A. Secretary of the Literary and Philosophical Society of Halifax.
Alison, William P., M.D. F.R.S.E. Professor of the Institutes of Medicine in the University of Edinburgh.
Allan, Robert, F.G.S. &c. Edinburgh.
Allan, Thomas, F.R.S. L. & E. Edinburgh.
Allen, Rev. William, Peel, near Bolton, Lancashire.
Allen, William, jun., Peel, near Bolton, Lancashire.
*Allis, Thomas, York.
*Allman, William, Professor of Botany, Trinity College, Dublin.
Anderson, Samuel, Edinburgh.
Anstis, J. B. Magdalen Hall, Oxford.
Apjohn, James, M.D. Professor of Chemistry, Royal College of Surgeons, Dublin.
[page] 608
Ashhurst, Rev. Thomas Henry, D.C.L. All Souls College, Oxford.
Ashley, Rt. Hon. Lord, M.P. F. Ast. S. 20, New Norfolk Street, London.
*Atkinson, James, Vice President of the Yorkshire Philosophical Society, York.
Atkinson, William, B.A. University College, Oxford.
Auchmuty, S. F., Brazennose College, Oxford.
Ayres, P. B. Oxford.
*Babbage, Charles, F.R.S. Lucasian Professor of Mathematics in the University of Cambridge, 1, Dorset Street, London.
Bachelor, Thomas, Magdalen Hall, Oxford.
Baily, Samuel, President of the Literary and Philosophical Society, Sheffield.
Bandinel, Rev. B., D.D. Bodleian Librarian, Oxford.
Bandinel, James, Foreign Office, London.
Barker, Edward, Sheffield.
Barker, Francis, M.D. Professor of Chemistry, Trinity College, Dublin.
Barnes, Rev. Frederick, D.D. Canon of Christ Church, Oxford.
*Barnes, Joseph W., M.A. Trinity College, Cambridge.
Baron, John, M.D. F.R.S. Gloucester.
*Bayldon, John, York.
Beach, W. Hicks, B.A. Oriel College, Oxford.
*Belcombe, Henry Stephens, M.D. York.
*Belgrave, Rev. Thomas, M.A. North Kilworth, Leicestershire.
*Bell, Thomas, F.R. & L.S. New Broad Street, London.
Berry, J. W. M. Brazennose College, Oxford.
Beynon, Rev. David, B.D. Jesus College, Oxford.
Bigge, E., University College, Oxford.
Bingham, William, B.A. St. Mary Hall, Oxford.
Bird, C. S., M.A. F.L.S. Reading.
Birkbeck, George, M.D. F.G.S. President of the London Mechanics' Institution, 50, Broad Street, London.
Birkbeck, William Lloyd, A.B. Fellow of Trinity College, Cambridge, 5, Gray's Inn Square, London.
*Black, James, M.D. Bolton, Lancashire.
Blackburn, Rev. J., Attercliffe, Sheffield.
*Blackburn, Charles, A.B. Portsmouth.
Blakesley, Joseph Williams, B.A. Fellow of Trinity College, Cambridge.
Blane, Henry, Brazennose College, Oxford.
* Blanshard, William, York.
* Bliss, Rev. P., D.C.L. Registrar of the University of Oxford.
* Blood, Bindon, F.R.S.E. M.R.I.A. Edinburgh.
* Blore, Edward, F.S.A. 62, Welbeck Street, London.
Boase, Henry S., Memb. Royal G.S. Cornwall, Penzance.
Boddington, Benjamin, Badger Hall, Shiffnall, Shropshire.
[page] 609
* Bond, Henry J. H., M.D. Cambridge.
Booth, John, M.D. F.G.S. Brush House, near Sheffield.
Bostock, John, M.D. F.R.S. F.L.S. G.S. &c., 22, Upper Bedford Place, Russell Square, London.
Bowstead, Rev. James, M.A. Corpus Christi College, Cambridge.
Boyd, William, B.A. University College, Oxford.
Brabant, Robert Herbert, M.D. Devizes.
Bragge, William, M.A. New College, Oxford.
Brancker, Thomas, Wadham College, Oxford.
Brayley, E. W. jun., A.L.S., of the London Institution, Teacher of the Physical Sciences in Bruce Castle School.
Brewster, Sir David, D.C.L. Oxon. LL.D. K.H. F.R.S.L. & E. Corresponding Member of the Institute of France, Allerly, Melrose.
Bricknell, Rev. W. Simcox, M.A. Worcester College, Oxford.
Bridges, Rev. Thomas Edward, D.D. President of Corpus Christi College, Oxford.
* Bridstock, W. P., F.G.S. Stoke Hill, Guildford.
* Brisbane, Sir Thomas Macdougal, K.C.B. F.R.S. L. & E. Corresponding Member of the Institute of France, Makerston, Kelso.
* Brooke, Henry James, F.R. & G.S. Mawbey Place, South Lambeth, London.
Broster, John, F.A.S.E. M.R.S.L. Brook Lodge, near Chester.
Brougham, William, M.A. M.P. 66, Mount Street, Grosvenor Square.
Broughton, Samuel Daniel, F.R. & G.S. 12, Great Marlborough Street, London.
Browell, Rev. W. Robert, M.A. Pembroke College, Oxford.
Brown, Rev. W. L., M.A. Christ Church, Oxford.
* Brown, G. B., Halifax.
Brown, Robert, D.C.L. Oxon. F.R.S. L. & E. V.P.L.S. M.R.I.A. &c. &c. Corresponding Member of the Institute of France, 17, Dean Street, Soho, London.
Brown, Rev. J., M.A. Trinity College, Cambridge.
Browne, Rev. Robert W., B.A. St. John's College, Oxford.
Brunel, Marc Isambard, F.R.S. Bridge Street, Blackfriars, London.
Brunel, Isambard Kindom, F.R.S. Bridge Street, Blackfriars, London.
Bryce, James, Belfast.
Brydges, Sir Harford Jones, D.C.L. Oxford.
Buckingham, His Grace the Duke of, 91, Pall Mall, London.
Buckland, Rev. William, D.D. F.R.S. Canon of Christ Church, Professor of Geology, &c. Oxford.
Buckle, Rev. H. G., M.A. Wadham College, Oxford.
Bull, Rev. J., D.D. Canon of Christ Church, Oxford.
Bull, Rev. H., M.A. Christ Church, Oxford.
Burchell, William John, F.L.S. Fulham, Middlesex.
Burgon, John William, 11, Brunswick Square, London.
Burnett, Gilbert, Professor of Botany, King's College, London.
Burrows, Rev. Joseph, B.D. Brazennose College, Oxford.
Burton, Rev. Edward, D.D. Regius Professor of Divinity, Oxford.
2 Q
[page] 610
Buxton, Edward North, Esq., 54, Devonshire Street, Portland Place, London.
*Canterbury, His Grace William, Archbishop of.
Cardwell, Rev. Edward, D.D. Principal of Alban Hall, Oxford.
*Carne, Joseph, F.R. & G.S. M.R.I.A. Penzance, Cornwall.
Carpenter, Rev. Lant, LL.D. Bristol.
Carpenter, Philip, Optician, 24, Regent Street, London.
Carter, Rev. Joseph, B.D. St. John's College, Oxford.
*Cattle, Robert, York.
*Cayley, Sir George, Bart., Brompton, near Malton.
*Cayley, Digby, Brompton, near Malton.
*Cayley, Edward Stillingfleet, Wydale, Malton.
*Chantrey, Francis, D.C.L. R.A. F.R.S. F.G.S. &c. Pimlico, London.
Cheek, Henry H., F.L.S. Edinburgh.
Children, J. George, Sec. R.S. F.R.S.E. F.S.A. F.L. & G.S. British Museum, London.
Christison, Robert, M.D. F.R.S.E. Professor of Materia Medica in the University of Edinburgh.
Chudleigh, Nicholas, Magdalen Hall, Oxford.
Churton, Rev. T. T., M.A. Brazennose College, Oxford.
Clark, George, B.A. University College, Oxford.
*Clark, James, M.D. F.R.S. George Street, Hanover Square, London.
Clark, William B., University College, Oxford.
Clarke, Rev. William, M.A. F.G.S. Professor of Anatomy in the University of Cambridge.
Cleoburey, Rev. C., M.A. Pembroke College, Oxford.
Cleoburey, W., Surgeon, Oxford.
Clerke, Rev. Francis, M.A. All Souls College, Oxford.
Clerke, Rev. C. C., B.D. Archdeacon of Oxford.
Clerke, Major Shadwell, K.H. F.G.S. F.R. Ast. Soc. Athenaeum Club, Knightsbridge.
Clift, William, F.R. & G.S. College of Surgeons, Lincoln's Inn Fields, London.
Clough, Rev. A.B., B.D. Jesus College, Oxford.
Cloyne, Right Rev. the Lord Bishop of.
Cockey, Edward, B.A. Wadham College, Oxford.
Cockin, W., Brazennose College, Oxford.
Coddington, Rev. H., M.A. F.R. & G.S. Trinity College, Cambridge.
Coker, Rev. John, B.C.L. Ratcliffe Rectory, Bucks.
Colby, Major, F.R.S. Tower, London.
Cole, Viscount, M.P. F.R.S. F.G.S.
Collier, G. F., M.D. 32, Spring Gardens, London.
*Connell, Arthur, F.R.S.E. Edinburgh.
Conolly, John, M.D. Warwick.
*Conybeare, Rev. William Daniel, M.A. F.R.S. V.P.G.S. Visitor of Bristol College, Corresponding Member of the Institute of France, Sully Rectory, Cardiff.
[page] 611
Conybeare, J., Sully, near Cardiff.
Cooke, Rev. G. Lee, B.D. Sedleian Professor, Oxford.
Cooke, Rev. T. Lee, M.A. Magdalen College, Oxford.
Cooke, William H., Brazennose College, Oxford.
Copleston, R. E., Exeter College, Oxford.
Corrie, Rev. John, F.R.S. President of the Birmingham Philosophical Institution, Woodville, near Birmingham.
Corrie, John Read, M.D. of Caius College, Cambridge, F.G.S. Birmingham.
Cotton, Benjamin, Freshwater, Isle of Wight.
Cowper, William Macquarrie, B.A. Dartmouth.
Cox, George, B.A. New College, Oxford.
Cox, George Valentine, M.A. New College, Oxford.
Cramer, Rev. John Anthony, D.D. Principal of New Inn Hall, Oxford.
*Creyke, Rev. Stephen, M.A. York.
Croke, Sir Alexander, D.C.L. Oriel College, Oxford.
Crompton, Dickinson Webster, F.L.S. Birmingham.
Crooke, William Henry, LL.D. 1, Lisson Grove, London.
*Croome, Rev. John, M.A. St. Mary Hall, Oxford; Bourton-on-the-Water, near Moreton, Gloucestershire.
Cumming, Rev. James, M.A. F.R.S. F.G.S. Professor of Chemistry in the University of Cambridge.
Cumming, George, M.D. Chester.
Cureton, Rev. William, B.A. Christ Church, Oxford.
Curtis, John, F.L.S. Grove Place, Lisson Grove, London.
Dale, James Charles, M.A. F.L.S. Glanvilles Wootton, Dorsetshire.
Dalton, Charles, Wadham College, Oxford.
*Dalton, Edward, LL.D. F.S.A. Bristol.
Dalton, John, D.C.L. Oxon. F.R.S. Corresponding Member of the Institute of France, Manchester.
Daniell, J. F., Professor of Chemistry in King's College, London.
D'Arblay, Rev. A., Christ College, Cambridge.
*Daubeny, Charles M.D. F.R.S. L.S. G.S. Professor of Chemistry in the University of Oxford.
Davies, Rev. T., B.D. Fellow of Jesus College, Oxford.
Davies, Thomas Stephens, F.R.S.E. F.R. Ast. Soc. Bath.
Dawson, George, M.A. Exeter College, Oxford.
Dean, Rev. J., D.D. Principal of St. Mary Hall, Oxford.
Deane, J. P., St. John's College, Oxford.
De la Beche, H. T., F.R.S. F.L.S. F.G.S. London.
Denison, J. E., M.P. Christ Church, Oxford; Portman Square, London.
Denton, Eaton D., B.A. 3, Middle Temple, London.
*Donkin, Thomas, York.
Donovan, —, Dublin.
Dornford, Rev. Joseph, M.A. Oriel College, Oxford.
2 Q 2
[page] 612
Douglas, James, Cavers, Roxburghshire.
Drake, Rev. James, Kirkthorpe, Wakefield.
Duboulay, John, Exeter College, Oxford.
* Dugard, Thomas, M.D. Shrewsbury.
Duncan, John, D.C.L. New College, Oxford.
Duncan, Philip B., M.A. Keeper of the Ashmolean Museum, Oxford.
*Dundas, Major-General Robert L., Arlington Street, London.
Dunn, Rev. James, M.A. Henbury, near Bristol.
Dunn, Robert, B.A. Exeter College, Oxford.
Dury, Rev. Theodore, Keighley.
Dyne, J. B., B.A. Wadham College, Oxford.
Dynham, William Burton, M.A. Magdalen Hall, Oxford.
Edwards, J., Oxford.
Egerton, Sir Philip de Malpas Grey, Bart., F.R.S. F.G.S. Oulton Park, near Tarporley, Cheshire.
Egerton, Thomas, F.G.S. Christ Church, Oxford.
Egerton, W., Brazennose College, Oxford.
*Ellis, Rev. Robert, M.A. York.
*Ellis, T. F., F. Camb. P.S. 15, Bedford Place, London.
Ellis, F. J., B.A. Merton College, Oxford.
England, Rev. —, Pembroke College, Cambridge.
Erle, Rev. Christopher, M.A. New College, Oxford.
Erle, H., Oxford.
*Estcourt, T. G. B., M.P. D.C.L. F.S.A. Estcourt, near Tetbury, Gloucestershire.
*Estcourt, W. J., Balliol College, Oxford.
Exeter, Right Rev. the Lord Bishop of.
*Eyre, Rev. Charles Wasteneys, M.A. Carlton, Notts.
Falconer, William, M.A. F.R.G.S. Exeter College, Oxford.
*Faraday, Michael, D.C.L. Oxon. F.R.S. Royal Institution, London.
Faulds, Andrew, Worsbrough, Barnsley, Yorkshire.
Faussett, Rev. G., D.D. Magdalen College, Oxford.
*Fawcett, Henry E., M.A. F.C.P.S. New Temple Buildings, Mitre Court, Temple, London.
Feild, Rev. Edward, M.A. Queen's College, Oxford.
Fellowes, Sir James, F.R.S. London.
Ferguson, Lieut.-Colonel, Huntley Burn, near Melrose.
Field, John, M.D. London.
*Fielding, George, Hull.
*Fielding, George H., Hull.
Fieldsend, John Charles, Surgeon, Oxford.
Fincham, John, Student N.A. Portsmouth.
*Fisher, Rev. John Hutton, M.A. F.G.S. Kirkby Lonsdale.
Fitton, W. H., M.D. F.R. & G.S. 53, Upper Harley Street, London.
*Fitzwilliam, Charles William, Earl, F.R. & G.S. President of the Yorkshire Philosophical Society.
[page] 613
* Flower, Rev. William, jun., M.A. York.
Forbes, Charles, Green Hill, Edinburgh
* Forbes, George, F.R.S. Edinburgh.
*Forbes, James David, F.R.S. L. & E. F.G.S. Professor of Natural Philosophy in the University of Edinburgh.
*Forbes, John, M.D. F.R.S. Chichester.
*Forbes, Sir John Stuart, Bart., Edinburgh.
*Forshall, Rev. Josiah, M.A. F.R.S. F.S.A. M.R.S.L. British Museum, London.
Forster, P., B.M. F.L.S. Chelmsford.
Foulkes, Rev. Henry, D.D. Principal of Jesus College, Oxford.
Foulkes, John Jocelyn, Jesus College, Oxford.
Fox, Hon. Colonel, M.P. Holland House, London.
Fox, Rev. J., D.D. Provost of Queen's College, Oxford.
Fox, Robert Were, Falmouth.
*Fox, George T., F.L. &G.S. Durham.
Fraser, William, Wilton Crescent, Belgrave Square, Pimlico, London.
Fremantle, Rev. W. R., M.A. Fellow of Magdalen College, Oxford.
Frend, William, 31, Upper Bedford Place, Russell Square, London.
Frost, Charles, F.S.A. President of the Hull Literary and Philosophical Society, Hull.
Gaisford, The Very Rev. Thomas, D.D. Dean of Christ Church, Oxford.
Garbett, James, A.M. Brazennose College, Oxford.
Garnier, Thomas, St.C.L. All Souls College, Oxford.
*Garnons, Rev. W. Lewes P., F.L.S. Sidney College, Cambridge.
Garratt, John, Christ Church, Oxford.
Gaselee, B., Balliol College, Oxford.
Gilbert, Rev. Ashurst T., D.D. Principal of Brazennose College, Oxford.
*Gilbert, Davies G., D.C.L. Oxon. V.P.R.S. F.G.S. Eastbourn, Sussex.
Gilbert, John D., M.A. Pembroke College, Oxford.
*Gilbertson, William, Preston, Lancashire.
Gilby, W. H., M.D. Clifton, near Bristol.
Giles, Rev. William, Barton Hall, near Manchester.
Gill, William, Engineer, Portsea.
Gillies, John, M.D. Edinburgh.
Glaister, Rev. W., M.A. Fellow of University College, Oxford.
Goddard, Rev. William, A.M. Jesus College, Oxford.
Goldie, George, M.D. Shrewsbury.
Gooden, James, F.S.A. M.R.G.S. 33, Tavistock Square, London.
Goolden, R. H., Queen's College, Oxford.
Gould, John, F.L.S. 20, Broad Street, Golden Square, London.
*Graham, R., M.D. F.R.S.E. Professor of Botany in the University of Edinburgh.
Graham, William, B.A. Christ Church, Oxford.
Graham, Thomas, Esq., F.R.S.E. Glasgow.
[page] 614
Grant, A., B.C.L. New College, Oxford.
Grantham, Rev. George, B.D. Magdalen College, Oxford.
Gray, John Edward, F.R.S. British Museum, London.
*Gray, Jonathan, V,P. Yorkshire Philosophical Society, York.
*Gray, William, jun.,Secretary of the Yorkshire Philosophical Society, York.
Gray, Rev. Walker, A.M. Henbury, near Bristol.
*Green,Joseph Henry, F.R. & G.S. Professor of Surgery, King's College; and Professor of Anatomy to the Royal Academy, London.
Greenough, George Bellas, F.R.S. & L.S. P.G.S. Regent's Park, London.
Gregory, William, M.D. F.R.S.E. Edinburgh.
Grenville, Right Hon. Lord, Chancellor of the University of Oxford.
*Greswell, Rev. Richard, M.A. F.R.S. Worcester College, Oxford.
Greville, Robert Kaye, M.D. F.R.S.E. Edinburgh.
Griffith, Richard John, F.G.S. Dublin.
Griffiths, John, B.A. Fellow of Wadham College, Oxford.
Grindall, Sturt, Captain R.N., Tubney House, near Wantage, Berkshire.
Grooby, Rev. James, B.A. F.Astr. Soc. Swindon, Wiltshire.
Guillemard, John, F.R. & G.S. 27, Gower Street, London.
*Halford, Sir Henry, Bart., London.
*Hallam, Henry, M.A. F.R.S. V.P.S.A. 67, Wimpole Street, London.
Halswell, Edmund, M.A. M.R.I. Gore Lodge, Brompton, near London.
Hamilton, Rev. Henry Parr, M.A. F.R.S.L. & E. F.G.S. Wath, Ripon.
Hamilton, Thomas, F.R.S.E. Edinburgh.
*Hamilton, W. R., M.R.I.A. Astronomer Royal of Ireland, Dublin.
*Hamilton, W. R., F.R.S. V.P.G.S. 22, Grafton Street, London.
Hamilton, W. J., Sec. G.S. 66, South Audley Street, London,
Hampden, Rev. R. D., M.A. St. Giles's, Oxford.
Hanmer, Thomas, Brazennose College, Oxford.
Hansell, Rev. Peter, M.A. University College, Oxford; and Fulham, near London.
*Harcourt, George, M.P. Nuneham, Oxfordshire.
*Harcourt, Rev. Charles G. Vernon, M.A. Rothbury, Northumberland.
*Harcourt, Egerton Vernon, Bishopsthorpe, York.
Harcourt, Octavius Vernon, Capt. R.N., Nuneham, Oxford.
Harcourt, Rev. William Vernon, F.R.S. F.G.S. Wheldrake, York.
Harding, John, Bridlington Quay, Yorkshire.
Harington, Rev. Richard, M.A. Brazennose College, Oxford.
Harper, John, York.
Harris, W. Snow, F.R.S. Plymouth.
Harris, Hon. Charles, B.A. Oriel College, Oxford.
Harrison, Benjamin, M.A. Christ Church, Oxford.
[page] 615
Hartop, Henry, Hoyland Hall, hear Barnsley.
Harvey, George, F.R.S.E. F.G.S. Plymouth.
Hastings, Charles, M.D. Worcester.
Haviland, John, M.D. F.G.S. St. John's, Cambridge, Regius Professor of Physic.
Hawkins, Rev. Edward, D.D., Provost of Oriel College, Oxford.
Hawkins, Rev. E., Exeter College, Oxford.
Hawkins, Francis, M.D. Curzon Street, May Fair, London.
Hawkins, John Heywood, M.P. Athenaeum, London; Bignor Park, Sussex.
Hawkins, John Isaac, Civil Engineer, Pancras Vale, Hampstead Road, London.
*Hay, Sir John, Bart., F.R.S.E. Edinburgh.
Head, E. W., M.A. Merton College, Oxford.
Heale, William James, B.A. Wadham College, Oxford.
Hemming, John, Brecknock Crescent, Camden Town, London.
Henry, Charles, M.D. Manchester.
*Henslow, Rev. John Stevens, M.A. F.L.S. F.G.S. Professor of Botany in the University of Cambridge.
Henwood, W. J., F.G.S. Perran Wharf, Truro.
Herapath, John, 2, Upper Bedford Place, Notting-hill, near London.
*Herschel, Sir J. F. W., F.R.S. F.G.S. Slough.
*Hey, John, Curator of the Leeds Literary and Philosophical Society, Leeds.
*Hey, Richard, York.
Hibbert, Samuel, M.D. F.R.S.E. F.G.S. Edinburgh.
Higgins, Godfrey, F.S.A. Skellow Grange, near Doncaster.
Hill, Edward, B.A. Christ Church, Oxford.
*Hill, Rowland, F.R.A.S. Bruce Castle, near London.
Hillyard, Temple, M.A. Thorpelands, near Northampton.
Hitchings, George, Surgeon, Oxford.
Hoare, Gurney, B.A. Trinity College, Cambridge.
Hodgkin, Thomas, M.D. New Broad Street, London.
Hodgkinson, Eaton, Member of the Philosophical Society, Manchester.
Hodgkinson, Rev. Henry, M.A. Rector of Arborfield, near Reading.
Hodgson, Joseph, F.R.S. Birmingham.
Hony, Rev. William Edward, B.D. F.G.S. Baverstock, Salisbury.
*Hope, Thomas Charles, M.D. F.R.S. V.P.R.S.E. Professor of Chemistry in the University of Edinburgh.
*Hopkins, William, Cambridge.
Hopkinson, W. L., M.D. Stamford, Lincolnshire.
Horne, Henry, M.A. Magdalen College, Oxford.
Howard, Luke, F.R.S. Ackworth, Pontefract.
Howse, Joseph, Cirencester.
*Hudson, James, Assistant-Secretary to the Royal Society, London.
Hudson, James, Oxford.
Hunter, Adam, M.D. Edinburgh.
Hunter, Rev. Joseph, F.S.A. Bath.
[page] 616
Hunter, Perceval, Leamington, Warwickshire.
Hunter, John,
*Hussey, Rev. Robert, M.A. Christ Church, Oxford.
Hutton, William, F.G.S. Newcastle-upon-Tyne.
Ingle, Rev. Charles, M.A. York.
*Inglis, Sir Robert, Bart., D.C.L. M.P. for the University of Oxford.
Jackson, Erasmus, Portsmouth.
Jackson, J., M.A. Brazennose College, Oxford.
Jacob, Arthur, M.D. Dublin.
James, J. P. R., M.R.S.L. Melrose.
James, Rev. William, M.A. Vice-Principal of Magdalen Hall, Oxford.
Jarrett, Professor, Cambridge.
Jeffreys, Hon. A., B.A. Christ Church, Oxford.
Jenkyns, Rev. Henry, M.A. Oriel College, Oxford.
*Jenyns, Rev. Leonard, M.A. F.L.S. Swaffbam Bulbeck, Newmarket.
Jeune, Rev. F., M.A, Pembroke College, Oxford.
Johnson, G. D., B.A. F.Z.S. St. John's College, Oxford.
Johnston, George, M.D. Berwick-upon-Tweed.
*Johnston, James W. F., M.A. F.R.S.E. Porto Bello, Edinburgh.
*Johnstone, Sir John V., M.A. M.P. President of the Scarborough Philosophical Society, Hackness, near Scarborough.
Jordan, Rev. John, B.A. Handborough, Ensham, Oxfordshire.
Joseph, Samuel Frederick, Magdalen Hall, Oxford.
Joy, John Home, of Trinity College, Dublin, Belfast.
Kay, Rev. William, M.A. Lincoln College, Oxford.
*Kennedy, John, Manchester.
Ker, Henry Bellenden, Esq. F.G.S., London.
Kerry, Earl of, M.P. F.G.S. Lansdowne House, London.
Kidd, John, M.D. F.R.S. Regius Professor of Medicine in the University of Oxford.
Kingsborough, Right Hon. Lord, Exeter College, Oxford.
Knight, Arnold James, M.D. Sheffield.
Knox, A. E., B.A. F.Z.S. Brazennose College, Oxford.
Kynnersley, E. Sneyd, Trinity College, Oxford.
Lacy, Rev. Charles, M.A, Tring, Hertfordshire.
Lane, John, R.N. 30, High Street, Oxford.
Lane, John, jun., Worcester College, Oxford.
*Lauder, Sir T. Dick, F.R.S.E. Edinburgh.
Lavie, Germain, B.A. Christ Church, Oxford.
Lawrence, William, F.R.S. Whitehall Place, London.
*Lawson, William, Liverpool.
Leggatt, Rev. Samuel, B.A. Portsmouth.
Lemon, Sir Charles, M.P. F.R. & G.S.
Lewin, T., M.A. Corpus Christi College, Oxford.
[page] 617
Lightfoot, Rev. John, M.A. Fellow of Exeter College, Oxford.
Lincoln, Earl of, M.P. Clumber, Nottinghamshire.
Lindley, John, F.G.S. &c. Professor of Botany in the University of London.
Linton, Rev. Robert, M.A. Magdalen College, Oxford.
*Lister, Joseph Jackson, F.R.S. Tokenhouse Yard, London.
Littledale, Henry, Halton Place, Skipton.
Littledale, William Dawson, Halton Place, Skipton.
Llandaff, Right Rev. Edward Copleston, D.D. Lord Bishop of.
*Lloyd, Rev. Bartholomew, LL.D. Provost of Trinity College, Dublin.
Lloyd, George, B.A. St. John's College, Oxford.
*Lloyd, Rev. H., F.T.C.D. Professor of Natural and Experimental Philosophy in the University of Dublin.
Lloyd, Joseph S., S.C.L. St. John's College, Oxford.
*Lloyd, William Horton, F.L.S. 18, Bedford Place, Russell Square, London.
*Lock, Edward, Oxford.
*Lock, Sir Joseph, Oxford.
*London, Right Rev. Charles James Blomfield, D.D. Lord Bishop of.
Loudon, Charles, M.D. Leamington.
Lowe, Robert, University College, Oxford.
Luard, Peter F., M.D. Warwick.
Luby, Rev. Thomas, Fellow of Trinity College, Dublin.
Luney, Rev. R., B.A. Plymouth.
Lyall, J. E., Balliol College, Oxford.
*Lyell, Charles, jun., M.A. F.R.S. L.S. G.S. Professor of Geology in King's College, London.
Macartney, James, M.D. F.R.S. M.R.I.A. Professor of Anatomy in Trinity College, Dublin.
Macbride, John David, D.C.L. Principal of Magdalen Hall, Oxford.
Macdonnell, Alexander, M.A. 2, Grosvenor Street West, London.
Macdonnell, Rev. Dr., Fellow of Trinity College, Dublin.
Mackenzie, William Bell, Magdalen Hall, Oxford.
Mackenzie, W. Stewart, of Seaforth, M.P.
Mackie, Rev. J. W., M.A. Christ Church, Oxford.
*Maclagan, David, M.D. F.R.S.E. Edinburgh.
Mac Lauchlan, J., F.G.S. Bicester, Oxfordshire.
Magrath, E., Secretary to the Athenaeum Club, Waterloo Place, London.
Mantell, Gideon, F.R. L. & G.S. Lewes.
Marsden, Charles, Esq., A.B. Hull.
*Marshall, John, Headingly, Leeds.
*Marshall, John, jun., M.P. Headingly, Leeds.
*Marshall, J. G., Headingly, Leeds.
Marshall, William, F.G.S. Newton Kyme, Tadcaster.
Marsham, Robert, D.C.L. Warden of Merton College, Oxford.
*Martin, F., Cambridge.
[page] 618
Martin, John, 30, Allsop's Terrace, New Road, London.
Martin, Joseph, Jesus College, Oxford.
*Mason, Thomas, York.
Massie, Edward, B.A. Wadham College, Oxford.
Menteath, C. G. S., F.R.S.E. Edinburgh.
Meredith, Rev. C. J., M.A. Lincoln College, Oxford.
Meynell, Thomas, jun., Friarage, Yarm.
Michell, Thomas, M.A. Buscot, Berks.
*Miller, W. H., M.A. F.G.S. F.C.P.S. Professor of Mineralogy in the University of Cambridge.
Mills, Rev. William, B.D. Professor of Moral Philosophy in the University of Oxford.
Milman, Rev. H., M.A. Brazennose College, Oxford.
*Milne, Sir David, K.C.B. F.R.S.E. Edinburgh.
*Milne, David, M.A. F.R.S.E. Edinburgh.
*Milton, William Charles Wentworth, Viscount, M.P. F.G.S. London.
Minto, Gilbert, Earl of, Minto House, Roxburghshire.
Moberley, Rev. George, M.A. Balliol College, Oxford.
*Money, Rev. K. E., M.A. Oriel College, Oxford; Much Marcle Parsonage, near Ledbury.
Montgomery, Robert, Lincoln College, Oxford.
Mordaunt, Sir John, B.A. Walton, near Stratford-on-Avon.
*More, J. S. Advocate, F.R.S.E. Edinburgh.
Morgan, Octavius, F.R.S. L.S. G.S. 70, Pall Mall, London.
Morgan, William, M.A. Magdalen College, Oxford.
*Morpeth, Viscount, M.P. Castle Howard, Yorkshire.
Morrell, Baker, Oxford.
Morrell, George, St. John's College, Oxford.
Morrell, Rev. R. P., M.A. Magdalen College, Oxford.
Morrell, F.G., St. Giles's, Oxford.
Murchison, Roderick Impey, F.R.S. & L.S. 3, Bryanstone Place, Bryanstone Square, London.
*Murphy, Robert, Caius College, Cambridge.
Murray, John, jun., F.G.S. 50, Albemarle Street, London.
*Nairne, James, F.R.S.E. Edinburgh.
Neill, Patrick, F.R.S.E. Edinburgh.
Nelson, Rev. G. M., M.A. Magdalen College, Oxford.
*Newman, William Lewin, York.
Nicholl, Iltid, F.R.S. Usk.
Nicoll, Whitlock, M.D. F.R.S. M.R.I.A. London.
Nolan, Frederick, D.C.L. Exeter College, Oxford; Prittlewell, Essex.
Norreys, Lord, M.P. F.G.S. 5, Great Stanhope Street, May Fair, London.
*Norris, Charles, Halifax.
*Norris, William, Halifax.
Northampton, Spencer Joshua Alwyne, Marquis of, F.G.S. Castle Ashby, Northamptonshire.
[page] 619
*Northen, Richard, Secretary to the Literary and Philosophical Society of Hull.
Oakes, Charles Henry, B. A. Merton College, Oxford.
O'Brien, Edward, B.A. Trinity College, Cambridge; Oxford and Cambridge University Club, London.
Ogle, J. A., M.D. F.R.S. Aldrichian Professor of Medicine, Oxford.
O'Neill, John, Upton, Essex.
Orlebar, Arthur B., Lincoln College, Oxford.
Ormerod, Thomas, M.A. Brazennose College, Oxford.
Overend, Wilson, Sheffield.
Owen, Jeremiah, Portsmouth.
Oxford, Right Rev. the Lord Bishop of.
Oxmantown, Lord, M.P. Magdalen College, Oxford; 46, Clarges Street, London.
Palfreyman, Luke, Secretary to the Literary and Philosophical Society of Sheffield.
Palmer, Roundell, Trinity College, Oxford.
*Palmer, William, M.A. 5, Essex Court, Temple, London.
Palmer, William, B.A. Magdalen College, Oxford.
Parker, Edward, B.A. Oriel College, Oxford.
Parsons, George, Secretary of the Birmingham Philosophical Institution, Birmingham.
Paxton, James, Surgeon, Oxford.
*Peacock, Rev. George, M.A. F.R. & G.S. Trinity College, Cambridge.
Pearsall, Thomas John, Literary and Philosophical Society, Hull.
Pearson, Rev. Thomas, M.A. Fellow of Queen's College, Oxford.
*Pearson, Rev. William, LL.D. F.R.S. V.P. Astronomical Society, South Kilworth, Leicestershire.
*Peel, Sir Robert, Bart., London.
Peers, Charles, Chislehampton.
Pendarves, William, M.A. M.P. F.R.S. & G.S. Pendarves, Cornwall; 36, Eaton Place, London.
Perceval, Robert, Brazennose College, Oxford.
Perkins, Rev. B. R., B.C.L. Christ Church, Oxford.
Perry, Charles, Cambridge.
Phillipps, Sir Thomas, Bart., M.A. F.L. & G.S. F.S.A. 14, Stratford Place, London.
Phillips, H. Worcester College, Oxford.
*Phillips, John, F.G.S. Secretary of the Yorkshire Philosophical Society, Museum, York.
*Philpot, Rev. H., Cambridge.
Pillans, James, F.R.S.E. Professor of Humanity in the University of Edinburgh.
Plumptre, Rev. T., M.A. University College, Oxford.
Portlock, Captain, Dublin.
Potter, Richard, jun., Smedley Hall, Manchester.
[page] 620
Powell, Rev. Baden, F.R.S. Savilian Professor of Geometry, Oxford.
Pratt, Samuel P., F.L. & G.S. Bath.
Price, Bonamy, M.A. Rugby.
Prichard, J. C., M.D. F.R.S. Bristol.
Pringle, J. W., F.G.S.
Prout, William, M.D. F.R.S. 40, Sackville Street, London.
Radcliffe, Rev. J., M.A. Vice-Principal of St. Mary Hall, Oxford.
Radford, Rev. John, B.D. Lincoln College, Oxford.
*Rathbone, William, Liverpool.
*Rawson, Christopher, F.G.S. President of the Literary and Philosophical Society of Halifax.
Read, Thomas F., University College, Oxford.
*Reade, Rev. Joseph B., M.A. Halifax.
Reeves, Frederick J. K., Merton College, Oxford.
Reid, David Boswell, M.D. F.R.S.E. Edinburgh.
Rennie, James, M.A. Professor of Natural History in King's College, London.
Reynolds, Rev. H., M.A. Jesus College, Oxford.
Rhoades, Rev. J., M.A. Wadham College, Oxford.
Richards, Rev. J., M.A. Exeter College, Oxford.
Richards, Thomas, Wadham College, Oxford.
Richardson, William, B.A. Wadham College, Oxford.
Rickards, R. F. B., Balliol College, Oxford.
*Rickman, Thomas, Architect, Ann Street, Birmingham.
*Rigaud, S. P., M.A. F.R.S. Savilian Professor of Astronomy, Oxford.
Riggs, Rev. George, M.A. Queen's College, Oxford.
Ritchie, Rev. William, LL.D. F.R.S. Professor of Natural Philosophy in the University of London.
Robinson, Rev. T. R., D.D. Professor of Astronomy, Armagh.
*Robison, John, Sec. R.S.E. Edinburgh.
Rogers, Rev. E., M.A. Wadham College, Oxford.
*Roget, Peter Mark, M.D. Sec. R.S. F.L. & G.S. 39, Bernard Street, Russell Square, London.
*Rotch, Benjamin, M.P. 1, Furnival's Inn, London.
*Rothman, Richard W., F.R.A.S. F.G.S. Trinity College, Cambridge.
Roundell, Rev. H. D., B.D. Magdalen College, Oxford.
Rushbrooke, H., Trinity College, Cambridge.
Rushout, George, Christ Church, Oxford.
Russell, James, V.P.R.S.E. Professor of Clinical Surgery in the University of Edinburgh.
Rutter, J. O. N., Curator of the Literary and Scientific Institution, Lymington.
Sabine, Joseph, F.R.S. 15, Mill Street, Conduit Street, London.
*Sadleir, Rev. Dr., Senior Fellow of Trinity College, Dublin.
Salmond, William, F.G.S. York.
Sandon, Lord Viscount, B.A. M.P. 41, Grosvenor Street, London.
[page] 621
Saull, William Devonshire, F.G.S. 15, Aldersgate Street, London.
Saumarez, Richard, F.R.S.E. Bath.
Saunders, Rev. Augustus, M.A. Christ Church, Oxford.
*Scoresby, Rev. William, F.R.S.L. & E. Corresponding Member of the Institute of France, Exeter.
Scudamore, John, Kentchurch Court, near Hereford.
*Sedgwick, Rev. Adam, F.R. & G.S. Woodwardian Professor of Geology in the University of Cambridge. Sedgwick, Rev. James, Freshwater, Isle of Wight.
Selkirk, Earl of, Christ Church, Oxford.
*Serle, Rev. Philip, B.D. Oddington, Oxfordshire.
Sewell, J. E., B.A. New College, Oxford.
Sewell, Rev. William, M.A. Fellow of Exeter College, Oxford.
Seymer, Henry Ker, S.C.L. All Souls College, Oxford.
Seymer, John Gunning, B.A. Alban Hall, Oxford.
Sharpe, Rev. Samuel, M.A. Wakefield.
Shirley, Evelyn P., Magdalen College, Oxford.
Shore, Offley, Sheffield.
*Short, Rev. Augustus, M.A. Christ Church, Oxford.
Short, Rev. Thomas, B.D. Trinity College, Oxford.
Shuttleworth, Rev. P. N., D.D. Warden of New College, Oxford.
Sigmond, George, M.D. 24, Dover Street, London.
Simcox, T. G., B.A. Wadham College, Oxford.
*Sinclair, Right Hon. Sir John, Bart.
Sinclair, Rev. John, F.R.S.E. Edinburgh.
Skene, James, F.R.S.E. Edinburgh.
Slatter, Rev. William, M.A. Christ Church, Oxford.
Smart, Rev. Newton, M.A. M.R.S.L. University College, Oxford.
Smith, B., Magdalen College, Oxford.
Smith, George, Queen's College, Oxford.
Smith, Gerard Edward, B.A. Strowting, near Hythe, Kent.
Smith, Samuel, 16, Duke Street, Westminster.
Smith, William, Hackness, Scarborough.
Smyth, William, B.A. Wadham College, and of Little Houghton, Northamptonshire.
Sneyd, Rev, Lewis, M.A. F.G.S. Warden of All Souls, Oxford.
*Somerset, His Grace the Duke of, F.R. & L.S. President of the Royal Institudon, Park Lane, London.
South, Sir James, F.R.S. Kensington, near London.
Sowerby, G. B., F.L. & Z.S. Regent Street, London.
*Stanley, Rev. Edward, M.A. F.L. & G.S. Alderley Rectory, Congleton, Cheshire.
*Stark, John, F.R.S.E. Edinburgh.
*Stevenson, Robert, F.R.S.E. F.G.S. Edinburgh.
*Steventon, Edwin, B.A. Corpus Christi College, Cambridge.
Stirling, Charles, St. Mary Hall, Oxford.
Stokes, Whitley, M.D. Professor of Mineralogy, Trinity College, Dublin.
[page] 622
Storey, A. M., M. A. F.R.S. Bassetdown House, Marlborough; and 86, Jermyn Street, London.
Stowe, William, Member of the Royal College of Surgeons, Buckingham.
Strangwayes, Edward Swainston, Alne, Easingwold, Yorkshire
*Strafon, Major-General, Sir Joseph, Edinburgh.
Strickland, Arthur, Boynton, Bridlington.
Strickland, Eustachius, York.
Strickland, Henry Eustachius, F.Z.S. Cracomb House, near Evesham, Worcestershire.
Strickland, Hugh, B. A. Oriel College, Oxford.
Strickland, Nathaniel E., M.A. Lincoln College, Oxford.
Stroud, Rev. Joseph, M.A. Wadham College, Oxford.
Stroud, William, M.D. 20, Great Coram Street, London.
Sturgeon, William, Artillery Place, Woolwich.
Sturt, Captain, Tubney House, near Oxford.
Sykes, Lieut.-Colonel, F.L. &G.S. 47, Albion Street, Hyde Park, London.
Symons, Rev. B. P., D.D. Warden of Wadham College, Oxford.
Talboys, D. A., Bookseller, Oxford.
Tancred, Thomas, B.A. Merton College, Oxford.
Tawney, Rev. R., M.A. Fellow of Magdalen College, Oxford.
Tayler, Rev. John James, B.A. Manchester.
*Taylor, John, F.R.S. Treas. G.S. Member of the Royal Geological
Society of Cornwall, 12, Bedford Row, London.
*Taylor, Richard, Assist. Sec. L.S. F.G.S. F.S.A. Red Lion Court, Fleet Street.
*Taylor, Richard, jun., Member of the Council of the Royal G.S. of Cornwall, Perran Arworthal, near Truro.
*Taylor, Rev. William, S. C.L. York.
Thistlethwaite, George, B.A. Brazennose College, Oxford.
Thomas, Rev. Vaughan, B.D. Corpus Christi College, Oxford.
Thomas, Rev. George, M.A. Worcester College, Oxford.
Thompson, Charles, M.D. Sheffield.
Thompson, Richard John, Kirby Hall, Boroughbridge.
*Thomson, Anthony Todd, M.D. F.L. & G.S. Professor of Materia Medica in the University of London.
*Thomson, George, Banker, Oxford.
Thomson, Thomas, M.D. Bedford.
*Thorp, Rev. Thomas, M.A F.G.S. Trinity College, Cambridge.
Tiddeman, Rev. Richard P. G., M.A. Magdalen Hall, Oxford.
Tite, William, Hon. Secretary of the London Institution, Finsbury Circus, London.
Townsend, John Charles, M.A. Milton, Oxfordshire.
*Townsend, Richard Edward, Doctors' Commons, London.
*Tracy, Edward Hanbury, Exeter College, Oxford; and Toddington, Gloucestershire.
[page] 623
*Trevelyan, Walter Calverley, M.A. F.R.S.E. F.L. & G.S. Wallington, Northumberland.
Trotter, Robert, F.G.S. Borde Hill, Cuckfield, Sussex.
Tuckett, Frederick, Frenchay, near Bristol.
Tupper, Daniel, Brazennose College, Oxford.
Turnbull, Peter, F.R. & S.A. 68, Baker Street, London.
*Turnbull, Rev. T. S., F.R.S. Caius College, Cambridge.
Turner, Edward, M.D. F.R.S.L. & E. Sec. G.S. Professor of Chemistry in the University of London.
*Turner, Samuel, F.R.S. & G.S. Liverpool.
Turner, Rev. William, Secretary to the Literary and Philosophical Society of Newcastle-upon-Tyne.
Twining, Aldred, Oriel College, Oxford.
Twiss, Travers, M.A. University College, Oxford.
*Tyrconnel, John Delaval, Earl of, F.R. & G.S. Kiplin, Catterick, Yorkshire.
*Upton, James Samuel, F.G.S. Trinity College, Cambridge.
Vandeleur, Henry, Worcester College, Oxford.
Vaughan, James, Worcester College, Oxford.
*Veysie, Rev. Daniel, B.D. Christ Church, Oxford.
Vigors, N. A., M.P. F.L. & G.S. Sec. Z.S. 16, Chester Terrace, Regent's Park, London.
Vores, Rev. Thomas, M.A. Wadham College, Oxford.
Vyvyan, Sir Richard, Bart., M.P. F.R. & G.S. 26, Great George Street, Westminster.
Walker, Henry, Christ Church, Oxford.
Walker, Joseph, Wadham College, Oxford.
Walker, Rev. Robert, M.A. F.R.S. Wadham College, Oxford.
Wall, Rev. Sandys, M.A. Christ Church, Oxford.
Wall, Whitmore, New College, Oxford.
Wallace, William, M.A. F.R.S.E. Professor of Mathematics in the University of Edinburgh.
Wallinger, Rev. William, M.A. Hastings.
*Wasse, Jonah, M.D. Moat Hall, Boroughbridge.
*Waterhouse, John, Halifax.
Watkins, Francis, Charing Cross, London.
Watkins, James R., Bolton, Lancashire.
Webb, C., Surgeon, Oxford.
Webb, Rev. Thomas William, M.A. Ross.
Wellbeloved, Rev. Charles, V.P. of the Yorkshire Philosophical Society, York.
West, William, Secretary of the Leeds Literary and Philosophical Society.
Westwood, John Obadiah, F.L.S. the Grove, Hammersmith, London.
Wheatstone, C., London.
[page] 624
* Whewell, Rev. William, M.A. F.R. & G.S. Trinity College, Cambridge.
White, Rev. Robert, B.D. Magdalen College, Oxford.
Whitley, C., M.A. St. John's College, Cambridge.
Whitmore, Sir George, K.C.H. Col. of Engineers, Cheltenham.
Whitmore, W. Woolrych, M.P.
Whitwell, Stedman, Architect, 22, Sussex Street, University, London.
*Williams, David, F.G.S. Bleaden, near Cross, Somerset.
*Williams, G., M.D. Professor of Botany in the University of Oxford.
Williams, Rev. John, M.A. Christ Church, Oxford.
Williams, R., Bridehead, Dorsetshire.
Williams, Robert, Oriel College, Oxford.
*Willis, Rev. Robert, M.A. F.G.S. Caius College, Cambridge.
Willis, Thomas, Magdalen Hall, Oxford.
Wilson, James, F.R.S.E. Edinburgh.
Wilson, Rev. J., M.A. Queen's College, Oxford.
Wilson, Rev. John, B.D. Trinity College, Oxford.
Wilson, Major D., M. R. Asiatic Society, B 4, Albany, London.
Wingfield, Charles, Surgeon, Oxford.
*Winterbottom, J. E., M.A. F.L. & G.S. St. John's College, Oxford.
Wintle, Rev. Thomas, B.D. St. John's College, Oxford.
Wintle, F. T., F.L.S. Oxford.
Witham, Henry, F.G.S. Lartington, Barnard Castle.
Wood, Sir Alexander, K.C. St. M. &St. G. M.R. Geog. Society, Colonial Office, London.
*Wood, George William, M.P. F.L. & G.S. Singleton Lodge, near Manchester.
Wood, J. F., Surgeon, Oxford.
Woodcock, Rev. H., D.D. Canon of Christ Church, Oxford.
Woolcombe, Henry, President of the Plymouth Institution, Plymouth.
*Woolgar, John Webb, F.R.A.S. Lewes.
*Woolley, William, Solicitor, Hull.
Wootten, John, M.D. Balliol College, Oxford.
Wright, T. G., M.D. Beverley, Yorkshire.
Wright, William, Richmond, Yorkshire.
Wynter, Rev. P., D.D. President of St. John's College, Oxford.
Yates, John Ashton, F.G.S. Dinglehead, near Liverpool.
*Yates, Joseph Brooks, F.S.A. West Dingle, near Liverpool.
*Yates, James, M.A. F.L. & G.S. Secretary of the Philological Society, 49, Upper Bedford Place, Russell Square, London.
York, His Grace Edward, Archbishop of, Bishopthorpe, York.
Young, Rev. John, D.D. M. R. Asiatic Society, Warwick House, Cheltenham.
Young, Richard, M.A. New College, Oxford.
Younge, Robert, F.L.S. Sheffield.
Printed by RICHARD TAYLOR, Red Lion Court, Fleet Street.